Abstract
The harmful algal bloom (HAB)-forming dinoflagellate Karenia mikimotoi was exposed to different nitrogen (N) conditions, in order to study the population growth, temporary cyst production and cellular biochemical compositions in laboratory. The results indicated the population growth of K. mikimotoi was inhibited by different levels of N starvation but showed similar fast recovery after the resupplement of N, and temporary cysts were induced in the period of N starvation. K. mikimotoi grew well in inorganic (NO3-, NO2- and NH4+) and organic (urea) nitrogen sources, but the growth parameters (K, Tp, r) showed differences when simulated by Logistic model regressions. When the cellular organic compounds were measured simultaneously, K. mikimotoi cultured in urea produced more short-chained fatty acids while K. mikimotoi cultured in NH4+ produced more non-fatty acids compounds, indicating the potential change of toxins production cultured by various N sources. We concluded that K. mikimotoi could adapt to fluctuating N environments typical of coastal environments including total N concentration (deficiency or recovery) and relative compositions (different N sources).
Introduction
In the past several decades, the increased frequency and distribution of harmful algal blooms (HABs) in marine ecosystems has attracted worldwide research attention [1, 2, 3]. The heightened occurrence of HABs is primarily caused by eutrophication, which closely links human activities such as increased energy demand of the human population, elevated usage of nitrogen (N) and phosphate (P) fertilizers and expanded aquaculture industries [4, 5]. As the most essential and frequent limiting nutrients, the concentration of N, P and N/P ratios will influence the population dynamics and the physiology of harmful algae at the species level [3, 6]. It is commonly accepted that N limitation (NO3-, NO2-, NH4+ and organic N (e.g., urea and amino acids)) occurs more frequently in marine ecosystems compared to freshwater ecosystems [7, 8, 9]. In coastal waters the increased concentration of the urea and reduced N forms is primarily due to human influence. Urea can account for over 50% of the total N input in various coastal areas, thus the role of multiple N forms on the proliferation of HABs becomes more important than a single N form [3, 10, 11, 12].
Dinoflagellates are specialized to adapt to unfavorable environments, by forming cysts, leading to diverse competitive abilities for these species [13, 14, 15]. Dinoflagellates are capable of forming both resting and temporary cysts, with resting cysts formed via sexual reproduction and temporary cysts from asexual reproduction. Both cyst types may be induced by physical disturbance, extreme temperature fluctuations, darkness, bacteria, nutrient stress etc. [15, 16, 17]. Compared to resting cysts, temporary cysts can quickly transition to vegetative cells when the ambient environments become favorable [14]. The encystment of dinoflagellates has been considered an important role in the maintenance of algal blooms during fluctuating environmental factors [18, 19, 20]. Marasović (1989) found temporary cysts of Gonyaulax polyedra Stein formed in Kaštela Bay (located at the eastern Adriatic coast) when environmental conditions changed dramatically, which was important for the persistence and recurrence of the blooms of this species. Garcés et al. (1998) reported that temporary cysts could play a pivotal role in the maintenance of massive blooms of Alexandrium taylori Balech in the north-west Mediterranean coast [21, 22].
K. mikimotoi is a common harmful algal species in worldwide marine ecosystems, especially in Asian areas [23, 24]. The occurrence of K. mikimotoi blooms have been reported along the west coast of Japan, east and south coasts of China, south Atlantic and east coast of USA and European coastse [1, 23, 25]. K. mikimotoi is evidenced to release hemolytic toxins and ichthyotoxins which threaten local fisheries and the health of the food web [26, 27]. Former studies indicated different nitrogen sources for HAB species could lead to the release of different toxins [28, 29]. As mentioned above, phytoplankton in coastal areas are not only experiencing enhanced eutrophication, but also changes in nutrient composition. The adaptation of K. mikimotoi to varying N conditions in relation to the occurrence of K. mikimotoi blooms, requires further investigation. In laboratory controlled conditions, we studied the response of K. mikimotoi to N starvation and recovery utilizing different forms of N. To determine how changing N conditions influence the K. mikimotoi bloom formation and toxin production, we analyzed population dynamics, formation of temporary cysts and fatty acid composition.
Material and methods
Experimental microalgal species of K. mikimotoi was provided by the Algal Center of the Institute of Oceanography, Chinese Academy of Sciences. This strain of K. mikimotoi was originally isolated from East China Sea (ECS) and it is clonal. K. mikimotoi was cultured with pre-filtered natural seawater from coastal Qingdao (pH: 8.0±0.2; salinity: 30), enriched with f/2 nutrients, trace elements and vitamins and sterilized (30 minutes, 121°C) before innoculation [30]. The irradiance and temperature in the incubator were 75 μmol photons m-2 s-1 and 19±1°C (respectively), under a light: dark cycle of 12h: 12h. 250-mL Erlenmeyer flasks were used for culturing species and the population was maintained at exponential growth phase via semi-continuous culture mode before experiments.
N starvation and recovery experiments
Six treatments were designed with different initial nitrogen (NO3-) concentrations for the N starvation experiment (Table 1). All the treatments were performed in triplicate. The initial cell concentrations for K. mikimotoi were 1×104 cells mL-1, and the culturing period to the stationary phase was around 20 days. The recovery experiment began at the termination of the starvation experiment. At the end of the N starvations experiment cultures were taken out and diluted to 1×104 cells mL-1 with nutrient replete f/2 medium. Cell densities were counted daily and cellular chl a concentration were measured at different time points during both the starvation and recovery experiments.
Table 1. Different N: P rations (mol: mol) in N starvation experiments.
| Treatment | NO3- (μmol L-1) | PO43- (μmol L-1) |
|---|---|---|
| 0 | 0 | 36 |
| N:P = 1:1 | 55 | 36 |
| N:P = 2:1 | 110 | 36 |
| N:P = 4:1 | 220 | 36 |
| N:P = 8:1 | 440 | 36 |
| control | 880 | 36 |
Microalgal cells were counted using a hemocytometer under a microscope OLYMPUS CX31, shape and size were observed and recorded using an inverted microscope (IX51 Olympus, Japan) to monitor the formation of temporary cysts. For spectrophotometric chl a measurements, 20 mL cultures were filtered onto GF/F glass fiber filters (Whatman) and pigments were extracted with 90% cold acetone overnight in the dark at -20°C [31].
Varying N source experiments
For this experiment, we applied four different forms of N sources, including NaNO3, NaNO2, NH4Cl and (NH2)2CO (urea). The N, P, trace metals and vitamins concentrations were set to that of f/20 media (N: 88.23μmol L-1, P: 3.6μmol L-1) [32]. All treatments were performed in triplicate and cultured to the stationary phase (around 20 days). The initial cell density and the culture volume were set to 1.0×104 cells mL-1 and 250 mL, respectively. Cell numbers were counted daily using a hemocytometer.
At the end of the experiment cells were collected to measure cellular fatty acids (FA) compositions. For FA analysis, 150–200 mL of culture was centrifuged to form pellets. The pellets were collected and stored in -20°C until Gas Chromatography—Mass Spectrometer (GC-MS) analysis. The cell pellets were broken by ultra—sonication (33kHz, 300W, 30 mins) in a solvent of ethyl acetate and petroleum ether (v: v = 1:1) to extract the FAs. This procedure was repeated three times and the extract was transferred to thick-walled glass tubes with Teflon-lined screw caps. Samples were then concentrated by centrifugation (4500 rpm, 15 mins). The supernatant was gently removed and the remaining liquid was evaporated under a stream of nitrogen gas. The resulting pellets containing algal FAs were converted to FA methyl esters (FAMEs) with the addition of 1 mL KOH-CH3OH (0.5 mol L-1), sealed with Teflon-lined screw caps under a stream of nitrogen gas and saponified for 15 mins at 70°C. After cooling, 1 mL boron trifluoride was added to each sample, followed by sealing, shaking and methanolysis for 30 mins at 70°C. 2.0 mL hexane and 2.0 mL saturated sodium chloride were added to each sample after the additional cooling step. The supernatant was collected and evaporated with nitrogen gas. The resulting 1 mL samples were stored at -80°C until GC-MS was conducted with an Agilent 5975C-7890A GC-MS instrument.
Statistics
All the statistical analyses were performed with SPSS16.0 and figures were plotted by Sigmaplot 12.3. The means and standard errors (S.E.) in the figures and tables were calculated from the triplicates for each treatment. Growth curves were regressed by the Logistic growth model [33] (Eq 1). Temporary cysts production rate (PC) and cell inhibition rate (PD) under N starvation were calculated according to the method of Fistarol et al. [34] from Eqs 2–5.
One-way analysis of variance (one-way ANOVA) was used to determine significance among treatments, in which LSD test was used to group-paired significance after the test of variance homogeneity.
| Equation 1 |
where Nt is the cell density at time t (×104 cells·mL-1); K is the carrying capacity of the population (×104 cells·mL-1); t is the time (day); a is a constant equalling to ln (K-N0)/ln (N0);N0 is the initial cell density of the population (×104 cells·mL-1); r is the intrinsic growth rate or the maximum specific growth rate (day-1).
| Equation 2 |
where NIcont is the intact cell number in the control; Nnde is the intact cell number in the treated group; TA is the affected cell number because of the environmental nutrient changes.
| Equation 3 |
where C is the number of temporary cysts; D is the decreased amount of cell number due to N deficiency.
| Equation 4 |
where PD is the cell inhibition rate.
| Equation 5 |
where PC is percentage of cysts production.
Results
Population growth when exposed to N starvation and recovery conditions
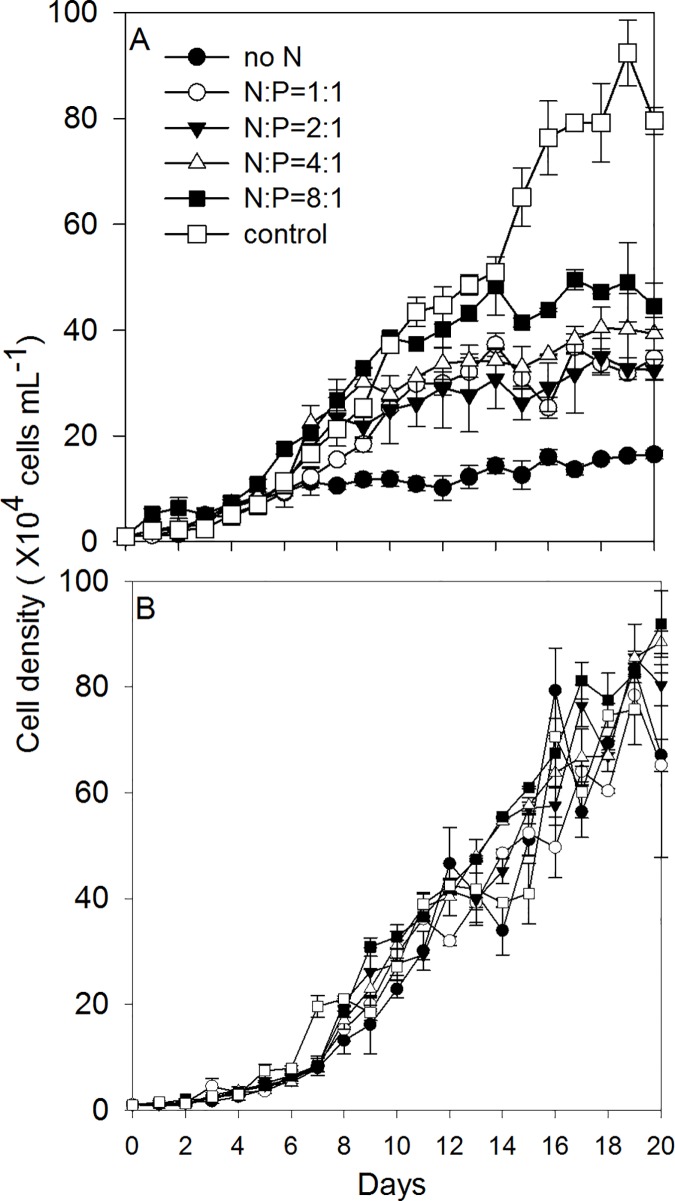
The population growth of K. mikimotoi under different N/P ratios and the recovery growth after the resupplement of N are shown in Fig 1. It took 20 days for the population to reach the stationary phase in both experiments. The population growth was significantly inhibited by N deficiency with the initial N concentration ranged from 0 μmol L-1 to 440 μmol L-1. The lower initial N concentration exhibited more inhibited population growth (Fig 1A). We calculated the regressed parameters by the Logistic model to quantify the growth dynamics in different treatments (Table 2). Based on the calculated Logistic parameters, the carrying capacity (K) values in all N deficient treatments were significantly lower (14.8 cells mL-1) than the control group (94.5 cells mL-1) (one-way ANOVA, F = 5.923, P = 0.006) (Table 2). The inflection time point (Tp; where population growth rate started to decline), showed a decrease in the lower initial N concentration treatments (day 5.7-day 7.3) compared to the control treatment (day 12.2) (Table 2). The maximum specific growth rate (r) did not vary significantly between treatments (one-way ANOVA, F = 1.933, P = 0.162) (Table 2).
Fig 1.
The population growth of K. mikimotoi under the N starvation condition (a) and the recovery condition (b) in different N: P ratios.
Table 2. The regressions of the Logistic model on K. mikimotoi population growth when exposed to N starvation conditions.
| Population growth parameters | Growth equation | Carrying capacity K (×104 cells mL-1) | r (d-1) | Tp (d) | R2 |
|---|---|---|---|---|---|
| No N | N = 14.82/(1+e(3.364–0.591t)) | 14.82* | 0.591 | 5.7 | 0.914 |
| N:P = 1:1 | N = 31.36/(1+e(3.623–0.577t)) | 31.36* | 0.578 | 6.2 | 0.975 |
| N:P = 2:1 | N = 33.89/(1+e(3.577–0.515t)) | 33.89* | 0.515 | 6.9 | 0.961 |
| N:P = 4:1 | N = 37.33/(1+e(3.533–0.498t)) | 37.33* | 0.498 | 7.0 | 0.975 |
| N:P = 8:1 | N = 47.05/(1+e3.263–0.444t)) | 47.05* | 0.444 | 7.3 | 0.985 |
| N:P = 16:1 | N = 94.51(1+e(3.767–0.308t)) | 94.51 | 0.308 | 12.2 | 0.984 |
* indicated there was a statically significant difference between this treatment and the corresponding control group (P<0.05).
After the replenishment of N, cells in different treatments showed similar recovery growth patterns (Fig 1B). Although the cells were under different levels of N starvation, the calculated Logistic parameters (K, r and Tp) after the N replenishment, were similar across the six treatments (Table 3). Compared to the parameters in the N starvation phase, K values in N = 0, N:P = 1:1, N:P = 2:1, N:P = 4:1 and N:P = 8:1 treatments increased 442.87%,171.23%, 184.64%, 144.82% and 94.25% in the recovery phase, respectively.
Table 3. The regressions of the Logistic model on K. mikimotoi population growth when exposed to N recovery conditions.
| Population growth parameters | Growth equation | Carrying capacity K (×104 cells mL-1) | r (d-1) | Tp (d) | R2 |
|---|---|---|---|---|---|
| No N | N = 80.43/(1+e(4.469–0.352t)) | 80.43 | 0.352 | 12.7 | 0.943 |
| N:P = 1:1 | N = 85.06/(1+e(3.811–0.285t)) | 85.06 | 0.285 | 13.4 | 0.971 |
| N:P = 2:1 | N = 96.47/(1+e(3.898–0.283t)) | 96.47 | 0.283 | 13.8 | 0.982 |
| N:P = 4:1 | N = 91.40/(1+e(3.987–0.310t)) | 91.40 | 0.310 | 12.9 | 0.986 |
| N:P = 8:1 | N = 91.40/(1+e(3.982–0.309t)) | 91.40 | 0.309 | 12.9 | 0.969 |
| N:P = 16:1 | N = 94.51/(1+e(3.581–0.261t)) | 94.51 | 0.261 | 13.7 | 0.998 |
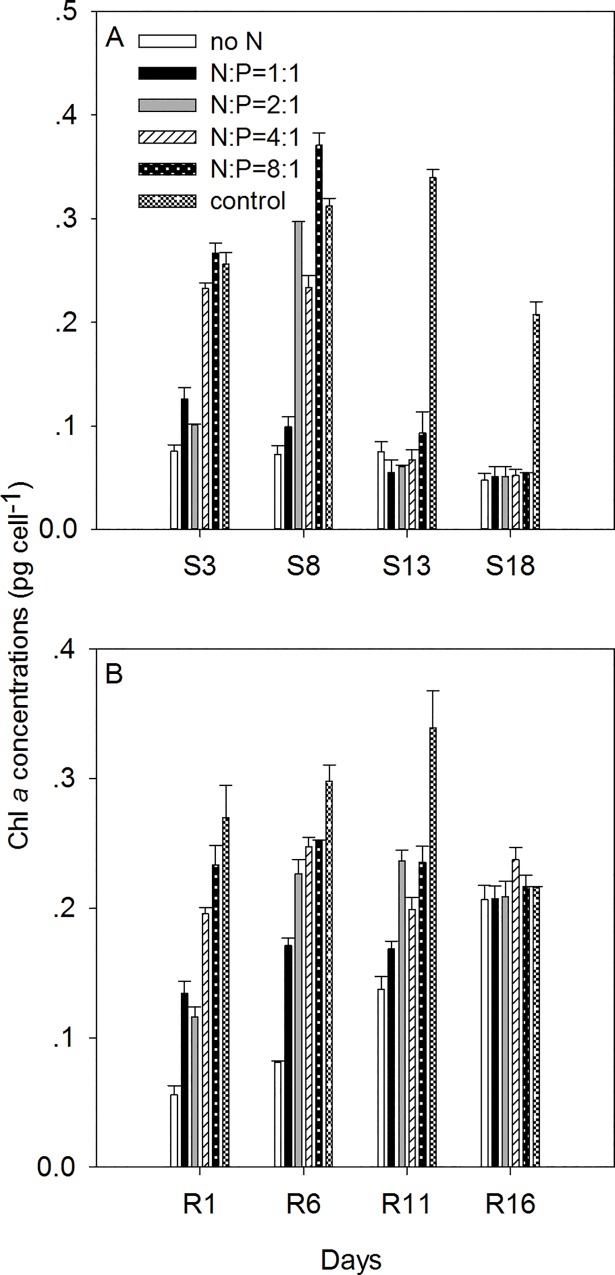
The chl a concentrations of individual cells were measured under the N starvation phase (S1-S18) and the recovery phase (R1-R16) (Fig 2). Chl a concentrations of K. mikimotoi cells were 0.28±0.05 pg cell-1 under normal conditions. In the N starvation phase, chl a concentrations started to decline on day S3 in the treatments N = 0, N:P = 1:1, N:P = 2:1 and N:P = 4:1 (P<0.001 for N = 0, N:P = 1:1, N:P = 2:1 treatments and P = 0.001 for N:P = 4:1 treatment, Fig 2A), while there was no significant change of cellular chl a content in the N:P = 8:1 treatments on day S3 (P = 0.078, Fig 2A). At the end of the experiment (day S18), the cellular chl a concentrations showed similar values in all N starvation treatments, which averaged 74.5% less than the control (P<0.01). In the recovery phase, all treatments recovered to values similar to the control after 16 days. After the replenishment of N, cellular chl a in the treatments of N:P = 1:1, N:P = 2:1, N:P = 4:1 and N:P = 8:1 showed an increase within 1 day (day R1), while chl a contents in the N = 0 treatment did not start to increase until day R6. On day R16, cellular chl a content in all treatments recovered to values similar to the control group (one-way ANOVA, F = 1.097, P = 0.411).
Fig 2. The changes of cellular chl a concentrations in the N starvation phase and the recovery phase under different N/P ratios.
S3-S18 indicated the N starvation phase while R1-R16 indicated the recovery phase.
The production of temporary cysts
The formation of temporary cysts were found in the N = 0 treatment beginning on day 8. Fig 3 showed an example of temporary cysts observed under the microscope, the temporary cysts were much bigger than normal cell size and became round in shape (Fig 3B). Although the cellular structure was not indicated by this photo, it was observed that the color of the cellular contents in the temporary cyst was darker than the normal cell and the cell wall became thick, smooth and transparent (Fig 3). The cell volume was calculated considering the cells as round. The average cellular volume of the temporary cysts was 2485.303±541.6348 μm3, which is ~ 3.5 times bigger than normal cells (730.0461±305.3773 μm3). Cell inhibition rate (PD, %) and temporary cysts production rate (PC, %) on day 8, 11, 14, 17, 20 in the no N treatment were calculated (Table 4). As the culture time increased, the cell inhibition rate increased and cyst production rate decreased (Table 4) resulting in a negative linear relationship (P<0.01, R2 = 0.9848).
Fig 3. The size and shape of temporary cysts of K. mikimotoi in the no N treatment on day 8.
The photo was taken by the inverted microscope (×40 magnification, IX51 Olympus, Japan).
Table 4. The cell inhabition rate (PD, %) and the temporary cysts production rate (PC, %) of K. mikimotoi in the no N treatment during the culture period.
| Time(day) | PD(%) | PC(%) |
|---|---|---|
| 8 | 80.66 | 9.12 |
| 11 | 89.38 | 5.19 |
| 14 | 89.12 | 5.44 |
| 17 | 93.56 | 2.68 |
| 20 | 92.96 | 2.74 |
Population growth under different N sources
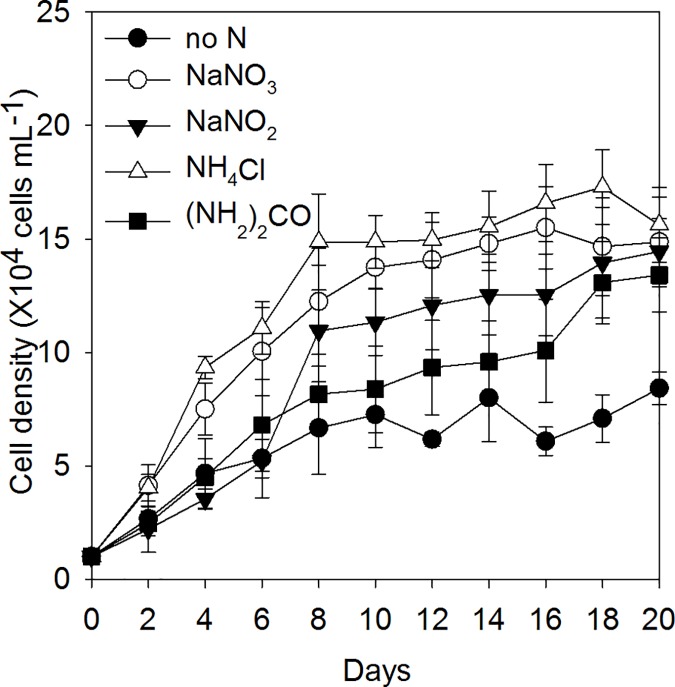
The population growth of K. mikimotoi under various N sources was shown in Fig 4 and the regression of Logistic model is shown in Table 5. Compared to the no N treatment, K. mikimotoi cells grew well in the four N forms, K values were highest in the NH4+ treatment (16.2×104 cells mL-1) and similar to the other three treatments (13.3–14.9×104 cells mL-1) (one-way ANOVA, F = 0.0028, P = 0.998). The NH4+ treatment showed the lowest Tp values and the urea treatment showed the highest Tp values (Table 5). Maximum growth rates (r) in the different treatments of N forms were significantly lower than the no N treatment (one-way ANOVA, F = 6.095, P = 0.009, Table 5). The urea treatment showed the slowest r values and the longest Tp values among the four treatments, indicating a slow growing pattern (Table 5).
Fig 4. The population growth of K. mikimotoi under different N sources.
Table 5. The regression of the logistic model under different nitrogen sources.
| Population growth parameters | Growth equation | Carrying capacity K (×104 cells mL-1) | r (d-1) | Tp (d) | R2 |
|---|---|---|---|---|---|
| No N | N = 7.23/(1+e(2.321–0.727t)) | 7.23 | 0.727 | 3.2 | 0.906 |
| N-NO3- | N = 14.89/(1+e(1.941–0.453t)) | 14.89 | 0.453* | 4.3 | 0.992 |
| N-NO2- | N = 13.93/(1+e(1.964–0.370t)) | 13.93 | 0.370* | 5.3 | 0.925 |
| N-NH4+ | N = 16.21/(1+e(2.052–0.515t)) | 16.21 | 0.515 | 4.0 | 0.978 |
| N-urea | N = 13.35/(1+e(1.612–0.216t)) | 13.35 | 0.216* | 7.6 | 0.937 |
* indicated there was a statically significant difference between this treatment and the corresponding control group (P<0.05).
Cells under different N sources were collected in the stationary phase and analyzed for FA composition by GC-MS. The results indicated both the types and relative compositions of fatty acids were variable under different N sources. We found Tetradecanoic acid (14:0), Hexadecanoic acid (16:0), Octadecapentaenoic acid (18:5ω3) and Octadecanoic acid (18:0) in all the treatments, accounting for 8.29%-11.89%, 24.70%-33.32%, 3.62%-16.61% and 4.38%-6.05% (respectively) of the total measured compounds. Except for the four major fatty acids, we also found Docosahexaenoic acid (DHA), Eicosapentaenoic acid (EPA), other unsaturated fatty acids (eg: 16:1ω7c and 18:2ω6), long-chain alkanes or alkenes, sterols and ketones.
The measured compounds were divided into four groups for easy comparison among the different treatments: 1) free fatty acids, 2) long-chain alkanes and alkenes, 3) sterols and ketones, and 4) other compounds (Table 6). Overall, the group of free fatty acids was the dominant compound in all the treatments but the proportion was much less in the NH4+ treatment (40.61%) compared to the other three treatments (82.13%-84.31%). The free fatty acids include saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA). In the four treatments, SFA accounted for the highest proportion of the total measured compounds, where the proportion was the lowest in the NH4+ treatment. MUFA was not found in the NH4+ treatment but was found in the other three treatments ranging from 6.02%-7.29%. The proportion of PUFA in the NH4+ treatment was ~10 times smaller than the other three treatments, while the proportion of long-chain alkanes or alkenes, sterols or ketones and the other compounds in the NH4+ treatment were much higher than the other three treatments.
Table 6. The classification of organic compounds measured by GC-MS under different nitrogen sources conditions.
| Organic compounds | NaNO3 | NaNO2 | NH4Cl | (NH2)2CO | |
|---|---|---|---|---|---|
| Free fatty acids | SFA | 49.43% | 44.02% | 37.67% | 44.31% |
| MUFA | 6.02% | 7.11% | 0.00% | 7.29% | |
| PUFA | 26.85% | 31.00% | 2.94% | 32.71% | |
| Total | 82.30% | 82.13% | 40.61% | 84.31% | |
| Long-chain alkane or alkene | 14.58% | 15.70% | 38.72% | 13.65% | |
| Sterols or ketone | 0.91% | 0.66% | 4.06% | 0.73% | |
| Others | 2.21% | 1.51% | 16.61% | 1.31% | |
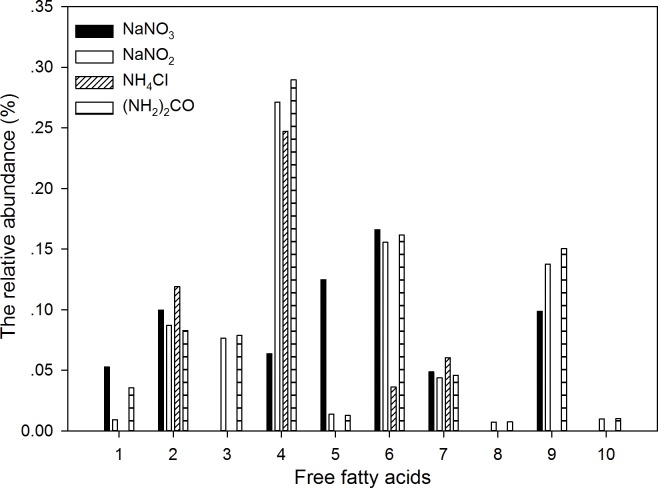
Fig 5 shows the proportions of 10 major free fatty acids out of the total measured organic compounds. In the 10 kinds of free fatty acids, the abundance of Hexadecanoic acid (16:0), Octadecapentaenoic acid (18:5ω3) and 4, 7, 10, 13, 16, 19-Docosahexaenoic acid (22:6ω3) were the highest among all free fatty acids, and the abundance was lower in the NH4+ treatment than the other three treatments. All 10 types of free fatty acids were detected in the NO2- and urea treatments, while only 5 types of free fatty acids were detected in the NH4+ treatment. The proportions of Dodecanoic acid and 9, 12-Octadecadienoic acid, 18:2ω6 in the NO3- treatment were much higher than the other three treatments. Cells in the NO2- and urea treatments had similar proportions for most of the free fatty acids.
Fig 5. The relative abundance (%) of the major free fatty acids in the total organic compounds measured by GC-MS under different nitrogen sources.
The numbers 1–10 indicated 10 kinds of free fatty acids: 1. Dodecanoic acid, 12:0; 2. Tetradecanoic acid, 14:0; 3. 9-Hexadecenoic acid, 16:1ω7c; 4. Hexadecanoic acid 16:0; 5. 9,12-Octadecadienoic acid, 18:2ω6; 6. Octadecapentaenoic acid, 18:5ω; 7. Octadecanoic acid, 18:0; 8. Eicosanoic acid 20:0; 9. 4,7,10,13,16,19-Docosahexaenoic acid 22:6ω3; 10. 5, 8, 11, 14, 17-Eicosapentaenoic acid 20:5ω3.
Discussion
K. mikimotoi blooms often occur in coastal areas where N limitation occurs frequently and N forms are complicated [3, 35–37]. We examined the response of K. mikimotoi to the variable ambient N conditions, and found: 1) the population growth of K. mikimotoi was limited by N starvation and recovered quickly after the replenishment of N; 2) temporary cysts were induced by N starvation; 3) K. mikimotoi grew well in four kinds of N source, but the population growth of K. mikimotoi varied in different forms of N, as well as the relative compositions of cellular organic compounds.
The effects of N starvation and resupplement
Under N starvation, we found inhibition of population growth and degradation of cellular chl a (Figs 1 and 2). As the initial N concentrations in the media decreased, the growth rates of K. mikimotoi population started to decrease earlier in the experiment (entered to Tp earlier), and the upper limit of population capacity (K) decreased (Table 2). The decline of population growth rates was linked to the degradation of proteins under N deficiency [38]. The loss of cellular chl a was also caused by the degradation of proteins (like D1 protein), which contained up to 20% of N [38, 39]. The loss of chl a in the cells, resulted in the decline of photosynthetic rates and carbon fixation abilities thus inhibiting energy production and cell division [40]. Under similar N deficient conditions, Zhao et al. [40] did not find apparent population decrease in the diatom Phaeodactylum tricornutum, indicating the K. mikimotoi population was sensitive to changes of ambient N.
After the replenishment of N, cells in all the treatments showed similar recovery growth patterns with cells under normal conditions (similar logistic growth although the treatments with less initial N in the starvation experiment started to recover later.) On day R1, there was no apparent increase of cellular chl a content in no N treatment but did increase 2.8 times in N: P = 8:1 treatment, indicating more severe N deficiency in cells leading to slower chl a re-synthesis. Different recovery patterns of population growth and cellular chl a indicated the recovery of cell division might be independent of re-synthesis of chl a at the beginning of the recovery phase. The priority to recover cell divisions over cellular constituents was also found in other species, and this is considered a competitive advantage in the natural environment when nutrient status shifts from deficiency to replete [39, 41, 42]. Although fast recovery is found in both population growth and chl a synthesis, neither of them are higher than control values at the end of the recovery experiments, indicating overcompensated growth was not found in our study.
The production of temporary cysts
Limited by the recording technology in the lab, the transformation of temporary cysts to vegetative cells was not recorded, but the expression of cyst formation related genes are planned to be measured in next step in order to provide more precise evidence for the temporary cysts formations. Although dinoflagellate genomes remain largely unknown, Lin et al. [43] found genes involved in sexual reproduction, cyst formation and germination of dinoflagellate Symbiodinium kawagutii, which provided the possibility for us to measure the cyst formation related genes of K. mikimotoi in the future.Temporary cysts were observed in the no N treatment and there was a negative relationship between the temporary cysts production rate and the cell inhibition rate. The induction of K. mikimotoi temporary cysts in lab experiments were reported by Uchida et al. [44] and Ma and Pan [45] in which temporary cysts were induced by the allelopathic effect from other microalgal species.Our study indicated N deficiency also could lead to the production of K. mikimotoi temporary cysts.
In the exponential growth phase (e.g. day 8), the temporary cysts production rate was high while the cell inhibition rate was low, indicating the ability for a fast recovery after the addition of N. On the contrary, there were fewer temporary cysts produced and more cells were inhibited in the stationary phase (e.g. day 20), indicating more a more severe impact to the N deficient cells and less possibility of a fast recovery. Therefore, the process of encystment could be induced by nutrient deficiency but still requires a minimum amount of nutrients. The “threshold effect” of nutrient stress on the encystment of dinoflagellates was also reported by Chen et al. [46] in a study of Akashiwo sanguinea. Cysts production rates in our study were between 2.74% and 9.12%, which are within the ranges of encystment rates as reported in previous studies [18, 46].
The effects of different N sources
Our results showed that K. mikimotoi cultures grew well when treated with four different forms of N sources, all of which reached cell density observed in K. mikimotoi blooms in the natural environment (105 cells mL-1)[47]. Different phytoplankton groups have different uptake rates and affinities to various forms of N, which are related to the enzyme activities and cellular energy budgets [48, 49]. Due to the influence of human activities, N forms are more variable in coastal areas than in the open ocean [6]. It is possible that the increased proportions of reduced N forms could change the phytoplankton community structure and favor the species with high affinities for reduced N forms [3]. Li et al. [37] reported the Vmax-urea of K. mikimotoi was higher than the HABs species Prorocentrum donghaiense. While the Vmax-NH4 of both species were higher than the diatom species in ECS, thus the increase of reduced ambient N forms would play an important role on triggering the proliferation of HABs of these two species [37].
In our study, K. mikimotoi population growth showed a slower growth pattern with the addition of urea compared to the other three N sources. The maximum cell densities of K. mikimotoi population (K) did not vary to other three N sources (one-way ANOVA, F = 0.0028, P = 0.998), indicating K. mikimotoi cells could effectively use urea. The utilization of urea in phytoplankton is a process of active uptake, which involves the participation of specific enzymes. These enzymes are not present in all species [50] as shown in Levasseur et al. [51] who reported cellular N deficiency in several species when urea was used as the only N source.
Similar to other dinoflagellate species, K. mikimotoi population showed the earliest exponential growth and highest carry capacity under NH4+ among four different N sources [37]. Indicating phytoplankton cells consumed the least energy under NH4+ compared to NO3- and urea, which may lead to the fastest population growth in our results [51]. Phytoplankton can directly utilize NH4+ to synthesize amino acids, while the use of NO3- and NO2- requires the participation of nitrate reductase and nitrite reductase, respectively [7]. In the treatments with three inorganic N sources, cells cultured by NO2- showed the smallest K value and the largest Tp value. Sciandra and Amara [52] pointed out the existence of NO3- was necessary to the synthesis of nitrite reductase, therefore the lack of NO3- in the NO2- treatment may lead to slow population growth in the K. mikimotoi cultures. The ability to use four different kinds of N provides further evidence that this population has a high competitive ability in changing coastal environments when the proportions of urea and NH4+ are increasing.
In the treatments of NO3-, NO2- and urea, free fatty acids took account for more than 80% of the total measured organic compounds, when considering the 10 types of major fatty acids measured herein. Cells in the NO2- and urea treatments produced more types of fatty acids than the other two treatments, such as 16:1ω7c, 20:0 and 22:6ω3. Free fatty acids in K. mikimotoi are considered to be sources of toxins, some of which have hemolytic toxicities [53, 54]. Sellem et al. [55] tested the toxic effect of four kinds of PUFA produced by K. mikimotoi, whose results indicated the toxic effects of 18:4ω and 18:5ω were higher than 20:5ω and 22:6 and they concluded that the toxicities of PUFA were inversely proportional to the length of the carbon chain. The proportion of fatty acids less than 18C (group 1–7) in NO3-, NO2-, NH4+ and urea treatments were 55.57%, 65.71%, 46.26% and 70.73%, respectively, indicating the highest potential toxicities of cells when using urea as N sources. This finding is consistent with former studies: Leong et al. [56] who found higher and different toxin concentrations in urea-grown cells of Alexandrium tamarense comparing to NO3—or NH4+-grown cells. Shimizu et al. [57] found the toxin concentrations of Karenia brevis cells with the addition of urea was 6-fold compared to the addition of NO3-. Therefore, the increased input of urea in coastal areas might not only result in increasing the frequency of K. mikimotoi blooms, but also result in the increasing toxicities of K. mikimotoi cells. On the contrary to culturing with the addition of urea, cells cultured with the addition of NH4+ had low proportions of fatty acids and high proportions of small molecular organic compounds, indicating potentially low toxicity of cells compared to culturing with the addition of the other three N sources.
Conclusion
In conclusion, the enhancement of coastal eutrophication changes N dynamics in terms of N: P (N: Si) ratios and its various forms, leading to severe (or alleviated) N limitation and the increased proportions of reduced N and organic N [6, 12]. The formation of temporary cysts under N starvation, fast recovery of population growth after relief of N starvation and the ability to utilize various N sources provide evidence that this strain of K. mikimotoi could take advantage of changing N conditions in coastal areas. Moreover, we found increased urea proportions in total N concentrations have the potential to lead to slow growing but more toxic K. mikimotoi cells, while increased NH4+ might lead to less toxic K. mikimotoi cells but higher cell densities. All the above results can be taken as evidence of evaluation or applied to models in order to evaluate and predict K. mikimotoi blooms in dynamical coastal environments. This study was only based on one stain of K. mikimotoi, whether similar patterns happen to other stains of K. mikimotoi still needs further investigation.
Supporting information
(XLSX)
Acknowledgments
We would like to thank the suggestions for the manuscript writing of Dr. Jamie Steichen, who is from Texas A&M University. Also, we thank the two reviewers whose comments greatly improved the presentation of the findings.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Science Foundation of China, grant number: U 1406403, (http://www.nsfc.gov.cn/). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hallegraeff GM (1993) A review of harmful algal blooms and their apparent global increase. Phycologia 32: 79–99. [Google Scholar]
- 2.Anderson DM, Glibert PM and Burkholder JM (2002) Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuar 25: 704–726. [Google Scholar]
- 3.Glibert PM and Burkholder JM (2011) Harmful algal blooms and eutrophication: “strategies” for nutrient uptake and growth outside the Redfield comfort zone. Chin J Oceanol Limnol 29: 724–738. [Google Scholar]
- 4.Heisler J, Glibert PM, Burkhilder JM, Anderson DM, Cochlan WP, Dennison WC, et al. (2008) Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J, Parker AE, Wilkerson FP and Dugdale RC (2015) Uptake and inhibition kinetics of nitrogen in Microcystis aeruginosa: Results from cultures and field assemblages collected in the San Francisco Bay Delta, CA. Harmful Algae 47: 126–140. [Google Scholar]
- 6.Glibert PM, Harrison J, Heil C and Seitzinger S (2006) Escalating worldwide use of urea-a global change contributing to coastal eutrophication. Biogeochemistry 77: 441–463. [Google Scholar]
- 7.Miller CB (2003) Biological Oceanography. Massachusetts: Blackwell Press. 480p. [Google Scholar]
- 8.Arrigo KR (2005) Marine microorganisms and global nutrient cycles. Nature 437: 349–455. 10.1038/nature04159 [DOI] [PubMed] [Google Scholar]
- 9.Howarth RW and Marino R (2006) Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: evolving views over three decades. Limnol Oceanogr 51: 364–376. [Google Scholar]
- 10.McCarthy JJ (1977) Nitrogenous nutrition of the plankton in the Chesapeake Bay. I. Nutrient availability and phytoplankton preferences. Limnol Oceanogr 22: 996–1011. [Google Scholar]
- 11.Furnas MJ (1983) Nitrogen dynamics in lower Narragansett Bay, Rhode Island. I. Uptake by size-fractionated phytoplankton population. J Plankton Res 5: 657–576. [Google Scholar]
- 12.Glibert PM, Garside C, Fuhrman JA and Roman MR (1991) Time dependent coupling of inorganic and organic nitrogen uptake and regeneration in the plume of the Chesapeake Bay estuary and its regulation by large heterotrophs. Limnol Oceanogr 36: 896–909. [Google Scholar]
- 13.Pfiester LA, and Anderson DM. Dinoflagellate reproduction In: Taylor FJR, editor. The Biology of Dinoflagellates. Oxford: Blackwell Scientific Publications; 1987. pp. 611–648. [Google Scholar]
- 14.Fistarol GO, Legrand C, Rengefors K and Granéli E (2004) Temporary cyst formation in phytoplankton: a response to allelopathic competitors. Envrion Microbiol 6: 791–798. [DOI] [PubMed] [Google Scholar]
- 15.Olli K (2004) Temporary cyst formation of Heterocapsa triquetra (Dinophyceae) in natural populations. Mar Bio 145: 1–8. [Google Scholar]
- 16.Nagasaki K, Yamaguchi M and Imai I (2000) Algicidal activity of a killer bacterium against the harmful red tide dinoflagellate Heterocapsa circularisquama isolated from Ago Bay, Japan. Bull Jpn Soc Sci Fish 66: 666–673. [Google Scholar]
- 17.Garcés E, Masό M and Camp J (2002) Role of temporary cysts in the population dynamics of Alexandrium taylori (Dinophyceae). J Plankton Res 24: 681–686. [Google Scholar]
- 18.Anderson DM and Lindquist NL (1985) Time-course measurements of phosphorus depletion and cyst formation in the dinoflagellate Gonyaulax tamarensis Lebour. J Exp Mar Bio Ecol 86: 1–13. [Google Scholar]
- 19.Olli K and Anderson DM (2002) High encystment success of the dinoflagellate Scrippsiella cf. lachrymosa in culture experiments. J Phycol 38: 145–156. [Google Scholar]
- 20.Tang YZ and Gobler CJ (2012) The toxic dinoflagellate Cochlodinum polykrikoides (Dinophyceae) produces resting cysts. Harmful Algae 20: 71–80. [Google Scholar]
- 21.Marasović I (1989) Encystment and excystment of Gonyaulax polyedra during a red tide. Estuar Coast Shelf Sci 28: 35–41. [Google Scholar]
- 22.Garcés E, Delgado M, Masó M and Camp J (1998) Life history and in situ growth rates of Alexandrium taylori (Dinophyceae, Pyrrophyta). J Phycol 34: 880–887. [Google Scholar]
- 23.Takayama H and Adachi R (1984) Gymnodinium nagasakiense sp. now, a red tide forming dinophyte in the adjacent waters of Japan. Bull Plankton Soc Jpn 31: 7–14. [Google Scholar]
- 24.Nézan E, Siano R, Boulben S, Six C, Bilien G, Chѐze K, et al. (2015) Genetic diversity of the harmful family Kareniaceae (Gymnodiniales, Dinophyceae) in France, with the description of Karlodinium gentienii sp. nov.: A new potentially toxic dinoflagellate. Harmful Algae 40: 75–91. [Google Scholar]
- 25.Gentien P, Lunven M, Lazure P, Youenou A and Crassous P (2007) Motility and autotoxicity in Karenia mikimotoi (Dinophyceae). Phil Trans R Soc Lond B Biol Sci 362: 1937–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang ZB and Hodgkiss IJ (2004) Hong Kong’s worst “red tide”: causative factors reflected in a phytoplankton study at Port Shelter station in 1998. Harmful Algae 3: 149–361. [Google Scholar]
- 27.Lei QY and Lu SH (2011) Molecular ecological responses of the dinoflagellate Karenia mikimotoi to phosphate stress. Harmful Algae. 12: 39–45. [DOI] [PubMed] [Google Scholar]
- 28.Parrish CC, Bodennec G and Gentien P (1998) Haemolytic glycoglycerolipids from Gymnodinium species. Phytochemistry 47: 783–787. [DOI] [PubMed] [Google Scholar]
- 29.Leong S, Murata A, Nagashima Y, Taguchi S (2004) Variability in toxicity of the dinoglagellate Alexandrium tamarense in response to different nitrogen sources and concentration. Toxicon 43(4): 407–415. 10.1016/j.toxicon.2004.01.015 [DOI] [PubMed] [Google Scholar]
- 30.Guillard RRL. Culture of phytoplankton for feeding marine invertebrates In: Smith WL, Chanley MH, editors. Culture of Marine Animals. New York: Plenum Press; 1975. pp. 26–60. [Google Scholar]
- 31.Parsons TR, Maita Y and Lalli CM (1984) A Manual of Chemical and Biological Methods for Seawater Analysis 1st ed. Oxford: Pergamon Press. 184p. [Google Scholar]
- 32.Thessen AE, Bowers HA and Stoecker DK (2009) Intra- and interspecies differences in growth and toxicity of Pseudo-nitzschia while using different nitrogen sources. Harmful algae 8: 792–810. [Google Scholar]
- 33.Wang Y and Tang X (2008) Interactions between Prorocentrum donghaiense Lu and Scrippsiella trochoidea (Stein) Loeblich III under laboratory culture. Harmful Algae 7: 65–75. [Google Scholar]
- 34.Fistarol GO, Legrand C, Rengefors K and Granélil E (2004) Temporary cyst formation in phytoplankton: a response to allelopathic competitors? Environmen Microbiol 6: 791–798. [DOI] [PubMed] [Google Scholar]
- 35.Zhou MJ, Zhu MY and Zhang J (2001) Status of harmful algal blooms and related research activities in China. Chin Bull Life Sci 13: 54–59. (in Chinese with English abstract). [Google Scholar]
- 36.Li J, Glibert PM, Zhou M, Lu S and Lu D (2009) Relationships between nitrogen and phosphorus forms and ratios and the development of dinoflagellate blooms in the East China Sea. Mar Eco Pro Ser 383: 11–26. [Google Scholar]
- 37.Li J, Glibert PM and Zhou M (2010) Temporal and spatial variability in nitrogen uptake kinetics during harmful dinoflagellate blooms in the East China Sea. Harmful Algae 9: 531–539. [Google Scholar]
- 38.Geider RJ, La Roche J, Greene RM and Olaizola M (1993) Response of the photosynthetic apparatus of Phaeodactylum tricornutum (Bacillariophyceae) to nitrate, phosphate, or iron starvation. J Phycol 29: 755–766. [Google Scholar]
- 39.Zhao Y, Quigg A and Wang Y (2015) The 24 hour recovery kinetics from N starvation in Phaeodactylum tricornutum and Emiliania huxleyi. J Phycol 51: 726–738. 10.1111/jpy.12314 [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y, Quigg A and Wang Y (2015) Comparison of population growth and photosynthetic apparatus changes in response to different nutrient status in a diatom and a coccolithophore. J Phycol 51: 872–884. 10.1111/jpy.12327 [DOI] [PubMed] [Google Scholar]
- 41.Greene RM, Geider RJ, Kolber Z and Falkowski PG (1992) Iron-induced changes in light harvesting and photochemical energy conversion process in eukaryotic marine algae. Plant Physiol 100: 565–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young EB and Beardall J (2003) Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and recovery cycle. J. Phycol. 39: 897–905. [Google Scholar]
- 43.Lin S, Cheng S, Song B, Zhong X, Lin X, Li W, et al. (2015) The Symbiodinium kawagutii genome illuminates dinoflagellate gene expression and coral symbiosis. Science 350(6261): 691–694. 10.1126/science.aad0408 [DOI] [PubMed] [Google Scholar]
- 44.Uchida T, Toda S, Matsuyama Y and Honjo T (1999) Interaction between the red tide dinoflagellates Heterocapsa circularisquama and Gymnodinium minimotoi in laboratory culture. J Exp Mar Bio Eco 241(2): 285–299. [Google Scholar]
- 45.Ma L and Pan K (2010) Inhibitory effects of three microalgae on the growth of Karenia mikimotoi. Mar Envrion Sci 29(6):794–798 (in Chinese with English abstract). [Google Scholar]
- 46.Chen Y, Tian Y, Yu R and Zhou M (2011) Toxic effects of Karenia mikimotoi extracts on mammalian cells. Chin J Oceanol Limnol 29: 860–868. [Google Scholar]
- 47.Long H and Du Q (2005) Primary research on Karenia mikimotoi bloom in Fujian coast. J Fujian Fish 4: 22–26 (in Chinese with English abstract). [Google Scholar]
- 48.Fan C, Glibert P, Alexander J and Lomas MW (2003) Characterization of urease activity in three marine phytoplankton species, Aureococcus anophagefferens, Prorocentrum minimum, and Thalassiosira weissflogii. Mar Bio 142: 949–958. [Google Scholar]
- 49.Develi E, Kidey A and Tugrul S (2006) Effect of nutrients on culture dynamics of marine phytoplankton. Aquat Sci 68: 28–39. [Google Scholar]
- 50.Antia NJ, Harrison PJ and Oliveira L (1991) The role of dissolved organic nitrogen in phytoplankton nutrition, cell biology, and ecology. Phycologia 30: 1–89. [Google Scholar]
- 51.Levasseur M, Thompson PA and Harrison P (1993) Physiological acclimation of marine phytoplankton to different nitrogen sources. J Phycol 29: 587–595. [Google Scholar]
- 52.Sciandra A and Amara R (1994) Effects of nitrogen limitation on growth and nitrite excretion rates of the dinoflagellate Prorocentrum minimum. Mar Ecol Prog Ser 105: 301–309. [Google Scholar]
- 53.Parrish CC, Bodennec G, Gentien P (1998) Haemolytic glycoglycerolipids from Gymnodinium species. Phytochemistry 47: 783–787. [DOI] [PubMed] [Google Scholar]
- 54.Arzul G, Gentien P, Bodennec G, Toularastel F, Youenou A and Crassous M.P. Comparison of toxic effects in Gymnodinium cf. nagasakiense polyunsaturated fatty acids In: Lassus G, Arzul G, Erard E, Gentine P, Marcaillou C, editors. Harmful Marine Algal Blooms. Technique et Documentation. Paris: Lavoisier, Intercept Ltd, 1995, pp. 395–400. [Google Scholar]
- 55.Sellem F, Pesando D, Bodennec G, Abed AE and Girard J (2000) Toxic effects of Gymnodinium cf. Mikimotoi unsaturated fatty acids to gametes and embryos of the sea urchin Paracentrotus lividus. Wat Res 34: 550–556. [Google Scholar]
- 56.Leong SCY, Murata A, Nagashima Y and Taguchi S (2004) Variability in toxicity of the dinoflagellate Alexandrium tamarense in response to different nitrogen sources and concentrations. Toxicon 43(4): 407–415. 10.1016/j.toxicon.2004.01.015 [DOI] [PubMed] [Google Scholar]
- 57.Shinizu Y, Watanabe N and Wrensford G. Biosynthesis of brevetoxins and heterotrophic metabolism in Gymnodinium brevis In: Lassus P, Arzul G. Erard-Le-Denn E, Gentien P, Marcaillou C, editors. Paris: Harmful Marine Algal Blooms, Paris: Lavoisier Publishing; 1993. pp. 351–357. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.