The main hot spots of marine biodiversity are also the areas that are affected the most by climate and, potentially, fishing impacts.
Keywords: climate change, climate and human threats, environmental variability, human fisheries, marine biodiversity, remote sensing
Abstract
Human activities drive environmental changes at scales that could potentially cause ecosystem collapses in the marine environment. We combined information on marine biodiversity with spatial assessments of the impacts of climate change to identify the key areas to prioritize for the conservation of global marine biodiversity. This process identified six marine regions of exceptional biodiversity based on global distributions of 1729 species of fish, 124 marine mammals, and 330 seabirds. Overall, these hot spots of marine biodiversity coincide with areas most severely affected by global warming. In particular, these marine biodiversity hot spots have undergone local to regional increasing water temperatures, slowing current circulation, and decreasing primary productivity. Furthermore, when we overlapped these hot spots with available industrial fishery data, albeit coarser than our estimates of climate impacts, they suggest a worrying coincidence whereby the world’s richest areas for marine biodiversity are also those areas mostly affected by both climate change and industrial fishing. In light of these findings, we offer an adaptable framework for determining local to regional areas of special concern for the conservation of marine biodiversity. This has exposed the need for finer-scaled fishery data to assist in the management of global fisheries if the accumulative, but potentially preventable, effect of fishing on climate change impacts is to be minimized within areas prioritized for marine biodiversity conservation.
INTRODUCTION
The exponential rise of atmospheric greenhouse gas concentrations over the past 30 years has increased the average global temperature by 0.2°C per decade (1). Most of this extra heat is being absorbed by the world’s oceans, particularly by their upper layers (2), with the mean global sea surface temperature (SST) increasing by approximately 0.4°C since the 1950s (3). The warming of the oceans drives greater stratification of the water column, thereby reducing mixing in some parts of the ocean, which affects oxygen (4) and nutrient availability (5) and, hence, primary production (2, 6, 7) and the ecophysiology of water-breathing organisms (8). The increase in water temperatures is, however, unevenly distributed spatially (9, 10) and, together with increased meltwater and discharged ice from terrestrial glaciers and ice sheets, influences the behavior of ocean currents, which play critical roles in the dynamics, local climates, and biology of the ocean (11, 12). Coincidentally with these environmental changes, industrial fisheries have resulted in the overexploitation and decimation of about 70% of world fish stocks (13), resulting in changes to fish communities and marine ecosystems since the Second World War (14, 15). Both climatic and human pressures can lead to shifts in the size, structure, spatial range, and seasonal abundance of populations (9, 16–18), which, in turn, may alter trophic pathways from primary producers to upper-trophic levels, propagating changes throughout ecosystems in both bottom-up and top-down directions (8, 18–20). Accordingly, climate and fishing impacts should not be treated in isolation from each other when it comes to conservation of marine biodiversity (21).
Despite the scale of these perturbations, our understanding of how environmental variability—driven by climate change and human activities—affects marine ecosystems has lagged far behind our knowledge of their impacts in terrestrial ecosystems (22). In part, this is because there is considerable uncertainty regarding the spatial and temporal details of these impacts in marine environments (23, 24). Satellite remote-sensing records have emerged as important tools for studying the most recent (up to three decades) and striking trends and patterns in both environmental (for example, SST or ocean currents) and biological (marine productivity) variables in the world’s oceans at unprecedented spatiotemporal resolutions (7, 10, 25). Additionally, worldwide fishing records (for example, annual landings) are available from the 1950s onward (see www.fao.org/fishery/en), albeit at a poorer spatial resolution [but see the study by Watson et al. (26) and www.seaaroundus.org for spatial disaggregation of the Food and Agriculture Organization (FAO) fishery data], providing important information to assist in a global assessment of the major human harvest that affects marine ecosystems (13, 15, 26). Analyzed together, these data provide the most detailed insights, to date, into the spatiotemporal distribution of environmental and human stressors threatening marine communities. However, few studies to date have analyzed the data on a fine-enough scale to identify the specific marine areas that, globally, are most at risk from climate change (9, 10) and exploitation from fisheries (26); none of them have combined these measurements of climate and human impacts with those for species distribution globally to identify hot spots of marine diversity that can be targeted for conservation at the local, regional, and global scale.
By overlaying the most spatially explicit measurements of marine biodiversity available globally with the finest-scale measurements for the cumulative impacts from climate change and the FAO data on fishing pressure, we aimed to identify the areas of highest conservation priority within our planet’s marine environment. In particular, (i) we compiled a species-level database recording the global distribution of 2183 marine species to identify hot spots of marine biodiversity; (ii) we derived spatially explicit information on the cumulative impact of climate change by combining more than three decades’ worth of information on SST, oceans currents, and marine productivity [that is, chlorophyll a concentration (CHL)]; (iii) we evaluated changes in fishing captures over the last 60 years to examine temporal trends in the exploitation of marine resources worldwide. We also identified countries that have contributed the most to fishing pressure at marine hot spots over the last decade, highlighting those fishing outside their Exclusive Economic Zone (EEZ).
RESULTS AND DISCUSSION
Temporal trends for SST, CHL, and ocean currents (table S1) were explored through least-squares linear regressions of annual information on a pixel basis covering the world’s oceans (fig. S1). Absolute values for the obtained slopes (a proxy to the magnitude of environmental changes occurring on a pixel basis) were combined to produce a single index of cumulative impacts of equally weighted changes in targeted oceanographic features (hereafter Cumulative Impact Index) with values ranging from 0 (no change) to 1 (maximum change). Overall, our Cumulative Impact Index indisputably reveals the uneven distribution of environmental changes in Earth’s oceans, with the most striking changes occurring at the poles and the tropics (Fig. 1). In northern regions, the main changes concern not only the North Sea but also those areas connected by the cold waters of the Labrador Current flowing southward along the eastern coast of Greenland and North America. These changes are largely driven by an increase in SST (fig. S1), likely caused by the increasing temperatures of the northward-flowing Atlantic waters arriving in the Arctic (27). As expected for warmer waters with increased vertical stratification and lower nutrient supply (7), we observed a general reduction in CHL values (fig. S1), a trend that may be exacerbated in the polar region by an increased influx of fresh water from melting sea ice (2, 28, 29) [but see the study by McQuatters-Gollop et al. (24)]. Similarly, large areas enclosing coastal waters of the North Pacific Ocean Basin from the Bering Sea to the East China Sea have also recorded large increases in SST with consequent decreases in CHL [fig. S1; see also the studies by Behrenfeld et al. (7) and Boyce et al. (29)]. Overall, the South Atlantic Ocean Basin has apparently experienced a gradual decline in marine productivity (29). However, we detect a large degree of spatial variability mainly as a consequence of the high rate of increase in CHL observed for the southeastern and southwestern Atlantic (fig. S1), patterns that might be associated with seafronts prevalent at these areas (7, 30). Spatial heterogeneity is also apparent with regard to changes in SST of this ocean basin. Water temperature has experienced a positive, but marginal, change in temperate regions. Southernmost seawater has become slightly colder, particularly in areas close to Tierra del Fuego and the Antarctic continent (31). Increasing water temperatures have also been very pronounced around the Australian continent (32), a trend that is probably driven by changes in the East Australian Current transporting hot water southward from the tropics to mid-latitudes (fig. S1).
Fig. 1. Global distribution of cumulative environmental impacts.
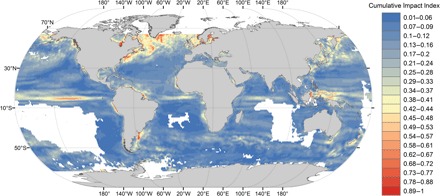
Index of cumulative impact of equally weighted changes in SST, CHL (a proxy to primary productivity), and ocean currents. Colors represent a dimensionless index of global impact (Cumulative Impact Index) ranging from 0 (no change) to 1 (maximum change), providing a measure of spatial heterogeneity in the magnitude of environmental changes and highlighting those marine areas that have undergone the largest changes in their environmental conditions.
Overall, observed changes in ocean circulation agree with the slowing of the global thermohaline circulation, resulting from the disproportionate heating at Earth’s polar regions: This encompasses all ocean basins and likely influences global climate (23) and marine productivity (7, 11, 28, 33). Accordingly, with a reorganization of the Atlantic Meridional Overturning Circulation (34), the substantial decrease in water speed (for both the eastern and northern components) that we detected for the Labrador Current (fig. S1) may contribute to the observed decline in marine productivity in the North Atlantic (28). Further, we detected a deceleration in the South Atlantic Gyre that affects both sides of the South Atlantic Ocean Basin (that is, the Brazil Current and the Benguela Current) and flows counterclockwise between the 15°S and the 40°S latitudes. The Malvinas Current that flows northward from Cape Horn along the Patagonian coast of Argentina has also decelerated, as has also occurred for the Antarctic Circumpolar Current flowing eastward ca. 40°S throughout the South Atlantic Ocean and the Indian Ocean. In the tropics, there have also been striking changes to ocean circulation, with increasing water speeds for the eastward Equatorial Countercurrent and with reversed trends for the North Equatorial Current and South Equatorial Current flowing westward ca. 10°N and 10°S latitudes, respectively (fig. S1).
Similar to the observed changes in oceanographic features, marine species are heterogeneously distributed (35–38). This raises the question: Do environmental stressors affect marine areas of enhanced biodiversity? On the basis of the worldwide distribution (the presence within an area) of 1729 species of fish, 124 marine mammals, and 330 seabirds, we produced a dimensionless index of biodiversity based on the equally weighted distribution of species richness for targeted taxa. This index allowed us to identify six different hot spots of marine biodiversity that concentrate in the Southern Hemisphere and include marine areas in temperate and tropical regions of the Atlantic Ocean, Indian Ocean, and Pacific Ocean (Fig. 2). The westernmost area includes the central-western Pacific waters of Peru and the Galápagos Archipelago. In the southwestern Atlantic Ocean, a marine hot spot occurs in the Patagonian waters of Argentina and Uruguay. The coasts of South Africa, Mozambique, Tanzania, Kenya, and Madagascar were included in a hot spot at the western side of the Indian Ocean. In the central-western Pacific Ocean, a large area including water masses surrounding Indonesia, Malaysia, Philippines, Papua New Guinea, Taiwan, and the south of Japan was grouped into one single hot spot. Waters surrounding New Zealand and Eastern and Southern Australia were considered the fifth hot spot at the southwestern Pacific; the sixth hot spot included marine areas in Oceania and the central Pacific Ocean. Overall, all these areas have experienced environmental perturbations. However, we also detected a large degree of spatial variability in observed environmental impacts at the local scale and the mesoscale (Fig. 2 and fig. S1). The most striking changes have occurred in the central-western Pacific and the southwestern Atlantic hot spots as a consequence of significant changes in SST and CHL (Fig. 2). While the first hot spot has largely undergone local to regional increases in both SST and CHL, the latter hot spot has been characterized by a huge decrease in SST over the last three decades (Fig. 2 and fig. S1), a phenomenon that may be partially driven by natural modes of climate variability, that is, El Niño/Southern Oscillation (7, 39, 40). In general, most of the other hot spots for marine biodiversity have experienced rising trends in SST but remain quite stable in terms of CHL or ocean circulation (Fig. 2).
Fig. 2. Environmental stressors affecting hot spots of marine biodiversity.
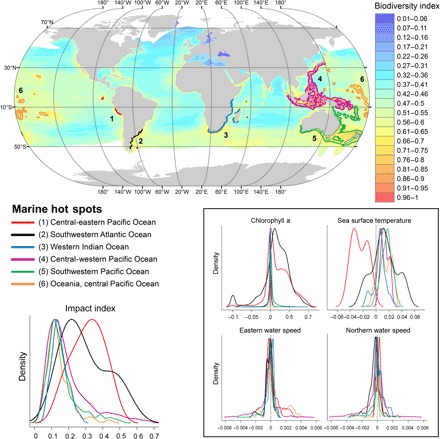
Marine hot spots (top) were identified on the basis of the spatially explicit information on the equally weighted distribution of fish (1729), marine mammal (124), and seabird (330) species. Colors represent a dimensionless index of biodiversity ranging from 0 (absence of species) to 1 (maximum species richness). Hot spots enclose 0.5° pixels with values of biodiversity over the upper 95th percentile. Density plots represent the distribution of environmental changes occurring within marine biodiversity hot spots and the derived Cumulative Impact Index, that is, the number of 1° pixels within each hot spot and with a given value for the estimated magnitudes of each environmental change. In this way, we aimed to highlight the idea that climate impacts may vary locally within defined hot spots as density plots extend over a range of values below and above zero, which denotes no change. Further details on the spatial distribution of these local impacts are provided in fig. S1.
All these environmental stressors likely interact in a number of ways, but little is known about the potential for synergetic or antagonistic interactions that may exacerbate or counteract deleterious effects on marine communities inhabiting these hot spots of marine biodiversity (41). Marine species may also respond differentially to changes in environmental conditions (42–44). Some species may benefit from shifts toward environmental conditions outside the normal range of variability (45), but in most cases, these environmental changes will prove suboptimal, and this will be made apparent through changes to populations and communities (2). In particular, there is overwhelming evidence that ocean warming in temperate regions, such as the southwestern Pacific Ocean or the western Indian Ocean, can affect marine species through a reduction in primary productivity (4–6) and also through trophic disruptions due to shifts in species distributions (18, 46–48) and changes in the timing of ecosystem-level processes (17, 49, 50). Small changes in water temperature and pH may also result in coral bleaching (51) and a severe simplification of tropical communities from the central-western Pacific Ocean and Oceania (52, 53). Changes in ocean circulation, which largely control marine patterns of productivity and food availability (54), may also have important global consequences for biological communities. Climate impacts on marine communities might vary spatially from the local to the regional scale according to the heterogeneous distribution of environmental stressors; thus, we should expect that consequences of changing climatic variables will be species-specific and even site-specific. Fine-scale, spatially explicit measurements on the distribution of environmental stressors, such as those provided in this study, are therefore crucial to effectively depict those local to regional areas of special concern for the conservation of marine biodiversity in the face of climate change.
Industrial fisheries may pose another serious threat for the conservation of species inhabiting marine biodiversity hot spots when those areas overlap with areas of intense human fishing activity (19, 55, 56). However, fishery data are available in such poor spatial resolution [57, but see the study by Watson et al. (26)] that analyses of the overlap of fishing intensity with climate change effects are necessarily limited. FAO fisheries landings are available for the last 60 years, enabling us to at least evaluate trends in the exploitation of marine resources. Although fishing has been practiced for centuries, fishing pressure has intensified in recent decades (15, 58) as a consequence of technical developments in fishing techniques and the demands of a rapidly increasing human population, leading to overexploitation and even collapse of many fish stocks (21). The world’s marine fisheries resources are under enormous pressure, with global fishing effort exceeding optimum and sustainable levels by an estimated factor of 3 to 4 (58). Observed trends showing annual increases in fishing captures (Fig. 3) suggest that this harvest pressure will continue and further exacerbate pressure on fish stocks well into the future (21, 59).
Fig. 3. Fishing impact on the marine environment.
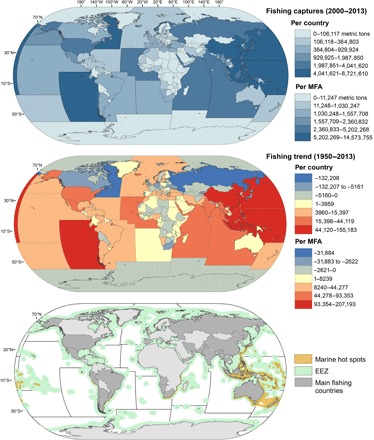
Average fishing captures (in metric tons) for the 2000–2013 period are represented per country and MFA (top). Trends in fishing captures (slope of the linear trend for the 1950–2013 period) are also represented per country and MFA (middle). Those countries contributing the most to fishing captures at those MFA that overlap to a large extent with marine biodiversity hot spots are highlighted in dark gray (bottom). All of them have sovereign EEZs overlapping marine hot spots, with the exception of Spain.
Fishing activities are particularly intense at Major Fishing Areas (MFAs, according to their FAO categorization) that overlap with marine biodiversity hot spots (Fig. 3). This is particularly true for the tropical regions of the Indian Ocean and the western Pacific Ocean, where the highest increasing rates in fishing pressure have been recorded at both the regional (Fig. 3) and the local scale (26; see also www.seaaroundus.org). Although biodiversity conservation is an issue of global concern, fishing policies are most commonly derived from decisions taken at a national level, particularly with regard to those occurring within EEZs (Fig. 3) where bordering sovereign states have special rights regarding the use of marine resources (United Nations Convention on the Law of the Sea, 1982). Fishing pressure differs among countries. China and Peru contribute the most to global captures (ca. 20%) and are likely to continue to do so according to the observed trends in fishing captures (Fig. 3). However, many other countries also contribute substantially to fishing captures within MFAs that overlap with marine hot spots (Fig. 3 and fig. S3). We identified 30 different coastal countries that collectively account for 80.5% of fishing captures in the areas of high biodiversity (fig. S2). All of them have sovereign EEZs overlapping marine hot spots, with the exception of Spain, which currently maintains huge distant fishing fleets in both the Indian Ocean and the southwestern Atlantic Ocean (fig. S2). Fishing policies that promote sustainable fishing practices are required if the aggravating effect of fishing intensity on climate change impacts is to be minimized. Although commercial fishery data are limited and our analysis does not take into account the impacts of artisanal and subsistence fisheries (from which data are not available), our results can be used to assess whether and how fishing activities should be managed spatially to minimize their negative impacts on species-rich ecosystems that are also affected by climate change (55, 60).
Uncertainty will always be a factor in research on marine organisms and their open environments. The challenge is to use the available data to produce scientifically sound approaches to identifying issues of marine conservation (61) given that the data are of variable quality and, in particular, deficient for fisheries [but see the study by Watson et al. (26)]. Our analyses provide a framework that allows the evaluation of environmental and human impacts on marine communities from local to global scales. Further, this adaptable framework can be continuously updated and enhanced by incorporating additional information on fishing pressure [for example, finer-scaled data on fishing captures (26)] and other human stressors (55, 62) or finer-scaled data on oceanographic features whenever these become available. What is clear from our current analyses is that the world’s areas of highest marine biodiversity are threatened by the impacts from both global warming and human fishing pressure. Thus, it behooves the international community to find solutions that go beyond the interests and borders of sovereign states if we are to conserve the biodiversity in these marine hot spots, in a similar way to which the world must tackle the associated causes of climate change itself.
MATERIALS AND METHODS
Experimental design
We used the longest time series of remote-sensing records—annual composites—on SST (1980–2014), CHL (1979–2014), and marine currents (1980–2014) available to provide finest-scale measurements of the impacts of climate change on the marine environment globally (table S1). We then observed and described these environmental changes within hot spots of marine biodiversity determined from the world distributions of more than 2000 species of seabirds, marine mammals, and fish.
Furthermore, the overall picture of human-related impacts on the marine environment would be incomplete without consideration of industrial fisheries. Using FAO data over the last 60 years, we investigated spatiotemporal variation in fishing captures. FAO is the only institution that maintains and provides long-term global fisheries statistics from the 1950s onward. On the basis of a modeled disaggregation of fish landing information from FAO (26), finer-scaled, spatially explicit data on fishing captures (with a particular emphasis on EEZ) can be found at www.seaaroundus.org. Although these modeled data may provide a better description of the local to regional impacts of human fisheries on marine biodiversity hot spots, here we used the raw data on fish landings from FAO to identify those countries that contribute the most to overall fishing pressure particularly within areas that overlap with marine biodiversity hot spots.
Statistical analysis
Pixel basis, least-squares linear regression of annual information from SST, CHL, and the eastern and northern components of water speed was used for deriving the significance of temporal trends (P < 0.05) and its magnitudes (slopes; that is, annual changes in target features) over the past three decades (fig. S1). CHL data were sourced online as annual composites, whereas data on SST and marine currents were obtained on a monthly basis and averaged yearly to generate a linear relationship to extract slopes. This calculation was repeated for every pixel of the globe. These magnitudes were combined to obtain our spatially explicit Cumulative Impact Index. Different layers were first resampled following a bilinear interpolation procedure to match the spatial resolution of the coarsest SST product (that is, 1° grid). Absolute values for the slopes of obtained trends (a proxy to the magnitude of environmental changes) were then standardized to the maximum value to make all variables comparable. These relative values [ranging from 0 (no change) to 1 (maximum recorded change)] were subsequently added on a pixel basis and standardized again to the maximum value. In this way, we obtained a dimensionless index ranging from 0 (no change) to 1 (maximum change), thereby providing information about spatial heterogeneity in the magnitude of environmental changes and highlighting those marine areas that have undergone the largest changes in their environmental conditions (Fig. 1).
We used information on the global distribution of 2183 species of vertebrates composed of 1729 fish, 124 marine mammals, and 330 seabirds to derive spatially explicit information on species richness (number of species) and to identify hot spots of marine biodiversity. The worldwide distribution of species was sourced online from the International Union for Conservation of Nature (IUCN) (www.iucnredlist.org/) and BirdLife International (www.birdlife.org) as ESRI (Environmental Systems Research Institute) supported geodatabases, including spatial information on species-specific occurrences (presence/absence). Occurrence shapefiles were transformed to 0.5° dichotomous grid features, with “1” denoting presence and “0” indicating absences. Obtained grid features for different fish, marine mammal, and seabird species were added to obtain three maps indicating the number of species per group occurring in each pixel. Each map was standardized by the total number of species per group to obtain a relative measure of the spatial distribution of fish, marine mammal, and seabird species richness. These relative values were subsequently added on a pixel basis and standardized again to the maximum value to obtain a dimensionless index ranging from 0 (absence of species) to 1 (maximum species richness), providing information about the spatial heterogeneity in species richness and highlighting hot spots of marine biodiversity without biases toward those groups (for example, fish) with a larger number of species (Fig. 2). We considered hot spots of marine biodiversity to be those marine areas enclosing pixels with values of species richness over the upper 95th percentile, which was a threshold that identified relatively small and compact marine areas of special concern within the main ocean basins (that is, Pacific Ocean, Indian Ocean, and Atlantic Ocean; fig. S3).
Finally, we evaluated changes in landings of fish over the last 60 years to look for temporal trends in the exploitation of marine resources. Further, we identified those countries that have contributed the most to fishing captures in general and at the derived hot spots of marine diversity during the last decade in particular. Long-term information (1950–2013) on marine fishing captures (in metric tons) was sourced from FAO using the FishStatJ software. Captures were restricted to marine fish (1149 species) and grouped per year, country, and MFA (according to FAO categories). Least-squares linear regressions of annual information on fish landing were used for deriving the temporal trend per country and MFA (Fig. 3).
Acknowledgments
GODAS data were provided by the National Oceanic and Atmospheric Administration/Oceanic and Atmospheric Research/Earth System Research Laboratory Physical Sciences Division, Boulder, CO, USA, from their website at www.esrl.noaa.gov/psd/. IUCN (www.iucnredlist.org/) and BirdLife International (www.birdlife.org) provided the world distribution of species included in the study. Data on fishing captures were provided by FAO. Funding: We thank the continued support of the Phillip Island Nature Parks, Penguin Ecosystem Research Centre. Grants were received from the Penguin Foundation, the Australian Research Council, and the European Union Horizon 2020 research and innovation program under grant agreement no. 641762 to the ECOPOTENTIAL project. Author contributions: F.R. initially designed the study and drafted the manuscript. F.R. and I.A. carried out statistical analyses. All authors helped in the drafting of and contributed substantially in revising the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: Spatially explicit outputs produced in this work are provided in .asc for easy visualization using GIS (geographic information system) software. This information is hosted on the Spanish National Research Council [Consejo Superior de Investigaciones Científicas (CSIC)] digital repository (http://hdl.handle.net/10261/142056).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/2/e1601198/DC1
fig. S1. Significance and magnitudes of observed environmental changes.
fig. S2. Major contributors to fishing pressure.
fig. S3. Identifying hot spots of marine biodiversity.
table S1. Long-term, remote-sensing records of oceanographic features.
REFERENCES AND NOTES
- 1.Hansen J., Sato M., Ruedy R., Lo K., Lea D. W., Medina-Elizade M., Global temperature change. Proc. Natl. Acad. Sci. U.S.A. 103, 14288–14293 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doney S. C., Ruckelshaus M., Duffy J. E., Barry J. P., Chan F., English C. A., Galindo H. M., Grebmeier J. M., Hollowed A. B., Knowlton N., Polovina J., Rabalais N. N., Sydeman W. J., Talley L. D., Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 4, 11–37 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Levitus S., Antonov J. I., Boyer T. P., Locarnini R. A., Garcia H. E., Mishonov A. V., Global ocean heat content 1955–2008 in light of recently revealed instrumentation problems. Geophys. Res. Lett. 36, L07608 (2009). [Google Scholar]
- 4.Keeling R. F., Körtzinger A., Gruber N., Ocean deoxygenation in a warming world. Ann. Rev. Mar. Sci. 2, 199–229 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Polovina J. J., Howell E. A., Abecassis M., Ocean’s least productive waters are expanding. Geophys. Res. Lett. 35, L03618 (2008). [Google Scholar]
- 6.Gregg W. W., Conkright M. E., Ginoux P., O’Reilly J. E., Casey N. W., Ocean primary production and climate: Global decadal changes. Geophys. Res. Lett. 30, 1809 (2003). [Google Scholar]
- 7.Behrenfeld M. J., O’Malley R. T., Siegel D. A., McClain C. R., Sarmiento J. L., Feldman G. C., Milligan A. J., Falkowski P. G., Letelier R. M., Boss E. S., Climate-driven trends in contemporary ocean productivity. Nature 444, 752–755 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Cheung W. W. L., Sarmiento J. L., Dunne J., Frölicher T. L., Lam V. W. Y., Deng Palomares M. L., Watson R., Pauly D., Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Chang. 3, 254–258 (2013). [Google Scholar]
- 9.Burrows M. T., Schoeman D. S., Buckley L. B., Moore P., Poloczanska E. S., Brander K. M., Brown C., Bruno J. F., Duarte C. M., Halpern B. S., Holding J., Kappel C. V., Kiessling W., O’Connor M. I., Pandolfi J. M., Parmesan C., Schwing F. B., Sydeman W. J., Richardson A. J., The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Cheung W. W. L., Watson R., Pauly D., Signature of ocean warming in global fisheries catch. Nature 497, 365–368 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Winton M., Griffies S. M., Samuels B. L., Sarmiento J. L., Frölicher T. L., Connecting changing ocean circulation with changing climate. J. Climate 26, 2268–2278 (2012). [Google Scholar]
- 12.Alheit J., Bakun A., Population synchronies within and between ocean basins: Apparent teleconnections and implications as to physical–biological linkage mechanisms. J. Mar. Syst. 79, 267–285 (2010). [Google Scholar]
- 13.Food and Agriculture Organization, The State of World’s Fisheries and Aquaculture 2008 (Food and Agriculture Organization of the United Nations, 2009). [Google Scholar]
- 14.Worm B., Barbier E. B., Beaumont N., Duffy J. E., Folke C., Halpern B. S., Jackson J. B. C., Lotze H. K., Micheli F., Palumbi S. R., Sala E., Selkoe K. A., Stachowicz J. J., Watson R., Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Anticamara J. A., Watson R., Gelchu A., Pauly D., Global fishing effort (1950–2010): Trends, gaps, and implications. Fish. Res. 107, 131–136 (2011). [Google Scholar]
- 16.Durant J. M., Hjermann D., Anker-Nilssen T., Beaugrand G., Mysterud A., Pettorelli N., Stenseth N. C., Timing and abundance as key mechanisms affecting trophic interactions in variable environments. Ecol. Lett. 8, 952–958 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Hipfner J. M., Matches and mismatches: Ocean climate, prey phenology and breeding success in a zooplanktivorous seabird. Mar. Ecol. Prog. Ser. 368, 295–304 (2008). [Google Scholar]
- 18.Molinos J. G., Halpern B. S., Schoeman D. S., Brown C. J., Kiessling W., Moore P. J., Pandolfi J. M., Poloczanska E. S., Richardson A. J., Burrows M. T., Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Chang. 8, 83–88 (2016). [Google Scholar]
- 19.Cury P. M., Boyd I. L., Bonhommeau S., Anker-Nilssen T., Crawford R. J. M., Furness R. W., Mills J. A., Murphy E. J., Österblom H., Paleczny M., Piatt J. F., Roux J.-P., Shannon L., Sydeman W. J., Global seabird response to forage fish depletion—One-third for the birds. Science 334, 1703–1706 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Baum J. K., Worm B., Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 78, 699–714 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Brander K. M., Global fish production and climate change. Proc. Natl. Acad. Sci. U.S.A. 104, 19709–19714 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenzweig C., Karoly D., Vicarelli M., Neofotis P., Wu Q., Casassa G., Menzel A., Root T. L., Estrella N., Seguin B., Tryjanowski P., Liu C., Rawlins S., Imeson A., Attributing physical and biological impacts to anthropogenic climate change. Nature 453, 353–357 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Hoegh-Guldberg O., Bruno J. F., The impact of climate change on the World’s marine ecosystems. Science 328, 1523–1528 (2010). [DOI] [PubMed] [Google Scholar]
- 24.McQuatters-Gollop A., Reid P. C., Edwards M., Burkill P. H., Castellani C., Batten S., Gieskes W., Beare D., Bidigare R. R., Head E., Johnson R., Kahru M., Koslow J. A., Pena A., Is there a decline in marine phytoplankton? Nature 472, E6–E7 (2011). [DOI] [PubMed] [Google Scholar]
- 25.McClain C. R., A decade of satellite ocean color observations. Ann. Rev. Mar. Sci. 1, 19–42 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Watson R., Kitchingman A., Gelchu A., Pauly D., Mapping global fisheries: Sharpening our focus. Fish Fish. 5, 168–177 (2004). [Google Scholar]
- 27.Spielhagen R. F., Werner K., Sørensen S. A., Zamelczyk K., Kandiano E., Budeus G., Husum K., Marchitto T. M., Hald M., Enhanced modern heat transfer to the Arctic by warm Atlantic water. Science 331, 450–453 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Schmittner A., Decline of the marine ecosystem caused by a reduction in the Atlantic overturning circulation. Nature 434, 628–633 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Boyce D. G., Lewis M. R., Worm B., Global phytoplankton decline over the past century. Nature 466, 591–596 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Acha E. M., Mianzan H. W., Guerrero R. A., Favero M., Bava J., Marine fronts at the continental shelves of austral South America: Physical and ecological processes. J. Mar. Syst. 44, 83–105 (2004). [Google Scholar]
- 31.Deser C., Phillips A. S., Alexander M. A., Twentieth century tropical sea surface temperature trends revisited. Geophys. Res. Lett. 37, L10701 (2010). [Google Scholar]
- 32.Wu L., Cai W., Zhang L., Nakamura H., Timmermann A., Joyce T., McPhaden M. J., Alexander M., Qiu B., Visbeck M., Chang P., Giese B., Enhanced warming over the global subtropical western boundary currents. Nat. Clim. Chang. 2, 161–166 (2012). [Google Scholar]
- 33.Doney S. C., Oceanography: Plankton in a warmer world. Nature 444, 695–696 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Boulton C. A., Allison L. C., Lenton T. M., Early warning signals of Atlantic Meridional Overturning Circulation collapse in a fully coupled climate model. Nat. Commun. 5, 5752 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karpouzi V. S., Watson R., Pauly D., Modelling and mapping resource overlap between seabirds and fisheries on a global scale: A preliminary assessment. Mar. Ecol. Prog. Ser. 343, 87–99 (2007). [Google Scholar]
- 36.Tittensor D. P., Mora C., Jetz W., Lotze H. K., Ricard D., Berghe E. V., Worm B., Global patterns and predictors of marine biodiversity across taxa. Nature 466, 1098–1101 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Pompa S., Ehrlich P. R., Ceballos G., Global distribution and conservation of marine mammals. Proc. Natl. Acad. Sci. U.S.A. 108, 13600–13605 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson A. D., Boyer A. G., Kim H., Pompa-Mansilla S., Hamilton M. J., Costa D. P., Ceballos G., Brown J. H., Drivers and hotspots of extinction risk in marine mammals. Proc. Natl. Acad. Sci. U.S.A. 109, 3395–3400 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beaugrand G., Edwards M., Raybaud V., Goberville E., Kirby R. R., Future vulnerability of marine biodiversity compared with contemporary and past changes. Nat. Clim. Chang. 5, 695–701 (2015). [Google Scholar]
- 40.Barber R. T., Chavez F. P., Biological consequences of El Niño. Science 222, 1203–1210 (1983). [DOI] [PubMed] [Google Scholar]
- 41.Darling E. S., Côté I. M., Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Gangoso L., Márquez-Ferrando R., Ramírez F., Gomez-Mestre I., Figuerola J., Understanding phenotypic responses to global change. BioEssays 35, 491–495 (2013). [Google Scholar]
- 43.Loya Y., Sakai K., Yamazato K., Nakano Y., Sambali H., van Woesik R., Coral bleaching: The winners and the losers. Ecol. Lett. 4, 122–131 (2001). [Google Scholar]
- 44.Fulton E. A., Interesting times: Winners, losers, and system shifts under climate change around Australia. ICES J. Mar. Sci. 68, 1329–1342 (2011). [Google Scholar]
- 45.Weimerskirch H., Louzao M., de Grissac S., Delord K., Changes in wind pattern alter albatross distribution and life-history traits. Science 335, 211–214 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Mueter F. J., Litzow M. A., Sea ice retreat alters the biogeography of the Bering Sea continental shelf. Ecol. Appl. 18, 309–320 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Spencer P. D., Density-independent and density-dependent factors affecting temporal changes in spatial distributions of eastern Bering Sea flatfish. Fish. Oceanogr. 17, 396–410 (2008). [Google Scholar]
- 48.Hare J. A., Alexander M. A., Fogarty M. J., Williams E. H., Scott J. D., Forecasting the dynamics of a coastal fishery species using a coupled climate–population model. Ecol. Appl. 20, 452–464 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Durant J. M., Hjermann D., Ottersen G., Stenseth N. C., Climate and the match or mismatch between predator requirements and resource availability. Clim. Res. 33, 271–283 (2007). [Google Scholar]
- 50.Ramírez F., Afán I., Tavecchia G., Catalán I. A., Oro D., Sanz-Aguilar A., Oceanographic drivers and mistiming processes shape breeding success in a seabird. Proc. Biol. Sci. 283, 20152287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoegh-Guldberg O., Mumby P. J., Hooten A. J., Steneck R. S., Greenfield P., Gomez E., Harvell C. D., Sale P. F., Edwards A. J., Caldeira K., Knowlton N., Eakin C. M., Iglesias-Prieto R., Muthiga N., Bradbury R. H., Dubi A., Hatziolos M. E., Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Jones G. P., McCormick M. I., Srinivasan M., Eagle J. V., Coral decline threatens fish biodiversity in marine reserves. Proc. Natl. Acad. Sci. U.S.A. 101, 8251–8253 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Idjadi J. A., Edmunds P. J., Scleractinian corals as facilitators for other invertebrates on a Caribbean reef. Mar. Ecol. Prog. Ser. 319, 117–127 (2006). [Google Scholar]
- 54.Afán I., Chiaradia A., Forero M. G., Dann P., Ramírez F., A novel spatio-temporal scale based on ocean currents unravels environmental drivers of reproductive timing in a marine predator. Proc. Biol. Sci. 282, 20150721 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halpern B. S., Walbridge S., Selkoe K. A., Kappel C. V., Micheli F., D’Agrosa C., Bruno J. F., Casey K. S., Ebert C., Fox H. E., Fujita R., Heinemann D., Lenihan H. S., Madin E. M. P., Perry M. T., Selig E. R., Spalding M., Steneck R., Watson R., A global map of human impact on marine ecosystems. Science 319, 948–952 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Ramírez F., Afán I., Hobson K. A., Bertellotti M., Blanco G., Forero M. G., Natural and anthropogenic factors affecting the feeding ecology of a top marine predator, the Magellanic penguin. Ecosphere 5, 1–21 (2014). [Google Scholar]
- 57.Hinz H., Murray L. G., Lambert G. I., Hiddink J. G., Kaiser M. J., Confidentiality over fishing effort data threatens science and management progress. Fish Fish. 14, 110–117 (2013). [Google Scholar]
- 58.Pauly D., Christensen V., Guénette S., Pitcher T. J., Sumaila U. R., Walters C. J., Watson R., Zeller D., Towards sustainability in world fisheries. Nature 418, 689–695 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Cheung W. W. L., Lam V. W. Y., Sarmiento J. L., Kearney K., Watson R., Zeller D., Pauly D., Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Glob. Chang. Biol. 16, 24–35 (2010). [Google Scholar]
- 60.Witherell D., Pautzke C., Fluharty D., An ecosystem-based approach for Alaska groundfish fisheries. ICES J. Mar. Sci. 57, 771–777 (2000). [Google Scholar]
- 61.Lewison R. L., Crowder L. B., Read A. J., Freeman S. A., Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 19, 598–604 (2004). [Google Scholar]
- 62.Halpern B. S., Frazier M., Potapenko J., Casey K. S., Koenig K., Longo C., Lowndes J. S., Rockwood R. C., Selig E. R., Selkoe K. A., Walbridge S., Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nat. Commun. 6, 7615 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/2/e1601198/DC1
fig. S1. Significance and magnitudes of observed environmental changes.
fig. S2. Major contributors to fishing pressure.
fig. S3. Identifying hot spots of marine biodiversity.
table S1. Long-term, remote-sensing records of oceanographic features.


