Abstract
Importance
Clinical features are unreliable for distinguishing Ocular Surface Squamous Neoplasia (OSSN) from benign conjunctival lesions.
Objective
To evaluate the adverse effects, accuracy and inter-observer variation of Toluidine Blue 0.05% vital staining in distinguishing OSSN, confirmed by histopathology, from other conjunctival lesions.
Design, Setting and Participants
Cross-sectional study in Kenya from July 2012 through July 2014 of 418 adults with suspicious conjunctival lesions. Pregnant and breastfeeding women were excluded.
Exposures
Comprehensive ophthalmic slit-lamp examination was conducted. Vital staining with Toluidine Blue 0.05% aqueous solution was performed before surgery. Initial safety testing was conducted on large tumours scheduled for exenteration looking for corneal toxicity on histology before testing smaller tumours. We asked about pain or discomfort after staining and evaluated the cornea at the slit lamp for epithelial defects. Lesions were photographed before and after staining. Diagnosis was confirmed by histopathology. Six examiners assessed photographs from a sub-set of 100 consecutive participants for staining and made a diagnosis of OSSN vs Non-OSSN.
Main Outcomes and Measures
Staining was compared with histopathology to estimate sensitivity, specificity and predictive values. Adverse effects were enumerated. Inter-observer agreement was estimated using the kappa statistic (k).
Results
143/419 (34%) participants had OSSN by histopathology. The median (interquartile range) age of the 419 was 37 (32-45) years and 278 (66%) were female. 322/419 participants had positive staining while 2/419 were equivocal. There was no histological evidence of corneal toxicity. Mild discomfort was reported by 88 (21%) and mild superficial punctate keratopathy seen in 7 (1.7%).For detecting OSSN, Toluidine blue had a sensitivity of 92% (95%CI, 87%-96%), specificity 31% (95%CI, 25%-36%), positive predictive value 41% (95%CI, 35%-46%), and negative predictive value 88% (95%CI, 80%-94%). Inter-observer agreement was substantial for staining (k=0.8) and moderate for diagnosis (k=0.4).
Conclusion and Relevance
With the high sensitivity and low specificity for OSSN compared with histopathology among patients with conjunctival lesions, Toluidine Blue 0.05% vital staining is a good screening tool, but not a good diagnostic tool due to a high frequency of false positives. The high negative predictive value suggests that a negative staining result indicates that OSSN is relatively unlikely.
Background
Ocular surface squamous neoplasia (OSSN) is an aggressive eye cancer, particularly affecting young adults in Africa, causing visual disability, high morbidity and mortality. The diagnosis is problematic. In most African countries pathology services are limited; most clinicians depend on their clinical judgment.1, 2 However, the appearance of OSSN overlaps with several benign conditions making a clinical impression unreliable. Surgical excision is the mainstay of OSSN treatment. A simple diagnostic test would help clinicians plan management, for example, by better delineating the boundaries of the lesion during excision. The test may also help in distinguishing early recurrent tumour from non-malignant abnormal tissue such as fibrosis, possibly avoiding the need for additional surgery.
Vital stains are used to colour living tissues. Several dyes are used extensively in ophthalmic surgery.3, 4 Toluidine blue (ToB) is an acidophilic metachromatic dye that stains abnormal tissue dark royal blue by penetrating into the nuclei of cancerous cells where it has a selective affinity for nucleic acids and by accumulating in the intercellular spaces.5 Malignant tissues stain more frequently than healthy epithelia because of their abundant nuclear material from increased mitoses and poor cell-to-cell adhesion.6, 7 Mucin and inflammatory cells also take up ToB.5, 7 ToB has been used safely for many years to aid the clinical diagnosis of oral and oropharyngeal cancer and to demarcate tumours during surgical excision.8, 9
A case report from Japan described the first use of topical ToB 0.05% vital staining for OSSN.10 The dye was reported to clearly demarcate the abnormal tissue, assisting the excision. The authors commented that ToB did not stain other conjunctival lesions such as pterygium (no data presented) and it was not toxic to the ocular surface. Two relatively small studies recently evaluated vital staining for OSSN using ToB 1% in Brazil and methylene blue 1% in South Africa.11, 12 However, given the variation in clinical phenotype and prevalence of conjunctival lesions it is necessary to test this in the local setting.
The aim of this study was to investigate the utility of Toluidine Blue 0.05% solution in detecting neoplastic tissue by evaluating its safety, accuracy and inter-observer variation.
Methods
Ethical Approval
This study was formally reviewed and approved by the Kenyatta National Hospital – University of Nairobi Ethics and Research Committee (KNH-UON ERC) and the London School of Hygiene and Tropical Medicine Ethics Committee. This study adhered to the tenets of the Declaration of Helsinki. All participants gave informed written consent to take part in the study before enrolment and did not receive a stipend to participate.
Participants
The study was conducted between July 2012 and July 2014 in four eye care centres in different parts of Kenya; Kenyatta National Hospital in Nairobi the capital city, PCEA Kikuyu Eye Unit in Central Kenya, Kitale District Hospital in the north Rift Valley and Sabatia Eye Hospital in western Kenya bordering Lake Victoria. It was part of a larger project on the epidemiology and management of OSSN in Kenya. These centres receive referral cases from the surrounding hospitals.
Consecutive adult patients (at least 18 years of age) seen in these four eye clinics with conjunctival lesions (first presentation or recurrence) suspected to be OSSN scheduled for surgery who gave consent to participate in the study were included. Pregnant women and breastfeeding mothers were excluded.
Toluidine Blue Eye Drops
Toluidine Blue 0.05% aqueous solution was prepared in the Kikuyu Eye Unit eye drop production facility. Toluidine powder (Sigma Aldrich, UK) 0.05g was diluted in 100ml of freshly distilled water and aliquoted into 5ml eye drop bottles. The bottles were sterilised in a water bath at 98°C for 30 minutes and checked for particles. Any with particles was discarded. A bottle was used for up to 28 days once opened. New batches were prepared every 6 months.
Clinical Assessment
A comprehensive ophthalmic examination was conducted using a slit lamp. Clinical features of lesions were assessed including inflammation, leukoplakia and involvement of adjacent structures Vital staining with Toluidine Blue 0.05% solution was performed at the slit lamp before surgery. One drop of the dye was applied to the ocular surface waiting for 30 seconds before wiping off the excess spillover from the eyelids with a soft tissue paper. Topical anaesthetic was not applied before staining in order to evaluate if ToB was painful. Staining with fluorescein was not done to avoid interference with the ToB dye.
Surgery and Histopathology
All lesions were excised under infiltration local anaesthetic using an operating microscope with a 3mm clear margin. The defect was reconstructed by primary closure. Cryotherapy was not applied as the participants with OSSN were invited to enroll in an additional treatment trial post-operatively. Specimens were placed directly into buffered formalin and subsequently examined at the histopathology laboratory at the MP Shah Hospital, Nairobi. One pathologist examined all the histology slides. Participants with mild, moderate or severe conjunctival intraepithelial neoplasia (CIN I, II, III), carcinoma-in-situ (CIS) or invasive squamous cell carcinoma (well, moderately and poorly differentiated) were classified as having OSSN. The diagnosis of actinic keratosis was based on the presence of elastotic stromal degeneration, acanthosis, hyperkeratosis and parakeratosis in the presence of normal cellular polarity. By the accepted criteria for dysplasia, such lesions were classified as CIN only if there was loss of polarity.
a). Safety study
There is extensive experience on the safety of using ToB in the oral cavity but only relatively limited data on the eye.9, 11–14 Therefore, we conducted initial testing on large tumours scheduled for exenteration. The exenteration specimens were examined by a histopathologist for evidence of corneal toxicity such as necrosis or inflammatory cells and dye penetration into the stroma (free or engulfed in macrophages).
The results of the safety data and information from previous published series were reported to the ethics committee and permission was granted to extend testing to participants with smaller lesions. Participants were asked about pain or discomfort, and we evaluated the cornea at the slit lamp for epithelial changes such as punctate staining.
b). Accuracy study
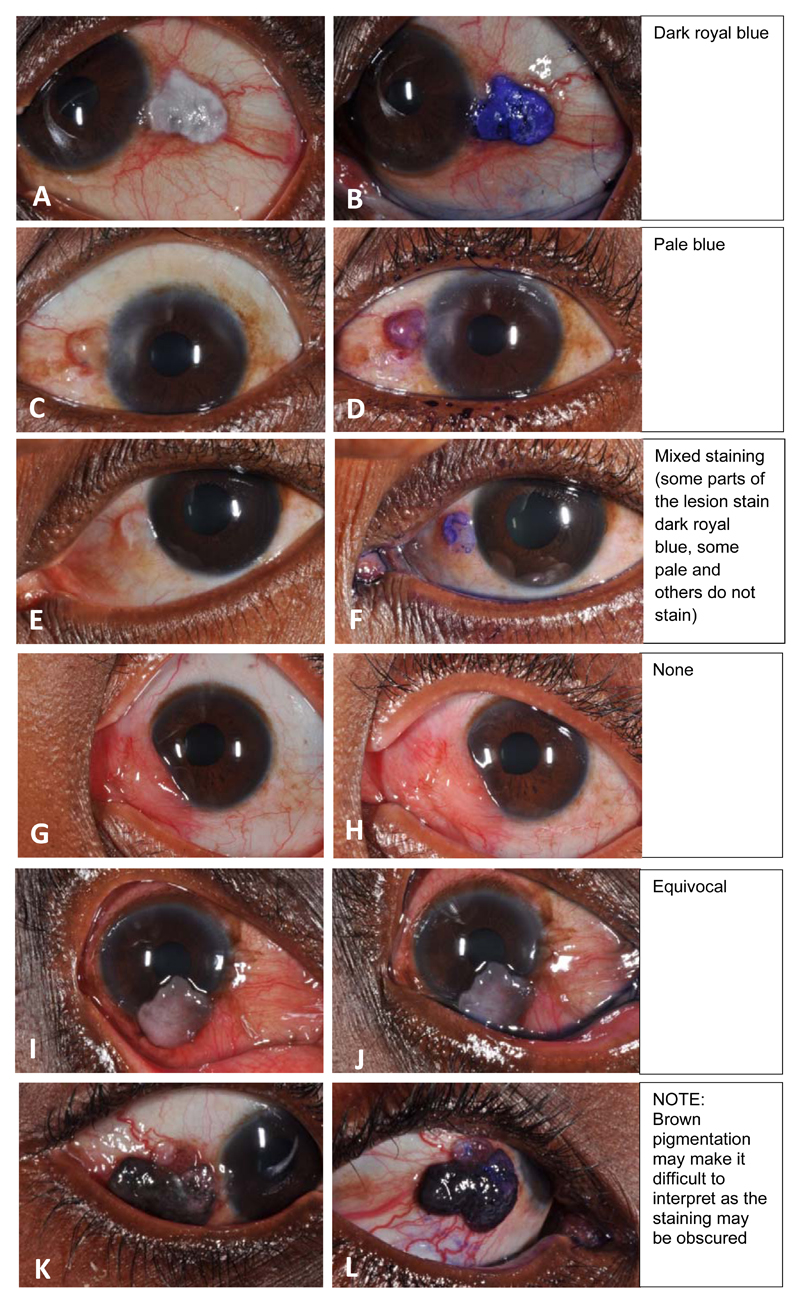
Staining was recorded using a 5-point system as: none, equivocal (if it was too pale to be sure there was staining), pale blue, mixed pattern (pale and deep blue) or deep royal blue (Figure 1). For the purpose of analysis, any blue staining was considered positive and equivocal staining excluded from the analysis. A stratified analysis by degree of staining was also conducted. Since it would be unlikely that a clinician would be in doubt about the likely diagnosis in patients with large orbital tumours, orbital cases were excluded from this analysis. Staining (positive vs negative) was compared to histopathology (OSSN vs not OSSN).
Figure 1. Toluidine blue 0.05% staining intensities and patterns; a 5-point scale.
c). Inter-observer variation study
The eye was photographed before and about 30 seconds after staining for subsequent independent grading of the staining pattern. A pair of photographs was taken, one in primary gaze and the other with the lesion in the centre using a Nikon D90 digital camera with 105mm lens.
Six final year residents in the Department of Ophthalmology, University of Nairobi at Kenyatta National Hospital were trained by one author (SG) using projected slides showing different degrees of Toluidine blue staining. They were informed that previous studies suggested that generally OSSN stained positive and benign lesions were negative, but this may not be invariably the case. A week later the same group independently assessed photographs from the last 100 consecutive participants enrolled into the study from one centre. Cases with features that are highly suggestive of malignancy, such as very large tumours invading the orbit were excluded. The trainer (SG) projected the images on a screen. None of the slides had been shown in the training session. The residents were masked to the diagnosis and did not discuss the cases. They were asked to grade the staining and suggest a diagnosis (OSSN vs non-OSSN), taking into account the clinical features of the lesion. The clinical case-mix in this sample of patients was comparable to the whole dataset that included patients from all four study centres.
Statistical Analysis
Data was managed in Access (Microsoft Windows 2010) and transferred into STATA version 12.1 (StataCorp, College Station, Texas, USA) for analysis. Sensitivity, specificity and predictive values of ToB vital staining were computed based on subsequent histological diagnosis.
For the inter-observer component, the scores for each clinician were compared to a reference standard using the kappa (k) statistic and graded using the Landis & Koch method.15 The examiners’ staining score was compared to the lead author’s assessment while their clinical diagnosis was compared to the histopathology report. The proportions they scored as positive or negative for stain and OSSN or non-OSSN for diagnosis were reported. To calculate an average value, the kappa statistics for each grader were transformed to Z scores using the Fisher Z transformation, averaged, and then back-transformed to a kappa statistic.
Results
Study Participants and Histological Diagnosis
A STARD diagram is shown in eFigure 1. Five hundred and thirty-seven (537) participants with conjunctival lesions were recruited to the larger OSSN project and 447 (83%) underwent Toluidine blue staining. There were 90 people recruited into the larger study while awaiting completion of the initial ToB safety phase. The final analysis consisted of 419 participants whose median (interquartile range) age was 37 (32-45) years and 277 (66%) were female. There were 143 (34%) OSSN and 276 (66%) non-OSSN lesions (Table 1).
Table 1. Toluidine blue staining patterns of 418 conjunctival lesions.
| Histopathology | Staining result | Total | ||||
|---|---|---|---|---|---|---|
| None | Pale blue | Mixed | Dark royal blue | Equivocal | ||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||
| OSSN | 11 (7.7) | 23 (16.2) | 17 (11.9) | 91 (64.1) | 1 (0.7) | 143 |
| Pterygium | 55 (34.8) | 24 (15.2) | 31 (19.6) | 48 (30.4) | 0 (0) | 158 |
| Actinic Keratosis | 20 (23.8) | 15 (17.9) | 19 (22.6) | 30 (35.7) | 0 (0) | 84 |
| Nevus | 4 (33.3) | 1 (8.3) | 4 (33.3) | 2 (16.7) | 1 (8.3) | 12 |
| Squamous papilloma | 1 (10.0) | 4(40.0) | 1(10.0) | 4 (40.0) | 0 (0) | 10 |
| Pyogenic granuloma | 2 (66.7) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 3 |
| Haemangioma | 0 (0) | 0 (0) | 0 (0) | 2 (100.0) | 0 (0) | 2 |
| Ocular rhinosporidiosis | 0 (0) | 0 (0) | 0 (0) | 2 (100.0) | 0 (0) | 2 |
| Chronic conjunctivitis | 0 (0) | 0 (0) | 1 (50) | 1 (50) | 0 (0) | 2 |
| Epidermoid cyst | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 |
| Sarcomatoid spindle cell tumour a | 1 (100.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Sebaceous hyperplasia of the caruncle | 1 (100.0) | 0 (0) | 0 (0) | 0(0) | 0 (0) | 1 |
| Total | 95 (22.5) | 67 (16.0) | 73 (17.5) | 182 (43.5) | 2 (0.5) | 419 |
Abbreviations: OSSN, ocular surface squamous neoplasia
this was a non-OSSN malignancy
a). Safety study
Seven participants with very large tumours (all were squamous cell carcinoma) were enrolled in the pilot toxicity study. None showed evidence of corneal toxicity on histology. Seven participants out of the 419 (1.7%) had a mild superficial punctate keratopathy around the lesion after vital staining possibly due to disruption of the tear film by the raised lesion and the associated drying. These were distributed as follows; 4 pterygium, 1 carcinoma in situ, 1 moderately differentiated squamous cell carcinoma and 1 capillary haemangioma. Most participants tolerated the stain well; 88/419 (21%) reported some mild discomfort immediately after application, all of which resolved rapidly.
b). Accuracy study
Different patterns and intensities of Toluidine Blue staining were seen (Figure 1). The seven orbital tumours in the safety phase all stained deep royal blue. Two participants out of 419 showed equivocal staining and were removed from the analysis. One had moderate intraepithelial dysplasia and the other a nevus.
Overall 322 of 417 (77%) smaller lesions stained with ToB, and staining was more frequent in the OSSN group (Table 2). Any blue ToB staining had a high sensitivity (92%; 95%CI, 87%-96%), low specificity (31%; 95%CI, 25%-36%), high negative predictive value (88%; 95%CI, 80%-94%) and low positive predictive value (41%; 95%CI, 35%-46%) compared to histology (Table 3). The low specificity was attributable to a high proportion (69.5%) of benign lesions staining positive (65% of pterygia and 76% of actinic keratosis).
Table 2. The association between vital staining with Toluidine Blue 0.05% and histological category (OSSN or Not OSSN).
| Staining result | OSSN n(%) | Not OSSN n(%) | Total | OR (95% CI) | p-value |
|---|---|---|---|---|---|
| Any blue staining | 131 (92.3%) | 191 (69.5%) | 322 | 5.2 (2.6-10.4) | <.001 |
| No staining | 11 (7.8%) | 84 (30.6%) | 95 | 1.0 | - |
| Total | 142 (100.0%) | 275 (100.0%) | 417 a | ||
| Stratified analysis | |||||
| Dark royal blue | 91 (64.1%) | 91 (33.1%) | 182 | 7.6 (3.6-16.2) | <.001 |
| Pale blue | 23 (16.2%) | 44 (16.1%) | 67 | 4.0 (1.7-9.3) | <.001 |
| Mixed pattern b | 17 (12.0%) | 56 (20.4%) | 73 | 2.3 (1.0-5.4) | .04 |
| Not staining | 11 (7.8%) | 84 (30.6%) | 95 | 1.0 | - |
| Total | 142 (100.0%) | 274 (100.0%) | 417 | ||
Abbreviations: OSSN, ocular surface squamous neoplasia; OR, odds ratio
The 2 participants who had equivocal staining results in table 1 are excluded from this table
Some areas of the lesion were deep royal blue and others pale
Individual percentages may have a rounding error
Table 3. Test performance indices for various levels of toluidine blue vital staining and a comparison of this Kenyan study with studies from South Africa12 and Brazil11.
| Staining result | Sensitivity (95%CI) | Specificity (95%CI) | Positive predictive value (95%CI) | Negative predictive value (95%CI) | Area under the ROC curve (95%CI) |
|---|---|---|---|---|---|
| Any blue (dark, pale or mixed) | 92% (87% - 96%) | 31% (25% - 36%) | 41% (35% - 46%) | 88% (80% - 94%) | .61 (.58 - .65) |
| Dark royal blue or pale blue | 80% (73% - 87%) | 51% (45% - 57%) | 46% (40% - 52%) | 83% (77% - 89%) | .66 (.61 - .70) |
| Dark royal blue | 64% (56% - 72%) | 67% (61% - 72%) | 50% (43% - 58%) | 78% (72% - 83%) | .66 (.61 - .70) |
| Pale blue | 16% (11% - 23%) | 84% (79% - 88%) | 34% (23% - 47%) | 66% (61% - 71%) | .50 (.46 - .54) |
| Mixed patterna | 12% (7% - 19%) | 80% (74% - 84%) | 23% (14% - 35%) | 64% (58% - 69%) | .46 (.42 - .49) |
| Comparison with other studies | |||||
| Parameter | Kenya | S. Africa | Brazilb | ||
| Vital stain dye | toluidine blue 0.05% | methylene blue 1% | toluidine blue 1% | ||
| Number of participants | 419 | 75 | 47 | ||
| Gender, Female No. (%) | 277 (66) | 45 (60) | 18 (38) | ||
| Age, median (IQR), y | 37 (32-45) | 35c | 58c | ||
| OSSN prevalence by histopathology No. (%) | 142 (34) | 33 (44)d | 27 (57) | ||
| Sensitivity (95%CI) | 92% (87% - 96%) | 97% (85% - 100%) | 100% (87% - 100%) | ||
| Specificity (95%CI) | 31% (25% - 36%) | 50% (36% - 65%) | 50% (27% - 73%) | ||
| Positive predictive value (95%CI) | 41% (35% - 46%) | 60% (47% - 72%) | 73% (56% - 86%) | ||
| Negative predictive value (95%CI) | 88% (80% - 94%) | 95% (78% - 99%) | 100% (69% - 100%) | ||
Abbreviations: ROC, receiver operator characteristic; IQR, interquartile range
Some areas of the lesion were deep royal blue and others pale
intervals were calculated from the data presented in the paper
The interquartile range was not reported in S. Africa and Brazil.
In the South African study, 49 (65%) were on the OSSN spectrum, however, in the analysis that was presented CIN was combined in with benign lesions for the calculation of the test parameters.
Deep royal blue staining demarcated the extent of the lesion well. A mixed staining pattern was observed in which only parts of the lesion would stain particularly actinic keratosis (Figure 1F). Mucus discharge also stained blue and should ideally be wiped away before staining. Also 133/275 (48%) of benign lesions had leukoplakia which stained blue. Brown pigmentation was found in 194 (47%) lesions. These included 12 cases of conjunctival naevi. Pigmentation made interpretation of staining more difficult (Figure 1L).
c). Inter-observer variation study
Staining results were easy to interpret. The scores of the six graders were similar to the lead author’s (agreement 91.3%) (Table 4). The lead author found 79% of the lesions stained with ToB, compared to an average of 76.5% for the six graders. The average kappa for staining scores was substantial (k=0.76). The six graders scored more lesions as OSSN compared to histopathology (53% vs 32%). The average kappa for diagnosis was moderate (k=0.40).
Table 4. Inter-observer agreement for the evaluation of ToB staining in 100 patients.
| Feature | Reference Standard No. (%) | Six Graders Median % | Agreement Median % | Average Kappa k (95%CI)c |
|---|---|---|---|---|
| Staining resulta | ||||
| Positive | 79 (79.0) | 76.5% | 91.3% | 0.76 (0.68 - 0.82) |
| Negative | 21 (21.0) | 23.5% | ||
| Diagnosisb | ||||
| OSSN | 32 (32.0) | 53.0% | 70.7% | 0.40 (0.31 - 0.48) |
| Non-OSSN | 68 (68.0) | 47.0% | ||
The reference standard for staining was the lead author
The reference standard for diagnosis was the histopathology result
The average kappa statistic was obtained by transforming the kappa statistics for each grader to Z scores using the Fisher Z transformation, averaging and performing a back-transformation.
Discussion
This is the largest study to date to evaluate ocular surface vital staining for the diagnosis of OSSN. It confirms findings from earlier studies that topical toluidine blue 0.05% is not associated with any significant adverse effects and found that the large majority of OSSN tumours stain.11, 12 The intensity of dark blue staining seen with the 0.05% preparation was similar to 1% solutions reported in other studies in South Africa and Brazil.
There were minimal side effects of vital staining. The mild superficial punctate keratopathy we observed may be attributable to dry eye due to disturbance of the tear film by the raised lesion. Dry eye is the most common ocular surface manifestation of HIV with a prevalence of up to 54%.16, 17 The mild discomfort reported on application of ToB may be aggravated by dry eye syndrome. The use of topical anaesthetic before vital staining may prevent this. Safety studies in animals found that intraocular injection (as opposed to topical use) of 1% and 2% ToB caused irreversible damage to all the corneal layers; 0.5% damaged the stromal keratocytes and corneal endothelium but 0.25% stained the lens capsule and did not damage any corneal layer or the trabecular meshwork.18 Wander et al conducted animal safety studies in rabbits and guinea pigs by applying eye drops of 0.01%, 0.1%, 0.25%, 0.5%, and 1.0% toluidine blue to stain corneal epithelial cells. The cells picked up the vital dye within 5 minutes. Wash out time was rapid and no toxic effects were observed.19
The diagnostic accuracy results of our study are similar to the ones from Brazil and South Africa showing high sensitivity and only low to moderate specificity (Table 3).11, 12 However, our study indicates that the sensitivity and specificity may not be quite as high as the two earlier, smaller studies had suggested. From the clinical standpoint however, the measure of accuracy that is more important than sensitivity or specificity is the predictive value. The positive predictive value in our study (41%) was lower than the South African (60%) and Brazilian (73%) studies. A caveat to such comparison is that the estimates of predictive values are only valid for the actual study population and similar populations with the same disease prevalence.
The South African study had a similar patient profile to ours with regard to age, sex and HIV infection.12 However, there were some of key differences: they used Methylene blue 1% dye and had a higher proportion of OSSN. Importantly, they combined the CIN lesions with benign lesions in their analysis, while we classified all CIN as part of the OSSN spectrum. They do not report how the CIN cases stained or whether there were different patterns of staining (pale or mixed). The patients in the Brazilian study were older than the Kenyan and South African study participants and were predominantly male (62%). Their HIV prevalence was not reported. This probably reflects different patterns of disease. The classification of OSSN and the grading systems for describing the staining in the Brazilian and Kenyan studies were similar. However, in the Brazilian study the concentration of Toluidine blue (1%) was 20 times that used in Kenya.
The differences observed in the test performance between these three studies could have a number of explanations. Firstly, it may reflect the larger sample size. There could be differences in patient populations, lesions included and diagnostic methods. We found lesion pigmentation made it more difficult to be certain about staining. The other two studies do not report on pigmentation. Our study had a higher proportion and wider variety of non-OSSN lesions than the other two. In addition, the gold standard for diagnosis of OSSN, histopathology, is open to interpretation.20
ToB has a higher sensitivity and specificity for oral cancers than we observed in our study. A Cochrane systematic review showed variable sensitivity of 50% to 97% and a more uniform specificity of 98% to 99%.8 However the prevalence of disease varied widely (1.4% to 50.9%). The difference between ocular and oral performance of ToB is unclear. Oral rinsing with 1% acetic acid before staining removes the surface debris (glycoprotein layer) and dehydrates cells.21 This may remove keratotic surface plaques which stained deep blue in our study (Figure 1). False positives may be further explained by the fact that ToB also stains inflammatory cells and mucin.7 Also OSSN, pterygia and actinic keratosis may be on the same causal pathway due to their association with ultraviolet radiation, p53 mutation and HPV with some regarding actinic keratosis and pterygia as pre-malignant lesions.22–24 They may therefore stain similarly.
The interpretation of staining results was relatively straightforward and had higher inter-observer agreement (k=0.76) than clinical diagnosis (k=0.40). Clinical diagnosis is more difficult due to the overlap in clinical features of OSSN and Non-OSSN.
This study had various limitations. Firstly, the concentration, purity and stability of ToB may have changed over time once a bottle was opened. A rising concentration may have increased the staining of all lesions. Secondly, in the analysis using 2x2 contingency tables, staining is treated as a dichotomous variable while in fact there are different degrees of intensity of blue staining. There was a high frequency of lesion pigmentation in this population but the use of more concentrated ToB may be toxic given its cell nucleus entry and may increase the false positive rates by staining more benign lesions.
If a test has a high sensitivity, a negative result has a high chance of ruling out the disease.25 Toluidine blue had a high sensitivity so a negative result makes OSSN unlikely does not completely rule it out.
In conclusion, ToB staining is safe and easily interpreted by different observers. Very few OSSN lesions did not stain with ToB. If ToB staining is negative then OSSN is unlikely. Positive staining demarcates conjunctival lesions well which could help in delineating the surgical excision margin particularly for circumlimbal OSSN lesions where both the corneal and conjunctival extents would otherwise not be clearly seen. Staining also detected small recurrences of OSSN (eFigure 2). ToB vital staining would not replace histopathology. The high sensitivity and low specificity makes ToB a good screening tool where there is an important penalty for missing a disease. In populations with limited histopathology services, an algorithm combining ToB staining with other clinical features may raise the composite specificity for OSSN.
Supplementary Material
Acknowledgments
The authors have no conflict of interest disclosures. SG received funding from the British Council for Prevention of Blindness (BCPB) fellowship programme. MJB is supported by The Wellcome Trust (Grant Number 098481/Z/12/Z). The funding organizations had no role in the design or conduct of this research. SG and MJB had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Author contributions:
Study concept and design: Gichuhi, Jaoko, Sagoo, Weiss, Burton
Acquisition, analysis, or interpretation of data: All authors
Drafting of the manuscript: Gichuhi, Sagoo, Weiss, Burton
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Gichuhi, Weiss, Burton
Obtained funding: Gichuhi, Weiss, Burton
Administrative, technical, or material support: Macharia, Kabiru, Zindamoyen, Rono, Ollando, Wanyonyi, Wachira, Munene, Jaoko, Sagoo, Weiss, Burton
Study supervision: Burton, Weiss
Conflict of interest disclosures:
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
References
- 1.Adesina A, Chumba D, Nelson AM, et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013 Apr;14(4):e152–157. doi: 10.1016/S1470-2045(12)70598-3. [DOI] [PubMed] [Google Scholar]
- 2.Rambau PF. Pathology practice in a resource-poor setting: Mwanza, Tanzania. Arch Pathol Lab Med. 2011 Feb;135(2):191–193. doi: 10.5858/135.2.191. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues EB, Costa EF, Penha FM, et al. The use of vital dyes in ocular surgery. Surv Ophthalmol. 2009 Sep-Oct;54(5):576–617. doi: 10.1016/j.survophthal.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Haritoglou C, Yu A, Freyer W, et al. An evaluation of novel vital dyes for intraocular surgery. Invest Ophthalmol Vis Sci. 2005 Sep;46(9):3315–3322. doi: 10.1167/iovs.04-1142. [DOI] [PubMed] [Google Scholar]
- 5.Herlin P, Marnay J, Jacob JH, Ollivier JM, Mandard AM. A study of the mechanism of the toluidine blue dye test. Endoscopy. 1983 Jan;15(1):4–7. doi: 10.1055/s-2007-1018595. [DOI] [PubMed] [Google Scholar]
- 6.Gandolfo S, Pentenero M, Broccoletti R, Pagano M, Carrozzo M, Scully C. Toluidine blue uptake in potentially malignant oral lesions in vivo: clinical and histological assessment. Oral Oncol. 2006 Jan;42(1):89–95. doi: 10.1016/j.oraloncology.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Sridharan G, Shankar AA. Toluidine blue: A review of its chemistry and clinical utility. J Oral Maxillofac Pathol. 2012 May;16(2):251–255. doi: 10.4103/0973-029X.99081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh T, Liu JL, Brocklehurst P, et al. Clinical assessment to screen for the detection of oral cavity cancer and potentially malignant disorders in apparently healthy adults. Cochrane Database Syst Rev. 2013;11:CD010173. doi: 10.1002/14651858.CD010173.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patton LL, Epstein JB, Kerr AR. Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J Am Dent Assoc. 2008 Jul;139(7):896–905. doi: 10.14219/jada.archive.2008.0276. quiz 993-894. [DOI] [PubMed] [Google Scholar]
- 10.Kaji Y, Hiraoka T, Oshika T. Vital staining of squamous cell carcinoma of the conjunctiva using toluidine blue. Acta Ophthalmol Scand. 2006 Dec;84(6):825–826. doi: 10.1111/j.1600-0420.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- 11.Romero IL, Barros Jde N, Martins MC, Ballalai PL. The use of 1% toluidine blue eye drops in the diagnosis of ocular surface squamous neoplasia. Cornea. 2013 Jan;32(1):36–39. doi: 10.1097/ICO.0b013e318243f61f. [DOI] [PubMed] [Google Scholar]
- 12.Steffen J, Rice J, Lecuona K, Carrara H. Identification of ocular surface squamous neoplasia by in vivo staining with methylene blue. Br J Ophthalmol. 2014 Jan;98(1):13–15. doi: 10.1136/bjophthalmol-2013-303956. [DOI] [PubMed] [Google Scholar]
- 13.Rashid A, Warnakulasuriya S. The use of light-based (optical) detection systems as adjuncts in the detection of oral cancer and oral potentially malignant disorders: a systematic review. J Oral Pathol Med. 2014 Sep 3; doi: 10.1111/jop.12218. [DOI] [PubMed] [Google Scholar]
- 14.Allegra E, Lombardo N, Puzzo L, Garozzo A. The usefulness of toluidine staining as a diagnostic tool for precancerous and cancerous oropharyngeal and oral cavity lesions. Acta Otorhinolaryngol Ital. 2009 Aug;29(4):187–190. [PMC free article] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- 16.Lestari YD, Sitompul R, Edwar L, Djoerban Z. Ocular diseases among HIV/AIDS patients in Jakarta, Indonesia. Southeast Asian J Trop Med Public Health. 2013 Jan;44(1):62–71. [PubMed] [Google Scholar]
- 17.Bekele S, Gelaw Y, Tessema F. Ocular manifestation of HIV/AIDS and correlation with CD4+ cells count among adult HIV/AIDS patients in Jimma town, Ethiopia: a cross sectional study. BMC Ophthalmol. 2013;13(1):20. doi: 10.1186/1471-2415-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamal Eldin SA, el Mehelmy EM, el Shazli EM, Mostafa YM. Experimental staining of the anterior lens capsule in albino rabbits. J Cataract Refract Surg. 1999 Sep;25(9):1289–1294. doi: 10.1016/s0886-3350(99)00153-4. [DOI] [PubMed] [Google Scholar]
- 19.Wander AH, Neumeister RD, Tschismadia I, Choromokos EA, Masukawa T. In vivo corneal and conjunctival epithelial nuclear stain. Cornea. 1985;4(1):8–13. [PubMed] [Google Scholar]
- 20.Margo CE, Harman LE, Mulla ZD. The reliability of clinical methods in ophthalmology. Surv Ophthalmol. 2002 Jul-Aug;47(4):375–386. doi: 10.1016/s0039-6257(02)00312-0. [DOI] [PubMed] [Google Scholar]
- 21.Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008 Jan;44(1):10–22. doi: 10.1016/j.oraloncology.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gichuhi S, Ohnuma SI, Sagoo MS, Burton MJ. Pathophysiology of ocular surface squamous neoplasia. Exp Eye Res. 2014 Dec;129C:172–182. doi: 10.1016/j.exer.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu T, Liu Y, Xie L, He X, Bai J. Progress in the pathogenesis of pterygium. Curr Eye Res. 2013 Dec;38(12):1191–1197. doi: 10.3109/02713683.2013.823212. [DOI] [PubMed] [Google Scholar]
- 24.Di Girolamo N. Association of human papilloma virus with pterygia and ocular-surface squamous neoplasia. Eye (Lond) 2012 Feb;26(2):202–211. doi: 10.1038/eye.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straus SE, Richardson WS, Glasziou P, Haynes BR. Evidence-based medicne - how to practice and teach EBM. 3rd ed. Elsevier; Churchill Livingstone: 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.