Abstract
Importance
There is a trend towards treating conjunctival lesions suspected to be ocular surface squamous neoplasia (OSSN) based on the clinical impression.
Objectives
To describe the presentation of OSSN and identify clinical features which distinguish it from benign lesions and subsequently evaluate their recognisability.
Design, Setting and Participants
Prospective multi-centre study in Kenya from July 2012 through July 2014 of 496 adults presenting with conjunctival lesions.
Exposures
Comprehensive history, slit lamp examination and photography before excision biopsy. Frequency of clinical features in OSSN and benign lesions recorded. One histopathologist examined all specimens. Six additional masked ophthalmologists independently examined photographs from 100 participants and assessed clinical features.
Main Outcomes and Measures
Proportions and means were compared using Chi-square, Fisher’s exact test or t-test as appropriate. Inter-observer agreement was estimated using Kappa statistic. Examiners’ assessments were compared to a reference.
Results
Among 496 participants, OSSN was the most common (38%) histological diagnosis, followed by pterygium (36%) and actinic keratosis (19%). OSSN cases were slightly older and tended to have lower levels of education than benign ones. Females predominated (67% of OSSN vs 64% of benign lesions; P = .65). HIV-infection was common among OSSN cases (74%). The commonest location was the nasal limbus (61% OSSN vs 78% benign lesions; P < .001). Signs more frequent in OSSN included; feeder vessels, odds ratio [OR], 5.8 [95%CI, 3.2-10.5]; moderate inflammation, OR, 3.5 [95%CI,1.8-6.8]; corneal involvement, OR, 2.7 [95%CI,1.8-4.0]; leukoplakia, OR, 2.6 [95%CI,1.7-3.9]; papilliform surface, OR, 2.1 [95%CI,1.3-3.5]; pigmentation, OR, 1.5 [95%CI, 1.0-2.2]; temporal location, OR, 2.0 [95%cI, 1.2-3.2]; circumlimbal location, (7.0% vs 0.3%; P<.001); severe inflammation (6.0% vs 0.3%; P <.001) and larger mean [SD] diameter (6.8 [3.2]mm vs 4.8[2.8]mm; P < .001). All OSSN signs were also observed in benign lesions. There was slight to fair inter-observer agreement in assessment of most signs and diagnosis (Kappa, 0.1-0.4). The positive predictive value of clinical appearance in identifying OSSN was 54% (interquartile range, 51-56) from photographs where prevalence was 32%.
Conclusions and Relevance
With overlapping phenotypes and modest inter-observer agreement, OSSN and benign conjunctival lesions are not reliably distinguished clinically. Point-of-care diagnostic tools may help.
Background
Ocular surface squamous neoplasia (OSSN) is a spectrum of pathology ranging from non-invasive intra-epithelial dysplasia of the conjunctiva and cornea (CCIN) to invasive squamous cell carcinoma.1 Worldwide, the incidence rate of OSSN is highest in the southern hemisphere (160S) with the peak occurring in Africa.2
The gold standard for diagnosis of OSSN is histopathology; however, the availability of this service is limited in Africa.3, 4 The decision to excise conjunctival lesions usually depends on the clinical impression. Most lesions are excised without subsequent histopathological confirmation of the diagnosis or information on tumour involvement of the excision margins. Even in countries with good access to pathology services, many lesions suspected of being OSSN are treated without histological confirmation of the diagnosis. In 2003 a standard of care survey in the USA showed that 51% of respondents always perform biopsies before instituting therapy for suspected OSSN lesions.5 This proportion was unchanged when the same survey was repeated in 2012.6 There are several reports from other regions where primary treatment for suspected OSSN tumours is provided using topical agents (mitomycin C, 5-fluorouracil, and interferon α2b) without excision for histopathological diagnosis.7–10 The rationale for this practice is to reduce the complications of excision such as limbal stem cell deficiency with large lesions or symblepharon. Population surveys to determine the prevalence of pinguecula or pterygium also rely on a clinical diagnosis to distinguish them from OSSN and other benign lesions.11–13
Several studies have tried to identify clinical features that may distinguish OSSN. A study in Tanzania found that OSSN lesions had a shorter mean duration than benign lesions (3.7 months vs 8.8 months; P = .03) while feeder vessels were more frequently associated with OSSN than benign lesions (P = .03).14 Male gender, temporal and superior locations, lack of corneal involvement, papillomatous and nodular appearance were associated with higher-grade OSSN lesions in a US study.15 OSSN lesions in HIV-infected individuals may be more likely to be of a higher grade of malignancy than HIV-negative patients.16
The aim of this study was to describe the clinical presentation of OSSN in Kenya and determine what clinical features might help to distinguish it from benign lesions. The main focus was on the frequency of clinical features in OSSN that could help to differentiate OSSN from other benign conjunctival lesions in this setting and the inter-observer variability in the recognition of these features.
Methods
Ethical Approval
This study was part of an integrated set of investigations into OSSN in Kenya. It was formally reviewed and approved by the Kenyatta National Hospital – University of Nairobi Ethics and Research Committee and the London School of Hygiene and Tropical Medicine Ethics Committee. This study adhered to the tenets of the Declaration of Helsinki. All participants gave informed written consent to take part in the study before enrolment and did not receive a stipend to participate.
Participants
Recruitment was between July 2012 and July 2014 in four eye care centers: Kenyatta National Hospital in Nairobi, PCEA Kikuyu Eye Unit about 25 kilometres (km) from Nairobi in Central Kenya, Kitale district hospital in the north Rift Valley 490km from Nairobi and Sabatia Eye Hospital 300km from Nairobi in the western highlands bordering Lake Victoria. We prospectively recruited all consenting, consecutive self-presenting adult patients (at least 18 years of age) with any conjunctival lesion (first presentation or a recurrence) suspected to be OSSN scheduled for surgery. Pregnant women and breastfeeding mothers were excluded.
Clinical Assessment
A comprehensive history was taken using a structured questionnaire and the eyes were examined with a slit lamp. The widest diameter of the lesion was measured using the slit lamp beam and scale. A pair of photographs of each lesion was taken, one in primary gaze and the other with the lesion in the center of the field. We used a Nikon D90 digital camera with Micro Nikkor 105mm F2.8 AFS zoom lens and the R1 close up speedlight. All photos were taken at 1:1 magnification ratio.
Surgery and Histopathology
All lesions were excised under local anaesthetic using an operating microscope with a 3mm clear margin. Cryotherapy was not applied as the participants were further invited to enroll in a treatment trial post-operatively. Specimens were placed directly into buffered formalin and subsequently examined at the histopathology laboratory at the MP Shah Hospital, Nairobi. One pathologist examined all the histology slides. Participants with mild, moderate or severe conjunctival intraepithelial neoplasia (CIN I, II, III respectively) together with any who had carcinoma-in-situ (CIS) and invasive squamous cell carcinoma were classified as having OSSN. A three-grade system was used to classify carcinomas histologically as well, moderately and poorly differentiated after the American Joint Committee on Cancer (AJCC).17 Benign lesions included pterygium, actinic keratosis, papillomas, pyogenic granulomas, nevi and rhinosporidiosis. The diagnosis of actinic keratosis was based on the presence of elastotic stromal degeneration, acanthosis, hyperkeratosis and parakeratosis in the presence of normal cellular polarity. By the accepted criteria for dysplasia, such lesions were classified as CIN only if there is loss of polarity.
Cases of OSSN were invited to enroll in a case-control study that involved testing for HIV and CD4 count. HIV was initially tested using Vironostika antigen/antibody kit then later changed to rapid tests using Alere Determine HIV-1/2 Ag/Ab and Trinity Unigold. CD4 count was tested using FacsCount (Becton Dickinson) USA. Those with benign lesions were not tested. Voluntary testing and counselling was offered at the health facility.
Inter-observer Study
To determine the inter-observer variability in the assessment of the clinical features six final year ophthalmology residents in the University Of Nairobi Department Of Ophthalmology at Kenyatta National Hospital independently assessed photographs from the last 100 consecutive participants enrolled into the study from one center. They were masked to the diagnosis. Images were projected onto a screen. The clinical case-mix was the same in this sample of patients compared to the whole dataset that included patients from all the four study centers. Cases with features that may suggest malignancy such as very large tumours filling the orbit were excluded from this assessment. The graders were asked to determine if each feature was either present, absent or difficult to determine.
Statistical Analysis
Data was managed in an Access database (Microsoft), cleaned and transferred into STATA version 12.1 (StataCorp, College Station, Texas, USA) for analysis. In this analysis we compared the clinical features of OSSN and benign lesions. Large orbital tumours and non-OSSN malignancies were excluded. Categorical variables were compared using the Pearson’s chi-square test, odds ratios (ORs) or Fisher’s exact test where appropriate. Logistic regression was used to obtain adjusted ORs. To determine whether continuous variables were normally distributed we generated Q-Q plots and compared the variances in both groups using the standard deviation test. Where the deviations differed the t-test was conducted with unequal variances.
The inter-observer agreement between graders was compared using the kappa (Κ) statistic without weighting and graded using the Landis & Koch method as poor, slight, fair, moderate, substantial or almost perfect.18 To calculate an average value, the kappa statistics for each grader were transformed to Z scores using the Fisher Z transformation, averaged, and then back-transformed to kappa.
Results
Five hundred and thirty-seven participants with conjunctival lesions were enrolled. Histology reports were available for 496 participants. Eighteen tissue specimens were autolysed on arrival at the pathology lab perhaps from poorly reconstituted formalin (one was a batch of 16 from one center) and 22 were presumed lost in transit. Seven (1.4%) were large orbital tumours. A total of 488 participants were therefore included in the analysis of clinical features.
Histopathological Diagnosis
OSSN was the most common type of ocular surface lesion (38%) (eTable 1 in the supplement). This was followed by pterygium (36%) and actinic keratosis (19%), which were the most common benign lesions. All stages of OSSN were seen with the most frequent being moderately differentiated squamous cell carcinoma. There was one case of sarcomatoid spindle cell carcinoma and a wide range of benign lesions.
Demographic Characteristics
The demographic characteristics of participants, subdivided by the pathology type are shown in Table 1. About two-thirds were female (65%), with no difference between OSSN and benign lesions. Most individuals presenting with conjunctival lesions were young to middle aged adults (mean [SD] age, 39 [11.3] years). Participants with OSSN were slightly older than those with benign lesions (P = .002), more likely to be widowed, and to have a lower level of education. Those who did not have any formal education had the highest risk of OSSN after adjusting for age and marital status.
Table 1. Demographic characteristics of participants with OSSN and benign conjunctival lesions. This includes orbital disease.
| Demographic feature | OSSN | Benign | OSSN vs Benign lesions |
|||
|---|---|---|---|---|---|---|
| N=187 | N=308 | Crude OR (95% CI) | P value | Adj ORa (95% CI) | P value | |
| Sex, No. (%) | ||||||
| Male | 62 (33.0) | 110 (36.0) | 1 [Reference] | 1 [Reference] | ||
| Female | 125 (67.0) | 198 (64.0) | 1.1 (0.8 - 1.6) | .56 | 1.1 (0.8 - 1.6) | .65 |
| Age in years, mean (SD), y | 41 (11.6) | 38 (10.9) | NA | .002b | NA | NA |
| Marital status, No. (%) | ||||||
| Single | 30 (16.0) | 42 (14.0) | 1 [Reference] | 1 [Reference] | ||
| Married | 123 (66.0) | 231 (75.0) | 0.8 (0.4 - 1.3) | .04 | 0.5(0.3 - 0.9) | .05 |
| Divorced or Separated | 11 (6.0) | 18 (6.0) | 0.9 (0.4 - 2.1) | 0.5 (0.2 - 1.3) | ||
| Widowed | 23 (12.0) | 17 (6.0) | 1.9 (0.9 - 4.2) | 0.9 (0.4 - 2.2) | ||
| Highest education level, No. (%) | ||||||
| More than secondary | 17 (9.0) | 66 (21.0) | 1 [Reference] | 1 [Reference] | ||
| Completed secondary school | 58 (31.0) | 85 (28.0) | 2.7(1.4 - 5.0) | 2.7 (1.4 - 5.1) | ||
| Some secondary school | 13 (7.0) | 37 (12.0) | 1.4 (0.6 - 3.1) | .001 | 1.4 (0.6 - 3.4) | <.001 |
| Completed primary school | 57 (30.0) | 74 (24.0) | 3.0 (1.6 - 5.8) | 3.1 (1.6 - 5.9) | ||
| Some primary school | 24 (13.0) | 38 (12.0) | 2.5 (1.2 - 5.2) | 2.4 (1.1 - 5.3) | ||
| None | 18 (10.0) | 8 (3.0) | 8.7 (2.9 - 26.5) | 10.8 (3.3 - 34.8) | ||
Abbreviations: OSSN, ocular surface squamous neoplasia; SD, standard deviation; NA, not applicable
adjusted for education, age group and marital status. Sex did not change the multivariable model so it was not included.
t test
Clinical History
The primary symptoms at presentation are shown in eTable 2 in the supplement. Overall, the presenting symptoms were similar by disease group (P = .14). The most frequent presenting complaint was a lump or swelling (67%) followed pain (12%), redness (6%) and itchiness (5%).
Additional information on the clinical history is presented in eTable 3 in the supplement. Median duration from first developing symptoms to presentation was longer for OSSN than benign tumours (8 months vs 5 months; P = .03) and a history of prior conjunctival excision was more frequent in OSSN than benign lesions (18% vs 6%; P < .001). The mean [SD] number of prior excision surgeries where this had taken place was however similar in both groups (1.4 [0.8] vs 1.3 [0.7]; P = .66). There was no evidence of a difference between OSSN and benign lesions in terms of a family history of eye cancer or cancer at another site.
There was strong evidence that participants with OSSN had longer sun exposure in their current (P = .02) and previous (P = .003) occupation, but little evidence that they had a current predominantly outdoor occupation (64% vs 57%; P = .14), or worked outdoors in previous employment (57% vs 48%; P = .22). There was no difference in the proportion who wore hats or sunglasses, or who smoked cigarettes. However, among smokers, the mean [SD] number of cigarettes smoked daily was higher among OSSN patients (12 [11] vs 7[6], P = .03).
Of 133 OSSN patients tested for HIV, 98 (74%) were positive. Median CD4 count of 91 patients with OSSN was 265 cells/mm3 (interquartile range, 125-670 cells/mm3). Some participants did not return for histology results after surgery and thus were not tested for HIV or CD4. Participants with OSSN were more likely to be on ART than those with benign lesions (38% vs 15%; P < .001). There was little evidence of a difference (P = .30) in mean [SD] duration of ART use in those with OSSN (2.9 [3.0] years) compared to those with benign lesions (3.5[2.9] years). According to the Kenya Ministry of Health HIV guidelines, HIV-infected patients with CD4≤350 cells/mm3 at first contact would be eligible for ART.19 It is difficult to know how many of our patients were eligible for ART as they were already in various stages of HIV care.
Clinical Features
Clinical features are described in Table 2 and illustrated in Figure 1&2. There were a wide variety of presentation patterns for each type of OSSN. We illustrate this with a range of moderately differentiated squamous cell carcinoma tumours in Figure 1, F-O. Overall, OSSN lesions were larger than benign lesions (mean [SD] diameter 6.8 [3.2] mm vs 4.8mm [2.8], P < .001). All the features seen in OSSN also occurred in benign lesions (Table 2) and this overlap is illustrated in Figure 2. OSSN lesions were more likely to be at the temporal limbus (28% vs 16%; P = .002), circumlimbal (7.0% vs 0.3%; P < .001), to have severe inflammation (P < .001) and leukoplakia (72% vs 50%; P < .001). A gelatinous appearance occurred with almost equal frequency in both groups, while a fibrovascular appearance was more frequent in benign lesions and a papilliform appearance in OSSN. OSSN was more likely to be pigmented, have a feeder vessel and involve the cornea. Regional lymphadenopathy was rare (n=7, 1.5%) in OSSN even in those with large orbital tumours.
Table 2. Comparison of the clinical features of OSSN with benign conjunctival lesions on slit lamp examination.
| Clinical feature | OSSN | Benign lesions | OSSN vs benign |
|
|---|---|---|---|---|
| N=180 | N=308 | |||
| No. (%) | No. (%) | OR (95% CI) | P value | |
| Lesion location | ||||
| nasal limbus | 110 (61.0) | 241 (78.0) | 0.4 (0.3 - 0.7) | <.001 |
| temporal limbus | 50 (28.0) | 50 (16.0) | 2.0 (1.2 - 3.2) | .002 |
| superior limbus | 2 (1.0) | 2 (0.7) | 1.7 (0.1 - 23.9) | .59 |
| inferior limbus | 1 (0.6) | 4 (1.3) | 0.4 (0.0 - 4.3) | .43 |
| circumlimbal | 12 (7.0) | 1 (0.3) | 21.9 (3.2 - 940.2) | <.001 |
| mostly corneal | 1 (0.6) | 0 (0.0) | ∞ | .19 |
| both nasal & temporal limbus | 3 (2.0) | 1 (0.3) | 5.2 (0.4 - 274.0) | .11 |
| caruncle | 0 (0.0) | 3 (1.0) | 0 (0.0 - 2.2) | .18 |
| lid | 1 (0.6) | 6 (2.0) | 0.3 (0.0 - 2.4) | .21 |
| Inflammation at the lesion site | ||||
| none | 21 (13.0) | 74 (24.0) | 1[Reference] | |
| minimal | 50 (28.0) | 111 (36.0) | 1.6 (0.9 - 2.9) | |
| mild | 46 (25.0) | 71 (23.0) | 2.3 (1.2 - 4.3) | <.001 |
| moderate | 51 (28.0) | 51 (17.0) | 3.5 (1.8 - 6.8) | |
| severe | 12 (6.0) | 1 (0.3) | 42.3 (3.7 - 478.3) | |
| Leukoplakia | 129 (72.0) | 152 (50.0) | 2.6 (1.7 - 3.9) | <.001 |
| Erythroplakia | 30 (17.0) | 53 (17.0) | 1.0 (0.6 - 1.6) | .88 |
| Gelatinous appearance | 121 (67.0) | 188 (61.0) | 1.3 (0.9 - 2.0) | .19 |
| Fibrovascular appearance | 18 (10.0) | 81 (26.0) | 0.3 (0.2 - 0.6) | <.001 |
| Papilliform appearance | 41 (23.0) | 38 (12.0) | 2.1 (1.3 - 3.5) | .003 |
| Brown lesion pigmentation | 96 (53.0) | 133 (44.0) | 1.5 (1.0 - 2.2) | .04 |
| Lesion feeder vessels | 163 (91.0) | 195 (64.0) | 5.8 (3.2 - 10.5) | <.001 |
| Corneal involvement | 115 (65.0) | 121 (40.0) | 2.7 (1.8 - 4.0) | <.001 |
| Lesion diameter, mean (SD), mm | 6.8 (3.2) | 4.8 (2.8) | NA | <.001 b |
Abbreviations: OSSN, ocular surface squamous neoplasia; SD, standard deviation; NA, not applicable; ∞ stands for infinite
The numbers assessed may vary in different cells if the item assessed did not apply to all participants
t-test
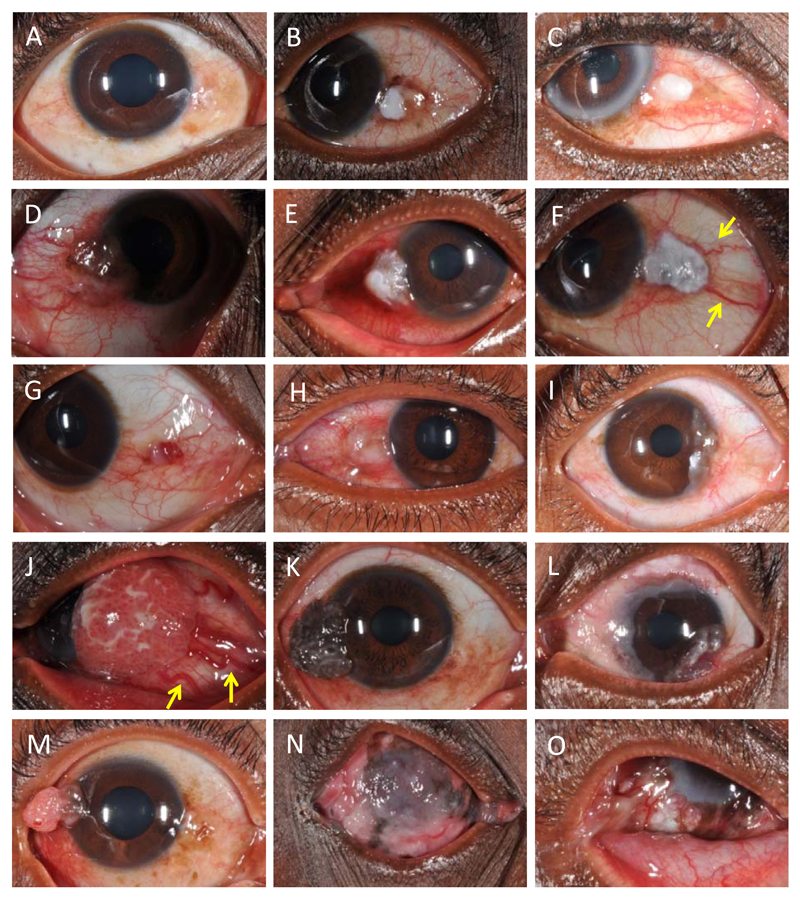
Figure 1. Five grades of inflammation associated with OSSN are shown in A-E. Various clinical features seen in moderately differentiated squamous cell carcinoma are shown from F to O. F-K shows different tumour surface appearances and various growth patterns are seen in L-O.
(A) No inflammation; (B) Minimal inflammation with leukoplakia and brown pigmentation;n (C) Mild inflammation with leukoplakia; (D) Moderate inflammation with leukoplakia; (E) Severe inflammation with leukoplakia; (F) Leukoplakia – patches of keratosis visible as white adherent plaques. Feeder vessels (distinctly dilated blood vessels larger than the rest of conjunctival vessels) are also shown by yellow arrows; (G) Erythoplakia – a red subconjunctival popular haemorrhage-like appearance; (H) Gelatinous appearance; (I) Fibrovascular appearance; (J) Papilliform appearance with markedly large feeder vessels (yellow arrows); (K) Brown pigmentation; (L) circumlimbal lesion; (M) pedunculated lesion;(N) Extensive corneal involvement with orbital disease; (O) Symblepharon.
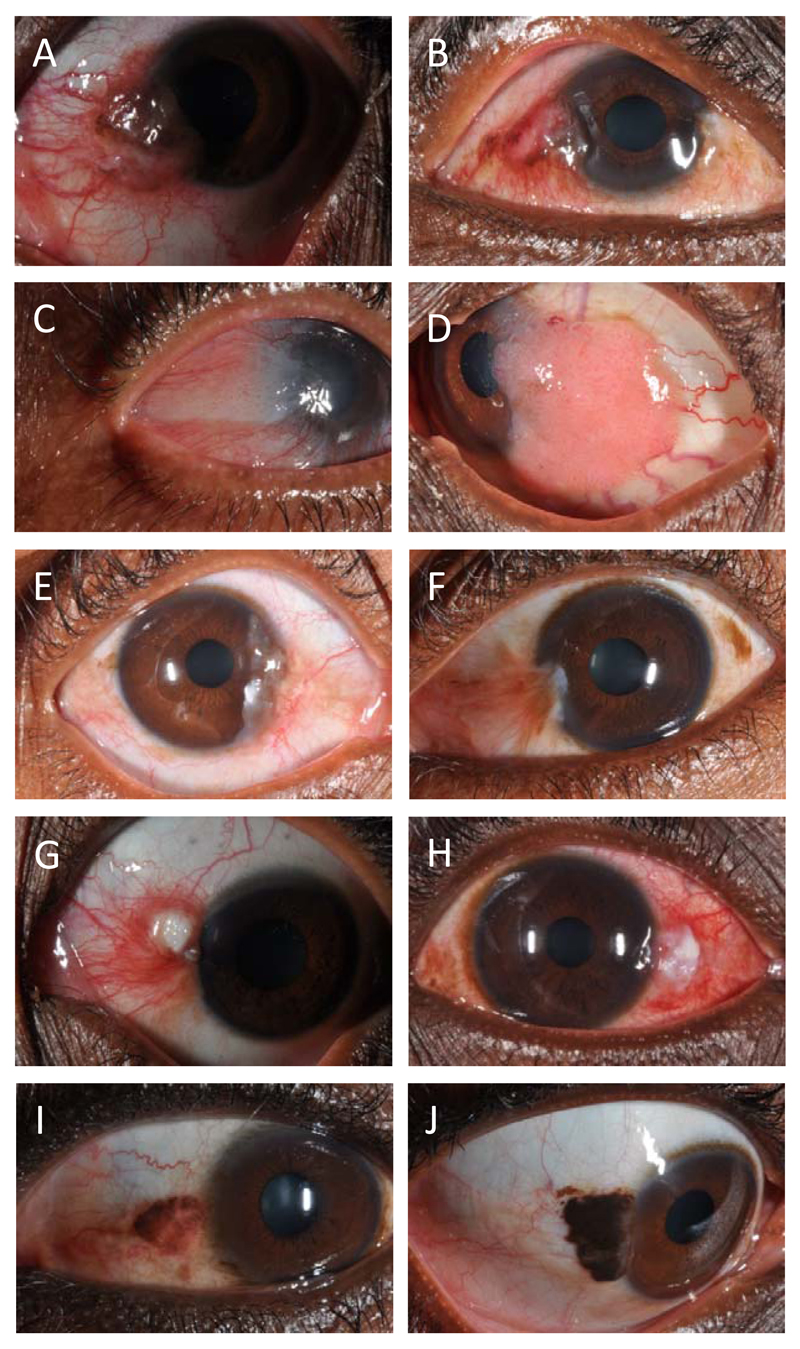
Figure 2. Overlapping clinical features of OSSN and benign/pre-malignant lesions.
A gelatinous surface with pigmentation in (A) a moderately differentiated squamous cell carcinoma and (B) a pterygium. A papilliform surface in (C) CIN2 and (D) a squamous papilloma. Note that the squamous papilloma in addition shows multiple feeder vessels. A fibrovascular appearance in (E) a moderately differentiated squamous cell carcinoma and (F) a pterygium. The pterygium has some brown pigmentation also seen on the temporal side of the same eye. Leukoplakia with moderate inflammation in (G) a well differentiated squamous cell carcinoma and (H) an actinic keratosis showing. Brown pigmentation in (I) CIN3 and (J) a nevus.
Patients with large orbital tumours
All seven participants with large orbital tumours had squamous cell carcinoma. Four were female and 3 were male. Their age ranged from 30 to 85 years. Only one had prior excision surgery, although no histology report was available. The tumours had been first noted 7 months to 15 years earlier. Five had HIV infection and 3 were on ART. Despite having large tumours for a long time only 2 of them had regional lymphadenopathy.
Inter-observer variation in recognition of clinical features
Inter-observer variation is described in eTable 4 in the supplement. Overall there was fair to moderate agreement in assessment of most signs and the clinical diagnosis. Most features were easily recognized by the graders. The proportions of features they recognized were fairly similar to an experienced examiner. Using clinical features to make a diagnosis of OSSN had a median sensitivity of 86% (interquartile range, 81-88), specificity of 60% (interquartile range, 53-69) and positive predictive value of 54% (interquartile range, 51-56) among the six examiners (eTable 5 in the supplement).
Discussion
There appears to be a tendency to treat presumed OSSN without a tissue diagnosis. However, we found a high degree of overlap in the clinical features of OSSN and benign lesions. Although some features were more frequent in OSSN than the benign group, they still occurred at a fairly high frequency in the benign group. In our view, the differences are insufficient to depend upon clinical features as an indicator of the underlying diagnosis. Moreover, there was only modest (k=0.4) inter-observer agreement in the assessment of the diagnosis and a positive predictive value (54%) no better than chance when using clinical features to make the diagnosis. The difficulty observed in determining surface appearance may be partly attributed to the lack of a stereoscopic view from photographs. The agreement in determining the presence of most clinical features was better than that for overall diagnostic classification into OSSN or benign.
The age and sex distribution of OSSN patients was consistent with prior series from Africa, where young adults and especially women predominate.2, 20 In temperate regions it is predominantly a disease of older males.21, 22 There was no difference in the sex distribution of OSSN and benign lesions. Higher education may increase awareness and earlier health seeking behaviour. Median duration before presentation did not however conform to this trend, and possibly showed the opposite of what has been previously reported.
The medical history of patients with OSSN and benign lesions is essentially similar. The difference in occupational history with a longer exposure to solar ultraviolet radiation (UVR) in those with OSSN than benign lesions is consistent with UVR being a major risk factor for OSSN.2 There was also a heavier exposure to cigarette smoking with OSSN lesions, which has so far not been clearly described as a risk factor for OSSN.
Although some clinical features showed differences between OSSN and benign lesions, it may be difficult to tell the two apart. For instance, OSSN lesions were larger than benign lesions but a 2.0mm difference between 6.8mm and 4.8mm is relatively small. A circumlimbal pattern was more frequent in OSSN; however, it only occurred in 3% of the conjunctival lesions. While OSSN was twice as likely to be temporal, 16% of benign lesions were located temporally, compared to 28% of OSSN lesions. Such a difference in proportion is difficult to rely on in the clinical setting.
The preponderance of nasal conjunctival lesions is consistent with earlier reports, and may be due to the previously described observation that incident temporal sunlight is focused nasally with a 20-fold magnification in intensity.23 Pterygia and actinic keratosis are considered pre-malignant and have some similarities with OSSN in their pathophysiology including association with solar UV radiation, p53 gene mutation and human papilloma virus (HPV).24–27 Being on the same causal pathway may also explain the overlap of clinical features. Further, we would also expect benign changes to occur before malignant ones. This may explain why OSSN cases were older than the benign cases most of whom had pterygia or actinic keratosis.
Differences between OSSN and benign lesions in the proportions of moderate and severe inflammation (P <.001) may not in isolation be easily applied in the clinical setting. OSSN was more likely to show leukoplakia than benign lesions, however 50% of benign lesions also had it. This situation is also seen with other features like the lesion surface appearance, pigmentation, feeder vessels and corneal involvement in Table 2 & Figure 2.
This study has a number of limitations. The six examiners in the inter-observer component did not have a full history, which may help to inform the clinical diagnosis, nor did they assess the lesions at the slit lamp as this would have been logistically impossible. Secondly, this was a hospital-based study, which may introduce selection bias in the types of patients seen. However the objective of the study was to compare OSSN and benign lesions presenting to clinicians in a healthcare facility setting, so this potential bias would not affect comparability of the two types of disease. Finally, distinguishing pterygia and OSSN by histopathology is sometimes difficult. Studies in Australia and USA found histopathological features of OSSN in 9.8% and 1.7% respectively of lesions previously classified as pterygia.28, 29
In conclusion the clinical features of OSSN and benign conjunctival lesions overlap. Both disease groups have common pathophysiological mechanisms and this may explain their overlapping clinical appearance. Although individual features are identified by different examiners with reasonable consistency, they do not reliably distinguish the two disease groups. Examination of photographs alone cannot replace clinical examination and biopsy, indicating that teleophthalmology approaches for the diagnosis of OSSN require more study. Therefore in the African context where the range of risk factors is perhaps wider and the clinical behaviour of the disease more aggressive compared to temperate regions we conclude that biopsy should be performed before treatment. The occurrence of malignant changes described in pterygia and other benign lesions further underscores the need for histopathology.
Supplementary Material
Acknowledgments
The authors have no conflict of interest disclosures. SG received funding from the British Council for Prevention of Blindness (BCPB) fellowship programme. MJB is supported by The Wellcome Trust (Grant Number 098481/Z/12/Z). The funding organizations had no role in the design or conduct of this research. SG and MJB had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Author contributions:
Study concept and design: Gichuhi, Sagoo, Weiss, Burton
Acquisition, analysis, or interpretation of data: All authors
Drafting of the manuscript: Gichuhi, Sagoo, Weiss, Burton
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Gichuhi, Weiss, Burton
Obtained funding: Gichuhi, Weiss, Burton
Administrative, technical, or material support: Macharia, Kabiru, Zindamoyen, Rono, Ollando, Wanyonyi, Wachira, Munene, Sagoo, Weiss, Burton Study supervision: Weiss, Burton
Conflict of interest disclosures:
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
References
- 1.Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39(6):429–450. doi: 10.1016/s0039-6257(05)80054-2. [DOI] [PubMed] [Google Scholar]
- 2.Gichuhi S, Sagoo MS, Weiss HA, Burton MJ. Epidemiology of ocular surface squamous neoplasia in Africa. Trop Med Int Health. 2013;18(12):1424–1443. doi: 10.1111/tmi.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adesina A, Chumba D, Nelson AM, et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013;14(4):e152–157. doi: 10.1016/S1470-2045(12)70598-3. [DOI] [PubMed] [Google Scholar]
- 4.Rambau PF. Pathology practice in a resource-poor setting: Mwanza, Tanzania. Arch Pathol Lab Med. 2011;135(2):191–193. doi: 10.5858/135.2.191. [DOI] [PubMed] [Google Scholar]
- 5.Stone DU, Butt AL, Chodosh J. Ocular surface squamous neoplasia: a standard of care survey. Cornea. 2005;24(3):297–300. doi: 10.1097/01.ico.0000138834.42489.ba. [DOI] [PubMed] [Google Scholar]
- 6.Adler E, Turner JR, Stone DU. Ocular surface squamous neoplasia: a survey of changes in the standard of care from 2003 to 2012. Cornea. 2013;32(12):1558–1561. doi: 10.1097/ICO.0b013e3182a6ea6c. [DOI] [PubMed] [Google Scholar]
- 7.Nutt RJ, Clements JL, Dean WH. Ocular surface squamous neoplasia in HIV-positive and HIV-negative patients and response to 5-fluorouracil in Angola. Clin Ophthalmol. 2014;8:2435–2440. doi: 10.2147/OPTH.S70459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell HC, Chadha V, Lockington D, Kemp EG. Topical mitomycin C chemotherapy in the management of ocular surface neoplasia: a 10-year review of treatment outcomes and complications. Br J Ophthalmol. 2010;94(10):1316–1321. doi: 10.1136/bjo.2009.176099. [DOI] [PubMed] [Google Scholar]
- 9.Besley J, Pappalardo J, Lee GA, Hirst LW, Vincent SJ. Risk factors for ocular surface squamous neoplasia recurrence after treatment with topical mitomycin C and interferon alpha-2b. Am J Ophthalmol. 2014;157(2):287–293 e282. doi: 10.1016/j.ajo.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Nanji AA, Moon CS, Galor A, Sein J, Oellers P, Karp CL. Surgical versus medical treatment of ocular surface squamous neoplasia: a comparison of recurrences and complications. Ophthalmology. 2014;121(5):994–1000. doi: 10.1016/j.ophtha.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Q, Xiang J, Cui X, Zhou X, Xu J. Prevalence and associated factors of pinguecula in a rural population in Shanghai, Eastern China. Ophthalmic Epidemiol. 2015;22(2):130–138. doi: 10.3109/09286586.2015.1012269. [DOI] [PubMed] [Google Scholar]
- 12.Marmamula S, Khanna RC, Rao GN. Population-based assessment of prevalence and risk factors for pterygium in the South Indian state of Andhra Pradesh: the Andhra Pradesh Eye Disease Study. Invest Ophthalmol Vis Sci. 2013;54(8):5359–5366. doi: 10.1167/iovs.13-12529. [DOI] [PubMed] [Google Scholar]
- 13.Viso E, Gude F, Rodriguez-Ares MT. Prevalence of pinguecula and pterygium in a general population in Spain. Eye (Lond) 2011;25(3):350–357. doi: 10.1038/eye.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguena MB, van den Tweel JG, Makupa W, et al. Diagnosing ocular surface squamous neoplasia in East Africa: case-control study of clinical and in vivo confocal microscopy assessment. Ophthalmology. 2014;121(2):484–491. doi: 10.1016/j.ophtha.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kao AA, Galor A, Karp CL, Abdelaziz A, Feuer WJ, Dubovy SR. Clinicopathologic correlation of ocular surface squamous neoplasms at bascom palmer eye institute: 2001 to 2010. Ophthalmology. 2012;119(9):1773–1776. doi: 10.1016/j.ophtha.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 16.Makupa II, Swai B, Makupa WU, White VA, Lewallen S. Clinical factors associated with malignancy and HIV status in patients with ocular surface squamous neoplasia at Kilimanjaro Christian Medical Centre, Tanzania. Br J Ophthalmol. 2012;96(4):482–484. doi: 10.1136/bjophthalmol-2011-300485. [DOI] [PubMed] [Google Scholar]
- 17.American Joint Committee on Cancer. Carcinoma of the conjunctiva. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. Cancer staging handbook. 7 ed. New York: Springer; 2010. pp. 600–602. [Google Scholar]
- 18.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 19.National AIDS/STI Control Program (NASCOP) Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: A rapid advice. 2014.
- 20.Tiong T, Borooah S, Msosa J, et al. Clinicopathological review of ocular surface squamous neoplasia in Malawi. Br J Ophthalmol. 2013;97(8):961–964. doi: 10.1136/bjophthalmol-2012-302533. [DOI] [PubMed] [Google Scholar]
- 21.Kim BH, Kim MK, Wee WR, Oh JY. Clinical and pathological characteristics of ocular surface squamous neoplasia in an Asian population. Graefes Arch Clin Exp Ophthalmol. 2013;251(11):2569–2573. doi: 10.1007/s00417-013-2450-0. [DOI] [PubMed] [Google Scholar]
- 22.Tunc M, Char DH, Crawford B, Miller T. Intraepithelial and invasive squamous cell carcinoma of the conjunctiva: analysis of 60 cases. Br J Ophthalmol. 1999;83(1):98–103. doi: 10.1136/bjo.83.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coroneo M. Ultraviolet radiation and the anterior eye. Eye Contact Lens. 2011;37(4):214–224. doi: 10.1097/ICL.0b013e318223394e. [DOI] [PubMed] [Google Scholar]
- 24.Liu T, Liu Y, Xie L, He X, Bai J. Progress in the pathogenesis of pterygium. Curr Eye Res. 2013;38(12):1191–1197. doi: 10.3109/02713683.2013.823212. [DOI] [PubMed] [Google Scholar]
- 25.Chalkia AK, Spandidos DA, Detorakis ET. Viral involvement in the pathogenesis and clinical features of ophthalmic pterygium (Review) Int J Mol Med. 2013;32(3):539–543. doi: 10.3892/ijmm.2013.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Girolamo N. Association of human papilloma virus with pterygia and ocular-surface squamous neoplasia. Eye (Lond) 2012;26(2):202–211. doi: 10.1038/eye.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dushku N, Hatcher SL, Albert DM, Reid TW. p53 expression and relation to human papillomavirus infection in pingueculae, pterygia, and limbal tumors. Arch Ophthalmol. 1999;117(12):1593–1599. doi: 10.1001/archopht.117.12.1593. [DOI] [PubMed] [Google Scholar]
- 28.Hirst LW, Axelsen RA, Schwab I. Pterygium and associated ocular surface squamous neoplasia. Arch Ophthalmol. 2009;127(1):31–32. doi: 10.1001/archophthalmol.2008.531. [DOI] [PubMed] [Google Scholar]
- 29.Oellers P, Karp CL, Sheth A, et al. Prevalence, treatment, and outcomes of coexistent ocular surface squamous neoplasia and pterygium. Ophthalmology. 2013;120(3):445–450. doi: 10.1016/j.ophtha.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.