Abstract
Fracture liaison services are recommended as a model of best practice for organising patient care and secondary fracture prevention for hip fracture patients, although variation exists in how such services are structured. There is considerable uncertainty as to which model is most cost-effective and should therefore be mandated. This study evaluated the cost-effectiveness of orthogeriatric (OG) and nurse-led fracture liaison service (FLS) models of post-hip fracture care compared to usual care. Analyses were conducted from a healthcare and personal social services payer perspective, using a Markov model to estimate the lifetime impact of the models of care. The base-case population consisted of men and women aged 83 years with a hip fracture. The risk and costs of hip and non-hip fractures were derived from large primary and hospital care datasets in the UK. Utilities were informed by a meta-regression of 32 studies. In the base-case analysis, the orthogeriatric-led service was the most effective and cost-effective model of care at a threshold of £30,000 per quality-adjusted life years gained (QALY). For women age 83 years, the OG-led service was the most cost-effective at £22,709/QALY. If only healthcare costs are considered, OG-led service was cost-effective at £12,860/QALY and £14,525/QALY for women and men aged 83 years, respectively. Irrespective of how patients were stratified in terms of their age, sex, and Charlson co-morbidity score at index hip fracture, our results suggest that introducing an orthogeriatrician-led or a nurse-led FLS is cost-effective when compared to usual care. Although, considerable uncertainty remains concerning which of the models of care should be preferred, introducing an orthogeriatrician-led service seems to be the most cost-effective service to pursue.
Keywords: cost-effectiveness, osteoporosis, hip fracture, secondary prevention, fracture liaison service, natural experiment, health economics, cost-utility, hip fracture, cost
Introduction
Hip fractures are a major public health problem, with high morbidity, mortality, and health and social care costs.(1–3) In the UK, hip fractures account for £1.1 billion per year in hospital costs,(4) which is expected to rise to £1.5 billion by 2025(4) when 104,000 annual cases are predicted.(3) A previous study showed that acute hospital admission due to hip fracture was the largest component of hospital costs, with higher costs and length of stay for second compared with index hip fractures.(4) There is therefore a strong economic incentive to identify and implement cost-effective measures for the provision of timely patient care and secondary fracture prevention following an index hip fracture.
Fracture liaison services are recommended as a model of best practice for patients with hip fracture. This is supported by international guidance,(5,6) patient organisations, the UK Department of Health,(7,8) and several UK national bodies (British Orthopaedic Association,(9) NICE,(10) National Osteoporosis Society, and Age UK(11)). The proposed model consists of two main stages of care: 1) orthogeriatric services focusing on achieving optimal recovery following hip fracture, and 2) nurse-led fracture liaison services focusing on secondary fracture prevention of fragility fractures.
The Glasgow Fracture Liaison Service(12) reported that for every 1000 patients with a fragility fracture assessed by that FLS, 18 fragility fractures (including 11 hip fractures) were prevented. However, no firm evidence or evidence-based consensus exists as to which care model should be mandated across the NHS. As a result, current practice reflects significant variations across NHS hospital providers in the adoption and organisation of FLS.(13)
We used large healthcare datasets based on de-identified computerised records, together with a detailed evaluation of hospital hip fracture services in a UK region(14) to estimate the ‘real world’ impact of the different models of care in terms of morbidity, survival and costs, and to determine the cost-effectiveness of orthogeriatric (OG) and nurse-led fracture liaison service (FLS) models of care following hip fracture compared to usual care in the English NHS.
Materials and Methods
Decision model and models of care
We estimated the cost-effectiveness of the following models of care for all patients with a hip fracture admitted to a NHS hospital: 1) Introduction of an orthogeriatrician-led (OG) model of post-hip fracture care; 2) Introduction of a nurse-led fracture liaison (FLS) model of post-hip fracture care; 3) Standard post-hip fracture care.
These models of care reflect the services provided in one regional area in the UK that comprises 11 hospitals receiving patients with acute hip fractures. Details on the OG and FLS models delivered within the region from 2003 to 2013 has been published elsewhere (14) (15). Briefly, although there was variation across hospitals, the introduction of the OG model involved the appointment of an orthogeriatrician as the clinical lead, responsible for case finding, pre-operative assessment, patient assessment and treatment initiation as well as having involvement in post-operative care. The average staff level of an OG for secondary fracture prevention services was estimated at 0.75 whole time equivalents (WTE) within region. The FLS model involved the appointment of a Nurse Specialist (Osteoporosis or Trauma) responsible for case finding, assessment, treatment recommendations and medication assessment, preparing the follow up plan as well as providing additional support for management of bone health in hospital. In this region of the UK, treatment adherence monitoring was predominately delegated to primary care. Finally, standard care post-hip fracture care reflects care provided without the introduction and/or expansion of OG and FLS models of care.
The perspective adopted was that of the NHS in England and personal social services, including primary and secondary healthcare and care home costs. Primary care costs included GP and practice nurse contacts, visits to other community healthcare professionals (e.g. health visitor, physiotherapist), laboratory tests and drugs. Secondary healthcare costs included outpatient visits, accident and emergency contacts, day cases and inpatient admissions. Primary and secondary care costs captured hip fracture related costs as well as all other costs. We did not include the cost of walking aids, home adaptation costs or home care costs funded by councils or local organisations (e.g. live-in help, meals, nursing care, domestic help, etc.).
Given the natural history of hip fracture progression with recursive events a Markov model was built to evaluate the lifetime costs, (quality-adjusted) life expectancy, and cost-effectiveness. The model structure was defined using an iterative process involving discussions with clinical experts and epidemiologists involved in the REFRESH study and supplemented by a literature review of economic models in the disease area.
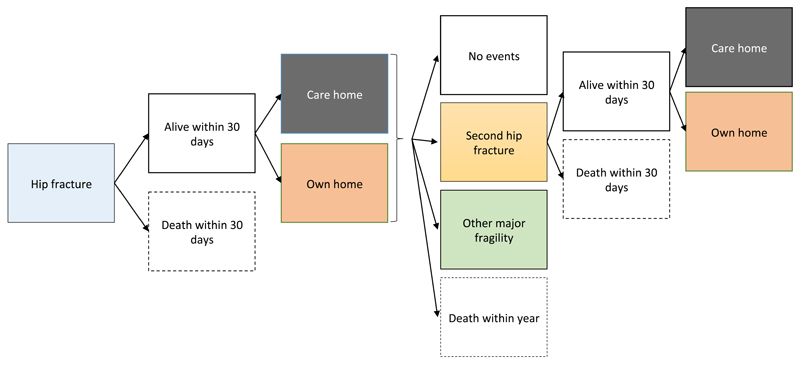
The Markov model, developed in Excel (Microsoft, Redmond, WA), was used to simulate the natural history of individuals following an index hip fracture across health states representing: history of index hip fracture, second hip fracture; major non-hip fracture(s) (pelvic, spine, wrist, humerus and rib) requiring hospitalisation; living in patient’s own home or in a care home; and dead (within 30 days post-hip fracture or within year) (Figure 1 and Supplementary Figure 1). We assumed that if a patient transitioned to a care home they would not go back to their own home for remainder of their life. A cycle length of one year was considered appropriate given the natural history of hip fracture patients, and half-cycle correction was performed.(16) All costs and outcomes were discounted using the recommended annual rate of 3.5%. Costs are quoted in 2012/2013 prices.
Figure 1.
Model structure and allowed transitions in the first year of simulation. The model simulated the transition of a cohort of patients with an index hip fracture through the health states over time, to estimate expected costs and outcomes. At the start of the simulation, patients with a hip fracture could die within 30 days or be discharged home or to a care home (nursing or residential care home). In the same cycle, patients could then develop a second hip fracture, other major fragility fracture requiring hospitalisation (non-hip such as pelvic, spine, wrist, humerus and rib), have no further events or die. If patients experienced a second hip fracture, they could die within 30 days or, if alive, be discharged to a care home or their own home.
Derivation of model inputs
Study subjects in Hospital Episode Statistics (HES) dataset
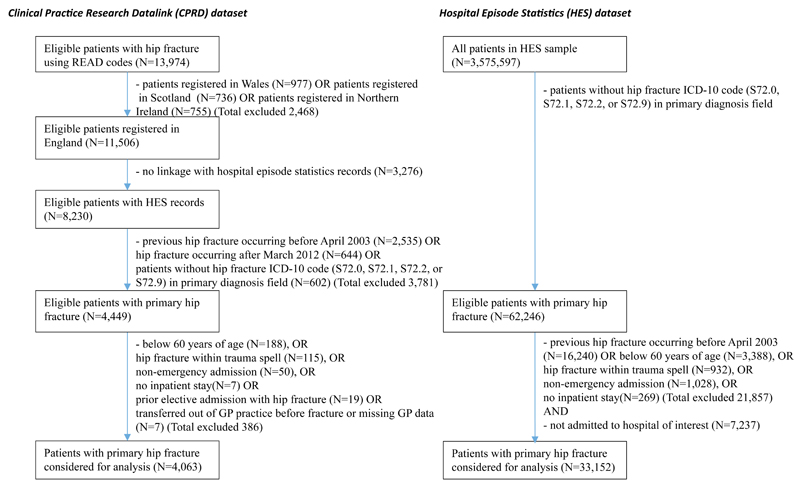
We extracted all hospital records for 33,152 patients over 60 years of age who had had an emergency hospital admission with a primary ICD-10 diagnosis code for hip fracture (S72.0, S72.1, S72.2 and S72.9) between April 2003 and March 2013 for a representative region of the UK (see Figure 2 and supplementary Table 1).(4) The HES data were used to develop the risk equations for the following events: time to second hip fracture, time to major non-hip fragility fracture requiring hospitalisation, discharge to care home (nursing or residential) following hip fracture, and time to death. The data were also used to estimate the annual hospitalisation costs for each health state of the model. See supplementary material and Leal et al. (2016)(4) for details on the valuation of hospital resource use.
Figure 2.
Identification of patients with hip fracture in the HES and CPRD datasets
Study subjects in the Clinical Practice Research Datalink (CPRD) dataset
We extracted all primary care contacts, laboratory tests and prescribed drugs for 4,063 patients registered in the CPRD GOLD database between 1st April 2003 and 31st March 2012 who had linked hospital use records indicating a hip fracture (see Figure 2 and Supplementary Table 2). Hip fracture was identified in primary care records using pre-defined READ codes (see Supplementary Table 3). The dataset was used to estimate the annual primary care costs for each health state of the model. Resource use was valued using national cost databases for the year 2012/13(17–19) (see Supplementary Table 4 for more details).
Relative effectiveness of OG and FLS
Previous work using the HES cohort(15) informed the relative effectiveness of the models of care measured by time to second hip fracture (hazard ratio: HR) and time to death (HR) adjusted for confounding factors such as age, sex, deprivation and Charlson-comorbidity index (see Table 1). In the base case, we assumed the effect of introducing OG or FLS relative to usual care on mortality following hip fracture was present only for the index fracture and in the year of the fracture; and the effect on second hip fracture was present only in the first two years post index hip fracture. These assumptions were explored in sensitivity analysis.
Table 1.
Model parameters
| Parameter | Mean (95%CI) | Source |
|---|---|---|
| Relative effectiveness (hazard ratio) | ||
| Introducing OG relative to usual care | ||
| 30-day mortality following index hip fracture | 0.73 (0.65-0.82) | (15) |
| 1-year mortality following index hip fracture | 0.81 (0.75-0.87) | (15) |
| 2-year risk of developing second hip fracture | 0.95 (0.79-1.15) | (15) |
| Introducing FLS relative to usual care | ||
| 30-day mortality following index hip fracture | 0.80 (0.71-0.91) | (15) |
| 1-year mortality following index hip fracture | 0.84 (0.77-0.93) | (15) |
| 2-year risk of developing second hip fracture | 1.03 (0.85-1.26) | (15) |
| Discharge to care home after index hip fracture (previously not in care home) | Supplementary Table 8 | HES |
| Discharge to care home after second hip fracture (previously not in care home) | Supplementary Table 8 | HES |
| Risk of second hip fracture | Supplementary Table 9 | HES |
| Risk of major non-hip fracture requiring hospitalisation | Supplementary Table 9 | HES |
| 30-day mortality after index hip fracture | Supplementary Table 9 | HES |
| 30-day mortality after 2nd hip fracture | Supplementary Table 9 | HES |
| All-cause mortality post 30 days of fracture | Supplementary Table 9 | HES |
| Intervention cost* | ||
| Fracture liaison nurse (Grade 7) per hip fracture patient | £200 | (18) |
| Orthogeriatrician (Consultant) | £420 | (18) |
| Proportion discharged to a care home that go to a nursing home | 0.48 (0.47-0.49) | (20) |
| Primary care costs in year of index fracture | Supplementary Table 10 | CPRD |
| Primary care costs in years after index fracture | Supplementary Table 10 | CPRD |
| Hospitalisation costs in year of index hip fracture | Supplementary Table 11 | HES |
| Hospitalisation costs in year of 2nd hip fracture | Supplementary Table 11 | HES |
| Hospitalisation costs in years following fracture | Supplementary Table 11 | HES |
| Hospitalisation costs if non-hip fracture occurs | Supplementary Table 12 | HES |
| Hospitalisation costs if death occurs | Supplementary Table 12 | HES |
| Cost of institutionalisation (per year)** | ||
| Nursing home | £39,000 | (18) |
| Residential home | £27,664 | (18) |
| Utility of hip fracture patients | ||
| Within 1 month of index fracture | 0.46 (0.38-0.55) | Lit review |
| At 12 months | 0.53 (0.47-0.61) | Lit review |
| At 24 months and after | 0.66 (0.60-0.74) | Lit review |
| Discount rate for costs and outcomes | 3.5% | HM Treasury |
includes salary, salary oncosts, qualification costs, management and non-staff overheads and capital overheads
Nursing home at £39,000 per year (£750 per week times 52 weeks) and residential home at £27,664 per year (£532 per week times 52 weeks); HES: Hospital Episode Statistics database; CPRD: Clinical Practice Research Datalink database
Cost of introducing OG and FLS
Based on the survey of 11 hospitals in the UK region(14) reporting staffing levels and non-clinical activities (e.g. clinical leadership roles) of an orthogeriatrician and a nurse leading the FLS within the same region, a FLS was assumed to operate at 100% capacity (whole time equivalent – WTE) whereas an OG was assumed to operate at 75% capacity. The annual costs of an OG and a FLS per hip fracture patient were estimated by multiplying the respective WTE by the total annual costs(18) and dividing these by 450 hip fracture patients (average patients seen per year across the 11 hospitals in the survey(20)) (see Table 1). The OG and FLS specific costs were assumed to occur in the year of the index hip fracture, which was explored in sensitivity analysis.
Statistical analysis
The rate of second hip fracture or of major non-hip fracture requiring hospitalisation following the index fracture was estimated using parametric survival models. Time to event was determined in continuous time from the onset of first hip fracture, using the censor date of death or the date of administrative censoring (31st March 2013). All-cause mortality was derived using two logistic models to capture the high mortality in the first 30 days after first and second hip fracture (separate models), and a Gompertz proportional hazards survival model for the subsequent years. Time to death was modelled in continuous time, using each patient’s current age as time at risk to better extrapolate beyond the observed follow-up period.(21) Logistic models estimated the probability of admission to care home (nursing or residential) following hip fracture (index and second). A generalised linear model (GLM), with a gamma distribution and identify link function, was used to predict the annual primary and hospital care costs by health state. Time-invariant covariates included gender, age and Charlson co-morbidity index at first hip fracture; and time-variant factors included occurrence or history of second hip fracture, occurrence or history of major non-hip fracture, and admission from care home or own home. The proportion of those discharged to a care home that go to a nursing home was derived from the National Hip Fracture Database report(20) and costed using a national cost database(18) (see Table 1). Statistical analysis was carried out using STATA v.12. See supplementary material for more methodological details.
Quality of life in patients with hip fracture
We used a meta-regression approach, with a linear mixed-effects model, to synthesise absolute utility data from 32 studies (21,085 patients) identified by literature review which reported preference-based quality of life for patients with hip fracture (e.g. EQ-5D, EQVAS, HUI2, etc.) (Supplementary Figure 2 and Supplementary Table 5). The resulting model was used to predict the EQ-5D utility values of hip fracture patients at several time points (onset, and 1, 6, 12, 18, 24 months) to estimate utility up to 2 years post hip fracture using the area under the curve (see Table 1). We assumed that utilities remained constant after the first year post hip fracture (i.e. 0.66), and that second hip fractures and major non-hip fractures requiring hospitalisation had the same utility in the year of the event as those at the onset of hip fracture (0.46). These assumptions were varied in sensitivity analyses.
Analysis
Two cohorts of 1,000 identical men and women were used to simulate a representative patient aged 83 years at hip fracture, with an average pre-admission Charlson-comorbidity score of 1.2 and living in their own home before the fracture. We further assessed the cost-effectiveness of the models of care in several subgroups of hip fracture patients defined according to their age, sex, and Charlson co-morbidity score at index hip fracture. A model of care was deemed to be cost-effective if the incremental cost-effectiveness ratio (ICER) was below £30,000 per QALY gained.(22) The ICER was estimated by dividing the difference in mean costs by the difference in mean effects (life years and QALYs) for a given model of care compared to its next best alternative. Internal validity of the model was checked using sensitivity analysis (extreme values) and by comparing the model outputs with the data used to build the model. Model parameters and structural assumptions were evaluated in one-way and probabilistic sensitivity analysis and quantified using a Cost-Effectiveness Acceptability Curve (CEAC)(23) and analysis of covariance methods (ANCOVA)(24) (see supplementary material). Key uncertainties in the model structure were identified when developing the conceptual framework.
Results
Risk and cost equations
The mean follow-up time of patients in the HES dataset was 2.6 years (SD 2.5) post index hip fracture and 84,717 patient-years of data were available to estimate the risk equations. The number of patients reporting second hip fracture and major non-hip fractures were 2,206 and 1,464, respectively (Supplementary Table 7). Supplementary Tables 8 and 9 report the risk models, and Supplementary Tables 10, 11 and 12 report the cost models. These models allow simulating the natural history of individuals and costs as well as patient heterogeneity. For example, being female was associated with higher risk of second hip fracture, higher risk of major non-hip fracture, lower risk of mortality (Supplementary Table 9), lower risk of hospitalisation and lower hospital costs (Supplementary Table 10), adjusting for other covariates. Patients living in a care home were associated with a higher risk of major non-hip fracture, higher risk of mortality (Supplementary Table 9), higher risk of hospital admission and higher hospital costs if admitted, as well as higher primary care costs in the year of the hip fracture (Supplementary Tables 10 and 11), adjusting for other covariates.
Representative patient - male
Table 2 reports the total life years, QALYs, and costs associated with each of the three models of care. For our male cohort of 1,000, the introduction of an OG and a FLS would result in a reduction of 26 (95% CI: 17-35) and 19 (95% CI: 9-29) deaths within 30 days of primary hip fracture, respectively, compared to usual care (see Supplementary Table 13). Within 1 year of primary hip fracture, the reduction in deaths by introducing an OG and a FLS, compared to usual care, would be 58 (95% CI: 44-71) and 46 (95%CI: 28-63), respectively. On average, over the lifetime of a patient, when compared to usual care, we would expect each patient to experience an increase of 0.18 (95%CI: 0.14-0.23) and 0.14 (95%CI: 0.08-0.19) life years (undiscounted) spent in their own home if an OG or a FLS were to be introduced, respectively.
Table 2.
Mean discounted costs and outcomes of the differing models of secondary prevention care
| Representative male* | Usual care | Fracture liaison nurse | Orthogeriatrician |
| Total costs | £39,069 (£37,798-£40,514) |
£41,044 (£39,495-£42,621) |
£41,679 (£40,265-£43,262) |
| Intervention | 0 | £200 | £420 |
| Hospital care | £23,025 (22406-23679) |
£23,678 (22988-24384) |
£23,814 (23132-24525) |
| Primary care | £3,276 (3046-3490) |
£3,471 (3234-3714) |
£3,523 (3285-3762) |
| Care home | £12,767 (11893-13817) |
£13,695 (12689-14837) |
£13,922 (12962-15039) |
| Total Life years | 2.68 (2.56-2.79) |
2.83 (2.70-2.96) |
2.88 (2.75-3.00) |
| Total QALYs | 1.64 (1.46-1.83) |
1.74 (1.54-1.96) |
1.77 (1.56-1.98) |
| Representative female* | |||
| Total costs | £50,534 (49226-52276) |
£52,444 (50935-54340) |
£53,081 (51559-54974) |
| Intervention | 0 | £200 | £420 |
| Hospital care | £23,893 (23390-24471) |
£24,387 (23804-25040) |
£24,478 (23918-25073) |
| Primary care | £4,721 (4417-5016) |
£4,902 (4571-5224) |
£4,955 (4635-5265) |
| Care home | £21,921 (20972-23134) |
£22,955 (21925-24301) |
£23,229 (22191-24524) |
| Total Life years | 3.89 (3.77-4.03) |
4.04 (3.91-4.19) |
4.09 (3.95-4.24) |
| Total QALYs | 2.42 (2.15-2.72) |
2.52 (2.23-2.82) |
2.54 (2.26-2.85) |
Aged 83 years at hip fracture, with an average pre-admission Charlson-comorbidity score of 1.2 and living in their own home before the fracture
Relative to usual care, the mean discounted healthcare and care home costs would be £2,610 (95%CI £2,109-£3,166) and £1,975 (95%CI 1,265-2,591) higher when an orthogeriatrician or a FLS were introduced, respectively. Higher care home costs accounted for 35% and 44% of the additional costs of FLS and OG, respectively. This was a result of increased longevity due to OG or FLS being introduced. The discounted average QALYs gained by male patients, relative to usual care, were 0.13 (95%CI 0.09-0.16) and 0.10 (95%CI 0.06-0.14) if an OG or a FLS were to be introduced, respectively.
At a £30,000 per QALY threshold, the most cost-effective model of care was introducing an orthogeriatrician, with an ICER of £23,407/QALY (Table 3). There was considerable uncertainty regarding the comparison between OG and FLS models of care with statistically non-significant difference in costs and QALYs being £635 (95%CI -£207 to £1,496) and 0.03 (95%CI -0.02 to 0.07), respectively (Table 3 and Supplementary Figure 3). However, the probability that introducing OG-led FLS was the most cost-effective option was estimated at 69%.
Table 3.
Cost-effectiveness of the differing models of secondary prevention care of hip fractures
| Representative male* | Difference in costs | Difference in LYs | Difference in QALYs | ICER (£/LY) | ICER (£/QALY) | Prob that is the most cost-effective at £30,000/QALY |
| Usual care | - | - | - | - | - | 0% |
| FLS vs. usual care | £1,975 (1297 to 2620) |
0.159 (0.095 to 0.218) |
0.099 (0.058 to 0.140) |
£12,458 | £19,955 | 31% |
| OG vs. fracture liaison nurse | £635 (-207 to 1496) |
0.043 (-0.031 to 0.116) |
0.027 (-0.019 to 0.074) |
£14,898 | £23,407 | 69% |
| Representative female | ||||||
| Usual care | - | - | - | - | - | 0% |
| FLS vs. usual care | £1,909 (1271 to 2562) |
0.149 (0.094 to 0.209) |
0.093 (0.057 to 0.133) |
£12,837 | £20,421 | 28% |
| OG vs. fracture liaison nurse | £638 (-207 to 1418) |
0.044 (-0.032 to 0.110) |
0.028 (-0.020 to 0.071) |
£14,618 | £22,709 | 72% |
Representative patient - female
For our female cohort of 1,000, mortality at 30 days and within 1 year was lower compared to the male cohort in the usual care (Supplementary Table 13). Hence, the introduction of an OG and a FLS would result in a reduction of 16 (95% CI: 11-22) and 12 (95% CI: 5-18) deaths within 30 days of primary hip fracture, respectively, compared to usual care. Within 1-year of primary hip fracture, the reduction in deaths by introducing an OG and a FLS, compared to usual care, were 42 (95% CI: 31-51) and 33 (95%CI: 21-45), respectively. On average, over a lifetime, when compared to usual care, we would expect each female to experience an increase of 0.17 (95%CI: 0.13-0.21) and 0.13 (95%CI: 0.08-0.18) life years (undiscounted) spent in their own home if an OG or a FLS were to be introduced, respectively.
The mean discounted healthcare and care home costs were £2,547 (95%CI 1,993-3,035) and £1,909 (95%CI 1,272-2,515) higher by introducing an OG and FLS, respectively, relative to usual care. As a result of longer longevity relative to males, care home costs now accounted for 41% and 51% of the additional costs of FLS and OG, respectively. The discounted average QALYs gained by female patients, relative to usual care, were 0.12 (95%CI 0.09-0.15) and 0.09 (95%CI 0.06-0.13) if an OG or a FLS were to be introduced, respectively.
At a £30,000 per QALY threshold, the most cost-effective model of care for females was introducing an orthogeriatrician, with an ICER of £22,709/QALY (Table 3). As with males, considerable uncertainty exists when comparing OG and FLS models of care with again non-significant differences in costs and QALYs: £638 (95%CI -£207 to £1,418) and 0.03 (95%CI -0.02 to 0.07) respectively (Table 3 and Supplementary Figure 4). Finally, the probability that introducing OG-led service was the most cost-effective option was estimated at 72%.
Sensitivity analysis
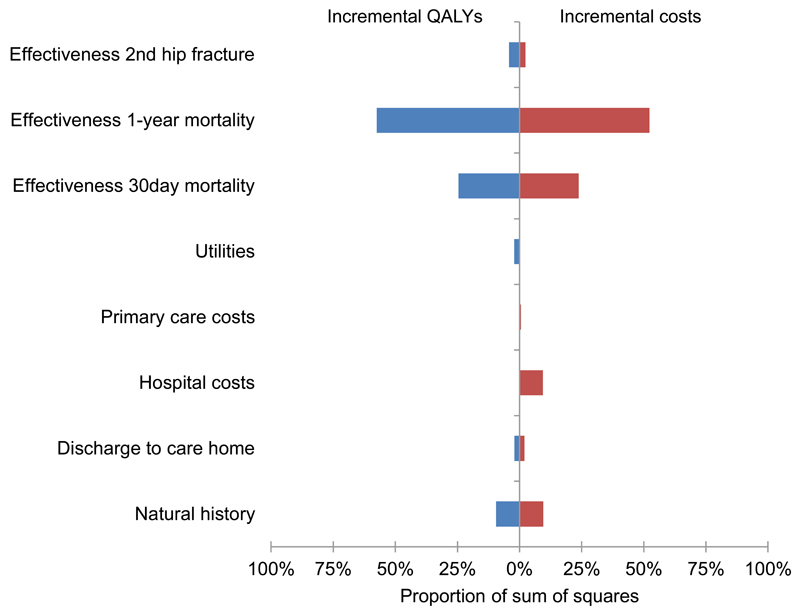
Figure 3 reports the impact of uncertainty in different parameters on the uncertainty of incremental costs and QALYs of introducing an orthogeriatrician compared to FLS. This was largely associated with the uncertainty in the relative effectiveness of an OG-led model of care compared to FLS on 30-day (accounting for 25% of all uncertainty in incremental costs and QALYs) and one-year mortality (52% to 58% of all uncertainty) following index hip fracture. The uncertainty in the natural history components of the model (e.g. absolute mortality, probability of second hip fracture or other major fragility fracture) was associated with 10% of all uncertainty in the incremental costs and QALYs. In terms of model structural assumptions, we tested the impact of changes to these assumptions on the cost-effectiveness results (Supplementary Table 14). Overall, at a £30,000/QALY threshold, the introduction of OG or FLS relative to usual care remained the most cost-effective options when the assumptions were changed. For example, assuming the effect of the interventions concerning second hip fracture extended over lifetime rather than 2 years resulted in OG becoming more cost-effective (£20,036/QALY). Also, assuming that the effect of the interventions on mortality extended over lifetime rather than one year post hip fracture resulted in OG being more cost-effective (£18,052/QALY). Excluding care home costs resulted in OG becoming more cost-effective (£13,039/QALY and £14,733/QALY for women and men, respectively). Assuming that the OG and FLS services resulted in additional £1000 per patient in management and test costs during the hip fracture admission relative to usual care resulted in OG becoming borderline cost-effective (£29,573/QALY). Finally, using the smallest hospital in the UK region (220 hip fractures per year) to estimate the intervention cost per patient (OG at £859 per patient and FLS at £409 per patient) resulted in FLS becoming the most cost-effective option (ICER of £22,922/QALY – Supplementary Table 14).
Figure 3.
Orthogeriatrician vs. nurse-led fracture liaison service: ANCOVA analysis of proportion of sum of squares for incremental QALYs saved and incremental costs explained by the uncertainty in the model. The horizontal axis represents the variation in incremental costs and QALYs that is associated with the uncertainty in the model inputs.
Subgroup analysis
For patients up to age 80 years old, introducing an OG was the most cost-effective option (see Supplementary Table 15). In contrast, introducing a FLS became the most cost-effective option for patients aged 90 years if their Charlson co-morbidity score at index hip fracture was 5 or above.
Discussion
Our cost-effectiveness analysis of models of hip fracture care found that the introduction and/or expansion of orthogeriatric and nurse-led FLS was more effective and cost-effective than usual care.
Two recent systematic reviews assessed the economic evidence concerning the prevention of osteoporotic fractures(25,26). Consistent with our findings, three previous economic evaluations, using Markov models, reported secondary fracture prevention interventions (i.e. hospital osteoporosis case manager, outpatient-based FLS, and hospital nurse-led FLS) to be cost-effective in populations with fragility fracture(27–29). These analysis were limited by the use of data from single institutions and by a relatively short follow up period (up for 1 year), or by the use of disparate data sources covering different populations of patients and time periods. Our study benefitted from the availability of large primary and hospital datasets to robustly estimate the impact of the models of care across a large representative population in terms of survival, prevention of second hip fracture, primary care and hospital care costs and cost-effectiveness. For example, having incorporated in our modelling robust estimates of the time to second hip fracture and death and the short and long-term costs associated with patients with hip fracture, we did not find the introduction and/or expansion of OG or a nurse-led services to be cost-saving compared to usual care, in contrast with previous work(27,28). This is largely explained by the added longevity that translates into higher costs and re-fractures compared to usual care.
Irrespective of how patients were stratified in terms of their age, sex, and Charlson co-morbidity score at index hip fracture, our results suggest that it is cost-effective to introduce an orthogeriatrician or FLS compared to usual care. We find that these models of care produce greater gains in life years than QALYs, suggesting that the positive impact on survival was not necessarily accompanied by proportional gains in quality of life. This is an area that would repay closer investigation.
There is considerable uncertainty in the evidence informing the model, particularly concerning the relative effectiveness of an OG compared to a nurse-led FLS on survival and prevention of second hip fracture, and also concerning the natural history of hip fracture. The large number of hip fractures every year for which these interventions are potentially relevant suggests that caution is required about decisions based on the model results. Our findings highlight the need for further research to reduce decision uncertainty, with particular emphasis on undertaking clinical trials to obtain unbiased comparisons of the different models of secondary care services.
The translation of our findings into other types of acute fragility fractures requiring inpatient care is possible. However, the management of vertebral fractures requires additional components, different mechanisms for case finding (routine VFA, text mining radiology reports, re-reading axial imaging) as well treatment (analgesia, vertebral augmentation) relative to hip fractures. Although these types of care pathway can be delivered within an FLS system they require layering of additional work flows and remain a priority for future service development(30).
Furthermore, the case-mix of patients seen by the OG and nurse-led FLS services may vary by type of fracture and frailty of patient, however, our qualitative evaluation of hip fracture services within the region did not detect differences in the types of patients that were seen by OG and FLS models of care(14).
Our study had several limitations. First, although we improved on previous studies by basing our estimates of effectiveness on a very large administrative dataset supported by a careful survey of the services provided in each hospital(14,15), there were no effectiveness data informed by clinical trials, and the limited range and number of services currently provided in England precluded considering some models of care, such as introducing a combined orthogeriatrician and nurse-led FLS. Second, we did not separate the different types of fragility-related fractures that patients could suffer post-discharge. We focused on major non-hip fractures requiring hospitalisation given their relative large impact in terms of healthcare costs. Furthermore, we did not separate non-hip fractures by type so that we could benefit from a larger sample to estimate the costs. Nonetheless, other fragility fractures not incurring hospitalisation were still captured in the model in the primary and hospital care costs. Third, we did not have quality of life data from individual patient records, and relied instead on a systematic review and meta-regression of published literature. This provided estimates of changes in EQ-5D - based utility conditional on time since hip fracture, but we could not reliably estimate utility values for non-hip fractures or the additional impact these may have on the quality of life of individuals with a history of hip fracture. The consequent assumptions were explored in sensitivity analysis but future research could address this limitation. Furthermore, the use of a cohort-based Markov model made it difficult to capture the trajectory of utility values taking into account both time since primary hip fracture and history of events such as non-hip fractures and second hip fracture. An individual-based (i.e. microsimulation) Markov model would have facilitated tracking of each individual’s history and time-varying utility values, but would have been more time-consuming to construct and computationally intensive to run(31). Finally, we only included healthcare and care home costs, and so excluded some important economic considerations for people with hip fracture and their families, such as unpaid care provided by friends and family, walking aids and home adaptation costs as a consequence of the fracture as well as locally funded home social care (e.g. provision of meals, nursing care, live-in help, etc.). Future research using UK-based populations of hip fracture patients is needed to assess the use and costs of these resources as well as the impact on informal care.
In conclusion, our work suggests that it is cost-effective to introduce an orthogeriatrician or a FLS secondary care service for patients with a hip fracture, predominantly because of their effects on mortality rather than on re-fracture. Further research is needed to make more informed decisions with a focus on estimating the effectiveness of these models of care informed by clinical trials.
Supplementary Material
Acknowledgements
This project was funded by the NIHR Health Services and Delivery Research programme (project number 11/1023/01). Support was received from the Oxford NIHR Musculoskeletal Biomedical Research Unit, Nuffield Orthopaedic Centre, University of Oxford. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HS&DR programme, NIHR, NHS or the Department of Health. This study is based in part on data from Hospital Episode Statistics and the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. However, the interpretation and conclusions contained in this study are those of the authors alone. The ReFRESH study group consists of Dr Andrew David Judge, Dr Muhammad Kassim Javaid, Professor Nigel Arden, Professor Cyrus Cooper, Professor Andrew Farmer, Dr Daniel Prieto-Alhambra, Dr Jose Leal, Professor Michael Goldacre, Professor Alastair Gray, Dr Janet Lippett, Dr Rachael Gooberman-Hill and Laura Graham.
Footnotes
Declaration of interest
JL, AM, DPA, NKA, CC, MKJ and AJ received grants from NIHR HS&DR during the conduct of the study. Outside the submitted work, MKJ reports personal fees from Lilly UK, Amgen, Sevier, Merck, Medtronic, Internis, Consilient Health and serves on the Scientific Committee of the National Osteoporosis Society and International Osteoporosis Foundation; DPA received grants from Bioiberica S.A. and Amgen Spain S.A.; CC received personal fees from Servier, Amgen, Eli Lilly, Merck, Medtronic and Novartis. NKA reports personal fees from Merck, Smith and Nephew, Q-Med, Nicox, Flexion, Bioiberica, Servier and grants and personal fees from Roche. AJ has received consultancy, lecture fees and honoraria from Servier, UK Renal Registry, Oxford Craniofacial Unit, IDIAP Jordi Gol, Freshfields Bruckhaus Deringer, has held advisory board positions (which involved receipt of fees) from Anthera Pharmaceuticals, INC., and received research sponsorship from ROCHE. SH and AD has no competing financial interests relevant to the submitted work.
Contributors
All authors had full access to all statistical reports and tables in the study. JL take responsibility for the integrity and accuracy of the economic analysis. All authors revised and approved the final version of the article. AJ and MKJ supervised the study and are joint senior authors.
References
- 1.Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20(10):1633–50. doi: 10.1007/s00198-009-0920-3. [DOI] [PubMed] [Google Scholar]
- 2.Cooper C, Mitchell P, Kanis JA. Breaking the fragility fracture cycle. Osteoporos Int. 2011;22(7):2049–50. doi: 10.1007/s00198-011-1643-9. [DOI] [PubMed] [Google Scholar]
- 3.Hernlund E, Svedbom A, Ivergard M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8(1-2):136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leal J, Gray AM, Prieto-Alhambra D, et al. Impact of hip fracture on hospital care costs: a population-based study. Osteoporos Int. 2016;27(2):549–558. doi: 10.1007/s00198-015-3277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsh D, Akesson K, Beaton DE, et al. Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int. 2011;22(7):2051–65. doi: 10.1007/s00198-011-1642-x. [DOI] [PubMed] [Google Scholar]
- 6.Eisman JA, Bogoch ER, Dell R, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012;27(10):2039–46. doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- 7.Department of Health. Falls and fractures: Effective interventions in health and social care. Department of Health; 2009. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@pg/documents/digitalasset/dh_109122.pdf. [Google Scholar]
- 8.Department of Health. Prevention Package for Older People. www.dh.gov.uk/en/Publicationsandstatistics/Publications/dh_103146.
- 9.British Orthopaedic Association. The care of patients with fragility fracture. http://wwwfracturescom/pdf/BOA-BGS-Blue-Book.pdf.
- 10.National Institute for Health and Care Excellence. Hip fracture: The management of hip fracture in adults. NICE guidelines CG124. https://www.nice.org.uk/guidance/cg124. [PubMed]
- 11.NOS and AGE UK. Report to the Minister of State for Care Services: Breaking Through: Building Better Falls and Fracture Services in England. https://www.nos.org.uk/documentdoc?id=987.
- 12.McLellan AR, Wolowacz SE, Zimovetz EA, et al. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int. 2011;22(7):2083–98. doi: 10.1007/s00198-011-1534-0. [DOI] [PubMed] [Google Scholar]
- 13.Royal College of Physicians. Falling standards, broken promises: report of the National Audit of Falls and Bone Health in Older People 2010. https://www.nos.org.uk/document.doc?id=1516.
- 14.Drew S, Sheard S, Chana J, Cooper C, Javaid MK, Judge A. Describing variation in the delivery of secondary fracture prevention after hip fracture: an overview of 11 hospitals within one regional area in England. Osteoporos Int. 2014;25(10):2427–33. doi: 10.1007/s00198-014-2775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barendregt JJ. The life table method of half cycle correction: getting it right. Med Decis Making. 2014;34(3):283–5. doi: 10.1177/0272989X13519863. [DOI] [PubMed] [Google Scholar]
- 16.Department of Health. NHS reference costs 2012 to 2013. https://www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013.
- 17.Curtis LH. Unit costs of health and social care 2013. Personal Social Services Research Unit. 2013 [Google Scholar]
- 18.Health and Social Care Information Centre. Prescription Cost Analysis. England: 2013. [Google Scholar]
- 19.Hawley S, Javaid MK, Prieto-Alhambra D, et al. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing. 2016;45(2):236–42. doi: 10.1093/ageing/afv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Hip Fracture Database. National report 2013. www.nhfd.co.uk.
- 21.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–33. doi: 10.1007/s00125-013-2940-y. [DOI] [PubMed] [Google Scholar]
- 22.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. http://publications.nice.org.uk/pmg9. [PubMed]
- 23.Fenwick E, C K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Economics. 2001;10(8):779–87. doi: 10.1002/hec.635. [DOI] [PubMed] [Google Scholar]
- 24.Briggs A, S M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford University Press; 2006. [Google Scholar]
- 25.Si L, Winzenberg TM, Palmer AJ. A systematic review of models used in cost-effectiveness analyses of preventing osteoporotic fractures. Osteoporos Int. 2013;25(1):51–60. doi: 10.1007/s00198-013-2551-y. [DOI] [PubMed] [Google Scholar]
- 26.Ganda K, Puech M, Chen JS, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int. 2013;24(2):393–406. doi: 10.1007/s00198-012-2090-y. [DOI] [PubMed] [Google Scholar]
- 27.McLellan A, Wolowacz S, Zimovetz E, et al. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporosis Int. 2011;22(7):2083–98. doi: 10.1007/s00198-011-1534-0. [DOI] [PubMed] [Google Scholar]
- 28.Majumdar SR, Lier DA, Beaupre LA, et al. Osteoporosis case manager for patients with hip fractures: results of a cost-effectiveness analysis conducted alongside a randomized trial. Arch Intern Med. 2009;169(1):25–31. doi: 10.1001/archinte.169.1.25. [DOI] [PubMed] [Google Scholar]
- 29.Cooper MS, Palmer AJ, Seibel MJ. Cost-effectiveness of the Concord Minimal Trauma Fracture Liaison service, a prospective, controlled fracture prevention study. Osteoporos Int. 2012;23(1):97–107. doi: 10.1007/s00198-011-1802-z. [DOI] [PubMed] [Google Scholar]
- 30.Javaid MK, Kyer C, Mitchell PJ, et al. Effective secondary fracture prevention: implementation of a global benchmarking of clinical quality using the IOF Capture the Fracture(R) Best Practice Framework tool. Osteoporos Int. 2015;26(11):2573–8. doi: 10.1007/s00198-015-3192-0. [DOI] [PubMed] [Google Scholar]
- 31.Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--3. Value Health. 2012;15(6):812–20. doi: 10.1016/j.jval.2012.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.