Abstract
Background
The mould Alternaria alternata is a major elicitor of allergic asthma. Diagnosis and specific immunotherapy (SIT) of Alternaria allergy are often limited by the insufficient quality of natural mould extracts.
Objective
To investigate whether recombinant Alt a 1 can be used for reliable diagnosis of Alternaria alternata allergy and to develop a safe, non-allergenic vaccine for SIT of Alternaria allergy.
Methods
The qualitative sensitization profile of 80 Alternaria-allergic patients from Austria and Italy was investigated using an allergen micro-array and the amount of Alternaria-specific IgE directed to rAlt a 1 was quantified by ImmunoCAP measurements.
Peptides spanning regions of predicted high surface accessibility of Alt a 1 were synthesized and tested for IgE reactivity and allergenic activity, using sera and basophils from allergic patients. Carrier-bound peptides were studied for their ability to induce IgG antibodies in rabbits which recognize Alt a 1 and inhibit allergic patients’ IgE reactivity to Alt a 1.
Results
rAlt a 1 allowed diagnosis of Alternaria allergy in all tested patients, bound the vast majority (i.e. >95%) of Alternaria-specific IgE and elicited basophil activation already at a concentration of 0.1 ng/mL. Four non-allergenic peptides were synthesized which, after coupling to the carrier protein keyhole limpet hemocyanin, induced Alt a 1-specific IgG and inhibited allergic patients’ IgE binding to Alt a 1.
Conclusions and clinical relevance
rAlt a 1 is a highly allergenic molecule allowing sensitive diagnosis of Alternaria allergy. Carrier-bound non-allergenic Alt a 1 peptides are candidates for safe SIT of Alternaria allergy.
Keywords: Alternaria alternata, allergen, Alt a 1, diagnosis, peptides, specific immunotherapy
Introduction
Moulds are one of the most important inducers of allergy worldwide [1, 2]. The clinical manifestations of mould allergy range from rhinitis and conjunctivitis to severe asthma [3–5]. The ascomycete Alternaria alternata is one of the most important causes of fungal allergy and may represent a risk factor for asthma [6, 7]. It can be found throughout the world, especially in the outdoor environment, but it has also been isolated from the indoor environment, e.g. from house dust [8, 9]. A study performed in 4962 allergic patients with respiratory symptoms showed that up to 12% were skin prick test positive to Alternaria alternata, whereas only 2.5% or less reacted to other common fungi (Aspergillus, Cladosporium and Penicillium) [10]. Diagnosis and specific immunotherapy (SIT) of Alternaria allergy are difficult to perform because of the insufficient quality of natural Alternaria allergen extracts [11]. The poor quality of mould extracts results from strain variabilities, varying culturing conditions and extracting protocols as well as degradation of allergens by fungal proteases [12–15].
Several Alternaria allergens have been identified [2, 16], but Alt a 1, a protein of 157 amino acids, has been suggested as the major Alternaria alternata allergen [17, 18]. A cDNA coding for Alt a 1 has been isolated and the corresponding recombinant allergen was shown to react with serum IgE from Alternaria-allergic patients [19].
Here, we used micro-arrayed rAlt a 1 as a diagnostic marker for Alternaria allergy and studied the percentage of Alternaria-specific IgE, which is directed against rAlt a 1 by quantitative IgE assays in two European populations of Alternaria-allergic patients. The allergenic activity of rAlt a 1 was studied using basophils from allergic patients. We then identified and synthesized non-allergenic peptides of Alt a 1 and used them to formulate a carrier-bound peptide vaccine. The coupled peptides were studied regarding their ability to induce upon immunization of rabbits Alt a 1-specific IgG antibodies, which inhibit allergic patients’ IgE reactivity to Alt a 1. Our study thus provides means for the diagnosis and safe immunotherapy of Alternaria allergy.
Methods
Characterization of allergic patients
Eighty Alternaria-allergic patients from Austria and Italy suffering from allergic rhinitis, conjunctivitis and/or asthma were identified based on a positive case history and the determination of specific IgE antibodies to Alternaria alternata (m6; as determined by CAP-FEIA, Phadia, Uppsala, Sweden). In addition, specific IgE antibodies to a mould mix (mx1: Alternaria, Cladosporium, Aspergillus, Penicillium) and to rAlt a 1 (m229) were measured by CAP-FEIA. An overview of the demographic and clinical features as well as total and specific IgE levels is given in Table 1. Sera from the allergic and from non-allergic individuals were analysed in an anonymous manner with approval by the Ethics committee of the Medical University of Vienna.
Table 1.
Demographic, clinical and serological characteristics of Alternaria alternata-sensitized patients from Austria and Italy
| Nat. | n | f/m | Age | ImmunoCAP (kUA/L) |
ISU | Symptoms |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total IgE | Mould mix | Alternaria | rAlt a 1 | ISAC | r | rc | a | ad | ||||
| A | 54 | 19/35 | 22.9 ± 11.4 | 723.3 ± 747.9 | 29.2 ± 19.3 | 33.6 ± 19 | 34.9 ± 21.7 | 36.6 ± 22.1 | 18 | 19 | 25 | 5 |
| I | 26 | 12/14 | 22.2 ± 13.9 | 634.7 ± 816.2 | 18 ± 14.2 | 21 ± 15.7 | 26.5 ± 20.2 | 27.3 ± 19.2 | 13 | 5 | 5 | 0 |
Nat., Nationality; A, Austria; I, Italy; n, number; f, female; m, male; kUA/L, kilounits of antigen per litre; ISAC, immuno solid-phase allergen chip; ISU, ISAC Standardized Units; r, rhinitis; rc, rhinoconjunctivitis; a, asthma; ad, atopic dermatitis; CAP and ISAC values are given as mean values.
Qualitative allergen profile by micro-array and quantitative analysis of Alt a 1-specific IgE levels
The IgE reactivity profiles of Alternaria-allergic patients to 103 micro-arrayed purified, recombinant and natural allergens including rAlt a 1 were determined using ImmunoCAP ISAC (Phadia Multiplexing Diagnostics, Vienna, Austria). Reactivities to the individual allergens and to rAlt a 1 were determined for patients suffering only from rhinitis/rhinoconjunctivitis and/or from asthma. The percentage of Alternaria-specific IgE directed against rAlt a 1 was calculated based on the quantitative IgE levels as determined by ImmunoCAP. The correlation of IgE levels determined by ImmunoCAP and ISAC was tested by the Pearson’s correlation test using SPSS 18.0 for Windows, and was considered as statistically significant for P ≤ 0.05.
Natural and recombinant Alt a 1 and synthetic Alt a 1-derived peptides
For purification of nAlt a 1, the Alternaria strain CBS 103.33 (Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands) was grown for 4 weeks in 400 mL Sabauroud glucose medium at room temperature. Fungal tissue was removed by filtration and the filtrate was loaded on a 5 mL HiTrap DEAE column (GE Healthcare, Munich, Germany), after dialysis against 10 mM NaH2PO4 and sterile-filtration (Steritop, 0.45 μm; Millipore, Billerica, MA, USA). Natural Alt a 1 was eluted with a linear salt gradient (0–0.5 M NaCl in 10 mM NaH2PO4) at approximately 25 mM NaCl. Fractions containing nAlt a 1 were pooled, dialysed against 10 mM NaH2PO4 (pH 7.5) and applied on a Superdex 200 gel filtration column (GE Healthcare). The purity of nAlt a 1 was assessed by SDS-PAGE [20] and the protein concentration was determined with the Micro BCA™ Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA).
Recombinant Alt a 1 expressed in Escherichia coli was purchased from BIOMAY (Vienna, Austria). The lyophilized protein was dissolved in double-distilled water at a concentration of 1 mg/mL and stored at −20 °C.
Four non-overlapping peptides of a length between 32 and 39 amino acids covering the sequence of Alt a 1 were synthesized, using a Fmoc (9-fluorenyl-meth-oxy-carbonyl)-strategy with 2-(1H-Benzotriazol-1-yl) 1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU)-activation (0.1 mmol small-scale cycles), on the Applied Biosystems peptide synthesizer model 433A (Foster City, CA, USA) [21]. Peptides were characterized by mass-spectrometry and further purified to >90% purity by preparative HPLC (piChem, Graz, Austria).
Peptides were selected according to predictions of hydrophilicity and surface exposure to be rich in surface-exposed amino acids (using the ProtScale bioinformatic tool on the ExPASY server and the Kyte & Doolittle algorithm). The hydrophobic leader peptide (amino acids 1–18) which is cleaved from the mature protein was not included.
Circular dichroism (CD) analysis of rAlt a 1, nAlt a 1 and of the Alt a 1-derived peptides
CD measurements were carried out on a Jasco J-810 spectropolarimeter (JASCO Corporation, Tokyo, Japan). Far-ultraviolet CD spectra were measured in a 2 mm path-length rectangular quartz cuvette (Hellma, Mullheim, Baden, Germany) at a protein concentration of 0.1 mg/mL. Spectra were recorded from 190 to 260 nm with 0.5 nm resolution at a scan speed of 50 nm/min, and resulted from averaging three scans. All measurements were performed in double-distilled water. The final spectra were corrected by subtracting the corresponding base line spectrum obtained under identical conditions. Results were expressed as the mean residue ellipticity (Θ) at a given wavelength.
IgE reactivity and allergenic activity of rAlt a 1 and Alt a 1-derived peptides
IgE antibody reactivity of rAlt a 1 and the synthetic peptides was determined in non-denaturing RAST-based dot blot experiments. Aliquots containing 1 μg/dot of rAlt a 1 or equimolar amounts of the peptides (0.25 μg/dot) were dotted onto nitrocellulose membranes (Protran® Nitrocellulose Transfer Membrane; Schleicher & Schuell, Dassel, Germany). Membranes were dried and afterwards incubated with allergic patients’ sera diluted 1 : 10 in PBST (PBS, 0.5% v/v Tween 20, pH 7.4). For control purposes, serum from a non-atopic individual or PBST was used. Bound IgE antibodies were detected with 1:15 diluted 125Iodine-labelled anti-human IgE antibodies (IBL, Hamburg, Germany), and visualized by autoradiography using Kodak XOMAT films and intensifying screens (Kodak, Heidelberg, Germany).
In addition, IgE reactivity to rAlt a 1 and Alt a 1-derived peptides (coating concentrations: rAlt a 1: 4 μg/mL; peptides 1–4: 1 μg/mL) was tested by ELISA essentially as described [22].
For measuring CD203c expression on basophils, heparinized blood samples of Alt a 1-allergic patients were incubated for 15 min with serial dilutions of rAlt a 1, or an equimolar mixture of uncoupled or KLH-coupled peptides (0.0001–10 μg/mL) at 37 °C. An anti-IgE antibody (1 μg/mL) (Immunotech, Marseille, France) or PBS (phosphate buffered saline) was used for control purposes. The up-regulation of CD203c by rAlt a 1 or Alt a 1-derived peptides was calculated from mean fluorescence intensities (MFIs) obtained with stimulated (MFIstim) and unstimulated (MFIcontrol) cells, and expressed as stimulation index (MFIstim: MFIcontrol) as reported [23]. All experiments were performed in triplicates.
Immunization of rabbits with rAlt a 1 or KLH-coupled peptides and measurement of specific IgG antibodies
Alt a 1-derived peptides were coupled to KLH (Keyhole limpet hemocyanin; Pierce, Rockford, IL, USA), purified with a Conjugation Kit (Sigma-Aldrich, St. Louis, MO, USA) and used for immunization of rabbits with 200 μg of the KLH-conjugated peptides or with rAlt a 1 using Freund’s complete and incomplete adjuvant (Charles River, Kisslegg, Germany). Serum samples were obtained before immunization (pre-immune sera) and in 4-week intervals after immunization and stored at −20 °C.
To determine rabbit IgG immune responses, ELISA plates were coated with 4 μg/mL rAlt a 1 overnight. Plates were washed with TBST (TBS, 0.05% v/v Tween 20, pH 8), blocked for 2.5 h at 37 °C with 1% w/v BSA in TBST and thereafter incubated with serial dilutions of rabbit anti-peptide or rabbit anti-rAlt a 1 anti-sera (1 : 5 × 10-3, 1 : 5 × 10-4, 1 : 5 × 10-5 and 1 : 5 × 10-6). Bound rabbit IgG antibodies were detected with a 1 : 1000 diluted HRP-labelled donkey anti-rabbit anti-serum (Amersham Biosciences, Little Chalfont, UK) [22].
ELISA competition assay for analysing the inhibition of allergic patients’ IgE binding to rAlt a 1 by peptide-specific IgG antibodies
The ability of peptide-induced rabbit antibodies to inhibit the binding of Alternaria-allergic patients’ IgE to rAlt a 1 was examined in ELISA competition assays [21]. ELISA plates, coated overnight with 1 μg/mL rAlt a 1, were washed with TBST, blocked and incubated with 1 : 100 diluted anti-peptide anti-sera, a mixture of equal amounts of the anti-peptide antisera, anti-rAlt a 1 anti-serum or, for control purposes, the corresponding pre-immune sera. The inhibition of allergic patients’ IgE binding was measured as described [22].
Results
rAlt a 1 binds most of the Alternaria-specific IgE in Alternaria-allergic patients
The 80 patients from Europe (Austria: n = 54; Italy: n = 26) with a case history and SPT results indicative of mould allergy were all positive when tested for IgE reactivity to a mould mix in the ImmunoCAP. Each of these patients also showed positive test results when tested for serum IgE reactivity to Alternaria extract. All, but two patients showed IgE reactivity to rAlt a 1. The levels of rAlt a 1-specific IgE in the rAlt a 1-positive patients were equally high and sometimes even higher than those determined with the Alternaria extract CAP, indicating that almost all of the Alternaria-specific IgE in these patients was directed against Alt a 1 (Table 1).
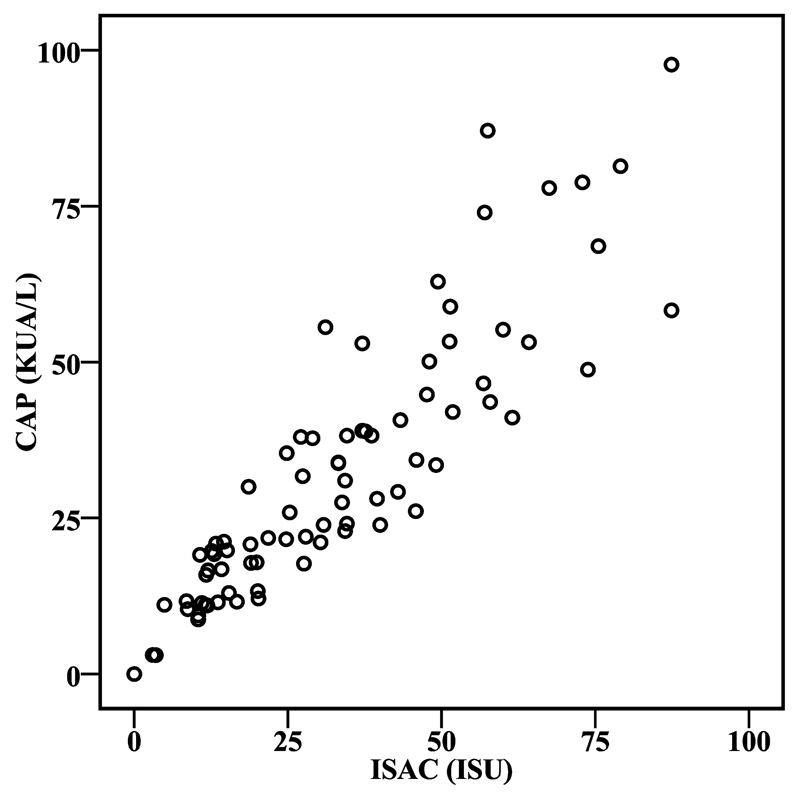
We then tested patients’ sera with micro-arrayed allergens, including rAlt a 1, by ImmunoCAP ISAC and found that all patients who had reacted with rAlt a 1 also reacted with micro-arrayed rAlt a 1. A high correlation (r = 0.891; P < 0.01) with the IgE levels determined by ImmunoCAP and ISAC was observed (Fig. 1). Interestingly, the two patients who were negative for rAlt a 1, but positive with the Alternaria extract reacted with several recombinant Aspergillus allergens indicating that these patients were not genuinely sensitized to Alternaria, but to Aspergillus.
Fig. 1.
Correlation of rAlt a 1-specific IgE antibodies determined by ImmunoCAP and ISAC. Alt a 1-specific IgE antibodies from 80 Alternaria-sensitized patients are indicated as kUA/L (y-axis) or ISAC Standardized Units (ISU) (x-axis), respectively.
No significant differences were noted regarding the rAlt a 1-specific mean IgE levels as determined by CAP and ISAC between Alternaria-allergic patients suffering only from rhinitis and/or conjunctivitis (CAP: 32.4 kUA/L; ISAC: 34.3 ISU) and those suffering from asthma and/or rhinitis (CAP: 32.1 kUA/L; ISAC: 31.4 ISU). We also did not note significant differences between Austrian and Italian Alternaria-allergic patients regarding rAlt a 1-specific IgE levels (Table 1).
The respiratory allergens recognized on the micro-array by the Austrian (A) and Italian (I) Alternaria-allergic patients were as follows: rPhl p 1 (A: 57.4%, I: 61.5%), rPhl p 2 (A: 29.6, I: 50.0%), rPhl p 4 (A: 48.1%, I: 38:5%), rPhl p 5 (A: 46.3, I: 46.2%), Phl p 6 (A: 33.3%, I: 30.8%), rPhl p 11 (A: 16.7%, I: 23.1%), nCup a 1 (A: 14.8%, I: 42.3%), nCry j 1 (A: 13.0%, I: 26.9%), rPar j 2 (A: 1.9%, I: 23.1%), nArt v 1 (A: 18.5%, I: 3.8%), nAmb a 1 (A: 14.8%, I: 3.8%), rBet v 1 (A: 33.3%, I: 26.9%), nOle e 1 (A: 44.4%, I: 46.2%), rFel d 1 (A: 42.9%, I: 42.3%), nDer p 1 (A: 20.4%, I: 9.3%) and nDer p 2 (A: 20.4%, I: 30.8%).
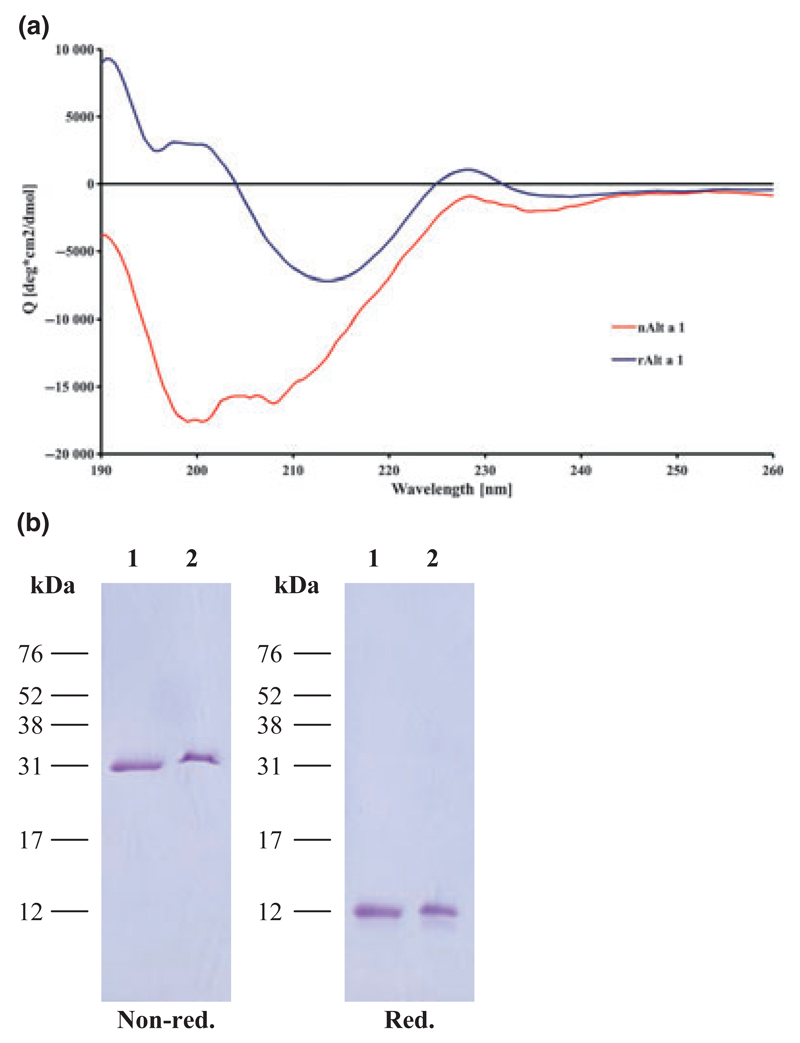
rAlt a 1, a folded protein containing predominantly β-sheet structure forms dimers via disulphide bridges
The far-ultraviolet circular dichroism (far-UV CD) spectrum of rAlt a 1 (Fig. 2A) shows a broad minimum at 213 nm indicating that the recombinant protein is folded and contains a considerable amount of β-sheets. Secondary structure analysis using the programme CDSSTR with the reference dataset 4, yielded 4% α-helix, 46% β-sheets, 18% β-turns and 30% random coils. The NRMSD value of 0.029 demonstrated a good fit between the calculated and the experimentally derived spectra. Also nAlt a 1 was a folded protein containing considerable amounts of ß-sheets similar to rAlt a 1 (8% α-helices, 33% ß-sheets, 26% ß-turns and 31% random coils), but showed a different CD spectrum.
Fig. 2.
Far-UV CD analysis and SDS-PAGE of rAlt a 1 and nAlt a 1. (A) The far-UV CD spectrum of rAlt a 1 (blue line) and nAlt a 1 (red line) is expressed as mean residue ellipticity Θ (y-axis) at a given wavelength (x-axis). (B) Recombinant Alt a 1 (lane 1) and natural Alt a 1 (lane 2) were separated by SDS-PAGE under non-reducing (non-red.) and reducing (red.) conditions and Coomassie-Blue stained. The molecular weight marker is indicated in the left margin.
When subjected to SDS-PAGE under reducing and non-reducing conditions, we found that rAlt a 1 migrates under reducing conditions as a band with a molecular mass of approximately 13 kDa, which corresponds to the calculated molecular weight (Fig. 2B). Under non-reducing conditions, rAlt a 1 migrates as a single band of 31 kDa, which corresponds to the molecular mass of a dimer (Fig. 2B). Natural purified Alt a 1 showed exactly the same behaviour as rAlt a 1. It migrated as monomer under reducing conditions and as dimer under non-reducing conditions in SDS-PAGE, and exhibited almost the same molecular weight as rAlt a 1 indicating that the allergen is not post-translationally modified (Fig. 2B).
Characterization of non-allergenic Alt a 1 peptides containing surface-exposed amino acids
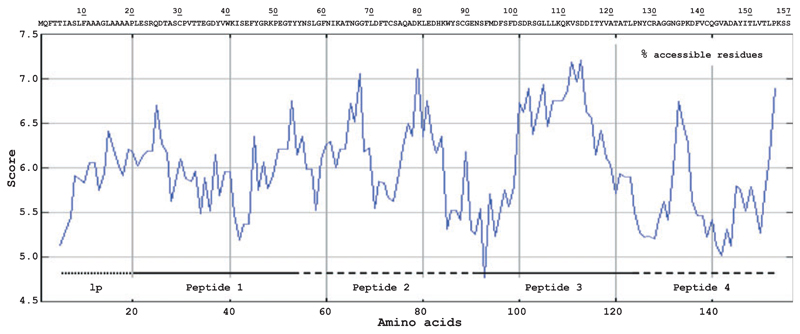
Alt a 1 is a protein comprising 157 amino acids of which the first 18 amino acids represent a leader peptide which is absent in the mature allergen (Fig. 3). With the aim to develop a non-allergenic peptide-based vaccine for Alt a 1, the protein was disrupted into four peptides of approximately 30–40 amino acids which were selected to be rich in surface-exposed amino acids. Fig. 3 shows the prediction of surface accessible regions of Alt a 1 aligned with the Alt a 1 sequence and the position of the four peptides which were synthesized (peptide 1: aa 19–52; peptide 2: aa 53–91; peptide 3: aa 92–122; peptide 4: aa 123–157). Following the hapten carrier principle described by Benacerraf [24, 25], we defined non-allergenic peptides of Alt a 1 which, after coupling to a carrier protein, can be used for immunization to induce IgG antibodies which should recognize Alt a 1 and block allergic patients’ IgE reactivity to surface exposed areas on Alt a 1. The coupling was thought to be achieved through disulphide bonds formed via naturally occurring cysteines in the peptide sequences. In case of peptide 3 which did not contain cysteines, a cysteine residue was added to the N-terminus of the peptide to allow coupling in the natural orientation of the peptide. The molecular weights of the peptides ranged from 3575 (peptide 3) to 4349.7 (peptide 2) dalton and the calculated isoelectric points were between 4.3 (peptide 3) and 7.7 (peptide 4).
Fig. 3.
Amino acid sequence and hydrophilicity plot of Alt a 1. The position of the leader peptide (lp) is represented by a dotted line. Alt a 1-derived peptides (peptide 1-4) are displayed as solid or dashed line. Scores above 5.5 (y-axis) represent regions (x-axis) of high surface accessibility.
Peptides were analysed for secondary structure by far-UV CD measurements. In contrast to rAlt a 1 which showed the typical spectrum of a folded, beta-sheet protein, spectra of the four peptides all had minima at 200 nm or below, indicating the lack of secondary structure elements (data not shown).
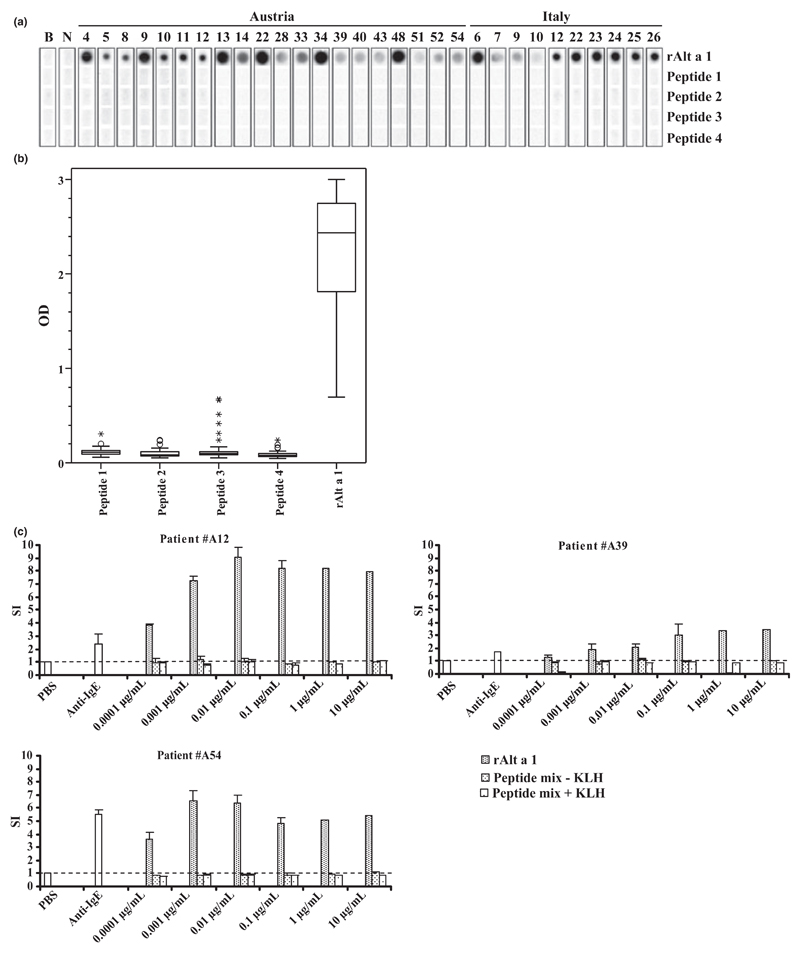
The IgE binding capacities of the four Alt a 1-derived peptides were compared with that of rAlt a 1 in a non-denaturing, RAST-based dot blot assay and by ELISA in those patients, where sufficient amounts of serum were available. Fig. 4A shows representative results obtained for sera from 20 Austrian and 10 Italian patients. We found that each of the sera showed IgE reactivity to rAlt a 1, but none of the peptides exhibited any detectable IgE reactivity. When tested for IgE reactivity with sera from 60 Alternaria-allergic patients by ELISA (Austria: n = 48; Italy: n = 12), we found that only few sera showed weak IgE reactivities to the Alt a 1-derived peptides (Fig. 4B).
Fig. 4.
IgE reactivity and allergenic activity of Alt a 1-derived peptides and rAlt a 1. (A) Nitrocellulose-dotted Alt a 1-derived peptides and rAlt a 1 were incubated with sera from 30 Alternaria-sensitized patients and the serum of a non-atopic individual (lane N) or buffer (lane B). (B) IgE reactivity tested by ELISA in 60 patients. OD values correspond to bound IgE and are represented in box plots, where boxes mark the interquartile range containing 50% of the data and lines across the boxes indicate the median. ○ represent outliers and asterisks are extreme values. (C) Basophils from three Alt a 1-sensitized patients were incubated with increasing concentrations (x-axis) of rAlt a 1 or an equimolar mixture of KLH-coupled or uncoupled Alt a 1-derived peptides. Stimulation indices (SI) displayed on the y-axis correspond to induced CD203c up-regulation on basophils. Results represent the mean ± SD from triplicates. The dashed line indicates the unspecific basophil activation after incubation with PBS.
To investigate the possible allergenic activity of the Alt a 1 peptides, basophil activation experiments were performed in Alternaria-allergic patients. Fig. 4C shows that rAlt a 1 induces a dose-dependent up-regulation of CD203c expression on basophils of Alternaria-allergic patients starting at 0.1 ng/mL, whereas mixes of the uncoupled and KLH-coupled peptides did not induce basophil activation up to the highest tested concentration (i.e. 10 μg/mL).
Immunization of rabbits with KLH-coupled Alt a 1 peptides induces Alt a 1-specific IgG antibodies, which inhibit allergic patients’ IgE binding to Alt a 1
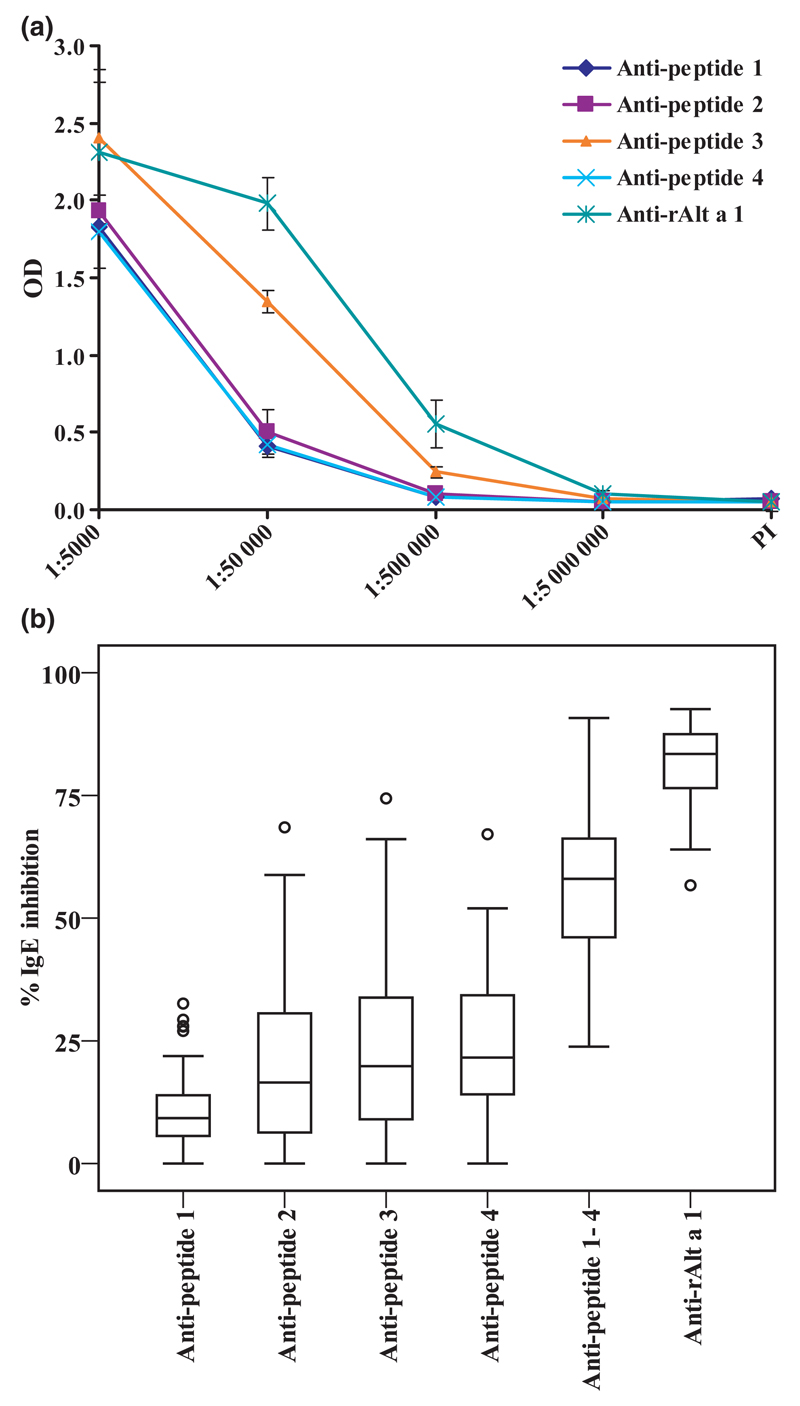
Fig. 5A displays the titration of Alt a 1-specific IgG antibodies which were induced by immunization with KLH-coupled peptides and rAlt a 1. All anti-peptide anti-sera recognized complete rAlt a 1, but the highest anti-rAlt a 1 IgG levels, which were almost comparable to those obtained with the anti-rAlt a 1 anti-serum, were observed for the anti-peptide 3 anti-serum (Fig. 5A).
Fig. 5.
(A) Induction of peptide- and rAlt a 1- specific IgG antibodies in rabbits. Rabbits were immunized with KLH-coupled Alt a 1-derived peptides or rAlt a 1. IgG reactivity of the final sera to ELISA plate bound rAlt a 1 was determined for different dilutions (x-axis) of the anti-sera and is displayed as optical density (OD) values (y-axis). (B) Anti-peptide anti-sera and anti-rAlt a 1 anti-serum inhibit Alternaria-sensitized patients’ IgE binding to rAlt a 1. Pre-incubation of ELISA plate bound rAlt a 1 with rabbit anti-peptide anti-sera, mixtures of equal amounts of anti-peptide anti-sera or anti-rAlt a 1 anti-serum reduces patients’ IgE binding (n = 38) to rAlt a 1. The percentage inhibition of IgE binding was calculated and is represented in box plots, where boxes mark the interquartile range containing 50% of the data, and lines across the boxes indicate the median. ○ represent outliers.
We then tested rabbit anti-peptide antibodies and rabbit anti-Alt a 1 antibodies for their ability to inhibit the binding of allergic patients’ (n = 38) IgE to Alt a 1 (Fig. 5B). With the single anti-peptide antibodies a modest inhibition of IgE binding was achieved. Mean inhibitions with anti-peptide 1, 2, 3 and 4 antibodies were 11.0%, 21.4%, 24.3% and 25.1%, respectively. When mixes of two anti-peptide antibodies were used, higher mean inhibitions in the range of 40% were obtained (data not shown). With a mix of all four anti-peptide antibodies (i.e. anti-peptide 1-4) a mean inhibition of IgE binding of 57.4% was achieved. A mean inhibition of 80.9% was obtained with the antibodies raised against the complete Alt a 1 allergen (Fig. 5B).
Discussion
Our results demonstrate that Alt a 1 is not only the major allergen in Alternaria alternata but also contains the majority of Alternaria-specific IgE epitopes and exhibits an extremely high allergenic activity. Micro-arrayed rAlt a 1 allows the sensitive diagnosis of allergy to Alternaria. In a population of 80 patients with suspected Alternaria allergy, genuine sensitization to Alternaria was confirmed in 78 patients, whereas two patients were sensitized to Aspergillus and thus gave false positive test results with Alternaria extract, most likely because of the presence of cross-reactive allergens, such as enolases, MnSODs or cross-reactive carbohydrates [18, 26]. Micro-arrayed rAlt a 1 can therefore be used for the diagnosis of allergy to Alternaria and for the identification of genuinely sensitized patients for allergen-specific immunotherapy (SIT). The micro-array approach may be particularly useful because it also allowed establishing co-sensitizations to several other respiratory allergens which need to be considered as potential elicitors of respiratory symptoms and asthma. Indeed, we found that Alternaria-allergic patients were frequently co-sensitized to a variety of pollen allergens (e.g. Phl p 1: 58.8%; Phl p 2: 36.6%; Phl p 5: 46.3%; Phl p 6: 32.5%; Bet v 1: 31.3%; Ole e 1: 45%) and to certain indoor allergens (e.g. Fel d 1: 43.8%). Interestingly, Der p 1 (20%) and Der p 2 (25%) were less frequently recognized in the mould populations studied. It may therefore be necessary to confirm the clinical relevance of the individual sensitizations by refinement of the case history, confirmation of allergen exposure and provocation testing.
As rAlt a 1 contained the majority of Alternaria-specific IgE epitopes and represented a folded allergen with presumably conformational IgE epitopes, we selected peptides containing surface-exposed amino acids of Alt a 1. These peptides showed only weak IgE reactivity in a few patients possibly because of the loss of structure and fold. The fact that none of the peptides showed any allergenic activity in basophils of allergic patients, although occasional IgE binding was observed also in previous studies [27, 28], may be explained by hapten-like IgE binding which does not cause cross-linking of IgE and basophil activation [29, 30].
In contrast to rAlt a 1 which according to CD measurements represented a folded protein with ß-sheet structure, none of the peptides had any secondary structure. We therefore assume that Alt a 1, such as most other respiratory allergens, contains primarily conformational IgE epitopes. Differences observed for the CD spectra of natural and the recombinant Alt a 1 might be caused by the presence of unfolded Alt a 1 in the natural protein preparation and/or by binding of a ligand to nAlt a 1 that influences the CD signal. In fact, we noted that the nAlt a 1 preparation always had a brownish colour which may be derived from melanin-containing particles to which Alt a 1 could be localized by immunogold electron microscopy [31].
According to the hapten-carrier concept [24, 25], antigen-specific IgG responses can be induced with carrier-bound antigen-derived peptides [32]. We therefore investigated whether it may be possible to induce Alt a 1-specific IgG antibodies upon immunization with the carrier-bound Alt a 1 peptides. For experimental purposes we used KLH as carrier protein because it has already been safely used in humans for the therapy of allergy and cancer [33–35]. Alternatively, recombinant fusion proteins consisting of allergen-derived peptides and viral carrier proteins may used for the production of the final vaccine [36, 37]. We found that also the carrier-bound peptides lacked allergenic activity, but nevertheless, induced Alt a 1-specific IgG antibodies. The individual peptide-induced anti-sera caused a modest inhibition of the polyclonal IgE response of Alternaria-allergic patients to Alt a 1 which indicates that the IgE epitopes are distributed evenly over the Alt a 1 structure. This wide distribution of IgE epitopes on Alt a 1 is different from other respiratory allergens (e.g. Bet v 1, Bet v 2, Phl p 1, Phl p 2, Phl p 5) [38–41] for which a clustering of IgE epitopes at certain areas of the molecules was found. Support for the assumption of a rather even distribution also comes from the observation that a mixing of antibodies with specificities to different peptides increased the inhibitory activity of the antibodies which was not the case for the birch pollen allergen Bet v 1 and the grass pollen allergen Phl p 1 [21, 42]. The blocking of allergic patients’ IgE binding to Alt a 1 by antibodies induced with the coupled peptides was lower than that achieved with the Alt a 1 allergen, but the peptide vaccine has the big advantage of lacking allergenic activity. rAlt a 1 induced basophil activation already at 0.1 ng/mL whereas none of the peptides was reactive up to highest tested dose of 10 μg/mL. The peptide vaccine may therefore be safely administered to patients at much higher doses than vaccines based on the rAlt a 1 wild-type allergen. In fact, vaccines based on purified recombinant major birch pollen allergen Bet v 1 and hypoallergenic derivatives thereof have already been successfully used in clinical trials [43]. Our pre-clinical data indicate that a vaccine based on carrier-bound non-allergenic Alt a 1 peptides may be safe and clinically effective for SIT of Alternaria allergy.
Acknowledgements
We thank Vera Civaj and Sabine Meyerweck for excellent technical assistance.
Funding
This work was supported by a research grant from the Christian Doppler Association and Biomay (Vienna, Austria) and in part by SFB grant F1820 of the Austrian Science Fund (FWF) and by the Italian Ministry of Health, Current Research program 2008.
Abbreviations
- Aa
amino acids
- CD
circular dichroism
- ISAC
immuno solid-phase allergen chip
- ISU
ISAC Units
- KLH
keyhole limpet hemocyanin
- N
natural
- R
recombinant
- SIT
specific immunotherapy
Footnotes
Conflict of interest
Teresa E Twaroch has no conflict of interest to declare.
Margit Focke has no conflict of interest to declare.
Karin Fleischmann has no conflict of interest to declare.
Nadja Balic has no conflict of interest to declare.
Christian Lupinek has no conflict of interest to declare.
Katharina Blatt has no conflict of interest to declare.
Rosetta Ferrara has no conflict of interest to declare.
Adriano Mari has no conflict of interest to declare.
Christoph Ebner has no conflict of interest to declare.
Peter Valent received a research grant from Biomay, Vienna, Austria.
Susanne Spitzauer has no conflict of interest to declare.
Ines Swoboda has no conflict of interest to declare.
Rudolf Valenta has acted as a paid consultant to Bio-may, Vienna, Austria and Phadia, Uppsala, Sweden, and has received funding for research carried out in this work.
References
- 1.Kurup VP, Shen HD, Vijay H. Immunobiology of fungal allergens. Int Arch Allergy Immunol. 2002;129:181–8. doi: 10.1159/000066780. [DOI] [PubMed] [Google Scholar]
- 2.Simon-Nobbe B, Denk U, Pöll V, Rid R, Breitenbach M. The spectrum of fungal allergy. Int Arch Allergy Immunol. 2008;145:58–86. doi: 10.1159/000107578. [DOI] [PubMed] [Google Scholar]
- 3.D’Amato G, Chatzigeorgiou G, Corsico R, et al. Evaluation of the prevalence of skin prick test positivity to Alternaria and Cladosporium in patients with suspected respiratory allergy. A European multicenter study promoted by the Subcommittee on Aerobiology and Environmental Aspects of Inhalant Allergens of the European Academy of Allergology and Clinical Immunology. Allergy. 1997;52:711–6. doi: 10.1111/j.1398-9995.1997.tb01227.x. [DOI] [PubMed] [Google Scholar]
- 4.Zureik M, Neukirch C, Leynaert B, Liard R, Bousquet J, Neukirch F European Community Respiratory Health Survey. Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community respiratory health survey. BMJ. 2002;325:411–4. doi: 10.1136/bmj.325.7361.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salo PM, Arbes SJ, Jr, Sever M, et al. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 2006;118:892–8. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez H, Bush RK. A review of Alternaria alternata sensitivity. Rev Iberoam Micol. 2001;18:56–9. [PubMed] [Google Scholar]
- 7.Neukirch C, Henry C, Leynaert B, Liard R, Bousquet J, Neukirch F. Is sensitization to Alternaria alternata a risk factor for severe asthma? A population-based study. J Allergy Clin Immunol. 1999;103:709–11. doi: 10.1016/s0091-6749(99)70247-2. [DOI] [PubMed] [Google Scholar]
- 8.D’Amato G, Spieksma FT. Aerobiologic and clinical aspects of mould allergy in Europe. Allergy. 1995;50:870–7. doi: 10.1111/j.1398-9995.1995.tb02492.x. [DOI] [PubMed] [Google Scholar]
- 9.Salo PM, Yin M, Arbes SJ, Jr, et al. Dustborne Alternaria alternata antigens in US homes: results from the National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 2005;116:623–9. doi: 10.1016/j.jaci.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mari A, Schneider P, Wally V, Breitenbach M, Simon-Nobbe B. Sensitization to fungi: epidemiology, comparative skin tests, and IgE reactivity of fungal extracts. Clin Exp Allergy. 2003;33:1429–38. doi: 10.1046/j.1365-2222.2003.01783.x. [DOI] [PubMed] [Google Scholar]
- 11.Helbling A, Reimers A. Immunotherapy in fungal allergy. Curr Allergy Asthma Rep. 2003;3:447–53. doi: 10.1007/s11882-003-0082-x. [DOI] [PubMed] [Google Scholar]
- 12.Vailes L, Sridhara S, Cromwell O, Weber B, Breitenbach M, Chapman M. Quantitation of the major fungal allergens, Alt a 1 and Asp f 1, in commercial allergenic products. J Allergy Clin Immunol. 2001;107:641–6. doi: 10.1067/mai.2001.114118. [DOI] [PubMed] [Google Scholar]
- 13.Ibarrola I, Suárez-Cervera M, Arilla MC, et al. Production profile of the major allergen Alt a 1 in Alternaria alternata cultures. Ann Allergy Asthma Immunol. 2004;93:589–93. doi: 10.1016/S1081-1206(10)61268-9. [DOI] [PubMed] [Google Scholar]
- 14.Martínez J, Gutiérrez A, Postigo I, Cardona G, Guisantes J. Variability of Alt a 1 expression by different strains of Alternaria alternata. J Investig Allergol Clin Immunol. 2006;16:279–82. [PubMed] [Google Scholar]
- 15.Sáenz-de-Santamaría M, Guisantes JA, Martínez J. Enzymatic activities of Alternaria alternata allergenic extracts and its major allergen (Alt a 1) Mycoses. 2006;49:288–92. doi: 10.1111/j.1439-0507.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 16.Rid R, Onder K, MacDonald S, et al. Alternaria alternata TCTP, a novel cross-reactive ascomycete allergen. Mol Immunol. 2009;46:3476–87. doi: 10.1016/j.molimm.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Asturias JA, Ibarrola I, Ferrer A, et al. Diagnosis of Alternaria alternata sensitization with natural and recombinant Alt a 1 allergens. J Allergy Clin Immunol. 2005;115:1210–7. doi: 10.1016/j.jaci.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Postigo I, Gutieérrez-Rodríguez A, Fernández J, Guisantes JA, Suñén E, Martínez J. Diagnostic value of Alt a 1, fungal enolase and manganese-dependent superoxide dismutase in the component-resolved diagnosis of allergy to pleosporaceae. Clin Exp Allergy. 2011;41:443–51. doi: 10.1111/j.1365-2222.2010.03671.x. [DOI] [PubMed] [Google Scholar]
- 19.De Vouge MW, Thaker AJ, Curran IH, et al. Isolation and expression of a cDNA clone encoding an Alternaria alternata Alt a 1 subunit. Int Arch Allergy Immunol. 1996;111:385–95. doi: 10.1159/000237397. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Focke M, Mahler V, Ball T, et al. Non-anaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–4. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- 22.Twaroch TE, Focke M, Civaj V, et al. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J Allergy Clin Immunol. 2011;128:178–84. doi: 10.1016/j.jaci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Hauswirth AW, Natter S, Ghannadan M, et al. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J Allergy Clin Immunol. 2002;110:102–9. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- 24.Siskind GW, Paul WE, Benacerraf B. Studies on the effect of the carrier molecule on antihapten antibody synthesis I. Effect of carrier on the nature of the antibody synthesized. J Exp Med. 1966;123:673–88. doi: 10.1084/jem.123.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul WE, Katz DH, Goidl EA, Benacerraf B. Carrier function in anti-hapten immune responses II. Specific properties of carrier cells capable of enhancing anti-hapten antibody responses. J Exp Med. 1970;132:283–99. doi: 10.1084/jem.132.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leino M, Reijula K, Mäkinen-Kiljunen S, Haahtela T, Mäkelä MJ, Alenius H. Cladosporium herbarum and Pityrosporum ovale allergen extracts share cross-reacting glycoproteins. Int Arch Allergy Immunol. 2006;140:30–5. doi: 10.1159/000091841. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Curran IH, Muradia G, De Vouge MW, Rode H, Vijay HM. N-terminus of a major allergen, Alt a I, of Alternaria alternata defined to be an epitope. Int Arch Allergy Immunol. 1995;108:254–9. doi: 10.1159/000237161. [DOI] [PubMed] [Google Scholar]
- 28.Kurup VP, Vijay HM, Kumar V, Castillo L, Elms N. IgE binding synthetic peptides of Alt a 1, a major allergen of Alternaria alternata. Peptides. 2003;24:179–85. doi: 10.1016/s0196-9781(03)00024-x. [DOI] [PubMed] [Google Scholar]
- 29.Ball T, Vrtala S, Sperr WR, et al. Isolation of an immunodominant IgE hapten from an epitope expression cDNA library. Dissection of the allergic effector reaction. J Biol Chem. 1994;269:28323–8. [PubMed] [Google Scholar]
- 30.Westritschnig K, Horak F, Swoboda I, et al. Different allergenic activity of grass pollen allergens revealed by skin testing. Eur J Clin Invest. 2008;38:260–7. doi: 10.1111/j.1365-2362.2008.01938.x. [DOI] [PubMed] [Google Scholar]
- 31.Twaroch TE, Arcalís E, Sterflinger K, Stöger E, Swoboda I, Valenta R. Predominant localization of the major Alternaria allergen Alt a 1 in the cell wall of airborne spores. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.10.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Arnon R, Van Regenmortel MH. Structural basis of antigenic specificity and design of new vaccines. FASEB J. 1992;6:3265–74. doi: 10.1096/fasebj.6.14.1385242. [DOI] [PubMed] [Google Scholar]
- 33.Harris JR, Markl J. Keyhole limpet hemocyanin (KLH): a biomedical review. Micron. 1999;30:597–623. doi: 10.1016/s0968-4328(99)00036-0. [DOI] [PubMed] [Google Scholar]
- 34.Gilewski T, Adluri S, Ragupathi G, et al. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6:1693–701. [PubMed] [Google Scholar]
- 35.Demoly P, Persi L, Dhivert H, Delire M, Bousquet J. Immunotherapy with keyhole limpet hemocyanin-conjugated decapeptide vaccine in cypress pollen allergy. Clin Exp Allergy. 2002;32:1071–6. doi: 10.1046/j.1365-2222.2002.01392.x. [DOI] [PubMed] [Google Scholar]
- 36.Edlmayr J, Niespodziana K, Linhart B, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009;182:6298–306. doi: 10.4049/jimmunol.0713622. [DOI] [PubMed] [Google Scholar]
- 37.Niespodziana K, Focke-Tejkl M, Linhart B, et al. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol. 2011;127:1562–70. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fedorov AA, Ball T, Mahoney NM, Valenta R, Almo SC. The molecular basis for allergen cross-reactivity: crystal structure and IgE-epitope mapping of birch pollen profilin. Structure. 1997;5:33–45. doi: 10.1016/s0969-2126(97)00164-0. [DOI] [PubMed] [Google Scholar]
- 39.Flicker S, Steinberger P, Ball T, et al. Spatial clustering of the IgE epitopes on the major timothy grass pollen allergen Phl p 1: importance for allergenic activity. J Allergy Clin Immunol. 2006;117:1336–43. doi: 10.1016/j.jaci.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Padavattan S, Flicker S, Schirmer T, et al. High-affinity IgE recognition of a conformational epitope of the major respiratory allergen Phl p 2 as revealed by X-ray crystallography. J Immunol. 2009;182:2141–51. doi: 10.4049/jimmunol.0803018. [DOI] [PubMed] [Google Scholar]
- 41.Flicker S, Vrtala S, Steinberger P, et al. A human monoclonal IgE antibody defines a highly allergenic fragment of the major timothy grass pollen allergen, Phl p 5: molecular, immunological, and structural characterization of the epitope-containing domain. J Immunol. 2000;165:3849–59. doi: 10.4049/jimmunol.165.7.3849. [DOI] [PubMed] [Google Scholar]
- 42.Gieras A, Cejka P, Blatt K, et al. Mapping of conformational IgE epitopes with peptide-specific monoclonal antibodies reveals simultaneous binding of different IgE antibodies to a surface patch on the major birch pollen allergen, Bet v 1. J Immunol. 2011;186:5333–44. doi: 10.4049/jimmunol.1000804. [DOI] [PubMed] [Google Scholar]
- 43.Pauli G, Larsen TH, Rak S, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–60. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]