Abstract
Many human glandular cancers metastasize along nerve tracts, but the mechanisms involved are generally poorly understood. The calcium-binding protein S100A4 is expressed at elevated levels in human cancers, where it has been linked to increased invasion and metastasis. Here we report genetic studies in a Drosophila model to define S100A4 effector functions that mediate metastatic dissemination of mutant Ras-induced tumors in the developing nervous system. In flies overexpressing mutant RasVal12 and S100A4, there was a significant increase in activation of the stress kinase JNK and production of the matrix metalloproteinase MMP1. Genetic or chemical blockades of JNK and MMP1 suppressed metastatic dissemination associated with S100A4 elevation, defining required signaling pathway(s) for S100A4 in this setting. In clinical specimens of human breast cancer, elevated levels of the mammalian paralogs MMP2, MMP9, and MMP13 are associated with a 4- to 9-fold relative decrease in patient survival. In individual tumors, levels of MMP2 and MMP13 correlated more closely with levels of S100A4, whereas MMP9 levels correlated more closely with the S100 family member S100P. Overall, our results suggest the existence of evolutionarily conserved pathways used by S100A4 to promote metastatic dissemination, with potential prognostic and therapeutic implications for metastasis by cancers that preferentially exploit nerve tract migration routes.
Introduction
Certain tumor cells have a propensity to invade the neighboring tissue and eventually establish new secondary tumors or metastases while others cannot (1, 2). These results suggest that a specific set of genes, different from those involved in the production of the neoplasia, are involved in promoting a complex series of steps to form metastases (3). The protein products of such genes have been termed metastasis-inducing proteins. One such gene/protein is S100A4 (4), a member of the S100-calcium-binding protein family (5). Although S100A4 cannot promote tumor formation directly, it can stimulate the remaining steps in the metastatic cascade in model rodent systems by combining with oncogenes such as RasVal12 and neu (4, 6). Moreover, S100A4 is overexpressed in human primary tumors and is associated with the premature death of patients with different types of metastatic carcinomas, including those from the breast (7), oral mucosa, bladder, pancreas, prostate, colorectum, esophagus, lung, stomach, and thyroid glands (8). The elevation of S100A4 can trigger multiple biological functions, including cell migration, invasion, extracellular matrix remodeling, and angiogenesis (8, 9). However, it is unknown what are the biologically relevant molecular events from the plethora triggered by S100A4 in cultured mammalian cells (10).
To generate a genetically tractable experimental model to investigate the molecular events triggered by S100A4, we have for the first time expressed human S100A4 in the fruit fly, Drosophila melanogaster by targeting its expression to the developing eye lobes (11) and not elsewhere in the brain or CNS (12, 13). Drosophila has conserved signal transduction pathways for cell cycle, growth control, and cell-to-cell communication (14) and a larval phase of only a few days, which can be interrogated by inhibitory chemicals applied directly to the growth medium. In addition, 70% of human cancer genes are conserved in the Drosophila model, but importantly none of the S100 family proteins are present (15). Overexpression of oncogenic Ras (RasVal12) causes the formation of tumors in the epithelial tissues of Drosophila (16), and these can be transformed into a malignant phenotype by disruption of suppressor genes such as scribble (scrib) and lethal2 (17). In this new model we have for the first time generated transgenic flies capable of conditionally-expressing the open-reading frame of human S100A4 under GAL4/UAS control (18, 19). We show, after multiple crosses, that the S100A4 gene is required to disseminate RasVal12 tumor cells from the optic lobes to the ventral nerve cord (VNC) and further afield in fly larvae. The combination of RasVal12 and loss of scrib in Drosophila activates the JNK pathway and this activation induces the matrix metalloproteinase MMP1, to allow dissemination of the cancer cells in the Ras oncogenic system (16). Therefore, we have assessed whether c-Jun and Drosophila MMP1 are downstream targets for promoting dissemination in our RasVal12/S100A4 novel larval model using fly genetics and inhibitory chemicals, and whether there is a uniquely similar association between S100A4 and mammalian MMPs in human breast cancer.
Materials and Methods
Expression of S100A4 in Drosophila melanogaster
HumanS100A4 wild-type (S100A4wt; ref. 6) and inactive mutant S100A4Δ2 (20) were cloned and expressed in transgenic flies (19) as described in Supplementary Methods. Stable transgenic lines were checked for S100A4 expression by crossing with da-GAL4 flies (18) and Western blotted. Resultant S100A4wt and S100A4Δ2 progeny produced (mean ± SE) similar 7.7 ± 0.6 ng and 8.8 ± 0.7 ng S100A4 protein per 20 flies, respectively, compared with undetectable levels (<0.1 ng) in parental controls (Student t test, P = 0.29). Remaining details are in Supplementary Methods. All initial Drosophila strains were described previously (21), remaining details are in Supplementary Methods. The flies were maintained in standard yeast agar medium at 25°C in a 12-hour light–dark incubator (21).
Metastatic assay
The eyeless-FLP–induced recombination of the FRT-flanked y linker in Act>y>GAL4 results in reconstitution of Actin-GAL4 and expression of UAS-GFP, and other UAS elements, in the developing eye (22). Dissemination of GFP from its original site of eye-antennal discs to VNCs was scored for each genotype/experimental condition on a scale of 0 to 3 (16). GFP localized in the optic lobes scored zero (stage 0), GFP on one side of the VNC scored 1 (stage I), on two sides of VNC scored 2 (stage II), and dissemination further into the VNC scored 3 (stage III). Average stage score of metastasis (ASSM) ±SE was recorded for each genotype/experimental condition. Fluorescent staining is described in Supplementary Methods. Confocal images of GFP were captured (21) and analyzed using ImageJ software (23), as described in Supplementary Methods. Corrected integrated fluorescence intensity (CIFI) = integrated intensity – (area of selected background brain × mean fluorescence density of background) (23). Mean CIFI ± SE was recorded.
Western blot analysis
This is described in Supplementary Methods. To correct for any loading differences, original intensity of each band was divided by that of actin. Intensity of each band for a particular larval group was then expressed as a ratio of that in the RasVal12 larvae. Mean value of three experiments ±SE was recorded. To ensure the intensity of band signals lay within the linear range, a plot of band intensity against μg GAPDH was drawn (y = 43947x − 42398, r2 = 0.997) and band intensity for any protein outside the linear range was excluded from the data and if necessary the gel was rerun with higher or lower levels of total protein.
Drug treatment
Inhibitors, JNK-IN-8 (kindly provided by Nathanael S. Gray, Harvard Medical School, Boston, MA; ref. 24) and batimastat (cat. no.: SML0041; Sigma-Aldrich; ref. 25) were added directly from 1 mg/mL stock dissolved in DMSO to larval medium preheated to 55–60°C. Same concentration of DMSO was added to controls without inhibitors. The drugs were incubated with the larvae continuously until harvesting at the third instar stage, equivalent to 7 days.
Statistical analyses
The significance of the difference between two categorical groups for each genotype, those with and those without metastases was determined by Fisher exact test, recording two-sided values of P. The significance of the difference in ASSM, in CIFI for GFP and MMP1, and in mean corrected intensity of each protein band in Western blots were calculated using two-sided Student t test (Stats Direct). Differences were considered significant when P < 0.05.
Patients and specimens
A retrospective study was undertaken using samples of 183 primary tumors from unselected breast cancer patients, as described previously (26, 27). Ethical approval was obtained from NRES Committee, North West REC Ref. 12/NW/0778, Protocol no. UoL000889, IRAS no. 107845. Samples were preserved in neutral buffered-formalin and embedded in paraffin-wax, as described previously (7).
IHC staining
This is described in Supplementary Methods. Western blots of breast cell lines verified the specificity of all three mAbs to MMPs yielding apparent molecular weights of 73,99,75 kDa for secreted latent MMP2, 9, 13, respectively, consistent with those reported recently (28). Remainders were verified previously (27). IHC-stained sections were analyzed and scored (7, 26, 28, 29), as recorded in Supplementary Methods. Association of staining for MMP2, 9, and 13 with patient survival time is reported in Supplementary Methods.
Results
Cooperation of RasVal12 and S100A4 in producing metastases in recombinant Drosophila
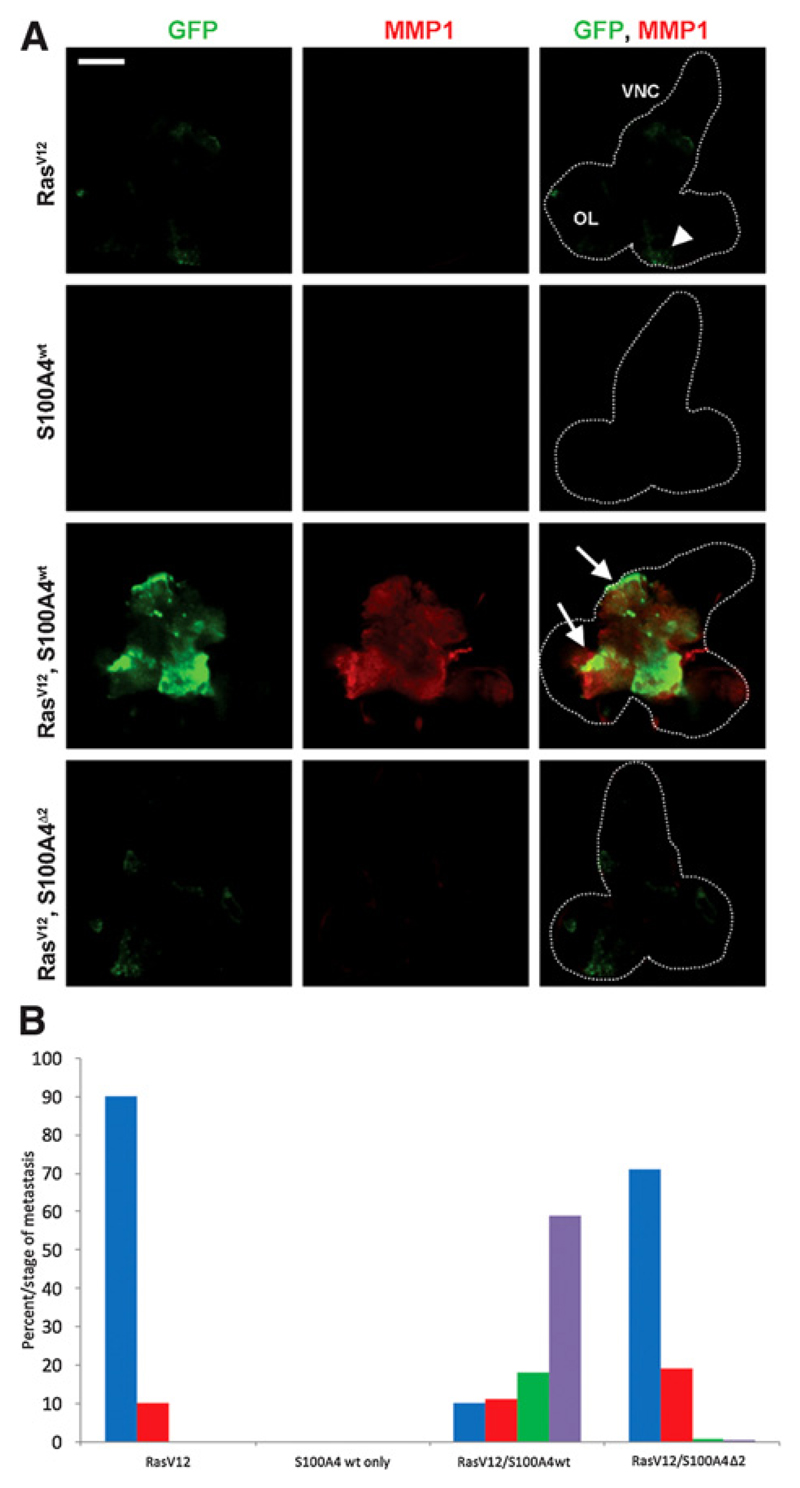
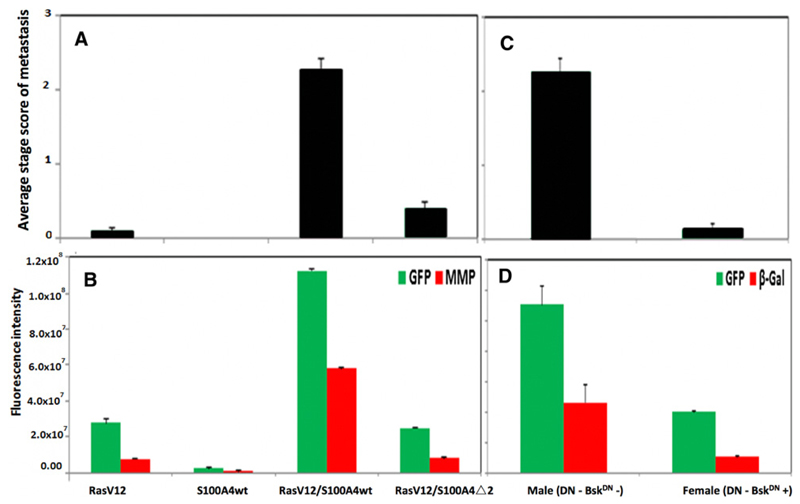
The brain containing the CNS was dissected from at least fifty, third instar larvae of different recombinant Drosophila. Male larvae with the genetic background RasVal12 alone (RasVal12 larvae) produced GFP-fluorescent tumors almost exclusively in the eye lobes (Fig. 1A) of 48 of 53 cases, with only 5 of 53 cases extending into one or the other side of the VNC (Fig. 1B). Extent of metastasis was semiquantified as described in Materials and Methods to produce an ASSM. There were no GFP-tumor deposits in the eye lobes or elsewhere in S100A4 larvae (Figs. 1A and B and 2A). The RasVal12/S100A4wt recombinant larvae produced metastasis to the VNC (Fig. 1C) in a significantly higher number of 53 of 59 cases (Fisher exact test, P < 0.0001; Fig. 1B), increasing the ASSM by a significant 24-fold over RasVal12 larvae (Student t test, P < 0.0001; Fig. 2A). There was also extensive metastasis to other organs, particularly to the gut and gonads (Supplementary Fig. S1). The RasVal12/S100A4Δ2 inactive mutant larvae (Materials and Methods) produced a significantly lower number of 16 of 56 with metastasis to the VNC (Fisher exact test, P < 0.0001; Fig. 1B), with significant 5.7-fold reduction in ASSM compared to RasVal12 larvae (Student t test, P = 0.0001; Figs. 1A and 2A). The CIFI of GFP (Materials and Methods) for images taken of the dissected CNS of RasVal12larvae was increased by a significant 4.1-fold in RasVal12/S100A4wt larvae (Student t test, P < 0.0001; Fig. 2B), but there was no significant difference in CIFI of RasVal12/S100A4Δ2 mutant larvae compared with RasVal12 larvae (Student t test, P = 0.49; Fig. 2B).
Figure 1.
A, Fluorescent images of GFP and MMP1 in larval CNS of different male recombinant Drosophila. CNS was dissected from third instar larvae of Drosophila with the following backgrounds: RasVal12 (RasV12) only; S100A4 wild type (S100A4wt) only; RasVal12, S100A4 wild type (RasV12/S100A4wt); and RasVal12, S100A4Δ2 (RasV12/S100A4Δ2). Representative CNS images show green fluorescence due to endogenous GFP, red fluorescence due to fluorescently labeled antibodies to MMP1, and merged fluorescent images are due to GFP and anti-MMP1. The outline of the relevant structures of the brain including optic lobes (OL) and VNC are indicated by the broken white line. The region that clearly expresses MMP in RasVal12, S100A4 transgenics (arrows) and the same region in RasVal12 transgenics (arrowhead) are shown (Scale bar, 100 μm). B, Histogram of resultant recombinant larvae. The percentage larvae with different stages (0–III) of metastasis from the optic lobes to the VNCs is shown for the recombinant Drosophila. The VNC of at least 50, third instar larvae were scored for the extent of metastasis on a sliding scale (Materials and Methods): from stage 0 (blue), stage I (red), stage II (green), and stage III (purple). Larvae containing RasVal12/S100A4 were significantly different from those containing RasVal12 alone, S100A4 alone, and RasVal12/S100A4Δ2 (Fisher exact test, P < 0.0001) and between larvae containing RasVal12 and RasVal12/S100A4Δ2 (P = 0.012).
Figure 2.
Tumor dissemination in different recombinant Drosophila larvae. A, Average stage of metastatic spread in RasVal12/S100A4 flies. ASSM of the primary tumor in the optic lobe spreading to the VNC is shown for male recombinant Drosophila with genetic backgrounds of RasVal12(RasV12), S100A4 wild type only (S100A4wt), RasVal12plus S100A4 wild type (RasV12/S100A4wt), and RasVal12 plus S100A4mutantΔ2 (RasV12/S100A4Δ2). At least 50 larvae were scored (Materials and Methods) and results are expressed as mean ± SE. Both double transgenic flies were significantly different from flies with RasVal12genotype (Student t test, P ≤ 0.005) and RasVal12/S100A4wt from RasVal12/S100A4Δ2 genotype (P = 0.0001). B, Fluorescence intensity of CNS images of RasVal12/S100A4 flies. Endogenous fluorescence from GFP (green) and from exogenously added labeled antibody to MMP1 (red) were recorded. CIFI of images of the dissected CNS from the same larvae in A were computed as described in Materials and Methods. Mean ± SE is shown. For GFP green fluorescence, RasVal12/S100A4wt versus RasVal12only, S100A4wt only, or RasVal12/S100A4Δ2 (Student t test, P < 0.0001); RasVal12 vs. RasVal12/S100A4Δ2 (P = 0.49). For MMP-1 red fluorescence, RasVal12/S100A4wt versus RasVal12only, S100A4wt only, and RasVal12/S100A4Δ2 (Student t test, P < 0.0001), but RasVal12 versus RasVal12/S100A4Δ2 larval CNS (P = 0.15). C, Average stage of metastatic spread in male and female JNK-suppressed RasVal12/S100A4 flies. ASSM of the primary tumor in the optic lobe spreading to the VNC is shown for recombinant Drosophila male and female larvae with the genetic backgrounds of RasVal12/S100A4wt, in which the BskDN dominant suppressor of JNK is expressed only in female flies. At least 20 larvae were scored as in A. Results are expressed as mean ± SE and there was a highly significant difference (Student t test, P < 0.0001). D, Quantification of the levels of fluorescent GFP and β-galactosidase in JNK suppressed flies. Endogenous fluorescence from GFP (green) and from exogenously added labeled antibody to β-galactosidase (β-Gal; red) were recorded. CIFI of images of the dissected CNS from the same larvae as in C were computed for male and female Drosophila with the RasVal12/S100A4wt genetic background. Mean ± SE is shown. The β-galactosidase is a marker of the activity of JNK (Materials and Methods). For GFP green fluorescence and β-galactosidase red fluorescence, significant reduction for female versus male RasVal12/S100A4wt larvae (Student t test, P < 0.0001 and P = 0.004, respectively).
Quantification of Ras, GFP, and S100A4 levels by Western blot analysis
Antibodies to S100A4 detected a specific band of the correct apparent molecular weight of 9 kDa in all fly lines containing the S100A4wt or S100A4Δ2 mutant gene, but no corresponding band in larvae containing RasVal12 alone (Supplementary Fig. S2). Larvae containing the RasVal12/S100A4wt and RasVal12/S100A4Δ2 genes produced a significant increase in Ras and a similar increase in GFP over that in larvae containing RasVal12 alone (Student t test, P < 0.001; Table 1). Protein bands of Ras and GFP were observed at the correct molecular weights (21 and 27 kDa, respectively; Supplementary Fig. S2 and Table 1). There was also highly significant increases of 220 ± 5- and 85 ± 11-fold in S100A4 protein in larvae containing the RasVal12 /S100A4wt and RasVal12/S100A4Δ2 genes, respectively (P < 0.0001; Table 1), when normalized to GFP. Thus, there is a significant association of expression of active S100A4 and metastasis in this model system.
Table 1.
Quantification of Western blots of different Drosophila lines
| Mean relative abundanceb |
||||
|---|---|---|---|---|
| Antibody toa | RasVal12 | S100A4wt | RasVal12/S100A4wt | RasVal12/S100A4Δ2 |
| (A) Ras | 1 ± 0.05 | 0.0097 ± 0.001 | 3.56 ± 0.08c | 1.96 ± 0.16c |
| (B) GFP | 1 ± 0.04 | 0.011 ± 0.002 | 3.50 ± 0.05c | 1.60 ± 0.10c |
| (C) S100A4 | 1 ± 0.06 | 9.62 ± 2.1 | 742 ± 19d | 136 ± 18d |
| (D) P-JNK | 1 ± 0.01 | 0.19 ± 0.02e | 40.5 ± 0.2d | 1.32 ± 0.08 |
| (E) Total JNK | 1 ± 0.04 | 0.23 ± 0.01e | 1.15 ± 0.07 | 1.04 ± 0.10 |
| (F) MMP | 1 ± 0.04 | 0.40 ± 0.07f | 13.1 ± 0.3 | 0.498 ± 0.001 |
| MMP | 1 ± 0.04 | nd | 2.17 ± 0.08g | nd |
Abbreviation: nd, not determined.
Ten μg protein larval extracts were treated with the antibody shown in Western blots of Supplementary Fig. S2.
Mean relative abundance after scanning the blots by densitometry (Materials and Methods) and the area under the peak corresponding to each protein was first normalized to that of actin and then ratioed to the level of that protein in the RasVal12 male larvae, which was arbitrarily set at 1. Mean relative abundance ± SE from three separate experiments.
Student t test P < 0.001 over RasVal12 male larvae.
Student t test P < 0.0001 over RasVal12 male larvae or S100A4wt male larvae.
Student t test P < 0.0001 over RasVal12 male larvae.
Student t test P = 0.02 over RasVal12 male larvae.
Student t test P < 0.0001, for female over male larvae.
Increased levels of activated JNK and MMP1 in Ras and S100A4-overexpressing larvae
Levels of JNK in RasVal12 and RasVal12/S100A4wt larvae were not significantly different in Western blot analysis (Student t test, P = 0.50; Table 1). However, levels of activated phospho-JNK and MMP1 at the reported molecular weights of 46 and 52 kDa, respectively (30), rose significantly by 13.1 ± 0.6- and 3.8 ± 0.1-fold, respectively, when normalized to GFP, in RasVal12/S100A4wt compared to RasVal12larvae (P < 0.0001; Supplementary Fig. S2 and Table 1). There was no significant increase in phospho-JNK, JNK, and MMP1 in RasVal12/S100A4Δ2 compared to RasVal12- larvae. In S100A4wt larvae alone, the levels of phospho-JNK, JNK, and MMP1 were significantly lower (P < 0.0001, P < 0.0001, P = 0.02; Supplementary Fig. S2 and Table 1), probably reflecting the absence of any primary tumor (Figs. 1A and B and 2A). There was also a modicum of red fluorescence for MMP1 in the eye lobes of RasVal12larvae (Fig. 1A and B), which rose significantly in RasVal12/S100A4wt larvae (P < 0.0001; Fig. 2B) showing extensive staining of the VNC (Fig. 1A and B). There was no significant difference in CIFI for RasVal12 and RasVal12/S100A4Δ2 larvae (Fig. 2B).
Activated JNK and MMP are downstream effectors in Ras and S100A4-overexpressing larvae
To determine the requirement for JNK signaling in the metastatic phenotypes, we expressed dominant-negative JNK encoded by basket (BskDN), together with RasVal12 and S100A4. When female and male siblings with and without BskDN, respectively, (Materials and Methods) were examined, 15/15 male, but only 2/15 female larvae produced extensive metastases to the VNC (Fisher exact test, P < 0.0001; Supplementary Fig. S3). ASSM and CIFI were reduced by a significant 17- and 2.8-fold in female larvae, respectively (Student t test, P < 0.0001; Fig. 2C and D). The expression of a genetically-engineered marker of JNK activity, puc-LacZ was followed by its induction of β-galactosidase (Materials and Methods; Supplementary Fig. S3). The CIFI for red fluorescent antibody to β-galactosidase fell significantly by 4.7-fold in females (P = 0.004; Supplementary Fig. S3 and Fig. 2D). In Western blots analysis, the level of MMP1 protein normalized to that in male RasVal12larvae fell 6.0-fold from 13.1 ± 0.3 to 2.17 ± 0.08 in male versus female larvae (P < 0.0001; Table 1).
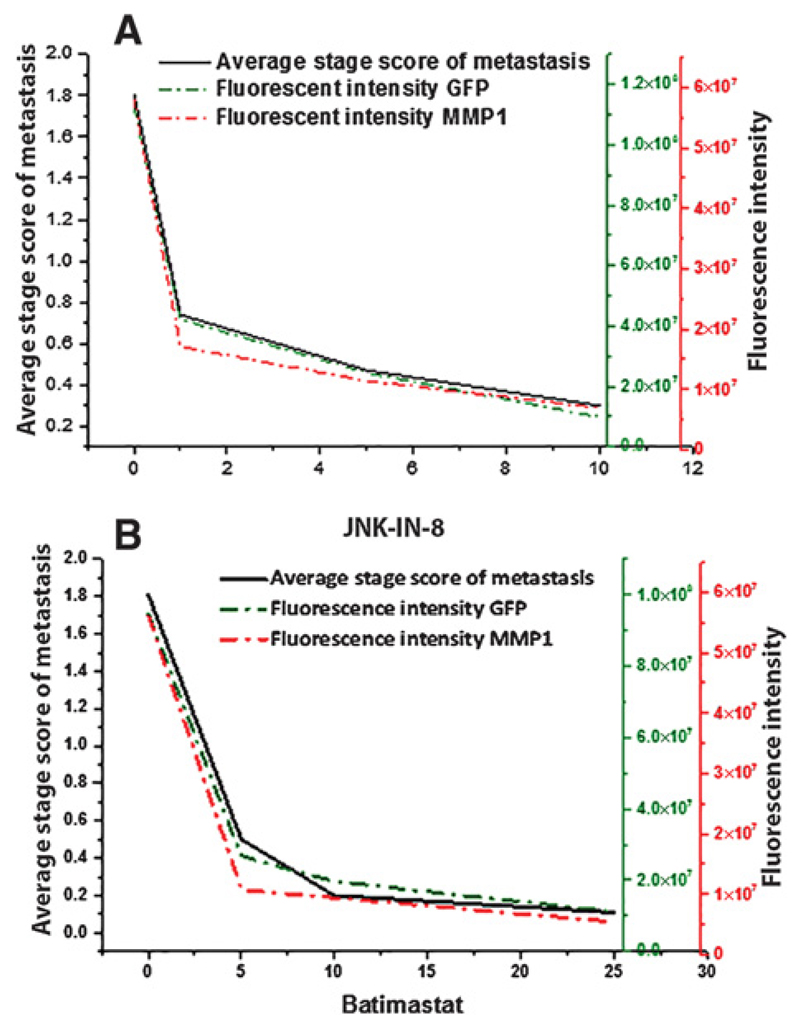
When increasing concentrations of the JNK-IN-8 inhibitor (24) were added to male RasVal12/S100A4wt larvae, there was a significant fall in ASSM of 2.5-fold for 1 μmol/L (Student t test, P < 0.0001), but thereafter a more gradual stepwise decline; the overall fall being 5.8-fold (P < 0.0001; Supplementary Fig. S4A and S4B; Fig. 3A). There was a similar significant decline in CIFI for GFP of 2.7-fold (P = 0.002) for 1 μmol/L inhibitor and then successive significant decreases; the overall fall being 11.4-fold (P < 0.0001; Fig. 3A). There was also a similar significant decline in CIFI for antibodies to endogenous MMP1 upon addition of 1 μmol/L JNK-IN-8 (Supplementary Fig. S4A and S4B; P = 0.0005), then further successive significant decreases; the overall fall being 8.3-fold (P < 0.0001; Fig. 3A).
Figure 3.
Tumor dissemination in recombinant flies treated with either JNK-IN-8 (A) or batimastat (B). Drosophila larvae with genetic background of RasVal12/S100A4wt were fed either 0, 1, 5, or 10 μmol/L of the JNK inhibitor JNK-IN-8 (A) or 0, 5, 10, or 25 μmol/L of the MMP1 inhibitor batimastat (B) in their medium (Materials and Methods). At least 20 larvae were scored and ASSM was computed as described in Materials and Methods. These same larvae were dissected, stained, and scored for endogenous green fluorescence from GFP and for red fluorescence from exogenously–added labeled antibody to MMP1. The CIFI was computed as described in Materials and Methods. Results are shown as mean ± SE. For ASSM, transgenic larvae fed 0 μmol/L of inhibitor were significantly higher than for larvae fed 1, 5, and 10 μmol/L JNK-IN-8 or for larvae fed 5, 10, and 25 μmol/L batimastat (Student t test, P ≤ 0.0001). For JNK inhibitor-treated larvae, decrease in CIFI for those fed 1, 5, and 10 μmol/L JNK-IN-8 of 2.7, 4.6, and 11.4 folds, respectively for GFP fluorescence (Student t test, P ≤ 0.002) and of 3.4, 5.1, and 8.3 folds, respectively, for MMP1-related fluorescence (Student t test, P ≤ 0.0005). For MMP1 inhibitor-treated larvae, decrease in CIFI for those fed on 5, 10, and 25 μmol/L batimastat of 3.5, 4.8, and 8.4 folds, respectively, for GFP fluorescence (Student t test, P ≤ 0.0002), and of 5.2, 5.9, and 10.3 folds, respectively, for MMP1-related fluorescence (Student t test, P = 0.02, 0.07, and 0.06, respectively).
When increasing concentrations of the inhibitor of MMP activity, Batimastat (25) was added to RasVal12/S100A4wt larvae, there were significant falls in ASSM of 3.4-fold for 5 μmol/L (P < 0.0001), but thereafter the decline was more gradual; the overall fall being 16.4-fold (P < 0.0001; Supplementary Fig. S4C and S4D; Fig. 3B). There was a similar significant decline in CIFI for GFP of 3.5-fold (P = 0.0002) for 5 μmol/L inhibitor and then successive nonsignificant decreases. The overall fall was 8.4-fold (P < 0.0001; Fig. 3B). There was also a rapid significant decline in CIFI for antibodies to endogenous MMP1 upon addition of 5 μmol/L batimastat (Supplementary Fig. S4C and S4D) of 5.2-fold (P = 0.028), then nonsignificant successive falls; the overall fall being 10.3-fold (P < 0.0001; Fig. 3B). Thus, a definite pathway has been established between S100A4 and MMP1 for induction of metastasis in this model system.
Association of MMPs with patient survival time in human breast cancer
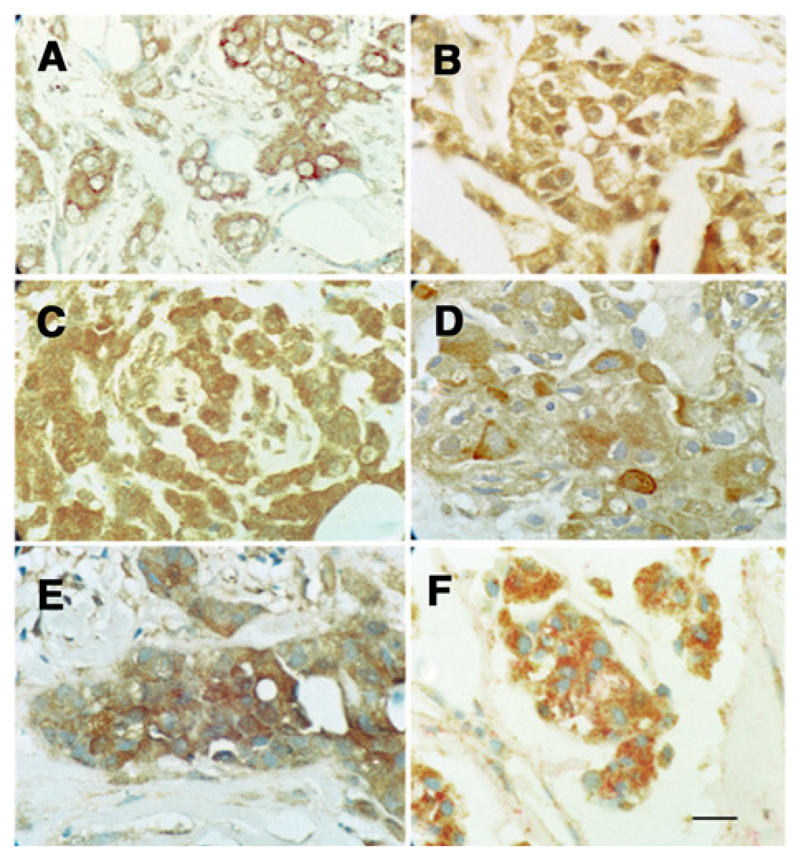
Next, we investigated the relationship in human breast cancer between the more commonly-occurring, mammalian MMPs, MMP2, 9, 13, and patient demise as a result of metastatic cancer (31). On examination of 183 breast carcinomas for IHC for these three MMPs, 32% to 67% contained carcinoma cells, which were negatively stained (<1% carcinoma cells stained), 19% to 26% were borderline stained (1–5% carcinoma cells stained), and the rest (15–47%) were stained to varying degrees (Fig. 4 and Supplementary Fig. S5; Supplementary Table S1). There were also some reactive stromal cells, mainly myofibroblasts, macrophages, and neutrophils that stained (Fig. 4). Assessment of staining class was made only for the malignant cells. Staining for individual MMPs was abolished by prior incubation of each antibody with the requisite MMP (Supplementary Fig. S5).
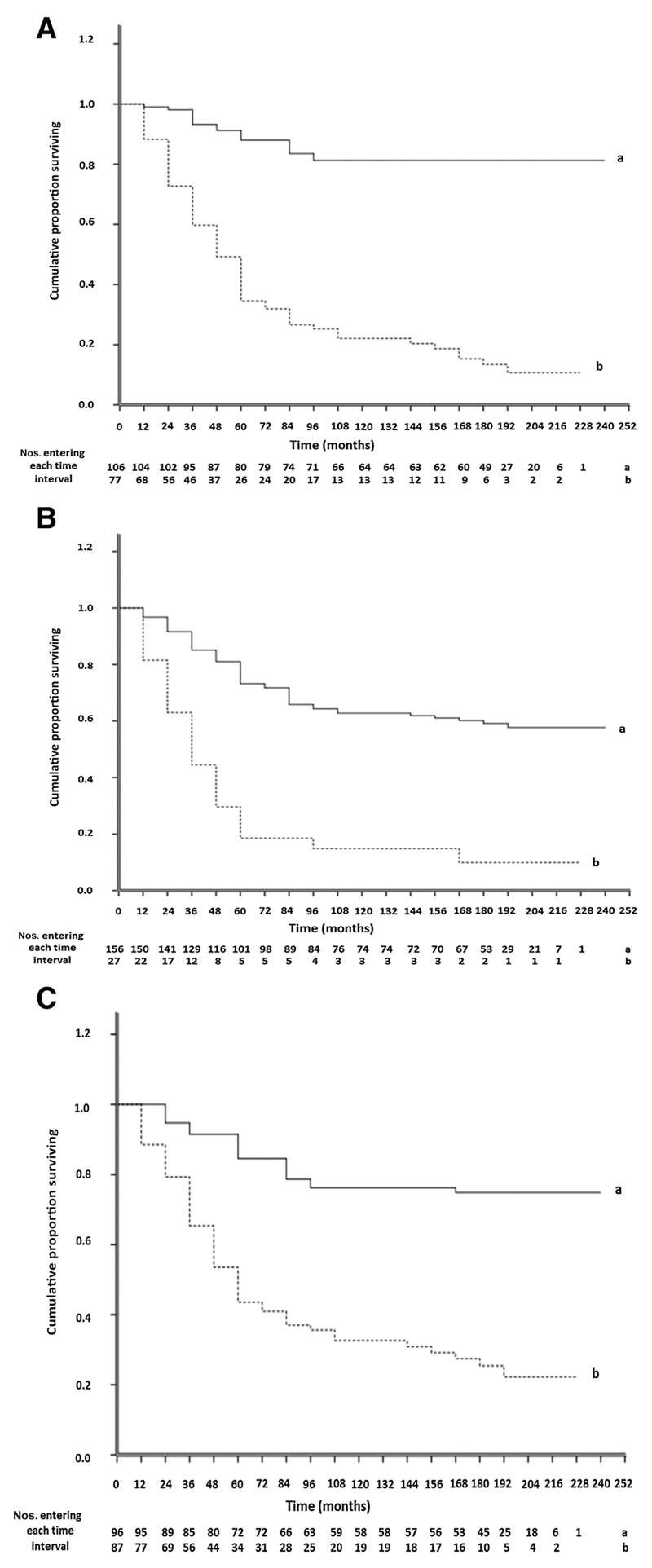
Figure 4.
Immunohistochemical staining of different breast carcinomas with antibody to MMP2 (A), MMP9 (B), or MMP13 (C) showing strong brown staining of the carcinoma cells' cytoplasm. Incubation with antibody to S100A4 (D) or S100P (E) showing strong, bead-like, cytoplasmic staining. F, Incubation with antibody to MMP2 with brown chromophore and to S100A4 with red chromophore showing most carcinoma cells were stained by both antibodies. Tumors were selected to show strong staining in A–C for their respective MMP, but the same tumor was stained in D–F as in A (×180; scale bar, 20 μm).
To determine whether there was any association between staining for the separate MMPs and of survival of patients, Kaplan–Meier survival curves were plotted for different staining groups. Overall, there was a significant difference in staining for each MMP (Wilcoxon Gehan Statistics, P < 0.001). However, the largest significant differences occurred between the (±) and (+) staining groups for MMP2, 9, and 13, respectively (Supplementary Table S2). The 183 patients were therefore separated into two categorical groups using a cutoff of 5% stained carcinoma cells for each MMP. Only 11 ± 4% survived with positively stained tumors, compared to 81 ± 4% with negatively stained tumors for MMP2; 10 ± 6% vs. 58 ± 4% for MMP9; and 22 ± 5% versus 75 ± 5% for MMP13 (Fig. 5). All differences were highly significant (P < 0.001) with median duration of survival of 47, 32, and 52 months for MMP2, 9, and 13 positively stained tumors versus 228 months in all cases of negatively stained tumors. These corresponded to relative risks (RR) of death of 9.04 (95% CI, 5.32–15.36), 4.69 (95% CI, 2.89–7.62), and 4.87 (95% CI, 2.98–7.97), respectively. Results for S100A4 with a cutoff of 5% were similar to that for individual MMPs, with only 9 ± 4% surviving versus 80 ± 4% for unstained tumors, median survival time of 46 months versus 228 months (χ2 = 71.8, P < 0.001), and RR of patient death of 9.96 (95% CI, 5.87–16.9; Supplementary Table S3). Patients with tumors stained positively for all three MMPs showed no significant increase in mortality (7% ± 6%), decrease in median survival time (30 months), or increase in RR (4.96; 95% CI, 2.99–8.24) than staining for either MMP2 or MMP9 separately (Supplementary Table S2). When all three MMPs were included in Cox's multivariate regression analysis (Materials and Methods), the individual contributions made to the time of patient demise showed that staining for MMP2 (P < 0.001) and that for MMP9 (P = 0.025) were independently significant while that for MMP13 was not (Supplementary Table S3).
Figure 5.
Association of immunohistochemical staining for MMPs with overall time of patient survival. Cumulative proportion of surviving patients as a fraction of the total for each year after presentation for patients with carcinomas classified as negatively-stained (set a, solid line) or positively-stained (set b, dotted line) is shown for MMP2 (A), MMP9 (B), and MMP13 (C). Numbers of patients entering each year are shown below. The two curves are highly significantly different in each case (Wilcoxon statistic χ2 = 71.81, 32.50, or 41.90 for A, B, or C, respectively, 1 df, P < 0.001). Further details are shown in Supplementary Materials.
Association of MMPs with S100A4 and patient survival
Results for IHC staining for the 3 MMPs using a 5% cutoff were cross-tabulated against pathologic variables and IHC staining for S100A4, S100P (29), estrogen receptor α (ERα), progesterone receptor (PgR), c-erbB-2 (Her2), cytokeratin 5/6 (CK5/6), and CK14 (32). All these variables have been reported to influence survival times in the same set of patients (26). Positive staining for each of MMP2, 9, and 13 was associated strongly with positive staining for S100A4 when using a 5% cutoff for S100A4. This association was slightly reduced with staining for S100P using a 5% cutoff (Table 2). Significance of association was much more marked for staining for S100A4 than for S100P when using a 1% cutoff. There was also a significant association with staining for CK5/6 and usually for CK14 (Table 2). Positive staining for any MMP was not significantly associated with involved lymph nodes, high tumor grade, large tumor size, nor with positive staining for ERα, PgR, or c-erbB-2 (Table 2). There was also a highly significant association of staining for each pair of MMPs (Table 2 and Supplementary Table S4).
Table 2.
Association of IHC staining for MMPs with other tumor variables
| Statistical significancec |
||||
|---|---|---|---|---|
| Tumor variablea | Patientb no. | MMP2 | MMP9 | MMP13 |
| Lymph nodes | 139 | 0.271 | 0.564 | 0.681 |
| Grade | 164 | 0.997 | 0.656 | 0.156 |
| Tumor size | 177 | 0.467 | 0.937 | 0.985 |
| MMP2 | 183 | – | 9.0 × 10−7 | 1.7 × 10−12 |
| MMP9 | 183 | 9.0 × 10−7 | – | 1.2 × 10−7 |
| MMP13 | 183 | 1.7 × 10−12 | 1.2 × 10−7 | – |
| S100A4 (5%) | 183 | 6.6 × 10−9 | 2.9 × 10−4 | 2.4 × 10−6 |
| S100A4 (1%) | 183 | 0d | 1.2 × 10−7 | 1.9 × 10−8 |
| S100P (5%) | 163 | 2.3 × 10−7 | 2.2 × 10−3 | 1.3 × 10−7 |
| S100P (1%) | 163 | 1.6 × 10−4 | 0.012 | 2.4 × 10−5 |
| CK14 | 172 | 5.7 × 10−7 | 2.8 × 10−3 | 0.372 |
| CK5/6 | 173 | 1.6 × 10−6 | 0.035 | 5.6 × 10−3 |
| ERα | 181 | 1.00 | 1.0 | 1.0 |
| PgR | 172 | 0.995 | 0.983 | 0.549 |
| C-erbB-2 | 183 | 0.660 | 1.00 | 0.809 |
Lymph nodes with or without tumor deposits; grade, histologic grades I and II vs. grade III; tumor size <5 cm vs. >5 cm in diameter; presence or absence of IHC staining for molecular variables using 5% cutoff for MMP2, MMP9, MMP13, S100A4 (5%), S100P (5%), ERα, PgR, c-erbB-2, and using a 1% cutoff for S100A4 (1%), S100P (1%), CK14, and CK5/6.
Number of patients from original 183.
Probability P from Fisher exact test using the Holm–Bonferroni correction calculated as 1 − (1 − P)n, where n = 12 (Materials and Methods).
Uncorrected P = 7.7 × 10−18.
When staining for S100A4 was tested for its relative probability of association (RA) with that for the three MMPs using binary logistic regression, that with MMP2 was strongest at 4.21 (P < 0.001), that with MMP9 of 2.41 was not significant, and that with MMP13 of 2.17 (P = 0.051) was very nearly significant. When staining for each of the MMPs, in turn, was assessed with staining for S100A4, CK14, ERα, PgR, and c-erb-2, only that for S100A4 and partially that for CK14 proved to be significant (Supplementary Table S5). When repeated using a different cutoff for S100A4 (1% instead of 5%; Table 2) and additionally including that for S100P, staining for MMP2 was most closely associated with that for S100A4 (Supplementary Table S5). To determine whether the three MMPs were independent of S100A4 when related to patient survival, they were included in a series of Cox's multivariate regression analyses (Materials and Methods; Supplementary Table S3). When a single MMP and S100A4 were only included, staining for S100A4 always emerged as the most significant association with patient survival time. Similar results were obtained if staining for S100A4 and all three MMPs were included in the same analysis, S100A4 emerged as the most significant association followed by MMP2 and then MMP9, whereas that due to MMP13 was not significant (Supplementary Table S3).
To determine whether there was coexpression of the MMPs and S100 proteins, two breast carcinomas were chosen that were either moderately or strongly stained for MMP2, and these were IHC restained for S100A4/P, 3 MMPs, CK5/6, and CK14. Exactly the same areas were examined for each antigen. The percentage of stained cells for S100A4 was not significantly different from that for MMP2 and MMP13, while staining for S100P was not significantly different from that for MMP9 (Supplementary Fig. S6; and Supplementary Table S6). Staining for S100A4 or MMP2 was also not significantly different from that for CK5/6, but only in the MMP2 moderately-stained carcinomas; all the other paired combinations were significantly different (Supplementary Fig. S6; and Supplementary Table S6). When serial sections from three breast carcinomas strongly-staining for MMP2 were doubly IHC-stained for S100A4 with red and for MMP2 with brown chromophores on the same section, there were (mean ± SE) 80.2 ± 2.2% doubly stained cells, 6.9% ± 0.9% cells stained red for S100A4, 2.9% ± 0.4% cells stained brown for MMP2 and 9.1% ± 1.5% unstained cells (ANOVA, F = 669.3, 3 df, P < 0.001; Supplementary Fig. S7). Thus, S100A4 is associated with and partially confounded for patient survival by the three MMPs to varying degrees.
Discussion
We have shown for the first time that S100A4 can induce metastasis in the Drosophila model and that the oncogene RasVal12 largely fails in this respect. The increase in number of larvae bearing VNC metastases (10-fold), in ASSM (24-fold), and in CIFI (4.1-fold) for RasVal12/S100A4 over RasVal12 larvae demonstrates clearly that S100A4 promotes extensive dissemination to the VNC, as well as elsewhere in the larvae (Supplementary Fig. S1). The reason for the differences in fold increases is due to the method of measurement, the CIFI included GFP fluorescence due to the primary as well as the metastases, whereas the first two parameters relate only to the metastases. That larvae containing RasVal12 and inactive S100A4Δ2 genes (20) show significantly less metastases (Fig. 1A, 2A, and B), demonstrates that the migratory/invasive ability of S100A4 (20) is required for its metastatic ability. That S100A4 larvae produce no tumors at all (Fig. 1A, 2A, and B) demonstrates that S100A4 alone is non-oncogenic, consistent with previous results in our S100A4 transgenic mice (33). The increases in Ras and GFP proteins of 3.5to 4-fold (Table 1) are consistent with the increase in GFP fluorescence of about 4-fold (Fig. 2B) and probably represent the increase in overall tumor mass between the RasVal12 and the RasVal12/S100A4 larvae.
In agreement with different genetically manipulated Ras oncogenic systems in Drosophila (16, 18), the levels of endogenous activated phospho-JNK and MMP1 rise significantly in RasVal12/S100A4 compared with RasVal12 larvae (Table 1). The rise in MMP1 protein is of the same order as the increase in fluorescently-labeled antibodies to MMP1. That JNK is indeed a downstream effector of RasVal12/S100A4 for metastasis is demonstrated by the reduction in the number with metastases and their ASSM in female BskDN-expressing larvae compared to the male unsuppressed larvae (Fig. 2C). That these suppressed values for RasVal12/S100A4 are not significantly different from those of the RasVal12 larvae (Fig. 2C) suggests that the predominant driver of the JNK–link to metastasis is the overexpression of S100A4. The 4.7-fold fall in the immunofluorescently-detectable β-galactosidase in the female, suppressed RasVal12/S100A4 larvae demonstrates that JNK needs to be activated to stimulate metastasis. Because the level of JNK protein is relatively constant between RasVal12 and RasVal12/S100A4 larvae (Table 1), S100A4 probably triggers activation of JNK by stimulating its increase in phosphorylation (24). Results using 10 μmol/L JNK-IN-8 confirm that JNK-induced phosphorylation of c-Jun is a necessary step in the S100A4–triggered pathway for metastasis. That there is a fall in CIFI for immunofluorescently detectable MMP1 (Fig. 3B) positions JNK before MMP in any pathway (16). Moreover, the fact that the MMP1 inhibitor, batimastat (25) inhibits ASSM and CIFI for GFP in the RasVal12/S100A4 larvae places MMP1 on the direct pathway to metastasis. The order of this novel S100A4-induced metastatic pathway is: S100A4→phospho-JNK→c-Jun→MMP1→metastasis. Thus, S100A4 appears to replicate the loss of function of suppressor genes scrib and lethal2 (17) or Her2 activation in the JNK/MMP pathway (16, 18). In transgenic mice or chemically transformed rat mammary cells, S100A4 combines with oncogenic Neu (Her2; ref. 6) or Ras (4), respectively to stimulate, via the cytoskeleton, cell migration, and then subsequent events for invasion/metastasis (34). However, the involvement of this novel pathway has hitherto been unreported.
The relevance of our unique Drosophila model for S100A4 has been pursued in human breast cancer. IHC staining of our cohort of 183 breast carcinomas for the individual MMPs2, 9, 13 demonstrates 15% to 47% primary tumors are stained positively using a cut-off of 5%, in approximate agreement with previous reports (35, 36). Here we show that the overall duration of survival of patients with positively-stained carcinomas is highly significantly worse than for those patients classified as not staining for one of MMP2, 9, or 13 (Fig. 5), in agreement with results for MMP2 in hepatocarcinoma (37), skin melanoma (38) and for MMP13 in breast (36) and colon cancer (39). In contrast, MMP9 has been reported to be a favorable indicator in lymph-node-negative breast cancer (40). This favorable prognosis may depend on the much higher cutoff employed, because our node-negative group showed no significant difference (Wilcoxon χ2 = 2.63, 1 df, P = 0.11). This difference was significantly greater for MMP9 staining in our node-positive patients (χ2 = 18.40, 1 df, P < 0.001). The other two MMPs showed similar significant differences in node-negative and node-positive patients (MMP2 χ2 = 25.46 and 25.39; MMP13 χ2 = 14.91 and 12.93, respectively). These results may suggest that MMP9 operates later than the other two MMPs at a post lymph-node-spreading stage in the disease process.
Overall, the RR of patient death in separate univariate analyses is greatest for patients with tumors stained for S100A4 (9.96), followed closely by those stained for MMP2 (9.04), then for MMP13 (4.87), and finally for MMP9 (4.69; Supplementary Table S3). However, the antibodies used here to detect the MMPs do not discriminate between inactive precursors or cleaved active MMPs and do not detect inhibitory TIMPs (41). Usually in cultured cells, S100A4 increases expression of MMP precursors and this results in an enhanced proteolytic activity and cell invasion/metastasis (42, 43). Moreover, S100A4 can act both intracellularly (43, 44) and extracellularly via RAGE receptors (45, 46) to stimulate MMP production. The fact that BskDN inhibits S100A4-induced MMP1 and metastasis to the VNC in our Drosophila model (Fig. 2C and D) suggests that MMP1 is produced by the tumor cells and not by reactive stromal cells (47), consistent with immunohistochemical results in our human breast cancers. In contrast to the Drosophila model, the three JNK proteins in human cancers can exert both pro- and anti-oncogenic effects depending on the cell type and cross-talk with other kinases (48, 49). Thus, the oncogenic effect of activated JNK cannot be determined in human cancers from the measurement of its level alone, and hence was not attempted here.
Upon manipulation in cultured cells, S100A4 has been reported to control production of a single MMP, one of MMP2, 9, or 13, depending on the source and sometimes the report (42–45). In contrast, we show here that positive staining for each MMP2, 9, or 13 is separately and in combination very strongly associated with S100A4 and to a lesser extent with S100P (Table 2). The significant association of staining for MMP2, 9 with the basal cell markers CK5/6, CK14 has been reported previously (50), predominantly placing these MMPs, together with S100A4 and S100P, in the most aggressive subtype of breast cancers (26). When tested for RA of staining for S100A4 with the other three MMPs together, S100A4 is more likely to occur with MMP2, and the higher significant RA of MMP2 for S100A4 over a combination of other proteins confirms this result (Supplementary Table S6). Thus, S100A4 is more associated with MMP2, 13, and S100P more with MMP9, at least at the cellular level (Supplementary Table S6). This differential association in the tumor raises the novel possibility of synergistic interactions between the S100 proteins (29) occurring via different target MMPs.
Multiple longitudinal comparisons with survival time for all three MMPs together in multivariate analysis shows that only MMP2 and MMP9 are independently significant, whereas the contribution of MMP13 is confounded by that due to the other two MMPs (Supplementary Table S3). These results suggest partial overlap occurs between MMP2/MMP9-related pathways and MMP13-related pathways, whereas those related to MMP2 and MMP9 are more separate. This result is consistent with their function, MMP13 is a collagenase, which is required to cut collagen fibrils first, before the two gelatinases, MMP2 or MMP9, can digest the remainder (51). When S100A4 and each MMP are tested in combination, the order of reduction in RR for S100A4 is MMP2 (42% reduction), then MMP13 (27% reduction) and finally MMP9 (11% reduction), whereas the reduction in RR for each MMP separately with S100A4 is similar (44%, 40%, and 43%, respectively; Supplementary Table S3). These results suggest the pathways that S100A4 may trigger leading to premature death from metastatic disease overlap, to some extent, with those triggered by the three MMPs, the most overlap being with MMP2-related and then with MMP13-related pathways. The results for the close association of S100A4 and MMP2 are confirmed at the level of the cell, where 91% of S100A4-containing cells also contain MMP2 and 96% of MMP2-containing cells also contain S100A4 (Supplementary Fig. S7). The considerable enhancing effect of S100P on S100A4-linked patient demise (29) may then be attributable, at least in part, to S100P targeting different MMPs from those targeted by S100A4 (Supplementary Table S6). This differential targeting of MMPs by S100 proteins is a novel mechanism for generation of the known synergy between different metastasis-inducing proteins in the development of many cancers.
Supplementary Material
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Acknowledgments
Grant Support
This work was supported by Cancer and Polio Research Fund (T.M. Ismail, A.M. Platt-Higgins, M. Al-Medhity, R. Barraclough, P.S. Rudland) and Medical Research Council G0801447 (A.M. Platt-Higgins, R. Barraclough, P.S. Rudland), and MR/K015931/1 (D. Bennett).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: T.M. Ismail, D. Bennett, R. Barraclough, P.S. Rudland
Development of methodology: T.M. Ismail, D. Bennett, A.M. Platt-Higgins, P.S. Rudland
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): T.M. Ismail, D. Bennett, P.S. Rudland
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): T.M. Ismail, D. Bennett, A.M. Platt-Higgins, M. Al-Medhity, P.S. Rudland
Writing, review, and/or revision of the manuscript: T.M. Ismail, D. Bennett, A.M. Platt-Higgins, M. Al-Medhity, R. Barraclough, P.S. Rudland
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): T.M. Ismail, A.M. Platt-Higgins, P.S. Rudland
Study supervision: T.M. Ismail, P.S. Rudland
References
- 1.Dunnington DJ, Hughes C, Monaghan P, Rudland PS. Phenotypic instability of rat mammary tumor epithelial cells. J Natl Cancer Inst. 1983;71:1227–40. [PubMed] [Google Scholar]
- 2.Dunnington DJ, Kim U, Hughes CM, Monaghan P, Ormerod EJ, Rudland PS. Loss of myoepithelial cell characteristics in metastasizing rat mammary tumors relative to their nonmetastasizing counterparts. J Natl Cancer Inst. 1984;72:455–66. [PubMed] [Google Scholar]
- 3.Steeg PS. Tumour metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 4.Davies BR, Davies MP, Gibbs FE, Barraclough R, Rudland PS. Induction of the metastatic phenotype by transfection of a benign rat mammary epithelial cell line with the gene for p9ka, a rat calcium-binding protein, but not with the oncogene EJ-ras-1. Oncogene. 1993;82:99–1008. [PubMed] [Google Scholar]
- 5.Barraclough R, Savin J, Dube SK, Rudland PS. Molecular cloning and sequence of the gene for p9Ka. A cultured myoepithelial cell protein with strong homology to S-100, a calcium-binding protein. J Mol Biol. 1987;198:13–20. doi: 10.1016/0022-2836(87)90453-0. [DOI] [PubMed] [Google Scholar]
- 6.Davies MP, Rudland PS, Robertson L, Parry EW, Jolicoeur P, Barraclough R, et al. Expression of the calcium-binding protein S100A4 (p9ka) in MMTV-neu transgenic mice induces metastasis of mammary tumours. Oncogene. 1996;13:1631–7. [PubMed] [Google Scholar]
- 7.Rudland PS, Platt-Higgins A, Renshaw C, West CR, Winstanley JH, Robertson L, et al. Prognostic significance of the metastasis-inducing protein S100A4 (p9ka) in human breast cancer. Cancer Res. 2000;60:1595–603. [PubMed] [Google Scholar]
- 8.Mazzucchelli L. Protein S100A4: too long overlooked by pathologists? Am J Pathol. 2002;160:7–13. doi: 10.1016/S0002-9440(10)64342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkinson SR, Barraclough R, West CR, Rudland PS. S100A4 regulates cell motility and invasion in an in vitro model for breast cancer metastasis. Br J Cancer. 2004;90:253–62. doi: 10.1038/sj.bjc.6601483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross SR, Sin CG, Barraclough R, Rudland PS. Joining S100 proteins and migration: for better or for worse, in sickness and in health. Cell Mol Life Sci. 2014;71:1551–79. doi: 10.1007/s00018-013-1400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazigou E, Apitz H, Johansson J, Loren CE, Hirst EM, Chen P-L, et al. Anterograde Jelly belly and Alk receptor tyrosine kinase signalling mediate retinal axon targeting in Drosophila. Cell. 2007;128:961–75. doi: 10.1016/j.cell.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring WJ. Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development. 1998;125:2181–91. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- 13.Hauck B, Gehring W, Walldorf U. Functional analysis of an eye specific enhancer of the eyeless gene in Drosophila. Proc Natl Acad Sci U S A. 1999;96:564–9. doi: 10.1073/pnas.96.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belvin MP, Anderson KV. A conserved signalling pathway: the Drosophila toll-dorsal pathway. Ann Rev Cell Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 15.Bennett D, Lyulcheva E, Cobbe N. Drosophila as a potential model for ocular tumours. Ocul Oncol Pathol. 2015;1:190–9. doi: 10.1159/000370155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uhlirova M, Bohmann D. JNK- and FOS-regulated Mmp1 expression cooperates with Ras to induce invasive tumours in Drosophila. EMBO J. 2006;25:5294–304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brumby AM, Richardson HE. Scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–79. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy JB. GAL4 system in Drosophila. A fly geneticist's Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 19.Evans PC, Smith TS, Lai MJ, Williams MG, Burke DF, et al. A novel type of deubiquitinating enzyme. J Biol Chem. 2003;278:23180–6. doi: 10.1074/jbc.M301863200. [DOI] [PubMed] [Google Scholar]
- 20.Ismail TM, Fernig DG, Rudland PS, Terry CJ, Wang G, et al. The basic C-terminal amino acids of calcium-binding protein S100A4 promote metastasis. Carcinogenesis. 2008;29:2259–66. doi: 10.1093/carcin/bgn217. [DOI] [PubMed] [Google Scholar]
- 21.Ciurciu A, Duncalf L, Jonchere V, Lansdale N, Vasieva O, Glenday P, et al. PNuTs/PP1 regulates RNAPII-mediated gene expression and is necessary for developmental growth. PLOS Genetics. 2013;9:e1003885. doi: 10.1371/journal.pgen.1003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baranski TJ, Cagan RL, inventors; Washington University , assignee. Transgenic Drosophila and methods of use thereof. WO2009055461A1. 2009 Apr 30;
- 23.McCloy RA, Rogers S, Caldon CE, Lorca T, Castro A, Burgess A, et al. Partial inhibition of cdk1 in G2 phase overrides the SAC and decouples mitotic events. Cell Cycle. 2014;13:1400–12. doi: 10.4161/cc.28401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang T, Inesta-Vaquera F, Niepel M, Zhang J, Ficarro SB, Machleidt T, et al. Discovery of selective covalent inhibitions of JNK. Chem Biol. 2012;19:140–54. doi: 10.1016/j.chembiol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sledge GW, Jr, Qulai M, Goulet R, Bone EA, Fife R. Effect of matrix metalloproteinase inhibitor batimastat on breast cancer regrowth and metastasis in athymic mice. J Natl Cancer Inst. 1995;87:1546–50. doi: 10.1093/jnci/87.20.1546. [DOI] [PubMed] [Google Scholar]
- 26.de Silva Rudland S, Platt-Higgins A, Winstanley JHR, Jones NJ, Barraclough R, West CR, et al. Statistical association of basal cell keratins with metastasis-inducing proteins in a prognostically unfavorable group of sporadic breast cancers. Am J Pathol. 2011;79:1061–72. doi: 10.1016/j.ajpath.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudland PS, Platt-Higgins A, Davies LM, de Silva Rudland S, Wilson JB, Aladwani A, et al. Significance of the Fanconi anemia FANCD2 protein in sporadic and metastatic human breast cancer. Am J Pathol. 2010;176:2935–47. doi: 10.2353/ajpath.2010.090779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orgaz JL, Pandya P, Dalmeida R, Karagiannis P, Sanchez-Loorden B. Diverse matrix metalloproteinase functions regulate cancer amoeboid migration. Nat Commun. 2015;5:4255–70. doi: 10.1038/ncomms5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G, Platt-Higgins A, Carrol J, de Silva Rudland S, Winstanley J, Barraclough R, et al. Induction of metastasis by S100P in a rat mammary model and its association with poor survival of breast cancer patients. Cancer Res. 2006;66:1199–207. doi: 10.1158/0008-5472.CAN-05-2605. [DOI] [PubMed] [Google Scholar]
- 30.Glasheen BM, Kabra AT, Page-McCaw A. Distinct functions for the catalytic and hemopexin domains of Drosophila matrix metalloproteinase. Proc Natl Acad Sci U S A. 2009;106:2659–64. doi: 10.1073/pnas.0804171106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q) SARs. Bioorg Med Chem. 2007;15:2223–68. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies M, Harris S, Rudland PS, Barraclough R. Expression of the rat, S-100-related, calcium-binding protein gene, p9Ka, in transgenic mice demonstrates different patterns of expression between these two species. DNA Cell Biol. 1995;14:825–32. doi: 10.1089/dna.1995.14.825. [DOI] [PubMed] [Google Scholar]
- 34.Du M, Wang G, Ismail TM, Gross S, Fernig DG, Barraclough R, et al. S100P dissociates myosin IIA filaments and focal adhesion sites to reduce cell adhesion and enhance cell migration. J Biol Chem. 2012;287:15330–44. doi: 10.1074/jbc.M112.349787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao X, Sun B, Hu L, Lahdesmaki H, Dunmire V, Feng Y, et al. Differential gene and protein expression in primary breast malignancies and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. Cancer. 2004;100:1110–22. doi: 10.1002/cncr.20095. [DOI] [PubMed] [Google Scholar]
- 36.Zhang B, Cao X, Liu Y, Cao W, Zhang F, Zhang S, et al. Tumour-derived matrix metalloproteinase-13 (MMP13) correlations with prognosis. BMC Cancer. 2008;8:183–84. doi: 10.1186/1471-2407-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sze KM, Wong KL, Chu GK, Lee JM, Yau TO, Ng OP-L, et al. Loss of phosphatase and tensin homolog enhances cell invasion and migration through AKT/Sp-1 transcription factor/matrix metalloproteinase 2 activation in hepatocellular carcinoma and has clincopathologic significance. Hepatology. 2011;53:1558–69. doi: 10.1002/hep.24232. [DOI] [PubMed] [Google Scholar]
- 38.Rotte A, Martinka M, Li G. MMP2 expression is a prognostic marker for primary melanoma patients. Cell Oncol. 2012;35:207–16. doi: 10.1007/s13402-012-0080-x. [DOI] [PubMed] [Google Scholar]
- 39.Yang B, Gao J, Rao Z, Shen Q. Clinicopathological significance and prognostic value of MMP-13 expression in colorectal cancer. Scand J Clin Lab Invest. 2012;72:501–5. doi: 10.3109/00365513.2012.699638. [DOI] [PubMed] [Google Scholar]
- 40.Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulous P, Trangas T, et al. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Br J Cancer. 2001;84:1488–96. doi: 10.1054/bjoc.2001.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deryugina EI, Quingley JP. Matrix metalloproteinases and tumour metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt-Hansen B, Klingelhofer J, Grum-Schwensen B, Christensen A, Andresen S, Kruse C, et al. Functional significance of metastasis-inducing S100A4 (Mts1) in tumor-stroma interplay. J Biol Chem. 2004;279:24498–504. doi: 10.1074/jbc.M400441200. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Zhang DL, Jiao XL, Dong Q. S100A4 regulates migration and invasion in hepatocellular carcinoma HepG2 cells via NF- κB-dependant MMP-9 signal. Eur Rev Med Pharmacol Sci. 2013;17:2372–82. [PubMed] [Google Scholar]
- 44.Jia W, Gao XJ, Zhang ZD, Yang ZX, Zhang G. S100A4 silencing suppresses proliferation, angiogenesis and invasion of thyroid cancer cells through downregulation of MMP-9 and VEGF. Eur Rev Med Pharmacol Sci. 2013;17:1495–508. [PubMed] [Google Scholar]
- 45.Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–11. doi: 10.1002/art.22042. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt-Hansen B, Ornås D, Grigorian M, Klingelhöfer J, Tulchinsky E, Lukanidin E, et al. Extracellular S100A4(mts1) stimulates invasive growth of mouse endothelial cells and modulates MMP-13 matrix metalloproteinase activity. Oncogene. 2004;23:5487–95. doi: 10.1038/sj.onc.1207720. [DOI] [PubMed] [Google Scholar]
- 47.Heppner KJ, Matrisian LM, Jensen RA, Rodgers WH. Expression of most metalloproteinase family members in breast cancer represents a tumor-induced host response. Am J Pathol. 1996;149:273–82. [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 49.Cellurale C, Gimuis N, Jiang F, Cavanagh-Kyros J, Lu S, Garlick DS, et al. Role of JNK in mammary gland development and breast cancer. Cancer Res. 2012;72:472–81. doi: 10.1158/0008-5472.CAN-11-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radenkovic S, Konjevic G, Jurisic V, Karadzic K, Nikitovic M, Gopcevic K, et al. Values of MMP-2 and MMP-9 in tumour tissue of basal-like breast cancer patients. Cell Biochem Biophys. 2014;68:143–52. doi: 10.1007/s12013-013-9701-x. [DOI] [PubMed] [Google Scholar]
- 51.Sela-Passwell N, Rosenblum G, Shoham T, Sagi I. Structural and functional bases for allosteric control of MMP activities: can it pave the path for selective inhibition? Biochim Biophys Acta. 2010;1803:29–38. doi: 10.1016/j.bbamcr.2009.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.