Abstract
Two new species of Thyronectria growing in Mediterranean vegetation are described from southern Spain; they are T. giennensis from Quercus ilex ssp. rotundifolia and T. pistaciae from Pistacia lentiscus. Both species are characterized by morphology of sexual and asexual morphs and by DNA data. They have olivaceous to green-brown muriform ascospores and are closely related to T. asturiensis and T. roseovirens, as determined by multigene phylogenetic analyses of a matrix containing six loci (ITS and 28S regions of nuc rDNA, ACT1, RPB1, RPB2, TEF1 and TUB2 genes). We also report that Cucurbitaria bicolor is a synonym of Thyronectria rhodochlora, the type species of Thyronectria.
Keywords: Ascomycota, Cucurbitaria, Hypocreales, Nectriaceae, phylogenetic analysis, Sordariomycetes, taxonomy
Introduction
The genus Thyronectria Sacc. was established by Saccardo (1875) for species with nectriaceous ascomata immersed in bark fissures with muriform ascospores similar to Thyridium Nitschke. The genus later was monographed by Seeler (1940), who characterized it by light-colored perithecia that are immersed in or are superficial on erumpent stromata, often covered with yellowish or greenish scales or powder, with evanescent pseudoparaphyses and hyaline, yellowish, green or brown, muriform ascospores. Because of the presence of paraphyses, Thyronectria, Thyronectroidea Seaver and Mattirolia Berl. & Bres. were included as members of the Thyridiaceae (Rossman et al. 1999, Checa et al. 2013) and most species of Thyronectria were classified by Rossman et al. (1999) in Nectria Fr. Hirooka et al. (2012) recognized that those species characterized by yellow scurf on ascomata or stromata belonged to the genera Allantonectria Earle, with non-septate, allantoid to short-cylindrical, hyaline ascospores, and Pleonectria, with one- to multiseptate variously shaped ascospores that may bud to produce hyaline, thin-walled, bacillar conidia in or outside asci, and transferred many Thyronectria spp. to Pleonectria.
Based on type studies, fresh material and molecular phylogenies, Jaklitsch and Voglmayr (2014) reinstated the genus Thyronectria (Nectriaceae, Hypocreales) and revealed that the type and other species of the genus do not contain true paraphyses but apical paraphyses, which are branched and anastomosing, and descend from an apical cushion to the bases of asci, just as in other members of the Nectriaceae but are in contrast more persistent in that they can be observed among mature asci. Based on multigene analyses they determined that Thyronectria and Pleonectria constitute the same genus and because Thyronectria is older they synonymized Mattirolia, Pleonectria and Thyronectroidea with Thyronectria, combining all respective epithets in Thyronectria. This was implemented by Lombard et al. (2015) in their overview of genera of the Nectriaceae. Jaklitsch and Voglmayr (2014) also included Thyridium vestitum (Fr.) Fuckel in phylogenetic analyses to illustrate the phylogenetic distance of Thyronectria from the Thyridiaceae. They recognized 32 species in Thyronectria and provided a key based on the Pleonectria tree of Hirooka et al. (2012).
During fieldwork in the Mediterranean area of Spain we encountered two species, which cannot be unequivocally identified by the above-mentioned key. Phylogenetic multigene analyses positioned these taxa close to T. roseovirens (Berl. & Bres.) Seeler but confirmed their status as distinct species. The main aim of this study is to describe these two new species in the context of the newly circumscribed genus Thyronectria.
Materials and Methods
Morphological observations
Microscopic preparations were mounted in water, 3 or 5% potassium hydroxide (KOH), lactophenol or lactic acid (LA) and observed through an oil immersion objective. The micrographs were made with a Nikon (Eclipse 80i) microscope with a Nikon (DS-5M) digital camera and OPTIKA B-350 microscope with Canon EOS 40D digital camera. The specimens are deposited in the herbarium of the University of Alcalá (AH).
Culture observations
A culture of Thyronectria giennensis was obtained by plating conidia from the natural substrate on CMD (cornmeal agar (Sigma, St Louis, Missouri) supplemented with 2% dextrose) and maintained as described by Jaklitsch et al. (2005) and also on 2% malt-extract agar (MEA; 2% malt extract, 2% agar-agar, both from Merck, Germany). Cultures of both Thyronectria giennensis and T. pistaciae were obtained from ascospores by excising an ascoma and by placing it into a drop of sterile water. The ascoma was opened in a second drop of sterile water; the extruded centrum was removed with a micropipette and spread with a sterile glass rod onto the surface of Petri dishes with PDA (potato-dextrose agar) or MEA (Lab. Conda-Pronadisa, Spain). The plates were sealed with laboratory film and incubated at room temperature. The cultures were deposited at CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands (CBS).
DNA extraction, PCR and sequencing
The extraction of genomic DNA, PCR and sequencing of segments of six loci, that is the nuc rDNA region encompassing internal-transcribed spacers 1 and 2, along with the 5.8S, and the D1-D2 domains of the 28S (ITS-28S), α-actin (ACT1) gene, RNA polymerase II subunit 1 (RPB1) and subunit 2 (RPB2) genes, translation elongation factor 1-α (TEF1) gene and β-tubulin (TUB2) gene, was performed as reported in Jaklitsch and Voglmayr (2014).
Phylogenetic analyses
GenBank accession numbers of sequences used in the phylogenetic analyses are provided in Jaklitsch and Voglmayr (2014). The GenBank accession numbers of the newly obtained sequences are given with the specimen data in the taxonomy section below.
All alignments were produced with the server version of MAFFT (www.ebi.ac.uk/Tools/mafft), checked and refined using BioEdit 7.0.4.1 (Hall 1999). To reveal the phylogenetic position of the two new Thyronectria species, the newly generated sequences were aligned with the six-gene matrix of Jaklitsch and Voglmayr (2014). Nectria asiatica Hirooka, Rossman & P. Chaverri, N. cinnabarina (Tode) Fr., N. dematiosa (Schwein.) Berk. and N. nigrescens Cooke were selected as outgroup taxa. The resulting combined sequence matrix contained 6208 alignment positions from six gene regions (630 from ACT1, 512 from ITS and 807 from 28S, 698 from RPB1, 1192 from RPB2, 1288 from TEF1 and 1081 from TUB2). Before phylogenetic analyses the approach of Wiens (1998) was applied to test for significant levels of localized incongruence among the six gene partitions, using the level of bootstrap support (Sung et al. 2007). For this the 70% maximum-parsimony (MP) bootstrap consensus trees calculated for each individual partition were compared, using the same parameters as for the combined analysis given below. No topological conflicts were observed between these bootstrap trees for each gene, indicating the absence of significant incongruence and combinability of the six loci (Wiens 1998).
Maximum parsimony (MP) analyses were performed with PAUP 4.0 b10 (Swofford 2002), using 1000 replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (Multrees option in effect, steepest descent option not in effect). All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data; the collapse command was set to minbrlen. Bootstrap analysis with 500 replicates was performed in the same way but using five rounds of random sequence addition and subsequent TBR branch swapping during each bootstrap replicate; in addition, each replicate was limited to 10 million rearrangements.
For ML analyses 500 fast bootstrap replicates were computed with RAxML (Stamatakis 2006) as implemented in raxmlGUI 1.3 (Silvestro and Michalak 2012) using the gtrcati substitution model. Substitution model parameters were calculated separately for the different gene regions included in the combined analyses. The multiple sequence alignment file and phylogenetic tree have been deposited in TreeBASE and are available at http://purl.org/phylo/treebase/phylows/study/TB2:S17892
Results
Molecular phylogeny
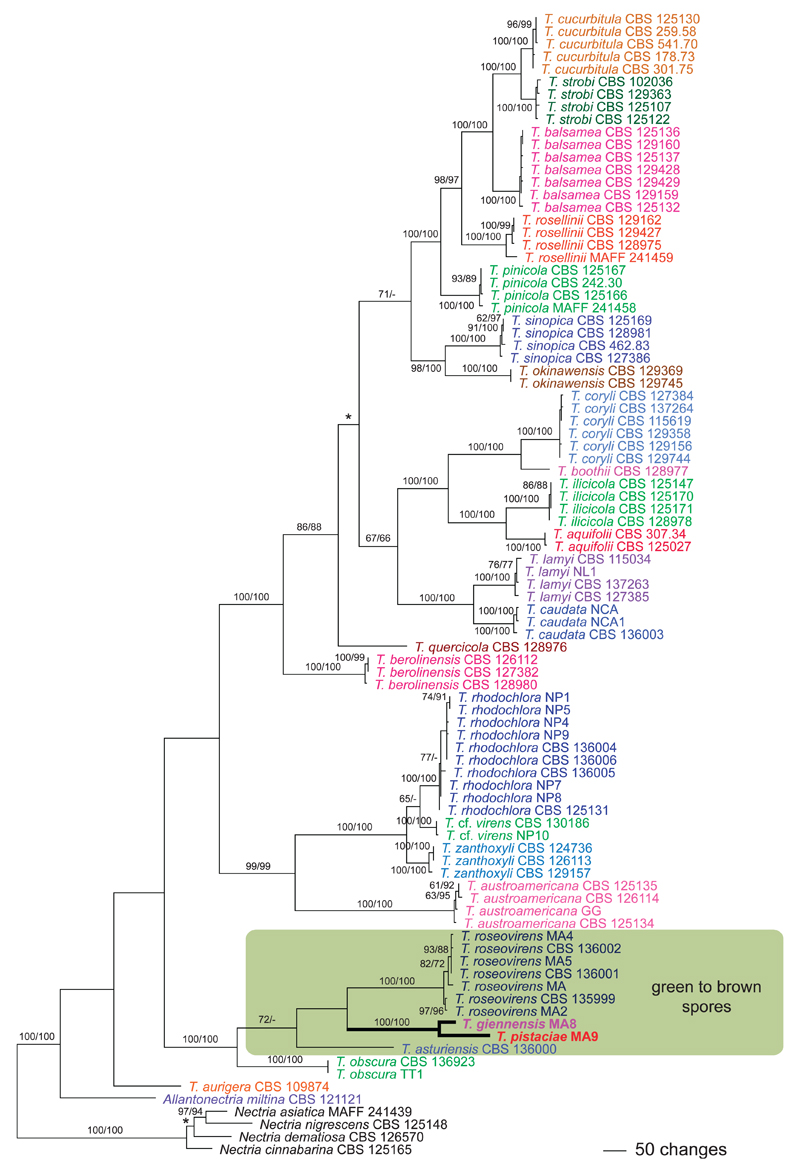
Apart from minor topological differences in the positions of T. aurigera (Berk. & Ravenel) Jaklitsch & Voglmayr, T. obscura Jaklitsch & Voglmayr and N. cinnabarina, the tree topology (Fig. 1) agrees with that (Fig. 2) in Jaklitsch and Voglmayr (2014). Thyronectria giennensis and T. pistaciae are encompassed in a strongly supported clade (100% MP and ML bootstrap support) together with T. asturiensis Jaklitsch & Voglmayr, T. obscura and T. roseovirens (Fig. 1). This clade is near basal to the rest of Thyronectria and contains all the species with green to brown spores in Thyronectria and the hyaline-spored T. obscura. Monophyly of the green- to brown-spored clade receives moderate MP bootstrap support (72%) but is unsupported in ML analyses. Within this clade T. asturiensis has a basal position, and T. giennensis and T. pistaciae form a strongly supported clade (100% MP and ML bootstrap support), which is sister to T. roseovirens but without bootstrap support. Sequences of T. giennensis and T. pistaciae show that they represent phylogenetically distinct species, in agreement with their distinct hosts, ascospores shapes and sizes.
Fig. 1.
Phylogram of one of 864 MP trees 7121 steps long revealed by PAUP from an analysis of the combined six-gene (ACT1, ITS-28S, RPB1, RPB2, TEF1, TUB2) matrix of Allantonectria and Thyronectria, with four species of Nectria sensu stricto selected as outgroup. MP and ML bootstrap support above 60% are given above or below branches. Strain/culture designations follow taxon names; the new clade containing the new species formatted in boldface. The asterisk denotes the nodes collapsed in the strict consensus tree of all MP trees.
Fig. 2.
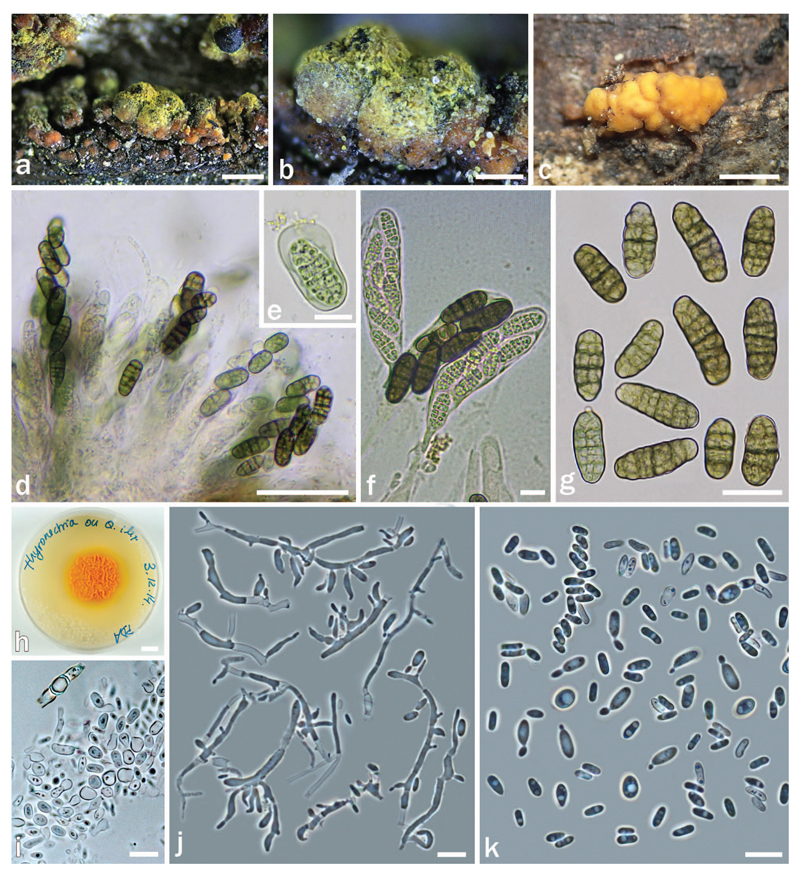
Thyronectria giennensis on Quercus ilex AH 47011 (HOLOTYPE). a–b. Stromata/ascomata. c. Conidiomata on natural substrate. d, f. Asci with biseriate ascospores. e. Immature ascospore. g. Mature ascospores. h. Colony on PDA. i. Detail of the mycelium forming chlamydospores. j. Conidiophores and phialides from PDA. k. Conidia. Bars: a = 500 μm; b, c = 200 μm; d = 50 μm; e, f, i–k = 10 μm; g = 20 μm; h = 1 cm.
A comprehensive key to all currently accepted species of Thyronectria, adapted from Voglmayr and Jaklitsch (2014), is provided (Supplementary file 1).
Taxonomy
Thyronectria giennensis Checa, M.N. Blanco, Jaklitsch, Voglmayr & G. Moreno, sp. nov. Fig. 2 MycoBank MB813051
Typification: SPAIN, JAÉN, Puerto de las Coberteras, Valdepeñas de Jaén, on dead branches of Quercus ilex ssp. rotundifolia, 27 May 2014, S. Tello (HOLOTYPE AH 47011). Ex-holotype culture MA8 = CBS 139474. Ex-holotype sequences: ACT1: KR057941; ITS-28S: KR057943; RPB1: KR057945; RPB2: KR057947; TEF1: KR057949; TUB2: KR057951.
Etymology: giennensis; from Gaiena (ancient Iberian Roman province, now called Jaén, Andalusia, Spain), the geographic area where this species was collected.
Stromata erumpent from bark fissures or formed on decorticated areas, up to 1.5 × 1 mm, KOH−. Stromatic tissue up to 100 μm thick above perithecia, composed of cells 4–6 μm diam forming textura globulosa to t. angularis, subhyaline to orange, covered by bright yellow scurf releasing a yellow pigment in KOH. Perithecia immersed in stromatic tissue, solitary or aggregated in groups of up to 10, subglobose to globose, 450–500 µm high × 300–400 µm diam, with black shining apical region, not collapsing when dry, KOH−, often formed on and surrounded by conidial masses. Perithecial wall 35–50 µm thick, of two layers: outer region 30–40 µm, cells 4–8 µm diam, forming textura globulosa to t. angularis, yellow; inner region 5–10 µm thick, of elongate, thin-walled, hyaline cells forming textura prismatica. Asci clavate, 85–130 × 20–25 µm (n = 25), with eight biseriately arranged ascospores and an inconspicuous inamyloid apical ring. Apical paraphyses abundant, 2–3 µm wide, septate and branched. Ascospores ellipsoidal or oblong, muriform, with 5–7 transverse septa, primary septum more pronounced, and 1–2 longitudinal septa, 22–28 (–30) × 9–10 µm (n = 60), smooth, at first hyaline, turning greenish and eventually green-brown, surrounded by an irregular sheath, mainly visible when immature.
Asexual morph on substrate: Conidiomata superficial on bark or erumpent, globose, 150–400 µm, orange outside, darkening with age, becoming almost black, isolated or forming aggregates up to 1.2 mm long, peridium pseudoparenchymatous, of pale yellowish, isodiametric to elongated cells 5–7 × 5–5.5 µm, interior cream or pale yellow; sometimes forming sexual morph. Phialides, mostly solitary, lateral or terminal, flask-shaped, 6–12 × 1.5–2 µm (n = 20). Conidia ellipsoidal to cylindrical, straight or slightly curved, unicellular, hyaline, smooth, 4–6 × 1.0–1.5 µm (n = 35).
Culture and asexual morph: On PDA colony 20–35 mm diam after 10 d at 25 C; surface whitish, turning orange due to conidial masses, centrally becoming roughened with a merulioid aspect, somewhat waxy, surrounded by a yellow and smooth marginal zone, 4 mm wide. Reverse orange, slightly paler than surface. On MEA colony up to 40 mm diam after 24 d at room temperature; surface orange to pinkish, merulioid in center, appearing more or less waxy; hyphae hyaline, septate, 2–4 µm diam. Reverse cream to orange. Phialides formed on ill-defined micronematous conidiophores, mostly solitary, lateral, 6–15 × 2–3 µm (n = 35), flask-shaped to cylindrical, mixed with small pegs. Conidia ellipsoidal to allantoid, unicellular, hyaline, smooth, 4–7(–11) × 1.5–2(–3.0) µm (n = 50), budding. After ca. 8 d hyphal cells becoming inflated and disarticulating to form chlamydospores.
Distribution: Southern Spain.
Habitat: Dead corticated branches of Quercus ilex ssp. rotundifolia (Fagaceae); possibly fungicolous, soc. Diplodia sp.
Notes: As in Thyronectria roseovirens, stromata of T. giennensis develop directly on conidiomata. This species grows on Quercus ilex ssp. rotundifolia in Spain, like the closely related T. asturiensis (actually, on dark subicular hyphae of a fungus) and the distantly related T. quercicola (Hirooka, Checa, Arenal & P. Chaverri) Jaklitsch & Voglmayr. Thyronectria quercicola is morphologically highly distinct with its hyaline, filiform ascospores with 8–15 transverse septa that bud to produce hyaline, thin-walled, slightly curved, bacillary ascoconidia filling the asci (Hirooka et al. 2012, Jaklitsch and Voglmayr 2014). Thyronectria giennensis differs from the similar green- to brown-spored T. asturiensis, T. roseovirens and T. pistaciae primarily by larger ascospores (22–30 × 9–10 µm vs. 14–22 × 6.3–9.3 µm in T. asturiensis, 13–25.5 × 7.5–13 µm in T. roseovirens and 17–22 × 10–13 µm in T. pistaciae). In addition, from T. asturiensis also by finely multiguttulate ascospore cells, which do not become dark brown at maturity and from T. roseovirens and T. pistaciae by different hosts. Another feature characteristic for T. giennensis is the lack of an asexual morph on PDA, the striking merulioid aspect of the colony and the disintegration of hyphae into chlamydospores.
Thyronectria pistaciae Checa, M.N. Blanco, Jaklitsch, Voglmayr & G. Moreno, sp. nov. Fig. 3 MycoBank MB 813052
Fig. 3.
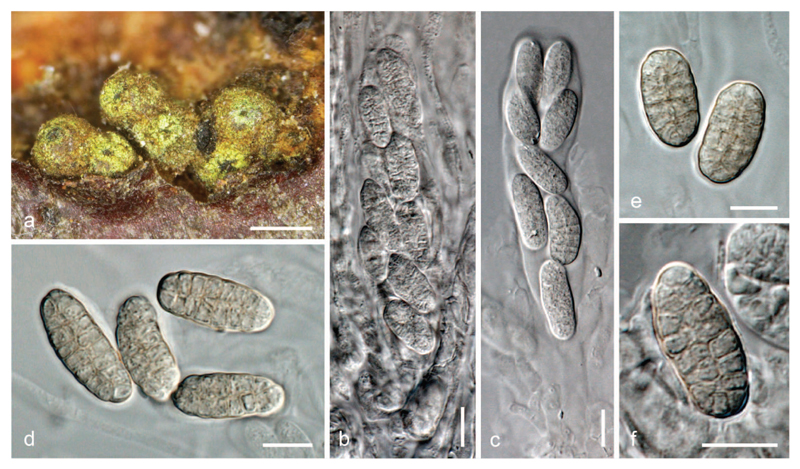
Thyronectria pistaciae on Pistacia lentiscus AH 45402 (HOLOTYPE). a–b. Stromata/ascomata. c. Stromata/ascomata and conidiomata on natural substrate (AH 47601). d–e. Asci with uniseriate ascospores. f. Ascospores. g. Colony on MEA. h. Detail of the elongated tufts formed on PDA. i. Strands, conidiophores and phialides on MEA. j. Conidia. Bars: a, b = 500 μm; c = 200 μm, d–e = 50 μm; f, i, j = 10 μm; g = 1 cm; h = 1 mm.
Typification: SPAIN, JAÉN, Sierra de la Grana, Fuensanta de Martos, on dead branches of Pistacia lentiscus, 30 Mar 2014, S. Tello (HOLOTYPE AH 45402). Ex-holotype culture MA9 = CBS 139475. Ex-holotype sequences: ACT1: KR057942; ITS-28S: KR057944; RPB1: KR057946; RPB2: KR057948; TEF1: KR057950; TUB2: KR057952).
Etymology: pistaciae; referring to the host, Pistacia.
Stromata up to 2 mm diam, immersed or erumpent from bark, often only visible through bark fissures. Stromatic tissue pseudoparenchymatous, yellowish, KOH−, LA+ yellow, consisting of 4–6 μm wide, thick-walled cells forming textura globulosa to t. angularis, at top above the perithecia up to 100 μm thick and covered by abundant greenish yellow to bright yellow amorphous scurf, surrounding perithecia that are scattered or aggregated in groups of up to 15, and extending under them as a hypostroma. Perithecia subglobose to globose, 250–450 μm high, 150–450 μm diam, with black, shining apical region, not collapsing when dry, KOH−, LA+ yellowish, smooth. Perithecial wall 30–40 μm thick, of two layers: outer region 25–35 μm thick, distinct from stroma, cells 4–8 μm wide, forming textura globulosa or t. angularis, yellow to reddish, inner region 5–6 μm thick, of elongate, thin-walled, hyaline cells, forming textura prismatica. Asci cylindrical, 90–120 × 13–16 μm (n = 25), containing eight uniseriately arranged ascospores and an inconspicuous non-amyloid apical ring. Apical paraphyses abundant, 2–3 μm wide, septate. Ascospores ellipsoidal to subglobose, 17–22 × 10–13 μm (n = 104), muri-form, with 3–5(–7) irregular transverse septa, with primary septum being more distinct than others; and 1–3 longitudinal septa, smooth, first hyaline, turning greenish to olivaceous, becoming more intensely green in KOH, surrounded by an irregular sheath, mainly visible when immature.
Asexual morph on natural substrate: Conidiomata observed only on one occasion (AH 47601), erumpent from bark, visible only through bark fissures, associated with ascomata, irregularly globose, 125–180 μm diam, orange, isolated or forming aggregates up to 1 mm long, peridium pseudoparenchymatous, orange, mainly with isodiametric cells, 5–7 μm diam, interior cream or pale yellow. Phialides aggregated, flask-shaped, (6–)7.5–9(–10) × 2.0–2.5 μm (n = 25). Conidia ellipsoidal to cylindrical, straight or slightly curved, unicellular, hyaline, smooth, (3–)3.5–4.5(–6) × 1.0–1.5 μm (n = 40).
Culture and asexual morph: On PDA colony 25–35 mm diam after 10 d at 25 C; surface yellowish to reddish, becoming covered by abundant whitish cottony aerial mycelium; sometimes surface appearing waxy. Reverse yellowish to orange or reddish. Odor reminiscent of honey. Colony forming abundant emerging elongated tufts tapering towards tip, up to 1.5 mm long and 0.3 mm wide, forming of aerial mycelium agglutinated in intertwining strands; horizontal strands attached to agar, up to 30 μm wide, in both cases bearing scant solitary lateral phialides and pegs. Hyphae 2–3 μm diam. Phialides flask-shaped to cylindrical, slightly tapering toward tip, 5–20 × 2–3 μm (n = 35). Conidia mainly formed on pegs, scant, ellipsoidal, hyaline, smooth, budding 5–10 × 1.5–3 μm. Conidia from phialides scant, 3–4 × 1–1.5 μm, budding (n = 40). On MEA colony without elongated tufts. Conidia mainly formed on phialides, similar to those from PDA.
Distribution: Southern Spain.
Habitat: Dead corticated branches of Pistacia lentiscus L. (Anacardiaceae); possibly fungicolous, soc. Diplodia sp.
Additional specimens examined: SPAIN, JAÉN, Sierra de la Grana, Fuensanta de Martos, on dead branches of Pistacia lentiscus, 5 Apr 2014, S. Tello (AH 45403); ibidem, 10 Jun 2014, S. Tello (AH 45404). Valdepeñas de Jaén, on dead branches of Pistacia lentiscus, 10 Apr 2015, S. Tello (AH 47601).
Notes: This species appears to be specific to Pistacia lentiscus, where we collected it on four occasions in nearby areas. On natural substrate the asexual morph was observed only in a single collection (AH 47601), where it was associated with ascomata. For comparison with other similar species see notes of T. giennensis. The ascospores are characteristic in their ellipsoidal to subglobose shape, reminiscent of a mulberry. In cultures, especially on PDA, erect elongated tufts are formed by hyphal strands, on which the conidiogenous cells are mainly located. These strands also appear parallel to the agar surface. Phylogenetically T. pistaciae is the closest relative of T. giennensis.
Additions to Thyronectria rhodochlora (Mont.) Seeler, Fig. 4
Fig. 4.
Thyronectria rhodochlora (LECTOTYPE of Cucurbitaria bicolor). a. Stromata/ascomata. b, c. Asci (b. immature). d–f. Ascospores. Bars: a = 500 μm, b–f = 10 μm.
In a revision of Fuckel’s Cucurbitaria type specimens we detected a synonym of Thyronectria rhodochlora. Fuckel (1871: 309) described Cucurbitaria bicolor Fuckel as having sulfur yellow perithecia with variable pale brownish muriform ascospores with 7–9 transverse septa. The material was issued by Fuckel as Fungi Rhenani 2451 and is thus accessioned in several herbaria. The material from G shows two coelomycetous fungi, one forming pycnidia containing minute unicellular rod-like hyaline conidia (3–5 × 1–1.5 μm; “spermogoniis” ss. Fuckel 1871) and a Diplodia with brown bicellular conidia 16–24 × 9–11.5 μm (“stylospores” ss. Fuckel 1871), the latter being the host of T. rhodochlora (Jaklitsch and Voglmayr 2014). Several groups of perithecia, 400–550 μm diam (including stroma), are surrounded by stromatic tissue overlain by yellow scurf; the peridium is red, turning orange in 3% KOH; ascomata contain numerous apical paraphyses and oblong to clavate asci (90–)94–112(–113) × (15.5–)16–20(–21) μm (n = 6) with eight ascospores in a biseriate arrangement. The ascospores are variable in shape, oblong, ellipsoidal to subglobose, have 5–9 mostly seven transverse septa and two longitudinal septa, measure (15.5–) 18.0–24.0(–28) × (7–)8–10.5(–11.0) μm, l/w (1.8–)2–2.7(–3) (n = 30). They are hyaline when immature and subhyaline to pale yellowish pinkish when mature. Many asci are immature. The material was incubated between autumn and spring in Fuckel’s observation garden. The morphological data and growth on Diplodia fit our concept of T. rhodochlora (Jaklitsch and Voglmayr 2014), leaving no doubt that Cucurbitaria bicolor is synonymous. This is also the first record of pycnidia for T. rhodochlora from the natural substrate; their conidia match those recorded for pycnidia from agar cultures (Jaklitsch and Voglmayr 2014).
Typification: GERMANY, Oestrich-Winkel, Schloß-park Reichartshausen, on Prunus padus, in May (without year), (G00127418 = LECTOTYPE of Cucurbitaria bicolor designated here; ISOLECTOTYPE = Nr. 31 [3965] in W). Typification reference: MBT 201799.
Discussion
The genus Thyronectria is mainly characterized by a yellowish scurf covering either the ascomata or stroma surface, seen at least in young ascomata, in combination with persistent hamathecial threads (Jaklitsch and Voglmayr 2014); ascospores are highly variable in shape and septation, from subglobose, ellipsoidal, fusiform to filiform, being one-septate, multiseptate or muriform, often budding to produce hyaline ascoconidia (Hirooka et al. 2012). Ascospore is mostly hyaline, yellowish to rosy, but a few species have green or even dark brown ascospores. Until recently the latter have been classified as the separate genera Mattirolia and Thyronectroidea in Thyridiaceae (Rossman et al. 1999), but molecular phylogenetic analyses and morphological investigations revealed that they belong to Thyronectria in the Nectriaceae (Jaklitsch and Voglmayr 2014).
Three Thyronectria species with green to brown ascospores were known: T. asturiensis and T. roseovirens from southern Europe and T. chryogramma from North America. We here describe two additional species, T. giennensis and T. pistaciae from Andalusia (southern Spain). Phylogenetically these species are closely related to the other green- to brown-spored Thyronectria species and form a moderately supported clade in MP analyses (Fig. 1). In Jaklitsch and Voglmayr (2014), although contained within the same highly supported clade, the two green- to brown-spored species did not form a monophylum, but these differences may be the result of increased taxon sampling of the current study.
Remarkably the biodiversity center of green- to brown-spored Thyronectria species appears to be the Iberian Peninsula in southwestern Europe, harboring four species, three of which are known only from there (T. asturiensis, T. giennensis, T. pistaciae). The fourth species, T. roseovirens, occurs on a wide range of fabaceous shrubs in Spain where it is not uncommon, whereas it appears to be rare and confined to Laburnum (Italy) and Ulex (England) elsewhere (Jaklitsch and Voglmayr 2014), indicating that it also might have originated from the Iberian Peninsula. Much of the Iberian Peninsula is characterized by dry Mediterranean climates, and thick-walled green to brown spores may be an adaptation to high insolation and drought.
The ascomata of Thyronectria species with green to brown spores are conspicuous by their bright yellow scurf, and it is surprising that they have only recently been collected and recorded from Spain (Checa et al. 2013). This demonstrates that species biodiversity of this area is still insufficiently studied, containing a substantial number of undescribed species. Due to its biohistorical, geographical, geological and orographic conditions, the Iberian Peninsula is a biodiversity hotspot of animals and plants, for example, with more than 8000 vascular plant species (Pineda and Montalvo 1995), and the evidence indicates this is true for fungi as well. It therefore can be assumed that additional undescribed Thyronectria species with green to brown spores will turn up in the course of more detailed studies in future.
The present report of Cucurbitaria bicolor as an additional synonym of Thyronectria rhodochlora shows the urgent need of critical taxonomic revisions based on type specimens. This is especially evident in old, large genera like Cucurbitaria, which contain numerous species that have never been critically reevaluated. It also demonstrates that species lists and numbers given for genera have to be treated with caution because they might be highly inaccurate without critical detailed evaluation. In the context of the one fungus-one name concept it should be noted also that many anamorph-teleomorph relationships are still based on co-occurrence of several forms in crowded on the same piece of substrate, as for example, interpreted by mycologists such as L. Fuckel in the 19th century.
Supplementary Material
Acknowledgments
The financial support by the Austrian Science Fund (FWF; project P25870-B16) to WJ is gratefully acknowledged. We thank Luis Monje and Ángel Pueblas of the Department of Drawing and Scientific Photography at the Alcalá University for their help in the digital preparation of the photographs. And we thank Javier Rejos, curator of the AH herbarium, for his assistance with the specimens examined in the present study.
Contributor Information
Julia Checa, Dpto. de Ciencias de la Vida, Facultad de Biología, Universidad de Alcalá, Alcalá de Henares, 28805 Madrid, Spain.
Walter M. Jaklitsch, Division of Systematic and Evolutionary Botany, Department of Botany and Biodiversity Research, University of Vienna, Rennweg 14, A-1030 Vienna, Austria; Institute of Forest Entomology, Forest Pathology and Forest Protection, Deptartment of Forest and Soil Sciences, BOKU-University of Natural Resources and Life Sciences, Peter Jordan-Straße 82, 1190 Vienna, Austria
Salvador Tello, Paseo del Obispo 7, 23150 Valdepeñas de Jaén, Jaén, Spain.
Hermann Voglmayr, Division of Systematic and Evolutionary Botany, Department of Botany and Biodiversity Research, University of Vienna, Rennweg 14, A-1030 Vienna, Austria.
Literature Cited
- Checa J, Blanco MN, Moreno G. Contributions to the family Thyridiaceae. New data on Sphaeria mutabilis. Mycotaxon. 2013;125:149–164. doi: 10.5248/125.149. [DOI] [Google Scholar]
- Fuckel KWGL. Symbolae mycologicae Beiträge zur Kenntniss der rheinischen Pilze. Erster Nachtrag. Jahrb Nassau Ver Naturk. 1871;25–26:287–346. (1871–1872) [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis, program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hirooka Y, Rossman AY, Samuels GJ, Lechat C, Chaverri P. A monograph of Allantonectria, Nectria and Pleonectria (Nectriaceae, Hypocreales, Ascomycota) and their pycnidial, sporodochial, and synnematous anamorphs. Stud Mycol. 2012;71:1–210. doi: 10.3114/sim0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia. 2005;97:1365–1378. doi: 10.3852/mycologia.97.6.1365. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. Persistent hamathecial threads in the Nectriaceae, Hypocreales: Thyronectria revisited and reinstated. Persoonia. 2014;33:182–211. doi: 10.3767/003158514X685211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, van der Merwe NA, Groenewald JZ, Crous PW. Generic concepts in Nectriaceae. Stud Mycol. 2015;80:189–245. doi: 10.1016/j.simyco.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda FD, Montalvo J. Dehesa systems in the western Mediterranean. In: Halladay P, Gilmour DA, editors. Conserving biodiversity outside protected areas—the role of traditional agro-ecosystems. Gland: IUCN; 1995. pp. 107–122. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, Lowen R. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes) Stud Mycol. 1999;42:1–248. doi: 10.5598/imafungus.2013.04.01.05. [DOI] [Google Scholar]
- Saccardo PA. Nova ascomycetum genera. Grevillea. 1875;4:21–22. [Google Scholar]
- Seeler EV. A monographic study of the genus Thyronectria. J Arnold Arboretum. 1940;21:429–460. [Google Scholar]
- Silvestro D, Michalak I. raxmlGUI: a graphical front-end for RAxML. Org Divers Evol. 2012;12:335–337. doi: 10.1007/s13127-011-0056-0. [DOI] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Sung GH, Sung JM, Hywel-Jones NL, Spatafora JW. A multigene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstrap approach. Mol Phylog Evol. 2007;44:1204–1223. doi: 10.1016/j.ympev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods) Sunderland, Massachusetts: Sinauer Associates; 2002. [Google Scholar]
- Wiens JJ. Combining datasets with different phylogenetic histories. Syst Biol. 1998;47:568–581. doi: 10.1080/106351598260581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.