Abstract
Quiescence is essential for long-term maintenance of adult stem cells. Niche signals regulate the transit of stem cells from dormant to activated states. Here we show that the E3-ubiquitin ligase Huwe1 (HECT, UBA and WWE domain containing 1) is required for proliferating stem cells of the adult mouse hippocampus to return to quiescence. Huwe1 destabilises pro-activation protein Ascl1 (achaete-scute family bHLH transcription factor 1) in proliferating hippocampal stem cells, which prevents accumulation of cyclin Ds and promotes the return to a resting state. When stem cells fail to return to quiescence, the proliferative stem cell pool becomes depleted. Thus, long-term maintenance of hippocampal neurogenesis depends on the return of stem cells to a transient quiescent state through the rapid degradation of a key activation factor.
Stem cells contribute to tissue homeostasis by generating new differentiated cells. Adult stem cells can enter a reversible state of quiescence that protects the cells from damage and the population from depletion. Niche signals determine the balance between quiescent and activated states. Excessive quiescence leads to too few differentiated progeny whereas excessive proliferation exhausts the stem cell population (1).
Neural stem cells (NSCs) in the dentate gyrus (DG) of the mouse hippocampus generate new granule neurons that integrate into the hippocampal circuit to modulate mood and memory (2, 3). Niche signals control expression of the transcription factor Ascl1 (achaete-scute family bHLH transcription factor 1), which in turn directs NSC proliferation (4). To identify factors that regulate Ascl1, we characterized proteins that co-immunoprecipitate with Ascl1 in cultured murine NSCs using mass spectrometry. We found that Huwe1 (HECT, UBA and WWE domain containing 1), a HECT domain E3 ubiquitin ligase associated with idiopathic intellectual disability and schizophrenia (5, 6), interacts with Ascl1 (Fig. S1). We generated embryonic telencephalon- and adult hippocampus-derived NSCs in which Huwe1 is expressed and can be inactivated by Cre recombinase (7) (Fig. S2). Inactivation of Huwe1 resulted in an accumulation of Ascl1 protein and an extension of its half-life from 38 minutes to 121 minutes (Fig. 1A to C and Fig. S2 B and G), while proteins destabilized by Huwe1 in other tissues were not affected (8–10) (Fig. S2 C). Ascl1 is degraded by the proteasome in NSCs (Fig. S2 D) and silencing of Huwe1 with shRNAs decreased the extent of poly-ubiquitinylation of Ascl1 (Fig. 1D). Therefore Huwe1 promotes the proteasomal degradation of Ascl1 protein.
Figure 1. Huwe1 controls Ascl1 stability in adult hippocampal stem cells.
(A, B) Huwe1 inactivation in embryonic telencephalon-derived cultured NSCs increases Ascl1 protein (A, western blot; Actin B (Actb) is used as loading control) but not Ascl1 mRNA levels (B, qPCR analysis of empty or CRE-expressing adenovirus-transduced cells). (C) Cells were treated with cycloheximide to stop protein synthesis for different times and processed for western blot to determine Ascl1 half-life. N = 4 independent experiments. (D) Ubiquitynilated Ascl1 (upper panel) and total Ascl1 levels (lower panel) were determined by immunoblotting with an anti-HA antibody after transfection with HA-Ascl1 and control or Huwe1 shRNA. (E to H) Huwe1 was inactivated in adult DG NSCs by 5 injections of tamoxifen at P60 followed by analysis at P90. Scale bar, 10 μm (F). The number of Ascl1-positive NSCs, identified by their position in the subgranular zone and the presence of a GFAP+ radial process (F, G), and the intensity of Ascl1 immunolabeling per cell (H), were quantified. N = 4 mice per condition (H) and n = 27 Ascl1-positive cells from 4 mice (control) and 42 Ascl1-positive cells from 4 mice (Huwe1cKO) (G). Yellow arrows in F point to Ascl1-positive cells.
Huwe1 is expressed throughout the brain, including in hippocampal NSCs and their progeny in the subgranular zone of the DG (Fig. S3). To study Huwe1 function in these cells, we generated mice in which administration of the small molecule tamoxifen inactivates the Huwe1 gene and initiates YFP expression in hippocampal NSCs (Huwe1fl;GLAST-CreERT2; Rosa-Stop-YFP mice (11), called Huwe1cKO mice hereafter). One month after tamoxifen administration, the intensity of Ascl1 immunolabel was enhanced in cells of the subgranular zone of Huwe1cKO mice compared to controls (Fig. 1E, F and G and Fig. S4). The number of GFAP+ radial NSCs expressing Ascl1 was also increased (Fig. 1H). We observed no difference in the expression of other known Huwe1 substrates (Fig. S5). Thus Huwe1 regulates Ascl1 stability in hippocampal NSCs. Since Ascl1 promotes NSC activation in the hippocampus (4), upregulation of Ascl1 in Huwe1cKO mice might stimulate NSC proliferation. Indeed, a higher proportion of NSCs in the DG of Huwe1cKO mice were cycling at P90 (Fig. 2A and B). Thus, Huwe1 suppresses hippocampal NSC proliferation in wild-type mice.
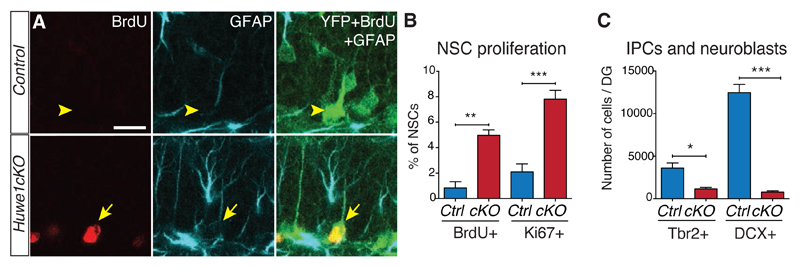
Figure 2. Huwe1 inactivation promotes hippocampal stem cell proliferation and blocks progenitor differentiation.
(A, B) Hippocampal stem cell proliferation was assessed by Ki67 staining (B) and BrdU incorporation after a 2-hour pulse (A and B). The total number of NSCs remained the same (Fig. S8 B). Yellow arrowheads point to BrdU-negative NSCs and yellow arrows point to BrdU-positive NSCs. Scale bar, 20 μm (A). N = 3 mice (BrdU) and 6 mice (Ki67) per condition. (C) The generation of neuronal precursors was assessed by counting the numbers of Tbr2-positive intermediate progenitors and of DCX-positive neuroblasts. N = 3 mice per condition.
Huwe1cKO mice also had too few intermediate progenitors and neuroblasts, and the remaining cells ectopically expressed Ascl1 (Fig. 2C and Fig. S6). The deletion of Huwe1 did not induce a switch towards gliogenesis, and intermediate progenitors were most likely eliminated by apoptosis (figs. S7 and S8). We suggest that persistence of Ascl1 protein in progenitors lacking Huwe1 maintains the proliferative state of NSCs and prevents differentiation of early intermediate.
To study the role of the interaction between Huwe1 and Ascl1 in the regulation of quiescence, we labeled quiescent NSCs by prolonged exposure to BrdU, followed by a chase (label-retention assay) (12). We then inactivated Huwe1 and analyzed the mice 3 weeks later (Fig. 3A). The numbers of BrdU-retaining progenitors were not significantly different in Huwe1cKO and control mice, indicating that the loss of Huwe1 did not lead to premature activation of quiescent stem cells, which would result in BrdU dilution (Fig. 3B and Fig. S9 A to F). Thus, Huwe1 is not required to maintain NSCs in quiescence.
Figure 3. Adult hippocampal stem cells fail to return to quiescence in Huwe1cKO mice.
(A and B) Mice received BrdU in the drinking water for 5 days, followed by Huwe1 inactivation. Analysis was performed three weeks later. N = 3 (control) and 6 (Huwe1cKO) mice. Figure S9 D-F shows an additional BrdU-retention experiment. (C and D). BrdU was administered after Huwe1 inactivation, when more Huwe1cKO NSCs than control NSCs proliferate (Fig. 2B). Analysis was performed three weeks later. N = 5 (control) and 3 (Huwe1cKO) mice. (E to G) EdU was injected 24 hours before analysis. EdU+ Ki67+ cells continue proliferating while EdU+ Ki67- cells have exited the cell cycle. No EdU+ NSCs expressed the astrocytic marker S100ß (Fig. S11 D to I). The yellow arrowhead points to an EdU+ Ki67- NSC and the yellow arrow to an EdU+ Ki67+ NSC. N = 47 (control) and 38 (Huwe1cKO) EdU+ NSCs from 6 and 5 mice, respectively. (H to K) Analysis was performed 5 months after Huwe1 inactivation. The overall number of NSCs was not changed but fewer NSCs proliferated in Huwe1cKO than control mice. N = 4 mice per condition. Scale bars, 20μm (G) and 50μm (I).
To determine whether Huwe1 is required for proliferating NSCs to return to quiescence, we marked cells exiting the cell cycle in the absence of Huwe1 by first inactivating Huwe1 and then performing a BrdU label-retention assay (Fig. 3C). BrdU-retaining radial cells in the subgranular zone of control mice were quiescent NSCs and not astrocytes as they did not express the astrocytic marker S100ß (Fig. S9 H). There were fewer BrdU-retaining NSCs in Huwe1cKO mice than in control mice (Fig. 3D and Fig. S9 I and J), indicating that without Huwe1, fewer NSCs returned to quiescence. We could not directly examine the divisions of Huwe1cKO NSCs by in vivo clonal analysis (13), because the low dose of tamoxifen required for this analysis was not sufficient to delete the Huwe1fl mutant allele (Fig. S10). To directly assess whether Huwe1 is required in proliferating NSCs for their return to quiescence, we marked instead a cohort of proliferating cells with a pulse of EdU and identified the fractions of NSCs that had either exited or re-entered the cell cycle 24 hours later by double labeling for EdU and Ki67 (Fig. 3E and Fig. S11). In control mice, 23.4% of EdU+ NSCs were negative for Ki67, suggesting that they had returned to quiescence after cycling and incorporating EdU (Fig. 3F and G). In Huwe1cKO mice only 2.6% of EdU+ NSCs were negative for Ki67, indicating that almost all Huwe1 mutant NSCs had re-entered the cell cycle (Fig. 3F and G). Thus, elimination of the activation factor Ascl1 from proliferating NSCs by Huwe1 in wild-type mice drives the cells into quiescence.
The long-term consequence of excessive proliferation of hippocampal NSCs in Huwe1cKO mice was examined five months after Huwe1 deletion, at P210 (Fig. 3H). The overall number of NSCs was unchanged, confirming that Huwe1 is not required for the maintenance of the predominant quiescent NSC population (Fig. 3I and J). In contrast, the number of proliferating NSCs was reduced (2.4 ± 0.1% Ki67+ NSCs in control mice; 0.3 ± 0.3% in Huwe1cKO mice; Fig. 3K), indicating that Huwe1 is required for the long-term maintenance of the proliferative NSC population in the hippocampus. This result also shows that stem cells that have proliferated and returned to quiescence are required to replenish the proliferative stem cell pool (Fig. S12).
Ascl1 activates the transcription of several cell cycle regulators in NSCs (4, 14). Huwe1-deficient NSCs showed higher expression of CcnD1 (Cyclin D1) and CcnD2 (Cyclin D2), two targets of Ascl1 (Figs. S13 A and S14). The elevation of CcnD1 and CcnD2 expression in Huwe1-mutant NSCs was due to the accumulation of Ascl1 since it was abolished after Ascl1 knockdown or deletion (Figs. S13 D and S14 A). The increase in CcnD1 expression in Huwe1cKO mice was seen in quiescent NSCs and to a greater extent in proliferating NSCs (Fig. 4B to E and Fig. S14 F). Thus, stabilization of Ascl1 in NSCs lacking Huwe1 promotes cell cycle re-entry by inducing the expression of CcnD genes.
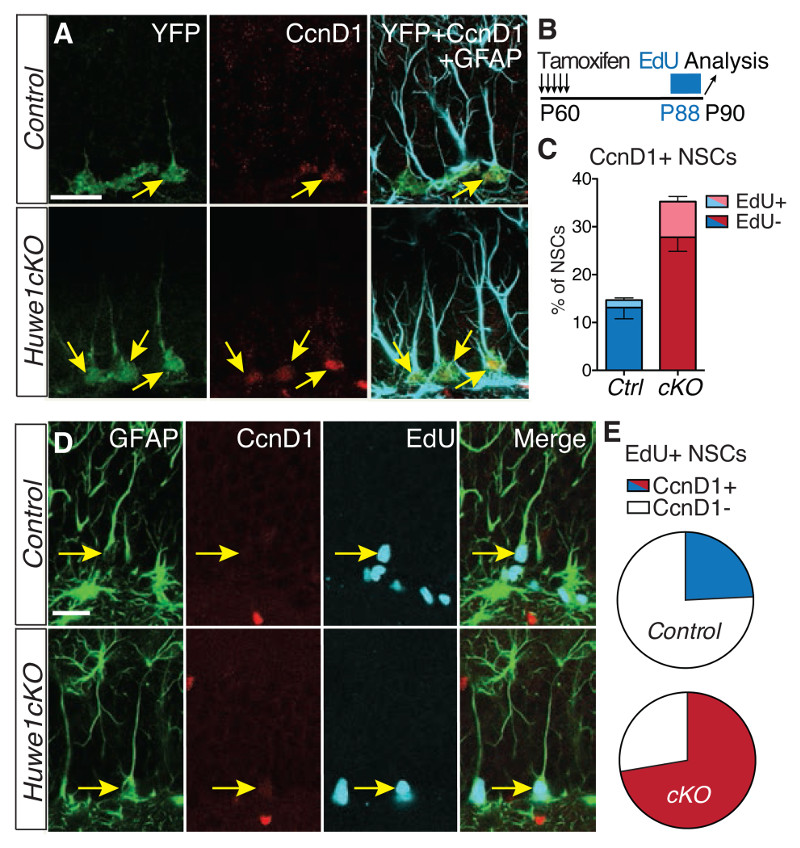
Figure 4. CcnD genes are abnormally upregulated in Huwe1cKO hippocampal stem cells.
(A to E) EdU was added to the drinking water for 48 hours prior to the analysis to mark cells that progressed through S-phase during this period. Co-labeling for EdU and CcnD1 identifies cells that have proliferated and still express CcnD1, required for proliferation of adult hippocampal stem cells (17, 18). Pie charts in E show the percentage of EdU+ NSCs that maintain CcnD1 expression. Yellow arrows in A point to CcnD1+ NSCs. Yellow arrowheads point to EdU+ CcnD1- and yellow arrows to EdU+ CcnD1+ NSCs in E. The elevation of CcnD1 and CcnD2 expression was not due to an increase in proliferation of Huwe1-mutant NSCs (4C and Fig. S13 B and C). N = 6 mice per condition (C) and N = 33 (control) and 47 (Huwe1cKO) EdU+ NSCs from 6 mice per condition (E). Scale bars, 20μm.
Posttranscriptional regulation controls stem cell activity, alongside transcriptional and epigenetic mechanisms (15). In the embryonic nervous system, Huwe1 promotes cell cycle exit and neuronal differentiation of progenitors by destabilizing N-myc (7). In the adult brain, we show here that Huwe1 targets the activation factor Ascl1 to promote the return of proliferating hippocampal NSCs to a resting state. Regulation of Ascl1 alone is not sufficient to promote quiescence exit, suggesting that additional signals are required to stimulate stem cell activity. Most NSCs continue to divide once activated and are eventually lost, thus contributing to the rapid attrition of the stem cell population over time (16). However, Huwe1 promotes the return to a resting state of a minority of dividing NSCs, which is essential for the long-term maintenance of the diminishing pool of proliferating stem cells (Fig. S12). Our results suggest that proliferating stem cells that return to quiescence form a pool of temporarily resting cells that is distinct from the main dormant pool and is the main contributor to neurogenesis in the adult hippocampus.
Supplementary Material
Supplement contains additional data.
One sentence summary.
The E3-ubiquitin ligase Huwe1 degrades the pro-activation factor Ascl1 in proliferating hippocampal stem cells, resulting in downregulation of Cyclin D genes and return to quiescence.
Acknowledgments
We gratefully acknowledge Lan Chen, Angeliki Achimastou and Elodie Lavedeze for technical support, Maria del Mar Masdeu, Rekha Subramaniams and Jacek Mor for managing the mouse colony, Antonio Iavarone and Magdalena Götz for providing transgenic mice, and Iván Crespo, Ryoichiro Kageyama and members of the Guillemot lab for discussions. The study was conceived and the manuscript was written by N.U. and F.G. Most of the work was performed by N.U., while D.L.C.B., J.A.D. and C.H. performed the MS analysis, J.A helped with experiments and A.F. and O.A. analyzed the polyubiquitination of Ascl1. N.U. was supported by fellowships from the MRC and the Francis Crick Institute, D. L.C.B. by fellowships from the FEBS and the Francis Crick Institute, J.A.D. was financed by the NWO project (184.032.201) and A.F. was financed by the Fondation de France. This work was supported by grants from the Wellcome Trust (106187/Z/14/Z), the BBSRC (BB/K005316/1), the MRC (U117570528) and the Francis Crick Institute (BZ10089) to F.G.
Footnotes
The authors declare no conflict of interest.
References and notes
- 1.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013 Jun;14:329. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010 May;11:339. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol. 2014 Sep 17;30C:51. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen J, et al. A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron. 2014;83:1085. doi: 10.1016/j.neuron.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froyen G, et al. Submicroscopic duplications of the hydroxysteroid dehydrogenase HSD17B10 and the E3 ubiquitin ligase HUWE1 are associated with mental retardation. Am J Hum Genet. 2008 Feb;82:432. doi: 10.1016/j.ajhg.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy SE, et al. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol Psychiatry. 2014 Jun;19:652. doi: 10.1038/mp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao X, et al. The HECT-domain ubiquitin ligase Huwe1 controls neural differentiation and proliferation by destabilizing the N-Myc oncoprotein. Nat Cell Biol. 2008 Jun;10:643. doi: 10.1038/ncb1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005 Jul 1;121:1085. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Hall JR, et al. Cdc6 stability is regulated by the Huwe1 ubiquitin ligase after DNA damage. Mol Biol Cell. 2007 Sep;18:3340. doi: 10.1091/mbc.E07-02-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, et al. Mule determines the apoptotic response to HDAC inhibitors by targeted ubiquitination and destruction of HDAC2. Genes Dev. 2011 Dec 15;25:2610. doi: 10.1101/gad.170605.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori T, et al. Inducible gene deletion in astroglia and radial glia--a valuable tool for functional and lineage analysis. Glia. 2006 Jul;54:21. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- 12.Terskikh VV, Vasiliev AV, Vorotelyak EA. Label retaining cells and cutaneous stem cells. Stem Cell Rev. 2012 Jun;8:414. doi: 10.1007/s12015-011-9299-6. [DOI] [PubMed] [Google Scholar]
- 13.Bonaguidi MA, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011 Jun 24;145:1142. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro DS, et al. A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev. 2011 May 1;25:930. doi: 10.1101/gad.627811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng LC, Tavazoie M, Doetsch F. Stem cells: from epigenetics to microRNAs. Neuron. 2005 May 5;46:363. doi: 10.1016/j.neuron.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Encinas JM, et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011 May 6;8:566. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowalczyk A, et al. The critical role of cyclin D2 in adult neurogenesis. J Cell Biol. 2004 Oct 25;167:209. doi: 10.1083/jcb.200404181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansorg A, Witte OW, Urbach A. Age-dependent kinetics of dentate gyrus neurogenesis in the absence of cyclin D2. BMC Neurosci. 2012;13:46. doi: 10.1186/1471-2202-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagace DC, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007 Nov 14;27:12623. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim EJ, Ables JL, Dickel LK, Eisch AJ, Johnson JE. Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS One. 2011;6:e18472. doi: 10.1371/journal.pone.0018472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schepers AG, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012 Aug 10;337:730. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 23.Conti L, et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005 Sep;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagihara H, Toyama K, Yamasaki N, Miyakawa T. Dissection of hippocampal dentate gyrus from adult mouse. Journal of visualized experiments: JoVE. 2009 doi: 10.3791/1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker TL, et al. Prominin-1 Allows Prospective Isolation of Neural Stem Cells from the Adult Murine Hippocampus. J Neurosci. 2013 Feb 13;33:3010. doi: 10.1523/JNEUROSCI.3363-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knobloch M, et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013 Jan 10;493:226. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010 Feb 25;463:1035. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hockemeyer D, et al. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell. 2008 Sep 11;3:346. doi: 10.1016/j.stem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forget A, et al. Shh signaling protects Atoh1 from degradation mediated by the E3 ubiquitin ligase Huwe1 in neural precursors. Dev Cell. 2014 Jun 23;29:649. doi: 10.1016/j.devcel.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11:1475. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Berg DL, et al. An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell. 2010 Apr 2;6:369. doi: 10.1016/j.stem.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao X, et al. The N-Myc-DLL3 cascade is suppressed by the ubiquitin ligase Huwe1 to inhibit proliferation and promote neurogenesis in the developing brain. Dev Cell. 2009 Aug;17:210. doi: 10.1016/j.devcel.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinals F, et al. BMP-2 decreases Mash1 stability by increasing Id1 expression. Embo J. 2004 Sep 1;23:3527. doi: 10.1038/sj.emboj.7600360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleinschmidt JA, Hugle B, Grund C, Franke WW. The 22 S cylinder particles of Xenopus laevis. I. Biochemical and electron microscopic characterization. Eur J Cell Biol. 1983 Nov;32:143. [PubMed] [Google Scholar]
- 35.Gebara E, et al. Heterogeneity of Radial Glia-Like Cells in the Adult Hippocampus. Stem Cells. 2016 Jan 4; doi: 10.1002/stem.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.