Abstract
Background
The role of Day 14 (D14) bone marrow (BM) assessment to detect increased blasts in patients undergoing induction for acute lymphoblastic leukemia (ALL) is not well-defined.
Methods
We evaluated 389 adolescent and adult patients with previously untreated Philadelphia chromosome (Ph)-negative ALL receiving frontline induction chemotherapy in whom D14 BM assessment was performed.
Results
D14 BM blasts <10% (including a blast-free aplastic BM) was observed in 319 patients (82%), 10–29% in 31 patients (8%), and ≥30% in 39 patients (10%). The composite complete remission (CR) and CR with inadequate platelet recovery (CRp) rate for these groups was 99.7%, 87% and 79%. The median event-free survival (EFS) was 49 months, 33 months, and 9 months, respectively (P<0.001). The median overall survival (OS) was 88 months, 37 months, and 21 months, respectively (P<0.001). D14 BM blast group was the only factor predictive for achievement of CR/CRp (P<0.001). By multivariate analysis, D14 BM blast group was independently prognostic for both EFS (HR 1.44, 95% CI 1.12–1.85, P=0.004) and OS (HR 1.45, 95% CI 1.14–1.85, P=0.003). However, when minimal residual disease (MRD) assessment at the time of CR was added to the model, D14 BM blast group was no longer prognostic for EFS or OS.
Conclusions
Assessment of residual D14 BM blasts in patients with ALL is highly predictive for achievement of CR with induction chemotherapy and for EFS and OS. However, the impact on long-term outcomes is less prognostic when MRD assessment is also available.
Keywords: Acute lymphoblastic leukemia, minimal residual disease, day 14, blasts, prognosis
Condensed Abstract
In patients with ALL receiving induction, increased blasts on Day 14 BM assessment is associated with lower CR rates and worse survival. The impact of Day 14 BM blast assessment on long-term outcomes is less prognostic when MRD information is also available.
Introduction
Although the vast majority of adults with acute lymphoblastic leukemia (ALL) achieve a complete remission (CR) with intensive chemotherapy, most ultimately relapse and die from their disease.1, 2 The prognosis of patients with relapsed ALL is poor3, 4; therefore, accurate risk stratification systems to identify high-risk patients who may benefit from more intensive post-remission therapies such as stem cell transplantation (SCT) or other innovative approaches, are imperative to improving outcomes in ALL. Pretreatment patient- and disease-related factors such as age, white blood cell (WBC) count at presentation, and cytogenetics, are helpful in estimating the risk of relapse and identifying patients who should be considered for SCT.5 However, for many patients, risk stratification based on pretreatment characteristics only fails to accurately predict for relapse.
Assessment of early morphologic response to chemotherapy may provide additional information about the disease chemosensitivity and underlying disease biology of a patient that is not available prior to the initiation of treatment. In patients with acute myeloid leukemia, the presence of residual bone marrow (BM) blasts on Day 14 (D14) of induction chemotherapy is associated with lower response rates and shorter survival.6–9 Several studies have suggested that interim assessment of BM blasts during induction for ALL identifies patients at high risk for relapse and death.10–15 These studies have varied significantly in their methodologies, some evaluating BM blasts from Day 7 to Day 15 of induction chemotherapy, and defining inadequate morphologic response as BM blasts ranging from ≥5% to ≥25%.
In recent years, assessment of minimal residual disease (MRD) has greatly improved the risk stratification in ALL.16–18 However, most ALL studies that evaluated the impact of early morphologic response to induction have not incorporated MRD information into their prognostic modeling. In the one study in which MRD information was considered, the presence of ≥10% BM blasts on D14 was not independently prognostic for either disease-free or overall survival (OS), suggesting that D14 BM evaluation may no longer be useful in the era of routine MRD assessment.19 To help clarify the role of D14 BM assessment in patients with ALL, we performed a retrospective analysis of 389 patients with untreated ALL who received induction chemotherapy at our institution and in whom a D14 BM assessment was performed. Most patients also had MRD information available.
Methods
Patients
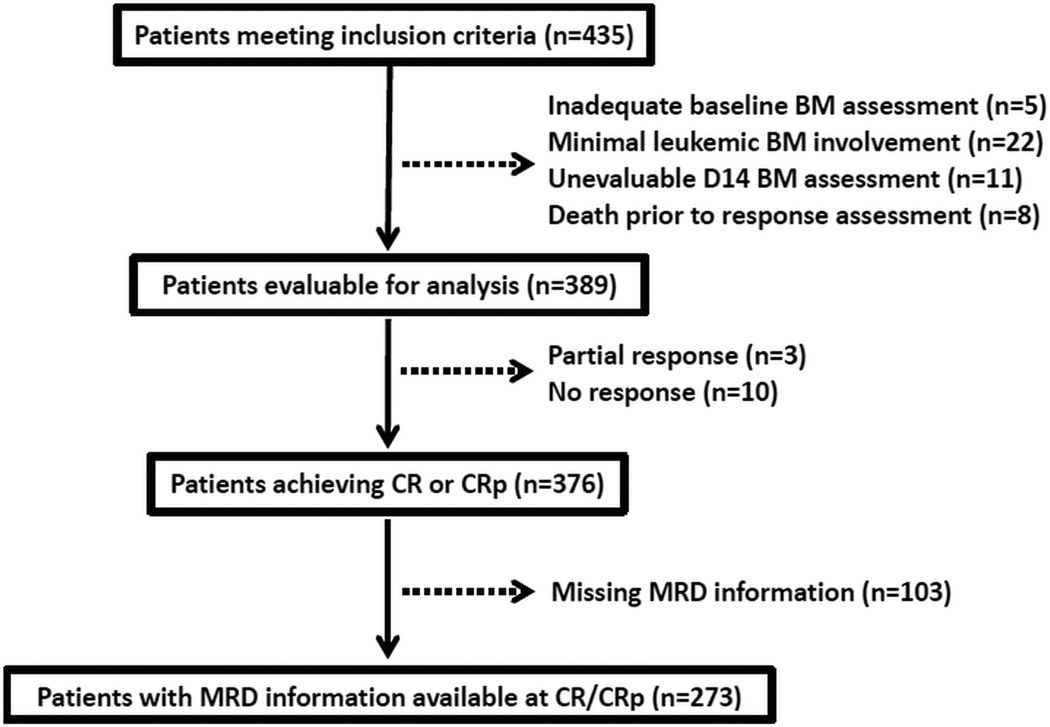
Between May 2000 and September 2015, 435 consecutive adolescent or adult patients at our institution with previously untreated Ph-negative B-ALL or T-ALL (excluding Burkitt or Burkitt-like leukemia) received induction with hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone alternating with methotrexate and high-dose cytarabine (hyper-CVAD) or with augmented Berlin-Frankfurt-Munster (Augmented BFM)-based chemotherapy, and had a BM assessment performed on D14 ± 2 of induction (Figure 1). Patients with an inadequate baseline BM evaluation, minimal leukemic BM involvement (defined as baseline BM blasts <25%), inevaluable D14 BM assessment, or who died prior to response assessment were excluded. A total of 389 patients met all inclusion criteria and were evaluable for analysis.
Figure 1.
Study population selection process.
Treatment Regimens
Of the 389 evaluable patients, 301 (77%) received a hyper-CVAD-based regimen and 88 (23%) received Augmented BFM. Augmented BFM was only used in patients <40 years of age. The details of these regimens were published elsewhere.10, 20 Hyper-CVAD was dose-reduced in elderly patients according to age and performance status. Most patients who received hyper-CVAD with CD20-positive ALL also received an anti-CD20 antibody, as has been standard practice at our institution for most of the study period. The specific treatment regimens used in this study are summarized in Supplementary Table 1. The treatment protocols were approved by the M.D. Anderson Cancer Center Institutional Review Board. Informed consent was obtained according to the Declaration of Helsinki and our institutional guidelines.
Response and Outcome Definitions
A CR was defined as the presence of <5% blasts in the BM aspirate, with >1 × 109/L neutrophils and >100 × 109/L platelets in the peripheral blood with no evidence of extramedullary disease. A CR with inadequate platelet recovery (CRp) was defined as meeting criteria for CR but with a platelet count ≤100 × 109/L. Relapse was defined by recurrence of ≥5% blasts in a BM aspirate, or the presence of extramedullary disease. Event-free survival (EFS) was calculated from the time of treatment initiation until treatment failure, relapse or death from any cause, and was censored at the time of SCT. OS was calculated from the time of treatment initiation until death from any cause.
Minimal Residual Disease
MRD by multiparameter flow cytometry (MFC) was performed on BM specimens at the time of CR or CRp and again 3 months later as previously described.21 Initially, a 15-marker, 4-color panel was used; later, a 6-color panel was used. MRD positivity was defined on MFC scatter plots as a cluster of at least 20 cells showing altered expression of ≥2 antigens. The sensitivity of this MRD assay is 0.01%.
Statistical Methods
Patient characteristics were summarized using median (range) for continuous variables and frequencies (percentages) for categorical variables. Associations between categorical and continuous variables were assessed using chi-square tests and one-way analysis of variance. Spearman ρ coefficient was used to analyze the correlation between D14 BM blasts and MRD status at CR or CRp. EFS and OS were calculated using Kaplan-Meier estimates, and survival estimates were compared using the log-rank test. The optimal D14 BM blast cutoff predicting for OS was determined by comparing hazard ratios (HR) of various cutoffs by log-rank test. Univariate Cox proportional hazards regression models were used to assess the association between patient characteristics and EFS or OS. Univariate logistic regression models were conducted to assess the relationship between the patient characteristics and achievement of CR or CRp. Patient characteristics with P values <0.10 in the univariate models were included in the multivariate model. Backward elimination was then used until all remaining predictors had a P value <0.05.
Results
D14 BM Blast Percentage and Cutoffs for Analysis
The optimal cutoff predicting for OS was identified as D14 BM blasts ≥30% vs. <30% (HR 2.91, 95% confidence interval [CI] 1.98–4.28, P<0.001). A separate cutoff was also identified at ≥10% vs. <10% BM blasts (HR 2.26, 95% CI 1.63–3.15, P<0.001), although this cutoff had less discriminatory power for OS than did ≥30% vs. <30%. Patients with 10–29% D14 BM blasts were found to have significantly better OS than those with ≥30% blasts (HR 1.96, 95% CI 1.11–3.45, P=0.03). Therefore, patients were divided into 3 groups based on D14 BM blast percentage (i.e. <10%, 10–29% and ≥30%) for the purpose of additional analyses.
Of the 280 patients with a quantifiable D14 BM blast count, the median percentage of D14 BM blasts was 2% (range 0–97%). Two hundred and ten patients (54%) had <10% blasts, 31 (8%) had 10–29% blasts and 39 (10%) had ≥30% blasts on D14 BM assessment, and 109 patients (28%) had a blast-free aplastic BM. These patients with an aplastic BM had similar OS to those with <10% D14 BM blasts, and these groups were therefore combined for analysis.
Baseline Characteristics and Predictors of D14 BM Blasts
The median age of the study population was 56 years (range, 13–86 years), median WBC 4.3 × 109/L (range, 0.4–602.4 × 109/L), and median baseline BM blasts 86% (range, 25%-100%). Three hundred twenty-nine patients (85%) had B-ALL, and 70 (18%) had poor-risk cytogenetics (defined as hypodiploidy or near triploidy, complex cytogenetics with ≥5 chromosomal abnormalities or presence of MLL rearrangement). Table 1 summarizes the associations of baseline characteristics with each D14 blast group. Baseline factors associated with inferior D14 morphologic response were older age (P=0.04), higher WBC count (P<0.01), and diagnosis of T-ALL (P=0.01).
Table 1.
Predictors of D14 BM blast clearance
| Characteristica | D14 BM blasts <10% (N= 319) |
D14 BM blasts 10–29% (N = 31) |
D14 BM blasts ≥30% (N = 39) |
P |
|---|---|---|---|---|
| Age (years) | 38 (13–86) | 31 (18–71) | 44 (19–79) | 0.04 |
| WBC (109/L) | 4.1 (0.4–420.0) | 6.9 (0.8–155.8) | 7.3 (0.9–602.4) | <0.01 |
| Hemoglobin (g/dL) | 9.4 (3.5–16.3) | 9.2 (6.8–14.8) | 9.0 (4.5–15.1) | 0.43 |
| Platelets (109/L) | 40 (1–513) | 41 (14–265) | 39 (7–188) | 0.68 |
| BM blasts (%) | 86 (25–100) | 85 (34–98) | 86 (39–98) | 0.32 |
| LDH (U/L) | 1060 (172–32029) | 855 (197–36630) | 1052 (339–4675) | 0.28 |
| PS | ||||
| 0–1 | 248 (81) | 27 (9) | 31 (10) | 0.78 |
| 2–4 | 45 (85) | 2 (4) | 6 (11) | |
| Diagnosis | ||||
| B-ALL | 276 (84) | 25 (8) | 28 (9) | 0.01 |
| T-ALL | 43 (72) | 6 (10) | 11 (18) | |
| Cytogenetics | ||||
| Poor-risk | 59 (84) | 8 (11) | 3 (4) | 0.32 |
| Others | 234 (82) | 20 (7) | 30 (11) | |
| Regimen | ||||
| Hyper-CVAD | 248 (82) | 19 (6) | 34 (11) | 0.61 |
| AugBFM | 71 (81) | 12 (14) | 5 (5) | |
Continuous variables are listed as median (range) and categorical variables as n (%)
D14, day 14; BM, bone marrow; WBC, white blood cells; BM, bone marrow; LDH, lactate dehydrogenase; PS, performance status; Hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone; AugBFM, augmented Berlin-Frankfurt-Munster
D14 BM Blasts Prediction of Response to Induction Chemotherapy
Response to induction chemotherapy and MRD status at CR and at 3 months are summarized in Table 2. Three hundred seventy-six patients (97%) achieved CR or CRp, 349 (93%) of whom achieved CR/CRp after 1 cycle of induction chemotherapy. MRD assessment was available at CR in 273 patients and at 3 months in 260 patients (73% and 69% of those achieving CR/CRp, respectively). The rates of MRD negativity at CR and at 3 months were 67% and 88%, respectively. MRD was also assessed at D14 in 143 patients. At D14, 67 patients (47%) were MRD-negative and 76 (53%) were MRD-positive. MRD status at D14 was not found to be significant for OS (P=0.24) and therefore for was not included in subsequent analyses.
Table 2.
Morphologic and MRD response by D14 BM blast percentage
| Characteristica | D14 BM blasts <10% (N= 319) |
D14 BM blasts 10–29% (N = 31) |
D14 BM blasts ≥30% (N = 39) |
P |
|---|---|---|---|---|
| Response | ||||
| CR | 312 (98) | 26 (84) | 31 (79) | |
| CRp | 6 (2) | 1 (3) | 0 (0) | <0.001 |
| PR | 0 (0) | 2 (6) | 1 (3) | |
| NR | 1 (0) | 2 (6) | 7 (18) | |
| Cycles to CR | ||||
| 1 | 301 (95) | 27 (100) | 21 (68) | <0.001 |
| ≥2 | 17 (5) | 0 (0) | 10 (32) | |
| MRD at CR/CRp | ||||
| Positive | 57 (25) | 16 (73) | 17 (89) | <0.001 |
| Negative | 175 (75) | 6 (27) | 2 (11) | |
| MRD at 3 months | ||||
| Positive | 19 (8) | 7 (37) | 6 (40) | <0.001 |
| Negative | 207 (92) | 12 (63) | 9 (60) | |
Variables are listed as n (%)
MRD, minimal residual disease; D14, day 14; BM, bone marrow; CR, complete response; CRp, CR with inadequate platelet recovery; PR, partial response; NR, no response
Patients with poorer D14 morphologic response had lower rates of CR/CRp (P<0.001). When stratified by age (i.e. ≤30, 30–59, and ≥60 years), D14 BM blasts remained predictive for achievement of CR/CRp (P<0.001 for all age groups). Median D14 BM blast percentage for patients not achieving CR/CRp was 59% (range, 10–96%); one patient with an apparently blast-free aplastic marrow on D14 failed to respond to induction. Patients with ≥30% D14 BM blasts were also significantly more likely to require more than 1 cycle of chemotherapy in order to achieve CR/CRp (P<0.001). In a univariate analysis of the pretreatment characteristics in Table 1, no characteristic was predictive for achievement of CR/CRp; only D14 BM blast group predicted for remission (odds ratio 0.15, 95% CI 0.07–0.32, P<0.001 for ≥30% vs. 10–29% vs. <10% blasts).
The rates of MRD negativity at the time of CR were lower with each successive increase in D14 BM blast group (75% vs. 27% vs. 11%, respectively, P<0.001). Similarly, 92% of patients with D14 BM blasts <10% achieved MRD negativity at 3 months, compared to 63% and 60% of patients with 10–29% and ≥30% blasts, respectively (P<0.001). As expected, there was a strong correlation between a higher D14 BM blast percentage and positive MRD status in CR (ρ=0.429, P<0.001).
D14 BM Blasts Prediction of EFS and OS
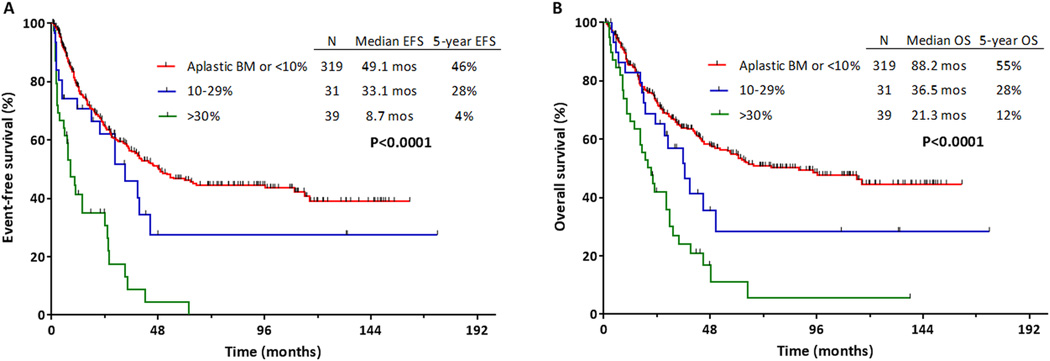
One hundred ninety-seven patients (51%) experienced treatment failure, relapsed or died at last follow-up. Median EFS for the entire cohort was 39 months, and the 5-year EFS rate was 42%. Table 3 shows the univariate analysis for EFS and OS. Median EFS for patients with D14 BM blasts <10% vs. 10–29% vs. ≥30% was 49 months, 33 months, and 9 months, respectively (P<0.001; Figure 2A). Five-year EFS rates for these groups were 46%, 28%, and 4%, respectively. Table 4 shows the multivariate analysis for predictors of EFS, including pretreatment characteristics and number of cycles to achieve CR/CRp. By multivariate analysis, D14 BM blasts were independently prognostic for EFS (HR 1.44, 95% CI 1.12–1.85, P=0.004 for ≥30% vs. 10–29% vs. <10% blast groups).
Table 3.
Univariate analysis for EFS and OS
| Characteristic | EFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (years): ≥60 vs. 30–59 vs. ≤30 | 1.64 | 1.35–1.98 | <0.001 | 1.82 | 1.49–2.22 | <0.001 |
| WBC (109/L): ≥40 vs. <40 | 2.00 | 1.36–2.92 | 0.001 | 1.91 | 1.32–2.76 | 0.001 |
| Hemoglobin (g/dL): <10 vs. ≥10 | 1.32 | 0.97–1.80 | 0.08 | 1.36 | 0.98–1.89 | 0.06 |
| Platelets (109/L): <100 vs. ≥100 | 1.33 | 1.10–1.60 | 0.003 | 1.28 | 1.06–1.55 | 0.01 |
| Baseline BM blasts: ≥60% vs. <60% | 0.72 | 0.46–1.13 | 0.15 | 0.65 | 0.40–1.06 | 0.08 |
| LDH: ≥2xULN vs. <2xULN | 1.23 | 0.93–1.63 | 0.14 | 1.11 | 0.83–1.48 | 0.48 |
| PS: 2–4 vs. 0–1 | 1.76 | 1.23–2.53 | 0.002 | 1.97 | 1.36–2.84 | <0.001 |
| Diagnosis: B-ALL vs. T-ALL | 1.18 | 0.81–1.71 | 0.40 | 1.24 | 0.85–1.81 | 0.26 |
| Cytogenetics: poor vs. standard | 1.64 | 1.15–2.33 | 0.006 | 1.70 | 1.20–2.42 | 0.003 |
| Treatment: Hyper-CVAD vs. AugBFM | 1.32 | 0.93–1.89 | 0.12 | 1.39 | 0.96–1.98 | 0.08 |
| Cycles to CR/CRp: ≥2 vs. 1 | 1.72 | 1.26–2.35 | 0.001 | 1.69 | 1.23–2.32 | 0.001 |
| MRD at CR/CRp: positive vs. negative | 2.00 | 1.39–2.89 | <0.001 | 2.33 | 1.61–3.37 | <0.001 |
| D14 BM blasts: ≥30%vs. 10–29% vs. <10% | 1.57 | 1.32–1.86 | <0.001 | 1.46 | 1.24–1.74 | <0.001 |
EFS, event-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; WBC, white blood cell; BM, bone marrow; LDH, lactate dehydrogenase; ULN, upper limit of normal; PS, performance status; Hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone; AugBFM, augmented Berlin-Frankfurt-Munster; CR, complete remission; CRp, CR with inadequate platelet recovery; MRD, minimal residual disease; D14, day 14
Figure 2.
Outcomes for patients by day 14 bone marrow (BM) blast percentage. (A) Event-free survival (EFS) and (B) overall survival (OS).
Table 4.
Multivariate analysis for EFS and OS by pretreatment characteristics, cycles to response and D14 BM blast percentage
| Characteristic | EFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (years): ≥60 vs. 30–59 vs. ≤30 | 1.56 | 1.24–1.95 | <0.001 | 1.64 | 1.30–2.07 | <0.001 |
| WBC (109/L): ≥40 vs. <40 | 2.41 | 1.53–3.78 | <0.001 | 2.09 | 1.37–3.19 | 0.001 |
| Platelets (109/L): <100 vs. ≥100 | 1.89 | 1.23–2.93 | 0.004 | 1.72 | 1.11–2.66 | 0.01 |
| PS: 2–4 vs. 0–1 | 1.87 | 1.23–2.83 | 0.003 | 2.11 | 1.38–3.22 | 0.001 |
| Cytogenetics: poor vs. standard | 1.53 | 1.02–2.28 | 0.04 | 1.54 | 1.04–2.27 | 0.03 |
| Cycles to CR/CRp: ≥2 vs. 1 | 1.77 | 1.24–2.52 | 0.002 | 1.87 | 1.30–2.69 | 0.001 |
| D14 BM blasts: ≥30%vs. 10–29% vs. <10% | 1.44 | 1.12–1.85 | 0.004 | 1.45 | 1.14–1.85 | 0.003 |
Univariate analysis for EFS and OS included the following variables: age, WBC count, platelets, hemoglobin, baseline BM blasts, lactate dehydrogenase, B-ALL vs. T-ALL, PS, cytogenetics, treatment regimen, cycles to CR, D14 BM blasts
EFS, event-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; WBC, white blood cell; PS, performance status; CR, complete remission; CRp, CR with inadequate platelet recovery; D14, day 14; BM, bone marrow
With a median follow-up of 59 months, 186 patients (48%) have died. The median OS for the entire cohort was 56 months, and the 5-year OS rate was 49%. Median OS for patients with D14 BM blasts <10% vs. 10–29% vs. ≥30% was 88 months, 37 months, and 21 months, respectively (P<0.001; Figure 2B; Table 3). Five-year OS rates for these groups were 55%, 28%, and 12%, respectively. By multivariate analysis, D14 BM blasts were independently prognostic for OS (HR 1.45, 95% CI 1.14–1.85, P=0.003 for ≥30% vs. 10–29% vs. <10% blast groups; Table 4).
Impact of MRD Assessment on Prognostic Significance of D14 BM Blasts
Median EFS for patients with MRD positive disease at CR was 27 months vs. 110 months for those who achieved MRD negativity (HR 1.99, 95% CI 1.47–3.33, P=0.001). Median OS for those with and without MRD at CR was 31 months and not reached, respectively (HR 2.30, 95% CI 1.76–4.04, P<0.001). MRD status at CR had overall better discrimination for EFS and OS than did MRD status at 3 months. To evaluate the impact of MRD on the prognostic significance of D14 BM blast percentage, MRD status at CR was added to the multivariate analysis of both EFS and OS (Table 5). By multivariate analysis, MRD status at CR was highly prognostic for both EFS (HR 1.96, 95% CI 1.23–3.12, P=0.005) and OS (HR 1.99, 95% CI 1.25–3.17, P=0.004). However, with the addition of MRD status to the analysis, baseline cytogenetics and D14 BM blasts were no longer independently prognostic for either EFS or OS.
Table 5.
Multivariate analysis for EFS and OS, including MRD status at CR
| Characteristic | EFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (years): ≥60 vs. 30–59 vs. ≤30 | 1.57 | 1.17–2.09 | 0.002 | 1.67 | 1.24–2.25 | 0.001 |
| WBC (109/L): ≥40 vs. <40 | 2.01 | 1.08–3.76 | 0.03 | 1.73 | 0.99–2.99 | 0.05 |
| Platelets (109/L): <100 vs. ≥100 | 2.54 | 1.32–4.90 | 0.005 | 2.09 | 1.12–3.91 | 0.02 |
| PS: 2–4 vs. 0–1 | 2.19 | 1.24–3.89 | 0.007 | 2.26 | 1.27–4.01 | 0.005 |
| Cycles to CR/CRp: ≥2 vs. 1 | 1.90 | 1.22–2.95 | 0.004 | 1.91 | 1.21–3.02 | 0.006 |
| MRD at CR/CRp: positive vs. negative | 1.96 | 1.23–3.12 | 0.005 | 1.99 | 1.25–3.17 | 0.004 |
Univariate analysis for EFS and OS included the following variables: age, WBC count, platelets, hemoglobin, baseline BM blasts, lactate dehydrogenase, B-ALL vs. T-ALL, PS, cytogenetics, treatment regimen, cycles to CR, D14 BM blasts, MRD status at CR/CRp
EFS, event-free survival; OS, overall survival; MRD, minimal residual disease; CR, complete remission; HR, hazard ratio; CI, confidence interval; WBC, white blood cell; PS, performance status; CRp, CR with inadequate platelet recovery
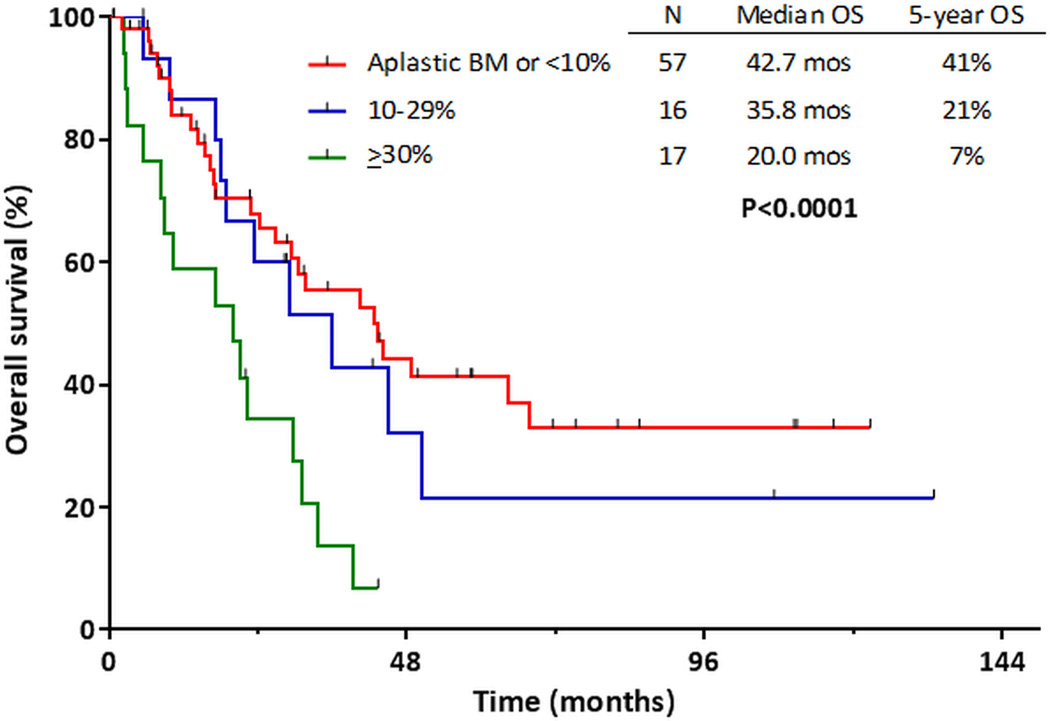
When only patients achieving CR/CRp were considered (n=273), the prognostic impact of D14 BM blast percentage varied by MRD status at CR. When patients with MRD negative disease were stratified by patients with D14 BM blasts <10% vs. ≥10%, OS was similar among both groups of patients (P=0.86). Only 2 patients with ≥30% D14 BM blasts achieved MRD negativity, so a 3-group comparison was not possible for this analysis. Among patients with MRD-positive disease at CR, OS was significantly longer in the patients with better morphologic response at D14 (Figure 3). The 5-year OS rates for patients with D14 BM blasts <10%, 10–29%, and ≥30% were 41%, 21%, and 7%, respectively (P<0.001).
Figure 3.
Overall survival of patients who were positive for minimal residual disease at complete response, stratified day 14 bone marrow blast percentage.
Impact of Regimen on Prognostic Significance of D14 BM Blasts
When patients were evaluated according to treatment regimen, D14 BM blast percentage did not have a differential prognostic effect among patients receiving either hyper-CVAD or Augmented BFM. In both groups of patients, D14 BM blast cutoffs of <10%, 10–29% and ≥30% identified 3 groups of patients with successively inferior OS. For patients treated with hyper-CVAD, median OS for these 3 groups was 61 months, 39 months and 21 months, respectively (P<0.001). For patients treated with Augmented BFM, median OS was not reached, 29 months, and 20 months, respectively (P=0.001). Additional stratification by age (i.e. ≤30, 30–59, and ≥60 years) showed that D14 BM blasts remained significant for both EFS and OS in all age groups.
Impact of SCT on Outcomes
SCT was performed in first CR/CRp in 33 patients (8% of the evaluable population and 9% of patients who achieved CR/CRp). MRD status was assessed prior to SCT in 30 patients, 25 of whom (83%) were MRD-negative at the time of SCT. Rates of MRD negativity were similar among the 3 D14 BM blast groups. Among the evaluable population, SCT was performed in 25 patients (11%) with <10% BM blasts, 4 (13%) with 10–29% blasts, and 4 (10%) with ≥30% blasts (P=0.43). Among 376 patients achieving CR/CRp with induction, SCT was performed in 8% of patients with <10% BM blasts, 15% of patients with 10–29% blasts, and 13% of patients with ≥30% blasts (P=0.19). When all evaluable patients were considered and censored for SCT in first remission, D14 blast group remained highly prognostic, with a median OS for patients with BM blasts <10%, 10–29% and ≥30% of 97 months, 45 months and 21 months, respectively (P<0.001).
Discussion
In this study evaluating the prognostic significance of D14 BM assessment in ALL, we found that patients with poorer morphologic response on D14 BM had lower rates of CR and poorer EFS and OS, with D14 BM blast percentage being the only factor that was predictive for achievement of CR. While D14 BM blast percentage was independently prognostic for EFS and OS when only pretreatment characteristics were considered, it did not provide additional prognostic information when MRD information was available.
Most previous studies of early morphologic response in ALL have been in children10, 13, 15, 22 or in adults treated with pediatric-inspired regimens.12, 14, 19 In contrast, the majority of adolescent and adult patients reported here (77%) received hyper-CVAD-based chemotherapy. Notably, only one previous study has evaluated D14 BM blasts in patients receiving hyper-CVAD.11 In this study, D14 BM blasts ≥5% was associated with worse EFS and OS only in patients who received the less intensive VAD regimen (vincristine, doxorubicin and dexamethasone) but not with patients who received hyper-CVAD. This suggests that the definition of morphologic clearance might vary based on the chemotherapeutic regimen administered, which highlights the importance of validating the utility of D14 BM assessment within the context of a particular regimen.
In this study, we identified three groups of patients based on D14 BM assessment (i.e. <10%, 10–29% and ≥30% blasts) with highly divergent remission and survival outcomes. A cut-off of 10% BM blasts on D14 has previously been used to define poor morphologic response and high-risk disease by the Spanish PETHEMA Group, and has been incorporated into risk-adapted strategies.12, 14, 19 A higher BM blast cut-off of 25% on either Day 722 or Day 1515 has also been used to define high-risk disease in two studies in childhood ALL. In our study, although 79% of patients with ≥30% blasts on D14 BM assessment were still able to achieve CR, their 3-year OS was only 21 months, significantly worse than those with 10–29% blasts on D14. These results suggest that morphologic response should not be viewed as a binary parameter, and that the degree of blast reduction should be taken into consideration when interpreting D14 BM results.
MRD has emerged as a powerful factor predicting for long-term outcomes in patients with ALL16–18. A number of methods for MRD determination are available, each with their own advantages and disadvantages.23, 24 While we found that D14 BM blasts were independently prognostic for survival when only pretreatment characteristics were considered, the incorporation of MRD status at CR superseded the prognostic impact of D14 BM assessment. This is consistent with the finding of the one previous study that evaluated the significance of D14 BM blasts in the context of MRD.19 However, this previous study evaluated only patients with high-risk ALL and prospectively assigned all patients with poor morphologic response on D14 BM to SCT. Our study evaluated an unselected population of varying baseline risk in which only 11% of patients with ≥10% D14 BM blasts underwent SCT in first remission. The present study therefore addressed the impact of D14 BM blast clearance in a significantly different patient population with a different pattern of post-remission therapy. Taken together with previously published results, our findings suggest that assessment of D14 BM blasts may not be very useful in guiding prognostication or risk-adapted strategies in patients with ALL in whom reliable MRD information is also available.
While MRD assessment is considered standard of care for the risk stratification of patients with ALL, the optimal platform for MRD detection (e.g. MFC, polymerase chain reaction or next generation sequencing) is not yet defined.23–25 The sensitivity of MRD assessment by MFC, for example, is limited by similarities between normal regenerating and malignant cells.24 There is a lack of standardization between treatment centers which results in suboptimal comparability of MRD assessment across different laboratories. Furthermore, the interpretation of MFC patterns requires significant expertise, which means that reliable and reproducible MRD assessment may not be available in some resource-poor settings or in settings where technician or pathologist experience is limited. In contrast, assessment of D14 BM morphologic response does not require significant resources or expertise. By multivariate analysis in which MRD information was not considered, we found that patients with ≥30% D14 BM blasts had an approximately 2-fold higher risk of death than did patients with <10% D14 BM blasts, which was comparable to the prognostic impact of MRD status in our analysis.
As expected, we found a significant correlation between D14 BM blasts and MRD status at CR, with 75% of patients with <10% D14 BM blasts achieving MRD negativity, while only 11% of those with ≥30% blasts became MRD negative at CR. Although D14 BM blasts were not independently prognostic for EFS or OS when MRD information was considered, it is notable that patients with MRD-positive disease at CR could be stratified according to D14 morphologic response in order to identify subgroups with divergent long-term survival rates. This finding suggests that, among patients with MRD-positive disease, poor early morphologic response identifies a group of patients with a particularly poor prognosis and a 5-year OS rate of only 7%. Prospective risk-adapted studies are needed to determine whether these patients may preferentially benefit from strategies using novel agents (for example CD19 or CD22 monoclonal antibodies), or from consideration of early SCT.
In contrast to the impact of D14 BM blasts on patients with MRD-positive disease, the outcomes of patients with MRD negativity did not differ when stratified by D14 BM blasts. Although MRD information was not available in 27% of patients, this finding is nevertheless significant and suggests that achievement of MRD negativity likely supersedes any prognostic information gained from assessment of early morphologic response to induction chemotherapy. It is important note however that only 8 patients (accounting for only 4.5% of those became MRD negative) had ≥10% BM blasts on D14. This low rate of poor morphologic response in the MRD negative group may have impaired the ability to detect a possible significant difference based on D14 BM blasts.
In conclusion, in patients with Ph-negative ALL undergoing frontline induction chemotherapy, D14 BM blasts were strongly prognostic for achievement of CR. D14 BM blasts were also prognostic for EFS and OS only when MRD status at CR was not considered. Assessment of D14 BM blasts may be useful for risk stratification and to guide post-induction treatment allocation in settings where reliable MRD information is not available.
Supplementary Material
Acknowledgments
Funding Source: Supported by the MD Anderson Cancer Center Support Grant CA016672,P30 CA016672
Footnotes
Disclosure of Conflicts of Interest: The authors report no conflicts of interest.
Authorship Contributions: N.J.S. designed the study, collected and analyzed the data, and wrote the manuscript; H.K. and E.J. designed the study, collected and analyzed the data, treated patients, and wrote the manuscript; K.S. designed the study and performed the statistical analysis; C.B-R. and J.D.K. assisted with pathologic interpretation; S.P. and G.C.I. collected and analyzed the data; F.R., D.T., J.E.C., T.M.K, M.K., N.J., G.G-M., I.K., P.K., R.E.C. and S.M.O treated patients. All authors reviewed and approved the manuscript.
References
- 1.Sive JI, Buck G, Fielding A, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol. 2012;157:463–471. doi: 10.1111/j.1365-2141.2012.09095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fielding AK, Rowe JM, Buck G, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123:843–850. doi: 10.1182/blood-2013-09-529008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oriol A, Vives S, Hernandez-Rivas JM, et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95:589–596. doi: 10.3324/haematol.2009.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Thomas D, Ravandi F, et al. Defining the course and prognosis of adults with acute lymphocytic leukemia in first salvage after induction failure or short first remission duration. Cancer. 2010;116:5568–5574. doi: 10.1002/cncr.25354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks DI, Alonso L, Radia R. Allogeneic hematopoietic cell transplantation in adult patients with acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2014;28:995–1009. doi: 10.1016/j.hoc.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Liso V, Albano F, Pastore D, et al. Bone marrow aspirate on the 14th day of induction treatment as a prognostic tool in de novo adult acute myeloid leukemia. Haematologica. 2000;85:1285–1290. [PubMed] [Google Scholar]
- 7.Kern W, Haferlach T, Schoch C, et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003;101:64–70. doi: 10.1182/blood-2002-02-0532. [DOI] [PubMed] [Google Scholar]
- 8.Hussein K, Jahagirdar B, Gupta P, Burns L, Larsen K, Weisdorf D. Day 14 bone marrow biopsy in predicting complete remission and survival in acute myeloid leukemia. Am J Hematol. 2008;83:446–450. doi: 10.1002/ajh.21133. [DOI] [PubMed] [Google Scholar]
- 9.Bertoli S, Bories P, Bene MC, et al. Prognostic impact of day 15 blast clearance in risk-adapted remission induction chemotherapy for younger patients with acute myeloid leukemia: long-term results of the multicenter prospective LAM-2001 trial by the GOELAMS study group. Haematologica. 2014;99:46–53. doi: 10.3324/haematol.2013.091819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachman J, Sather HN, Gaynon PS, Lukens JN, Wolff L, Trigg ME. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: a report from the Children’s Cancer Group. J Clin Oncol. 1997;15:2222–2230. doi: 10.1200/JCO.1997.15.6.2222. [DOI] [PubMed] [Google Scholar]
- 11.Cortes J, Fayad L, O’Brien S, Keating M, Kantarjian H. Persistence of peripheral blood and bone marrow blasts during remission induction in adult acute lymphoblastic leukemia confers a poor prognosis depending on treatment intensity. Clin Cancer Res. 1999;5:2491–2497. [PubMed] [Google Scholar]
- 12.Ribera JM, Ortega JJ, Oriol A, et al. Prognostic value of karyotypic analysis in children and adults with high-risk acute lymphoblastic leukemia included in the PETHEMA ALL-93 trial. Haematologica. 2002;87:154–166. [PubMed] [Google Scholar]
- 13.Sebban C, Browman GP, Lepage E, Fiere D. Prognostic value of early response to chemotherapy assessed by the day 15 bone marrow aspiration in adult acute lymphoblastic leukemia: a prospective analysis of 437 cases and its application for designing induction chemotherapy trials. Leuk Res. 1995;19:861–868. doi: 10.1016/0145-2126(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 14.Ribera JM, Oriol A, Bethencourt C, et al. Comparison of intensive chemotherapy, allogeneic or autologous stem cell transplantation as post-remission treatment for adult patients with high-risk acute lymphoblastic leukemia. Results of the PETHEMA ALL-93 trial. Haematologica. 2005;90:1346–1356. [PubMed] [Google Scholar]
- 15.Lauten M, Moricke A, Beier R, et al. Prediction of outcome by early bone marrow response in childhood acute lymphoblastic leukemia treated in the ALL-BFM 95 trial: differential effects in precursor B-cell and T-cell leukemia. Haematologica. 2012;97:1048–1056. doi: 10.3324/haematol.2011.047613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107:1116–1123. doi: 10.1182/blood-2005-07-2708. [DOI] [PubMed] [Google Scholar]
- 17.Ravandi F, Jorgensen JL, O’Brien SM, et al. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2016;172:392–400. doi: 10.1111/bjh.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel B, Rai L, Buck G, et al. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. Br J Haematol. 2010;148:80–89. doi: 10.1111/j.1365-2141.2009.07941.x. [DOI] [PubMed] [Google Scholar]
- 19.Ribera JM, Oriol A, Morgades M, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol. 2014;32:1595–1604. doi: 10.1200/JCO.2013.52.2425. [DOI] [PubMed] [Google Scholar]
- 20.Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 21.Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122:1214–1221. doi: 10.1182/blood-2012-11-466482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 23.Schrappe M. Detection and management of minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2014;2014:244–249. doi: 10.1182/asheducation-2014.1.244. [DOI] [PubMed] [Google Scholar]
- 24.van Dongen JJ, van der Velden VH, Bruggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. 2015;125:3996–4009. doi: 10.1182/blood-2015-03-580027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campana D. Minimal residual disease monitoring in childhood acute lymphoblastic leukemia. Curr Opin Hematol. 2012;19:313–318. doi: 10.1097/MOH.0b013e3283543d5c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.