Abstract
Some biological systems detect the rate of change in a stimulus rather than the stimulus itself only. We suggest that the immune system works in this way. According to the discontinuity theory of immunity, the immune system responds to sudden changes in antigenic stimulation and is rendered tolerant by slow or continuous stimulation. This basic principle, which is supported by recent data on immune checkpoints in viral infections, cancers, and allergies, can be seen as a unifying framework for diverse immune responses.
The discontinuity theory of immunity in the context of change-detection systems in biology
Imagine a toad sitting in the close vicinity of a fly. Whilst the fly remains still, the toad does not move, but as soon as the fly moves, the toad snaps at it. This natural pattern of behavior, which can be observed directly, indicates that the toad detects rapid changes in a visual stimulus, rather than the stimulus itself only. Many biological systems behave in a similar fashion, not perceiving the stimulus itself only, but detecting the rate of change in the stimulus, a feature related to Weber’s law (1, 2). According to this law, which was first proposed in 1905, our visual perception of a given stimulus depends not on the absolute level of the stimulus, but on its magnitude relative to background levels. It has recently been shown that several cellular signaling pathways, including the ERK2 response to EGF and the influence of β-catenin on Wnt signaling, involve the detection of changes in a stimulus (1).
Here, we argue that change-detection may also be a basic organizing principle underlying the workings of the immune system, constituting a pillar of the “discontinuity theory of immunity” (3). According to this theory, the immune system detects antigenic variations (Δa) over time (Δt): the Δa/Δt derivative. Consequently, the immune system can detect sudden changes, leading to the triggering of effector immune responses. Such a concept has been pioneered for adaptive immune cells by Grossman and Paul for decades (4, 5). The discontinuity theory of immunity applies to both innate and adaptive immunity. Importantly, the immune system can also adapt to slow or long-lasting modifications in the host, which it then treats as a new reference point. Thus, effector immune responses are induced by sudden antigenic changes, whereas chronic exposure to antigens leads to immune tolerance (3). High, but continuous antigen concentrations and low levels of variation of antigen concentration (Δa/Δt → 0) would, therefore, be predicted to lead to immune tolerance (Table 1). We explore the effects of chronicity, for which large amounts of data have recently been published, in more detail here.
Table 1. Weak or chronic stimulations lead to a tolerogenic immune response while sudden modifications lead to an effector immune response.
On the graphs, the blue line represents antigen concentration and the red line represents the immune response.
| Type of stimulation | Graph | Examples | Immune response | |
|---|---|---|---|---|
| Continuous | Weak stimulation and/or weak costimulation | 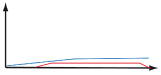 |
Anergy | Tolerogenic |
| Chronic stimulation |  |
- Intrinsic (e.g., CTLA-4 or PD-1) - Extrinsic (e.g., TRegs) |
||
| Discontinuous | Sudden appearance of a pathogen | 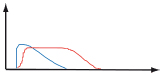 |
- Recognition of patterns - Recognition of the absence of a pattern - Recognition of tissue damage - Recognition of functional modifications |
Effector |
| Sudden appearance of tumor antigens | ||||
| Sudden metabolic modification | ||||
Recent experimental evidence supporting the discontinuity theory of immunity
The timing of exposure to a given immune stimulus, such as an antigen, plays a major role in the triggering of the immune response (4–8), together with other factors, such as the dose and molecular structure of the antigen. The discontinuity theory stems from the demonstration that chronic exposure to immune stimuli can desensitize both adaptive (9) and innate immune cells (10, 11). This theory is supported by an array of recent experimental evidence in three pathological contexts: viral infections, cancers, and allergies. In all these contexts, the immune effector response declines when the immune system is confronted with the chronic presence of an immune stimulus. The tolerogenic mechanisms at work in cases of persistent exposure of the immune system to an antigen have been dissected at length by many groups and may be intrinsic or extrinsic to the immune cell (Table 1). Intrinsic mechanisms include cell surface receptor internalization (e.g., antigen receptors expressed by T and B lymphocytes are internalized upon antigen recognition) and the degradation of key downstream signaling elements. Other cell intrinsic regulatory circuits include the engagement of co-inhibitory receptors that dampen cell activation. These receptors include immune receptor tyrosine-based inhibition motif (ITIM)-containing cell surface molecules such as Killer cell Ig-like receptors (KIRs), TIGIT, and CD47, and so-called immune checkpoints such as PD-1, CTLA-4, Lag-3, and Tim-3 (12). Although these receptors can be expressed in many different immunological situations (e.g., PD-1 is expressed on functional follicular T helper cells), they are often induced upon sustained cell activation, illustrating a molecular mechanism by which chronic stimulation leads to the desensitization of immune effectors (9). Tolerogenic mechanisms can also be cell-extrinsic, as exemplified by the production of immunoregulatory cytokines, such as IL-10 or TGF-β, by regulatory T cells induced by persistent antigenic stimulation (13). Exposure to tissue autoantigens leads to the activation of self-reactive regulatory T cells. These cells persist in tissues and suppress autoimmune responses (14). Continuous T-cell receptor signaling is critical for both the development of regulatory T cells in the thymus and for the maintenance and function of these cells in the periphery (15).
In chronic viral infections, the immune system often deteriorates to “exhaustion” (16), a state in which T lymphocytes are first primed and develop effector functions, but then prolonged stimulation leads to a gradual loss of function over time (13). In chronic lymphocytic choriomeningitis virus infections in mice, cytotoxic lymphocytes adapt to the persistent infection via a positive feedback pathway between PD-1 and the transcription factor FoxO1, leading to their desensitization (17).
This concept has also been clinically validated for tumors, in which chronic exposure to tumors also induces exhaustion (18). Immune checkpoints prevent overt activation of the immune system and protect normal tissues from collateral damage. Tumor cells often evade immune attack, as the ligands for these inhibitory receptors are expressed by the tumor cells themselves or by cells in the tumor microenvironment. There has been a recent paradigm shift in cancer immunotherapy, with the introduction of treatments based on antibodies called immune checkpoint inhibitors, which block the interaction between checkpoints and their ligands. These inhibitors make immune cell activation possible, by blocking a dominant inhibitory mechanism. Immune checkpoint inhibitors have been shown to be effective and to have manageable safety profiles for several types of cancer (19). The demonstration that such treatments can abolish immunological tolerance to the tumor indicates that it is possible to re-introduce discontinuity in tumor antigen perception.
Another confirmation of the induction of immune tolerance by chronic antigen exposure has been provided by advances in allergen immunotherapy over the last decade, with some degree of desensitization being induced by repeated allergen injections in patients. This method has proved effective for allergic rhinitis, allergic asthma, and food allergies (20).
In many situations, immune tolerance is thus induced by interactions with antigens to which the body is chronically exposed, consistent with the discontinuity theory of immunity (Table 1). However, no induction of immune tolerance is observed in chronic inflammatory disorders. Immunological stimulation is thought to be persistent in these conditions, but this has yet to be demonstrated. There may be oscillations in the exposure of the immune system to immunological stimuli, preventing the induction of tolerance (3). Further studies are required to determine whether chronic inflammatory conditions can develop when the immune system is faced with the intermittent presentation of a persistent stimulus.
The discontinuity theory of immunity can also serve as the basis of innovative therapeutic strategies. This theory is consistent with the occurrence of flare-ups in several autoimmune disorders, and suggests that these flare-ups may reflect intermittent interactions between immune receptors and autoantigens. It also suggests that desensitization strategies might be useful in the treatment of autoimmune diseases, to induce tolerance by subjecting the immune system to continuous exposure to the autoimmune stimulus. These predictions generate novel, testable research hypotheses.
A unifying framework for immunology
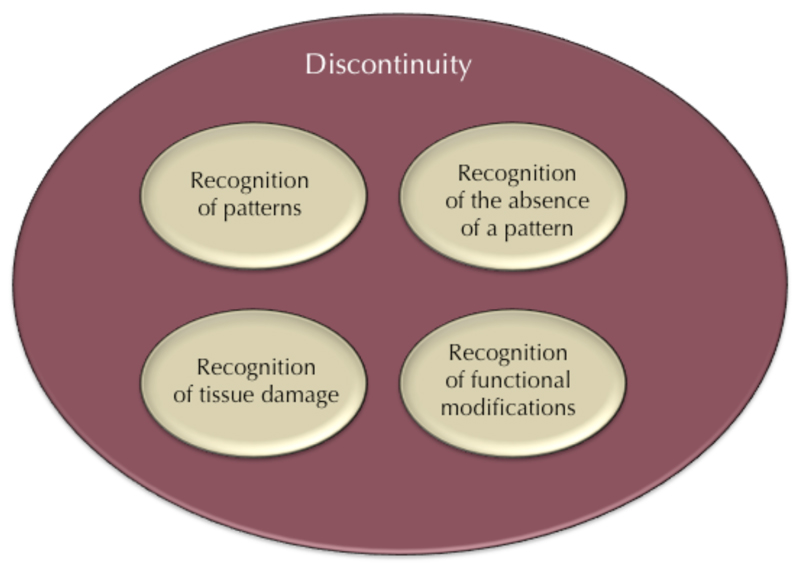
The discontinuity theory of immunity is based on two founding principles: the important role of time in the immune response, and the frequent detection, by the immune system, of modifications internal to the host. Indeed, the triggering of an immune response is a complex process based on the integration of many different signals, including antigen structure and the context in which recognition occurs (21). Several different modes of immune recognition can, thus, be distinguished (21, 22): recognition of patterns (either intracellularly or extracellularly, by pattern recognition receptors), recognition of the absence of a pattern (“missing self” as with NK cells, macrophages or complement), recognition of tissue damage, or recognition of functional modifications (as exemplified by the “guard hypothesis” in plants (23)).
We suggest that all these modes of recognition are instances of the general rule that the immune system senses sudden changes in the organism (Figure 1). It is now well-established that, in addition to their classical role in the sensing of “nonself” or “danger”, many immune cells detect internal disturbances. This is the case for many “innate” immune cells, including NK cells and macrophages (3), as well as “adaptive” immune cells (5). More generally, the detection of structural modifications to the host seems to have played a major role in the evolution of immune systems. Many organisms seem to make use of such an economical system for detecting internal change rather than a more costly system based on the detection of structural features of pathogens. Several examples of “effector-triggered immunity” have now been described in studies of the so-called “guard hypothesis” in plants (23), and in invertebrates (24). Primitive chordates also have NK-like systems (detecting missing or modified self) (25), lending support to speculations that the immune recognition of major changes in the host has been a driving force in the evolution of the immune system, and that the perception of missing or modified self in vertebrates is a remnant of this evolutionarily ancient system. Many other recent data tend to the same conclusion, including, for example, the demonstrations that an endogenous caspase-11 ligand elicits the release of interleukin-1 from living dendritic cells (26), that an innate pathway activated by disturbance of the mucus layer exerts an antiviral effect preceding the action of interferon (27), and that a guard-like mechanism of pyrin regulation exists in human (28).
Figure 1.
The discontinuity theory provides a unifying framework for known immunological mechanisms. The mechanisms underlying the recognition of patterns, absence of a pattern, tissue damage, and functional modifications have been considered separately, but the discontinuity theory explains these mechanisms as instances of the more general rule that immune systems have been selected by evolution to respond to sudden modifications within the host.
Immune mechanisms of change-detection are well known, and some were first described decades ago. However, the processes underlying the recognition of patterns, an absence of pattern, tissue damage, and functional modifications have generally been considered to be separate phenomena, with different explanations. The discontinuity theory of immunity encompasses all these different modes of recognition in a single explanatory framework, according to which immune systems have been selected during evolution for their capacity to sense sudden modifications in the body. The question whether mechanisms of immune recognition diverged from a common mechanism of change recognition or many mechanisms of immune recognition converged on a common strategy of change recognition remains open.
Herbert Feigl (29) once pointed out that “the aim of scientific explanation throughout the ages has been unification, i.e. the comprehending of a maximum of facts and regularities in terms of a minimum of theoretical concepts and assumptions.” In a similar vein, the discontinuity theory of immunity seeks to provide a single, simple explanation for many different immunological phenomena.
One key lesson to be drawn from the framework suggested here is the need for more detailed studies of the time course of the immune response. Along this line, a pivotal element in the organization of the immune system resides in its capacity to adapt to slow or long-lasting modifications in the host, which it then treats as a new reference point. The duration of the stimulation needed for inducing immune responses must be thus compared to the time required for the immune system to adapt to the same stimulation. An illustration of the importance of these kinetics parameters resides in the killing of MHC class I-negative targets by NK cells raised in a MHC class I-positive environment, and the incapacity of NK cells raised in a MHC class I-negative environment to kill the same targets. Adoptive transfer experiments indicate that it takes several days to a whole population of NK cells to adapt to the chronic loss of MHC Class I in their environment, although it takes a few hours for a whole population of NK cells to kill MHC Class I-negative cells in vivo (30, 31). Quantitative investigations on the impact of time and antigenic dose would be facilitated by much needed collaboration between immunologists and biomathematicians, extending beyond traditional disciplinary boundaries (32).
Key bullet points.
Like many other biological systems, the immune system can be seen as a change-detection system.
The immune system detects sudden modifications, but is rendered tolerant to slow changes or chronic antigen expression.
This view is supported by many recent data and opens up new avenues for research and treatment.
Further studies of the timing of the immune response, at the interface between immunology and biomathematics, are required.
Acknowledgments
T.P.’s work is supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme - grant agreement n° 637647 (IDEM Starting Grant) and by the IDEX program of the University of Bordeaux. The E.V. laboratory is supported by the European Research Council (THINK Advanced Grant), the Ligue Nationale contre le Cancer (Equipe Labellisée) and by institutional grants from INSERM, CNRS and Aix-Marseille University to CIML. E.V. is a scholar of the Institut Universitaire de France. E.V. is a co-founder and a shareholder of Innate-Pharma. We thank Sébastien Jaeger for stimulating discussions.
References
- 1.Casci T. Signalling: Sensing a change. Nat Rev Genet. 2010;11:92–93. [Google Scholar]
- 2.Trautmann A. From kinetics and cellular cooperations to cancer immunotherapies. Oncotarget. 2016 doi: 10.18632/oncotarget.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pradeu T, Jaeger S, Vivier E. The speed of change: towards a discontinuity theory of immunity? Nat Rev Immunol. 2013;13:764–769. doi: 10.1038/nri3521. [DOI] [PubMed] [Google Scholar]
- 4.Grossman Z, Paul WE. Adaptive cellular interactions in the immune system: the tunable activation threshold and the significance of subthreshold responses. Proc Natl Acad Sci. 1992;89:10365–10369. doi: 10.1073/pnas.89.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman Z, Paul WE. Dynamic tuning of lymphocytes: physiological basis, mechanisms, and function. Annu Rev Immunol. 2015;33:677–713. doi: 10.1146/annurev-immunol-032712-100027. [DOI] [PubMed] [Google Scholar]
- 6.Johansen P, et al. Antigen kinetics determines immune reactivity. Proc Natl Acad Sci. 2008;105:5189–5194. doi: 10.1073/pnas.0706296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han S, Asoyan A, Rabenstein H, Nakano N, Obst R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc Natl Acad Sci. 2010;107:20453–20458. doi: 10.1073/pnas.1008437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumjohann D, et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 10.Tripathy SK, et al. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205:1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, et al. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc Natl Acad Sci. 2007;104:13744–13749. doi: 10.1073/pnas.0702881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Rosenblum MD, et al. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vahl JC, et al. Continuous T Cell Receptor Signals Maintain a Functional Regulatory T Cell Pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staron MM, et al. The Transcription Factor FoxO1 Sustains Expression of the Inhibitory Receptor PD-1 and Survival of Antiviral CD8+ T Cells during Chronic Infection. Immunity. 2014;41:802–814. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topalian SL, Drake CG, Pardoll DM. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jutel M, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–568. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chovatiya R, Medzhitov R. Stress, Inflammation, and Defense of Homeostasis. Mol Cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Der Biezen EA, Jones JDG. Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem Sci. 1998;23:454–456. doi: 10.1016/s0968-0004(98)01311-5. [DOI] [PubMed] [Google Scholar]
- 24.Stuart LM, Paquette N, Boyer L. Effector-triggered versus pattern-triggered immunity: how animals sense pathogens. Nat Rev Immunol. 2013;13:199–206. doi: 10.1038/nri3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khalturin K, Becker M, Rinkevich B, Bosch TCG. Urochordates and the origin of natural killer cells: Identification of a CD94/NKR-P1-related receptor in blood cells of Botryllus. Proc Natl Acad Sci. 2003;100:622–627. doi: 10.1073/pnas.0234104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanoni I, et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352:1232–1236. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iversen MB, et al. An innate antiviral pathway acting before interferons at epithelial surfaces. Nat Immunol. 2016;17:150–158. doi: 10.1038/ni.3319. [DOI] [PubMed] [Google Scholar]
- 28.Masters SL, et al. Sci Transl Med. doi: 10.1126/scitranslmed.aaf1471. in press. [DOI] [PubMed] [Google Scholar]
- 29.Feigl H. In: Minnesota Studies in the Philosophy of Science. Radner M, Winokur S, editors. IV. University of Minnesota Press; Minneapolis: 1970. pp. 3–15. [Google Scholar]
- 30.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207:2065–2072. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott JM, Wahle JA, Yokoyama WM. MHC class I–deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I–sufficient environment. J Exp Med. 2010;207:2073–2079. doi: 10.1084/jem.20100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.May RM. Uses and Abuses of Mathematics in Biology. Science. 2004;303:790–793. doi: 10.1126/science.1094442. [DOI] [PubMed] [Google Scholar]