Abstract
Human studies have shown that heterotopic nociceptive conditioning stimulation (HNCS) applied to a given body location reduce the percept and brain responses elicited by noxious test stimuli delivered at a remote body location. It remains unclear to what extent this effect of HNCS relies on the spinal-bulbar-spinal loop mediating the effect of diffuse noxious inhibitory controls (DNIC) described in animals, and/or on top-down cortical mechanisms modulating nociception. Importantly, some studies have examined the effects of HNCS on the brain responses to nociceptive input conveyed by Aδ-fibers. In contrast, no studies have explored the effects of HNCS on the responses to selective nociceptive C-fiber input and non-nociceptive Aβ fiber input. In this study, we measured the intensity of perception and event related potentials (ERPs) to stimuli activating Aδ-, C- and Aβ-fibers, before, during and after HNCS, obtained by immersing one foot in painful cold water. We observed that (i) the perceived intensity of nociceptive Aδ- and C- stimuli was reduced during HNCS, (ii) the ERPs elicited by Aδ- and Aβ-, and C- stimuli were also reduced during HNCS. Importantly, because Aβ-ERPs are related to primary afferents that ascend directly through the dorsal columns without being relayed at spinal level, the modulation of these responses may not be explained by an influence of descending projections modulating the transmission of nociceptive input at spinal level. Therefore, our results indicate that, in humans, HNCS should be used with caution as a direct measure of DNIC-related mechanisms.
Keywords: Nociception, pain, cold pressor test, CO2 laser, laser-evoked potentials, event related potentials (ERPs), heterotopic noxious conditioning stimuli, HNCS, DNIC
Introduction
Pain can be modulated by inhibitory and facilitatory mechanisms acting both at spinal and supra-spinal levels (Millan, 2002; Porreca et al., 2002). These modulatory mechanisms are increasingly considered to play an important role in the individual susceptibility to pain.
In animals, modulatory mechanism can involve a spinal-bulbar-spinal loop in which noxious stimuli applied to a given body part inhibit, at the level of the dorsal horn, transmission of nociceptive input originating from all other body parts (see (Le Bars, 2002) for a review). The neural substrates of this mechanism, termed “diffuse noxious inhibitory control" (DNIC), remain yet to be fully understood. Lesions of the periaqueductal grey (PAG) do not modify DNIC. In contrast, the mechanism is disrupted by lesions of the subnucleus reticularis dorsalis in the caudal medulla (Bouhassira et al., 1992; Villanueva & Le Bars, 1995). Recent data also suggest that, contrary to what has been previosly proposed, lesions of the rostral ventromedial medulla (RVM), including the nucleus raphe magnus, can affect DNIC (Chebbi et al., 2014). Furthermore, the parabrachial nucleus (PB) would also be involved in triggering DNIC (Lapirot et al., 2009). Finally, a role of descending dopaminergic controls has been recently identified (Lapirot et al., 2011).
Human studies have shown that nociceptive conditioning stimuli applied to a given body location reduce the percept and brain responses elicited by test stimuli delivered at a remote location (De Broucker et al., 1990; Willer et al., 1990; Bouhassira et al., 1993). Furthermore, human studies have shown that heterotopic noxious conditioning stimulation (HNCS) inhibits the spinal RIII reflex, suggesting that at least part of the effect of HNCS on pain perception may be due to descending inhibitory influences on spinal transmission (Willer et al., 1984; Roby-Brami et al., 1987; Willer et al., 1989; De Broucker et al., 1990; Danziger et al., 1998; Scheuren et al., 2014). For this reason, the effects of HNCS have often been considered as a direct human correlate for DNIC (see (Pud et al., 2009)).
Importantly, descending modulatory effects are thought to operate predominantly at the level of Wide Dynamic Range (WDR) neurons, onto which nociceptive C-fibers, Aδ-fibers and Aβ-fibers converge. Using laser-evoked potentials, studies have demonstrated that HNCS can reduce the magnitude of the brain responses to nociceptive input conveyed by Aδ-fibers (Plaghki et al., 1994; Watanabe et al., 1996).
So far, no studies have examined whether HNCS affects the brain responses to inputs conveyed selectively by nociceptive C-fibers. One of the reasons for this lack of knowledge is that selective activation of C-fibers is difficult to achieve. However we (Jankovski et al., 2013; van den Broeke & Mouraux, 2014a) and other groups (Magerl et al., 1999) have shown that laser-evoked brain responses and percepts (Churyukanov et al., 2012) related to the selective activation of C-fibers can be obtained using a temperature-controlled infrared CO2 laser stimulator to selectively activate heat-sensitive C-fiber afferents that have a lower thermal activation threshold than Aδ-fiber afferents (Jankovski et al., 2013; van den Broeke & Mouraux, 2014a). Therefore, the first objective of our study was to assess the effects of HNCS on the perception and brain responses to selective C-fiber input.
Furthermore, no study has yet examined whether HNCS also affects the perception and event-related potentials (ERPs) to sensory input conveyed by non-nociceptive Aβ-fibers (Somatosensory evoked potentials, SEPs). Considering that these brain responses are related to Aβ-fiber primary afferents which are not relayed in the spinal cord but ascend directly through the dorsal columns to reach second-order neurons in the dorsal column nuclei, one would expect that these responses would not be under the influence of descending spinal modulatory mechanisms. Therefore, the second objective of our study was to assess the effects of HNCS on the perception and brain responses to Aβ-fiber input, in order to examine whether the effects of HNCS can be attributed to changes occurring exclusively at spinal level and/or to changes also occurring at supra-spinal level.
Methods
Experiment 1
Participants
Experiment 1 was performed on eight healthy volunteers recruited among students and staff of the Université catholique de Louvain (3 female and 5 male; all right-handed, aged 23 to 35 years). The phase of the menstrual cycle in the main experiment was not taken into consideration in the participating women, although previous studies have suggested that during the ovulatory phase, HNCS may trigger a greater modulation of nociceptive responses (Tousignant-Laflamme & Marchand, 2009). Participants had no history of neurological, psychiatric, dermatological or chronic pain disorders, and no recent history of psychotropic or analgesic drug use. During a preliminary session, volunteers were given a fully detailed explanation concerning the experimental procedures and were familiarized with the experimental setup and task, the nociceptive stimuli and the rating procedures. Participants were comfortably seated in a chair with forearms resting on the armrests. Written informed consent was obtained from all participants. The study was approved by the Ethics Committee of the Université catholique de Louvain (B40320096449).
Experimental design
Each participant underwent three blocks: before, during and after the HNCS conditioning procedure. Within each block, behavioural (reaction times, intensity of perception) and electrophysiological (event-related brain potentials) responses to 20 infrared CO2 laser stimuli activating nociceptive C- and Aδ-fibers free nerve endings (Aδ-stimulus, C-stimulus) and 20 transcutaneous electrical stimuli activating non-nociceptive Aβ-fibers (Aβ-stimulus) were recorded. The laser stimuli were applied to the right hand dorsum. The electrical stimuli were applied over the superficial branch of the right radial nerve. Within each block, the two types of stimuli were presented in separate runs, whose order was randomized across participants.
Thermal CO2 laser stimulation (Aδ-stimulus and C-stimulus)
Thermal stimuli were applied to the right hand dorsum using a CO2 laser in which the power of the laser beam is regulated continuously during stimulation using an online measurement of skin temperature optically in line with the laser beam. This feedback control of power output allowed defining specific skin temperature heating profiles (LSD, SIFEC, Belgium). Because the CO2 laser has a wavelength of 10.6 µm, 99% of the delivered energy was confined within the most superficial layer of the skin (<100 µm beneath the skin surface), i.e. where most of the free nerve endings of nociceptors terminate (Meyer et al., 1976). The laser beam was conducted through a 10 m optical fiber. An optical fiber vibrator was used to introduce a small amount of variable lateral or angular displacement in the fiber, altering internal reflections and, thereby, resulting in a homogeneous spatial distribution of power within the stimulated area. At the end of the fiber, optics collimated the beam in a 6 mm beam diameter at target site.
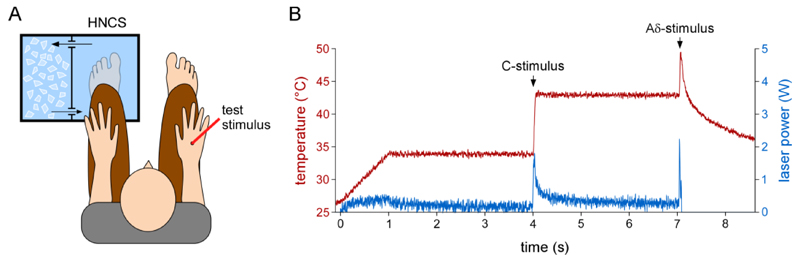
An approach similar to Magerl et al. (Magerl et al., 1999) was used to temporally dissociate the activation of nociceptive C-fibers and Aδ-fibers and, thereby, allow the recording of behavioral and electrophysiological responses related to the activation of each fiber type. The procedure is represented in Figure 1B. First, skin temperature was brought to 34°C using a 1-second heating ramp and maintained at that temperature for 3 seconds (baseline skin temperature). Second, the skin temperature was increased to 43°C in 10 ms. In a previous study, we showed that such temperatures are above the thermal activation threshold of C-fibers but below the thermal activation threshold of Aδ-fibers (Churyukanov et al., 2012). Hence, this first step increase allowed activating C-fibers selectively (C-stimulus). Finally, after maintaining the skin temperature at 43°C for 3 seconds, the skin temperature was increased to 49°C in 10 ms, and maintained at that temperature for 40 ms before interrupting laser power output. This second target temperature activated Aδ-fibers. After each trial, the target of the laser was displaced to a random position on the hand dorsum, in order to avoid nociceptor sensitization and/or habituation. The inter-trial interval varied randomly between 5-10 seconds.
Figure 1. Experimental setup.
Left panel (A). Twenty thermal stimuli activating successively C- and Aδ-fibers and twenty electrical stimuli activating Aβ fibers were applied on the right hand dorsum before, during and after applying a heterotopic noxious conditioning stimulus (HNCS) to the left foot (immersion in a 5°C circulating water bath). The order of administration of the test stimuli was counterbalanced across participants. Right panel (B) details of how activation of C- and Aδ-fibers was obtained with the laser stimulator. The ordinate represents skin surface temperature, the abscissa represents time in seconds.
Transcutaneous electrical stimulation (Aβ-stimulus)
Transcutaneous electrical stimuli consisted of constant current square-wave electrical pulses (0.5 ms duration; DS7A, Digitimer Ltd, UK) delivered through a pair of feltpad skin electrodes (0.5 cm diameter, 2 cm inter-electrode distance) placed at the right wrist, over the superficial branch of the radial nerve. Prior to the experimental session, the intensity of the electrical stimulus was set to twice the detection threshold (2.30 ± 0.80 mA). The inter-trial interval varied randomly between 5-10 seconds.
Heterotopic noxious conditioning stimulation (HNCS)
During the first block (before HNCS), participants immersed their left foot into a water bath containing water at 30.9 ± 0.8°C, that was perceived as neither warm nor cold. During the second block (during HNCS), participants immersed their foot in another water bath of circulating water whose temperature was set to 5°C (measured at 5.1 ± 0.3°C at the beginning of the block and 5.7 ± 0.3°C at the end of the block). This procedure elicited a perception of pain after about 10-15 sec, which peaked after about 30-45 sec and then stabilized. The intensity of the elicited pain was just below the limit of tolerance for most subjects. During the third block (after HNCS), participants re-immersed the foot in the water bath of the first block (temperature 30.0 ± 0.8°C). In each block, recordings began 30-seconds after immersing the foot in the water bath. A 5-minutes rest period separated each block. The foot was dried and covered with a towel during the rest period.
Behavioral measures
Reaction times
During nociceptive stimulation, participants were asked to respond as quickly as possible by pressing a button held in the left hand when perceiving the first (43°C) and second (49°C) step-increase in skin temperature, corresponding to the C-stimulus and the Aδ-stimulus, respectively. Reaction times were measured relative to the onset of the step-increase. Based on the large difference between the conduction velocities of Aδ- and C- fibers, responses <650 ms were considered related to the detection of Aδ-fiber input, whereas responses ≥ 650 ms were considered related to the detection of C-fiber input (Churyukanov et al., 2012). Only trials with a reaction time between 650-2000 ms were included for the analysis of C-fiber ERPs. This represented 85 ±3 % of the total number of trials. Only trials with a reaction time <650 ms were included for the analysis of Aδ-fiber ERPs, representing 91 ±5% of the total number of trials. During non-nociceptive stimulation, participants were also asked to respond as quickly as possible by pressing the same button used to detect nociceptive stimuli.
Intensity of perception
After each stimulus, participants were asked to rate the intensity of the perception elicited by C-, Aδ- and Aβ-fiber stimuli using a numerical rating scale (NRS) presented at eye level, approximately 1.5 m in front of the participant. The extremities of the scale were annotated with the words “no detection” (NRS = 0) and “maximum pain” (NRS = 100), respectively. An anchor at the middle of the scale (NRS = 50) defined the borderline between the non-painful and painful domains of perception. Participants provided a verbal report of the perceived intensity of perception. Only ratings to perceived stimuli (>0) were included in the analysis.
Electrophysiological measures
The EEG was recorded using 64 Ag-AgCl electrodes placed on the scalp according to the international 10-20 system (Waveguard64 cap, Cephalon A/S, Denmark). Scalp signals were recorded using an average reference. Impedance was kept below 10 kΩ. Ocular movements and eye blinks were recorded using two surface electrodes placed at the upper-left and lower-right sides of the right eye. All signals were amplified and digitized at a 1 kHz sampling rate (64-channel ASA-LAB EEG/ERP system, Advanced Neuro Technologies, The Netherlands).
Continuous EEG signals were band-pass filtered (0.5-30 Hz) using a Butterworth zero-phase filter and segmented into 3 s epochs ranging from -1 to +2 s relative to the onset of the stimulus (C-stimulus, Aδ-stimulus and Aβ-stimulus). Artifacts produced by eye blinks or eye movements were subtracted using a validated method based on an Independent Component Analysis (Jung et al., 2000). Baseline correction was performed by subtracting the average voltage of the prestimulus interval ranging from -1 to 0 s. In addition, epochs with amplitude values exceeding ±100 µV (i.e., epochs likely to be contaminated by an artifact) were rejected.
For each type of stimulus (C-stimulus, Aδ-stimulus, Aβ-stimulus), separate average waveforms were computed for each block (before, during and after), resulting in three average waveforms for the C-stimulus (C-LEP), three average waveforms for the Aδ-stimulus (Aδ-LEP) and three average waveforms for the Aβ-stimulus (Aβ-SEP). In the C-LEP waveforms, no significant signal deflection could be identified, and the signals were not further analyzed using this approach (see following paragraphs). In the Aδ-LEP waveforms, three distinct ERP components were identified as follows. The Aδ-N2 and Aδ-P2 waves were identified at electrode Cz (average reference) as the most negative and positive deflections occurring between 100-600 ms after stimulus onset or step-increase in skin temperature. The Aδ-N1 wave was identified at electrode C3 re-referenced to electrode Fz, as the first negative deflection preceding the Aδ-N2 wave, which appears as a positive wave in this montage. In the Aβ-ERP waveforms, three distinct ERP components were identified as follows. The Aβ-N2 and Aβ-P2 waves were identified at electrode Cz (average reference) as the most negative and positive deflections occurring between 100-350 ms after stimulus onset. The Aβ-N1 was identified at electrode C3 re-referenced to electrode Fz and defined as the first negative deflection preceding the Aβ-N2.
Experiment 2
Considering that we could not obtain reliable C-fiber responses with the experimental paradigm used in experiment 1, we conducted a second experiment in which we tested selectively the effects of HNCS on C-LEPs. This experiment was performed on 8 healthy volunteers recruited among students and staff of the Université catholique de Louvain (2 female and 6 male; one left handed, aged 22 to 37 years). We recruited mainly men to avoid potentially confounding effects of the phase of the menstrual cycle. Participants had no history of neurological, psychiatric, dermatological or chronic pain disorders, and no recent history of psychotropic or analgesic drug use. During a preliminary session, volunteers were given a fully detailed explanation concerning the experimental procedures. Participants were comfortably seated in a chair with forearms resting on the armrests. Written informed consent was obtained from all participants. The study was approved by the Ethics Committee of the Université catholique de Louvain.
Experimental design
As in experiment 1, each participant underwent three blocks: before HNCS, during HNCS, and after HNCS. Water temperature was 30.5 ± 0.9°C before HNCS, 5.9 ± 0.5 °C during HNCS and 30.3 ± 0.8°C after HNCS.
Thermal CO2 laser stimulation (C-stimulus)
Thermal stimuli were applied using the same device as in experiment 1. The laser beam was 6 mm. The stimuli consisted in 100 ms of radiant heat, of which 10 ms corresponded to the rapid heating ramp during which the skin was brought to the target temperature, and 90 ms corresponded to the plateau of the target temperature. In order to increase the number of trials in which the thermal stimulus activated C-fibers selectively, we used the approach recently proposed by van den Broeke and Mouraux (van den Broeke & Mouraux, 2014a). At the beginning of the experiment, the thermal detection threshold of Aδ- and C-fibers was determined using two staircase algorithms. Reactions times >650 ms were considered compatible with the detection of input conveyed by C-fibers, and reaction times ≤650 ms were considered compatible with the detection of input conveyed by faster Aδ-fibers (Churyukanov et al., 2012). Participants were asked to respond as soon as they perceived a stimulus. The staircase procedure used to estimate the C-fiber thermal detection threshold started at 41°C. The temperature was then increased by 1°C when the stimulus was not detected, and decreased by 1°C when the stimulus was detected. The staircase procedure used to estimate the Aδ-fiber thermal detection threshold started at 46°C. The temperature was increased by 1°C when the stimulus was detected with a reaction time ≤650 ms, and decreased when the stimulus was detected with a reaction time ≥650 ms. The staircases were interrupted after the occurrence of 3 reversals. The target temperature of the test stimulus was defined as the average of the C- and Aδ-fibers thresholds. This ensured that the test stimulus was above the threshold of C-fibers, but below the threshold of Aδ-fibers (i.e., that the test stimulus activated C-fibers selectively). In each block, the test stimuli were repeated until we obtained at least 20 trials in which the stimulus was detected with a reaction time >650 ms, with a maximum of 40 trials. After each trial, the target of the laser was displaced to a random position on the hand dorsum, in order to avoid nociceptor sensitization and/or habituation. The inter-trial interval varied randomly between 5-10 seconds.
Intensity of perception
After each stimulus, participants were asked to rate the intensity of the perception elicited by the stimulus using a Numerical Rating Scale (NRS). The extremities were described as “no detection” (NRS = 0) and “maximum pain” (NRS = 100), respectively. An anchor at the middle of the scale (NRS = 50) defined the transition between the non-painful and painful domains of perception. Such as in experiment 1, only ratings to perceived stimuli (>0) were included in the analysis.
Electrophysiological measures
Previous studies showed that N2 and P2 peaks of the C-LEP are maximal over the midline centro-parietal electrodes. Therefore, we recorded the EEG using 5 channels (Cz, Cpz, Pz, M1, M2) of a 64 Ag-AgCl cap (Waveguard64 cap, Cephalon A/S, Denmark). Impedance was kept below 10 kΩ. Ocular movements and eye blinks were recorded using two surface electrodes placed at the upper-left and lower-right sides of the right eye. All signals were amplified and digitized at a 4 kHz sampling rate (64-channel ASA-LAB EEG/ERP system, Advanced Neuro Technologies, The Netherlands).
Continuous EEG signals were band-pass filtered (0.5-30 Hz) using a Butterworth zero-phase filter and segmented into 3 s epochs ranging from -1 to +2 s relative to the onset of the stimulus. The Gratton-Coles method was used to correct eye-movements and blinks (Gratton et al., 1983). Contrary to ICA, this method can be applied also with a limited number of channels. Baseline correction was performed by subtracting the average voltage of the prestimulus interval ranging from -1 to 0 s. In addition, epochs with amplitude values exceeding ±70 µV (i.e., epochs likely to be contaminated by an artifact) were rejected. The signal was rereferenced to the mastoids. The maximal peak was observed at Cz in 6 subjects and in Cpz in 2 subjects.
Statistical analyses
To assess possible differential effects of HNCS on the intensity of perception and ERPs elicited by each of the three types of stimuli, we used non-parametric repeated measures Friedman’s tests. Post-hoc analyses with Wilcoxon signed-rank tests were conducted to follow up significant effects. All statistical analyses were performed in SPSS 19 (IBM Armonk, NY).
Results
Experiment 1
Intensity of perception
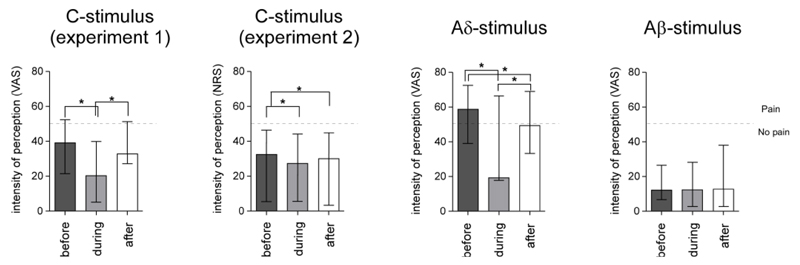
The Friedman test revealed a significant effect of ‘block’ (before, during, and after HNCS) on the perception of Aδ and C stimuli (Aδ-NRS: χ2=14.2, p=0.001; C-NRS χ2=12.2, p=0.002) but not Aβ stimuli (Aδ-NRS: χ2=4.2, p=0.122). Post-hoc analyses with Wilcoxon tests showed that Aδ-NRS ratings were reduced during HNCS as compared to before HNCS (z=-2.5, p=0.012), but also as compared to after HNCS (z=-2.5, p=0.012) (Table 1). Aδ-NRS ratings after HNCS were also significantly reduced as compared to before HNCS (z=-2.3, p=0.017). C-NRS ratings were reduced during HNCS as compared to before HNCS (z=-2.5, p=0.012), and returned to baseline level after HNCS (during vs. after HNCS: z=-2.5, p=0.012; after vs. before HNCS: z=-1.2, p=0.208) (Table 1).
Table 1. Perceived intensity and ERPs magnitude.
Statistical values of the non-parametric analysis on the perceived intensity and on the magnitude of the ERPs. Post-hoc comparisons (Wilcoxon test) are reported when the overall analysis (Friedman Test) achieved the significance threshold.
| Friedman Test | Post-hoc comparisons (Wilcoxon) | ||||
|---|---|---|---|---|---|
| Before vs.During |
During vs. After |
Before vs. After |
|||
| Ratings | |||||
| Aδ | p=0.001 | p=0.012 | p=0.012 | p=0.017 | |
| C (experiment 1) | p=0.002 | p=0.012 | p=0.012 | p=0.208 | |
| C (experiment 2) | p=0.036 | p=0.012 | p=0.208 | p=0.050 | |
| Aβ | n.s. | ||||
| ERPs | |||||
| Aδ–N2 | p=0.008 | p=0.017 | n.s. | p=0.012 | |
| Aδ–P2 | n.s. | ||||
| Aβ–N2 | p=0.021 | n.s. | 0.012 | n.s. | |
| Aβ–P2 | p=0.030 | p=0.017 | p=0.036 | p=0.484 | |
| C-N2 | p=0.034 | p=0.012 | n.s. | p=0.036 | |
| C-P2 | n.s. | ||||
ERPs elicited by C-, Aδ- and Aβ-stimuli
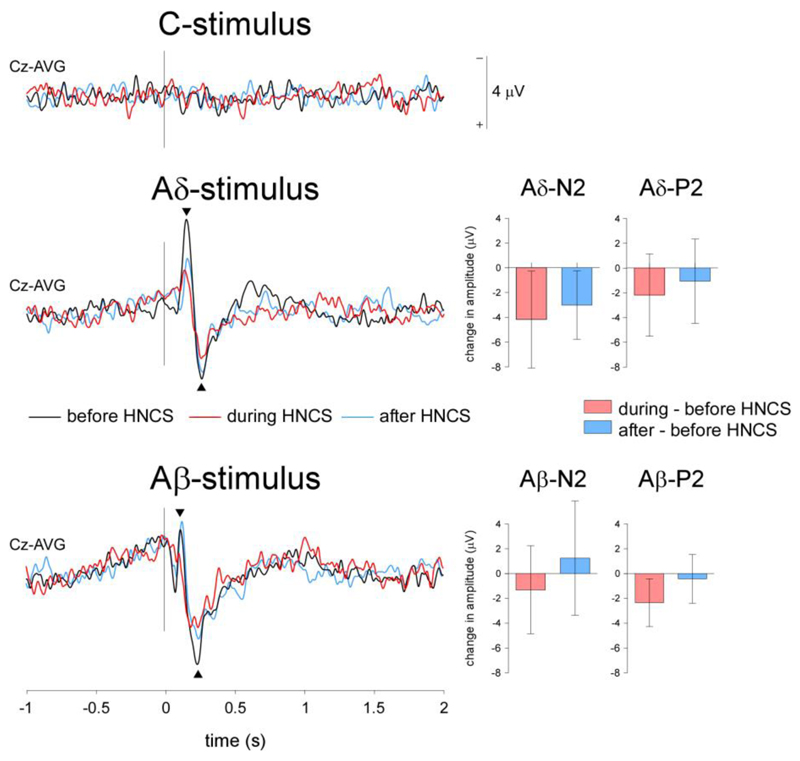
Group-level average waveforms are shown in Figure 3. As explained in Methods, C-stimuli did not elicit consistent ERPs, and were not further analyzed.
Figure 3. Event-related potentials (N2-P2).
Grand-average waveforms of the vertex ERPs elicited by C-, Aδ- and Aβ- stimuli. Note that the magnitude of both the Aδ-N2 and Aβ-P2 evoked responses was reduced during HNCS as compared to before and after HNCS. In contrast, no clear ERP could be identified following C-fiber stimulation. The bar graphs represent the difference in amplitude between conditions. Negative changes in amplitude correspond to a decrease in amplitude as compared to before HNCS.
N2 waves elicited by Aδ- and Aβ-stimuli
There was a significant effect of ‘block’ on the vertex negative peak of the Aδ-ERP (Aδ-N2: χ2=9.7, p=0.008), which was reduced during HNCS as compared to before HNCS (z=-2.3, p=0.017), and remained reduced after HNCS (after vs. during HNCS: z=-0.280, p=0.779, after vs. before HNCS: z=-2.5 p=0.012) (Table 1). The magnitude of the Aβ-N2 was also influenced by ‘block’ (χ2=7.7, p=0.021). However, this effect was mainly driven by an enhancement of the negative peak after HNCS, rather than by a reduction during HNCS (before vs. during HNCS: z=-1.1, p=0.263; before vs. after HNCS: z=-1.2, p=0.208; during vs. after HNCS: z=-2.5, p=0.012).
P2 waves elicited by Aδ- and Aβ-stimuli
No significant effects of ‘block’ were observed for the vertex positive peak of the Aδ-ERP (χ2=3.2, p=0.197). In contrast, the magnitude of the Aβ-P2 was significantly modulated by ‘block’ (χ2=7, p=0.030). Post-hoc comparisons showed that the Aβ-P2 was significantly reduced during HNCS as compared to before HNCS (z=-2.3, p=0.017). Importantly, the amplitude of the Aβ-P2 returned to baseline levels after HNCS (during vs. after HNCS: z=-2.1 p=0.036; after vs. before HNCS: z=-0.7, p=0.484) (Table 1).
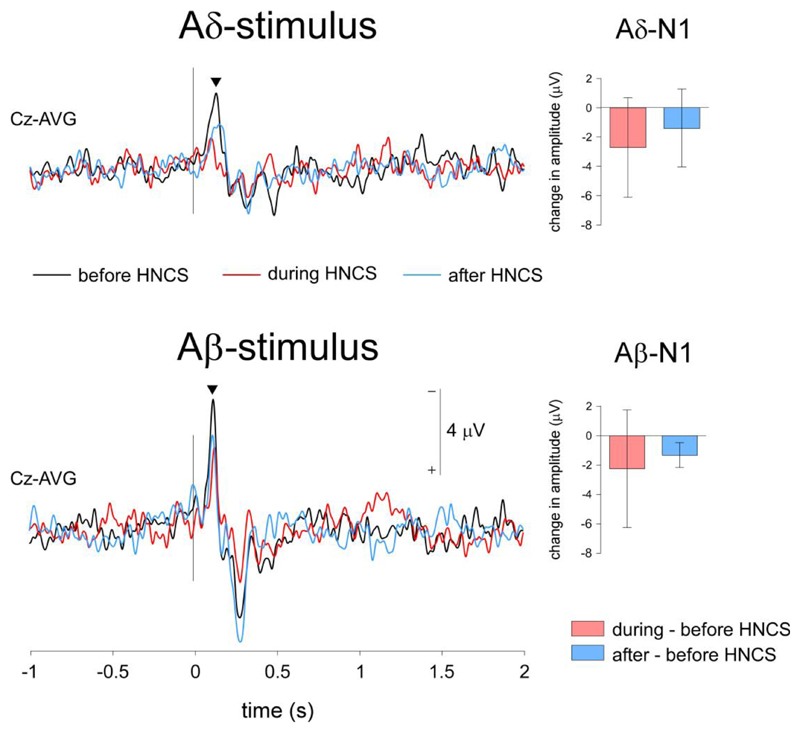
N1 waves elicited by Aδ- and Aβ-stimuli
The amplitude of the Aδ-N1 did not change as a function of ‘block’ (χ2=4, p=0.135). In contrast, the Aβ-N1 was significantly modulated by ‘block’ (χ2=6.7, p=0.034). However, and in line with the effect of ‘block’ on the magnitude of the negative vertex component, this effect was not driven by a significant reduction during HNCS, but by a significant enhancement after HNCS (before vs. during HNCS: z=-1.6, p=0.093; during vs. after HNCS: z=-0.840 p=0.401; before vs. after HNCS: z=-2.5 p=0.012).
Experiment 2
Intensity of perception
Such as in experiment 1, the Friedman test revealed a significant effect of ‘block’ (before, during, and after HNCS) on the perception of C- stimuli (C-NRS χ2=7.750, p=0.021). Post-hoc analyses with Wilcoxon tests showed that C-NRS ratings were reduced during HNCS as compared to before HNCS (z=-2.1, p=0.036), but remained on average lower after HNCS (during vs. after HNCS: z=-1.2, p=0.208; before vs. after HNCS: z=-1.9, p=0.050) (Table 1 and Figure 2).
Figure 2. Perceptual ratings.
Median intensity of perception ratings for C, Aδ, and Aβ stimuli presented before, during and after HNCS. Bars represent the interquartile range. Asterisks indicate significant differences between conditions at p<0.05.
ERPs elicited by C-stimuli
The group-level average waveforms obtained in experiment 2 are shown in Figure 5.
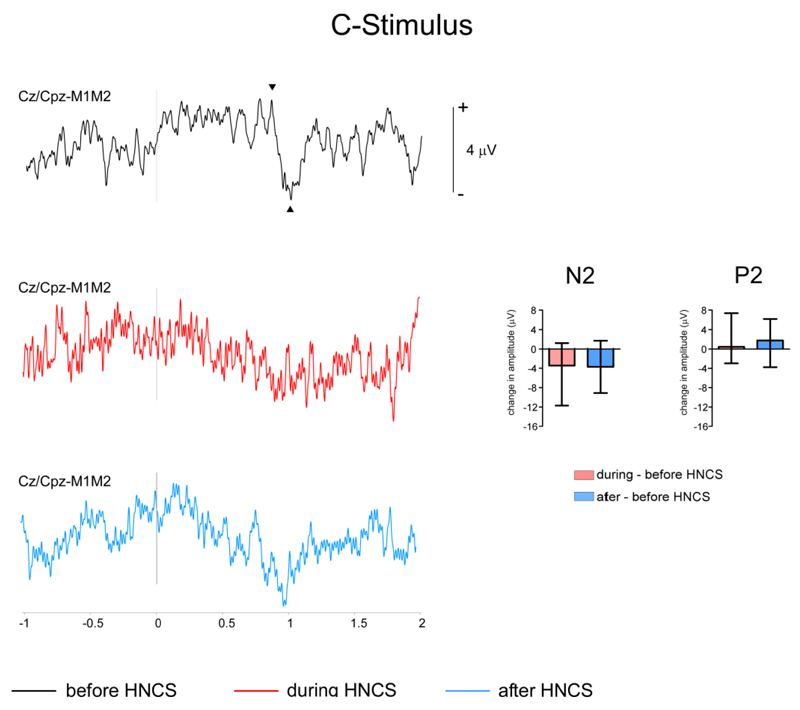
Figure 5. Event-related potentials elicited by selective C-fiber stimulation.
Grand-average waveforms of the vertex ERPs elicited by selective C-stimuli. There was a significant reduction of the vertex negative peak of the C-LEP during and after HNCS as compared to before HNCS. No significant effect of ‘block’ was observed for the vertex positive peak of the C-ERP. The bar graphs represent the difference in amplitude between conditions. Negative changes in amplitude correspond to a decrease in amplitude as compared to before HNCS.
N2 waves elicited by C-stimuli
There was a significant effect of ‘block’ on the vertex negative peak of the C-LEP (C-N2: χ2=6.750, p=0.034), which was reduced during HNCS as compared to before HNCS (z=-2.5, p=0.012), and remained reduced after HNCS (after vs. during HNCS: z=-0.28, p=0.779, after vs. before HNCS: z=-2.1 p=0.036).
P2 waves elicited by C-stimuli
No significant effect of ‘block’ was observed for the vertex positive peak of the C-LEP (χ2=1, p=0.607).
Discussion
In this study, we investigated the effects of HNCS on the perception and ERPs elicited by the stimulation of nociceptive Aδ- and C-fiber afferents, and non-nociceptive Aβ-fiber afferents.
We observed that HNCS reduced the perceived intensity of both Aδ- and C-fiber nociceptive inputs. In addition, HNCS reduced the magnitude of the Aδ-N2, the C-N2, and the Aβ-P2. Importantly, this latter effect could not be attributed only to habituation as the amplitude of the Aβ-P2 decreased during HNCS, but returned to baseline levels after HNCS.
Notably, the ERPs elicited by Aβ-stimuli are primarily related to inputs which are not relayed in the spinal cord but ascend directly through the dorsal columns to reach second-order neurons in the dorsal column nuclei. Therefore, by showing that HNCS modulates the brain responses to non-nociceptive Aβ-fiber inputs, our results indicate that the effects of HNCS should be cautiously solely attributed to descending projections modulating transmission at spinal level.
HNCS modulates perception of C- and Aδ-stimuli
In human studies, it has been shown that HNCS is able to modulate consistently the perceived intensity of noxious test stimuli, irrespective of the nociceptive conditioning procedure and the nature of the nociceptive test stimulus (for reviews see (Pud et al., 2009; van Wijk & Veldhuijzen, 2010)). This effect has been attributed to descending modulatory projections which are thought to operate predominantly at the level of wide dynamic range (WDR) neurons of the dorsal horn, onto which nociceptive C-fibers and Aδ-fibers, as well as non-nociceptive Aβ-fibers converge (Le Bars, 2002).
We observed that HNCS reduced the pain evoked by both nociceptive C-fiber input and nociceptive Aδ-fiber input. Such as in previous studies (Treister et al., 2010), the reduction in perception was stronger during HNCS as compared to after HNCS, indicating that the reduction observed during HNCS was not solely due to perceptual habituation. Indeed, had it been the case, one would have expected the intensity of the percepts elicited after HNCS to be further reduced. Importantly, the finding that the intensity of the percept elicited by Aδ-fiber and C-fiber input remained lower 5-10 minutes after HNCS could be due to perceptual habituation, but also to a long-lasting after-effect of HNCS. Indeed, Willer et al. (Willer et al., 1990) showed that the RIII reflex can be suppressed up to 6-9 minutes after HNCS.
Contrasting with the effects of HNCS on the perception of nociceptive Aδ- and C-fiber inputs, we observed no effect of HNCS on the perception of non-nociceptive Aβ-fiber input. This lack of modulation should be interpreted with caution, as it could be explained by different factors. Indeed, several studies including the present one have observed that experimental manipulations can modulate the brain responses elicited by transcutaneous stimulation of non-nociceptive Aβ-fibers without modulating subjective reports of intensity of perception (Torta et al., 2013; Van Den Broeke & Mouraux, 2014b; Torta et al., 2015). This could be explained by the fact that transcutaneous electrical stimulation elicits a robust percept, identical from trial to trial. Hence, the modulatory effect of HNCS on the processing of Aβ-fiber input could be insufficient to result in a noticeable change of perception.
HNCS modulates ERPs elicited by Aδ, Aβ and C stimuli
In line with previous studies showing a reduction of the vertex negativity (Aδ-N2) after CO2 laser stimulation (Plaghki et al., 1994) and of the N2-P2 vertex component elicited by contact-heat stimulation (Moont et al., 2011), we found that HNCS reduces the magnitude of the ERPs elicited by nociceptive Aδ-fiber input (experiment 1), as well as the ERPs elicited by C-fiber input (experiment 2). However, because this reduction remained present after HNCS, the observed reduction could be explained by a sustained effect of HNCS, or by an unspecific effect of habituation.
Most notably, we observed that HNCS also modulates the ERPs elicited by non-nociceptive Aβ fiber input. Indeed, the Aβ-P2 was significantly more reduced during HNCS as compared to after HNCS, indicating that the reduction of Aβ-P2 amplitude observed during HNCS was not simply due to response habituation (Figure 3).
The finding that HNCS exerts a similar effect on the brain responses to nociceptive inputs conveyed by Aδ- and C-fibers and non-nociceptive input conveyed by Aβ-fibers is important. Indeed, the ERPs elicited non-nociceptive Aβ-fiber input reflect activity, which is not mainly relayed in the dorsal horn of the spinal cord, but ascends predominantly through the dorsal columns to reach second-order neurons in the dorsal column nuclei. Therefore, the effect of HNCS on the brain responses to Aβ-fiber input may not be explained by mechanisms involving descending spinal modulatory projections, in particular, the spinal-bulbar-spinal loop underlying DNIC. It has to be considered that WDR neurons of the dorsal horn also receive input from non-nociceptive Aβ-fiber afferents, and that the ascending Aβ information through the dorsal column also has an indirect component (postsynaptic dorsal column) with relay in the spinal cord. However, the contribution of these Aβ-fiber projections onto the spinothalamic tract to the middle-latency N1, N2 and P2 waves of Aβ-ERPs is probably negligible. This is demonstrated by the results of studies showing that lesions of the spinothalamic tract lead to the dissociated observation of (1) altered or absent Aδ-ERPs and (2) preserved Aβ-ERPs (Kakigi et al., 1991; Treede et al., 1991; Iannetti et al., 2001; Iannetti et al., 2013).
What then are the mechanisms that could explain the effects of HNCS on the evoked brain responses to both nociceptive and non-nociceptive inputs? Using functional magnetic resonance imaging (fMRI), Sprenger et al. (Sprenger et al., 2011) found that HNCS leads to a tonic increase of BOLD signal in the anterior cingulate cortex, as well as to increased functional coupling between the cingulate cortex and brain stem regions hypothesized to be involved in the control of descending spinal pain-modulatory pathways. Interestingly, using fMRI of the spinal cord, Sprenger et al. (Sprenger et al., 2012) also showed that mental distraction can reduce the BOLD response to nociceptive stimuli at the level of the dorsal horn. Therefore, the effects of HNCS on the brain responses to nociceptive and non-nociceptive inputs might be also explained by top-down cognitive-attentional mechanisms distinct from DNIC. Regarding nociceptive input, there is convincing evidence that at least part of these effects is related to a top-down modulation occurring already at the level of the first relay in the dorsal horn. Whether similar corticofugal mechanisms could modulate the transmission of non-nociceptive somatosensory input at the level of dorsal column nuclei should be envisaged. Supporting this hypothesis, it has been shown that corticofugal projections can modulate the responses of dorsal column nuclei to tactile stimuli (Nunez & Malmierca, 2007; Malmierca et al., 2014). This effect has been suggested to contribute to the mechanisms of selective attention. Obviously, the fact that top-down mechanisms triggered by HNCS may modulate the transmission of nociceptive and non-nociceptive somatosensory inputs at the level of their first synaptic relay in the dorsal horn or brainstem does not preclude the fact that somatosensory processing may be further modulated at higher-order relays such as the thalamus and cortex (Dockstader et al., 2010).
Brain responses related to the selective activation of C fibers
In the first experiment we were unable to identify consistent ERPs related to the activation of C-fibers, in contrast with the results obtained by Magerl et al. (Magerl et al., 1999). This could be due to the fact that assessing the effects of HNCS on the responses elicited by Aβ-, Aδ- and C-stimuli required collecting EEG responses within very limited time periods. Therefore, we were able to collect only a small number of trials, and this could explain why no reproducible C-LEPs were identified in the average waveforms (van den Broeke & Mouraux, 2014a). In order to overcome this issue, we conducted a second experiment, which focused exclusively on C-LEPs. In this second experiment, we were able to increase the number of trials in which the stimulus selectively activated C fibers, and we were able to collect more C-LEPs in a more reliable fashion.
Conclusion
Our results suggest that HNCS indifferently inhibits the responses to nociceptive Aδ and C-fiber inputs, but also to non-nociceptive Aβ-fiber input. This finding has important clinical implications as it indicates that the effects of HNCS on the perception or brain responses to noxious stimuli should be used cautiously as a direct measure of DNIC mechanisms and their possible involvement in patients with chronic pain.
Figure 4. Event-related potentials (N1).
Grand-average waveforms of the ERPs recorded at the contralateral central electrode (C3) following Aδ- and Aβ-stimulation (N1). Both stimuli elicited an early-latency negative wave (N1). The magnitude of the Aβ-N1, but not the Aδ-N1 was reduced significantly during HNCS. The bar graphs represent the difference in amplitude between conditions. Negative changes in amplitude correspond to a decrease in amplitude as compared to before HNCS.
Acknowledgements
We thank Daniel Le Bars and Emanuel Van Den Broeke for their insightful comments. MC received support from bursary of President of the Russian Federation for an internship abroad. DMT is an Academie Universitaire de Louvain-Marie Curie Incoming Post-doc. AM received support from an ERC starting grant (“PROBING-PAIN”).
Footnotes
The authors have no conflict of interest.
References
- Bouhassira D, Le Bars D, Bolgert F, Laplane D, Willer JC. Diffuse noxious inhibitory controls in humans: a neurophysiological investigation of a patient with a form of Brown-Sequard syndrome. Annals of neurology. 1993;34:536–543. doi: 10.1002/ana.410340406. [DOI] [PubMed] [Google Scholar]
- Bouhassira D, Villanueva L, Bing Z, le Bars D. Involvement of the subnucleus reticularis dorsalis in diffuse noxious inhibitory controls in the rat. Brain research. 1992;595:353–357. doi: 10.1016/0006-8993(92)91071-l. [DOI] [PubMed] [Google Scholar]
- Chebbi R, Boyer N, Monconduit L, Artola A, Luccarini P, Dallel R. The nucleus raphe magnus OFF-cells are involved in diffuse noxious inhibitory controls. Exp Neurol. 2014;256:39–45. doi: 10.1016/j.expneurol.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Churyukanov M, Plaghki L, Legrain V, Mouraux A. Thermal detection thresholds of Adelta- and C-fibre afferents activated by brief CO2 laser pulses applied onto the human hairy skin. PloS one. 2012;7:e35817. doi: 10.1371/journal.pone.0035817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger N, Rozenberg S, Bourgeois P, Charpentier G, Willer JC. Depressive effects of segmental and heterotopic application of transcutaneous electrical nerve stimulation and piezo-electric current on lower limb nociceptive flexion reflex in human subjects. Archives of physical medicine and rehabilitation. 1998;79:191–200. doi: 10.1016/s0003-9993(98)90299-4. [DOI] [PubMed] [Google Scholar]
- De Broucker T, Cesaro P, Willer JC, Le Bars D. Diffuse noxious inhibitory controls in man. Involvement of the spinoreticular tract. Brain : a journal of neurology. 1990;113(Pt 4):1223–1234. doi: 10.1093/brain/113.4.1223. [DOI] [PubMed] [Google Scholar]
- Dockstader C, Cheyne D, Tannock R. Cortical dynamics of selective attention to somatosensory events. NeuroImage. 2010;49:1777–1785. doi: 10.1016/j.neuroimage.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Baumgartner U, Tracey I, Treede RD, Magerl W. Pinprick-evoked brain potentials: a novel tool to assess central sensitization of nociceptive pathways in humans. Journal of neurophysiology. 2013;110:1107–1116. doi: 10.1152/jn.00774.2012. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Truini A, Galeotti F, Romaniello A, Manfredi M, Cruccu G. Usefulness of dorsal laser evoked potentials in patients with spinal cord damage: report of two cases. Journal of neurology, neurosurgery, and psychiatry. 2001;71:792–794. doi: 10.1136/jnnp.71.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovski A, Plaghki L, Mouraux A. Reliable EEG responses to the selective activation of C-fibre afferents using a temperature-controlled infrared laser stimulator in conjunction with an adaptive staircase algorithm. Pain. 2013;154:1578–1587. doi: 10.1016/j.pain.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Shibasaki H, Tanaka K, Ikeda T, Oda K, Endo C, Ikeda A, Neshige R, Kuroda Y, Miyata K, et al. CO2 laser-induced pain-related somatosensory evoked potentials in peripheral neuropathies: correlation between electrophysiological and histopathological findings. Muscle & nerve. 1991;14:441–450. doi: 10.1002/mus.880140510. [DOI] [PubMed] [Google Scholar]
- Lapirot O, Chebbi R, Monconduit L, Artola A, Dallel R, Luccarini P. NK1 receptor-expressing spinoparabrachial neurons trigger diffuse noxious inhibitory controls through lateral parabrachial activation in the male rat. Pain. 2009;142:245–254. doi: 10.1016/j.pain.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Lapirot O, Melin C, Modolo A, Nicolas C, Messaoudi Y, Monconduit L, Artola A, Luccarini P, Dallel R. Tonic and phasic descending dopaminergic controls of nociceptive transmission in the medullary dorsal horn. Pain. 2011;152:1821–1831. doi: 10.1016/j.pain.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain research. Brain research reviews. 2002;40:29–44. doi: 10.1016/s0165-0173(02)00186-8. [DOI] [PubMed] [Google Scholar]
- Magerl W, Ali Z, Ellrich J, Meyer RA, Treede RD. C- and A delta-fiber components of heat-evoked cerebral potentials in healthy human subjects. Pain. 1999;82:127–137. doi: 10.1016/S0304-3959(99)00061-5. [DOI] [PubMed] [Google Scholar]
- Malmierca E, Chaves-Coira I, Rodrigo-Angulo M, Nunez A. Corticofugal projections induce long-lasting effects on somatosensory responses in the trigeminal complex of the rat. Frontiers in systems neuroscience. 2014;8:100. doi: 10.3389/fnsys.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA, Walker RE, Mountcastle VB., Jr A laser stimulator for the study of cutaneous thermal and pain sensations. IEEE transactions on biomedical engineering. 1976;23:54–60. doi: 10.1109/tbme.1976.324616. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Progress in neurobiology. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Moont R, Crispel Y, Lev R, Pud D, Yarnitsky D. Temporal changes in cortical activation during conditioned pain modulation (CPM), a LORETA study. Pain. 2011;152:1469–1477. doi: 10.1016/j.pain.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Nunez A, Malmierca E. Corticofugal modulation of sensory information. Advances in anatomy, embryology, and cell biology. 2007;187:1–74. 1 p following table of contents. [PubMed] [Google Scholar]
- Plaghki L, Delisle D, Godfraind JM. Heterotopic nociceptive conditioning stimuli and mental task modulate differently the perception and physiological correlates of short CO2 laser stimuli. Pain. 1994;57:181–192. doi: 10.1016/0304-3959(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends in neurosciences. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain. 2009;144:16–19. doi: 10.1016/j.pain.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B, Willer JC, Le Bars D. An electrophysiological investigation into the pain-relieving effects of heterotopic nociceptive stimuli. Probable involvement of a supraspinal loop. Brain : a journal of neurology. 1987;110(Pt 6):1497–1508. doi: 10.1093/brain/110.6.1497. [DOI] [PubMed] [Google Scholar]
- Scheuren R, Anton F, Erpelding N, Michaux G. Beep tones attenuate pain following Pavlovian conditioning of an endogenous pain control mechanism. PloS one. 2014;9:e88710. doi: 10.1371/journal.pone.0088710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger C, Bingel U, Buchel C. Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain. 2011;152:428–439. doi: 10.1016/j.pain.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Buchel C. Attention modulates spinal cord responses to pain. Current biology : CB. 2012;22:1019–1022. doi: 10.1016/j.cub.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Torta DM, Legrain V, Algoet M, Olivier E, Duque J, Mouraux A. Theta burst stimulation applied over primary motor and somatosensory cortices produces analgesia unrelated to the changes in nociceptive event-related potentials. PloS one. 2013;8:e73263. doi: 10.1371/journal.pone.0073263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torta DM, Legrain V, Mouraux A. Looking at the hand modulates the brain responses to nociceptive and non-nociceptive somatosensory stimuli but does not necessarily modulate their perception. Psychophysiology. 2015 doi: 10.1111/psyp.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousignant-Laflamme Y, Marchand S. Excitatory and inhibitory pain mechanisms during the menstrual cycle in healthy women. Pain. 2009;146:47–55. doi: 10.1016/j.pain.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Treede RD, Lankers J, Frieling A, Zangemeister WH, Kunze K, Bromm B. Cerebral potentials evoked by painful, laser stimuli in patients with syringomyelia. Brain : a journal of neurology. 1991;114( Pt 4):1595–1607. doi: 10.1093/brain/114.4.1595. [DOI] [PubMed] [Google Scholar]
- Treister R, Eisenberg E, Gershon E, Haddad M, Pud D. Factors affecting - and relationships between-different modes of endogenous pain modulation in healthy volunteers. European journal of pain. 2010;14:608–614. doi: 10.1016/j.ejpain.2009.10.005. [DOI] [PubMed] [Google Scholar]
- van den Broeke EN, Mouraux A. Enhanced brain responses to C-fiber input in the area of secondary hyperalgesia induced by high-frequency electrical stimulation of the skin. Journal of neurophysiology. 2014a;112:2059–2066. doi: 10.1152/jn.00342.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Broeke EN, Mouraux A. High frequency electrical stimulation of human skin induces heterotopical mechanical and heat hyperalgesia and enhanced responses to vibrotactile input. Journal of neurophysiology. 2014b doi: 10.1152/jn.00651.2013. [DOI] [PubMed] [Google Scholar]
- van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. The journal of pain : official journal of the American Pain Society. 2010;11:408–419. doi: 10.1016/j.jpain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Villanueva L, Le Bars D. The activation of bulbo-spinal controls by peripheral nociceptive inputs: diffuse noxious inhibitory controls. Biological research. 1995;28:113–125. [PubMed] [Google Scholar]
- Watanabe S, Kakigi R, Hoshiyama M, Kitamura Y, Koyama S, Shimojo M. Effects of noxious cooling of the skin on pain perception in man. Journal of the neurological sciences. 1996;135:68–73. doi: 10.1016/0022-510x(95)00253-x. [DOI] [PubMed] [Google Scholar]
- Willer JC, De Broucker T, Le Bars D. Encoding of nociceptive thermal stimuli by diffuse noxious inhibitory controls in humans. Journal of neurophysiology. 1989;62:1028–1038. doi: 10.1152/jn.1989.62.5.1028. [DOI] [PubMed] [Google Scholar]
- Willer JC, Le Bars D, De Broucker T. Diffuse noxious inhibitory controls in man: involvement of an opioidergic link. European journal of pharmacology. 1990;182:347–355. doi: 10.1016/0014-2999(90)90293-f. [DOI] [PubMed] [Google Scholar]
- Willer JC, Roby A, Le Bars D. Psychophysical and electrophysiological approaches to the pain-relieving effects of heterotopic nociceptive stimuli. Brain : a journal of neurology. 1984;107( Pt 4):1095–1112. doi: 10.1093/brain/107.4.1095. [DOI] [PubMed] [Google Scholar]