Abstract
Background
The clinical efficacy of hyper-CVAD (HCVAD) + ponatinib has not been compared to that of HCVAD + dasatinib in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) in a randomized clinical trial.
Methods
We analyzed 110 patients with newly diagnosed Ph+ ALL enrolled in two consecutive prospective phase 2 clinical trials of frontline HCVAD with either dasatinib (n=63) or ponatinib (n=47). Propensity score analysis with 1:1 matching with the nearest neighbor matching method, and inverse probability of treatment weighting (IPTW) analysis based on the propensity scores were performed to assess response rates, event-free survival (EFS), and overall survival (OS) between cohorts.
Results
Propensity score matching identified 41 patients in each cohort. With propensity score matching, the 3-year EFS rates for HCVAD + ponatinib and HCVAD + dasatinib were 69% and 46%, respectively (p=0.04), and the 3-year OS rates were 83% and 56%, respectively (p=0.03). Inverse probability of treatment weighting analysis using pre-matching cohorts showed that HCVAD + ponatinib had significantly higher rates of minimal residual disease negativity by flow cytometry on day 21, complete cytogenetic response at complete response (CR), major molecular response at CR and 3 months, and complete molecular response at 3 months. IPTW confirmed that HCVAD + ponatinib was associated with longer EFS (p=0.003) and OS (p=0.001) compared to HCVAD + dasatinib.
Conclusion
The clinical outcome of HCVAD + ponatinib appears superior to that of HCVAD + dasatinib in patients with Ph+ ALL.
Keywords: Philadelphia chromosome, acute lymphoblastic leukemia, hyper-CVAD, ponatinib, dasatinib
Introduction
The addition of a BCR-ABL1 tyrosine kinase inhibitor (TKI) to chemotherapy has improved the outcomes of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).1-3 This combination is now considered standard of care frontline therapy for patients with Ph+ ALL.4 Despite the significant improvement in outcome with chemotherapy plus a TKI, relapses are common,1-2 with the acquisition of the T315I kinase domain mutation being the most common cause of relapse.5-7
Ponatinib is a third-generation TKI with more potent activity on BCR-ABL1 tyrosine kinase than other TKIs, and which also overcomes T315I mutations.7 The combination of ponatinib with hyper-CVAD (HCVAD) chemotherapy for patients with newly diagnosed Ph+ ALL produced encouraging results with 2-year event-free survival (EFS) and overall survival (OS) rates of 81% and 80%, respectively.8 Notably, these survival rates appear superior to those reported with HCVAD in combination with either imatinib9, 10 or dasatanib2, 5. This apparent improvement of outcomes observed with HCVAD + ponatinib may be in part due to the relatively high complete molecular response (CMR) rate achieved with this combination. In the phase II trial, HCVAD + ponatinib was associated with a CMR rate of 78%8, which is higher than that reported with other TKIs in combination with chemotherapy5, 10-13. Furthermore, previous studies have suggested that deeper molecular response in patients with Ph+ ALL are independently predictive for improved survival.14, 15
There have been no randomized clinical trials comparing various TKIs in combination with chemotherapy for Ph+ ALL, and therefore the optimal frontline TKI for Ph+ ALL is unknown. The aim of this study was therefore to compare clinical outcomes of patients with Ph+ ALL treated with frontline HCVAD + ponatinib to those who received HCVAD + dasatinib. A propensity score analysis was used in order to balance patient characteristics and reduce bias when performing a retrospective comparison of patients treated with each of these regimens.
Methods
Patients
Adult patients with newly diagnosed Ph+ ALL enrolled in consecutive prospective phase 2 clinical trials with either HCVAD + dasatinib or HCVAD + ponatinib were analyzed. The inclusion criteria were similar for both trials, and the treatment schedules have been previously reported.2, 8 In the HCVAD + ponatinib protocol, 2 patients with no cardiovascular risk factors experienced chest pain followed by sudden death. Due to the concern for potential vascular toxicity, the protocol was subsequently amended to use lower doses of ponatinib, and no further cardiovascular deaths have been observed.8 Ponatinib was administered at 45 mg daily for 2 weeks during induction phase only, then at 30 mg daily continuously from cycle 2 with further reduction to 15 mg daily continuously once a complete molecular response was achieved. Stem cell transplantation was performed based on donor availability and at the discretion of the treating physician. The treatment protocols were approved by the MD Anderson Cancer Center Institutional Review Board. Informed consent was obtained according to the Declaration of Helsinki and our institutional guidelines.
Response Assessment and Definitions
Minimal residual disease (MRD) by multiparameter flow cytometry (MFC) was performed on bone marrow specimens at day 21 with a sensitivity of 0.01% as previously described.14 Complete cytogenetic response was evaluated at the time of complete remission (CR) and was defined as the absence of t(9;22) or any other previously detected clonal abnormalities. Molecular responses were assessed at the time of CR and at 3 months. CMR was defined as the absence of a detectable BCR-ABL1 transcript with a sensitivity of 0.01%. Major molecular response (MMR) was defined as a BCR-ABL1/ABL1 ratio ≤0.1% on the International Scale for p210 BCR-ABL1 or a 3-log reduction in transcripts for p190 BCR-ABL1, but not meeting criteria for CMR. ABL1 kinase domain sequencing analysis was performed with polymerase chain reaction-based DNA sequencing of the BCR-ABL1 fusion transcript in codons 221 to 500 of the ABL1 kinase domain including codon 315. The lower limit of detection sensitivity was 20% mutation-bearing cells in the sample tested.
Statistical Methods
Multiple imputations were performed because exclusion of patients with at least one missing variable may cause bias.16 Logistic regression was used for propensity score calculation from baseline patient characteristics including age, performance status, white blood cell count, cytogenetic risk group, type of BCR-ABL1 transcript, administration of rituximab, percentage of CD20 positive blasts at diagnosis, and presence of central nervous system disease. Propensity score analysis with 1:1 matching was performed with the nearest neighbor matching method using calipers of width equal to 0.2 of the standard deviation of the logit of the propensity score.17 Inverse probability of treatment weighting (IPTW) analysis based on the propensity scores was performed on the pre-matched cohort to assess response rates with binary logistic regression and to assess EFS and OS between cohorts with the Cox proportional hazard model.18 EFS was defined as the time from treatment initiation to the date of relapse or death at any time. OS was defined as the time from treatment initiation to the date of death. The Kaplan-Meier method was used for survival analysis with the log-rank test. All the statistical data analyses were performed with SPSS version 23.0 software (SPSS, Chicago, IL) and R version 3.2.4.
Results
Patient Characteristics
Overall, 51 and 72 patients were treated with HCVAD + ponatinib and HCVAD + dasatinib, respectively. After excluding 4 patients treated with HCVAD + ponatinib and 9 with HCVAD + dasatinib who had received prior therapy, a total of 110 patients were evaluable for this analysis (HCVAD + ponatinib, n=47; HCVAD + dasatinib, n=63). Baseline patient characteristic before propensity score matching is described in Supplemental Table 1. EFS and OS for patients in the HCVAD + ponatinib cohort were superior to that of the HCVAD + dasatinib cohort (EFS: p=0.04; OS: p=0.03) (Supplemental Figure 1).
Response Rates
Propensity score matching identified 41 patients in each cohort (Table 1). The median follow-up was 30 and 65 months in the matched HCVAD + ponatinib and HCVAD + dasatinib groups, respectively. The differences of all clinical variables were minimized after propensity score matching. The median time to absolute neutrophil count recovery after induction therapy was 18 days (range, 13-29) and 18 days (range, 14-24) in the HCVAD + ponatinib and HCVAD + dasatinib cohort, respectively (p=0.55); the median days to platelet count recovery was 22 days (range, 17-35) and 22 days (range, 17-44), respectively (p=0.75). Using the IPTW method to compare response rates, HCVAD + ponatinib was associated with significantly higher rates of MRD negativity by MFC at day 21 (p=0.03), complete cytogenetic response (p=0.01), MMR or deeper at CR (p=0.04) and at 3 months (p=0.03), and CMR at 3 months (p=0.03) (Table 2). Of the responses assessed, only CMR at CR was not significantly different between the HCVAD + ponatinib and HCVAD + dasatinib groups (p=0.28). With propensity score matching, there was a tendency of more frequent negative MRD at D21 bone marrow by MFC (HCVAD + ponatinib vs. HCVAD + dasatinib; 73% vs 54%; p=0.101) and higher rates of CCyR at CR and both MMR and CMR at CR and at3 months, all favoring in the HCVAD + ponatinib over HCVAD + dasatinib (CCyR at CR 94% vs. 88%; MMR at CR 68% vs. 50%; CMR at CR 47% vs. 38%; MMR at 3 months 95% vs. 81%; CMR at 3 months 84% vs. 63%, respectively) (Table 3).
Table 1.
Baseline patient characteristics after propensity score matching
| No. (%) or Median (range) | P | ||
|---|---|---|---|
| HCVAD + ponatinib [n=41] | HCVAD + dasatinib [n=41] | ||
| Age, (y) | 57 (27-80) | 55 (22-75) | 0.68 |
| White blood cell count (×109/L) | 8.5 (0.9-629.4) | 12.2 (0.4-658.1) | 0.87 |
| Performance status | |||
| 0-1 | 35 (85) | 37 (90) | 0.74 |
| ≥2 | 6 (15) | 4 (10) | |
| Cytogenetic abnormalities | |||
| Isolated Ph+ | 6 (15) | 6 (15) | 0.98 |
| Ph+ and other | 29 (71) | 28 (68) | |
| Diploid, (Ph+ by FISH/PCR) | 3 (7) | 3 (7) | |
| Unknown, (Ph+ by FISH/PCR) | 3 (7) | 4 (10) | |
| Transcript subtype | |||
| B2A2 | 4 (10) | 5 (12) | 0.39 |
| B3A2 | 4 (10) | 1 (2) | |
| B2A2 + B3A2 | 1 (2) | 1 (2) | |
| E1A2 | 30 (73) | 34 (83) | |
| E1A3 | 2 (5) | 0 | |
| CD20 positivity, (%) | 8.9 (0-100) | 15.2 (0-98) | 0.61 |
| Rituximab therapy | 14 (34) | 12 (29) | 0.64 |
| CNS disease | 3 (7) | 3 (7) | 1.00 |
Abbreviations: HCVAD, hyper-CVAD; Ph+, Philadelphia chromosome-positive; FISH, fluorescence in situ hybridization; PCR, polymerase chain reaction; CNS, central nervous system
Table 2.
Inverse probability of treatment weighted analysis for responses and survival outcomes
| HCVAD + ponatinib vs. HCVAD + dasatinib | HR | 95% CI | P |
|---|---|---|---|
| Negative MRD on D21 by FCM | 0.516 | 0.281-0.947 | 0.03 |
| Complete cytogenetic response at CR | 0.238 | 0.078-0.722 | 0.01 |
| Molecular response at CR | |||
| CMR | 0.634 | 0.278-1.449 | 0.28 |
| MMR or deeper | 0.409 | 0.175-0.954 | 0.04 |
| Molecular response at 3 months | |||
| CMR | 0.352 | 0.137-0.904 | 0.03 |
| MMR or deeper | 0.195 | 0.044-0.854 | 0.03 |
| Survival outcomes | |||
| EFS | 0.486 | 0.303-0.778 | 0.003 |
| OS | 0.374 | 0.208-0.673 | 0.001 |
Abbreviations: HCVAD, hyper-CVAD; HR hazard ratio; CI, confidence interval; MRD, minimal residual disease; FCM, flow cytometry; D21, day 21 of induction therapy; CR, complete response; CMR, complete molecular response; MMR, major molecular response; EFS, event-free survival; OS, overall survival.
Table 3.
Propensity score matching for responses and survival outcomes
| HCVAD + Ponatinib [n=41] | HCVAD + Dasatinib [n=41] | P | |
|---|---|---|---|
| Response after induction therapy, No. (%) | |||
| CR | 41 (100) | 39 (95) | 0.36 |
| CRp | 0 | 1 (2) | |
| Died | 0 | 1 (2) | |
| Negative MRD at D21 by FCM | 29/40 (73) | 19/35 (54) | 0.10 |
| Complete cytogenetic response at CR | 31/33 (94) | 28/32 (88) | 0.37 |
| Molecular response at CR, No. (%) | |||
| CMR | 9/19 (47) | 6/16 (38) | 0.56 |
| MMR or deeper | 13/19 (68) | 8/16 (50) | 0.27 |
| Molecular response at 3 months | |||
| CMR | 16/19 (84) | 10/16 (63) | 0.25 |
| MMR or deeper | 18/19 (95) | 13/16 (81) | 0.31 |
| Clinical outcome, (%) | |||
| 1-year EFS | 86 | 68 | 0.04 |
| 2-year EFS | 76 | 49 | |
| 1-year OS | 89 | 73 | 0.03 |
| 2-year OS | 83 | 61 | |
Abbreviations: HCVAD, hyper-CVAD; MRD, minimal residual disease; FCM, flow cytometry; D21, day 21 of induction therapy; CR, complete response; CRp, complete response with incomplete platelet recovery; CMR, complete molecular response; MMR, major molecular response; EFS, event-free survival; OS, overall survival.
Survival Outcomes
With propensity score matching, the 3-year EFS rates of HCVAD + ponatinib and HCVAD + dasatinib were 69% and 46%, respectively (p=0.04), and the 3-year OS rates were 83% and 56%, respectively (p=0.03) (Figure 1); with censoring at the time of stem cell transplantation, the 3-year EFS rates were 65% and 47%, respectively (p= 0.10), and 3-year OS rates were 84% and 60%, respectively (p=0.07) (Supplemental figure 2). Using IPTW analysis of pre-matched cohorts with the Cox proportional hazard model, HCVAD + ponatinib was associated with significantly longer EFS (HR 0.49 [95%CI 0.30-0.78], p=0.003) and OS (HR 0.37 [95% CI 0.21-0.67], p=0.001). When censoring at the time of allogeneic stem cell transplantation, HCVAD + ponatinib remained superior to HCVAD + dasatinib with significantly longer EFS (HR 0.61 [95% CI 0.37-1.00] p=0.0497) and OS (HR 0.45 [95% CI 0.24-0.85], p=0.012). Each cohort had 8 patients (20%) who proceeded to allogeneic stem cell transportation. Of 8 patients who underwent stem cell transplantation in the ponatinib + HCVAD cohort, 6 patients received maintenance TKI after transplantation (ponatinib, n=2; dasatinib, n=3; nilotinib, n=1): of 8 patients in the dasatinib cohort, 2 patients received maintenance TKI therapy with dasatinib after transplantation.
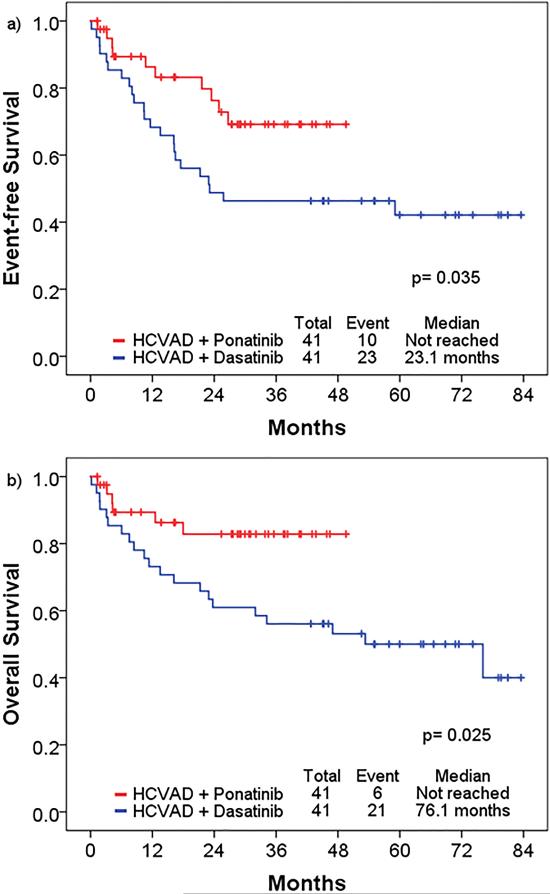
Figure 1.
Hyper-CVAD + ponatinib and hyper-CVAD + dasatinib after propensity score matching: a) event-free survival, b) overall survival. The 3-year EFS rates of hyper-CVAD + ponatinib and hyper-CVAD + dasatinib were 69% and 46%, respectively (p=0.04), and the 3-year OS rates were 83% and 56%, respectively (p=0.03).
Of 10 patients who relapsed in the HCVAD + dasatinib cohort, 4 underwent successful ABL1 kinase testing; 1 patient had a T315I mutation, 1 had a F359V mutation, and 2 did not have a detectable mutation. Of 5 patients who relapsed in the HCVAD + ponatinib cohort, only 2 relapsed while still on ponatinib; 1 of these patients had E255K mutation and 1 did not have a detectable mutation. Of the 10 patients who relapsed in the HCVAD + dasatinib cohort, 6 received multiagent chemotherapy plus a TKI, 2 received chemotherapy without a TKI, 1 received a TKI alone, and 1 received inotuzumab ozogamicin. Of the 5 patients who relapsed in the HCVAD + ponatinib cohort, 3 received multiagent chemotherapy plus a TKI, 1 received blinatumomab plus a TKI, and 1 received inotuzumab plus a TKI.
Of the 41 patients treated with HCVAD + ponatinib, 6 patients died; causes of death were cardiovascular disease (n=2), head injury (n=1), relapse (n=1), sepsis (n=1), complication after stem cell transplant (n=1). After the protocol amendment of the HCVAD + ponatinib, no further cardiovascular deaths were observed. Of the 41 patients treated with HCVAD + dasatinib, 21 patients died; causes of death were relapse (n=8), sepsis (n=6), complication after stem cell transplant (n=4), cardiovascular disease (n=1), early death (n=1), and unknown (n=1). Of 27 deaths in the entire cohort, no significant difference in the cause of death was observed between cohorts (p=0.209).
Discussion
This is the first report on clinical outcomes of HCVAD + ponatinib compared to that of HCVAD + dasatinib in patients with Ph+ ALL. We performed propensity score matching and IPTW to balance baseline patient characteristics, and demonstrated improved response rates, including MRD negativity by MFC and both cytogenetic and molecular responses in the HCVAD + ponatinib cohort. In both the pre-matched and matched cohorts, HCVAD + ponatinib was associated with prolonged EFS and OS compared to HCVAD + dasatinib. In the absence of a randomized prospective clinical trial, these results suggest that HCVAD + ponatinib is a superior frontline approach for patients with Ph+ ALL.
The higher rates of deep response observed in patients who received HCVAD + ponatinib likely contributed to improved rates of EFS and OS. In individual studies of chemotherapy plus a TKI, deeper molecular responses have been associated with improved survival.11, 12, 19 Similar findings have also been reported in pooled analyses of patients treated with chemotherapy plus one of several TKIs.14, 15 In one report of patients with Ph+ ALL who did not go stem cell transplantation in first remission, the achievement of CMR at 3 months was independently associated with improved OS and was associated with an impressive 4-year OS of 66%.15 Notably, the prognostic impact of CMR was independent of TKI received, suggesting that the apparent improved outcomes observed with higher potency TKIs such as ponatinib may be largely mediated through the ability to achieve CMR. Indeed, using IPTW analysis, the present study found that HCVAD + ponatinib was associated with a significantly higher CMR rate at 3 months, which translated to significantly improved survival outcomes for patients treated with this combination.
In addition to the greater potency and deeper responses obtained with ponatinib, its ability to overcome the T315I BCR-ABL1 kinase mutation also likely contributed to the improved EFS and OS observed with the HCVAD + ponatinib combination as compared to HCVAD + dasatinib. Although T315I mutations are rarely if ever present at the time of initial diagnosis, they have been identified in 57%-71% of patients with Ph+ ALL who relapse after initial treatment with dasatinib and are the putative driver of relapse in this setting. The lower relapse rates observed with HCVAD + ponatinib in the present study are thus likely in part due to the ability of ponatinib to overcome this potential driver of resistance.
These findings have several potential implications for the management of Ph+ ALL, especially for patients who are poor candidates for full-intensity chemotherapy. Although HCVAD + ponatinib achieves promising long-term outcomes in a selected population, this combination may not be ideal for elderly patients or those with significant comorbidities. HCVAD has been reported to have relatively high toxicity in elderly patients with ALL, with an induction mortality rate of 10% and death in CR rate of 34% in one study.20 Safer, effective regimens are therefore especially needed in this patient population. Given the exceptionally high CMR rate achieved with ponatinib as compared to those achieved with other earlier-generation TKIs, there is a rationale for combining ponatinib with other effective but less toxic agents, such as the anti-CD22 antibody-drug conjugate inotuzumab ozogamicin or the anti-CD19 bi-specific T-cell engager, blinatumomab. Interim results of blinatumomab in patients with Ph+ ALL have demonstrated significant clinical activity with this agent, with a remission rate of 36% in patients with relapsed/refractory disease, suggesting that this agent may become an important part of the armamentarium in the management of Ph+ ALL.21
This low-intensity therapy approach in combination with a TKI was also evaluated by the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) study, which showed 5-year EFS and OS rates of 37.1% and 45.6% without difference between the high-dose imatinib + reduced-intensity chemotherapy arm and standard-dose imatinib + HCVAD arm.22 Furthermore, the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) reported 20-month disease-free survival and OS rates of 51.1%, and 69.2% with dasatinib and steroids.6 These studies suggest that combination of a TKI with reduced intensity chemotherapy may be a feasible treatment option for patients with Ph+ ALL, especially for those who may not be able to tolerate full intensity chemotherapy. In this context, ponatinib may be able to further improve survival and reduce the rate of relapse, as ponatinib is associated with higher CMR rates than the other available TKIs. The study of ponatinib in combination with dose-reduced chemotherapy or new monoclonal antibodies like blinatumomab is therefore warranted, both in the frontline and the relapsed/refractory settings, particularly in elderly patients.
One potential limitation to propensity score analysis is that this type of analysis only balances known and selected variables. It is therefore possible that unrecognized risk factors might affect these findings. However, the patients enrolled in these trials had similar baseline characteristics, and the inclusion criteria were similar across trials. Furthermore, patients with significant comorbidities that might affect survival outcome were excluded from our study. Thus, in the absence of a randomized, controlled phase III trial, the present study offers convincing evidence for the superiority of HCVAD + ponatinib in the frontline setting.
In conclusion, patients treated with frontline HCVAD + ponatinib appear to have improved EFS and OS compared to those treated with HCVAD + dasatinib. Given the established potency of ponatinib in Ph+ ALL, prospective studies of ponatinib in combination with low-intensity chemotherapy or novel monoclonal antibodies are warranted in order to improve outcomes with reduced toxicity.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Osamu Miura at Tokyo Medical and Dental University for useful feedback.
Funding source
Supported by the MD Anderson Cancer Center Support Grant CA016672 and the “Charif Souki Cancer Research Fund” P30 CA016672, Ronald DePinho.
Footnotes
Authorship Contributions
K.S. treated patients, collected data, designed the study, analyzed the data, and wrote the manuscript. E.J. treated patients, designed the study, analyzed the data, wrote, and edited the manuscript. V.J., G.M., and R.G. managed the data. N.J.S. and G.C.I. treated patients and collected the data. H.K., F.R., D.T., N.D., T.K., M.K., N.J., G.G.M., J.C., and S.O. treated patients. All authors provided significant intellectual input, and reviewed and approved the final version of the manuscript.
Disclosure of Conflict of Interest
E.J. received consultancy for Ariad, BMS, and Pfizer, and research grants from Ariad, BMS, TEVA, and Pfizer. F.R. received research funding from Novartis and BMS. J.C. received research support from Ariad, BMS, Novartis, Pfizer, and Teva, and is a consultant for Ariad, BMS, Novartis and Pfizer. H.K. received research grants from Novartis, BMS, Pfizer, and Ariad. N.D. received research funding from BMS, Novartis, Sunesis, Incyte, and Bioline. Other authors have nothing to disclose.
References
- 1.Fielding AK, Rowe JM, Buck G, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123:843–850. doi: 10.1182/blood-2013-09-529008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravandi F, O'Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116:2070–2077. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz KR, Carroll A, Heerema NA, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children's Oncology Group study AALL0031. Leukemia. 2014;28:1467–1471. doi: 10.1038/leu.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarnas JC, Brown PA, Aoun P, et al. Acute Lymphoblastic Leukemia, Version 2.2015. J Natl Compr Canc Netw. 2015;13:1240–1279. doi: 10.6004/jnccn.2015.0153. [DOI] [PubMed] [Google Scholar]
- 5.Ravandi F, O'Brien SM, Cortes JE, et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121:4158–4164. doi: 10.1002/cncr.29646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foa R, Vitale A, Vignetti M, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118:6521–6528. doi: 10.1182/blood-2011-05-351403. [DOI] [PubMed] [Google Scholar]
- 7.O'Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabbour E, Kantarjian H, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. Lancet Oncol. 2015;16:1547–1555. doi: 10.1016/S1470-2045(15)00207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 10.Daver N, Thomas D, Ravandi F, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100:653–661. doi: 10.3324/haematol.2014.118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Kim DW, Cho BS, et al. Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2012;26:2367–2374. doi: 10.1038/leu.2012.164. [DOI] [PubMed] [Google Scholar]
- 12.Yoon JH, Yhim HY, Kwak JY, et al. Minimal residual disease-based effect and long-term outcome of first-line dasatinib combined with chemotherapy for adult Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann Oncol. 2016 doi: 10.1093/annonc/mdw123. [DOI] [PubMed] [Google Scholar]
- 13.Yanada M, Sugiura I, Takeuchi J, et al. Prospective monitoring of BCR-ABL1 transcript levels in patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia undergoing imatinib-combined chemotherapy. Br J Haematol. 2008;143:503–510. doi: 10.1111/j.1365-2141.2008.07377.x. [DOI] [PubMed] [Google Scholar]
- 14.Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122:1214–1221. doi: 10.1182/blood-2012-11-466482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Short NJ, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016 doi: 10.1182/blood-2016-03-707562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin DB. Multiple Imputation for Non-Response in Surveys. John Wiley; New York, NY: 1987. [Google Scholar]
- 17.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 18.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DY, Joo YD, Lim SN, et al. Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood. 2015;126:746–756. doi: 10.1182/blood-2015-03-636548. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien S, Thomas DA, Ravandi F, Faderl S, Pierce S, Kantarjian H. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer. 2008;113:2097–2101. doi: 10.1002/cncr.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinelli G, Dombret H, Chevallier P, et al. Complete Molecular and Hematologic Response in Adult Patients with Relapsed/Refractory (R/R) Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia (ALL) Following Treatment with Blinatumomab: Results from a Phase 2 Single-Arm, Multice. Blood. 2015;126:679–679. [Google Scholar]
- 22.Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125:3711–3719. doi: 10.1182/blood-2015-02-627935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.