Abstract
The formation of a nodule within a congenital melanocytic nevus (CMN) raises concerns about possible melanoma. Most new nodular growths that develop during childhood, however, are benign proliferative nodules (PN); melanoma is very rare. The distinction of melanoma from PN can at times be difficult clinically and histopathologically, requiring ancillary molecular tests for diagnosis. While the application of molecular methods has revealed new insights into the mutational and genomic landscape of childhood melanomas, little is known about epigenetic events that may drive the growth of a melanoma or PN in a CMN. In this study we compared the expression of H3K27me3, a key regulator in chromatin remodelling-controlled transcription, in PNs and pediatric nodular melanomas arising within medium-sized to large CMN by immunohistochemistry (IHC). Significant loss of H3K27me3 expression was seen in four of five melanomas, but not in any of the 20 PNs. This observation suggests that epigenetic events likely play a role in the pathogenesis of melanoma developing in the dermis or subcutis of CMN. Furthermore, assessing for H3K27me3 expression by IHC may be diagnostically useful for problematic cases.
Keywords: Melanoma, Congenital Melanocytic Nevus, Proliferative Nodule, Immunohistochemistry, Epigenetic, H3K27me3
Introduction
New nodules may form within a congenital melanocytic nevus (CMN). The majority of these are benign proliferative nodules (PNs), but on rare occasion a melanoma may develop within the dermal or subcutaneous compartment of a large melanocytic nevus 1–4. The incidence with which PNs develop has been estimated to range from 3 - 20% in giant CMN1, 5, 6. The incidence of cutaneous melanoma arising in CMN of all sizes and numbers considered together is lower, of the order of less than 1% to 3%7–10. However this varies substantially with the severity of the clinical phenotype.
In most children a PN can reliably be distinguished from melanoma by clinico-pathologic correlation. Clinically, a PN typically differs from melanoma by uniformity of color and outline, a tendency to stabilize, and lower likelihood to ulcerate. Histopathologically, the melanocytes of a PN tend to blend in with those of the background nevus, but nonetheless form a microscopically distinct nodule due to their hypercellularity. There is a range of histopathologic appearances of PNs from small to large epithelioid to fusiform, blue or Spitz nevus-like, pigmented or amelanotic6, 11. Sometimes a nodule may display mesenchymal features under the microscope12. In general, melanomas are more abruptly demarcated from the surrounding nevus, display a higher degree of nuclear atypia, and many more tumor cells are seen in mitosis11. In melanoma, there may be apoptotic bodies or even frank tumor necrosis.
Some melanomas display marked nuclear pleomorphism, numerous mitoses and tumor necrosis, making their recognition as a malignancy by light microscopic examination straightforward. However, especially on a partial biopsy, the distinction of a PN from melanoma can be difficult, in particular when the level of atypia and number of mitoses are in a grey zone, i.e., at a level that can be seen both in association with a benign as well as a malignant tumor, or the nodule has spitzoid or undifferentiated mesenchymal features. In those cases, ancillary genetic studies may assist in the diagnostic work-up. In the past decade, cytogenetic analysis has emerged as a useful adjunct method for the distinction of melanoma from PN11, 13. Melanomas tend to show segmental chromosomal gains and/or losses involving different chromosomes while PNs either may not contain any detectable cytogenetic aberrations or may carry whole chromosome gains or losses11, 13–15, but there are rare exceptions16. Furthermore interpretation of these techniques typically requires some experience or expertise in assessing cytogenetics in melanocytic neoplasia.
Much has been learned in recent years about the mutational landscape of melanomas17. Melanomas in adults, the vast majority of which arise at the dermal-epidermal junction, tend to be rich in mutations, with variations in mutation type and number depending on anatomic site and extent of sun-exposure18, 19. Preliminary evidence of mutational analyses of childhood melanomas indicates that conventional (“adult-type”) melanomas affecting the skin surface of children also carry UV-mutations and commonly harbor BRAFV600 mutations20. However, pediatric melanomas associated with CMN tend to lack UV-mutations and have a lower mutational burden 20(KJB, unpublished data). Given the distinct clinical setting and behavior (lack of UV exposure, onset of rapid tumor growth) we speculated that epigenetic events may play a role in the development of subepidermal childhood melanomas arising in CMN.
The purpose of this study was to explore the possible role of epigenetic changes in subsurface nodular melanomas arising in CMN. As a marker for epigenetic changes we chose immunohistochemical expression of H3K27me3 21, 22. This marker was chosen because the assay conditions are well established in our laboratory, and it has been tested on melanomas before.
Materials and Methods
The work in this study was approved by the institutional review boards of both institutions. Twenty benign PMs and five nodular melanomas that arose in the dermal component of a CMN during childhood were selected and analyzed immunohistochemically. We also evaluated 10 primary melanomas from adults. Five micron thick sections were taken from formalin-fixed and paraffin-embedded tissue.
An automated immunohistochemistry system (Leica Bond, polymer) was used, with a commercially available antibody to H3K27me3 (Cell Signaling, clone C36B11).
Labeling was scored according to the percentage of immunoreactive tumor cells per total number of tumor cells: 0 = no staining; 1+ = 1-25% of tumor cells are positive; 2+ = 26-50% of tumor cells are positive; 3+ = 51-75% of tumor cells positive and 4+ = 76-100% of tumor cells are positive.
Genomic Analysis
Sequence analysis was performed on all five melanomas for clinical purposes. Two tumors were analyzed by FoundationOneR; two tumors were analyzed at Imperial College in London, UK; one tumor was analyzed at the Northwestern University Feinberg School of Medicine in Chicago, IL, with the PGM ion torrent 50 gene cancer panel (ThermoFisher, Waltham, MA).
The five melanomas were also analyzed cytogenetically. Melanoma case 1 was analyzed by FoundationOneR. Case 1 and 2 were analyzed by SNP array (OncoScanR). In case 3 array comparative genomic hybridization (CGH) was performed as per the manufacturer’s instructions, using Roche Nimblegen 135K oligonucleotide arrays and a sex-matched commercial pooled control. Cases 4 and 5 were analyzed by fluorescent in situ hybridization analysis (FISH), using probes for 6p, 6q, 6cent, 11q, and 8q23, 24.
Results
Clinical Findings
Of the 20 children with PNs, 11 were female and 9 were male. The mean age was 4.2 years; the median age was 2 years. Of the five children with a nodular melanoma arising in a CMN, three were girls and two of them were boys. Three of the children with melanoma also had preceding or concurrent proliferative nodules at other sites. Two children died of metastatic melanoma. One of them was a boy (case 2) who died in his second year of life. He had a primary nodular melanoma that arose in perianal soft tissue in association with a giant bathing trunk CMN (measuring > 40 cm in largest dimension, 160 additional nevi). The other child with a lethal melanoma was a girl (case 3). She developed a nodular melanoma in a large CMN on the scalp (20cm projected adult size; multiple other melanocytic nevi from birth) at the age of 6 years. She died at age 7. The three other children are still alive, but with limited follow-up. One of them is a girl who developed a nodular melanoma on the scalp at the age of 8 years (case 1). Her melanoma arose in a CMN measuring 9 x 8 cm, with a predicted adult size of approximately 10 cm. The girl is still alive without evidence of disease 1 year after surgical removal of the primary tumor. The fourth child with a nodular melanoma is an infant girl. Her recently diagnosed melanoma was congenital and associated with a CMN of the scalp measuring 12 cm in greatest dimension. The sentinel lymph node and two of 28 non-sentinel regional lymph nodes were positive for tumor deposits. The girl is alive with follow-up of 5 months (October 2016). One patient is a teenage boy who developed a subsurface nodular melanoma at age 15 on the scalp at the site of a previously partially-excised CMN measuring 10 x 20 cm. He also had multiple other nevi. One of his regional lymph nodes was found to be positive for metastatic melanoma. He is currently alive and well with a follow-up of approximately 1 year.
Pathologic Findings
The histopathologic findings of the PNs are summarized in Table1 and representative examples are illustrated in Figures 1 - 3. Each PN was histopathologically characterized by a hypercellular expansile melanocyte proliferation in the dermis. Ten lesions were amelanotic. Focal melanin pigment was present in six nodules; four nodules were heavily pigmented throughout. Two of them resembled an epithelioid blue nevus. The dominant cell type in 12 cases was round to oval (epithelioid) in morphology (Fig. 2). In five cases there was a mix of both spindle and epithelioid-shaped melanocytes. Three tumors were predominantly fusiform. Two tumors were associated with stromal sclerosis.
Table 1.
Clinical and pathologic features of benign proliferative nodules
| Case | Age | Sex | Site | Cytology | Melanin | H3K27me3 - IHC |
|---|---|---|---|---|---|---|
| 1 | 2 weeks | F | Scalp | Epithelioid | Absent | 4+ |
| 2 | 4 years | M | Scalp | Epithelioid | Present | 4+ |
| 3 | 11 weeks | M | Back | Fusiform | Absent | 4+ |
| 4 | 10 months | F | Back | Mixed | Absent | 4+ |
| 5 | 16 months | F | Scalp | Epithelioid | Present | 4+ |
| 6 | 4 weeks | M | Back | Fusiform | Absent | 4+ |
| 7 | 13 years | M | Back | Epithelioid | Present | 4+ |
| 8 | 2 years | F | Abdomen | Epithelioid | Focal | 4+ |
| 9 | 18 years | M | Scalp | Epithelioid | Focal | 4+ |
| 10 | 5 years | M | Back | Epithelioid | Focal | 4+ |
| 11 | 2 years | F | Scalp | Mixed, sclerosing | Focal | 4+ |
| 12 | 3 years | F | Leg | Mixed, sclerosing | Absent | 4+ |
| 13 | 9 years | M | Leg | Epithelioid | Absent | 4+ |
| 14 | Infant | F | Scalp | Epithelioid | Present | 4+ |
| 15 | 14 years | F | Leg | Epithelioid | Focal | 4+ |
| 16 | 5 years | F | Leg | Epithelioid | Focal | 4+ |
| 17 | 5 years | F | Scalp | Epithelioid | Absent | 4+ |
| 18 | 4 weeks | M | Back | Fusiform | Absent | 4+ |
| 19 | 6 months | F | Back | Mixed | Absent | 4+ |
| 20 | 4 years | M | Back | Mixed | Absent | 4+ |
M = male; F = female
3 + = 51 – 75% of tumor cells are immunoreactive
4+ = more than 75% of tumor cells are immunoreactive
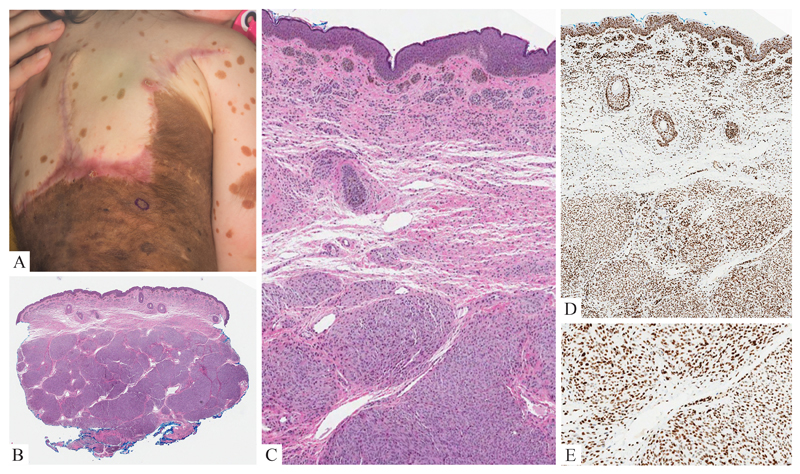
Figure 1. Benign Proliferative Nodule.
A: 4 year-old girl with nodular growth (circled) in a giant congenital nevus. B: A punch biopsy shows a densely cellular amelanotic nodule of melanocytes in the dermis. C: Transition area of nodule and background nevus: The nuclei are uniformly bland, but there is higher cellular density within the nodule. D: Both nevus and cellular nodule homogeneously label for H3K27me3.
Figure 3. Congenital Proliferative Nodule of the Scalp.
A: Polypoid nodule of epithelioid melanocytes. B: The nodular proliferation expresses H3K27me3. C: The nodular is composed of intermediate-sized epithelioid melanocytes. D: Most cells expresses H3K27me3.
Figure 2. Proliferative Nodule.
A: Polypoid cellular nodule within a CMN. B: The lesional melanocytes express H3K27me3. C: The nodule is composed of a dense population of small to intermediate-sized cytologically bland amelanotic epithelioid melanocytes. D: The melanocytes within and adjacent to the nodule homogeneously show uniform nuclear labeling for H3K27me3.
All melanomas presented as nodular dermal and/or subcutaneous tumors without associated in situ melanoma. The tumor cells displayed epithelioid cell features (Table 2, Figs. 4 – 7). In two cases they had a small round blue cell (melanoblastic) appearance with minimal visible cytoplasm (Fig. 4). In two cases the tumor cells were small to intermediate in size with enlarged nuclei and small amounts of pink cytoplasm. One of them contained a subpopulation of tumor cells with melanin pigment (Fig. 5); the other was predominantly amelanotic (Fig. 6). One case was composed of amelanotic large pleomorphic epithelioid tumor cells (Fig. 7) with pink cytoplasm. Mitoses were frequent (mitotic rate > 4/mm2) in all but one case. The mitotic rate of the large epithelioid cell nodule of the 15 year old boy (case 5) had a mitotic rate of only 1/mm2.
Table 2.
Clinical, pathologic and molecular findings of the nodular melanomas associated with a congenital melanocytic nevus
| Case # | Age (yrs) | Sex | Site | Tumor Diameter | Cytology | Cytogenetics | Mutations | H3K27 IHC | FU |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | F | Scalp | 9.4 mm | Amelanotic small round blue | Multiple gains, losses | NRAS c.182A>G, Q61R | 1+ | Alive (1 yr) |
| 2 | 1 | M | Perianal soft tissue | 14 mm | Amelanotic small round blue | Gain of 6p RAD18-BRAF fusion | None detected | 1+ | DOD |
| 3 | 6 | F | Scalp | 10 mm | Pigmented small epithelioid | Multiple gains, losses | NRAS c.181C>A, p.Q61K | 2+ | DOD |
| 4 | Newborn | F | Back | 7 mm | Amelanotic small epithelioid | Multiple gains, losses | NRAS c.37G>C, G13R | 1+ | Alive (4mo) |
| 5 | 15 | M | Scalp | 8 mm | Amelanotic large epithelioid | Multiple gains, losses | NRAS and BRAF wild type | 4+ | Alive (12 mo) |
Abbreviations:
Yrs = years; F = female; M = male; IHC = immunohistochemistry; FU = follow-up DOD – dead of disease; mo = months
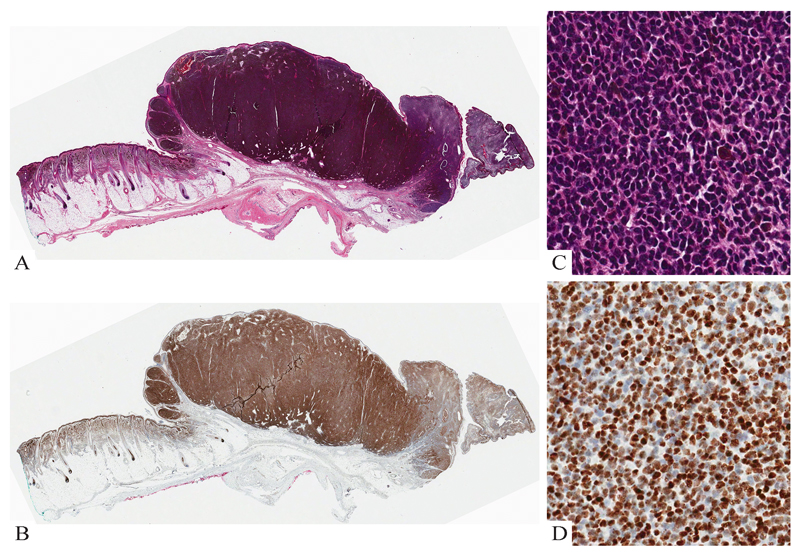
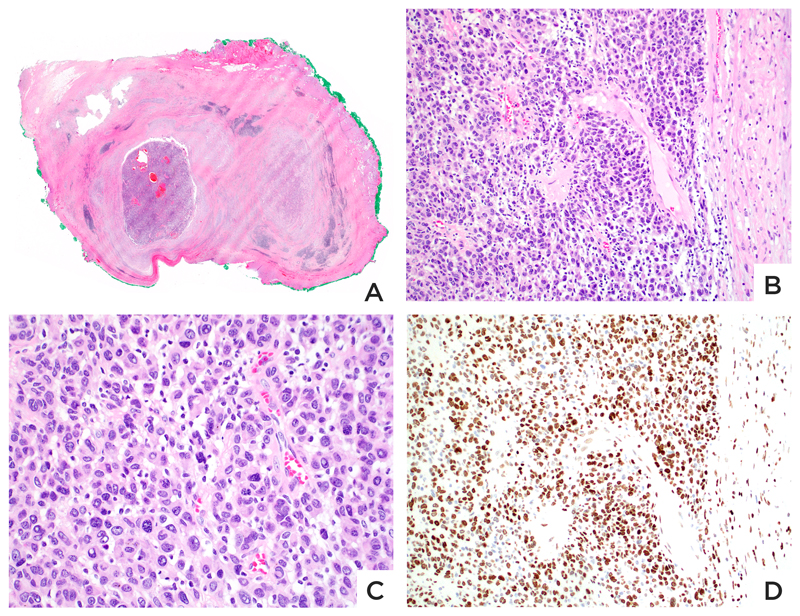
Figure 4. Lethal melanoma from a 15 month-old boy.
A: Congenital melanocytic nevus with a subcutaneous melanoma nodule. B: The compound melanocytic nevus involving epidermis and dermis is composed of cytologically bland cells with evidence of maturation. C: The nevus component retains expression of H3K27me3. D: The melanoma nodule is composed of discohesive round cells with little cytoplasm, mitoses and no maturation. D: Most melanoma cells lack expression of H3K27me3.
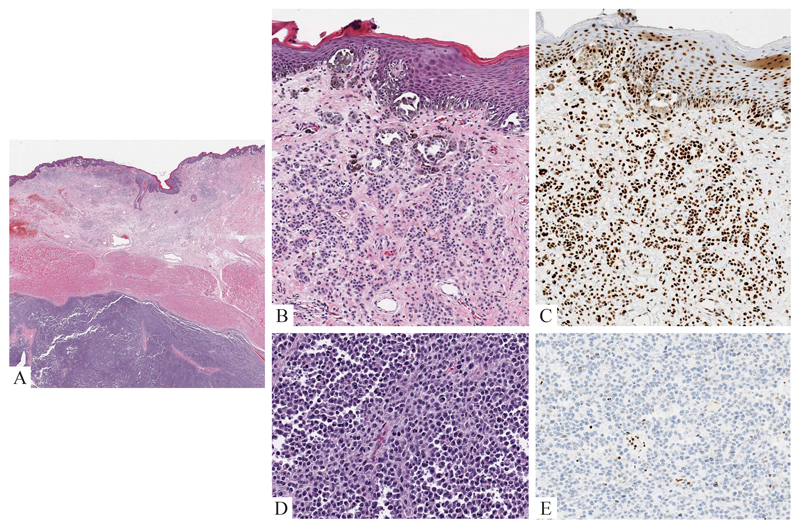
Figure 7. Nodular melanoma of the scalp at the site of a congenital nevus (15 year old boy).
A: Tumor nodule in soft tissue at the site of a previously incompletely excised congenital nevus. B: The tumor is sharply demarcated from the adjacent benign tissue. C: The tumor cells are large with pleomorphic nuclei and amelanotic pink cytoplasm. D: The majority of tumor cells express H3K27me3.
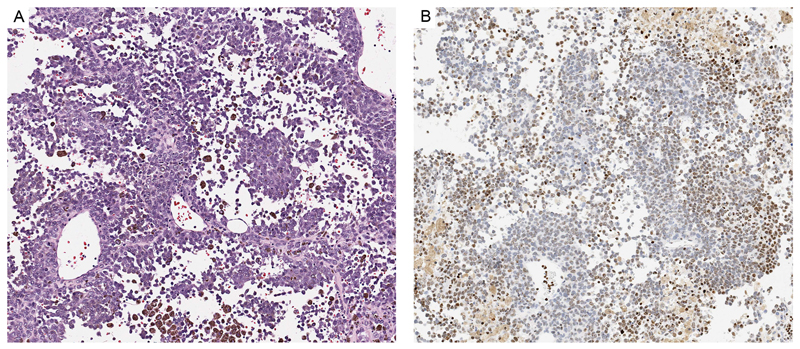
Figure 5. Lethal melanoma from a 6 year-old girl.
A: The tumor is composed of epithelioid melanocytes with atypical nuclei. Some of them are pigmented. B. Approximately half of the tumor cells lack expression of H3K27me3.
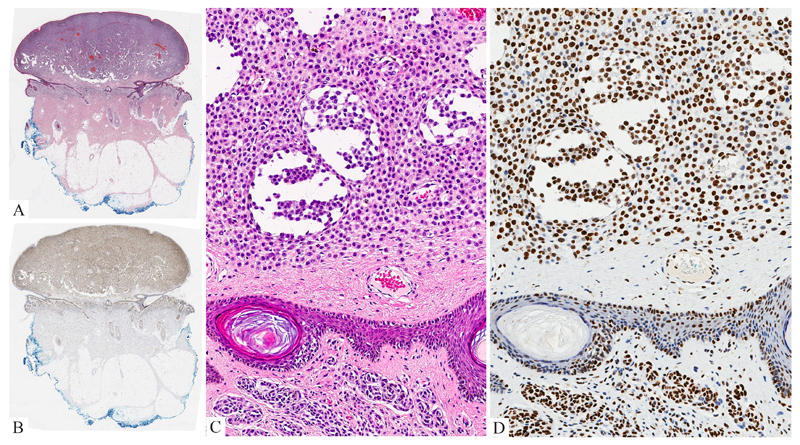
Figure 6. Congenital Melanoma of the Scalp (same patient as Figure 3).
A: Polypoid nodule of atypical epithelioid melanocytes with adjacent CMN. B: Little labeling for H3K27me3 is seen within the melanoma nodule while the adjacent melanocytic nevus is uniformly positive. C: The melanoma cells display an epithelioid cell appearance. The nuclei are enlarged and have irregular contours. Mitotic figures are present. D: The melanoma cells lack expression of H3K27me3.
Immunohistochemical Findings
All 20 PNs homogenously expressed H3K27me3. Nuclear expression was seen throughout the nodule as well as in the adjacent cells of the melanocytic nevus outside of the PN (Figs. 1 - 3). Positive labeling was seen in > 80% of lesional cells of all 20 PNs.
Marked reduction in labeling for H3K27me3 was seen in four prepubertal pediatric nodular melanomas that arose in a CMN (Table 2, Figs. 4 - 6), while the expression of H3K27me3 was retained in the adjacent nevus and normal tissue. Among these four tumors, the extent of loss of labeling for H3K27me3 within the melanoma ranged from nearly complete (Figs. 4 and 6) to approximately half of the tumor cells (Fig. 5). One nodular melanoma (case 5, Table 2), which developed on the scalp of a teenager, showed only minimal (approx. 10%) loss H3K27me3 expression (Fig. 7).
All ten adult melanomas retained expression of H3K27me3, but partial loss of labeling (< 50% of tumor cells were immuno-negative) was seen in one acral melanoma (not shown).
Molecular Aberrations of Melanomas
The molecular aberrations detected in the nodular melanomas are summarized in Table 2. Three of the five tumors harbored NRAS mutations: two had an NRASQ61R, one an NRASG13R mutation. In two cases (cases 3 and 5) Sanger sequencing analysis of NRAS and BRAF confirmed NRASQ61R and BRAF wild-type mutation status in one case (case 3). The other tumor (case 5) was confirmed to have wild-type mutation status for both NRAS and BRAF. In three cases, targeted next generation sequencing analysis was performed, which failed to reveal any mutation in case 2, detected a single NRASQ61R mutation in case 1, and documented a single NRASG13R mutation in case 4. All five melanoma cases had segmental chromosomal copy number aberrations. Genomic analysis documented numerous small segmental gains and losses involving different chromosomes in three of the four melanomas (cases 1, 3, 4 and 5). One lethal melanoma (case 2) contained only a single segmental gain of 6p by SNP array analysis. This melanoma was found to have a RAD18-BRAF fusion and small deletions involving PIK3CA, PIK3R1 and CTNNB1 by next generation sequence analysis (FoundationOneR).
Discussion
Much has been learned about the mutational landscape of melanomas17. The majority of melanomas in adults carry a high mutational burden, especially those tumors associated with chronic UV-damage18, 19. A recent study on a limited number of “childhood and adolescent” melanomas also identified a high number of mutations in conventional melanomas of teenagers and young adults (age range 11 to 20 years; median age = 16 years), with frequent BRAFV600 and TERT promoter mutations20. In contrast, each of the three melanomas associated with a CMN, which occurred in children younger than 5 years of age, contained an activating NRASQ61R mutation and no TERT promoter mutation20. It is of interest that the mutational burden of two of the three CMN melanomas was very low20. However, it is difficult to draw conclusions from this study about melanomas associated with CMN due to the small number of cases (only three) and lack of detail about the pathology of the melanomas20. It is not clear whether the melanomas associated with CMN developed at the dermal-epidermal junction or in the dermis/subcutis. The authors also failed to specify the phenotype of patients with the congenital nevi.
We know, however, from prior studies that NRAS mutations are associated with melanomas of large CMN 2, 25, 26. Three of the five melanomas tested in this series also had an NRAS mutation.
The apparent paucity of the total number of mutations and the commonly observed rapid clinical growth of melanoma in early childhood made us suspect that epigenetic drivers may play a role in their pathogenesis. We also reasoned that epigenetic changes relevant in the pathogenesis of melanomas are likely different from benign PNs and could potentially be used for diagnostic purposes.
Among epigenetic events, post-translational modifications of the N-terminal cores of histones, such as by methylation, influences chromatin configuration and thereby modulates accessibility of transcription factors and transcriptional activity21, 27, 28. H3K27 trimethylation (H3K27me3) is a well-known epigenetic gene silencer that can repress transcription. Polycomb repressive complexes 2 (PRC2) containing enhancer of zeste (EZH) 2, a methyltransferase and core component of PRC2, are responsible for trimethylation of H3K2721. Aberrant expression of H3K27me3 has been found in different types of cancers and has been correlated with prognosis 22, 29. H3K27me3 expression levels can be assessed in formalin-fixed and paraffin-embedded tissues by immunohistochemistry and its expression pattern has recently been found to be useful for the distinction of malignant peripheral nerve sheath tumors from histologic mimics, including melanomas30, 31. We explored the role of epigenetic events in childhood melanomas arising in CMN by comparing H3K27me3 expression in benign PNs and nodular melanomas of medium-sized and large CMN.
Despite the rarity of bona-fide nodular melanomas arising in CMN of young children, we were able to analyze five tumors. Four of five tumors showed marked reduction in labeling of H3K27me3, from approximately half of the tumor cells in one case to more than 80% in the other three. One tumor showed minimal (approximately 10%) loss of labeling. It differed from the other tumors histopathologically by a large epithelioid cell phenotype, low mitotic rate and clinically by its occurrence in a teenager. In contrast to the majority of melanomas, all PNs retained expression of H3K27me3 expression in more than 80% of tumor cells.
The loss of H3K27me3 staining in melanomas of young children is of interest, since prior studies on H3K27me3 expression in melanomas suggested that this marker is typically retained 30, 31. Those melanomas, however, were from adults, with no documentation of an associated melanocytic nevus. Future studies of a larger set of different types melanomas from different age groups are needed to determine the expression pattern of H3K27me3 and its significance in various types of melanomas and at different clinical stages.
In conclusion, we report herein reduction of H3K27me3 expression in four of five nodular melanomas arising in the subepithelial compartment of a CMN during childhood. In contrast, H3K27me3 expression was retained or only mildly reduced in 20 benign PNs and the nevus cells adjacent to the melanoma nodules. These observations document for the first time that H3K27me3 expression can be markedly reduced in primary cutaneous melanoma. They indicate a likely role for epigenetic events in the pathobiology of at least some pediatric nodular melanomas developing in CMN. Our results also suggest that H3K27me3 expression has the potential to become an ancillary diagnostic tool for the distinction of a melanoma from an unusual PN. Since no major reduction in labeling for H3K27me3 was seen in any of the 20 benign PNs examined herein, loss of labeling for this marker should make one hesitate establishing a diagnosis of PN. One the other hand, retention of H3K27me3 expression can be seen in both PN and melanoma. Examination of a larger set of cases is necessary to assess the biologic significance of H3K27me3 expression in melanomas and its potential diagnostic value.
Acknowledgement
The authors wish to thank Danielle Leng and Allyne Manzo for their excellent assistance in preparing this manuscript and figures and Alex Virasami for assistance with histopathological sections.
Disclosures: Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. VK is funded by the Wellcome Trust, grant award number WT104076MA.
References
- 1.Chen AC, McRae MY, Wargon O. Clinical characteristics and risks of large congenital melanocytic naevi: a review of 31 patients at the Sydney Children's Hospital. Australas J Dermatol. 2012;53:219–223. doi: 10.1111/j.1440-0960.2012.00897.x. [DOI] [PubMed] [Google Scholar]
- 2.Kinsler VA, Thomas AC, Ishida M, et al. Multiple congenital melanocytic nevi and neurocutaneous melanosis are caused by postzygotic mutations in codon 61 of NRAS. J Invest Dermatol. 2013;133:2229–2236. doi: 10.1038/jid.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price HN, Schaffer JV. Congenital melanocytic nevi-when to worry and how to treat: Facts and controversies. Clin Dermatol. 2010;28:293–302. doi: 10.1016/j.clindermatol.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Surrenti T, Diociaiuti A, Inserra A, et al. Melanoma in a 5-year-old child with a giant congenital melanocytic naevus. Acta Derm Venereol. 2012;92:607–608. doi: 10.2340/00015555-1280. [DOI] [PubMed] [Google Scholar]
- 5.Yun SJ, Kwon OS, Han JH, et al. Clinical characteristics and risk of melanoma development from giant congenital melanocytic naevi in Korea: a nationwide retrospective study. Br J Dermatol. 2012;166:115–123. doi: 10.1111/j.1365-2133.2011.10636.x. [DOI] [PubMed] [Google Scholar]
- 6.Phadke PA, Rakheja D, Le LP, et al. Proliferative nodules arising within congenital melanocytic nevi: a histologic, immunohistochemical, and molecular analyses of 43 cases. Am J Surg Pathol. 2011;35:656–669. doi: 10.1097/PAS.0b013e31821375ea. [DOI] [PubMed] [Google Scholar]
- 7.Krengel S, Hauschild A, Schafer T. Melanoma risk in congenital melanocytic naevi: a systematic review. Br J Dermatol. 2006;155:1–8. doi: 10.1111/j.1365-2133.2006.07218.x. [DOI] [PubMed] [Google Scholar]
- 8.Kinsler VA, Chong WK, Aylett SE, Atherton DJ. Complications of congenital melanocytic naevi in children: analysis of 16 years' experience and clinical practice. Br J Dermatol. 2008;159:907–914. doi: 10.1111/j.1365-2133.2008.08775.x. [DOI] [PubMed] [Google Scholar]
- 9.Ka VS, Dusza SW, Halpern AC, Marghoob AA. The association between large congenital melanocytic naevi and cutaneous melanoma: preliminary findings from an Internet-based registry of 379 patients. Melanoma Res. 2005;15:61–67. doi: 10.1097/00008390-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Bett BJ. Large or multiple congenital melanocytic nevi: occurrence of cutaneous melanoma in 1008 persons. J Am Acad Dermatol. 2005;52:793–797. doi: 10.1016/j.jaad.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Yelamos O, Arva NC, Obregon R, et al. A comparative study of proliferative nodules and lethal melanomas in congenital nevi from children. Am J Surg Pathol. 2015;39:405–415. doi: 10.1097/PAS.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 12.Christou EM, Chen AC, Sugo E, Barbaric D, Wargon O. Proliferative nodules of undifferentiated spindle cells arising in a large congenital melanocytic naevus. Australas J Dermatol. 2014;55:e24–28. doi: 10.1111/ajd.12017. [DOI] [PubMed] [Google Scholar]
- 13.Bastian BC, Xiong J, Frieden IJ, et al. Genetic changes in neoplasms arising in congenital melanocytic nevi: differences between nodular proliferations and melanomas. Am J Pathol. 2002;161:1163–1169. doi: 10.1016/S0002-9440(10)64393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh K, Moore S, Sandoval M, et al. Congenital malignant melanoma: a case report with cytogenetic studies. Am J Dermatopathol. 2013;35:e135–138. doi: 10.1097/DAD.0b013e318284a679. [DOI] [PubMed] [Google Scholar]
- 15.Murphy MJ, Jen M, Chang MW, Grant-Kels JM, Makkar H. Molecular diagnosis of a benign proliferative nodule developing in a congenital melanocytic nevus in a 3-month-old infant. J Am Acad Dermatol. 2008;59:518–523. doi: 10.1016/j.jaad.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Machan S, Molina-Ruiz AM, Fernandez-Acenero MJ, et al. Metastatic melanoma in association with a giant congenital melanocytic nevus in an adult: controversial CGH findings. Am J Dermatopathol. 2015;37:487–494. doi: 10.1097/DAD.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 17.Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krauthammer M, Kong Y, Bacchiocchi A, et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat Genet. 2015;47:996–1002. doi: 10.1038/ng.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shain AH, Garrido M, Botton T, et al. Exome sequencing of desmoplastic melanoma identifies recurrent NFKBIE promoter mutations and diverse activating mutations in the MAPK pathway. Nat Genet. 2015 doi: 10.1038/ng.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu C, Zhang J, Nagahawatte P, et al. The genomic landscape of childhood and adolescent melanoma. J Invest Dermatol. 2015;135:816–823. doi: 10.1038/jid.2014.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan AA, Lee AJ, Roh TY. Polycomb group protein-mediated histone modifications during cell differentiation. Epigenomics. 2015;7:75–84. doi: 10.2217/epi.14.61. [DOI] [PubMed] [Google Scholar]
- 22.He WP, Li Q, Zhou J, et al. Decreased expression of H3K27me3 in human ovarian carcinomas correlates with more aggressive tumor behavior and poor patient survival. Neoplasma. 2015;62:932–937. doi: 10.4149/neo_2015_113. [DOI] [PubMed] [Google Scholar]
- 23.Gerami P, Li G, Pouryazdanparast P, et al. A highly specific and discriminatory FISH assay for distinguishing between benign and malignant melanocytic neoplasms. Am J Surg Pathol. 2012;36:808–817. doi: 10.1097/PAS.0b013e31824b1efd. [DOI] [PubMed] [Google Scholar]
- 24.Gerami P, Jewell SS, Morrison LE, et al. Fluorescence in situ hybridization (FISH) as an ancillary diagnostic tool in the diagnosis of melanoma. Am J Surg Pathol. 2009;33:1146–1156. doi: 10.1097/PAS.0b013e3181a1ef36. [DOI] [PubMed] [Google Scholar]
- 25.Salgado CM, Basu D, Nikiforova M, et al. Amplification of mutated NRAS leading to congenital melanoma in neurocutaneous melanocytosis. Melanoma Res. 2015;25:453–460. doi: 10.1097/CMR.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 26.Salgado CM, Basu D, Reyes-Mugica M. NRAS Status in Giant Melanocytic Nevus With Metastatic Melanoma. Am J Dermatopathol. 2016 doi: 10.1097/DAD.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 27.Podlaha O, De S, Gonen M, Michor F. Histone modifications are associated with transcript isoform diversity in normal and cancer cells. PLoS Comput Biol. 2014;10:e1003611. doi: 10.1371/journal.pcbi.1003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon JA, Kingston RE. Occupying chromatin: Polycomb mechanisms for getting to genomic targets, stopping transcriptional traffic, and staying put. Mol Cell. 2013;49:808–824. doi: 10.1016/j.molcel.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleven AH, Sannaa GA, Briaire-de Bruijn I, et al. Loss of H3K27 tri-methylation is a diagnostic marker for malignant peripheral nerve sheath tumors and an indicator for an inferior survival. Mod Pathol. 2016 doi: 10.1038/modpathol.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer IM, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4–13. doi: 10.1038/modpathol.2015.134. [DOI] [PubMed] [Google Scholar]
- 31.Prieto-Granada CN, Wiesner T, Messina JL, Jungbluth AA, Chi P, Antonescu CR. Loss of H3K27me3 Expression Is a Highly Sensitive Marker for Sporadic and Radiation-induced MPNST. Am J Surg Pathol. 2016;40:479–489. doi: 10.1097/PAS.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]