Abstract
Intransitive competition networks, those in which there is no single best competitor, may ensure species coexistence. However, their frequency and importance in maintaining diversity in real-world ecosystems remains unclear. We used two large datasets from drylands and agricultural grasslands to assess: 1) the generality of intransitive competition, 2) intransitivity-richness relationships, and 3) effects of two major drivers of biodiversity loss (aridity and land-use intensification) on intransitivity and species richness. Intransitive competition occurred in >65% of sites and was associated with higher species richness. Intransitivity increased with aridity, partly buffering its negative effects on diversity, but was decreased by intensive land use, enhancing its negative effects on diversity. These contrasting responses likely arise because intransitivity is promoted by temporal heterogeneity, which is enhanced by aridity but may decline with land-use intensity. We show that intransitivity is widespread in nature and increases diversity, but it can be lost with environmental homogenization.
Keywords: aridity, biodiversity, coexistence, drylands, land use, mesic grasslands, rock-paper-scissors game
Introduction
Species coexistence is made possible by a range of mechanisms including differential resource uptake, frequency-dependent enemy attack or limited dispersal (Chesson 2000; HilleRisLambers et al. 2012). Most of these mechanisms reduce competitive exclusion; however, such reduction is not required for species coexistence because the absence of a competitive hierarchy may allow species coexistence even if they compete strongly (Gilpin 1975; Wootton 2001). This lack of competitive hierarchy within a community is nature’s equivalent to the rock-paper-scissors game: species A excludes B (A>B), B excludes C (B>C) but C excludes A (C>A; e.g., Kerr et al. 2002). Such networks of interactions are termed intransitive competition networks and may enhance species coexistence because no species is a universally weak competitor (Laird & Schamp 2006, Rojas-Echenique & Allesina 2010).
Intransitivity can emerge and allow species coexistence via different mechanisms. Niche differentiation can generate intransitivity if species compete for the same nutrients but have differential competitive abilities depending on their balance (e.g., N/P ratios) or on the presence of a third species (e.g., Huisman et al. 2001; Borer et al. 2007). Such intransitivity can be enhanced by temporal resource heterogeneity and/or spatial heterogeneity among different interaction neighborhoods (Allesina & Levine 2011). Alternatively, intransitivity may arise if the hierarchy in species’ ability to exploit resources differs from their ability to prevent resource uptake by others (Buss 1980; Laird & Schamp 2006). Intransitive competition networks may be common in nature, although studies empirically demonstrating them have generally focused on species-poor assemblages of, e.g., bacteria (Kerr et al. 2002), lizards (Sinervo & Lively 1996), or intertidal organisms (Buss 1980).
Mathematical models have provided further insights into the underlying mechanisms and ecological implications of intransitive competition networks (Gilpin 1975; Laird & Schamp 2006; Allesina & Levine 2011). However, modelling studies alone cannot reveal how frequent intransitivity is or how many species in natural communities are maintained by it. Indeed, the role of intransitive competition in structuring plant communities remains unclear despite years of research devoted to answering this question. Some studies have found that intransitivity is an important mechanism structuring plant communities (e.g., Freckleton et al. 2000), but others have suggested the opposite (e.g., Grace et al. 1993). A potential explanation for these contrasting results is that the degree of intransitivity depends on the species pool considered. As with many measures of community organization, considering an overall metric for all species in the community can render very different results than more detailed analyses of a particular subset of species (e.g., Stone & Roberts 1992; Ulrich & Gotelli 2007). Similarly, if competition is intransitive amongst dominant or amongst rare species, but strongly hierarchical (i.e., transitive) between such groups, communities would be organized by nested intransitive networks. Such nestedness could increase coexistence, but would result in no overall signal of intransitivity for the whole community. To test this idea field assessments quantifying intransitivity for different groups of species within a community are necessary.
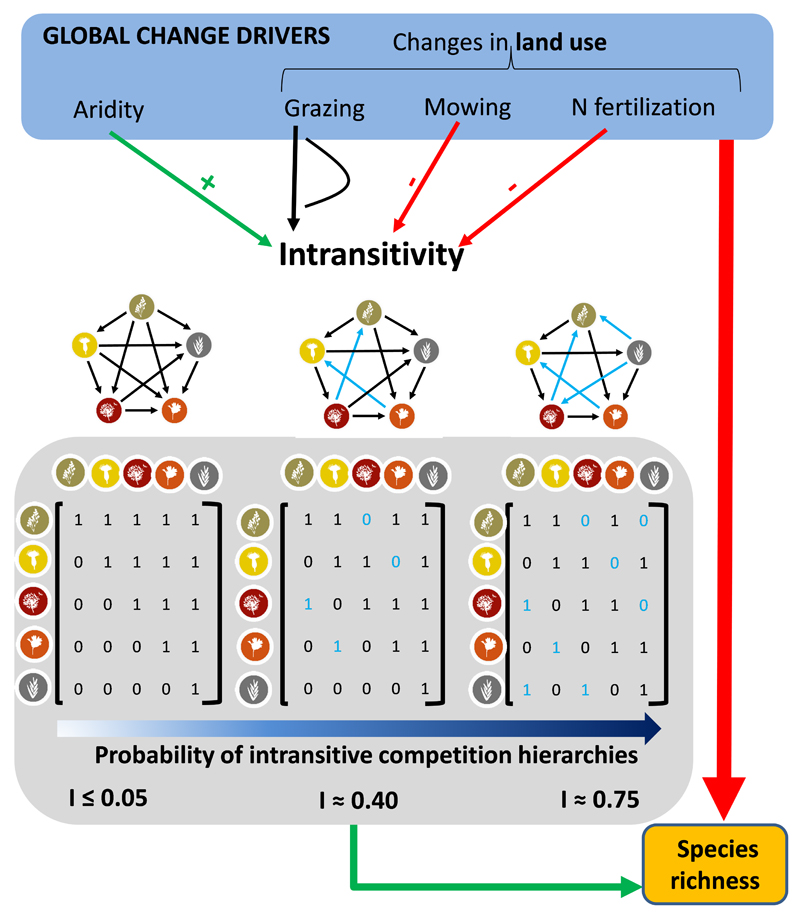
The degree of intransitivity in plant communities might also be altered by two of the major global change drivers (GCDs hereafter) threatening biodiversity in terrestrial ecosystems: land-use intensification and climate change (Sala et al. 2000). Both GCDs alter heterogeneity and productivity, which in turn are likely to affect intransitive competition networks. Intransitivity might be more common and important for coexistence in productive environments, because environmental filtering is relaxed and competition may be more important than disturbance or abiotic stress in structuring communities (e.g., Gilpin 1975; Bowker et al. 2010). Productivity increases with fertilization (Suding et al. 2005; Manning 2013), which would suggest more intransitivity at higher land-use intensity. Modelling and empirical evidence suggest that intransitive competition is more likely to occur in heterogeneous environments (Huisman et al. 2001). In these cases, niche specialization coupled with different limiting resources across local interaction neighbourhoods can generate, or interact with, intransitive competition enhancing species coexistence (Allesina & Levine 2011). In this regard, GCDs can modify the level of intransitivity in a community by altering not only the spatial, but also the temporal heterogeneity in resources. High land-use intensity (fertilization or overgrazing) can reduce variation in biomass over time (Osem et al. 2002; Grman et al. 2010), suggesting that temporal heterogeneity is reduced at high land use intensity. On the other hand, climate change could increase temporal heterogeneity, especially in drylands, where water availability is often more variable in drier than wetter environments (Whitford 2002). The well-known negative effects of GCDs on diversity may therefore be buffered or enhanced depending on their indirect effects on the degree of intransitivity (Fig. 1). However, the interrelationship between GCDs and the competitive hierarchy amongst coexisting species is poorly understood.
Figure 1.
Conceptual model outlining our theoretical framework. We address here the direct and indirect relationships between 1) global change drivers (GCDs), 2) intransitive competition networks and 3) species richness. Intransitivity is expected to increase richness. GCDs are expected to decrease species richness and have variable effects (positive = green, negative = red, unimodal = black) on intransitivity. Species-by-species transition matrices with different levels of intransitivity are shown. These matrices have an associated competition network (arrow pointing from winner to loser) and their changes in abundance across time or space (represented in different columns within the grid boxes). Competitive reversals from perfect hierarchical competition are in blue (numbers and arrows) and the changes expected in our intransitivity metric (I) are shown.
To address these research gaps we used a recently developed method to measure the degree of intransitivity from observational data (Ulrich et al. 2014a) using two large datasets describing plant diversity responses to changes in land use or aridity. We tested the following hypotheses: i) intransitive competition is frequent in plant communities, ii) intransitive networks are more common amongst species similar in dominance, but transitive competition (i.e., strong hierarchy among competitors) prevails between species with contrasting dominance levels (i.e., intransitive competition networks are nested), iii) the degree of intransitivity in plant communities is positively related to their species richness, and iv) increases in intransitivity in response to more temporally heterogeneous or fertile environments mitigate the impact of increasing aridity and intensive land uses on plant richness.
Materials and Methods
Study sites
We used two large-scale datasets: the occurrence of plant species in European grasslands along land-use intensity gradients (the German Biodiversity Exploratories; Fischer et al., 2010), and the occurrence of plant species along aridity gradients in global dryland ecosystems (the BIOCOM project; Maestre et al., 2012). These two datasets complement each other and allow us to assess the overall frequency and drivers of intransitivity across a wide range of communities varying in habitat type, species pool and environmental conditions as well as across datasets with different sampling methods (see details below).
The Biodiversity Exploratories include 1500 grassland plots, varying in land use and situated in three regions of Germany (Fischer et al., 2010; Blüthgen et al. 2012; Socher et al. 2013). In each of these 4 m × 4 m grassland plots, the relative cover of all plant species was recorded. In the center of each plot, a 10 cm-depth soil sample was taken to measure total soil nitrogen and soil organic carbon concentrations (Fischer et al. 2010). Information on land use was obtained via questionnaires sent to land owners; these asked about grazing type (permanent, rotational, none), livestock type (sheep, cattle or other), fertilization (fertilized or unfertilized), mowing (number of cuts per year), and the presence of water drainage or water retention structures (see Fischer et al., 2010; Blüthgen et al. 2012 for full methodological details). This classification resulted in 40 different levels of land-use intensity and management types. Hereafter, we refer to this dataset as “grasslands”.
Data from the BIOCOM project were gathered in 224 dryland sites (all with aridity index values [precipitation/potential evapotranspiration] < 0.65) scattered across all continents except Antarctica. These sites include a variety of habitat types (grasslands, shrublands and open woodlands). In each habitat type, the sites were placed spanning a natural gradient of aridity (full details in Maestre et al. 2012). At each site plant species and their relative cover were recorded in four 30m-long quadrats, divided into 80 1.5 m × 1.5 m quadrats. Climatic variables were extracted from the WorldClim database (Hijmans et al. 2005), and were used to derive an aridity index (precipitation/potential evapotranspiration). To ease interpretation, we use the complement of the aridity index (1-aridity index) so that higher levels of this metric indicate drier environments (Delgado-Baquerizo et al. 2013). Hereafter, we refer to this dataset as “drylands”.
Data organization and measurement of the degree of intransitivity
We measured the degree of intransitivity in the grassland and dryland datasets by using the Markov chain approach of Ulrich et al. (2014a). Under the assumption that observed species abundances represent the equilibrium abundances of the species forming the community, the method allows us to assess 1) to what degree competition predicts observed species abundances, and 2) the degree of intransitivity within a given competition network. As a measure of species abundances, we used the cover of each species within each quadrat (drylands) or plot within a cluster (grasslands; see how clusters were assembled below). Thus a single metric of intransitivity was calculated by each site (drylands) or cluster (grasslands). At equilibrium, observed species abundances should be equal to the dominant eigenvector of a hypothetical species × species transition matrix (i.e., the matrix that contains the probability that one species replaces another in a given quadrat [drylands] or plot [grasslands]); as used in Markov chain models. 100,000 patch-transition species by species matrices are randomly generated, of which the 100 best fitting ones (i.e. matrices where the dominant eigenvector is closest to the observed species abundances) are chosen. The match (R2) between simulated and observed abundances informs about the importance of competition, with higher values meaning higher importance of competition for community assembly. We used for further analyses those sites or clusters with match levels (R2) > 0.60, as their metrics of intransitivity are reliable. Results using a higher threshold (R2 > 0.70) were qualitatively similar to those presented here and are not shown.
If competition is fully transitive then one species will always have a higher probability of displacing the rest (represented as high transition coefficients between species in the matrix columns vs those in the matrix rows; Fig. 1). If however, there are competitive reversals (species in the rows displace species in the columns, blue numbers in Fig. 1) then this indicates intransitivity. The degree of intransitivity can be measured as the number of competitive reversals found in the best-fitting matrices (see also Laird & Schamp 2006). Our intransitivity metric (I) is the normalized count of these competitive reversals in the patch-transition matrix (equation 1; Ulrich et al. 2014a):
| (1) |
where pij is the probability that species i (in the column) replaces species j (in the row) in a given patch; j ranges from 1 to m (total number of species), i from m to m-1, and k from i+1 to m. Increasing values of I indicate higher levels of intransitivity within the community. Our metric (I), therefore, ranges from 1 (fully intransitive community) to 0 (fully transitive community; Fig. 1).
Although spatial heterogeneity between local neighbourhoods may enhance intransitive competition by providing more opportunities for niche differentiation, it may also complicate its measurement and make competition ranks more difficult to estimate from the observed abundances (e.g., Ulrich et al. 2014a). Thus, for our method to yield reliable results, quadrats within a given site (drylands) or plots within a given cluster (grasslands) should be as homogeneous as possible. To meet this requirement, and according to their different structure, the two datasets were organized differently (henceforth we refer to them as grassland clusters and dryland sites). As the grasslands dataset lacked within-plot replication, we organized the 1500 plots into 190 plot clusters with the same land-use type and region to have enough replication to calculate I. The high number of species found in the grasslands (318–365, depending on the region; Socher et al. 2013) made it impossible to produce clusters of plots which were relatively homogenous in their environmental conditions and contained a sufficient number of plots to analyze all possible interactions between species pairs. Therefore, we only considered the five dominant species within each cluster and divided the dataset in clusters of ~ 6–10 plots (always greater than 5, the number of species considered). When 12 or more plots were found within the same land-use type and region, we divided them into two clusters according to total soil nitrogen and organic carbon concentrations to create the most environmentally homogeneous, and the highest number, of clusters possible.
To allow comparison between both datasets, we also considered the five dominant species within each site in drylands. Using this database we assessed changes in our intransitivity metric as a function of the number of species considered, progressively including a larger number of subordinate species. This allowed us to determine if the probability of detecting intransitive competition varied depending on the target species pool, and therefore if intransitive competition networks were nested (present only amongst the dominant species) or not (see full results in Appendix S1).
The methodology used here has three important assumptions to which our results are reasonably robust. First, it assumes that the sampled communities are at equilibrium. The high match between observed and predicted abundances (see results), and the consistency of match levels across all land-use intensities (Appendix S2) suggest that violations of this assumption have not affected the results. Second, we assume that species are not dispersal limited within our sites or clusters. Dispersal limitation is unlikely because the selected species are abundant across the three regions (grasslands; see also Appendix S3), and sampling quadrats were close to each other (drylands). Third, we assumed our sites to be environmentally homogeneous. To further determine that environmental variation between sites did not drive intransitivity measures, we recalculated intransitivity whilst correcting for environmental conditions and this led to similar values (see Appendix S2).
Separating intransitive competition from other processes enhancing coexistence is difficult from observational, or even manipulative, studies. The main distinguishing characteristic between these mechanisms is that intransitivity relies on strong competition, i.e., it reduces co-occurrence of plant species within local interaction neighbourhoods (i.e., quadrat [drylands] or plot [grasslands] scale; Laird & Schamp 2006). The latter should lead to segregation of species between sampling quadrats. Those coexistence mechanisms relying on reduced competition (e.g., differential resource uptake), instead, should allow co-occurrence of different plant species at the local interaction neighbourhood scale. When applied to the matrices of the drylands dataset, our intransitivity metric was positively correlated with the level of species spatial segregation between quadrats (Spearman´s ρ = 0.59; Appendix S4). These results suggest that a high level of competitive exclusion within these local interaction neighbourhoods took place in the studied plots, and thus a strong confounding effect of other local-scale coexistence mechanisms that reduce competition in our results is unlikely.
Statistical analyses
Extent of intransitive competition in nature and its relationship with species richness
We evaluated whether average values of our intransitivity metric (I) differed from 0.05 (indicating fully transitive communities) by using Wilcoxon’s matched pairs test. The threshold of 0.05 was obtained from simulated matrices; those with intransitive loops always had predicted values of I > 0.05, whereas the 95% confidence limits of I in test matrices with no intransitivity always included the value of 0 (Ulrich et al. 2014a). Separate tests were performed to assess whether or not metrics calculated for each dataset (clusters in grasslands or sites in drylands) differed from this threshold. To compare the level of intransitivity between the two datasets, we used Mann-Whitney rank tests because the data departed from a normal distribution. The relationship between intransitivity and species richness was evaluated by performing OLS model II regressions using the lmodel2 package (Legendre 2008) for R version 3.0.2 (R Development Core Team 2013). Wilcoxon and Mann-Whitney rank tests were conducted with SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
Relationship between intransitivity, global change drivers and diversity
We used structural equation modeling (SEM; Grace 2006) to analyze the relationships between land use (grasslands) or aridity (drylands), intransitivity and species richness. Our a priori model followed the rationale stated in the introduction (see also Fig. 1): aridity and land use affect both species richness and intransitivity, and intransitivity affects species richness (see Appendix S5 for full details and rationale). Both datasets have strong spatial clustering (sites were sampled within regions in the grasslands and within countries in the drylands). To account for this, we introduced the geographic coordinates in the SEMs. Latitude sufficed to represent the spatial distribution of the grassland dataset (the three regions were distributed along a North-South axis) whereas both latitude and longitude were necessary to represent the spatial distribution of the dryland sites, which were globally distributed.
The different land-use categories (grazing and livestock types, number of cuts per year, fertilization or water management) from the grasslands dataset were simplified with a non-metric multidimensional scaling (NMDS), which can handle categorical and continuous variables (McCune & Grace 2002). A two-dimensional NMDS solution was sufficient to represent the data. High values along axis 1 indicated the more intense land-use practices of water drainage and permanent grazing (rather than rotational grazing). High values along axis 2 were associated with grazing by livestock other than sheep (mostly cattle, which have a larger impact in terms of biomass removal and plant diversity than sheep; Blüthgen et al. 2012; Socher et al. 2013) and more frequent mowing (axis 2; details in Appendix S5).
An additional set of analyses were performed as an alternative to data reduction with NMDS in the grasslands dataset. Land-use factors could vary in their effects (e.g., grazing vs. fertilization) and also in their effect within regions (Socher et al. 2013). Thus, separate SEMs using grazing, fertilization, number of cuts and water management as different land-use predictors were performed, and the same a priori model structure was used to analyze each region separately (details and results in Appendix S6). SEM analyses were performed using AMOS for windows (SPSS Inc., Chicago, IL, USA).
Results
Extent of intransitive competition in nature and its relationship with species richness
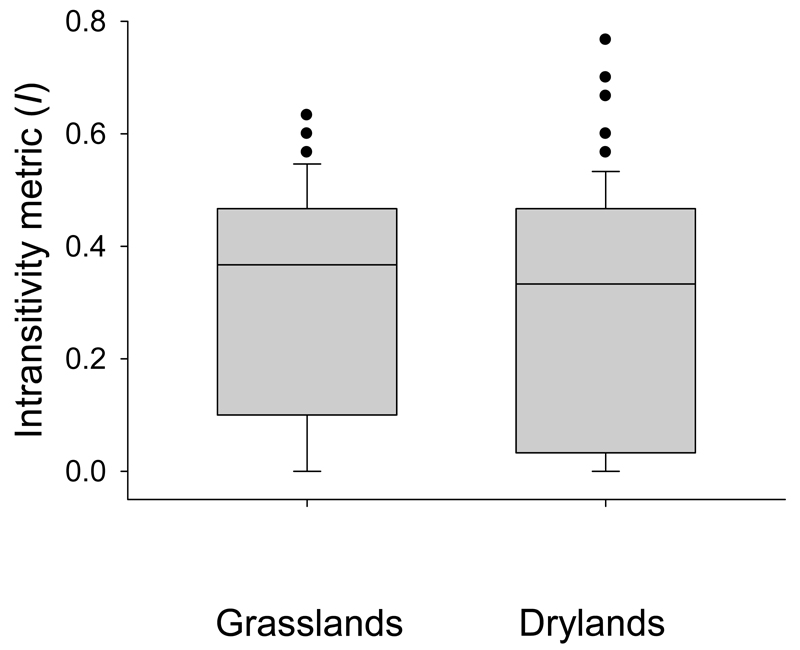
Intransitive competition networks (those in which I > 0.05) were detected at most study sites (Wilcoxon’s test: z < -8.9; P < 0.001; N > 150 in both datasets; Fig. 2). The simulated matrices satisfactorily reflected observed abundances in 92% of the grassland and 78% of the dryland sites (R2≥ 0.70 in both cases, although these percentages were smaller when including environmental variables; Appendix S2). Although the average degree of intransitivity did not change across the two datasets (Fig. 2), the frequency of sites displaying some degree of intransitivity did: 82% of grassland clusters had I values higher than 0.05, while this was the case in 68% of the dryland sites.
Figure 2.
Intransitivity (measured as metric I) observed in grasslands (n = 175 clusters of environmentally similar grasslands out of a set of 1500 sites) and drylands (n = 151, sites). Box plots show the median, 25% and 75% quartiles. The intransitivity metric was not significantly different between the two datasets (Mann-Whitney´s U = 12030; P = 0.16).
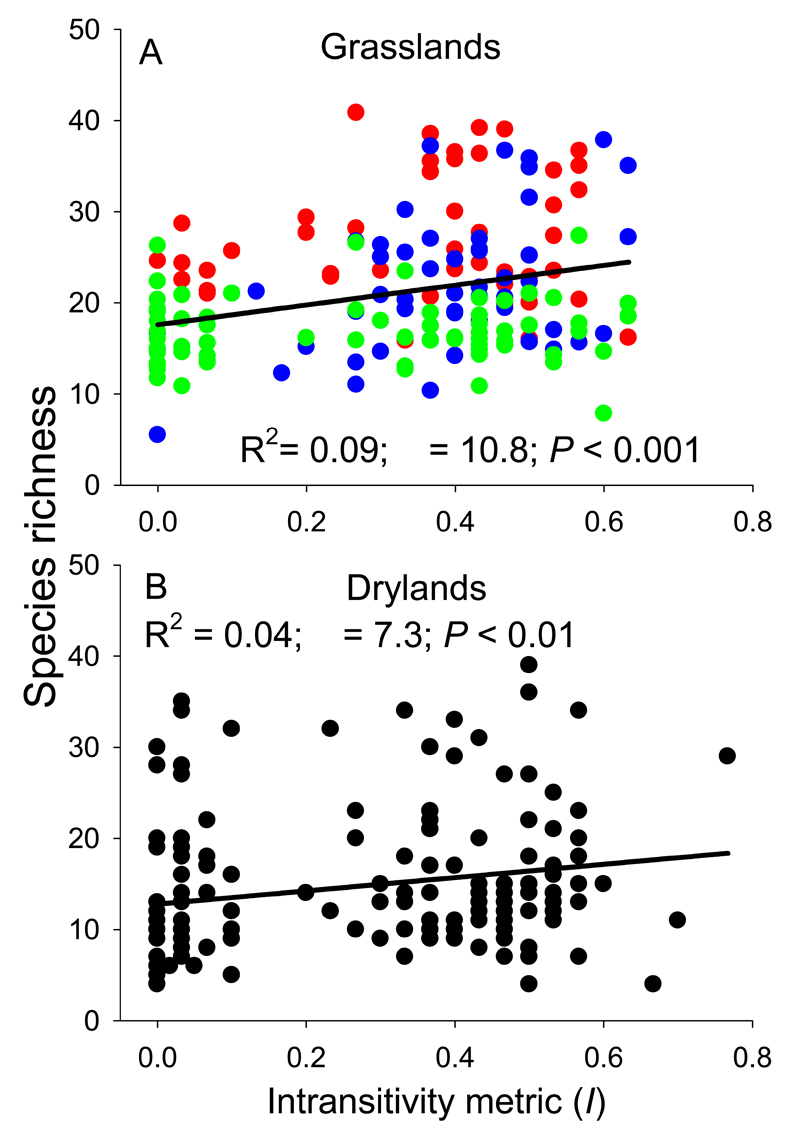
Furthermore, the strength of intransitivity (I value) was positively related to plant richness in both datasets (Fig. 3). The presence of intransitivity increased species richness by 6 species in the grasslands and by 4 in the drylands, based on comparing the lowest (I < 0.05) and highest (0.4 < I < 0.8) levels of intransitivity within the studied communities (Fig. 3). Although the overall relationship between intransitivity and richness was consistent across datasets (Fig. 3), within both datasets the level of intransitivity and its relationship with richness varied geographically. The degree of intransitivity decreased with increasing latitude in both grasslands and drylands (Fig. 4). Intransitivity-richness relationships were either positive (Central), neutral (North-east) or negative (South-west) depending on the grassland region considered (Fig. 3A; Appendix S6).
Figure 3.
Relationships between intransitivity (measured as metric I) and species richness in grasslands (A; mean for each cluster of sites) and drylands (B). Model II OLS regression results are shown. The different colors in the upper panel show the three different study regions: Southwest (red, n = 50), Central (blue, n = 54) and Northeast (green, n = 71).
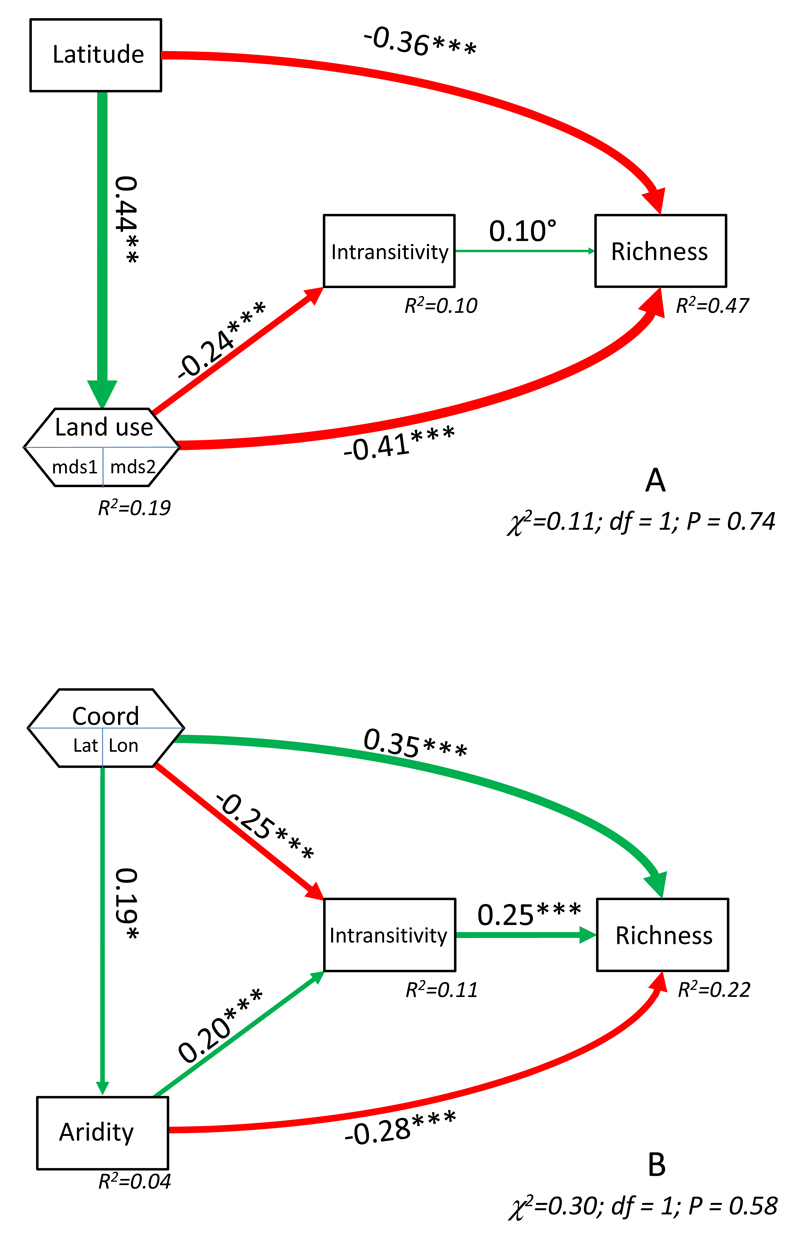
Figure 4.
Structural equation models depicting effects of geographic factors (region or latitude/longitude) and global change drivers (land-use or aridity) on intransitivity and species richness for grasslands (A) and drylands (B). Composite variables are shown with the variables forming them inside. The width of arrows is proportional to the standardized path coefficient, with green and red lines for positive and negative relationships, respectively. The overall goodness-of-fit test and the R2 for each variable introduced are given. P-values are: *** = P < 0.001; ** = P < 0.01; * = P < 0.05; º = P < 0.1. Lat = latitude, lon = longitude, mds= non-metric multi-dimensional ordination axes performed with the land-use variables.
We found an exponential decay in I values as more subordinate species were considered in our calculations (Appendix S1). This suggests strong nestedness of intransitive networks caused by high intransitivity amongst the dominant species, and strong competitive exclusion of rarer species by dominant ones. It must be noted that the positive relationship between intransitivity and species richness remained consistently positive regardless of the number of species considered (Appendix S1).
Effects of global change drivers on intransitivity and diversity
Land-use intensification and aridity reduced species richness, but had contrasting effects on intransitivity (Fig. 4). Aridity increased intransitivity in dryland communities (Fig. 4B), and this indirectly ameliorated the negative effects of aridity on species richness. In contrast, increasing land-use intensification reduced intransitivity, and this slightly enhanced the direct negative effects of land-use intensity on diversity. More detailed analysis of the land-use effects revealed that both fertilization and mowing decreased species richness and the degree of intransitivity (Table 1). Increased grazing intensity had a similar effect: switching from rotational to permanent grazing, or from sheep to cattle grazing, substantially reduced species richness and intransitivity. Nevertheless, a clear result was that the intransitivity-mediated effect of land use intensification on species richness was much weaker and variable than its direct negative effects (Table 1).
Table 1.
Summary results of the structural equation models performed with the different environmental factors (in rows) separately. Standardized total effects (STE; sum of direct and indirect effects) and standardized direct effects (SDE; equivalent to the path coefficient from the predictor to the response variable) for richness are shown. For intransitivity STE = SDE. Environmental factors were introduced as: Mowing (number of cuts per year), grazing (sheep/other, permanent/rotational/none) fertilization (yes/no), and water management (drainage/retention/none). Significant path coefficients are highlighted in bold.
| Intransitivity | Richness | ||
|---|---|---|---|
| SDE | SDE | STE | |
| Mowing | -0.16 | -0.29 | -0.31 |
| Grazing | 0.20 | 0.50 | 0.51 |
| Fertilization | -0.09 | -0.44 | -0.45 |
| Water management (only NE region) | 0.15 | -0.46 | -0.45 |
| Aridity | 0.20 | -0.28 | -0.23 |
Discussion
Extent of intransitive competition networks in natural plant communities
Intransitive competition networks have previously only been demonstrated in simple three-species systems (e.g., Buss 1980; Sinervo & Lively 1996; Kerr et al. 2002) and in mathematical models (Gilpin 1975; Wootton 2001; Laird & Schamp 2006; Allesina & Levine 2011). To date there has been little empirical evidence to suggest that they are widespread in nature (but see Bowker et al. 2010; Allesina & Levine 2011; Soliveres et al. 2011). Using field data from two large datasets and a novel methodology, we provide strong evidence that intransitive competition networks are both common in natural plant communities and are associated with higher species richness. This general pattern was robust and not influenced by the biome, sampling methodology or spatial scale considered.
Previous studies assessing the degree of intransitivity in plant communities have generated contrasting results and substantial debate (Aarsen 1988; Keddy & Shipley 1989; Silvertown & Dale 1991; Grace et al. 1993; Freckleton et al. 2000). Generally, these studies concluded that intransitivity is uncommon in plant communities and, therefore, sharply contrast with our results (Grace et al. 1993; but see Aarsen 1988; Freckleton et al. 2000; Allesina & Levine 2011). This contrast may be explained by the differences in the methodology used and the species pool considered. Pairwise competition experiments are often performed in the greenhouse, and do not consider multispecies assemblages or the context-dependency of competition under natural and changing environments (Herben & Krahulec 1990; Silvertown & Dale 1991; Chamberlain et al. 2014). Thus, the pairwise approach to estimating competition could underestimate the occurrence of intransitive loops (Grace et al. 1993; Laird & Schamp 2008; Allesina & Levine 2011). Indeed, competitive hierarchies identified using pairwise approaches fail to predict observed abundances in the field (Aarsen 1989; Weigelt et al. 2007; Engel & Wetzin 2008). In contrast, the patch-transition matrices used here implicitly account for competition under natural conditions and in multiple species assemblages (Ulrich et al. 2014a and references therein), and thus provide a truer reflection (according to the high match levels found between simulated and observed data) of competitive hierarchies and more accurate assessments of intransitivity in natural communities.
Regarding the role of the species pool when estimating intransitivity, and in agreement with our second hypothesis, we found strong nestedness in intransitive competition networks. Our results suggest high levels of intransitivity among the dominant species, but not between dominant and rare species (Appendix S1). Studies focusing on dominant species will, therefore, likely find high levels of intransitivity (e.g., Freckleton et al. 2000), whereas those including broader species pools will likely find the opposite pattern. While these contradictory results have fueled strong debate (e.g., Aarsen 1988; Grace et al. 1993), only by analyzing real-world data were we able to cast some light on the potential explanation for these contradictions. Our nestedness hypothesis requires experimental confirmation, but it suggests that coexistence of similarly abundant (or co-dominant) species could be promoted by nested intransitive competition networks.
The relationship between intransitivity and species richness
Our study is, to the best of our knowledge, the first to empirically show a positive relationship between the strength of intransitivity and species richness in natural communities, thus supporting previous mathematical and conceptual models (Huisman et al. 2001; Laird & Schamp 2006; Wootton 2001; Rojas-Echenique & Allesina 2010). This relationship suggests that the degree of intransitivity among the dominant species alone could explain 4–9% of the variance observed in plant species richness, which, given the wide range of environmental conditions, habitats and sampling procedures in our study, suggests that intransitivity is an important driver of species richness. We also find that intransitive competition boosted species richness considerably (Fig. 3). Future work is needed to fully integrate intransitive competition with coexistence theory (Chesson 2000; HilleRisLambers et al. 2012) and to determine whether intransitive loops equalize fitness between species (e.g., Laird & Schamp 2006) and/or stabilize niche differences (Rojas-Echenique & Allesina 2010). However, these first empirical results on the relationship between intransitivity and diversity suggest that it may be an important, but largely overlooked, coexistence mechanism. Our results also suggest that incorporating multi-species (rather than multiple pairwise) competition dynamics and nested competition networks, which have been largely neglected before, can contribute explaining species coexistence. More studies are needed to confirm whether the patterns we find are consistent across ecosystem types and different groups of organisms; our results and the methodology employed (Ulrich et al. 2014a) pave the way for such future research.
Effects of global change drivers on intransitivity and diversity
Could an increase in intransitivity offset the negative effects of global change drivers (GCDs) on diversity? This would require three conditions: i) GCDs (here, aridity or land-use intensity) directly decrease richness, ii) intransitivity increases richness, and iii) GCDs increase intransitivity. While i) and ii) were supported by our results, we found that iii) was largely dependent on the GCD studied (Table 1).
We speculate that the contrasting effects of aridity and land-use intensity on intransitivity are related to their different effects on temporal heterogeneity. We minimized the role of spatial heterogeneity on our intransitivity metric. Thus, although spatial heterogeneity across local interaction neighbourhoods would normally be an important driver of intransitivity and plant coexistence (Huisman et al. 2001; Sears & Chesson 2007; Allesina & Levine 2011), it should not affect intransitivity here. However temporal heterogeneity is still expected to increase opportunities for intransitivity. Temporal heterogeneity could enhance intransitivity in competition networks through temporal storage effects (Chesson 1983) as a given species will experience higher intra- than inter-specific competition during favorable time periods and this may hinder its ability to compete with others, enhancing the chances to form intransitive competition loops. It may also provide more opportunities for niche differentiation, where slightly different environmental conditions across time can generate different competition hierarchies and therefore enhance community-level intransitivity and allow coexistence (Allesina & Levine 2011). In this regard, aridity is known to increase temporal heterogeneity in water availability (e.g., Whitford 2002) which might explain the more pronounced effects of intransitivity in drylands. Land-use intensification (grazing and fertilization) instead, reduces temporal heterogeneity in biomass (Osem et al. 2002; Grman et al. 2010) and also asynchrony of species fluctuations in diverse communities (Hautier et al. 2014). Additionally, both grazing and mowing can compromise potential trade-offs between competition abilities (e.g., those between resource uptake and pollinator attraction) and reduce the chances for intransitive competition (Aarsen 1992). Overall, more intensive land uses can reduce temporal niche dimensionality (similarly as it does with spatial niche dimensionality; Harpole & Tilman 2007) and therefore shifts in competition hierarchy across time, preventing intransitive competition. This may explain why increasing temporal heterogeneity in land use has been shown to increase diversity (Allan et al. 2014) as it might also increase intransitivity and other coexistence mechanisms. Overall, our results point to another means by which GCDs alter competition between plants (see Tilman & Lehman 2001 for a review) and suggest that the effects of such GCDs depend on how they impact upon the temporal heterogeneity of resources. The unique nature of our data also allowed us to shed some light on other drivers of intransitivity within natural communities, which have been largely overlooked by previous studies and also are likely to be linked to changes in temporal heterogeneity. For example, the strong latitudinal gradient in intransitivity found in the drylands could be due to rainfall variability, which decreases from north to south in the studied sites (Ulrich et al. 2014b).
Our results provide weak support for the notion that intransitive competition networks should prevail in more productive environments (Gilpin 1975; Bowker et al. 2010). We found a higher frequency of intransitive communities in the more productive grasslands (~81%) than in the drylands (~67%; but see Appendix S2). However, productivity may not positively affect intransitivity at smaller scales: the more heavily fertilized grasslands had lower intransitivity (Table 1), as did those in the northern region in Germany, which is also the most productive (Fischer et al. 2010). Thus, it is unlikely that the negative effects of fertilization on diversity (Suding et al. 2005; Socher et al. 2013) will be counterbalanced by increased intransitivity associated with overall productivity (see Table 1). The latter result might be explained by a shift towards light competition with increased fertility and an increased dominance by some fast-growing species (Tilman & Lehman 2001; Suding et al. 2005). This is likely to increase fitness differences between species which would be expected to result in more asymmetric and therefore more transitive competition.
Conclusions
We found that intransitive competition networks are widespread in natural plant communities and explained 4-9% of the variance in species richness across a wide variety of habitat-types and environmental conditions. Additionally, different global change drivers had contrasting effects on intransitivity: aridity increased it, while land-use intensification generally reduced intransitivity. These differences are probably explained by their contrasting effects on temporal environmental heterogeneity. Thus, more intransitive competition could partially buffer diversity loss in natural communities, where the drivers of diversity loss increase this heterogeneity, but it is unlikely to buffer diversity loss resulting from environmental homogenization. Finally, we identified two properties of intransitive networks that have been previously overlooked: a strong geographical gradient and a nested structure in intransitive competition networks, both undetectable with previous modelling or local empirical studies. The latter suggests that intransitivity is prevalent between dominant species, but not between dominant and rarer species, and this could explain contrasting results between studies of differing species pool size. Forty years after its inclusion in ecology, we assessed for the first time the extent of intransitive competition in real-world plant communities. Our approach and findings pave the way for wider empirical evaluation of intransitivity in a range of systems, and highlight the links between intransitivity and other well-studied coexistence mechanisms.
Supporting Information
Additional Supporting Information may be downloaded via the online version of this article at Wiley Online Library (www.ecologyletters.com).
Acknowledgements
We thank three anonymous reviewers for comments on a previous version of this manuscript. We thank the managers of the Biodiversity Exploratories, Swen Renner, Sonja Gockel, Kerstin Wiesner, and Martin Gorke for their work in maintaining the plot and project infrastructure; Simone Pfeiffer and Christiane Fischer giving support through the central office, Michael Owonibi for managing the central data base, and Eduard Linsenmair, Dominik Hessenmöller, Jens Nieschulze, François Buscot and the late Elisabeth Kalko for their role in setting up the Biodiversity Exploratories project. We thank all the member of the EPES-BIOCOM network for their involvement in data collection. The work has been partly funded by the DFG Priority Program 1374 "Infrastructure-Biodiversity-Exploratories" (DFG- FI1246/6-1, DFG-FI1246/9-1) and by the BIOCOM project, funded by the European Research Council under the European Community's Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement 242658. Field work permits were issued by the responsible state environmental offices of Baden-Württemberg, Thüringen, and Brandenburg (according to § 72 BbgNatSchG). WU was supported by the Polish National Science Centre (grant 2014/13/B/NZ8/04681).
Footnotes
Authorship
SS, EA and FTM conceived the study, SS and WU analyzed the data, SS, MF, FTM, DP, MB, SB, JLQ, IS, MDB, WW, VO, PM, SAS and JM gathered data, SS wrote the first draft and all co-authors significantly contributed to improve it.
References
- 1.Aarssen LW. ‘Pecking order’ of four plant species from pastures of different ages. Oikos. 1988;51:3–12. [Google Scholar]
- 2.Aarssen LW. Causes and consequences of variation in competitive ability in plant communities. J Veg Sci. 1992;3:165–174. [Google Scholar]
- 3.Allan E, Bossdorf O, Dormann CF, Prati D, Gossner MM, Tscharntke T, et al. Interannual variation in land-use intensity enhances grassland multidiversity. Proc Natl Acad Sci USA. 2014;111:308–303. doi: 10.1073/pnas.1312213111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allesina S, Levine JM. A competitive network theory of species diversity. Proc Natl Acad Sci USA. 2011;108:5638–5642. doi: 10.1073/pnas.1014428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blüthgen N, Dormann CF, Prati D, Klaus VH, Kleinebecker T, Hölzel N, et al. A quantitative index of land-use intensity in grasslands: Integrating mowing, grazing and fertilization. Basic Appl Ecol. 2012;13:207–220. [Google Scholar]
- 6.Borer ET, Hosseini PR, Seabloom EW, Dobson AP. Pathogen-induced reversal of native dominance in a grassland community. Proc Natl Acad Sci USA. 2007;104:5473–5478. doi: 10.1073/pnas.0608573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowker MA, Soliveres S, Maestre FT. Competition increases with abiotic stress and regulates the diversity of biological soil crusts. J Ecol. 2010;98:551–560. [Google Scholar]
- 8.Buss LW. Competitive intransitivity and size-frequency distributions of interacting populations. Proc Natl Acad Sci USA. 1980;77:5355–5359. doi: 10.1073/pnas.77.9.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamberlain SA, Bronstein JL, Rudgers JA. How context dependent are species interactions? Ecol Lett. 2014;17:881–890. doi: 10.1111/ele.12279. [DOI] [PubMed] [Google Scholar]
- 10.Chesson P. Coexistence of competitors in a stochastic environment: The storage effect. Pop Biol. 1983;52:188–198. [Google Scholar]
- 11.Chesson P. Mechanisms of maintenance of species diversity. Ann Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 12.Delgado-Baquerizo M, Maestre FT, Gallardo A, Bowker MA, Wallenstein MD, Quero JL, et al. Aridity decouples soil C, N and P biogeochemical cycles in global drylands. Nature. 2013;502:672–676. doi: 10.1038/nature12670. [DOI] [PubMed] [Google Scholar]
- 13.Engel EC, Wetzin JF. Can community composition be predicted from pairwise species interactions? Plant Ecol. 2008;195:77–85. [Google Scholar]
- 14.Fischer M, Bossdorf O, Gockel S, Hänsel F, Hemp A, Hessenmöller D, et al. Implementing large-scale and long-term functional biodiversity research: The biodiversity exploratories. Basic Appl Ecol. 2010;11:473–485. [Google Scholar]
- 15.Freckleton RP, Watkinson AR, Dowling PM, Ley AR. Determinants of the abundance of invasive annual weeds: community structure and non-equilibrium dynamics. Proc R Soc B. 2000;267:1153–1161. doi: 10.1098/rspb.2000.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilpin ME. Limit cycles in competition communities. Am Nat. 1975;109:51–60. [Google Scholar]
- 17.Grace JB. Structural Equation Modeling and Natural Systems. Cambridge Univ Press; 2006. [Google Scholar]
- 18.Grace JB, Guntenspergen GR, Keough J. The examination of a competition matrix for transitivity and intransitive loops. Oikos. 1993;68:91–98. [Google Scholar]
- 19.Grman E, Lau JA, Schoolmaster DR, Gross KL. Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecol Lett. 2010;13:1400–1410. doi: 10.1111/j.1461-0248.2010.01533.x. [DOI] [PubMed] [Google Scholar]
- 20.Harpole WS, Tilman D. Grassland species loss resulting from reduced niche dimension. Nature. 2007;446:791–793. doi: 10.1038/nature05684. [DOI] [PubMed] [Google Scholar]
- 21.Hautier Y, Seabloom EW, Borer ET, Adler PB, Harpole WS, Hillebrand H, et al. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature. 2014;508:521–525. doi: 10.1038/nature13014. [DOI] [PubMed] [Google Scholar]
- 22.Herben T, Krahulec F. Competitive hierarchies, reversals of rank order and the de Wit approach: are they compatible? Oikos. 1990;58:254–265. [Google Scholar]
- 23.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Interntl J Climatol. 2005;25:1965–1978. [Google Scholar]
- 24.HilleRisLambers J, Adler PB, Harpole WS, Levine JM, Mayfield MM. Rethinking community assembly through the lens of coexistence theory. Ann Rev Ecol Evol Syst. 2012;43:227–248. [Google Scholar]
- 25.Huisman J, Johansson AM, Folmer EO, Weissing FJ. Towards a solution of the plankton paradox: the importance of physiology and life history. Ecol Lett. 2001;4:408–411. [Google Scholar]
- 26.Keddy PA, Shipley B. Competitive hierarchies in herbaceous plant communities. Oikos. 1989;54:234–241. [Google Scholar]
- 27.Kerr B, Riley MA, Feldman MW, Bohannan BJ. Local dispersal promotes biodiversity in a real-life game of rock–paper–scissors. Nature. 2002;418:171–174. doi: 10.1038/nature00823. [DOI] [PubMed] [Google Scholar]
- 28.Laird RA, Schamp BS. Competitive intransitivity promotes species co-existence. Am Nat. 2006;168:182–193. doi: 10.1086/506259. [DOI] [PubMed] [Google Scholar]
- 29.Laird RA, Schamp BS. Does local competition increase the coexistence of species in intransitive networks? Ecology. 2008;89:237–247. doi: 10.1890/07-0117.1. [DOI] [PubMed] [Google Scholar]
- 30.Legendre P. lmodel2: Model II Regression. 2008 Available: http://www.cran.r-project.org.
- 31.Maestre FT, Quero JL, Gotelli NJ, Escudero A, Ochoa V, Delgado-Baquerizo M, et al. Plant species richness and ecosystem multifunctionality in global drylands. Science. 2012;335:214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning P. The impact of nitrogen enrichment on ecosystems and their services. In: Wall DH, editor. The Oxford handbook of soil ecology and ecosystem services. Oxford University Press; UK: 2012. [Google Scholar]
- 33.McCune B, Grace JB. Analysis of ecological communities. MjM Software Design; 2002. [Google Scholar]
- 34.Osem Y, Perevolotsky A, Kigel J. Grazing effect on diversity of annual plant communities in a semi-arid rangeland: interactions with small-scale spatial and temporal variation in primary productivity. J Ecol. 2002;90:936–946. [Google Scholar]
- 35.Rojas-Echenique JR, Allesina S. Interaction rules affect species coexistence in intransitive networks. Ecology. 2011;92:1174–1180. doi: 10.1890/10-0953.1. [DOI] [PubMed] [Google Scholar]
- 36.Sala OE, Chapin FS, III, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 37.Sears ALW, Chesson P. New methods for quantifying the spatial storage effect: an illustration with desert annuals. Ecology. 2007;88:2240–2247. doi: 10.1890/06-0645.1. [DOI] [PubMed] [Google Scholar]
- 38.Silvertown J, Dale P. Competitive hierarchies and the structure of herbaceous plant communities. Oikos. 1991;61:441–444. [Google Scholar]
- 39.Sinervo B, Lively CM. The rock–paper–scissors game and the evolution of alternative male strategies. Nature. 1996;380:240–243. [Google Scholar]
- 40.Stone L, Roberts A. Competitive exclusion, or species aggregation? Oecologia. 1992;91:419–424. doi: 10.1007/BF00317632. [DOI] [PubMed] [Google Scholar]
- 41.Socher SA, Prati D, Boch S, Müller J, Baumbach H, Gockel S, et al. Interacting effects of fertilization, mowing and grazing on plant species diversity of 1500 grasslands in Germany differ between regions. Basic Appl Ecol. 2013;14:126–136. [Google Scholar]
- 42.Soliveres S, Eldridge DJ, Maestre FT, Bowker MA, Tighe M, Escudero A. Microhabitat amelioration and reduced competition among understorey plants as drivers of facilitation across environmental gradients: towards a unifying framework. Persp Plant Ecol Evol Syst. 2011;13:247–258. doi: 10.1016/j.ppees.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suding KN, Collins SL, Gough L, Clark C, Cleland EE, Gross KL, et al. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc Natl Acad Sci USA. 2005;102:4387–4392. doi: 10.1073/pnas.0408648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tilman D, Lehman C. Human-caused environmental change: impacts on plant diversity and evolution. Proc Natl Acad Sci USA. 2001;98:5433–5440. doi: 10.1073/pnas.091093198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulrich W, Gotelli NJ. Null model analysis of species nestedness patterns. Ecology. 2007;88:1824–1831. doi: 10.1890/06-1208.1. [DOI] [PubMed] [Google Scholar]
- 46.Ulrich W, Soliveres S, Kryszewski W, Maestre FT, Gotelli NJ. Matrix models for quantifying competitive intransitivity from species abundance data. Oikos. 2014a;123:1057–1070. doi: 10.1111/oik.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulrich W, Soliveres S, Maestre FT, Gotelli NJ, Quero JL, Delgado-Baquerizo M, et al. Climate and soil attributes determine plant species turnover in global drylands. J Biogeogr. 2014b doi: 10.1111/jbi.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weigelt A, Schumacher J, Walther T, Bartelheimer M, Steinlein T, Beyschlag W. Identifying mechanisms of competition in multi-species communities. J Ecology. 2007;95:53–64. [Google Scholar]
- 49.Whitford WG. Ecology of desert systems. Academic Press; California, USA: 2002. [Google Scholar]
- 50.Wootton JT. Causes of species diversity differences: a comparative analysis of Markov models. Ecol Lett. 2001;4:46–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.