Abstract
Good’s buffers ionic liquids (GB-ILs), composed of cholinium-based cations and Good’s buffers anions, display self-buffering characteristics in the biological pH range, and their polarity and hydrophobicity can be easily tuned by a proper manipulation of their ions chemical structures. In this work, the extraction ability for bovine serum albumin (BSA) of aqueous biphasic systems (ABS) formed by polypropylene glycol 400 (PPG 400) and several GB-ILs was evaluated. ABS formed by PPG 400 and cholinium chloride ([Ch]Cl), GBs, and sucrose were also investigated for comparison purposes. It is shown that BSA preferentially migrates for the GB-IL-rich phase, with extraction efficiencies of 100%, achieved in a single-step. Dynamic light scattering, and circular dichroism (CD) and Fourier transform infrared (FTIR) spectroscopies were employed to evaluate the effect of the investigated cholinium-based GB-ILs on the BSA stability, and compared with results obtained for the respective GBs precursors, [Ch]Cl and sucrose, a well-known protein stabilizer. Molecular docking studies were also carried out to investigate on the binding sites of GB-IL ions to BSA. The experimental results confirm that BSA has a higher stability in GB-ILs than in any of the other compounds investigated.
Keywords: Good’s buffer ionic liquids, aqueous two-phase extraction, protein stability, bovine serum albumin (BSA), protein extraction
Introduction
Proteins play crucial roles in many biological processes and are of fundamental value in biotechnological, therapeutic and diagnostic applications [1]. Their production has rapidly grown up in the last decade [1]. Usually, proteins are produced in highly complex media comprising cell wall materials and nucleic acids. Therefore, the development of cost-effective and sustainable separation and purification techniques for proteins is of major relevance. Nevertheless, these techniques should be of a biocompatible nature due to the poor stability of proteins when removed from their native environments. Proteins can lose their native structure by slight changes in the surrounding environment, such as pH, temperature, mechanical stress or by the addition of chemical denaturants [2]. The active state of proteins is the well-known folded state, whereas the denatured or the inactive state corresponds to the unfolded form.
In the past years, the most common techniques used for the separation and purification of proteins included precipitation, filtration-related techniques, reverse micelle-based approaches, gel chromatography, electrophoresis, affinity chromatography, among others [3, 4]. In general, to obtain highly pure proteins, several purification steps are required, which frequently cause losses on the protein yield and might represent high energy and solvents/materials consumption. Thus, most of these methods are not suitable for large-scale applications. In this aspect, aqueous biphasic systems (ABS) are an alternative and scalable technique for the separation and purification of proteins. ABS also allow proteins to maintain their biological activities due to their water-rich environment [5]. In most circumstances, ABS consist of a polymer-rich top phase and a second polymer- or salt-rich bottom phase [5]. Amongst the investigated polymers, polyethylene glycol (PEG), polypropylene glycol (PPG) and dextran are frequently used. When considering polymer–salt systems, ABS formed by phosphate-based salts are commonly employed due to the buffering capacity over the physiological pH (6-9) range afforded by the combination of different salts. This type of systems have been used in the extraction of proteins, enzymes and antibodies from their biological-containing medium, in single or two-staged primary purification processes [6].
In addition to the more deeply investigated polymer-based ABS, in the last decade, Gutowski et al. [7] demonstrated the formation of a novel class of ABS by combining inorganic salts and ionic liquids (ILs) in aqueous media. ILs are salts composed of large organic cations and organic or inorganic anions, which contribute to a decrease on their melting temperatures. Their application as media for maintaining the protein stability has been extensively studied, either employed as co-solvents with water, in biphasic systems, or as “neat” solvents [8–11]. Some reports also demonstrated that some ILs are able to maintain or even increase the proteins stability [11–13].
An attractive feature of ILs relays on the possibility of tuning their solvent properties by the manipulation of the ions chemical structures, and thus, IL-based ABS appear as liquid-liquid partitioning systems with tuneable features, such as extraction performance and selectivity [14–16]. IL-based ABS have been used for the extraction of proteins and enzymes, while demonstrating to provide higher extraction efficiencies than the traditional PEG-based ABS [17–34]. Alkylimidazolium-based ILs combined with phosphate-based salts were the most widely investigated phase-forming components of ABS [15]. However, such ILs display some toxicity and are poorly biodegradable [35]. On the other hand, the presence of high charge density salts is deleterious to proteins. To overcome these drawbacks, ABS composed of polypropylene glycol 400 (PPG 400) and cholinium-based ILs have been proposed, since both the cholinium-based ILs and PPG 400 are non-toxic and biodegradable [36]. Despite these systems being able to provide high extraction efficiencies for proteins, they still present some limitations, mainly related with the pH values of the coexisting phases, since no buffering compounds are used as phase-forming components. The use of carbohydrates to replace the highly charged salting-out salts to form IL-based ABS has also been proposed [37]; yet, these ABS are still unable to perform as buffered liquid-liquid extraction systems. Consequently, the need for self-buffering ILs has emerged. Until recently, few works have reported ILs with buffering characteristics, and those were mainly based on anions such as phthalate, tartrate and phosphate [38–40]. Nevertheless, these anions are not sufficiently “inert” for biochemical- and biotechnological-related processes. They may participate in the cellular metabolism and interact with some chelate metal ions, like calcium, zinc or magnesium, that are important for maintaining the cells functions; e.g., phosphate anions interact with calcium leading to the precipitation of calcium phosphate [41].
Recently, we have devoted our efforts aiming at synthesizing novel ILs with buffer characteristics, in the physiological pH range, comprising anions derived from biological buffers - Good’s buffers [42]. Nevertheless, the selection of a suitable buffer does not depend only on providing appropriate pKa values for attaining a desired pH of the medium, but also on the biocompatible nature of the buffer used. The buffer chemical structure, either zwitterionic or non-zwitterionic, plays a key role on proteins stability. In fact, it was already demonstrated that buffers with similar pKa values lead to different effects or results when dealing with proteins [43]. Most of the biological buffers used today were developed by Good and co-workers [41, 44]. Good’s buffers (GBs) are designed based on several criteria, guarantying their inertness, while able to stabilize proteins, especially at high concentrations [45–49]. They are based on zwitterionic amino acids, which can act as anions of ILs by neutralizing them with organic bases [42].
In previous works, GBs-based anions (Tricine, TES, CHES, HEPES, and MES) were paired with alkylimidazolium [42], tetraalkylammonium [42], and cholinium cations [50]. The developed GB-ILs were found to offer a great buffer capacity in the biological pH range, and exhibited marked stabilizing effect on the proteins structure [42]. The tetrabutylammonium-based GB-ILs formed aqueous biphasic systems (ABS) with high-charge density salts [42]. These ABS were shown to be able to extract BSA with an extraction efficiency of 100 % for the GB-IL-rich phase [42]. However, high-charge density salts should be avoided since a high ionic strength can be deleterious to proteins. To overcome this drawback, cholinium-based GB-ILs were recently proposed, both to substitute the more toxic imidazolium- and tetraalkylammonium-based ILs, and to create more biocompatible ABS combined with biodegradable polymers, namely polypropylene glycol (PPG 400, with a molecular weight of 400 g.mol-1) [50]. PPG 400 is a biocompatible and biodegradable polymer, with a lower critical solution temperature in binary mixtures with water (LCST ≈ 46 °C), and which could be recovered conveniently by heating at a temperature above the LCST [36]. However, these biocompatible ABS still need to be characterized in what concerns their extraction performance for a broad variety of proteins and on their effect upon the proteins’ stability. Therefore, in this work, a wide variety of ABS composed of GB-ILs and PPG 400 were initially ascertained for the extraction of bovine serum albumin (BSA). BSA was chosen since it is commonly used in the separation of enantiomers [51]. BSA has been used in counter-current resolution or chromatography to separate D,L-tryptophan [52], ofloxacin [53], and D,L-Kynurenine [54]. Still, the resolution obtained for enantiomers is poor since the extraction efficiencies of BSA obtained with more conventional polymer-based ABS are usually low. Therefore, the search on effective liquid-liquid extraction systems for BSA is of crucial value for such an attempt. The stability of BSA in the studied GB-ILs, respective GBs precursors, was investigated using dynamic light scattering (DLS) and attenuated total reflectance (ATR) Fourier transform infrared (FTIR) and circular dichroism (CD) spectroscopies. Moreover, the obtained results where compared with that of cholinium chloride, [Ch]Cl, and sucrose (common stabilizers of proteins).
Experimental Section
Materials
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES, purity > 99.5 wt%), N-[tris(hydroxymethyl)methyl]glycine (Tricine, purity > 99 wt%), 2-(cyclohexylamino)ethane sulfonic acid (CHES, purity > 99 wt%), 2-(N-morpholino)ethanesulfonic acid (MES, purity > 99 wt%), 2-[(2-hydroxy-1,1-bis(hydroxymethyl)ethyl)amino]ethane sulfonic acid (TES, purity > 99 wt%), and choline hydroxide ([Ch][OH], 45 wt% in methanol), cholinium chloride ([Ch]Cl, purity > 98 wt%), and PPG 400 were purchased from Sigma-Aldrich. BSA/fraction V, pH = 7.0, was obtained from Acros Organics. Methanol (HPLC grade, purity > 99.9) was obtained from Fisher Scientific (UK), and acetonitrile (purity > 99.7) was supplied from Lab-Scan (Ireland). Sucrose (purity > 99.5 wt%) was supplied by HiMedia Lab. Purified water was obtained using a reverse osmosis and a Milli-Q plus 185 water purifying system and was used thorough in all experiments.
Synthesis and characterization of Good’s buffers ionic liquids (GB-ILs)
The details of the synthesis of cholinium-based GB-ILs have been described in a previous work [50]. Briefly, the [Ch][OH] solution was added slowly to an aqueous solution of slightly excess equimolar buffer under constant stirring, at room temperature, and overnight. The mixture was then subjected to evaporation at 60°C under vacuum. The resultant residue (viscous liquid) was dissolved in a mixture of acetonitrile and methanol (1:1) and stirred vigorously for 1 h to precipitate the excess buffer and filtered off. The GB-IL product was then evaporated at room temperature under vacuum for 3 days. The water content of the investigated GB-ILs was determined by Karl–Fischer titration (Metrohm Ltd., model 831) and was found to be less than 0.05 wt%. The synthesized compounds were characterized by 1H and 13C NMR spectroscopy (Bruker AMX 300) operating at 300.13 and 75.47 MHz, respectively, and their melting points were measured by differential scanning calorimetry (DSC) using a Perkin Elmer DSC-7 instrument (Norwalk, CT) with a heating rate of 5 °C/min under a N2 flow of 40 mL·min-1. The NMR data and melting points are reported in Table S1 in the Supporting Information.
ABS phase diagrams and tie-lines
The binodal curve of each ABS phase diagram was determined using the cloud point titration method at (25 ± 1) ºC and at atmospheric pressure. Repetitive drop-wise addition of an aqueous GB, GB-IL, [Ch]Cl or sucrose solution to pure PPG 400 was carried out until the detection of a cloudy biphasic solution, followed by the drop-wise addition of water until detection of a monophasic region. The opposite addition was also conducted aiming at gathering more complete phase diagrams. This procedure was carried out under constant stirring. Each mixture composition was determined by the weight quantification of all components added within ± 10-4 g (using an analytical balance, Mettler Toledo Excellence XS205 DualRange). Some phase diagrams were previously reported by us [50] whereas others are here presented for the first time.
The tie-lines (TLs) of each phase diagram were determined by a gravimetric method described by Merchuk et al. [55]. The selected mixture, at the biphasic regime, was prepared by weighting the appropriate amount of GB, GB-IL, [Ch]Cl or sucrose + PPG 400 + water, vigorously stirred, and further submitted to centrifugation for 10 min and at a controlled temperature of (25 ± 1)ºC. After centrifugation, the sample was left in equilibrium for more 10 min at (25 ± 1)ºC to guarantee the equilibration of the coexisting phases at the target temperature. After this period, each phase was carefully separated and weighted. Finally, each individual TL was determined by application of the lever-arm rule to the relationship between the weight of the top and bottom phases and the overall system composition. Further details can be found elsewhere [55].
Extraction efficiencies of BSA
The ternary mixtures compositions used in the partitioning experiments of BSA were gravimetrically prepared at a fixed common mixture composition: 30 wt% of GB, GB-IL, [Ch]Cl or sucrose + 30 wt% of PPG 400 + 40 wt% of water. The aqueous solution added contained BSA at a concentration of circa 0.5 g·L-1. The fixed mixture composition was chosen based on a common point for which all the systems are able to form two-aqueous phases. This fixed mixture composition also allows introducing an equal amount of BSA in each system, and thus permit the direct comparison of the extraction efficiencies afforded by the different ABS under study. Each mixture was vigorously stirred, centrifuged for 10 min, and left to equilibrate for at least 10 min at (25 ± 1)ºC to achieve a complete BSA partitioning between the two phases. After, a careful separation of the phases was performed and the amount of BSA in each phase was quantified by SE-HPLC (Size Exclusion High-Performance Liquid Chromatography). Each phase was diluted at a 1:10 (v:v) ratio in a phosphate buffer solution before injection. A Chromaster HPLC (VWR, Hitachi) coupled with an UV-Vis detector was used. RP-HPLC was performed on an analytical column (25 cm × 2 mm i.d., 25 μm), Lichrospher 100 RP-18 (from Merck). A 100 mM phosphate buffer in MPPG400iQ water (mobile phase) was run isocratically with a flow rate of 0.8 mL·min-1. The column oven and autosampler temperatures were kept constant at 25°C. The injection volume was of 25 µL. The wavelength was set at 280 nm whereas the retention time of BSA was found to 10.6 min within an analysis time of 24 min. The quantification of the BSA was carried out by external standard calibration method in the range of 0.001 to 1.0 g·L-1 of protein. At least three independent biphasic mixtures for each PPG400-based system were prepared and 3 samples of each phase were quantified. The interference of all the phase-forming components with the quantification method was also ascertained and blank control samples were always initially analyzed.
The percentage extraction efficiency of BSA, EEBSA%, is the percentage ratio between the amount of protein in the GB-, GB-IL-, [Ch]Cl- or sucrose-rich aqueous phase to that in the total mixture.
Circular dichroism (CD)
Circular dichroism (CD) spectra were recorded on a JASCO J-720 spectropolarimeter (JASCO, Hiroshima, Japan) with a 175-800 nm photomultiplier (EXEL-308). CD spectra were recorded in the range from 200 to300 nm with quartz Suprasil ® CD cuvettes (0.1 cm) at room temperature (ca. 25 °C). Each CD spectrum is the result of three accumulations originally recorded in degrees and converted to [θ], molar ellipticity, with the spectropolarimeter software. The following acquisition parameters were used: data pitch, 0.5 nm; band width, 1.0 nm; response, 4 s; and scan speed, 50 nm/min.
The ternary mixtures were prepared using the same procedure as for the extraction efficiency experiments. After the careful separation of the phases, each phase was diluted at a 1:10 (v:v) ratio in phosphate buffer saline solution (PBS) and their CD spectra measured. The spectra of blank control samples, containing everything except BSA, were also measured and subtracted from the respective BSA CD spectra.
Dynamic light scattering (DLS)
The average hydrodynamic radius (RH) values of BSA were determined as a function of temperature using a Zetasizer Nano ZS (Malvern Instruments Ltd., UK) equipped with He-Ne laser light source (4mW) with a wavelength (λ) = 633 nm. The measurements were carried out at a fixed scattering angle, 173°. The instrument is equipped with a thermostatic chamber with a temperature controller that allows to perform measurements under a given temperature and from 0 °C to 90 °C. Around 1.3 cm3 of bubble free samples of 20 mg·cm-3 of BSA in aqueous solutions of 0.05 and 0.5 M of GBs, GB-ILs, [Ch]Cl or sucrose at pH = 7.4, in square glass cuvettes (PCS8501), were placed into the thermostatic chamber. The desired pH of the GB-ILs and GBs was achieved by adding HCl/NaOH concentrated aqueous solutions since they are self-buffering compounds. For [Ch]Cl and sucrose, the buffer HEPES (0.05 M, pH =7.4) was used in combination. The samples were incubated at 25 °C for 4 h prior to the measurements.
Infrared spectroscopy
ATR-FTIR spectra of 30 mg·mL-1 of BSA in aqueous solutions of 0.05 and 0.5 M of GBs, GB-ILs, [Ch]Cl or sucrose (at pH = 7.4 adjusted as described before for the DLS samples) were acquired with a resolution of 4 cm−1 in the 400–4000 cm−1 region, with an ABB MB3000 FTIR spectrometer, using a PIKE MIRacl and single refection diamond/ZnSe crystal plate. At least 5 repeated measurements were done for each sample. The second-derivative of the Gaussian curve-fitting analysis of the amide I region was determined using the PeakFit v4.0 (AISN software Inc.). The calculations of the percentage amount of the α-helices, β-sheets, and turns were performed by computing the ratios of the areas of the bands assigned to the respective substructure.
Molecular docking
The interaction sites of BSA with the cholinium cation were identified using the Auto-dock Tools vina 1.5.4 program [56]. The crystal structure of BSA (PDB, 3v03) [57] was used in the molecular docking. The natural bond orbital (NBO) charges of the cholinium cation were calculated using a polarizable continuum model (IEF-PCM) at the DFT/B3LYP/6-311++G(d,p) level, and then used in the docking. The NBO charges were computed with the Gaussian 09 software [58]. The grid center at the center of mass of BSA was (90.398 × 28.894 × 23.482) Å in the x-, y-, and z-axes, respectively. The grid dimension was (84 × 56 × 82) Å to cover the whole interaction surface of the protein. The binding model that has the lowest binding free energy was searched out from 9 different conformers for each ligand.
Results and Discussion
Phase diagrams and tie-lines
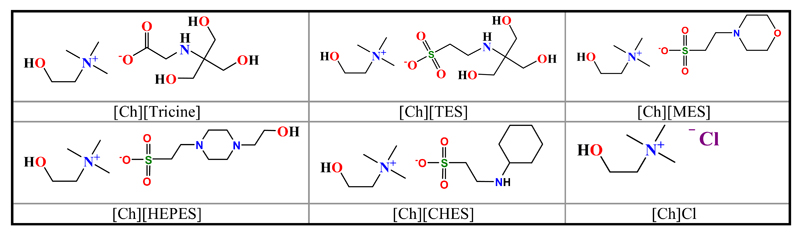
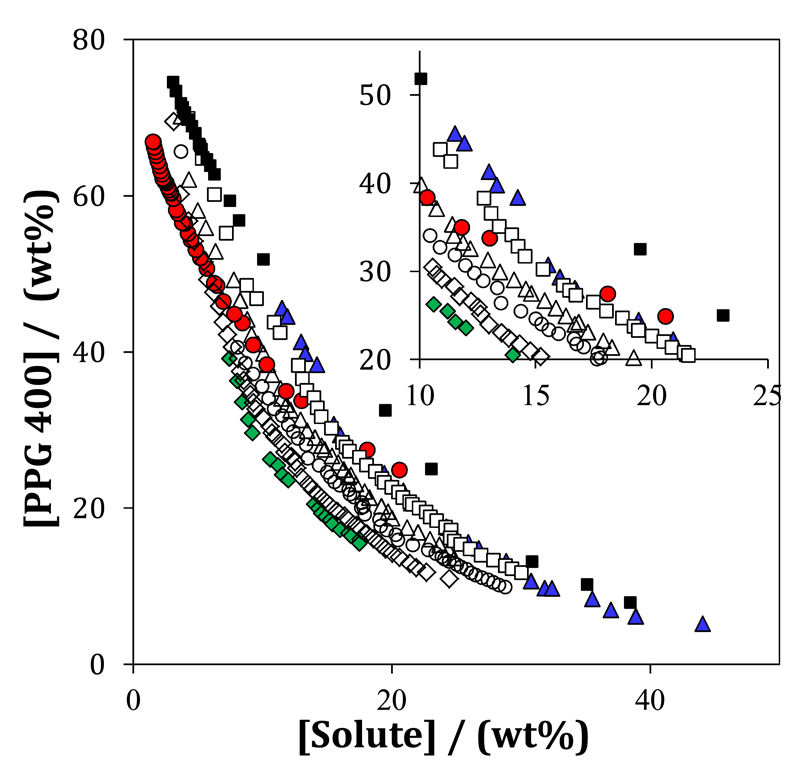
The use of GB-ILs as protein stabilizers could be very relevant because they are self-buffering in the biological pH range while sharing the ILs’ tuneable properties. The structures of the synthesized cholinium-based GB-ILs ([Ch][Tricine], [Ch][TES], [Ch][HEPES], [Ch][CHES], and [Ch][MES]), as well as [Ch]Cl, are shown in Fig. 1. The experimental data points corresponding to the ternary ABS phase diagrams composed of water, PPG 400 and [Ch][GB], [Ch]Cl, GBs or sucrose are illustrated in Fig. 2. The experimental data are shown in weight fraction. The respective phase diagrams in molality units are shown in Figs. S1 and S2 in the Supporting Information. The region above the saturation curve corresponds to solutions that form two phases while at compositions below the binodal curve only one phase exists. The GBs- and sucrose-based ABS are reported here for the first time while the GB-IL-based ABS have been previously reported [50].
Fig. 1.
Chemical structures of the cholinium-based GB-ILs and cholinium chloride.
Fig. 2.
Ternary phase diagrams at 25ºC for the systems composed of water + PPG 400 and: (■) Sucrose, (▲) TES, (●) HEPES, (♦) [Ch]Cl, (◊) [Ch][Tricine] (Δ) [Ch][TES], (○) [Ch][HEPES], and (□) [Ch][MES].
Amongst the studied phase-forming components, the buffers CHES, MES and Tricine, as well as [Ch][CHES], were not able to form ABS with PPG 400. For all the studied ABS, the top phase corresponds to the polymer-rich aqueous phase while the bottom phase is mainly composed of [Ch][GB], [Ch]Cl, GBs or sucrose and water. The detailed experimental weight fraction data for each phase diagram, and the parameters obtained by the regression of the experimental binodal curves determined at 25°C under atmospheric pressure are reported in the Supporting Information. In addition, some tie-lines (TLs) and tie-line lengths (TLLs) are presented in the Supporting Information.
The ability of the phase-forming components to form ABS in presence of PPG 400 is as follows: Ch]Cl > [Ch][Tricine] > [Ch][HEPES] > [Ch][TES] > [Ch][MES] > HEPES > TES > Sucrose (Fig. 2). In general, the cholinium-based ILs present a higher ability to create ABS when compared with GBs or sucrose. This is of high-value since lower amounts of phase-forming components are required to perform liquid-liquid extractions with GB-ILs. It seems thus that the presence of a cholinium cation increases the affinity of the GB-ILs for water turning them more able to induce the phase separation, i.e., to act as salting-out species. Considering the fact that all ILs share the same cation, the pattern obtained for the GB-ILs is related with the hydrophilic nature of the anion. ILs hydrating more easily are more able to induce the formation of a second liquid phase in presence of a moderately hydrophobic polymer, such as PPG 400 [15].
Extraction efficiencies of BSA
The ABS investigated in this work were ascertained in what concerns their extraction ability for proteins, namely BSA, from aqueous solutions. All the extractions were conducted at a fixed mixture composition: 30 wt% of GB, GB-IL, [Ch]Cl or sucrose + 30 wt% of PPG 400 + 40 wt% of water. The extraction efficiencies of BSA (EEBSA%) and the initial mixture compositions at 25 ºC are depicted in Table 1. By a proper weight balance analysis, as well as by visual inspection, it was possible to observe a significant amount of precipitated protein in the systems composed of HEPES and even more remarkable in the [Ch]Cl-based ABS. In the remaining systems, no precipitated protein was observed, and BSA was completely extracted (100% of extraction achieved in a single-step) for the [Ch][GB]-rich phase. The sucrose- and TES-based based ABS also provide 100% of extraction attained in a single-step. Nevertheless, it should be mentioned that the systems formed with sucrose or more traditional ILs require the use of an external buffer able to maintain the pH of the system. On the other hand, the phase-forming constituents of the GB-IL- and GB-based ABS do not require the addition of buffers since they are, by themselves, buffers and protein stabilizers.
Table 1.
Initial mixture compositions and percentage extraction efficiencies of BSA, EEBSA%, in the investigated ABS at 25ºC.
| Phase-forming compound | Weight fraction composition / (wt %) |
EEBSA% | |
|---|---|---|---|
| PPG400 | Phase-forming compound | ||
| [Ch][Tricine] | 30.27 ± 0.06 | 30.02 ± 0.00 | 100 |
| [Ch][MES] | 29.88 ± 0.04 | 29.80 ± 0.02 | 100 |
| [Ch][TES] | 29.71 ± 0.11 | 30.30 ± 0.11 | 100 |
| [Ch][HEPES] | 29.86 ± 0.11 | 30.16 ± 0.15 | 100 |
| [Ch]Cl | 29.99 ± 0.16 | 30.09 ± 0.21 | significant protein precipitation |
| TES | 29.94 ± 0.10 | 29.53 ± 0.10 | 100 |
| HEPES | 29.86 ± 0.08 | 30.16 ± 0.14 | moderate protein precipitation |
| Sucrose | 30.01 ± 0.07 | 29.96 ± 0.028 | 100 |
In general, the studied ABS display higher extraction efficiencies than other reported IL-based ABS. BSA is commonly used as a model protein to investigate the extraction efficiency afforded by IL-based ABS for proteins [23, 30, 31, 36, 59, 60]. The ABS formed by the pair [C4mim][N(CN)2]/K2HPO4 ABS was used to extract BSA from aqueous solution of saccharides (arabinose, glucose, sucrose, raffinose or dextran), with extraction efficiencies ranging between 82.7% and 100% for the IL-rich phase, while the saccharides are enriched in the salt-rich phase [23]. Moreover, several alkylimidazolium bromide-based ILs were investigated as phase-forming components of ABS, namely [C2mim]Br/[C4mim]Br/[C6mim]Br/[C8mim]Br + K2HPO4, with extraction efficiencies of BSA for the IL-rich phase in the order of 76.36 %, 87.4 %, 91.63 % and 96.60 %, respectively, indicating that the extraction yield increases with the increase of the alkyl chain length of the IL cation [30]. Zeng et al. [31] used an ABS constituted by different 1,1,3,3-tetramethylguanidine acrylate guanidinium-based ILs and K2HPO4 to extract BSA from aqueous media, and the extraction efficiency of BSA to the IL-rich phase ranged between 5.70 % and 99.45 %. Even with cholinium-based ILs, extraction efficiencies of BSA in the (tri-cholinium citrate + PPG 400) and (cholinium lactate + PPG 400) ABS only up to ~86.4 and ~88.5 % for the IL-rich phase, respectively, have been reported [36]. Therefore, the GB-ILs investigated in this work are potential candidates as phase-forming components of ABS for the extraction of proteins - they lead to the complete extraction of BSA, in a single-step, while being able to maintain the pH of the aqueous medium.
In all GB-IL-based systems it is observed the preferential partitioning of BSA for the IL-rich aqueous phase. It is well-accepted that the partitioning of proteins between the two phases of an ABS is a complex phenomenon, guided mainly by several competing interactions between the solute being partitioned and the phase-forming components. This phenomenon is highly complex since a protein can interact with the surrounding media/molecules through hydrogen-bonding, electrostatic interactions and dispersive forces. In addition, steric effects can also influence the protein preferential migration. In the studied systems, BSA is negatively charged (the isoelectric point of BSA is 4.8 [61]) and electrostatic interactions between the amino acid residues on the protein surface and the IL cation can occur – cf. the pH profiles given in the Supporting Information (Fig. S3). Nevertheless, all the studied ILs comprise the same cation. This means that while the GB-ILs are efficient to extract and to maintain the protein structure, [Ch]Cl is the worst candidate since a significant amount of precipitated protein was observed. GB-IL anions seem thus to be the best candidates - maybe due to preferential hydrogen-bonding and dispersive interactions established with the protein. Furthermore, in the ABS investigated, BSA preferentially migrates to the more hydrophilic phase (IL-rich phase). In previous studies of BSA extraction in ABS formed by GB-ILs and salts, a preferential partitioning of BSA for the IL-rich phase was observed [42]. Yet, in these salt-IL systems, the IL-rich layer corresponds to the most hydrophobic phase. Therefore, these combined results suggest that the BSA partition is not dominated by the relative hydrophobicity of the phases but actually by specific interactions with the IL, as discussed below.
Protein stability
The stability of proteins during extraction and purification procedures is an essential requirement for their further applications. Changes in the protein environment, such as temperature, pH, ionic force, and addition of deleterious solvents can alter the proteins native state. After the outstanding results presented before, with extraction efficiencies of 100% achieved in a single-step, it is crucial to evaluate the stability of BSA at the aqueous phases. Therefore, and aiming at understanding the impact of the phase-forming components on the protein stability, the thermal stability and the structure of BSA were measured in aqueous solutions of 0.05 and 0.5 M of some GB-ILs ([Ch][Tricine], [Ch][TES], and [Ch][HEPES]), as well as in the corresponding Good’s buffers precursors, [Ch]Cl, and sucrose, at pH 7.4. The GB-ILs/GBs selected for this study are those that exhibit high buffering capacity at pH 7.4 [50].
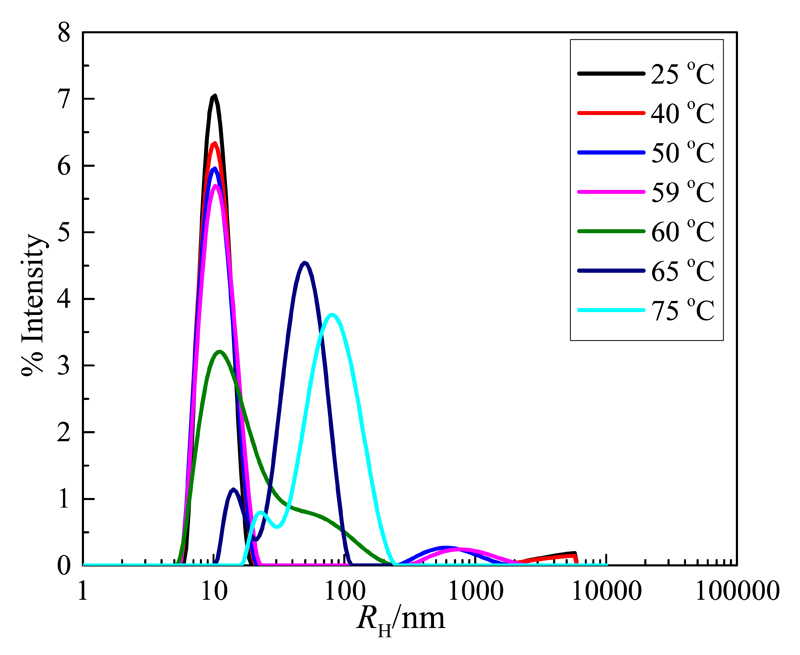
DLS measurements for BSA were carried out in (0.05 and 0.5) M of TES, HEPES, Tricine, sucrose, [Ch]Cl, [Ch][Tricine], [Ch][TES], and [Ch][HEPES] at pH 7.4. When BSA is heated above its denaturation temperature (~55 °C) [45–48], aggregation occurs due to the exposure of the hydrophobic amino acid residues to the solvent, and that phenomenon can be easily monitored by DLS. Fig. 3 shows the intensity-weighted DLS CONTIN plot at 173° for BSA in 0.5 M [Ch][TES], as an example, at different temperatures. For the sake of clarity, only the curves at 25, 53, 54, 55, 57, and 59 °C are plotted. As shown in Fig. 3, the native state of BSA exhibits one average peak that occurs at RH = 4.7 nm at 25 °C. There is another very small peak corresponding to a minor population resulting from the aggregation of BSA, with RH > 100 nm. Indeed, DLS is an ideal technique for studying the protein aggregation [45–48]. The intensity of the peak corresponding to the native structure decreases slightly with an increase in temperature from 25 °C to 59 °C. Nevertheless, the size of the protein remains almost constant up to 59 °C which indicates that BSA is in its native state up to this temperature. Above 60 °C, there is a significant increase on the protein size (6.7 nm). This result indicates that BSA starts to denature at 60 ºC (Td = 60 °C) reaching, for instance, RH = 18 nm at 65 °C. Moreover, by a cool back procedure until room temperature, the BSA size does not change (RH = 18 nm), revealing that the denaturation process is irreversible. The denaturation profiles of BSA in the investigated aqueous solutions (protein size vs. temperature) are plotted in Figs. S4 and S5 in the Supporting Information, where Td is the temperature at which the protein size significantly increases [45–48]. From the denaturation profiles, it is clearly seen that all the phase-forming components of ABS investigated increase the Td values of BSA along with their concentration. As an example, the Td values of BSA are 52, 53, 54, 53, 55, 57, 54, and 54 °C in 0.05 M of TES, HEPES, Tricine, sucrose, [Ch]Cl, [Ch][Tricine], [Ch][TES], and [Ch][HEPES], respectively. In general, the thermal stability of BSA in [Ch]Cl is greater than in GBs or sucrose. The three GBs and sucrose have the same impact on the protein stability. Nevertheless, the combination of the cholinium cation and GB-based anions, to form GB-ILs, induces an even higher BSA thermal stability. These results combined with the complete extraction in a single-step, as well as with the lower amount of phase-forming components to form ABS, clearly support the high potential of GB-ILs to be explored in the extraction and purification of proteins.
Fig. 3.
The intensity-weighted DLS CONTIN plot at 173° for BSA in 0.5 M [Ch][TES] at different temperatures and pH 7.4.
The IR spectra of the amide I and II groups of BSA in the presence of GB-ILs and in the remaining phase-forming components of ABS were also determined, at room temperature and pH 7.4, to evaluate the possible changes of the BSA secondary structure. The amide I band appears at ~1653 cm-1 due to the C=O stretching vibration, while the amide II arising from a combination of N—H bending with C—H stretching vibrations appears at ~1547 cm-1. A curve fitting was employed for the amide I band (Fig. S6 in the Supporting Information)) to determine the BSA secondary structure. In the buffer solution, the bands at (1615, 1631, 1653, 1675, and 1697) cm-1 are assigned to intermolecular β-sheet, intra-molecular β-sheet, α-helix, turn, and antiparallel β-sheet, respectively [62–64]. The secondary structure of BSA is known to be mostly composed of α-helices of nine helical loops connected with 17 disulfide bridges. The α-helix value in buffer solution is in good agreement with those previously reported [62–64]. The α-helix content of BSA in the studied protein stabilizers is given in Table 2. Interestingly, the helicity of BSA in GB-ILs is greater than in the conventional IL, sugar, and GBs. The α-helices content in BSA follows the order: [Ch][TES] > [Ch][Tricine] > [Ch][HEPES] > sucrose > TES > [Ch]Cl > HEPES > Tricine. Although sucrose was found to completely extract BSA with no visible precipitation at the studied conditions, cholinium-based GB-ILs are certainly better protein stabilizers than sucrose (a well-known and commonly employed protein stabilizer).
Table 2.
Secondary structure analysis (from FTIR spectra) for BSA in the studied protein stabilizers at room temperature and pH = 7.4.
| Protein stabilizers | Concentration | Amide I components / (%) |
||||
|---|---|---|---|---|---|---|
| inter β-sheet | intra β-sheet | α-helix | turn | antiparallel β-sheet | ||
| Tricine | 0.05 M[42] | 2.4[42] | 25.0[42] | 57.6[42] | 14.2[42] | 0.8[42] |
| 0.5 M | ― | 24.3 | 58.3 | 17.4 | ― | |
| TES | 0.05 M | ― | 24.3 | 59.8 | 15.9 | ― |
| 0.5 M | ― | 29.1 | 61.2 | 9.7 | ― | |
| HEPES | 0.05 M | ― | 26.4 | 58.3 | 25.3 | ― |
| 0.5 M | 3.0 | 20.9 | 59.7 | 16.4 | ― | |
| sucrose | 0.05 M | ― | 30.0 | 59.9 | 10.1 | ― |
| 0.5 M | ― | 24.8 | 61.5 | 13.7 | ― | |
| [Ch]Cl | 0.05 M | ― | 25.4 | 59.5 | 15.1 | ― |
| 0.5 M | ― | 25.3 | 60.0 | 14.7 | ― | |
| [Ch][Tricine] | 0.05 M | ― | 25.6 | 61.1 | 13.3 | ― |
| 0.5 M | ― | 27.3 | 62.9 | 9.8 | ― | |
| [Ch][TES] | 0.05 M | ― | 29.7 | 62.5 | 7.8 | ― |
| 0.5 M | ― | 25.7 | 63.1 | 11.2 | ― | |
| [Ch][HEPES] | 0.05 M | ― | 27.5 | 60.0 | 12.5 | ― |
| 0.5 M | ― | 29.9 | 61.1 | 9.0 | ― | |
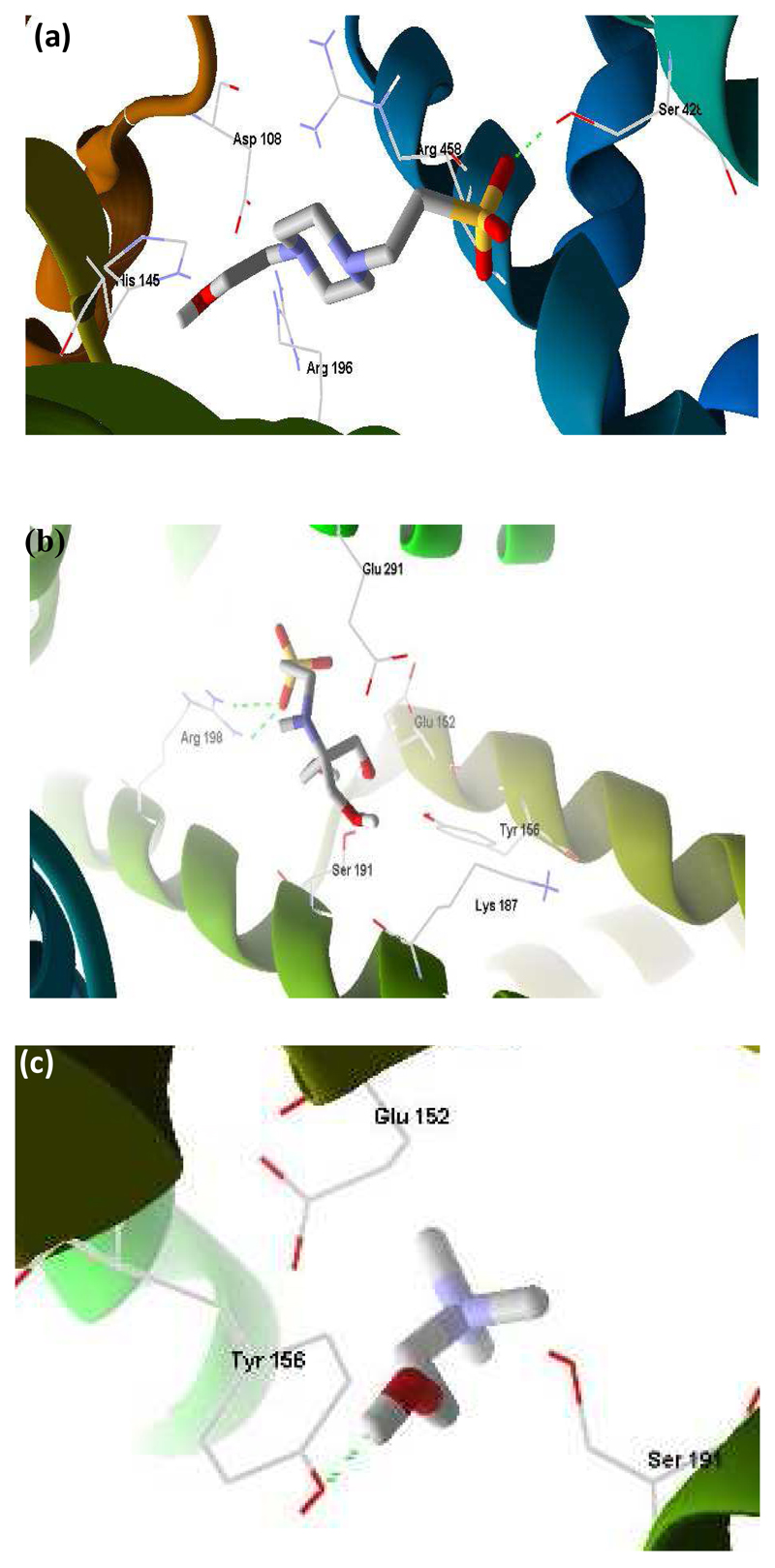
It can be noted from Figs. S4 and S5 in the Supporting Information, that the particle size (RH) of BSA at room temperature increases with the concentration of GBs, GB-ILs and [Ch]Cl, while in the case of sucrose solution it is essentially constant. This suggests that the GB-IL ions bind to the protein surface; nevertheless, the protein becomes more compact as observed from the increase of the helicity of BSA –Table 2. A molecular docking study was additionally carried out to ascertain on the binding sites of the GB-IL ions to BSA - Fig. 4. The cholinium cation forms one hydrogen bond with Tyr156. On the other hand, for the GB-IL anions the following binding sites were found: (i) the HEPES anion forms one hydrogen bond with Ser 428; (ii) the TES anion forms two hydrogen bonds with Arg198; and (iii) the Tricine anion forms five hydrogen bonds with Leu346, Glu353, and Arg208 amino acid residues, and as previously identified [42]. The binding free energies of [HEPES]¯, [Tricine]¯, [TES]¯, and [Ch]+ are (-5.1, -4.6 [42], -5.6, and -3.5) kcal·mol-1. These values suggest that, in general, GB anions are more favourable to establish hydrogen-bonds with BSA when compared with the cholinium cation. Since [Ch][GBs] have shown to improve the BSA stability when compared with [Ch]Cl, it seems that the stability afforded by GB-ILs is a major result of a direct binding of the GB-derived anions with the protein, which is shown to preferential occur by hydrogen-bonding. Besides the greater stability afforded by GB-ILs, as compared to traditional protein stabilizers, it should be mentioned that the pH range of these new stabilizers can also be tuned by choosing a proper GB-derived anion.
Fig. 4.
Molecular docking of BSA with [HEPES]ˉ (a), [TES]ˉ, and [Ch]+ (c).
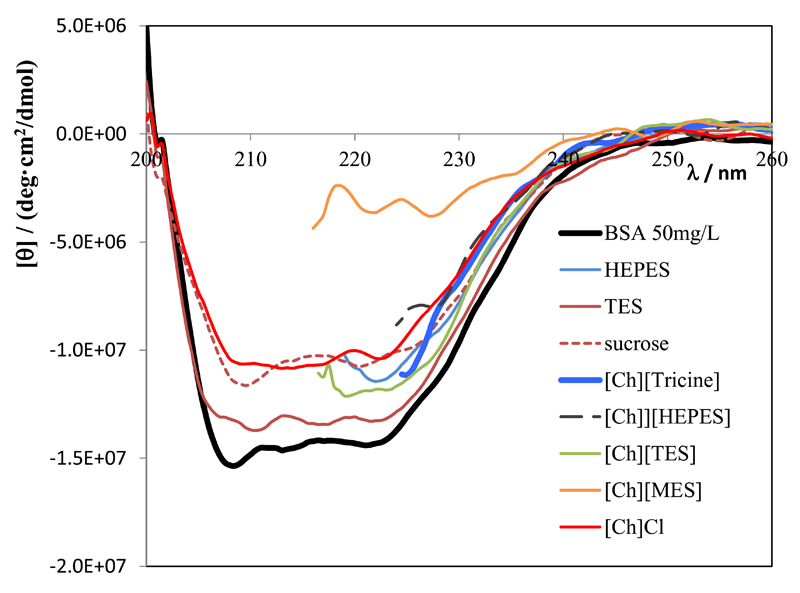
Finally, circular dichroism spectroscopy was used to analyse the secondary structure of BSA after each extraction. Circular dichroism is a spectroscopic tool used to study the conformation of biomolecules, such as proteins [65, 66]. Different secondary structures correspond to characteristic CD spectra, and it is considered that the protein CD spectrum is the sum of the spectra of the individual secondary structures present. According to the literature, the secondary structure of BSA is composed of 67% of α-helix, 10% of turn, and no β-sheet [67]. Furthermore, the α-helix spectrum is composed of two negative peaks at 208 nm and 222 nm [68]. In this work, circular dichroism was used to monitor the changes in the conformation of BSA after the extraction procedure, and the spectra recorded for the bottom phase are depicted in Fig. 5. It should be remarked that the analysis using standard algorithms [69] was attempted in this work, since the BSA concentration was not additionally determined and the anions of the GB-ILs have very high absorption in the UV range (MES, HEPES, TES and Tricine), precluding thus measurements of CD spectra below 220 nm. The spectrum of a standard solution of BSA (50mg/L in a PBS solution) was also measured and it is included for comparison purposes.
Fig. 5.
Circular Dichroism spectra (in [θ]) for the bottom phase measured in the far UV for all systems after the BSA extraction.
The CD spectra depicted in Fig. 5 clearly show that the secondary structure of BSA after the extraction procedure, and for all systems, is similar to the one of the freshly prepared solution of BSA. All spectra exhibit a maximum of negative ellipticity at ca. 222 and 208 nm (whenever it was possible to measure below 220 nm), and characteristic of α-helical proteins. Even when it was not possible to record the spectra bellow 220 nm it is quite clear that they have the shape characteristic of α-helical proteins. The only system which showed a different spectrum was the one obtained for [Ch][MES], maybe due to its lower buffer capacity at pH 7.0.
A rough estimate of the α-helical content can be obtained from the average residue ellipticity at 222 nm, [θ]222, using the expression: fraction of α-helix = - ([θ]222 + 3000)/33000 [70]. The values included in Table S6 in the Supporting Information show that the α-helical content is always higher than 45% (the value estimated for the standard BSA sample was 65%). However, these values have high associated uncertainties, since they depend on the BSA’s average residues concentration. The CD spectra of the top polymer-rich aqueous phase was also measured for samples containing [Ch][Cl] and sucrose (see Fig. S7 in the Supporting Information), confirming therefore the extraction efficiency values of 100%, as after subtraction of the blank spectra, the CD signal is null. In summary, we can conclude that after extraction with the investigated ABS, the conformation of BSA does not change, and the protein maintains its secondary structure.
Conclusions
In this work, we investigated the extraction ability of aqueous two-phase systems composed of GB-ILs, GBs, [Ch]Cl or sucrose + PPG 400 for BSA. The GBs- and sucrose-based ABS were here reported for the first time. All the investigated ABS are biocompatible and benign because they are majorly constituted by water and by non-toxic and biodegradable phase-forming components. For all systems, the complete extraction of BSA was achieved in a single-step. Nevertheless, moderate to significant amounts of precipitated protein were observed in the HEPES- and [Ch]Cl-based ABS. Moreover, lower amounts of GB-ILs are required to form ABS with PPG 400.
The stability of BSA in aqueous solutions of cholinium-based GB-ILs (Ch][TES], [Ch][Tricine], [Ch][HEPES]) have been investigated using ATR-FTIR and DLS, and the results were compared with those obtained with the respective GBs precursors (TES, Tricine, and HEPES), with a conventional IL ([Ch]Cl), and a well-known protein stabilizer (sucrose). The helicity of BSA was found to follow the order: [Ch][TES] > [Ch][Tricine] > [Ch][HEPES] > sucrose > TES > [Ch]Cl > HEPES > Tricine. The thermal stability of BSA in aqueous solutions of cholinium-based GB-ILs was shown to be greater than in the other aqueous solutions of the remaining phase-forming components, as evidenced by the DLS measurements. Circular dichroism spectroscopy also confirmed that the protein is stable and keeps its α-helical secondary structure in all studied systems, and after the extraction step. Molecular docking studies were also carried out to investigate on the binding sites of GB-IL ions to BSA. Either the cholinium cation or the GB-derived anions establish hydrogen-bonds with BSA, which seem to be favourable to maintain the protein stability. In summary, the investigated cholinium-based GB-ILs are potential candidates for the formation of ABS, able to lead to complete extractions of BSA while maintaining the protein structure.
Supplementary Material
Acknowledgements
This work was developed in the scope of the project CICECO - Aveiro Institute of Materials (Ref. FCT UID /CTM /50011/2013), financed by national funds through the FCT/MEC and co-financed by FEDER under the PT2020 Partnership Agreement. M. Taha acknowledges Fundação para a Ciȇncia e Tecnologia (FCT) for the post-doctoral grant SFRH/BPD/78441/2011. M.G. Freire acknowledges the European Research Council (ERC) for the Grant ERC-2013-StG-337753. I. Correia acknowledges FCT for Investigator FCT contract.
References
- [1].Pei Y, Wang J, Wu K, Xuan X, Lu X. Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep Purif Technol. 2009;64:288–295. [Google Scholar]
- [2].Canchi DR, García AE. Cosolvent Effects on Protein Stability. Annu Rev Phys Chem. 2013;64:273–293. doi: 10.1146/annurev-physchem-040412-110156. [DOI] [PubMed] [Google Scholar]
- [3].Johnson RD, Arnold FH. Review: Multipoint binding and heterogeneity in immobilized metal affinity chromatography. Biotechnol Bioeng. 1995;48:437–443. doi: 10.1002/bit.260480505. [DOI] [PubMed] [Google Scholar]
- [4].Scopes RK. Protein purification: principles and practice. Springer; 1994. [Google Scholar]
- [5].Ribeiro SC, Monteiro GA, Cabral JMS, Prazeres DMF. Isolation of plasmid DNA from cell lysates by aqueous two-phase systems. Biotechnol Bioeng. 2002;78:376–384. doi: 10.1002/bit.10227. [DOI] [PubMed] [Google Scholar]
- [6].Rito-Palomares M. Practical application of aqueous two-phase partition to process development for the recovery of biological products. J Chromatogr B. 2004;807:3–11. doi: 10.1016/j.jchromb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- [7].Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD, Rogers RD. Controlling the Aqueous Miscibility of Ionic Liquids: Aqueous Biphasic Systems of Water-Miscible Ionic Liquids and Water-Structuring Salts for Recycle, Metathesis, and Separations. Journal of the American Chemical Society. 2003;125:6632–6633. doi: 10.1021/ja0351802. [DOI] [PubMed] [Google Scholar]
- [8].Cantone S, Hanefeld U, Basso A. Biocatalysis in non-conventional media-ionic liquids, supercritical fluids and the gas phase. Green Chem. 2007;9:954–971. [Google Scholar]
- [9].Olivier-Bourbigou H, Magna L, Morvan D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Applied Catalysis A: General. 2010;373:1–56. [Google Scholar]
- [10].Walker AJ, Bruce NC. Cofactor-dependent enzyme catalysis in functionalized ionic solvents. Chem Commun. 2004:2570–2571. doi: 10.1039/b410467f. [DOI] [PubMed] [Google Scholar]
- [11].Kumar A, Venkatesu P. Overview of the Stability of α-Chymotrypsin in Different Solvent Media. Chem Rev. 2012;112:4283–4307. doi: 10.1021/cr2003773. [DOI] [PubMed] [Google Scholar]
- [12].Weingaertner H, Cabrele C, Herrmann C. How ionic liquids can help to stabilize native proteins. Physical Chemistry Chemical Physics. 2012;14:415–426. doi: 10.1039/c1cp21947b. [DOI] [PubMed] [Google Scholar]
- [13].Weaver KD, Vrikkis RM, Van Vorst MP, Trullinger J, Vijayaraghavan R, Foureau DM, McKillop IH, MacFarlane DR, Krueger JK, Elliott GD. Structure and function of proteins in hydrated choline dihydrogen phosphate ionic liquid. Physical Chemistry Chemical Physics. 2012;14:790–801. doi: 10.1039/c1cp22965f. [DOI] [PubMed] [Google Scholar]
- [14].Pereira JF, Rebelo LPN, Rogers RD, Coutinho JA, Freire MG. Combining ionic liquids and polyethylene glycols to boost the hydrophobic–hydrophilic range of aqueous biphasic systems. Phys Chem Chem Phys. 2013;15:19580–19583. doi: 10.1039/c3cp53701c. [DOI] [PubMed] [Google Scholar]
- [15].Freire MG, Claudio AFM, Araujo JMM, Coutinho JAP, Marrucho IM, Lopes JNC, Rebelo LPN. Aqueous biphasic systems: a boost brought about by using ionic liquids. Chem Soc Rev. 2012;41:4966–4995. doi: 10.1039/c2cs35151j. [DOI] [PubMed] [Google Scholar]
- [16].Freire MG, Pereira JFB, Francisco M, Rodriguez H, Rebelo LPN, Rogers RD, Coutinho JAP. Insight into the Interactions That Control the Phase Behaviour of New Aqueous Biphasic Systems Composed of Polyethylene Glycol Polymers and Ionic Liquids. Chem Eur J. 2012;18:1831–1839. doi: 10.1002/chem.201101780. [DOI] [PubMed] [Google Scholar]
- [17].Du Z, Yu Y-L, Wang J-H. Extraction of Proteins from Biological Fluids by Use of an Ionic Liquid/Aqueous Two-Phase System. Chem Eur J. 2007;13:2130–2137. doi: 10.1002/chem.200601234. [DOI] [PubMed] [Google Scholar]
- [18].Ruiz-Angel MJ, Pino V, Carda-Broch S, Berthod A. Solvent systems for countercurrent chromatography: An aqueous two phase liquid system based on a room temperature ionic liquid. J Chromatogr A. 2007;1151:65–73. doi: 10.1016/j.chroma.2006.11.072. [DOI] [PubMed] [Google Scholar]
- [19].Cao Q, Quan L, He C, Li N, Li K, Liu F. Partition of horseradish peroxidase with maintained activity in aqueous biphasic system based on ionic liquid. Talanta. 2008;77:160–165. doi: 10.1016/j.talanta.2008.05.055. [DOI] [PubMed] [Google Scholar]
- [20].Dreyer S, Kragl U. Ionic liquids for aqueous two-phase extraction and stabilization of enzymes. Biotech Bioeng. 2008;99:1416–1424. doi: 10.1002/bit.21720. [DOI] [PubMed] [Google Scholar]
- [21].Dreyer S, Salim P, Kragl U. Driving forces of protein partitioning in an ionic liquid-based aqueous two-phase system. Biochem Eng J. 2009;46:176–185. [Google Scholar]
- [22].Pei Y, Wang J, Wu K, Xuan X, Lu X. Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep Purif Technol. 2009;64:288–295. [Google Scholar]
- [23].Pei Y, Li Z, Liu L, Wang J, Wang H. Selective separation of protein and saccharides by ionic liquids aqueous two-phase systems. Sci China Chem. 2010;53:1554–1560. [Google Scholar]
- [24].Lu Y, Lu W, Wang W, Guo Q, Yang Y. Thermodynamic studies of partitioning behavior of cytochrome c in ionic liquid-based aqueous two-phase system. Talanta. 2011;85:1621–1626. doi: 10.1016/j.talanta.2011.06.058. [DOI] [PubMed] [Google Scholar]
- [25].Deive FJ, Rodriguez A, Pereiro AB, Araujo JMM, Longo MA, Coelho MAZ, Lopes JNC, Esperanca JMSS, Rebelo LPN, Marrucho IM. Ionic liquid-based aqueous biphasic system for lipase extraction. Green Chem. 2011;13:390–396. [Google Scholar]
- [26].Ventura SPM, Sousa SG, Freire MG, Serafim LS, Lima ÁS, Coutinho JAP. Design of ionic liquids for lipase purification. J Chromatogr B. 2011;879:2679–2687. doi: 10.1016/j.jchromb.2011.07.022. [DOI] [PubMed] [Google Scholar]
- [27].Ventura SPM, de Barros RLF, de Pinho Barbosa JM, Soares CMF, Lima AS, Coutinho JAP. Production and purification of an extracellular lipolytic enzyme using ionic liquid-based aqueous two-phase systems. Green Chem. 2012;14:734–740. [Google Scholar]
- [28].Deive FJ, Rodríguez A, Rebelo LPN, Marrucho IM. Extraction of Candida antarctica lipase A from aqueous solutions using imidazolium-based ionic liquids. Sep Purif Technol. 2012;97:205–210. [Google Scholar]
- [29].Novak U, Pohar A, Plazl I, Žnidaršič-Plazl P. Ionic liquid-based aqueous two-phase extraction within a microchannel system. Sep Purif Technol. 2012;97:172–178. [Google Scholar]
- [30].Lin X, Wang Y, Zeng Q, Ding X, Chen J. Extraction and separation of proteins by ionic liquid aqueous two-phase system. Analyst. 2013;138:6445–6453. doi: 10.1039/c3an01301d. [DOI] [PubMed] [Google Scholar]
- [31].Zeng Q, Wang Y, Li N, Huang X, Ding X, Lin X, Huang S, Liu X. Extraction of proteins with ionic liquid aqueous two-phase system based on guanidine ionic liquid. Talanta. 2013;116:409–416. doi: 10.1016/j.talanta.2013.06.011. [DOI] [PubMed] [Google Scholar]
- [32].zeng q, wang y, huang y, ding x, chen j, xu k. Deep Eutectic Solvent as a Novel Extraction Media for Protein Partitioning. Analyst. 2014;139:2565–2573. doi: 10.1039/c3an02235h. [DOI] [PubMed] [Google Scholar]
- [33].Desai RK, Streefland M, Wijffels RH, Eppink MHM. Extraction and stability of selected proteins in ionic liquid based aqueous two phase systems. Green Chem. 2014 [Google Scholar]
- [34].Ding X, Wang Y, Zeng Q, Chen J, Huang Y, Xu K. Design of functional guanidinium ionic liquid aqueous two-phase systems for the efficient purification of protein. Anal Chim Acta. 2014;815:22–32. doi: 10.1016/j.aca.2014.01.030. [DOI] [PubMed] [Google Scholar]
- [35].Petkovic M, Seddon KR, Rebelo LPN, Silva Pereira C. Ionic liquids: a pathway to environmental acceptability. Chem Soc Rev. 2011;40:1383–1403. doi: 10.1039/c004968a. [DOI] [PubMed] [Google Scholar]
- [36].Li Z, Liu X, Pei Y, Wang J, He M. Design of environmentally friendly ionic liquid aqueous two-phase systems for the efficient and high activity extraction of proteins. Green Chem. 2012;14:2941–2950. [Google Scholar]
- [37].Freire MG, Louros CLS, Rebelo LPN, Coutinho JAP. Aqueous biphasic systems composed of a water-stable ionic liquid + carbohydrates and their applications. Green Chem. 2011;13:1536–1545. [Google Scholar]
- [38].Ou G-n, Zhu M-x, She J-r, Yuan Y-z. Ionic liquid buffers: a new class of chemicals with potential for controlling pH in non-aqueous media. Chem Commun. 2006;0:4626–4628. doi: 10.1039/b611810k. [DOI] [PubMed] [Google Scholar]
- [39].Xu L, Ou G, Yuan Y. Ionic liquids as acid/base buffers in non-aqueous solvents for homogeneous catalysis: A case of selective hydrogenation of olefins and unsaturated aldehyde catalyzed by ruthenium complexes. J Organomet Chem. 2008;693:3000–3006. [Google Scholar]
- [40].Ou G, Yang J, He B, Yuan Y. Buffer-mediated activation of Candida antarctica lipase B dissolved in hydroxyl-functionalized ionic liquids. J Mol Catal B: Enzym. 2011;68:66–70. [Google Scholar]
- [41].Good NE, Winget GD, Winter W, Connolly TN, Izawa S, Singh RMM. Hydrogen Ion Buffers for Biological Research. Biochemistry. 1966;5:467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- [42].Taha M, e Silva F, Quental MV, Ventura SPM, Freire MG, Coutinho JAP. Good's buffers as a basis for developing self-buffering and biocompatible ionic liquids for biological research. Green Chem. 2014:3149–3159. doi: 10.1039/C4GC00328D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ugwu SO, Apte SP. The effect of buffers on protein conformational stability. Pharmaceutical Technology. 2004;28:86–109. [Google Scholar]
- [44].Ferguson WJ, Braunschweiger KI, Braunschweiger WR, Smith JR, McCormick JJ, Wasmann CC, Jarvis NP, Bell DH, Good NE. Hydrogen ion buffers for biological research. Anal Biochem. 1980;104:300–310. doi: 10.1016/0003-2697(80)90079-2. [DOI] [PubMed] [Google Scholar]
- [45].Taha M, Gupta BS, Khoiroh I, Lee M-J. Interactions of Biological Buffers with Macromolecules: The Ubiquitous “Smart” Polymer PNIPAM and the Biological Buffers MES, MOPS, and MOPSO. Macromolecules. 2011;44:8575–8589. [Google Scholar]
- [46].Taha M, Lee M-J. Interactions of TRIS [tris(hydroxymethyl)aminomethane] and related buffers with peptide backbone: Thermodynamic characterization. Phys Chem Chem Phys. 2010;12:12840–12850. doi: 10.1039/c0cp00253d. [DOI] [PubMed] [Google Scholar]
- [47].Gupta BS, Taha M, Lee M-J. Interactions of bovine serum albumin with biological buffers, TES, TAPS, and TAPSO in aqueous solutions. Process Biochem. 2013;48:1686–1696. [Google Scholar]
- [48].Gupta BS, Taha M, Lee M-J. Buffers more than buffering agent: introducing a new class of stabilizers for the protein BSA. Phys Chem Chem Phys. 2015;17:1114–1133. doi: 10.1039/c4cp04663c. [DOI] [PubMed] [Google Scholar]
- [49].Gupta BS, Taha M, Lee M-J. Superactivity of α-chymotrypsin with biological buffers, TRIS, TES, TAPS, and TAPSO in aqueous solutions. RSC Adv. 2014;4:51111–51116. [Google Scholar]
- [50].Taha M, Almeida MR, e Silva FA, Domingues P, Ventura SPM, Coutinho JAP, Freire MG. Novel Biocompatible and self-buffering ionic liquids for biopharmaceutical applications. Chem Eur J. 2015;21:4781–4788. doi: 10.1002/chem.201405693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Foucault AP. Enantioseparations in counter-current chromatography and centrifugal partition chromatography. Journal of Chromatography A. 2001;906:365–378. doi: 10.1016/s0021-9673(00)00499-4. [DOI] [PubMed] [Google Scholar]
- [52].Ekberg B, Sellergren B, Albertsson P-Å. Direct chiral resolution in an aqueous two-phase system using the counter-current distribution principle. Journal of Chromatography A. 1985;333:211–214. doi: 10.1016/s0021-9673(01)87344-1. [DOI] [PubMed] [Google Scholar]
- [53].Arai T, Kuroda H. Distribution behavior of some drug enantiomers in an aqueous two-phase system using counter-current extraction with protein. Chromatographia. 1991;32:56–60. [Google Scholar]
- [54].Shinomiya K, Kabasawa Y, Ito Y. Enantiomeric Separation of Commercial D,L-Kynurenine with an Aqueous Two-Phase Solvent System by Cross-Axis Coil Planet Centrifuge. Journal of Liquid Chromatography & Related Technologies. 1998;21:135–141. [Google Scholar]
- [55].Merchuk JC, Andrews BA, Asenjo JA. Aqueous two-phase systems for protein separation: Studies on phase inversion. J Chromatogr B. 1998;711:285–293. doi: 10.1016/s0378-4347(97)00594-x. [DOI] [PubMed] [Google Scholar]
- [56].Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comp Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Majorek KA, Porebski PJ, Dayal A, Zimmerman MD, Jablonska K, Stewart AJ, Chruszcz M, Minor W. Structural and immunologic characterization of bovine, horse, and rabbit serum albumins. Mol Immunol. 2012;52:174–182. doi: 10.1016/j.molimm.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Frisch MJ, T GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, et al. Gaussian 09. Gaussian, Inc; Wallingford CT: 2009. [Google Scholar]
- [59].Huang S, Wang Y, Zhou Y, Li L, Zeng Q, Ding X. Choline-like ionic liquid-based aqueous two-phase extraction of selected proteins. Analytical Methods. 2013;5:3395–3402. [Google Scholar]
- [60].Du Z, Yu Y-L, Wang J-H. Extraction of proteins from biological fluids by use of an ionic liquid/aqueous two-phase system. Chemistry-a European Journal. 2007;13:2130–2137. doi: 10.1002/chem.200601234. [DOI] [PubMed] [Google Scholar]
- [61].Shouren G, Kojio K, Takahara A, Kajiyama T. Bovine serum albumin adsorption onto immobilized organotrichlorosilane surface: Influence of the phase separation on protein adsorption patterns. Journal of Biomaterials Science, Polymer Edition. 1998;9:131–150. doi: 10.1163/156856298x00479. [DOI] [PubMed] [Google Scholar]
- [62].Ye J, Fan F, Xu X, Liang Y. Interactions of black and green tea polyphenols with whole milk. Food Res Int. 2013;53:449–455. [Google Scholar]
- [63].Charbonneau DM, Tajmir-Riahi H-A. Study on the Interaction of Cationic Lipids with Bovine Serum Albumin†. J Phys Chem B. 2009;114:1148–1155. doi: 10.1021/jp910077h. [DOI] [PubMed] [Google Scholar]
- [64].Murayama K, Tomida M. Heat-Induced Secondary Structure and Conformation Change of Bovine Serum Albumin Investigated by Fourier Transform Infrared Spectroscopy. Biochem Eng J. 2004;43:11526–11532. doi: 10.1021/bi0489154. [DOI] [PubMed] [Google Scholar]
- [65].Sreerama N, Woody RW. Protein Secondary Structure from Circular Dichroism Spectroscopy: Combining Variable Selection Principle and Cluster Analysis with Neural Network, Ridge Regression and Self-consistent Methods. J Mol Biol. 1994;242:497–507. doi: 10.1006/jmbi.1994.1597. [DOI] [PubMed] [Google Scholar]
- [66].Pelton JT, McLean LR. Spectroscopic Methods for Analysis of Protein Secondary Structure. Anal Biochem. 2000;277:167–176. doi: 10.1006/abio.1999.4320. [DOI] [PubMed] [Google Scholar]
- [67].Murayama K, Tomida M. Heat-Induced Secondary Structure and Conformation Change of Bovine Serum Albumin Investigated by Fourier Transform Infrared Spectroscopy. Biochemistry. 2004;43:11526–11532. doi: 10.1021/bi0489154. [DOI] [PubMed] [Google Scholar]
- [68].Hu X, Cui S, Liu Jq. Fluorescence studies of interaction between flavonol p-coumaroylglucoside tiliroside and bovine serum albumin. Spectrochim Acta A Mol Biomol Spectrosc. 2010;77:548–553. doi: 10.1016/j.saa.2010.06.016. [DOI] [PubMed] [Google Scholar]
- [69].Sreerama N, Woody RW. Protein Secondary Structure from Circular Dichroism Spectroscopy: Combining Variable Selection Principle and Cluster Analysis with Neural Network, Ridge Regression and Self-consistent Methods. J Mol Biol. 1994;242:497–507. doi: 10.1006/jmbi.1994.1597. [DOI] [PubMed] [Google Scholar]
- [70].Morrisett JD, David JSK, Pownall HJ, Gotto AM. Interaction of an apolipoprotein (apoLP-alanine) with phosphatidylcholine. Biochemistry. 1973;12:1290–1299. doi: 10.1021/bi00731a008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.