Abstract
We describe the construction of systems for expressing the cloned streptavidin gene in Escherichia coli. Although the streptavidin gene is extremely lethal to the host cells, because of the strong biotin binding of the gene product, the gene was expressed efficiently in E. coli by using T7 RNA polymerase/T7 promoter expression systems. The expressed streptavidin accumulated to more than 35% of the total cell protein. The expressed streptavidin was insoluble in the cell. However, after solubilization by dialysis against 6 M guanidine hydrochloride (pH 1.5) and removal of guanidine hydrochloride by dialysis, the protein became soluble and renatured. This simple procedure yielded streptavidin purified almost to homogeneity. The purified streptavidin bound 3.5-3.9 molecules of biotin per molecule, indicating that it had almost full biotin-binding ability. Some of the purified streptavidin molecules aggregated into oligomers, suggesting that the C-terminal region of the molecule, present in our material but absent in typical preparations, may be responsible for the aggregation.
Full text
PDF


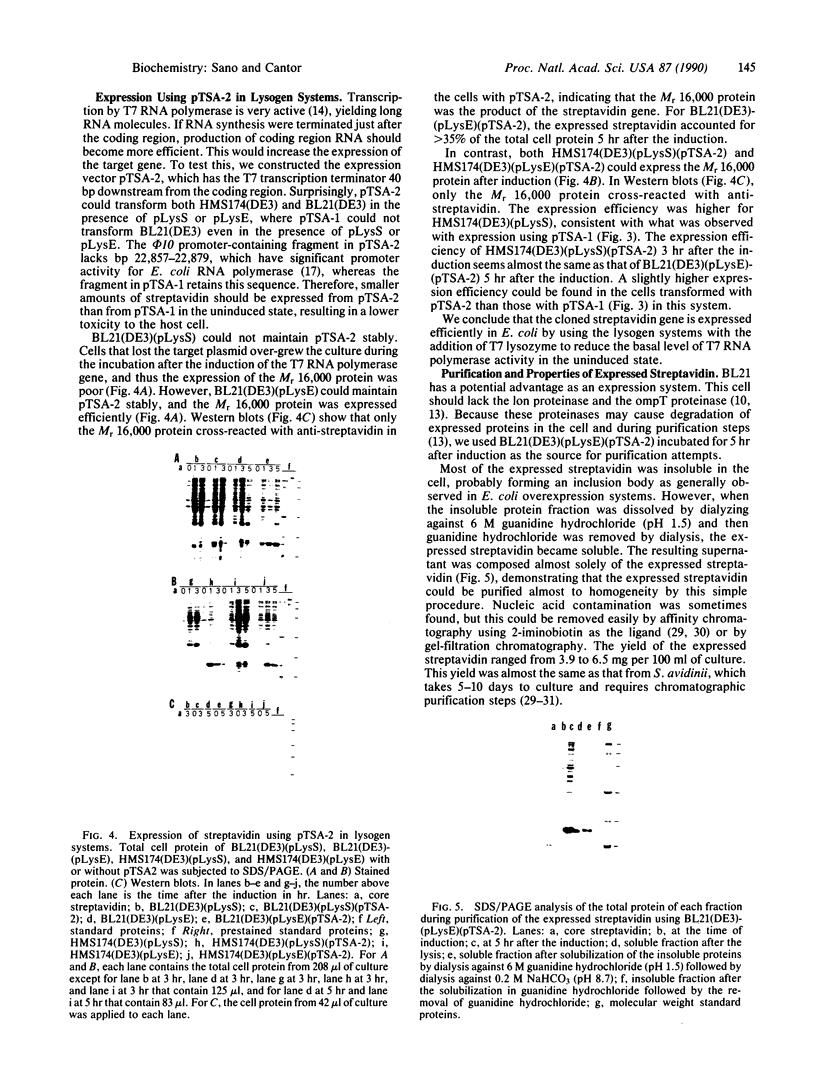
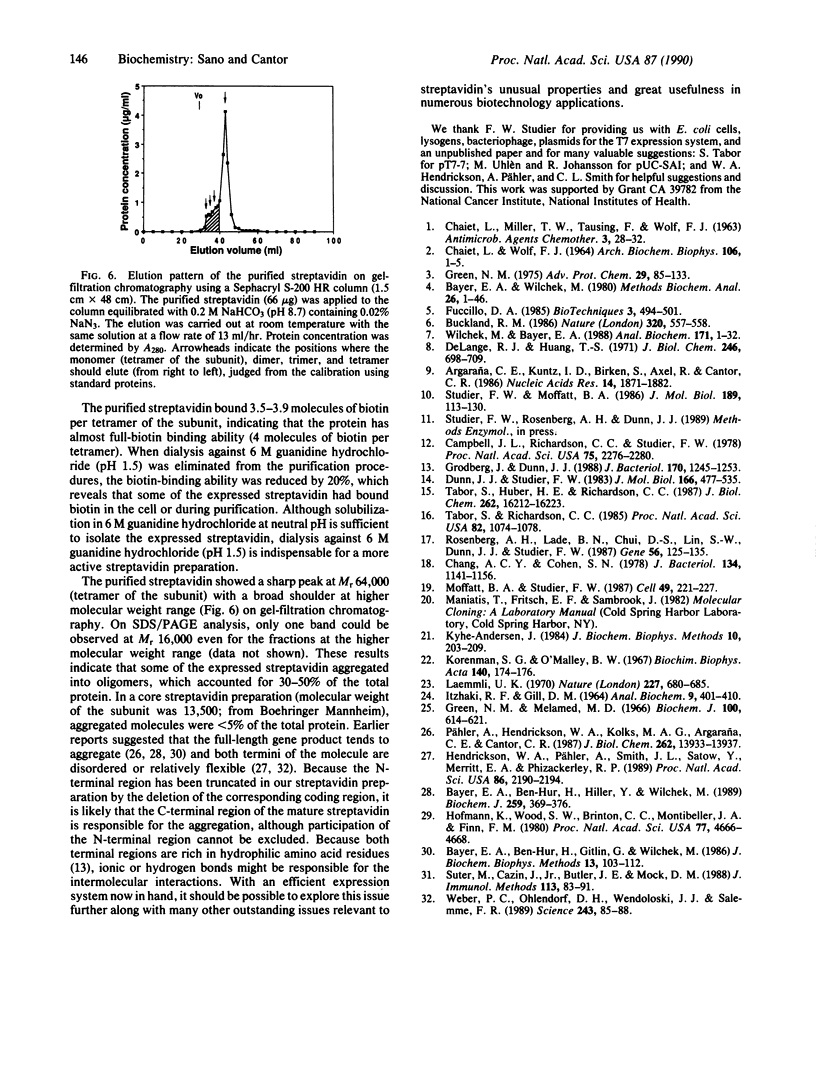
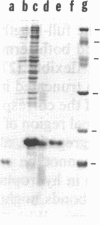
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argaraña C. E., Kuntz I. D., Birken S., Axel R., Cantor C. R. Molecular cloning and nucleotide sequence of the streptavidin gene. Nucleic Acids Res. 1986 Feb 25;14(4):1871–1882. doi: 10.1093/nar/14.4.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Ben-Hur H., Gitlin G., Wilchek M. An improved method for the single-step purification of streptavidin. J Biochem Biophys Methods. 1986 Sep;13(2):103–112. doi: 10.1016/0165-022x(86)90022-9. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Ben-Hur H., Hiller Y., Wilchek M. Postsecretory modifications of streptavidin. Biochem J. 1989 Apr 15;259(2):369–376. doi: 10.1042/bj2590369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- CHAIET L., MILLER T. W., TAUSIG F., WOLF F. J. ANTIBIOTIC MSD-235. II. SEPARATION AND PURIFICATION OF SYNERGISTIC COMPONENTS. Antimicrob Agents Chemother (Bethesda) 1963;161:28–32. [PubMed] [Google Scholar]
- CHAIET L., WOLF F. J. THE PROPERTIES OF STREPTAVIDIN, A BIOTIN-BINDING PROTEIN PRODUCED BY STREPTOMYCETES. Arch Biochem Biophys. 1964 Jul 20;106:1–5. doi: 10.1016/0003-9861(64)90150-x. [DOI] [PubMed] [Google Scholar]
- Campbell J. L., Richardson C. C., Studier F. W. Genetic recombination and complementation between bacteriophage T7 and cloned fragments of T7 DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2276–2280. doi: 10.1073/pnas.75.5.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Huang T. S. Egg white avidin. 3. Sequence of the 78-residue middle cyanogen bromide peptide. Complete amino acid sequence of the protein subunit. J Biol Chem. 1971 Feb 10;246(3):698–709. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Green N. M., Melamed M. D. Optical rotatory dispersion, circular dichroism and far-ultraviolet spectra of avidin and streptavidin. Biochem J. 1966 Sep;100(3):614–621. doi: 10.1042/bj1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodberg J., Dunn J. J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988 Mar;170(3):1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W. A., Pähler A., Smith J. L., Satow Y., Merritt E. A., Phizackerley R. P. Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2190–2194. doi: 10.1073/pnas.86.7.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K., Wood S. W., Brinton C. C., Montibeller J. A., Finn F. M. Iminobiotin affinity columns and their application to retrieval of streptavidin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4666–4668. doi: 10.1073/pnas.77.8.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moffatt B. A., Studier F. W. T7 lysozyme inhibits transcription by T7 RNA polymerase. Cell. 1987 Apr 24;49(2):221–227. doi: 10.1016/0092-8674(87)90563-0. [DOI] [PubMed] [Google Scholar]
- Pähler A., Hendrickson W. A., Kolks M. A., Argaraña C. E., Cantor C. R. Characterization and crystallization of core streptavidin. J Biol Chem. 1987 Oct 15;262(29):13933–13937. [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Suter M., Cazin J., Jr, Butler J. E., Mock D. M. Isolation and characterization of highly purified streptavidin obtained in a two-step purification procedure from Streptomyces avidinii grown in a synthetic medium. J Immunol Methods. 1988 Oct 4;113(1):83–91. doi: 10.1016/0022-1759(88)90384-5. [DOI] [PubMed] [Google Scholar]
- Tabor S., Huber H. E., Richardson C. C. Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J Biol Chem. 1987 Nov 25;262(33):16212–16223. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P. C., Ohlendorf D. H., Wendoloski J. J., Salemme F. R. Structural origins of high-affinity biotin binding to streptavidin. Science. 1989 Jan 6;243(4887):85–88. doi: 10.1126/science.2911722. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. The avidin-biotin complex in bioanalytical applications. Anal Biochem. 1988 May 15;171(1):1–32. doi: 10.1016/0003-2697(88)90120-0. [DOI] [PubMed] [Google Scholar]