Abstract
This review summarizes the state-of-the-art knowledge of the usage of poly(hydroxy alkanoate)s in medical and sanitary applications. Depending on the monomers incorporated into the polymers and copolymers, this class of polymers exhibits a broad range of (thermo-)plastic properties, enabling their processing by, e.g., solution casting or melt extrusion. In this review, strategies for the polymer analogous modification of these materials and their surfaces are highlighted and correlated with the potential applications of the corresponding materials and blends. While the commercial availability of purified PHAs is addressed in brief, special focus is put on the (bio-)degradability of these polymers and ways to influence the degradation mechanism and/or the duration of degradation.
Keywords: poly(hydroxy alkanoate), polymer analogous modification, polymer processing, bio-degradation, medical application
Introduction
In the past decades, mankind has produced more plastics than ever before, while simultaneously, due to the stagnation in oil production and the general demand for “greener” products, plastics from renewable resources have become more and more important1,2. Such materials often face significant challenges when compared to petrochemical plastics, particularly in terms of higher production costs and availability of the source materials3,4. Whilst competitive costs are still a very important factor for the commercial usage of polymers from renewable resources, advanced medical applications, such as tissue repair, polymer-based depots for controlled drug release or implants, have paved the way for biodegradable as well as biocompatible materials. Petroleum-derived products commonly cannot meet the requirements inherent to those applications. In the past years, polyurethanes and derivatives from poly(ethylene glycol) (PEG), which used to be the “golden standard” for polymers in medical applications, have been continuously replaced by different polymers and blends from natural resources due to their superior biocompatibility and biodegradability. Poly(hydroxy alkanoate)s (PHAs) (Fig. 1) are one of the new materials that have received a lot of attention since their discovery in 1926 by Lemoigne5.
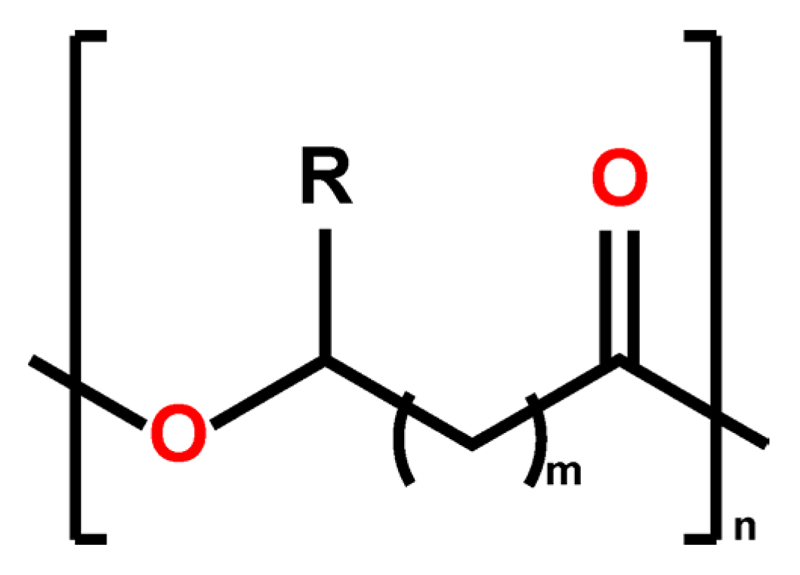
Fig. 1.
Generic structural formula of PHAs. The integer m typically has the value of 1 (with the exception of 4-hydroxy alkanoates such as 4-hydroxybutyrate), the integer n quantifies the degree of polymerization.
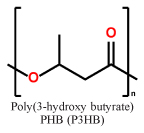
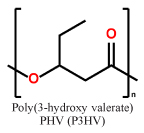
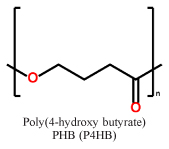
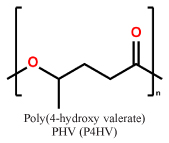
Short chain length (scl) PHAs (Table 1) with 3–5 carbon atoms per repetition unit, and medium chain length (mcl) with 6–14 carbon atoms per repetition unit are the two main representatives of the PHA congeners. These two types of PHAs differ tremendously in their mechanical behavior. Glass transition temperatures of scl-PHAs like PHB range around 5–10 °C and can be drastically lowered by copolymerization with PHV or P4HB (yielding the copolymers P(HB-HV) and P(HB-4HB), while mcl-PHAs have their glass-transition points at lower temperatures6,7. While scl-PHAs are brittle and tend to have high crystallinity, mcl-PHAs are more flexible, but exhibit a comparably low mechanical strength. Especially the brittle behavior of the scl-PHAs has limited its usage in industrial production.
Table 1.
Representative repetition units in scl-PHAs and mcl-PHAs
| scl-PHAs | 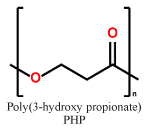 |
 |
 |
 |
 |
||
| mcl-PHAs | 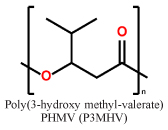 |
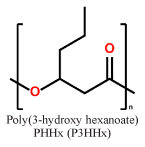 |
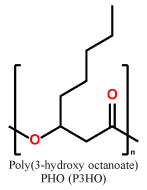 |
Blends and copolymers of PHAs have evolved into a desired strategy to overcome these shortcomings; this new material class is still under intense investigation4. The monomers most commonly considered in research are listed in Table 1. Apart from the investigation of new blends and copolymers, the investigations of new approaches for the functionalization of existing saturated and unsaturated PHAs is still a very hot topic8–15. The development of PHAs with tailor-made material qualities for direct usage in a broad spectrum of applications has become easier to achieve than ever before.
Production
In nature, PHAs are synthesized by a variety of different Gram-positive and Gram-negative bacteria. More than 300 different microorganisms that are able to synthesize and accumulate PHA intracellularly are known today16. The list includes wild type bacteria, such as Cupriavidus necator, Azotobacter species, Pseudomonas species, and Methylobacterium species as well as engineered strains of, e.g., Escherichia coli and Cupriavidus necator17–19. These types of bacteria synthesize PHA polymers or copolymers and store them in the form of granules in the cytoplasm. The ability for the cells to grow to high cell densities on the one hand, and to accumulate a high (total cell mass) percentage of PHA on the other, is the key criterion to look for in potential production strains. Hence, despite the fact that a high number of microorganisms capable of producing PHAs have been identified, only very few bacteria from this list are suited for industrial scale production.
Bacteria produce PHAs as storage polymers for carbon and energy under metabolism-limited conditions if carbon and energy sources are present, but one or more growth-essential nutrients, such as phosphate, nitrogen or oxygen are deficient. The biosynthesis of PHAs usually occurs with purified sugars or edible oils as feedstock, but alternative carbon sources, such as whey20, palm oil21, sunflower meal22, and different waste materials23–25 have been reported as well. In addition, strategies for the production from sugar molasses are under thorough investigation3,26,27. The main reason for the on-going search for cheap substrates as feedstock originates from the challenge to produce PHAs within the same range of costs like polyethylene (PE) or polypropylene (PP). Alternative carbon sources, more efficient processing and improved production strains are fields of active research to overcome this challenge28.
Cavalheiro et al. showed the possibility of PHB production by Cupriavidus necator using waste glycerol from the biodiesel industry29. They observed that the specific growth rate peaks with a glycerol feed in the range of 20–40 g L–1 due to the amounts of sodium still present in the feedstock are due to the precedent transesterfication processes.
Khosravi-Darani et al. reported the microbial production of PHB from C1 carbon sources such as methanol, methane or CO230. Although commercial PHB production from these carbon sources has not yet been established by many companies31, the consideration of using such cheap substrates would be very favorable. Mozejko recently showed that saponified waste palm oil can be used as an attractive renewable resource for mcl-PHA synthesis32. With a polymer productivity of 57.8 mg L-1 h-1 and a mass fraction of PHA in cell dry mass of 43 % of cell dry mass CDW after 17 h of fermentation, this method proved to be an efficient approach. Muhr et al. reported the usage of fractions of waste lipids from animal processing as a feedstock for PHA production24, showing that Pseudomonas citronellolis as well as Pseudomonas chlororaphis33 are prospective candidates for large-scale production of PHA. Tanadchangsaeng et al. reported the biosynthesis of a statistical copolymer of P(HB-HMV) with up to 38 mol-% of PHMV34. Cupriavidus necator expressing the PHA synthase from Pseudomonas species was fed with structural analogs that served as HMV precursors.
Engineered strains
Apart from the wild type strains of microorganisms such as Cupriavidus necator, there is an on-going trend to engineer suitable microorganisms to produce PHA35. In recent years, a number of strains were engineered for PHA accumulation rates of up to 90 wt.-% of CDW36. Jeon et al. produced engineered recombinant Ralstonia eutropha strains in a manner that the bacteria produced PHAs such as statistical P(HB-HHx) copolymers in quantities up to 40 wt.-%19. Notably, unrelated carbon sources such as glucose, fructose and gluconate were used as feedstock. Mifune et al. showed that engineered strains of Cupriavidus necator for the production of statistical P(HB-HHx) copolymers were able to reach over 80 wt.-% of PHA accumulation, using soy bean oil as carbon source18. Saika and coworkers recently published results concerning a recombinant E. coli for the production of statistical P(HB-HMV) copolymers from leucine by expressing leucine metabolism-related enzymes derived from Clostridium difficile17. Tripathi et al. reported a β-oxidation weakened Pseudomonas putida KT2442, able to produce a diblock copolymer of the composition PHB-block-PHHx37. The utilized recombinant strain of Pseudomonas putida consists of pha operon and therefore is able to produce mcl-PHA.
Mixed culture approaches
Purified and single strain cultures are by definition more expensive than the mixed culture approach, which can also provide the ability to use cheaper substrates3. Especially the cheap carbon sources of this approach make it an interesting possibility for an environmentally sustainable PHA production system. Fradinho et al. showed that a PHA production from individual and mixed volatile fatty acids (acetate, propionate, butyrate, lactate, malate and citrate) can be achieved38. Only acetate and butyrate led to PHB formation, propionate induced the synthesis of statistical P(HB-HV) copolymers. It was postulated that acetate is likely to act as a co-substrate for butyrate and propionate uptake, since uptake rates of both carbon sources were increased in the presence of acetate. Johnson et al. worked on the enrichment of a mixed bacterial culture with a high PHA storage capacity in order to compete with genetically engineered bacteria producing PHA from pure substrates like sugars39. These optimized processes led to high contents of PHA in the cell dry mass and high production rates, but expensive substrates, equipment, and high energy input were required. Within this study, a maximum PHB content of 89 wt.-% within 7.6 h under continuous feeding with acetate was achieved. Anterrieu et al. showed the integration of biopolymer production with process water treatment at a sugar factory40. The challenges in these studies were to find a way to combine nitrogen removal with PHA production.
Processing
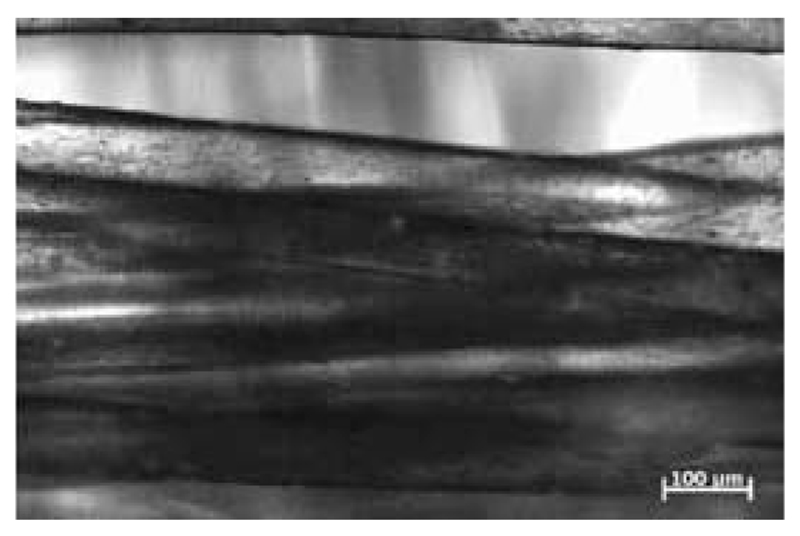
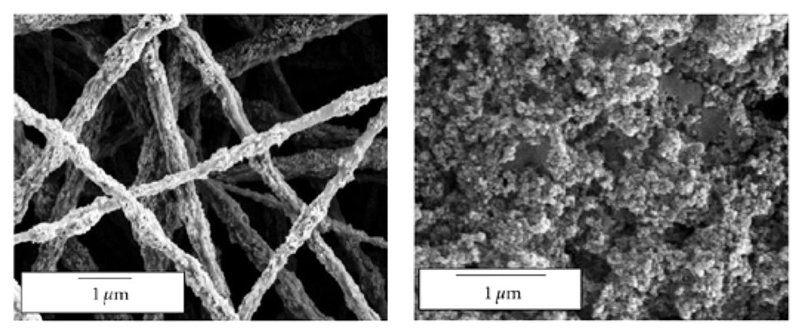
The development of new techniques in production processing has been a highly active research field in recent years41–45. Based on their mechanical properties and their (commercial) availability (see above), scl-PHAs (homopolymers as well as copolymers) are focused on. Due to the melting points of PHP (77 °C) and PHB (175 °C)46, as well as PHV (105 °C)47, scl-PHAs and derived copolymers can be processed by injection molding, extrusion, extrusion bubbles into films and hollow bodies, and fibre spinning. While polymer degradation of scl-PHAs starts from temperatures only slightly above their melting temperatures, namely from approx. 180 °C, the processing parameters have to be fine-tuned with high precision. For a better understanding of the degradation process and potential bottlenecks of the PHA class, several studies were made in the last decade32,33. Recently, Hufenus et al. reported the fiber melt-spinning of PLA and statistical copolymers of the composition P(HB-HV) for the production of new materials for biomedical purposes50. They were able to produce fibers with suitable mechanical properties, e.g. tensile strength and Young’s modulus to construct a textile fabric (Fig. 2).
Fig. 2.
Overlay of light and fluorescence micrographs showing cell adhesion on fibers composed of a PLA core and a coating of statistical copolymers of P(HB-HV). Reprinted from reference50 with permission from John Wiley and Sons.
Prior to any processing, PHAs have to be purified in order to remove bacterial components present in the crude polymers – for biomedical applications, in particular, the bacterial endotoxins have to be removed51. Sevastianov et al. reported the importance of proper endotoxin removal for in-vivo applications52. Notably, PHAs are most commonly soluble in dichloromethane, chloroform, and dichloroethane, although other solvents like ethyl acetate or methyl tert-butyl ether have recently been investigated as well53,54. Koller et al. reported the usage of the “anti-solvent” acetone for the purification of scl-PHAs in a novel closed system combining components for extraction, filtration and product work-up, compared to already established repeated dissolution-precipitation strategies55,56. By designing a new apparatus and optimizing the operating temperature and pressure control, a method was developed that can also be applied to mcl-PHAs.
Modification
The most common homopolymer PHB, and the most common statistical copolymer P(HB-HV), have significant shortcomings in terms of brittleness and high degree of crystallinity57. P4HB and copolymers containing 4HB are under investigation for their mechanical properties for further surgical applications58,59. The tensile strength of P4HB is close to ultrahigh molecular weight PE and is a very flexible material of high strength. However, PHB and the statistical copolymer P(HB-HV) are the only PHAs currently available in large scale. Post-synthetic and polymer analogous strategies were developed in order to overcome the shortcomings of the pristine PHAs.
Combining PHAs with other polymers in the form of a blend enables new substance classes with modified and tailor-made characteristics, such as brittleness, crystallinity, and degradation rates60,61. The scl-PHAs, due to their crystallinity, are more resistant to biodegradation than mcl-PHAs. By blending a different polymer, such as a mcl-PHA into the matrix of a scl-PHA, higher degradation rates can be achieved while having similar polymer properties. Martelli et al. reported the characterization of mcl-PHA-based blends, focusing on blends of the statistical copolymer P(HB-HV) and a mcl-PHA composed of PHO, poly(hydroxy decanoate), and poly(hydroxy dodecanoate)62. The addition of 5 wt.-% of the mcl-PHA resulted in an increased strain at break of 50 %, compared to non-modified statistical copolymers of P(HB-HV).
Wu and colleagues developed a simple and safe nanoparticle system by coating P(HB-HHx) (statistical copolymers) nanoparticles with poly(ethylene imine) for application in in-vitro and ex-vivo cellular manipulation. The particles were produced from Rhodamine-B-loaded P(HB-HHx) nanoparticles with a diameter of 154 ± 71 nm, which were subsequently coated with poly(ethylene imine) in order to facilitate the binding to and uptake by cells63. A similar strategy for the coating of nanoparticles of statistical copolymers of P(HB-HV) by poly(vinyl alcohol) was published by Masood et al.64 The particles contained the anti-cancer drug Ellipticine. Boyandin et al. reported blends composed of different tropical soils and their influence on the biodegradation of PHA homopolymers and blends65. Various factors such as differences in soil and climatic conditions were found to play a crucial role in the degradation. The major degraders of PHA could be identified as bacteria of the genera Burkholderia, Bacillus, Cupriavidus, Streptomyces, Nocardiopsis and Mycobacterium, as well as the fungi Gongronella butleri, Penicillium species, Acremonium recifei, Purpureocillium lilacinus, and Trichoderma pseudokoningii.
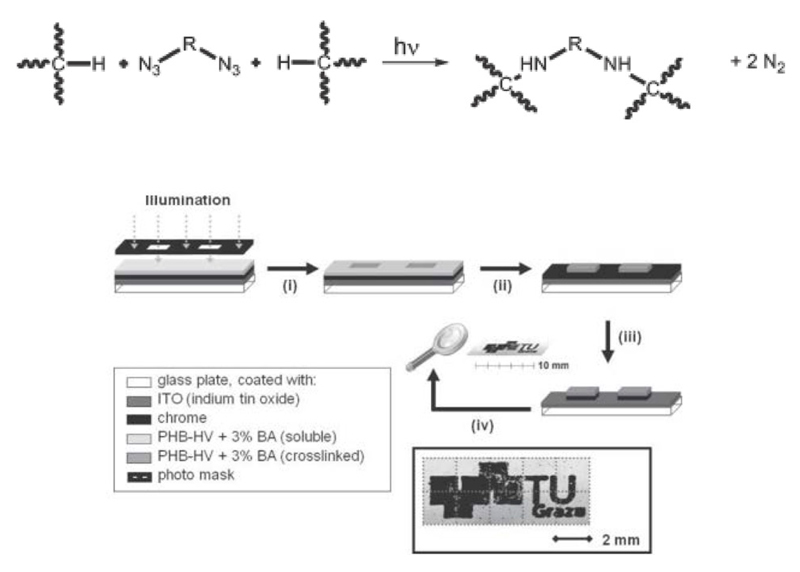
Polymer-analogous reactions have particularly focused on adding functionality to unsaturated PHAs involving the highly reactive double bond of the PHA’s side-chains. Reports comprise the carboxylation9, hydroxylation10, introduction of amine groups11, and combination with other (hydrophilic) polymers like PEG12. The polymer-analogous cross-linking of polymer chains is another strategy aiming at the increase of the mechanical strength of PHAs13. Rupp et al. reported the UV-induced crosslinking of a scl-PHA copolymer, namely films of P(HB-HV), with a bisazide capable of absorbing UV light14. By addition of up to 3 wt.-% of the bisazide (BA) to P(HB-HV), crosslinking degrees of more than 90 % could be achieved within irradiation times of less than 1 minute. This process was successfully employed in photolithographic processes that produced images with 50 µm resolution (Fig. 3).
Fig. 3.
Top: Crosslinking of polymer chains with a bisazide (BA) under UV irradiation. Bottom: Multi-step photolithographic processes based on P(HB-HV). Reproduced in part from reference14 with permission of The Royal Society of Chemistry.
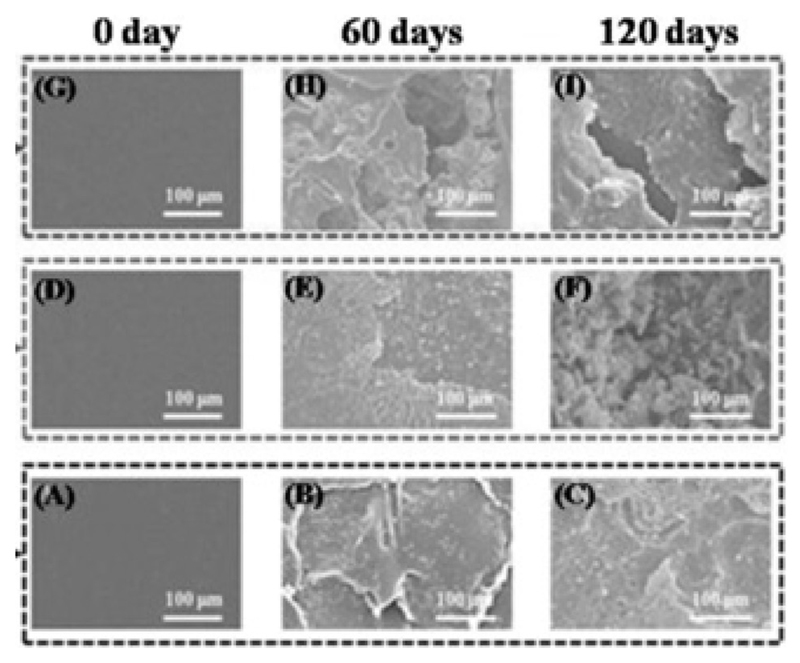
The thermally induced crosslinking of biopolyesters (comprising PHAs) by telechelic poly(ethylene glycol)-bisazides was summarized in a patent15, potentially overcoming the long degradation rates of pristine PHAs. Recent work by Wu et al. focused on the characterization of PHA composites that were either chemically crosslinked with cellulose acetate or blended with chestnut shell fibers, the latter for enhanced biodegradation66,67. The compounds produced were characterized, among others, by biodegradation rates, adhesion of cells, hydrophilicity and mechanical properties (Fig. 4). The results confirm the strategy, since the mechanical properties, especially the tensile strength, of both composites increased. The biodegradation rate was also higher in both composites compared to pure PHA, maintaining biocompatibility.
Fig. 4.
SEM images of the morphology of PHA composites after 0, 60, and 120 days of degradation. A-C: pristine PHA, D-F: PHA crosslinked with cellulose actetate, G-I: PHA grafted on acrylic acid/cellulose acetate. Biodegradation by Acetobacter pasteurianus can be monitored by the erosion in the films. Reprinted from reference66 with permission from Elsevier.
Applications
Certain microorganisms with the ability to produce PHA depolymerases can promote the biodegradation of PHA. Under aerobic conditions, they are degraded into water and carbon dioxide and, under anaerobic conditions, into methane and water68. The biodegradability and biocompatibility of PHAs makes them the ideal candidate for a broad spectrum of applications. However, the rates of degradation are not always suited to the purpose, and depend highly on the microbial environment of the PHA product69–73. Corresponding applications of PHAs include improved forms of food packaging74, waste bags, or agricultural plastics. Sudesh and colleagues reported about the synthesis of PHA from palm oil and new applications thereof21. In their approach, they investigated PHA as a potential facial oil blotting material as well as a potential dye remover from wastewater.
Recent work from Sridewi et al. showed that (blended) PHB-TiO2 electrospun nanocomposite fibers and films (Fig. 5) can be used for the removal of dyes, such as malachite green via decolorization, degradation, and detoxification75. Materials derived from the fiber melt-spinning of PLA and P(HB-HV) (reported by Hufenus et al., see above)50, were subjected to biodegradability studies on human fibroblasts that revealed no toxicity of the fibers and cells proliferated well under the condition provided by these new materials. Additionally, the molecular weight loss caused by biodegradation of the fibers reduced the tensile strength up to 33 % after 4 weeks of incubation, further proving the promise of this new material in medical applications.
Fig. 5.
SEM micrographs showing the morphological features of electrospun fibers (left) and spincast films (right) of PHB blended with TiO2. Reprinted from reference75 with permission from Hindawi Publishing Corporation.
Castro-Mayorga et al. showed the stabilization of antimicrobial silver nanoparticles (preventing their agglomeration) by a PHA obtained from mixed bacterial culture74. The study demonstrated that unpurified statistical copolymers of P(HB-HV) can be used as capping agent that helps prevent agglomeration.
The area most considered for possible application of PHAs and PHA blends is the area of medical applications1,8,58,76–84. The usage as a system for drug delivery is perhaps the longest investigated application in this area, but still a highly active field85,86. Naveen et al. synthesized nanofibers mats of PHB by electrospinning and loaded them with Kanamycin sulphate to test against Staphylococcus aureus. The drug release of the antibiotic showed more than 95 % release within 8 hours84. Xiong and colleagues prepared nanoparticles of PHB, statistical copolymers of P(HB-HHx) and PLA and loaded them with lipid-soluble colorant rhodamine B isothiocyanate as a model compound87. Due to their size, nanoparticles can penetrate deeply into tissue material and are more efficiently taken up by cells. They achieved a high loading efficiency of around 75 % with the PHB homo- and P(HB-HHx) statistical copolymers, and a drug release over a period of at least 20 days, while reference PLA nanoparticles only lasted 15 days. Chaturvedi et al. used blends of PHB with cellulose acetate phthalate (CAP) in different compositions for drug loading with 5-fluorouracil, an anticancer drug, and investigated the simulated colon delivery of the said drug88. The pH-sensitive property of the blend caused a higher in vitro release at alkaline pH than at acidic pH, suggesting its potential for colon delivery.
Tissue repair has become one of the major fields when combining PHA with medical applications77,81,83,89. The high biocompatibility of PHB, which is not surprising when considering the natural occurrence of 3HB in the blood stream1, makes it an ideal candidate for scaffolds, which can later be used for the repair of tissue damage. Results of animal testing clearly showed the high biocompatibility of implants of PHB and P(HB-HV) statistical copolymers. Shishatskaya et al. and Volova et al. investigated the physiological and biochemical characteristics of Wistar rats implanted with PHA sutures. Long-term (1 year) observations showed that the animals with PHB or P(HB-HV) threads were active and healthy throughout the experiment, and suggested that the implanted polymer threads did not affect the organism in a negative way56,90. For biodegradable implants, the high crystallinity is indeed a problem, rendering the attack of degrading enzymes more difficult91. Various blends of different PHAs, such as P(HB-HV), are therefore under investigation, combining the excellent biocompatibility of PHB with a lower degree of crystallinity provided by the incorporated PHV89.
Basnett et al. reported novel blends composed of PHO and PHB for medical applications. In their study, blends with various ratios of PHB and PHO in various ratios were created, and degradation as well as biocompatibility tests were carried out92. The degradation found in these blends occurred via surface erosion and not bulk degradation, which would lead to a more controlled degradation, while still maintaining the core structure. Combined with an increased biocompatibility with HMEC-1 cells, these blends showed a very promising perspective for the development of biodegradable materials.
Another strategy for tissue repair was reported by Ellis and colleagues who produced laser-perforated biodegradable PHA scaffold films93. The pores of the films of statistical copolymers of P(HB-HV) exhibited micrometer dimensions. Hence, once the cells were seeded onto the film surface, they could attach and proliferate on the upper surface as well as through the pores and into the region of the damaged tissue. In their study, they achieved an increased surface amorphicity at the pore edges, which may facilitate cell adhesion and could promote growth and migration of cells for regenerative medicine.
Sutures of PHB and P(HB-HV), respectively, were found to exhibit the mechanical strength required for use in muscle-facial wounds, and, hence, they were tested on animals intramuscularly90. The environmental tissue reacted to the PHAs by a transient post-traumatic inflammation, as well as in the formation of fibrous capsules with a thickness of up to 200 μm, which thinned upon prolonged exposure. If the sutures were implanted for periods of up to one year, they stimulated no suppurative inflammation or necrosis.
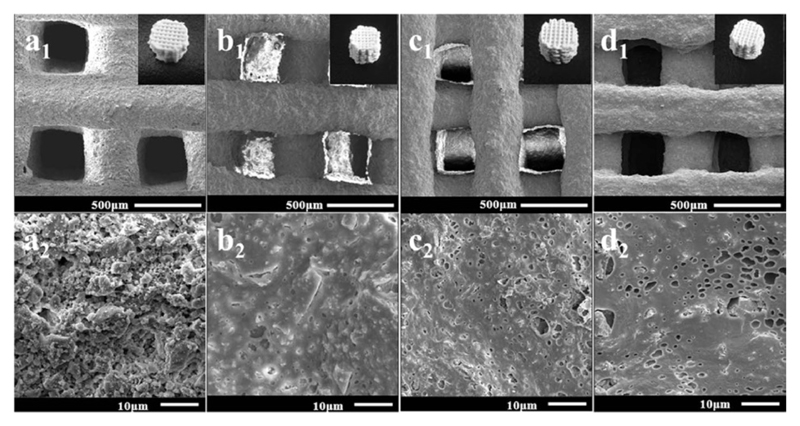
On the topic of nerve injury repair, a new strategy provided by Wang et al. described the usage of PHA as scaffolds for human bone marrow stromal cells94. A statistical terpolyester of the composition P(HB-HV-HHx) was compared with poly(lactic acid) and P(HB-HHx) for their function in differentiating the human bone marrow stromal cells into nerve cells. It could be shown that the terpolyester had stronger cell adhesion, proliferation and differentiation than the other two polymers. Three-dimensional scaffolds of a composite composed of P(HB-HHx) and mesoporous bioactive glass (in different mass ratios) were printed with a 3D bioplotter by the group of Zhao et al., aiming at the delivery of materials for enhanced bone regeneration95. These highly porous, yet robust scaffolds showed good bioactivity, stimulated human bone marrow stromal cells adhesion and stimulated bone regeneration in in-vivo experiments (Fig. 6).
Fig. 6.
SEM images of composite scaffolds of P(HB-HHx) and mesoporous bioactive glass (MBG) at different magnifications (top and bottom). A: reference material poly(vinyl alcohol):MBG = 1:7; B: P(HB-HHx):MBG = 1:7; C: P(HB-HHx):MBG = 1:5; D: P(HB-HHx):MBG = 1:3. Reproduced in part from reference95 with permission of The Royal Society of Chemistry.
Conclusions and outlook
New technologies and evolution of production modes (batch, feed batch, continuous) methods have allowed PHAs to become a recognizable player in the field of biodegradable polymers. Research in biotechnology and strain engineering has enabled the production of PHAs from cheap substrates, in-line with existing processing facilities such as waste treatment and easier-to-handle microorganisms compared to the wild type production strains reported in the past. Continuous improvements of PHA contents within the cells and growth rates are reported on a regular basis.
PHAs are still far from joining the competitive level of production costs compared to petroleum-based polymers such as PP or PE. Hence, currently, PHAs can be only considered for advanced applications, in which they cannot be replaced by petroleum-based polymers. Correspondingly, PHAs are well-suited for any application that requires biocompatibility and/or biodegradability of the polymer. Notably, purity requirements are high for such applications, and the removal of impurities, such as endotoxins, further increases the production costs. For medical applications, in particular the homopolymers PHB and PHV as well as the statistical copolymer PHB-stat-PHV have been investigated. These so-called scl-PHAs can be processed by melt processes as they are thermoplasts, or by solution processes due to their solubility in (a limited number of) organic solvents.
In order to adapt the polymers’ properties to the specific application details and requirements, several techniques can be applied to PHAs, not to mention the blending with other (biodegradable and biocompatible) polymers, and polymer-analogous modification by, e.g., crosslinking as prominent examples. Using these techniques, a broad spectrum of tailor-made mechanical and physical properties can be realized. In the area of medical applications, in particular tissue engineering could benefit from these recent developments and improvements. PHA blends as scaffolds for tissue repair are a highly active research area that has produced a lot of very promising results from in-vivo tests in recent years.
PHAs, quo vadis? Facing the need for advanced and/or alternative medical devices for tissue engineering, drug delivery, and implants in their broadest sense on the one hand, and the promising research results in recent years, the applicability of PHAs in those medical areas is more than plausible in the near future.
Acknowledgements
fW and kpL would like to thank the Austrian Science Fund FWF for financial support within the project I1123-N19 MimiFlow. fS would like to acknowledge funding by the Laura Bassi Centre of Expertise “BioResorbable Implants for Children - BRIC”, headed by A. Weinberg and managed by the Austrian Research Promotion Agency FFG. The research work was performed at the Polymer Competence Center Leoben GmbH (PCCL, Austria) within the framework of the COMET-program of the Federal Ministry for Transport, Innovation and Technology and Federal Ministry for Economy, Family and Youth with contributions by the Graz University of Technology and NAWI Graz. The PCCL is funded by the Austrian Government and the State Governments of Styria, Lower Austria and Upper Austria.
References
- 1.Zinn M, Witholt B, Egli T. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv Drug Deliv Rev. 2001;53:5. doi: 10.1016/S0169-409X(01)00218-6. [DOI] [PubMed] [Google Scholar]
- 2.Chen G-Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev. 2009;38:2434. doi: 10.1039/b812677c. [DOI] [PubMed] [Google Scholar]
- 3.Albuquerque MGE, Torres CAV, Reis MAM. Polyhydroxyalkanoate (PHA) production by a mixed microbial culture using sugar molasses: Effect of the influent substrate concentration on culture selection. Water Res. 2010;44:3419. doi: 10.1016/j.watres.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Chanprateep S. Current trends in biodegradable polyhydroxyalkanoates. J Biosci Bioeng. 2010;110:621. doi: 10.1016/j.jbiosc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Lemoigne M. Produits de dehydration et de polymerisation de l’acide oxobutyrique. Bull Soc Chim Biol. 1926;8:770. [Google Scholar]
- 6.Chanprateep S, Kulpreecha S. Production and Characterization of Biodegradable Terpolymer Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate-co-4-Hydroxybutyrate) by Alcaligenes sp. A-04. J Biosci Bioeng. 2006;101:51. doi: 10.1263/jbb.101.51. [DOI] [PubMed] [Google Scholar]
- 7.De Koning GJM, van Bilsen HMM, Lemstra PJ, Hazenberg W, Witholt B, Preusting H, van der Galiën JG, Schirmer A, Jendrossek D. A biodegradable rubber by crosslinking poly(hydroxyalkanoate) from Pseudomonas oleovorans. Polymer. 1994;35:2090. doi: 10.1016/0032-3861(94)90233-X. [DOI] [Google Scholar]
- 8.Hazer DB, Kiliçay E, Hazer B. Poly(3-hydroxyalkanoate)s: Diversification and biomedical applications A state of the art review. Mater Sci Eng C. 2012;32:637. doi: 10.1016/j.msec.2012.01.021. [DOI] [Google Scholar]
- 9.Lee MY, Park WH. Preparation of bacterial copolyesters with improved hydrophilicity by carboxylation. Macromol Chem Phys. 2000;201:2771. doi: 10.1002/1521-3935(20001201)201:18<2771::AID-MACP2771>3.0.CO;2-V. [DOI] [Google Scholar]
- 10.Hirt TD, Neuenschwander P, Suter UW. Telechelic diols from poly[(R)-3-hydroxybutyric acid] and poly{[(R)-3-hydroxybutyric acid]-co-[(R)-3-hydroxyvaleric acid]} Macromol Chem Phys. 1996;197:1609. doi: 10.1002/macp.1996.021970503. [DOI] [Google Scholar]
- 11.Sparks J, Scholz C. Synthesis and Characterization of a Cationic Poly(β-hydroxyalkanoate) Biomacromolecules. 2008;9:2091. doi: 10.1021/bm8005616. [DOI] [PubMed] [Google Scholar]
- 12.Kim HW, Chung CW, Rhee YH. UV-induced graft copolymerization of monoacrylate-poly(ethylene glycol) onto poly(3-hydroxyoctanoate) to reduce protein adsorption and platelet adhesion. Int J Biol Macromol. 2005;35:47. doi: 10.1016/j.ijbiomac.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Divyashree MS, Shamala TR. Effect of gamma irradiation on cell lysis and polyhydroxyalkanoate produced by Bacillus flexus. Radiat Phys Chem. 2009;78:147. doi: 10.1016/j.radphyschem.2008.08.010. [DOI] [Google Scholar]
- 14.Rupp B, Ebner C, Rossegger E, Slugovc C, Stelzer F, Wiesbrock F. UV-induced crosslinking of the biopolyester poly(3-hydroxybutyrate)-co-(3-hydroxyvalerate) Green Chem. 2010;12:1796. doi: 10.1039/c0gc00066c. [DOI] [Google Scholar]
- 15.Wiesbrock F, Ebner C, Stelzer F, Weinberg A, Kühn K-D. Hybrid polymeric materials for medical applications and preparation thereof. EP 2 537 540 A1. C.A: 2012 Dec 21;:2840115.
- 16.Steinbüchel A, Füchtenbusch B. Trends Biotechnol. 1998;16:419. doi: 10.1016/S0167-7799(98)01194-9. [DOI] [PubMed] [Google Scholar]
- 17.Saika A, Watanabe Y, Sudesh K, Tsuge T. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxy-4-methylvalerate) by recombinant Escherichia coli expressing leucine metabolism-related enzymes derived from Clostridium difficile. J Biosci Bioeng. 2014;117:670. doi: 10.1016/j.jbiosc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Mifune J, Nakamura S, Fukui T. Engineering of pha operon on Cupriavidus necator chromosome for efficient biosynthesis of poly(3-hydroxybutyrate-co-3hydroxyhexanoate) from vegetable oil. Polym Degrad Stab. 2010;95:1305. doi: 10.1016/j.polymdegradstab.2010.02.026. [DOI] [Google Scholar]
- 19.Jeon J-M, Brigham CJ, Kim Y-H, Kim H-J, Yi D-H, Kim H, Rha C, Sinskey AJ, Yang Y-H. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (P(HB-co-HHx)) from butyrate using engineered Ralstonia eutropha. Appl Microbiol Biotechnol. 2014;98:5461. doi: 10.1007/s00253-014-5617-7. [DOI] [PubMed] [Google Scholar]
- 20.Povolo S, Romanelli MG, Basaglia M, Ilieva VI, Corti A, Morelli A, Chiellini E, Casella S. Polyhydroxyalkanoate biosynthesis by Hydrogenophaga pseudoflava DSM1034 from structurally unrelated carbon sources. New Biotechnol. 2013;30:629. doi: 10.1016/j.nbt.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Sudesh K, Bhubalan K, Chuah J-A, Kek Y-K, Kamilah H, Sridewi N, Lee Y-F. Synthesis of polyhydroxyalkanoate from palm oil and some new applications. Appl Microbiol Biotechnol. 2011;89:1373. doi: 10.1007/s00253-011-3098-5. [DOI] [PubMed] [Google Scholar]
- 22.Kachrimanidou V, Kopsahelis N, Papanikolaou S, Kookos IK, De Bruyn M, Clark JH, Koutinas AA. Sunflower-based biorefinery: Poly(3-hydroxybutyrate) and poly(3- hydroxybutyrate-co-3-hydroxyvalerate) production from crude glycerol sunflower meal and levulinic acid. Bioresour Technol. 2014;172:121. doi: 10.1016/j.biortech.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 23.Hafuka A, Sakaida K, Satoh H, Takahashi M, Watanabe Y, Okabe S. Effect of feeding regimens on polyhydroxybutyrate production from food wastes by Cupriavidus necator . Bioresour Technol. 2011;102:3551. doi: 10.1016/j.biortech.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Muhr A, Rechberger EM, Salerno A, Reiterer A, Schiller M, Kwiecień M, Adamus G, Kowalczuk M, Strohmeier K, Schober S, Mittelbach M, et al. Biodegradable latexes from animal-derived waste: Biosynthesis and characterization of mcl-PHA accumulated by Ps. citronellolis. React Funct Polym. 2013;73:1391. doi: 10.1016/j.reactfunctpolym.2012.12.009. [DOI] [Google Scholar]
- 25.Hermann-Krauss C, Koller M, Muhr A, Fasl H, Stelzer F, Braunegg G. Archaeal Production of Polyhydroxyalkanoate (PHA) Co- and Terpolyesters from Biodiesel Industry-Derived By-Products. Archaea. 2013;2013:129268. doi: 10.1155/2013/129268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albuquerque MGE, Eiroa M, Torres C, Nunes BR, Reis MAM. Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J Biotechnol. 2007;130:411. doi: 10.1016/j.jbiotec.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Gouda MK, Swellam AE, Omar SH. Production of PHB by a Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiol Res. 2001;156:201. doi: 10.1078/0944-5013-00104. [DOI] [PubMed] [Google Scholar]
- 28.Urtuvia V, Villegas P, González M, Seeger M. Bacterial production of the biodegradable plastics polyhydroxyalkanoates. Int J Biol Macromol. 2014;70:208. doi: 10.1016/j.ijbiomac.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Cavalheiro JMBT, de Almeida MCMD, Grandfils C, da Fonseca MMR. Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem. 2009;44:509. doi: 10.1016/j.procbio.2009.01.008. [DOI] [Google Scholar]
- 30.Khosravi-Darani K, Mokhtari Z-B, Amai T, Tanaka K. Microbial production of poly(hydroxybutyrate) from C1 carbon sources. Appl Microbiol Biotechnol. 2013;97:1407. doi: 10.1007/s00253-012-4649-0. [DOI] [PubMed] [Google Scholar]
- 31.Criddle CS, Hart JR, Wu W-M, Sundstrom ER, Morse MC, Billington SL, Rostkowski KH, Frank CW. Production of PHA using Biogas as Feedstock and Power Source. US 2013/0071890 A1. 2012 Mar 21;
- 32.Możejko J, Ciesielski S. Saponified waste palm oil as an attractive renewable resource for mcl-polyhydroxyalkanoate synthesis. J Biosci Bioeng. 2013;116:485. doi: 10.1016/j.jbiosc.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Muhr A, Rechberger EM, Salerno A, Reiterer A, Malli K, Strohmeier K, Schober S, Mittelbach M, Koller M. Novel Description of mcl-PHA Biosynthesis by Pseudomonas chlororaphis from Animal-Derived Waste. J Biotechnol. 2013;165:45. doi: 10.1016/j.jbiotec.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Tanadchangsaeng N, Kitagawa A, Yamamoto T, Abe H, Tsuge T. Identification, Biosynthesis, and Characterization of Polyhydroxyalkanoate Copolymer Consisting of 3-hydroxybutyrate and 3-Hydroxy-4-methylvalerate. Biomacromolecules. 2009;10:2866. doi: 10.1021/bm900696c. [DOI] [PubMed] [Google Scholar]
- 35.Steinbüchel A. Perspectives for Biotechnological Production and Utilization of Biopolymers: Metabolic Engineering of Polyhydroxyalkanoate Biosynthesis Pathways as a Successful Example. Macromol Biosci. 2001;1:1. doi: 10.1002/1616-5195(200101)1:1<1::AID-MABI1>3.0.CO;2-B. [DOI] [Google Scholar]
- 36.Tappel RC, Pan W, Bergey NS, Wang Q, Patterson IL, Ozumba OA, Matsumoto K, Taguchi S, Nomura CT. Engineering Escherichia coli for Improved Production of Short-Chain- Length-co-Medium-Chain-Length Poly[(R)-3-hydroxyalkanoate] (SCL-co-MCL PHA) Copolymers from Renewable Nonfatty Acid Feedstocks. Sustain Chem Eng. 2014;2:1879. doi: 10.1021/sc500217p. [DOI] [Google Scholar]
- 37.Tripathi L, Wu L-P, Chen J, Chen G-Q. Synthesis of Diblock copolymer poly-3-hydroxybutyrate-block-poly-3--hydroxyhexanoate [PHB-b-PHHx] by a β-oxidation weakened Pseudomonas putida KT2442. Microb Cell Fact. 2012;11:44. doi: 10.1186/1475-2859-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fradinho JC, Oehmen A, Reis MAM. Photosynthetic mixed culture polyhydroxyalkanoate (PHA) production from individual and mixed volatile fatty acids (VFAs): Substrate preferences and co-substrate uptake. J Biotechnol. 2014;185:19. doi: 10.1016/j.jbiotec.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 39.Johnson K, Jiang Y, Kleerebezem R, Muyzer G, van Loosdrecht MCM. Enrichment of a Mixed Bacterial Culture with a High Polyhydroxyalkanoate Storage Capacity. Biomacromolecules. 2009;10:670. doi: 10.1021/bm8013796. [DOI] [PubMed] [Google Scholar]
- 40.Anterrieu S, Quadri L, Geurkink B, Dinkla I, Bengtsson S, Arcos-Hernandez M, Alexandersson T, Morgan-Sagastume F, Karlsson A, Hjort M, Karabegovic L, et al. Integration of biopolymer production with process water treatment at a sugar factory. New Biotechnol. 2014;31:308. doi: 10.1016/j.nbt.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Myung J, Strong NI, Galega WM, Sundstrom ER, Flanagan JCA, Woo S-G, Waymouth RM, Criddle CS. Disassembly and reassembly of polyhydroxyalkanoates: Recycling through abiotic depolymerization and biotic repolymerization. Bioresour Technol. 2014;170:167. doi: 10.1016/j.biortech.2014.07.105. [DOI] [PubMed] [Google Scholar]
- 42.Vargas A, Montaño L, Amaya R. Enhanced polyhydroxyalkanoate production from organic wastes via process control. Bioresour Technol. 2014;156:248. doi: 10.1016/j.biortech.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 43.Heimersson S, Morgan-Sagastume F, Peters GM, Werker A, Svanström M. Methodological issues in life cycle assessment of mixed-culture polyhydroxyalkanoate production utilising waste as feedstock. New Biotechnol. 2014;31:383. doi: 10.1016/j.nbt.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Montano-Herrera L, Pratt S, Arcos-Hernandez MV, Halley PJ, Lant PA, Werker A, Laycock B. In-line monitoring of thermal degradation of PHA during melt-processing by Near-Infrared spectroscopy. New Biotechnol. 2014;31:357. doi: 10.1016/j.nbt.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Koller M, Muhr A. Continuous Production Mode as a Viable Process-Engineering Tool for Efficient Poly(hydroxyalkanoate) (PHA) Bio-Production. Chem Biochem Eng Q. 2014;28:65. [Google Scholar]
- 46.Andreessen B, Steinbüchel A. Biosynthesis and Biodegradation of 3-Hydroxypropionate- Containing Polyesters. Appl Environ Microbiol. 2010;76:4919. doi: 10.1128/AEM.01015-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van de Velde K, Kiekens P. Biopolymers: overview of several properties and consequences on their applications. Polym Test. 2002;21:433. doi: 10.1016/S0142-9418(01)00107-6. [DOI] [Google Scholar]
- 48.Di Lorenzo ML, Sajkiewicz P, Gradys A, La Pietra P. Optimization of melting conditions for the analysis of crystallization kinetics of poly(3-hydroxybutyrate) e-polymers. 2009;27:313. [Google Scholar]
- 49.Chiellini E, Grillo Fernandes E, Pietrini M, Solaro R. Factorial design in optimization of PHAs processing. Macromol Symp. 2003;197:45. doi: 10.1002/masy.200350705. [DOI] [Google Scholar]
- 50.Hufenus R, Reifler FA, Maniura-Weber K, Spierings A, Zinn M. Biodegradable Bicomponent Fibers from Renewable Sources: Melt-Spinning of Poly(lactic acid) and Poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)] Macromol Mater Eng. 2012;297:75. doi: 10.1002/mame.201100063. [DOI] [Google Scholar]
- 51.Koller M, Niebelschütz H, Braunegg G. Strategies for recovery and purification of poly[(R)-3-hydroxyalkanoates] (PHA) biopolyesters from surrounding biomass. Eng Life Sci. 2013;13:549. doi: 10.1002/elsc.201300021. [DOI] [Google Scholar]
- 52.Sevastianov VI, Perova NV, Shishatskaya EI, Kalacheva GS, Volova TG. Production of purified polyhydroxyalkanoates (PHAs) for applications in contact with blood. J Biomater Sci Polym Ed. 2003;14:1029. doi: 10.1163/156856203769231547. [DOI] [PubMed] [Google Scholar]
- 53.Sabir MI, Xu X, Li L. A review on biodegradable polymeric materials for bone tissue engineering applications. J Mater Sci. 2009;44:5713. doi: 10.1007/s10853-009-3770-7. [DOI] [Google Scholar]
- 54.Wampfler B, Ramsauer T. Isolation and Purification of Medium Chain Length Poly(3-hydroxyalkanoates) (mcl-PHA) for Medical Applications Using Nonchlorinated Solvents. Biomacromolecules. 2010;11:2716. doi: 10.1021/bm1007663. [DOI] [PubMed] [Google Scholar]
- 55.Koller M, Bona R, Chiellini E, Braunegg G. Extraction of short-chain-length poly-[(R)-hydroxyalkanoates] (scl-PHA) by the “anti-solvent” acetone under elevated temperature and pressure. Biotechnol Lett. 2013;35:1023. doi: 10.1007/s10529-013-1185-7. [DOI] [PubMed] [Google Scholar]
- 56.Volova T, Shishatskaya E, Sevastianov V, Efremov S, Mogilnaya O. Production of purified polyhydroxyalkanoates (PHAs) for applications in contact with blood. Biochem Eng J. 2003;16:125. doi: 10.1016/S1369-703X(03)00038-X. [DOI] [PubMed] [Google Scholar]
- 57.Bugnicourt E. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym Lett. 2014;8:791. doi: 10.3144/expresspolymlett.2014.82. [DOI] [Google Scholar]
- 58.Williams SF, Martin DP. Biopolymers Online. John Wiley & Sons; Weinheim: 2005. Applications of Polyhydroxyalkanoates (PHA) in Medicine and Pharmacy; pp. 91–103. [Google Scholar]
- 59.Koller M, Hesse P, Bona R, Kutschera C, Atlić A, Braunegg G. Biosynthesis of High Quality Polyhydroxyalkanoate Co- and Terpolyesters for Potential Medical Application by the Archaeon Haloferax mediterranei. Macromol Symp. 2007;253:33. doi: 10.1002/masy.200750704. [DOI] [Google Scholar]
- 60.Ha C, Cho W. Miscibility, properties, and biodegradability of microbial polyester containing blends. Prog Polym Sci. 2002;27:759. doi: 10.1016/S0079-6700(01)00050-8. [DOI] [Google Scholar]
- 61.Chen LJ, Wang M. Production and evaluation of biodegradable composites based on PHB–PHV copolymer. Biomaterials. 2002;23:2631. doi: 10.1016/S0142-9612(01)00394-5. [DOI] [PubMed] [Google Scholar]
- 62.Martelli SM, Sabirova J, Fakhoury FM, Dyzma A, de Meyer B, Soetaert W. Obtention and characterization of poly(3-hydroxybutyricacid-co-hydroxyvaleric acid)/mcl-PHA based blends. LWT – Food Sci Technol. 2012;47:386. doi: 10.1016/j.lwt.2012.01.036. [DOI] [Google Scholar]
- 63.Wu L-P, Wang D, Parhamifar L, Hall A, Chen G-Q, Moghimi SM. Poly(3-hydroxybutyrate-co-R-3-hydroxyhexanoate) Nanoparticles with Polyethylenimine Coat as Simple, Safe, and Versatile Vehicles for Cell Targeting: Population Characteristics, Cell Uptake, and Intracellular Trafficking. Adv Healthcare Mater. 2014:817. doi: 10.1002/adhm.201300533. [DOI] [PubMed] [Google Scholar]
- 64.Masood F, Chen P, Yasin T, Fatima N, Hasan F, Hameed A. Encapsulation of Ellipticine in poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) based nanoparticles and its in vitro application. Mater Sci Eng C Mater Biol Appl. 2013;33:1054. doi: 10.1016/j.msec.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 65.Boyandin AN, Prudnikova SV, Karpov VA, Ivonin VN, Ðỗ NL, Nguyễn TH, Lê TMH, Filichev NL, Levin AL, Filipenko ML, Volova TG, et al. Microbial degradation of polyhydroxyalkanoates in tropical soils. Int Biodeterior Biodegradation. 2013;83:77. doi: 10.1016/j.ibiod.2013.04.014. [DOI] [Google Scholar]
- 66.Wu C-S. Mechanical properties, biocompatibility, and biodegradation of cross-linked cellulose acetate-reinforced polyester composites. Carbohydr Polym. 2014;105:41. doi: 10.1016/j.carbpol.2014.01.062. [DOI] [PubMed] [Google Scholar]
- 67.Wu C-S, Liao H-T. The mechanical properties, biocompatibility and biodegradability of chestnut shell fibre and polyhydroxyalkanoate composites. Polym Degrad Stab. 2014;99:274. doi: 10.1016/j.polymdegradstab.2013.10.019. [DOI] [Google Scholar]
- 68.Thompson RC, Moore CJ, vom Saal FS, Swan SH. Plastics, the environment and human health: current consensus and future trends. Philos Trans R Soc Lond B Biol Sci. 2009;364:2153. doi: 10.1098/rstb.2009.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Philip S, Keshavarz T, Roy I. Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chem, Techno Biotechnol. 2007;247:233. doi: 10.1002/jctb.1667. [DOI] [Google Scholar]
- 70.Gao X, Chen J-C, Wu Q, Chen G-Q. Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr Opin Biotechnol. 2011;22:768. doi: 10.1016/j.copbio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 71.Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32:762. doi: 10.1016/j.progpolymsci.2007.05.017. [DOI] [Google Scholar]
- 72.Li X-T, Zhang Y, Chen G-Q. Nanofibrous polyhydroxyalkanoate matrices as cell growth supporting materials. Biomaterials. 2008;29:3720. doi: 10.1016/j.biomaterials.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Shrivastav A, Kim H-Y, Kim Y-R. Advances in the Applications of Polyhydroxyalkanoate Nanoparticles for Novel Drug Delivery System. Biomed Res Int. 2013;2013:1. doi: 10.1155/2013/581684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castro-Mayorga JL, Martínez-Abad A, Fabra MJ, Olivera C, Reis M, Lagarón JM. Stabilization of anti-microbial silver nanoparticles by a polyhydroxyalkanoate obtained from mixed bacterial culture. Int J Biol Macromol. 2014;71:103. doi: 10.1016/j.ijbiomac.2014.06.059. [DOI] [PubMed] [Google Scholar]
- 75.Sridewi N, Lee Y-F, Sudesh K. Simultaneous Adsorption and Photocatalytic Degradation of MalachiteGreenUsing Electrospun P(3HB)-TiO2 Nanocomposite Fibers and Films. Int J Photoenergy. 2011;2011:1. doi: 10.1155/2011/597854. [DOI] [Google Scholar]
- 76.Brigham CJ, Sinskey AJ. Applications of Polyhydroxyalkanoates in the Medical Industry. Int J Biotechnol Wellness Ind. 2012;1:53. doi: 10.6000/1927-3037.2012.01.01.03. [DOI] [Google Scholar]
- 77.Chen G-Q, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005;26:6565. doi: 10.1016/j.biomaterials.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 78.Hazer B. Amphiphilic Poly(3-hydroxy alkanoate)s: Potential Candidates forMedical Applications. Int J Polym Sci. 2010;2010:1. doi: 10.1155/2010/423460. [DOI] [Google Scholar]
- 79.Tan L, Yu X, Wan P, Yang K. Biodegradable Materials for Bone Repairs: A Review. J Mater Sci Technol. 2013;29:503. doi: 10.1016/j.jmst.2013.03.002. [DOI] [Google Scholar]
- 80.Rai R, Keshavarz T, Roether JA, Boccaccini AR, Roy I. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater Sci Eng R Reports. 2011;72:29. doi: 10.1016/j.mser.2010.11.002. [DOI] [Google Scholar]
- 81.Williams SF, Martin DP, Horowitz DM, Peoples OP. PHA applications: addressing the price performance issue I. Tissue engineering. Int J Biol Macromol. 1999;25:111. doi: 10.1016/S0141-8130(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 82.Xu X-Y, Li X-T, Peng S-W, Xiao J-F, Liu C, Fang G, Chen KC, Chen G-Q. The behaviour of neural stem cells on polyhydroxyalkanoate nanofiber scaffolds. Biomaterials. 2010;31:3967. doi: 10.1016/j.biomaterials.2010.01.132. [DOI] [PubMed] [Google Scholar]
- 83.Yang Q, Wang J, Zhang S, Tang X, Shang G, Peng Q, Wang R, Cai X. The Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and its Applications in Tissue Engineering. Curr Stem Cell Res Ther. 2014;9:215. doi: 10.2174/1574888X09666140213160853. [DOI] [PubMed] [Google Scholar]
- 84.Naveen N, Kumar R, Balaji S, Uma TS, Natrajan TS, Sehgal PK. Synthesis of Nonwoven Nanofibers by Electrospinning – A Promising Biomaterial for Tissue Engineering and Drug Delivery. Adv Eng Mater. 2010;12:B380. doi: 10.1002/adem.200980067. [DOI] [Google Scholar]
- 85.Pouton C, Akhtar S. Biosynthetic polyhydroxyalkanoates and their potential in drug delivery. Adv Drug Deliv Rev. 1996;18:133. doi: 10.1016/0169-409X(95)00092-L. [DOI] [Google Scholar]
- 86.Kabilan S, Ayyasamy M, Jayavel S, Paramasamy G. Pseudomonas sp. as a Source of Medium Chain Length Polyhydroxyalkanoates for Controlled Drug Delivery: Perspective. Int J Microbiol. 2012;2012:1. doi: 10.1155/2012/317828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiong Y-C, Yao Y-C, Zhan X-Y, Chen G-Q. Application of Polyhydroxyalkanoates Nanoparticles as Intracellular Sustained Drug-Release Vectors. J Biomater Sci Polym Ed. 2010;21:127. doi: 10.1163/156856209X410283. [DOI] [PubMed] [Google Scholar]
- 88.Chaturvedi K, Kulkarni AR, Aminabhavi TM. Blend Microspheres of Poly(3-hydroxybutyrate) and Cellulose Acetate Phthalate for Colon Delivery of 5-Fluorouracil. Ind Eng Chem Res. 2011;50:10414. doi: 10.1021/ie2011005. [DOI] [Google Scholar]
- 89.Volova TG, Shishatskaya EI, Nikolaeva ED, Sinskey A. In vivo study of 2D PHA matrices of different chemical compositions: tissue reactions and biodegradations. J Mater Sci Technol. 2014;30:549. doi: 10.1179/1743284713Y.0000000470. [DOI] [Google Scholar]
- 90.Shishatskaya EI, Volova TG, Puzyr AP, Mogilnaya OA, Efremov SN. Tissue response to the implantation of biodegradable polyhydroxyalkanoate sutures. J Mater Sci Mater Med. 2004;15:719. doi: 10.1023/B:JMSM.0000030215.49991.0d. [DOI] [PubMed] [Google Scholar]
- 91.Brzeska J, Heimowska A, Janeczek H, Kowalczuk M, Rutkowska M. Polyurethanes Based on Atactic Poly[(R,S)-3-hydroxybutyrate]: Preliminary Degradation Studies in Simulated Body Fluids. J Polym Environ. 2014;22:176. doi: 10.1007/s10924-014-0650-2. [DOI] [Google Scholar]
- 92.Basnett P, Ching KY, Stolz M, Knowles JC, Boccaccini AR, Smith C, Locke IC, Keshavarz T, Roy I. Novel Poly(3-hydroxyoctanoate)/Poly(3-hydroxybutyrate) blends for medical application. React Funct Polym. 2013;73:1340. doi: 10.1016/j.reactfunctpolym.2013.03.019. [DOI] [Google Scholar]
- 93.Ellis G, Cano P, Jadraque M, Martín M, López L, Núñez T, de la Peña E, Marco C, Garrido L. Laser microperforated biodegradable microbial polyhydroxyalkanoate substrates for tissue repair strategies: an infrared microspectroscopy study. Anal Bioanal Chem. 2011;399:2379. doi: 10.1007/s00216-011-4653-8. [DOI] [PubMed] [Google Scholar]
- 94.Wang L, Wang Z-H, Shen C-Y, You M-L, Xiao J-F, Chen G-Q. Differentiation of human bone marrow mesenchymal stem cells grown in terpolyesters of 3-hydroxyalkanoates scaffolds into nerve cells. Biomaterials. 2010;31:1691. doi: 10.1016/j.biomaterials.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 95.Zhao S, Zhu M, Zhang J, Zhang Y, Liu Z, Zhu Y, Zhang C. Three dimensionally printed mesoporous bioactive glass and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) composite scaffolds for bone regeneration. J Mater Chem B. 2014;2:6106. doi: 10.1039/C4TB00838C. [DOI] [PubMed] [Google Scholar]