Abstract
Unlike any other polymer class, the (co-)poly(2-oxazoline)s have tremendously benefited from the introduction of microwave reactors into chemical laboratories. This review focuses on the research activities in the area of (co-)poly(2-oxazoline)s prepared by microwave-assisted syntheses and, correspondingly, summarizes the current-state-of the-art of the microwave-assisted synthesis of 2-oxazoline monomers and the microwave-assisted ring-opening (co-)polymerization of 2-oxazolines as well as prominent examples of post-polymerization modification of (co-)poly(2-oxazoline)s. Special attention is attributed to the kinetic analysis of the microwave-assisted polymerization of 2-oxazolines and the discussion of non-thermal microwave effects.
Keywords: microwave-assisted polymerization, ring-opening polymerization, poly(2-oxazoline), non-thermal microwave effects, post-polymerization modification
1. Introduction
Among all polymerizations and polymer classes investigated under MW irradiation, the CROP of poly(2-oxazoline)s is on top of the list in terms of the number of research findings published. This type of ROP, which has been reported by four research groups independently more than half a century ago [1–4], has been hibernating for decades due to the comparably low polymerization rates inherent to this type of polymerization. Performing these polymerizations in dedicated MW reactors provides access to autoclave conditions and, correspondingly, limitations of the reaction temperatures by boiling points of the reactants and/or solvents are overruled. Concomitant with the acceleration of the polymerizations of 2-oxazolines at elevated temperatures, a vivid discussion has arisen whether these accelerations can be retraced to common laws of physical chemistry (in particular the Arrhenius law) or also potential non-thermal MW effects should be considered [5] (cp. section 3.1).
Benefiting from ‘revived’ research activities in the area of poly(2-oxazoline)s, a plethora of review articles focussing on this polymer class and materials derived therefrom (in particular for medical and medicinal applications) has been published recently [6–16]. This review dedicatedly addresses the benefits of MW irradiation for the CROP of poly(2-oxazoline)s. Preceded by a part describing the MW-assisted synthesis of 2-oxazoline monomers (section 2), this review summarizes the current state-of-knowledge of reactions kinetics for the synthesis of homopoly(2-oxazoline)s (section 3), addresses the synthesis of copoly(2-oxazoline)s (section 4), and highlights examples of MW-assisted post-polymerization modifications (section 5).
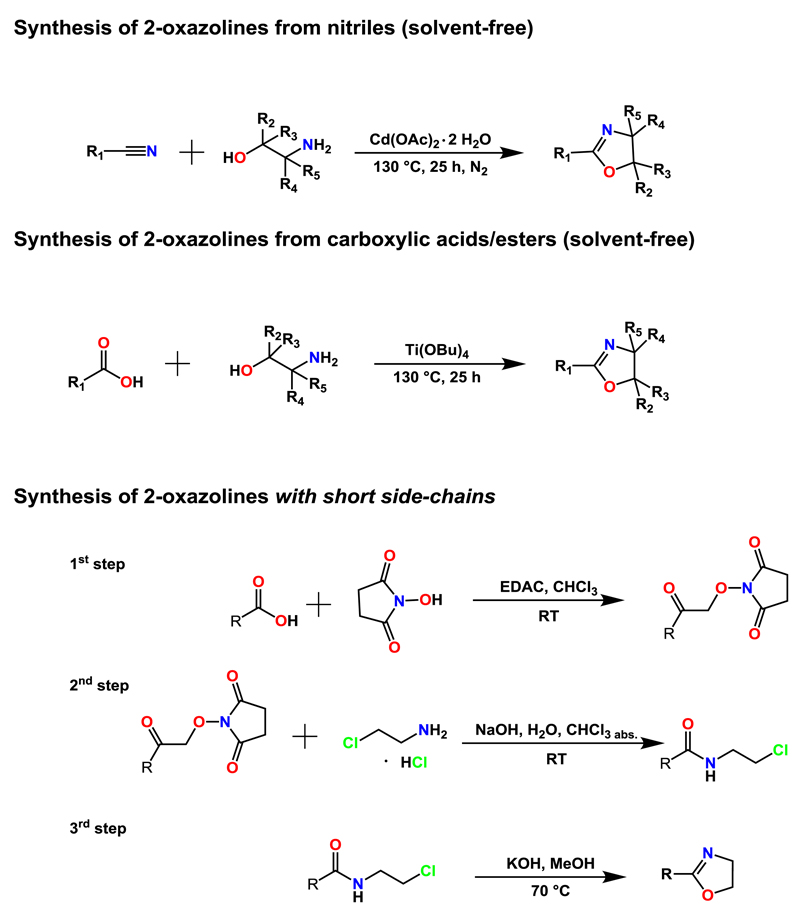
2. Synthesis of 2-oxazoline monomers
2-Oxazoline monomers are synthesized according to one of the three well-known standardized synthetic protocols, in particular from nitriles [17], carboxylic acids [18;19] or the corresponding acid chlorides/activated esters [18] (Scheme 1). These syntheses are commonly performed under CH or at r.t. So far, MW-assisted synthesis protocols have been reported for selected 2-oxazoline monomers only. A clear focus on carboxylic acids and derived compounds as reactants can be observed for the MW-assisted protocols as well.
Scheme 1.
Schematic representation of the common synthetic routes for the preparation of 2-oxazoline monomers involving amino alcohols.
2.1. MW-Assisted synthesis of 2-oxazolines from the reaction of carboxylic acids and derived compounds with amino alcohols
Carboxylic acids
Marrero-Terrero and colleagues performed a detailed synthetic study of the MW-assisted solvent-free syntheses of 2,4,4-trisubstituted 2-oxazolines from the reaction of carboxylic acids and adequately substituted ethanol amines [20]. The experiments were performed in a multi-mode domestic MW oven as well as in a single-mode MW reactor dedicated for chemical synthesis (modified reactor S402 of Prolabo). Zinc oxide was used as a catalyst; yields of more than 90% could be achieved from synthesis at temperatures in the range of 200 °C. Arsalani et al. reported the solvent-free MW-assisted preparation of 2,4-disubstituted bis-2-oxazolines, which were linked by a PEG-based chain in their 2-positions [21]. Telechelic PEG diacid was reacted with primary tris(hydroxylmethyl)methylamine. By applying MW irradiation of 600 W in a domestic microwave oven for approx. 0.5 h, yields of 79% could be obtained. Huang and colleagues described the solvent-free MW-assisted syntheses of 2-oxazolines carrying a hydroxyalkyl substituent in 2-position from the reaction of α-hydroxy-substituted carboxylic acids and β-amino alcohols [22].
Acid chlorides
The MW-assisted synthesis of chiral 2-oxazolines was reported by Mamaghani and coworkers [23]. Seven different benzoic acid chloride derivatives, which differed in the substitution pattern of the aromatic ring, were reacted with 2-amino-3-phenyl propanol at 0 °C to produce the corresponding amides in high yield. These amides were subsequently in-situ converted into tosylates, and ring-closed to obtain the corresponding 2,4-disubstituted 2-oxazolines was achieved under MW irradiation of 800 W (the type of MW reactor was not specified), notably in solvent-free syntheses.
Amides
The synthesis of 2-oxazolines from N-acylbenzotriazoles and 2-amino-2-methyl-1-propanol was reported by Katritzky et al. [24]. The experiments were performed from solutions of the reactants in chloroform in the single-mode MW reactor Discover by CEM as a two-step process with intermediate addition of thionyl chloride to the reaction mixture. The products were obtained in high yields, e.g. of 95% in the case of 1,4-phenylene-bis-2-oxazoline. Notably, this product can also be derived from PET bottle waste, the procedure of which was described by Shukla et al. [25]. Bis-(2-hydroxyethyl) terephthalamide, which was obtained in moderate to high yield from the aminolysis of PET waste according to CH or MW-assisted protocols, was subsequently chlorinated, brominated or nitrated. All of the corresponding products were heated conventionally or in MW reactors in N,N-dimethylformamide / potassium carbonate suspension, yielding the bis-2-oxazoline. The MW reactor used was a domestic oven.
Nitriles
Wood and colleagues described a high-yielding MW-assisted synthesis of 2-oxazolines from the reaction of alkyl and aryl nitriles and 2,2-dimethyl-ethanol amine [26]. A Lewis acid catalyst was required to enhance the yields of the 2-oxazolines. The MW-assisted reaction of amino alcohols with the so-called Pinner salts, imino ether hydrochlorides that can be obtained from the reaction of nitriles with hydrochloric acid and alcohols, as well as the MW-assisted reaction of amino alcohols with imino ethers, which can be derived from Pinner salts by removal of the hydrochloric acid, was reported by Garrigues et al. [27].
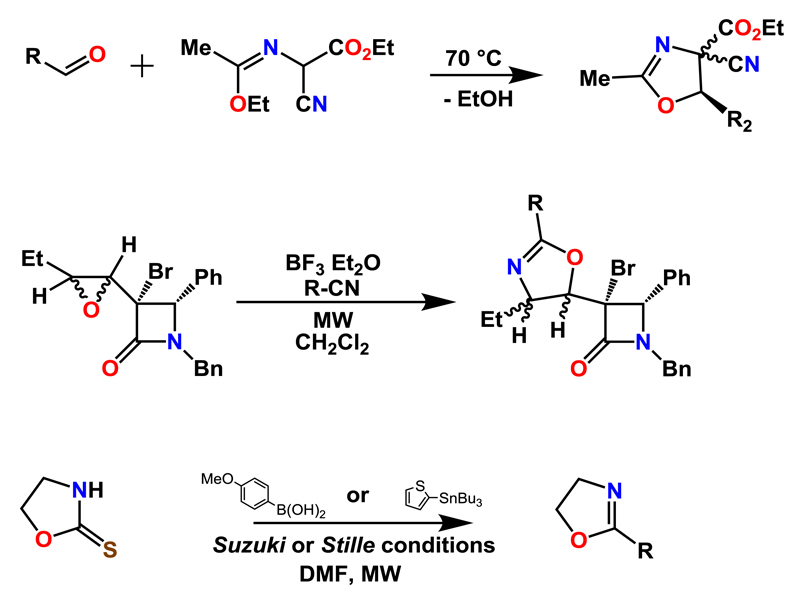
2.2. Other MW-assisted syntheses of 2-oxazoline monomers
Bazureau et al. reported the solvent-free MW-assisted synthesis of 4,4,5-trisubstituted 2-methyl-2-oxazolines; the substituents at the 4-position comprised one cyano and one carboxylate group, while the substituent in 5-position was varied [28]. All reactions were performed in a Synthewave 402 Prolabo MW reactor from the reaction of 2-cyano-2-(1-ethoxyethylidene)aminoethanoate and various aldehydes (Scheme 2). During the cycloaddition, trans:cis selectivities of up to 85:15 could be obtained.
Scheme 2.
Schematic representation of the synthetic routines for the preparation of 2-oxazoline monomers under MW irradiation: cycloadditions (top), conversion of epoxides (middle), and Suzuki- and Stille-type of reactions.
The conversion of β-lactam-containing peroxides into 2-oxazolines by the boron trifluoride etherate-catalyzed reaction with nitriles was performed by Cardillo et al. [29] (Scheme 2). Performing the reaction at r.t. yielded the products with a yield of 30 to 60%, while the same reaction in the multi-mode MW reactor Milestone Mycrosynth (80 °C, 2.5 min) yielded the 2-oxazolines in yields of 65 to 70%. The acidic hydrolysis of the 2-oxazolines was investigated under MW irradiation as well. Tatibouet and colleagues reported the MW-assisted synthesis of 2-aryl-2-oxazolines from 1,3-oxazolidine-2-thiones under Suzuki or Stille reaction conditions [30] (Scheme 2). The MW-assisted reactions were performed at 100 °C for 1 h, yielding the 2-aryl-2-oxazolines in yields of up to 86%.
3. Homopoly(2-oxazoline)s: Detailed investigation of the polymerization kinetics
Coming a long way from microwave reactors for domestic use, single- and multi-mode MW reactors capable of exact control over the temperature and pressure during the reaction were introduced approximately two decades ago. These new types of reactors rapidly found their way into chemical laboratories all over the world. The advantage of having a fully controllable reactors that can be operated under autoclave condition (eliminating the bottleneck of temperature limitations by the boiling points), makes them an ideal tool for chemical syntheses.
3.1. CROP of 2-oxazolines with aliphatic and aromatic side-chains
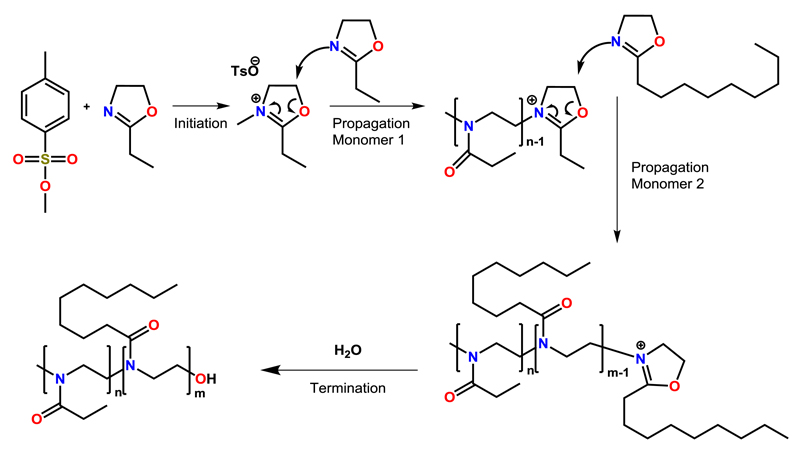
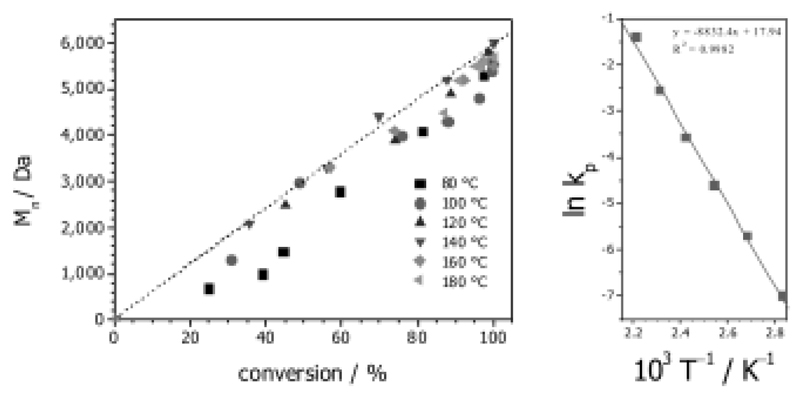
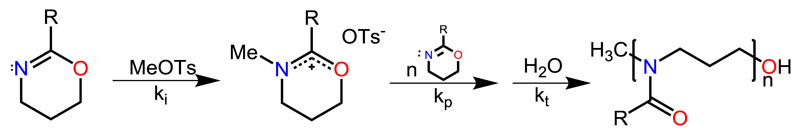
Schubert et al. started their work on accelerating the living polymerization of 2-oxazolines in 2004 with the readily available monomer EtOx (Scheme 3) [31]. Initiated by methyl tosylate, the monomer was polymerized in a Biotage single-mode MW reactor within a range of elevated temperatures, which were chosen for the kinetic analysis of the CROP of EtOx. At 190 °C and 11 bar, full conversion of the monomer was achieved in less than 1 min (DP = 60), compared to 6 h under conventional heating in acetonitrile (reflux at 82 °C). This acceleration of the ROP by factors of up to 350 was accompanied by (minor) side reactions, indicated by a slight change in the polymers’ color from transparent to yellowish. The optimum temperature was identified at 140 °C (Figure 1), showing an acceleration by factors of 70 without any observable side-reactions. All polymers showed remarkably low (molar mass) dispersity of around 1.1 as determined by SEC. The reaction rates kp were calculated and used in an Arrhenius plot, which showed no deviations from analogous CH polymerizations, revealing that no intrinsic MW effects were detectable (Figure 1).
Scheme 3.
Schematic representation of the mechanism of the CROP of 2-oxazolines, shown on the example of the synthesis of the diblock copoly(2-oxazoline) pEtOx-block-pNonOx.
Figure 1.
Kinetic analysis of the CROP of EtOx. Left side: Number-average molecular weights (Mn) plotted against the monomer conversion. Right side: Corresponding Arrhenius plot. Reprinted from reference [31] (doi: 10.1002/marc.200400369) with permission from John Wiley and Sons.
In a subsequent study, Schubert and coworkers expanded their kinetic studies of the CROP of 2-oxazolines by investigating MeOx, NonOx, and PhOx [32]. All monomers were polymerized in a range between 100 and 180 °C, and were further characterized in terms of monomer conversion during the CROP as well as the (molar mass) dispersity of the synthesized poly(2-oxazoline)s. It was found that the polymerizations could not only be carried out in highly concentrated solutions, but also in bulk, while a narrow (molar mass) dispersity of < 1.20 was maintained; all polymerizations followed the law of Arrhenius and did not exhibit any intrinsic (non-thermal) MW effects.
These reports were contradicted by a report from Ritter et al., who also investigated the MW-assisted CROP of PhOx [33]. In their study, they compared CH with MW-assisted heating on the example of the CROP of PhOx by using an open single-mode MW system under atmospheric pressure. From the kinetic measurements, they concluded that the reaction rates were clearly enhanced under MW-assisted heating and claimed the existence of non-thermal MW effects. This phenomenon was explained by the specific heating of the substrates due to the strong response of the oxazolinium cation and its tosylate counterpart in the rapidly changing electromagnetic field of the microwaves. This idea was further backed up by additional experiments with varying monomer:initiator ratios, yielding the same results. In order to conclude the scientific controversy, Schubert et al. re-investigated the CROP of PhOx in more detail: The authors compared the CROP of PhOx under CH and MW-assisted heating under the same conditions in a closed reaction vessel (autoclave conditions), and came to the conclusion that the rate of polymerization was identical, independent of the heating source and argued that non-thermal MW effects could not be observed [34].
Schubert and colleagues got into more detail with their investigations of different poly(2-oxazoline)s. In 2005, a study correlating the length and shape of the side-chain of various poly(2-oxazoline)s and the mechanical and thermal properties was published, enabling the desktop-planning of copoly(2-oxazoline)s with targeted properties [35]. Another study focused on the implementation of MW-assisted polymerizations in a high-throughput workflow [36]: An ASW 2000 synthetic platform was employed for the preparation of the reaction mixtures and, after MW-assisted polymerization, for the preparation of samples of the polymers for further analyses.
More recently, a comparative study on various alkyl sulfonate initiators by Hoogenboom and co-workers revealed that alkyl tosylates are relatively slow initiating species, with the exception of methyl tosylate [37]. Alkyl nosylates and triflates revealed fast initiation and, based on the higher stability of alkyl nosylates, these species were identified as most robust alkyl sulfonate initiating species. Furthermore, in search for a good polymerization solvent for MeOx, Hoogenboom and colleagues discovered that sulfolane is a common rate accelerating solvent for the CROP of 2-oxazolines [38].
Recent work of Shen and co-workers detailed the optimization of the initiation of the CROP of EtOx [39]. By employing rare-earth metal triflates such as Sc(OTf)3 and using otherwise the same reaction conditions as the already established protocols, a very efficient alternative to the initiator methyl tosylate was found. Concomitant with molar-mass dispersities of 1.15 (or lower) and similar conversion rates, the rare-earth catalyst could also be used very efficiently for the CROP of EtOx. Mechanistic details of the regioselectivity of the initiation of the CROP of 2-oxazolines were recently reported by Wiesbrock et al. [40], who showed that the π-electron delocalization along the N-C-O segment in 2-oxazolines is comparable to its analogue along the O-C-O segment in esters.
3.2. 2-Oxazolines with functionalized side-chains and higher homologues of 2-oxazolines
The versatility of the class of 2-oxazolines and their higher homologues such as 2-oxazines enabled research in the field of the synthesis and characterization of novel (co-)poly(2-oxazoline)s.
Schubert et al. reported the synthesis and polymerization of a difluorinated 2-phenyl-2-oxazoline, which was found to be the fastest reacting 2-oxazoline monomer so far [41–42]. ]. In order to verify the hypothesis that the CROP of 2-phenyl-2-oxazolines can be accelerated by substitution with electron-withdrawing substituents, which lower the electron density of the aromatic system, a set of experiments was designed: All mono-fluorinated PhOx as well as the ortho-difluoro-2-phenyl-2-oxazoline were synthesized. The para- and meso-substituted monofluorinated PhOx showed a lowered polymerization rate, while ortho-monofluorinated and ortho-difluorinated PhOx had significant higher rates. In subsequent studies, Schubert, Hoogenboom et al. further expanded the studies of the MW-assisted syntheses of homopoly(2-oxazoline)s, comprising the determination of secondary structure formation of main-chain chiral poly(2-oxazoline)s in solution [43]: Optically active 2-oxazolines were synthesized by the zinc acetatecatalyzed reaction of valeronitrile with chiral 2-amino-1-butanols. The polymers were characterized by solubility tests in various solvents and circular dichroism measurements. Polymers from diasterochemically pure monomers were found to form secondary structures such as helices in solution, while polymers and copolymers from racemic monomer mixtures formed random coils. This work was investigated in more detail considering the effects of the alkyl side-chains of 2-oxazolines on the secondary structure formation [44]: Five different chiral 2-oxazolines with varying side-chain lengths were polymerized, and their secondary structure in solution was investigated. It was observed that changes in the length of the alkyl side-chain significantly influenced the optical properties. Further work addressed the investigation of the thermo-responsive properties of poly(2-oxazoline)s [45]. In succeeding experiments, the behavior of 2-oxazolines and 2-oxazines (Scheme 4) with short side-chains was investigated in detail [46]. It was shown that the cloud-point temperature of the poly(2-oxazine)s decreased with decreasing hydrophilicity, and that the additional methyl group in the backbone of the poly(2-oxazine)s [with respect to poly(2-oxazoline)s] made it more hydrophobic in general. Kinetic studies revealed that the MW polymerization of 2-oxazolines was about four times slower than that of corresponding 2-oxazolines, which is similar as has been reported for CH. In contrast, Ritter and co-workers reported a 1.8-fold acceleration of the CROP of 2-phenyl-2-oxazine under MW irradiation in the open vessel mode of the CEM Discover [47].
Scheme 4.
Schematic presentation of the CROP of 2-oxazine monomers.
Very recently, the MW-assisted polymerization of methyl ester functionalized 2-oxazoline monomers was reported by Hoogenboom et al., revealing an unexpected acceleration of the polymerization of this monomer compared to MeOx and EtOx [48–49]. Detailed kinetic studies complemented by molecular modeling revealed that the observed acceleration results from the interaction of the methyl ester side-chains with the cationic oxazolinium chain end on the one hand while the methyl ester stabilizes the transitions state for monomer addition on the other hand.
3.3. MW-Assisted syntheses of homopoly(2-oxazoline)s: Special applications
Renewable resources
Motivated by the call for polymeric materials from renewable resources, a 2-oxazoline monomer from soy fatty acid with olefinic functionality in the side chain was synthesized by Schubert et al. and investigated in kinetic studies [50]. The double bonds within the monomers made them an ideal target for crosslinking by UV-irradiation. Notably, the unsaturated bonds were not affected by the CROP and were maintained in the polymer side-chains. During the CROP, full monomer conversion was reached within 8 min at 140 °C under bulk conditions (DP = 60). The resulting polymers were successfully crosslinked by UV-irradiation, yielding a product insoluble in organic solvents. The work on soy-based poly(2-oxazoline)s was continued and further focused on the micellization behavior of corresponding copolymers [51]. Combining 2-‘soy alkyl’-2-oxazoline and EtOx, novel copolymers were synthesized and characterized by SEC and 1H NMR analyses. Via DLS and AFM analyses, the micellization of these amphiphilic copolymers was investigated, and the increase in micellar size with increasing length of the hydrophobic block could be shown.
CROP of 2-oxazolines in ionic liquids
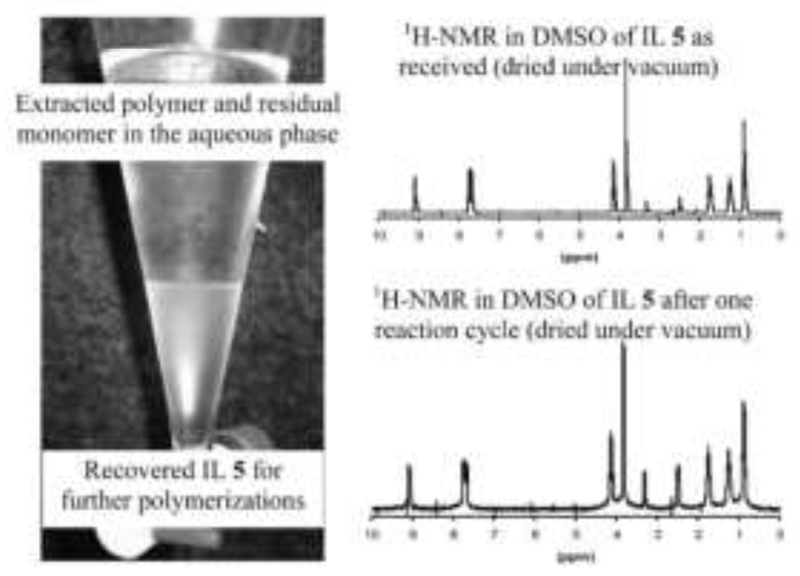
In order to meet the on-going demand for environmental awareness in the area of volatile organic solvents used during polymerizations, Schubert et al. investigated the MW-assisted CROP of 2-oxazolines in ILs [52]. ILs are commonly considered a favorable alternative to organic solvents because of their broad range of solubility properties and their inherent capability for recycling. In the area of MW-heated reactions, also their efficient uptake of MW energy is a key asset. Five different ILs were tested as reaction media for the CROP of EtOx. 1-Butyl-3-methyl-imidazolium hexafluorophosphate proved to be the best-suited candidate, exhibiting faster polymerization rates than those of the CROP of EtOx in acetonitrile. Recovery and recycling of the IL was highly efficient (Figure 2). This work was expanded towards different types and classes of monomers (including PhOx and MMA) as well as the establishment of a more convenient approach for the purification of the polymer and recovery of the IL [53]. The recovery of the polymers and copolymers from the reaction mixture was facilitated in those cases in which the polymer precipitated from the reaction mixture after the addition of water to the homogenous reaction mixtures. The IL could be removed from the polymer by filtration and later be recycled by MW-assisted flash distillation.
Figure 2.
CROP of EtOx in ILs. Left side: Easy and efficient recovery of the IL 1-butyl-3-methyl-imidazolium hexafluorophosphate and recovery of pEtOx by extraction with water. Right side: 1H-NMR spectra of the IL as received and after one cycle of polymerization. Reproduced from reference [52] (doi: 10.1039/B608364A) with permission of The Royal Society of Chemistry.
MW-Assisted scale-up
The range of work on the MW-assisted CROP of 2-oxazolines also included studies on the scale-up of the polymerization. Initial investigations of the scale-up of the MW-assisted CROP of 2-oxazolines detailed the batch type of reactions. Schubert et al. reported the scale-up of the CROP of EtOx from the 400 mg to the 100 g scale using novel MW reactors by Biotage [54]. It was found that the synthetic protocols, which were optimized on the small scale, did not need to be further optimized. In a subsequent study, Schubert and coworkers reported the first homopolymerizations of 2-oxazolines in continuous flow [55]. Using EtOx as a model monomer in different multimode MW systems, they investigated a set of experiments designed to find optimal reaction conditions in terms of pressure, MW penetration depth and flow speed. Under optimized conditions, polymers with a molar mass dispersity of 1.33 could be synthesized.
4. MW-Assisted copolymerizations of 2-oxazolines
Preceded by the first syntheses of homopoly(2-oxazoline)s in MW reactors, research also addressed copoly(2-oxazoline)s, in particular combining different types of poly(2-oxazoline) repetition units in order to form novel copolymers with elevated properties. Analogously, the combination of poly(2-oxazoline)s with entirely different polymer classes has become an active field of research. The ability to fine-tune the mechanical and thermal properties as well as the surface energies of these novel compounds makes them ideal materials for advanced applications.
4.1. Block copoly(2-oxazoline)s
Schubert and colleagues prepared a 16-membered library of diblock copolymers and chain-extended homopolymers consisting of the four monomers MeOx, EtOx, PhOx and NonOx [56]. Thermogravimetric analysis showed that all the polymers were stable up to temperatures of 300 °C. Glass-transition temperatures of the homo- and copoly(2-oxazoline)s were found to range between 57 and 107 °C; notably, no glass-transition temperatures could be measured for the copolymers containing block(s) of pNonOx. The E modules of these diblock copoly(2-oxazoline)s were determined in dedicated characterization study [57].
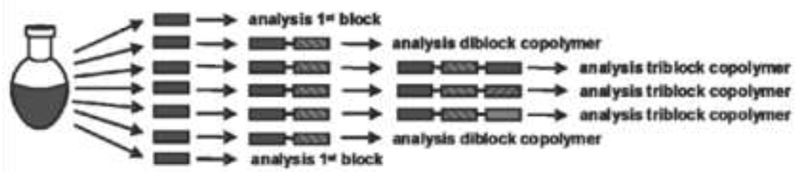
In a subsequent study, triblock copoly(2-oxazoline)s were reported: A 30-membered triblock copolymer library from MeOx, EtOx, NonOx, and PhOx was synthesized and characterized (Figure 3) [58]. Each copolymer consisted of 33 repetition units of the respective monomers. The (total) polymerization times were remarkably short, ranging from 13 to 62 min. Special focus was given to the micellization behavior of some amphiphilic triblock copolymers: Micelles from copolymers with a hydrophobic middle block were smaller than those with hydrophilic middle blocks. This work was continued in more detail by a subsequent study of ter- and quarterpoly(2-oxazoline)s [59]. These novel copoly(2-oxazoline)s revealed molecular weight dispersities of 1.38 or lower, strongly depending on the presence/absence of repetition units of NonOx. The size of the micelles was found to correlate with their composition and confirmed previously established hypotheses. Triblock copoly(2-oxazoline)s composed of EtOx, 2-(1-ethylheptyl)-2-oxazoline and 2-(2,6-difluorophenyl)-2-oxazoline were reported by Schubert et al. [60]. The triblock copolymers showed low molar mass dispersities of around 1.12; their composition was confirmed by fragmentation studies using MALDI-TOF.
Figure 3.
Synthetic procedure that was applied for the preparation of triblock copolymers with the same first and second blocks. Reprinted with permission from reference [58] (doi: 10.1021/ma060952a). Copyright (2006) American Chemical Society.
4.2. Statistical copoly(2-oxazoline)s
Schubert and coworkers reported a series of copoly(2-oxazoline)s with different molecular architectures, based on the monomers EtOx and NonOx [61]. Random and block copolymers of both monomers were compared in terms of molar mass dispersity, kinetics and surface energies. Contact-angle measurements revealed the enrichment of alkyl side-chains of the NonOx repetition units at the surface of polymer films, concomitant with a pronounced decrease of the surface energy, which had been analyzed in detailed manner for diblock copoly(2-oxazoline)s [62–63]. In the case of statistical copoly(2-oxazoline)s containing NonOx repetition units, this effect was less pronounced, and the tailor-made adaption of surface energies was enabled. Furthermore, random copoly(2-oxazoline)s had a lower degree of crystallinity and lower glass-transition temperature, resulting in higher mechanical energy dissipation, lower E modules and greater creep compliance, constituting viscoelastic properties.
Schubert and colleagues also prepared copolymers of EtOx and 2-(3-ethylheptyl)-2-oxazoline, the latter being a 2-oxazoline with a branched side-chain [64]. The amount of EtOx repetition units in the copoly(2-oxazoline)s was varied in the range of 0 to 100%. The glass-transition temperatures were found to depend linearly on the wt.-% content of 2-(3-ethylheptyl)-2-oxazoline in the copolymers. This enabled the simple fine-tuning of the glass-transition temperature for specific applications. The significant influence of the branching of the side-chains on the chain mobility was investigated in a subsequent study of the effects on glass-transition temperatures and mechanical properties [65]. Random copolymers from MW-assisted syntheses were also reported on the examples of copolymers consisting of EtOx and ‘SoyOx’, a soy based 2-oxazoline monomer with unsaturated side chains [66], as well as a set of different 2-oxazoline and 2-oxazine monomers, comprising the repetition units of MeOx, PhOx and a substituted 4-ethyl-2-butyl-2-oxazoline, from which it was observed that the polymerization rate decreased with increasing sterical hindrance [67].
4.3. Gradient copoly(2-oxazoline)s
Hoogenboom, Schubert et al. copolymerized representative combinations of MeOx, EtOx, NonOx and PhOx for the kinetic investigations of the terpolymerizations [68]. Due to (in particular) the low polymerization rates of PhOx, the one-pot synthesis of ‘gradient-random’, ‘gradient-block’ and ‘random-block’ terpolymers could be achieved, paving the way to ‘quasi-diblock’ copoly(2-oxazolines from one-pot/one-step copolymerization [69]. Gradient copolymers composed of NonOx and PhOx were the subject of a subsequent study [70], in which the reactivity ratios were determined. Gradient copolymers could be prepared, combining hydrophobic amorphous and semi-crystalline poly(2-oxazoline) segments in one gradient copolymer.
Schubert et al. prepared copolymers of PhOx (or difluoronated PhOx) and EtOx in one-pot/one-step syntheses [71]. The corresponding gradient copoly(2-oxazoline)s showed amphiphilic behavior, resulting in the self-assembly into micelles in aqueous solution. Due to the pronounced hydrophobic character of fluorinated PhOx, the corresponding copolymers were able to form larger micelles than those of the copolymers with (unsubstituted) PhOx.
5. Post-polymerization modifications
Based on the facilitated access to various types of copoly(2-oxazoline)s (eventually) with novel repetition units from MW-assisted syntheses, also the research area of (co-)poly(2-oxazoline)-based materials has benefited significantly from the advent of MW reactors dedicatedly designed for organic syntheses. Consequently, also this area will be addressed hereinafter in brevity, despite the fact that some of these post-polymerization modifications are not operated under MW irradiation – the copoly(2-oxazoline)s in the centers of those studies, nonetheless, have been derived from MW-assisted syntheses. The examples in this section comprise click chemistry reactions such as the UV induced thiol-ene reaction, the formation of hydrogels and negative photoresists via crosslinking of the polymer side-chains, and the hydrolysis of copoly(2-oxazoline)s. It should be noted that even though this part only comprises examples that are based on polymers that were prepared by MW irradiation, there is a wide variety of beautiful work reported for clickable and reactive functional poly(2-oxazoline)s for which the reader is referred to the relevant literature [7;14;19;72–77].
5.1. Click Chemistry
The concept of click chemistry has been shown in particular on the example of UV-induced thiol-ene click reactions. This type of reactions can be conveniently performed at r.t., reducing polymer degradation (and the degradation of eventually occluded organic molecules) to a minimum.
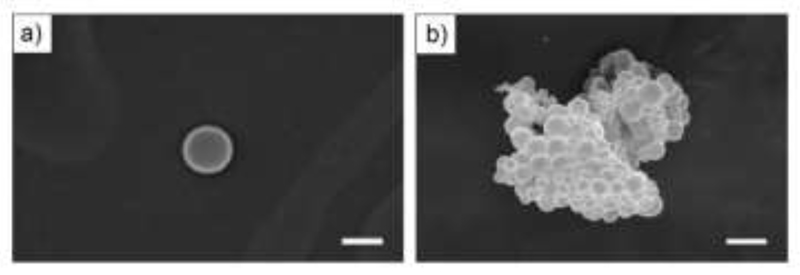
The cellular uptake of copoly(2-oxazoline)-based nanoparticles was studied on the example of a copoly(2-oxazoline) consisting of EtOx and Dc=Ox, endcapped with the dye fluorescein [78]. The double bond of the Dc=Ox repetition units enabled the post-polymerization modification, whereas the covalently bound fluorescin enabled the study of cellular uptake (Figure 4). Spherical nanoparticles with diameters in the range of 200 to 800 nm were obtained by nanoprecipitation of solutions of the copolymer into a non-solvent. Confocal laser scanning microscopy showed the cellular intake and the homogeneous distribution of the nanoparticles within the cytosol.
Figure 4.
Nanoparticles of copoly(2-oxazoline) consisting of EtOx and Dc=Ox, a) pEtOx-co-pDc=Ox-OH and b) pEtOx-co-pDc=Ox-fluorescine. Reprinted from reference [78] (doi: 10.1002/marc.201000283) with permission from John Wiley and Sons.
Veronese, Schubert, Hoogenboom and colleagues pursued a different approach in post-polymerization modifications [79]. By quenching the CROP of EtOx with sodium carbonate, they prepared semi-telechelic pEtOx with hydroxyl end-groups. These hydroxyl groups could be modified into a carboxylate function in post-polymerization fashion and, after activation by an active ester, be used for the covalent binding to proteins. It was concluded that the modified pEtOx could be used as an alternative to PEG.
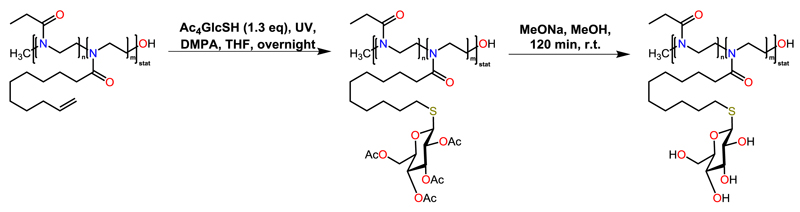
Glycopoly(2-oxazoline)s derived from a copoly(2-oxazoline) of EtOx and Dc=Ox were reported by Schubert et al. (Scheme 5) [80]. The olefinic functions of the Dc=Ox repetition units were used as ‘ene’ component in UV-induced thiol-ene click reactions with the thiol 2,3,4,6-tetra-O-acetyl-1-thio-β-d-glycopyranose. In the final step, the acetyl protection groups of the sugar were cleaved in alkaline media, yielding the targeted glycopoly(2-oxazoline)s. The cloud points of these (unprotected) glycopoly(2-oxazoline)s were found to decrease with the increasing number of sugar moieties, which was referred to hydrogen bonding between the hydroxyl sugar groups and the polymer backbone.
Scheme 5.
Schematic representation of the preparation of glycopoly(2-oxazoline)s using thiolene click chemistry.
Also the surface modification of silicon substrates by MW-assisted click reactions with dedicatedly functionalized copoly(2-oxaoline)s was shown by Schubert and colleagues [81]. The motivation of this work was the investigation of a novel heating process for the surface functionalization of self-assembled monolayers. Hence, an azide-functionalized silicon surface was reacted in a Huisgen 1,3-dipolar cycloaddition with a semi-telechelic acetlyene-functionalized pEtOx.
Schubert et al. also reported a green approach for the synthesis and modification of olefinic poly(2-oxazoline)s. Dc=Ox, which can be derived from renewable resources, was polymerized in bulk under MW irradiation. For the post-polymerization thiol-ene click functionalization, a ‘green’ solvent, namely 2-methyl-tetrahydrofuran, was chosen [82].
5.2. Crosslinking
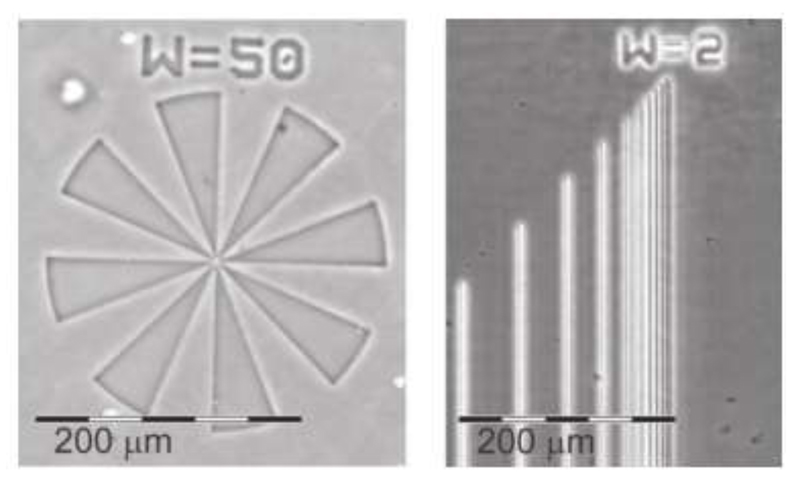
If the thiol-ene click reaction of ‘ene’-functionalized copoly(2-oxazoline)s is performed with multifunctional thiols, crosslinked copolymers can be obtained. Furthermore, if UV irradiation is applied through a mask, crosslinking only occurs at the transparent areas of the mask, and, hence, only the areas preset by transparent segments of the mask become insoluble, yielding a so-called negative photoresist. Wiesbrock and colleagues developed water-developable negative photoresists (Figure 5) based on, e.g., pEtOx-stat-pBu=Ox and pPhOx-stat-pDc=Ox [83–84], choosing a copoly(2-oxazoline) with short side-chains on one hand, and one with longer (hydrophobic) side-chains on the other hand. The photoresist showed resolutions higher than 2 µm. These findings were expanded in the context of MW-assisted ‘green’ polymer chemistry [85]: The copoly(2-oxazoline)s constituting the base polymer of the photoresist were derived from NonOx and Dc=Ox, both from renewable resources, in energy-efficient MW-assisted copolymerizations in recyclable ionic liquids. Preceded by crosslinking via UV induced thiol-ene click reactions, the photoresist could be developed in ethyl lactate. With a resolution of higher than 1 µm, this photoresist is an alternative to conventional products.
Figure 5.
Phase contrast image of pEtOx80-stat-pBu=Ox20 after UV-induced crosslinking with a tetrathiol for 60 s through a mask aligner and subsequent development in 1-methoxy-2-propanol for 30 s. Reprinted from reference [83] (doi: 10.1002/marc.201100717) with permission from John Wiley and Sons.
Schubert, Gohy and coworkers reported the solvent-induced morphological transition in core-crosslinked block copolymer micelles [86] based on a diblock copolymer consisting of EtOx and 2-‘soy alkyl’-2-oxazoline. The cores of the micelles were crosslinked under UV irradiation, yielding spherical micelles capable of swelling in acetone. Morphological changes within the micelles were observed.
Dargaville, Hoogenboom and coworkers investigated the cell attachment onto poly(2-oxazoline)-based hydrogels [87]. Hydrogels of poly(2-oxazoline)s, namely a copoly(2-oxazoline) of MeOx and Dc=Ox, were prepared and functionalized with the peptide CRGDSG and crosslinked with a dithiol by thiol-ene click chemistry. The interaction of the hydrogel and human fibroblast was investigated.
The swelling degrees of copoly(2-oxazoline)-based hydrogels were correlated with the gel composition by Wiesbrock et al. [88]. From a 32-membered library of poly(2-oxazoline)-based hydrogels of the composition pEtOx-pPhOx-pPBO, which were prepared in-situ during the polymerization, they determined the swelling degrees in the solvents water, dichloromethane, and ethanol. The hydrogels were capable of incorporating (small) organic molecules either in-situ during the (co-)polymerization or according to post-synthetic routines. Li et al. described the swelling and deswelling behavior of poly(2-isopropyl-2-oxazoline)-based hydrogels [89]. The hydrogels were prepared in-situ by copolymerization with bis-functional 1,4-phenylene-bis-2-oxazoline. Detailed data of the swelling behavior were obtained from combined FT-IR and two-dimensional correlation spectroscopy. Different dynamic transition mechanisms could be observed upon heating and cooling, resulting in different volume phase transition temperatures of 35 °C during heating and a range between 41 to 30 °C during cooling.
Hoogenboom et al. reported the crosslinking of poly(2-oxazoline)-based hydrogels via UV-induced thiol-ene reactions with a dithiol component [90]. The hydrogels were prepared by dissolving copolymers of Dc=Ox and either MeOx or EtOx (with a molar mass dispersity between 1.22 to 1.43) in ethanol and mixing the solution with a bis-functional thiol, either 2,2’-(ethylenedioxy)diethanethiol or ethylene glycol bis(3-mercaptopropionate). After the addition of the photoinitiator 2,2-dimethoxy-2-phenylacetophenone to the solution and low energy UV irradiation of 2.2 mW cm-2 for 4 min, hydrogel discs with a depth of 1 mm and a radius of 7 mm were obtained. All gels were characterized by swelling in phosphate buffered saline and in HEPES buffer of pH 8. After an initial swelling period, degradation could be observed, expanding the potential usage for poly(2-oxazoline) hydrogels in medic(in)al applications. The potential in this field was discussed by Hoogenboom also in another occasion [91].
The synthesis of a novel 2-oxazoline monomer with a Boc-protected amino group in the side-chain was described by Schubert and coworkers [92], in analogy to a synthesis previously described in literature [93]. Homopolymerizations as well as copolymerizations with EtOx yielded novel compounds. The obtained poly(amino-2-oxazoline)s coordinated with DNA in reversible fashion. The strength of these bonds correlated directly with the amount of amino groups within the copolymer. It was possible to crosslink the copolymers with epichlorohydrin, yielding hydrogels with adjustable swelling degrees and the ability to absorb DNA. Further work of Schubert et al. focused on the stabilization of Factor VIII, an essential human coagulation factor, through poly(2-oxazoline)-based hydrogels [94]. The hydrogels were prepared by in-situ by the copolymerization of EtOx and bis-functional 1,4-phenylene-bis-2-oxazoline.
In the field of medical applications, Wiesbrock et al. demonstrated the enhanced adhesion of poly(2-oxazoline) hydrogels to cancer cells by functionalization with the RGD-peptide motif [95]. Networks of different compositions of EtOx, NonOx, Dc=Ox and bis-functional 2,2’-tetramethylene-bis-2-oxazoline were characterized by swelling degrees in H2O, ethanol and dichloromethane. These gels, which were coupled with the RGD protein motif (from a cyclic pentapeptide carrying a thiol function) via post-polymerization UV-induced thiol-ene reaction, showed preferential cell adhesion towards human pancreatic cancer BON cells. Fluorescence microscopy enabled the recognition of the hydrogels, which were clearly attached to the BON cells.
5.3. Hydrolysis
Schubert and coworkers described a strategy for the MW-assisted quantitative hydrolysis of pEtOx, yielding PEI, while optimizing the reaction times and clean-up procedure [96]. The optimum hydrolysis conditions proved to be 1 h at 130 °C at a concentration of up to 0.33 g of pEtOx in 1 mL of 6 m HCl, leading to significantly reduced reaction times. By dissolving the crude PEI in methanol and then precipitating it in diethyl ether, impurities could be removed efficiently. The purity of the polymer was verified by 1H NMR spectroscopy and MALDI-TOF analysis.
Hoogenboom et al. addressed their investigations on the preparation of partially hydrolyzed pEtOx, namely the synthesis of pEtOx-stat-PEI, and the effects of temperature on the reaction [97]. The authors reported a maximum acceleration of the hydrolysis reaction at 180 °C using low-concentrated aqueous solutions of HCl. Further work on this topic focused on the influence of partial hydrolyzed pEtOx on in-vivo systems, on behalf of mucosal irritation and cytotoxicity [98]. No tissue damage was caused by polymers with hydrolysis degrees up to 25% according to the slug mucosal irritation test. Additionally, the enzyme-catalyzed hydrolysis of pEtOx by digestive enzymes was studied, revealing that this hydrolysis was quantitatively negligible (less than 0.2% hydrolysis after 6 h at 37 °C).
Hoogenboom and coworkers published a study concerning the acidic and alkaline mediated hydrolyses of gradient and diblock copolymers consisting of MeOx and PhOx [99]. It was observed that, under acidic conditions, both side-chains were cleaved from the polymer backbone, although the hydrolysis of the MeOx repetition units was preferred. Under alkaline conditions, however, the hydrolysis rates for both types of repetition units were significantly slower. Due to significantly lowered rates for the cleavage of the PhOx repetition units, specific hydrolysis of the MeOx repetition units could be observed. This selectivity could be further enhanced by utilizing an ethanol-water mixture for hydrolysis [100]. With this improvement, a 95% hydrolysis of the methyl side-chains of a pMeOx60-block-pPhOx15 copolymer could be achieved.
Wiesbrock et al. developed contact biocides derived from partially hydrolyzed (co)poly(2-oxazoline)s [101]. The copoly(2-oxazoline)-stat-PEIs were prepared by acid-mediated (partial) hydrolysis of pEtOx and pNonOx, respectively. The copolymers were compounded as additives in polypropylene plates containing 5 wt.-% of the copolymers. The antimicrobial activity against E. coli, P. aeruginosa, C. albicans and S. aureus was tested, showing a significant reduction of the microbes by mere contact with the compound plates. Notably, the hydrolysis of pEtOx could be performed at much higher rates than that of pNonOx under otherwise identical conditions.
6. Summary, Conclusions and Outlook
Due to the comparably low polymerization rates inherent to the CROP of 2-oxazoline monomers, this class of polymers has significantly benefited from the introduction of MW reactors dedicatedly designed for chemical syntheses. Concomitant with facilitated access to autoclave conditions and an unparalleled uniform heat distribution (at least for small-scale syntheses), MW reactors have become the reactor-of-choice for the CROP of 2-oxazolines. Like with numerous syntheses in organic chemistry, the discussion of the existence of non-thermal MW effects has also been led for the polymerization of 2-oxazolines; it could be shown by detailed kinetic analyses that intrinsic microwave effects do not exist.
Research in the areas of ‘poly(2-oxazoline)s’ and ‘poly(2-oxazoline)-based materials’ has benefitted enormously from MW-assisted chemistry, in particular:
Development and (co-)polymerization of novel 2-oxazoline monomers, such as fluorinated congeners.
Systematic synthesis and investigation of block copoly(2-oxazoline)s, comprising studies of the micellization behavior.
Introduction of aspects of ‘Green Chemistry’ to the synthesis of (co-)poly(2-oxazolines), to mention the performance of the CROP in (recyclable) ILs and the synthesis of 2-oxazoline monomers from renewable resources as prominent examples.
Novel poly(2-oxazoline)-based materials such as photoresists from renewable resources and (co-)poly(2-oxazoline-based gels for applications in the medical sector.
Almost exactly one decade after the first report of a MW-assisted CROP of a 2-oxazoline monomer [31], concomitant with on-going intensive research at the international level, numerous and sustainable developments in this research area are likely to continuously occur.
Acknowledgement
FW and KPL would like to gratefully acknowledge Austrian Science Fund FWF for funding of the project I 1123-N19 (MimiFlow). The work was performed at the PCCL in the context of the project PolyComp within the framework of the COMET-program of the Federal Ministry for Transport, Innovation and Technology and Federal Ministry for Economy, Family and Youth. The PCCL is funded by the Austrian Government and the State Governments of Styria, Lower Austria and Upper Austria. USS thanks the Carl-Zeiss-Foundation and the TMWWdG (State of Thuringia).
Abbreviations
- AFM
atomic force microscopy
- Bu=Ox
2-but-3’-enyl-2-oxazoline
- Dc=Ox
2-dec-9’-enyl-2-oxazoline
- CH
conventional heating
- CROP
cationic ring-opening polymerization
- EtOx
2-ethyl-2-oxazoline
- DLS
dynamic light scattering
- DP
degree of polymerization
- IL
ionic liquid
- MALDI-TOF
matrix-assisted laser desorption ionization - time-of-flight
- MeOx
2-methyl-2-oxazoline
- MW
microwave
- NonOx
2-nonyl-2-oxazoline
- PBO
1,3-phenylene-bis-2-oxazoline
- pPBO
poly(1,3-phenylene-bis-2-oxazoline)
- pBu=Ox
poly(2-but-3’-enyl-2-oxazoline)
- pDc=Ox
poly(2-dec-9’-enyl-2-oxazoline)
- PEG
poly(ethylene glycol)
- PEI
poly(ethylene imine)
- PET
poly(ethylene terephthalate)
- pEtOx
poly(2-ethyl-2-oxazoline)
- PhOx
2-phenyl-2-oxazoline
- pMeOx
poly(2-methyl-2-oxazoline)
- pNonOx
poly(2-nonyl-2-oxazoline)
- pPhOx
poly(2-phenyl-2-oxazoline)
- ROP
ring-opening polymerization
- r.t.
room temperature
- SEC
size-exclusion chromatography
- wt.-%
weight percent
References
- [1].Seeliger W, Aufderhaar E, Diepers W, Feinauer R, Nehring R, Thier W, Hellmann H. Recent syntheses and reactions of cyclic imidic esters. Angew Chem Int Ed. 1966;5:875–888. doi: 10.1002/anie.196608751. [DOI] [PubMed] [Google Scholar]
- [2].Tomalia DA, Sheetz DP. Homopolymerization of 2-alkyl- and 2-aryl-2-oxazolines. J Polym Sci Part A Polym Chem. 1966;4:2253–2265. [Google Scholar]
- [3].Bassiri TG, Levy A, Litt M. Polymerization of cyclic imino ethers. I. Oxazolines. J Polym Sci Part B Polym Lett. 1967;5:871–879. [Google Scholar]
- [4].Kagiya T, Narisawa S, Maeda T, Fukui K. Ring-opening polymerisation of 2-substituted 2-oxazolines. J Polym Sci Part B Polym Lett. 1966;4:441–445. [Google Scholar]
- [5].Herrero MA, Kremsner JM, Kappe CO. Nonthermal Microwave Effects Revisited: On the Importance of Internal Temperature Monitoring and Agitation in Microwave Chemistry. J Org Chem. 2008;73:36–47. doi: 10.1021/jo7022697. [DOI] [PubMed] [Google Scholar]
- [6].Hoogenboom R. Poly(2-oxazoline)s: A Polymer Class with Numerous Potential Applications. Angew Chem Int Ed. 2009;48:7978–7994. doi: 10.1002/anie.200901607. [DOI] [PubMed] [Google Scholar]
- [7].Rossegger E, Schenk V, Wiesbrock F. Design Strategies for Functionalized Poly(2-Oxazoline)s and Derived Materials. Polymers. 2013;5:956–1011. [Google Scholar]
- [8].Hoogenboom R. Poly(2-oxazoline)s: Alive and kicking. Macromol Chem Phys. 2007;208:18–25. [Google Scholar]
- [9].Kelly AM, Wiesbrock F. Strategies for the Synthesis of Poly(2-Oxazoline)-Based Hydrogels. Macromol Rapid Commun. 2012;33:1632–1647. doi: 10.1002/marc.201200333. [DOI] [PubMed] [Google Scholar]
- [10].Hartlieb M, Kempe K, Schubert US. Covalently cross-linked poly(2-oxazoline) materials for biomedical applications – from hydrogels to self-assembled and templated structures. J Mater Chem B. 2015;3:526–538. doi: 10.1039/c4tb01660b. [DOI] [PubMed] [Google Scholar]
- [11].de la Rosa VR. Poly(2-oxazoline)s as materials for biomedical applications. J Mater Sci Mater Med. 2014;25:1211–1225. doi: 10.1007/s10856-013-5034-y. [DOI] [PubMed] [Google Scholar]
- [12].Konradi R, Acikgoz C, Textor M. Polyoxazolines for Nonfouling Surface Coatings - A Direct Comparison to the Gold Standard PEG. Macromol Radpid Commun. 2012;33:1663–1676. doi: 10.1002/marc.201200422. [DOI] [PubMed] [Google Scholar]
- [13].Luxenhofer R, Han Y, Schulz A, Tong J, He Z, Kabanov AV, Jordan R. Poly(2-oxazoline)s as Polymer Therapeutics. Macromol Radpid Commun. 2012;33:1613–1631. doi: 10.1002/marc.201200354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guillerm B, Monge S, Lapinte V, Robin J-J. How to Modulate the Chemical Structure of Polyoxazolines by Appropriate Functionalization. Macromol Radpid Commun. 2012;33:1600–1612. doi: 10.1002/marc.201200266. [DOI] [PubMed] [Google Scholar]
- [15].Schlaad H, Diehl C, Gress A, Meyer M, Demirel AL, Nur Y, Bertin A. Poly(2-oxazoline)s as Smart Bioinspired Polymers. Macromol Radpid Commun. 2010;31:511–525. doi: 10.1002/marc.200900683. [DOI] [PubMed] [Google Scholar]
- [16].Sedlacek O, Monnery BD, Filippov SK, Hoogenboom R, Hruby M. Poly(2-Oxazoline)s - Are They More Advantageous for Biomedical Applications Than Other Polymers? Macromol Radpid Commun. 2012;33:1648–1662. doi: 10.1002/marc.201200453. [DOI] [PubMed] [Google Scholar]
- [17].Witte H, Seeliger W. Cyclische Imidsaeureester aus Nitrilen und Aminoalkoholen. Liebigs Ann Chem. 1974;6:996–1009. [Google Scholar]
- [18].Beck M, Birnbrich P, Eicken U, Fischer H, Fristad WE, Hase B, Krause H-J. Polyoxazoline auf fettchemischer Basis. Angew Makromol Chem. 1994;223:217–233. [Google Scholar]
- [19].Gress A, Voelkel A, Schlaad H. Thio-Click Modification of Poly[2-(3-butenyl)-2-oxazoline] Macromolecules. 2007;40:7928–7933. [Google Scholar]
- [20].García-Tellado F, Loupy A, Petit A, Marrero-Terrero AL. Solvent-Free Micro-wave-Assisted Efficient Synthesis of 4,4-Disubstituted 2-Oxazolines. Eur J Org Chem. 2003:4387–4391. [Google Scholar]
- [21].Arsalani N, Zare P, Namazi H. Solvent free microwave assisted preparation of new telechelic polymers based on poly(ethylene glycol) Express Polym Lett. 2009;3(7):429–436. [Google Scholar]
- [22].Wei W, Zhang D, Yuan Q, Shen D, Huang B. Synthesis of 2-(α-hydroxyalkyl)-2-oxazoline under microwave irradiation in bulk. Yingyong Huaxue. 2004;21(12):1329–1330. [Google Scholar]
- [23].Mamaghani M, Nahmoodi NO, Fallah Ghasemi S. An Efficient Synthesis of New Chiral Oxazolines. J Iran Chem Soc. 2010;7(4):972–977. [Google Scholar]
- [24].Katritzky AR, Cai C, Suzuki K, Singh SK. Facile Syntheses of Oxazolines and Thiazolines with N-Acylbenzotriazoles under Microwave Irradiation. J Org Chem. 2004;69:811–814. doi: 10.1021/jo0355092. [DOI] [PubMed] [Google Scholar]
- [25].Parab YS, Shah RV, Shukla SR. Microwave irradiated synthesis and characterization of 1,4-phenylene bis-oxazoline from bis-(2-hydroxyethyl) terephthalamide obtained by depolymerization of poly(ethylene terephthalate) bottle waste. Curr Chem Lett. 2012:81–90. [Google Scholar]
- [26].Clarke DS, Wood R. A facile one stage synthesis of oxazolines under microwave irradiation. Synth Comm. 1996;26(7):1335–1340. [Google Scholar]
- [27].Oussaid B, Berlan J, Soufiaoui M, Garrigues B. Improved synthesis of oxazoline under microwave irradiation. Synth Comm. 1995;25(5):659–665. [Google Scholar]
- [28].Fraga-Dubreuil J, Cherouvrier JR, Bazureau JP. Clean solvent-free dipolar cycloaddition reactions assisted by focused microwave irradiations for the synthesis of new ethyl 4-cyano-2-oxazoline-4-carboxylates. Green Chem. 2000;2:226–229. [Google Scholar]
- [29].Benfatti F, Cardillo G, Gentilucci L, Tolomelli A, Monari M, Piccinelli F. A Microwave-Enhanced, Lewis Acid-Catalyzed Synthesis of 1,3-Dioxanes and Oxazolines from Epoxides. Adv Synth Catal. 2007;349:1256–1264. [Google Scholar]
- [30].Silva S, Tardy S, Routier S, Suzenet F, Tatibouet A, Rauter AP, Rollin P. 1,3-Oxazoline and 1,3-oxazolidine-2-thiones as substrates in direct modified Stille and Suzuki cross-coupling. 2008;49(39):5583–5586. [Google Scholar]
- [31].Wiesbrock F, Hoogenboom R, Abeln CH, Schubert US. Single-mode microwave ovens as new reaction devices: Accelerating the living polymerization of 2-ethyl-2-oxaoline. Macromol Rapid Commun. 2004;25:1895–1899. [Google Scholar]
- [32].Wiesbrock F, Hoogenboom R, Leenen MAM, Meier MAR, Schubert US. Investigation of the living cationic ring-opening polymerization of 2-methyl-, 2-ethyl-, 2-nonyl-, and 2-phenyl-2-oxazoline in a single-mode microwave reactor. Macromolecules. 2005;38:5025–5034. [Google Scholar]
- [33].Sinnwell S, Ritter H. Microwave accelerated polymerization of 2-phenyl-2-oxazoline. Macromol Rapid Commun. 2005;26:160–163. [Google Scholar]
- [34].Hoogenboom R, Leenen MAM, Wiesbrock F, Schubert US. Microwave accelerated polymerization of 2-phenyl-2-oxazoline: Microwave or temperature effects? Macromol Rapid Commun. 2005;26:1773–1778. [Google Scholar]
- [35].Hoogenboom R, Fijten MWM, Thijs HML, Van Lankwelt BM, Schubert US. Microwave-assisted synthesis and properties of a series of poly(2-alkyl-2-oxazoline)s. Des Monomers Polym. 2005;8(6):659–671. [Google Scholar]
- [36].Hoogenboom R, Wiesbrock F, Leenen MAM, Meier MAR, Schubert US. Accelerating the living polymerization of 2-nonyl-2-oxazoline by implementing a microwave synthesizer into a high-throughput experimentation workflow. J Comb Chem. 2005;7(1):10–13. doi: 10.1021/cc049846f. [DOI] [PubMed] [Google Scholar]
- [37].Glassner M, D’hooge DR, Park JY, van Steenberge PHM, Monnery BD, Reyniers M-F, Hoogenboom R. Systematic investigation of alkyl sulfonate initiators for the cationic ring-opening polymerization of 2-oxazolines revealing optimal combinations of monomers and initiators. Eur Polym J. 2015;65:298–304. [Google Scholar]
- [38].Vergaelen M, Verbraeken B, Monnery BD, Hoogenboom R. Sulfolane as Common Rate Accelerating Solvent for the Cationic Ring-Opening Polymerization of 2-Oxazolines. ACS Macro Lett. 2015;4:825–828. doi: 10.1021/acsmacrolett.5b00392. [DOI] [PubMed] [Google Scholar]
- [39].Hu F, Xie S, Jiang L, Shen Z. Living cationic ring-opening polymerization of 2-oxazolines initiated by rare-earth metal triflates. RSC Adv. 2014;4:59917–59926. [Google Scholar]
- [40].Fimberger M, Luef KP, Payerl C, Fischer RC, Stelzer F, Kállay M, Wiesbrock F. The π-Electron Delocalization in 2-Oxazolines Revisited: Quantification and Comparison with Its Analogue in Esters. Materials. 2015;8:5385–5397. doi: 10.3390/ma8085249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lobert M, Köhn U, Hoogenboom R, Schubert US. Synthesis and microwave assisted polymerization of fluorinated 2-phenyl-2-oxazolines: the fastest 2-oxazoline monomer to date. Chem Commun. 2008:1458–1460. doi: 10.1039/b717455a. [DOI] [PubMed] [Google Scholar]
- [42].Lobert M, Thijs HML, Erdmenger T, Eckardt R, Ulbricht C, Hoogenboom R, Schubert US. Synthesis, Microwave-Assisted Polymerization, and Polymer Properties of Fluorinated 2-Phenyl-2-oxazolines: A Systematic Study. Chem Eur J. 2008;14:10396–10407. doi: 10.1002/chem.200800671. [DOI] [PubMed] [Google Scholar]
- [43].Bloksma MM, Rogers S, Schubert US, Hoogenboom R. Secondary structure formation of main-chain chiral poly(2-oxazoline)s in solution. Soft Matter. 2010;6:994–1003. [Google Scholar]
- [44].Bloksma MM, Rogers S, Schubert US, Hoogenboom R. Main-chain chiral poly(2-oxazoline)s: Influence of alkyl side-chains on secondary structure formation in solution. J Polym Sci Part A Polym Chem. 2011;49:2790–2801. [Google Scholar]
- [45].Bloksma MM, Weber C, Perevyazko IY, Kuse A, Baumgärtel A, Vollrath A, Hoogenboom R, Schubert US. Poly(2-cyclopropyl-2-oxazoline): From rate acceleration by cyclopropyl to thermoresponsive properties. Macromolecules. 2011;44:4057–4064. [Google Scholar]
- [46].Bloksma MM, Paulus RM, Van Kuringen HPC, Van der Woerdt F, Lambermont-Thijs HML, Schubert US, Hoogenboom R. Thermoresponsive Poly(2-oxazoline)s. Macromol Rapid Commun. 2012;33:92–96. doi: 10.1002/marc.201100587. [DOI] [PubMed] [Google Scholar]
- [47].Sinnwell S, Ritter H. Microwave Accelerated Polymerization of 2-Phenyl-5,6-dihydro-4H-1,3-oxazine: Kinetics and Influence of End-Groups on Glass Transition Temperature. Macromol Rapid Commun. 2006;27:1335–1340. [Google Scholar]
- [48].Bouten PJM, Hertsen D, Vergaelen M, Monnery BD, Boerman MA, Goossens H, Catak S, van Hest JCM, van Speybroeck V, Hoogenboom R. Accelerated living cationic ring-opening polymerization of a methyl ester functionalized 2-oxazoline monomer. Polym Chem. 2015;6:514–518. [Google Scholar]
- [49].Bouten PJM, Hertsen D, Vergaelen M, Monnery BD, Catak S, van Hest JCM, van Speybroeck V, Hoogenboom R. J Polym Sci Part A: Polym Chem. 2015 doi: 10.1002/pola.27733. (in press) [DOI] [Google Scholar]
- [50].Hoogenboom R, Schubert US. Microwave-assisted cationic ring-opening polymerization of a soy-based 2-oxazoline monomer. Green Chem. 2006;8:895–899. [Google Scholar]
- [51].Hoogenboom R, Leenen MAM, Huang H, Fustin C-A, Gohy J-F, Schubert US. Microwave-assisted synthesis and micellization behavior of soy-based copoly(2-oxazoline)s. Colloid Polym Sci. 2006;284:1313–1318. doi: 10.1007/s00396-006-1496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Guerrero-Sanchez C, Hoogenboom R, Schubert US. Fast and “green” living cationic ring opening polymerization of 2-ethyl-2-oxazoline in ionic liquids under microwave irradiation. Chem Commun. 2006:3797–3799. doi: 10.1039/b608364a. [DOI] [PubMed] [Google Scholar]
- [53].Guerrero-Sanchez C, Lobert M, Hoogenboom R, Schubert US. Microwave-assisted homogeneous polymerizations in water-soluble ionic liquids: An alternative and green approach for polymer synthesis. Macromol Rapid Commun. 2007;28:456–464. [Google Scholar]
- [54].Hoogenboom R, Paulus RM, Pilotti A, Schubert US. Scale-up of microwave-assisted polymerizations in batch mode: The cationic ring-opening polymerization of 2-ethyl-2-oxazoline. Macromol Rapid Commun. 2006;27:1556–1560. [Google Scholar]
- [55].Paulus RM, Erdmenger T, Becer CR, Hoogenboom R, Schubert US. Scale-up of microwave-assisted polymerizations in continuous-flow mode: Cationic ring-opening polymerization of 2-ethyl-2-oxazoline. Macromol Rapid Commun. 2007;28:484–491. [Google Scholar]
- [56].Wiesbrock F, Hoogenboom R, Leenen M, Van Nispen SFGM, Van der Loop M, Abeln CH, Van den Berg AMJ, Schubert US. Microwave-assisted synthesis of a 42-membered library of diblock copoly(2-oxazoline)s and chain-extended homo poly(2-oxazoline)s and their thermal characterization. Macromolecules. 2005;38:7957–7966. [Google Scholar]
- [57].Kranenburg JM, Tweedie CA, Hoogenboom R, Wiesbrock F, Thijs HML, Hendriks CE, Van Vliet KJ, Schubert US. Elastic moduli for a diblock copoly(2-oxazoline) library obtained by high-throughput screening. J Mater Chem. 2007;17:2713–2721. [Google Scholar]
- [58].Hoogenboom R, Wiesbrock F, Huang H, Leenen MAM, Thijs HML, Van Nispen SFGM, Van der Loop M, Fustin C-A, Jonas AM, Gohy J-F, Schubert US. Microwave-assisted cationic ring-opening polymerization of 2-oxazolines: A powerful method for the synthesis of amphiphilic triblock copolymers. Macromolecules. 2006;39:4719–4725. [Google Scholar]
- [59].Hoogenboom R, Wiesbrock F, Leenen MAM, Thijs HML, Huang H, Fustin C-A, Guillet P, Gohy J-F, Schubert US. Synthesis and aqueous micellization of amphiphilic tetrablock ter- and quaterpoly(2-oxazoline)s. Macromolecules. 2007;40:2837–2843. [Google Scholar]
- [60].Kempe K, Baumgärtel A, Hoogenboom R, Schubert US. Design of new amphiphilic triblock copoly(2-oxazoline)s containing a fluorinated segment. J Polym Sci Part A Polym Chem. 2010;48:5100–5108. [Google Scholar]
- [61].Fijten MWM, Kranenburg JM, Thijs HML, Paulus RM, Van Lankvelt BM, De Hullu J, Springintveld M, Thielen DJG, Tweedie CA, Hoogenboom R, Van Vliet KJ, et al. Synthesis and structure-property relationships of random and block copolymers: A direct comparison for copoly(2-oxazoline)s. Macromolecules. 2007;40:5879–5886. [Google Scholar]
- [62].Wijnans S, De Gans B-J, Wiesbrock F, Hoogenboom R, Schubert US. Characterization of a Poly(2-oxazoline) Library by High-Throughput, Automated Contact-Angle Measurements and Surface-Energy Calculations. Macromol Rapid Commun. 2004;25:1958–1962. [Google Scholar]
- [63].Hoeppener S, Wiesbrock F, Hoogenboom R, Thijs HML, Schubert US. Morphologies of spin-coated films of a library of diblock copoly(2-oxazoline)s and their correlation to the corresponding surface energies. Macromol Rapid Commun. 2006;27:405–411. [Google Scholar]
- [64].Kempe K, Jacobs S, Lambermont-Thijs HML, Fijten MWM, Hoogenboom R, Schubert US. Rational design of an amorphous poly(2-oxazoline) with a low glass-transition temperature: monomer synthesis, copolymerization and properties. Macromolecules. 2010;43:4098–4104. [Google Scholar]
- [65].Kempe K, Rettler EF-J, Paulus RM, Kuse A, Hoogenboom R, Schubert US. A systematic investigation of the effect of side chain branching on the glass transition temperature and mechanical properties of aliphatic (co-)poly(2-oxazoline)s. Polymer. 2013;54:2036–2042. [Google Scholar]
- [66].Hoogenboom R, Thijs HML, Fijten MWM, Schubert US. Synthesis, characterization, and cross-linking of a library of statistical copolymers based on 2-“soy alkyl”-2-oxazoline and 2-ethyl-2-oxazoline. J Polym Sci Part A Polym Chem. 2007;45:5371–5379. [Google Scholar]
- [67].Lambermont-Thijs HML, Fijten MWM, Van der Linden AJT, Van Lankvelt BM, Bloksma MM, Schubert US, Hoogenboom R. Efficient cationic ring-opening polymerization of diverse cyclic imino ethers: unexpected copolymerization behavior. Macromolecules. 2011;44:4320–4325. [Google Scholar]
- [68].Hoogenboom R, Wiesbrock F, Leenen MAM, Van der Loop M, Van Nispen SFGM, Schubert US. Kinetic investigations on microwave-assisted statistical ter-polymerizations of 2-oxazoline monomers. Aust J Chem. 2007;60:656–661. [Google Scholar]
- [69].Hoogenboom R, Thijs HML, Fijten MWM, Van Lankvelt BM, Schubert US. One-pot synthesis of 2-phenyl-2-oxazoline-containing quasi-diblock copoly(2-oxazoline)s under microwave irradiation. J Polym Sci Part A Polym Chem. 2007;45:416–422. [Google Scholar]
- [70].Lambermont-Thijs HML, Jochems MJHC, Hoogenboom R, Schubert US. Synthesis and properties of gradient copolymers based on 2-phenyl-2-oxazoline and 2-nonyl-2-oxazoline. J Polym Sci Part A Polym Chem. 2009;47:6433–6440. [Google Scholar]
- [71].Lobert M, Hoogenboom R, Fustin C-A, Gohy J-F, Schubert US. Amphiphilic gradient copolymers containing fluorinated 2-phenyl-2-oxazolines: microwave-assisted one-pot synthesis and self-assembly in water. J Polym Sci Part A Polym Chem. 2008;46:5859–5868. [Google Scholar]
- [72].Lava K, Verbraeken B, Hoogenboom R. Poly(2-oxazoline)s and click chemistry: A versatile toolbox toward multi-functional polymers. Eur Polym J. 2015;65:98–111. [Google Scholar]
- [73].Luxenhofer R, Jordan R. Click Chemistry with Poly(2-oxazoline)s. Macromolecules. 2006;39:3509–3516. [Google Scholar]
- [74].Lav T-X, Lemechko P, Renard E, Amiel C, Langlois V, Volet G. Development of a new azido-oxazoline monomer for the preparation of amphiphilic graft copolymers by combination of cationic ring-opening polymerization and click chemistry. React Funct Polym. 2013;73:1001–1008. [Google Scholar]
- [75].Le Fer G, Amiel C, Volet G. Copolymers based on azidopentyl-2-oxazoline: Synthesis, characterization and LCST behavior. Eur Polym J. 2015;71:523–533. [Google Scholar]
- [76].Taubmann C, Luxenhofer R, Cesana S, Jordan R. First Aldehyde-Functionalized Poly(2-oxazoline)s for Chemoselective Ligation. Macromol Biosci. 2005;5:603–612. doi: 10.1002/mabi.200500059. [DOI] [PubMed] [Google Scholar]
- [77].Legros C, de Pauw-Gillet M-C, Tam KC, Lecommandoux S, Taton D. Aldehyde-functional copolymers based on poly(2-oxazoline) for post-polymerization modification. Eur Polym J. 2015;62:322–330. [Google Scholar]
- [78].Kempe K, Vollrath A, Schaefer HW, Poehlmann TG, Biskup C, Hoogenboom R, Hornig S, Schubert US. Multifunctional poly(2-oxazoline) nanoparticles for biological applications. Macromol Rapid Commun. 2010;31:1869–1873. doi: 10.1002/marc.201000283. [DOI] [PubMed] [Google Scholar]
- [79].Mero A, Pasut G, Dalla Via L, Fijten MWM, Schubert US, Hoogenboom R, Veronese FM. Synthesis and characterization of poly(2-ethyl-2-oxazoline)-conjugates with proteins and drugs: Suitable alternatives to PEG-conjugates. J Control Release. 2008;125:87–95. doi: 10.1016/j.jconrel.2007.10.010. [DOI] [PubMed] [Google Scholar]
- [80].Kempe K, Neuwirth T, Czaplewska J, Gottschaldt M, Hoogenboom R, Schubert US. Poly(2-oxazoline) glycopolymers with tunable LCST behavior. Polym Chem. 2011;2:1737–1743. [Google Scholar]
- [81].Haensch C, Erdmenger T, Fijten MWM, Hoeppner S, Schubert US. Fast surface modification by microwave assisted click reactions on silicon substrates. Langmuir. 2009;25(14):8019–8024. doi: 10.1021/la901140f. [DOI] [PubMed] [Google Scholar]
- [82].Kempe K, Hoogenboom R, Schubert US. A green approach for the synthesis and thiol-ene modification of alkene functionalized poly(2-oxazoline)s. Macromol Rapid Commun. 2011;32:1484–1489. doi: 10.1002/marc.201100271. [DOI] [PubMed] [Google Scholar]
- [83].Schenk V, Ellmaier L, Rossegger E, Edler M, Griesser T, Weidinger G, Wiesbrock F. Water-developable poly(2-oxazoline)-based negative photoresists. Macromol Rapid Commun. 2012;33:396–400. doi: 10.1002/marc.201100717. [DOI] [PubMed] [Google Scholar]
- [84].Wiesbrock F, Stelzer F, Schenk V, Ellmaier L, Polymer Competence Center Leoben GmbH, Austria Technologie & Systemtechnik AG WO 2013/036979 2013
- [85].Petit C, Luef KP, Edler M, Griesser T, Kremsner JM, Stadler A, Grassl B, Reynaud S, Wiesbrock F. Microwave-assisted syntheses in recyclable ionic liquids: Photoresists based on renewable resources. ChemSusChem. 2015 doi: 10.1002/cssc.201500847. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Huang H, Hoogenboom R, Leenen MAM, Guillet P, Jonas AM, Schubert US, Gohy J-F. Solvent.induced morphological transistion in core-cross-linked block copolymer micelles. J Am Chem Soc. 2006;128:3784–3788. doi: 10.1021/ja057762k. [DOI] [PubMed] [Google Scholar]
- [87].Farrugia BL, Kempe K, Schubert US, Hoogenboom R, Dargaville TR. Poly(2-oxazoline) hydrogels for controlled fibroblast attachment. Biomacromolecules. 2013;14:2724–2732. doi: 10.1021/bm400518h. [DOI] [PubMed] [Google Scholar]
- [88].Kelly AM, Hecke A, Wirnsberger B, Wiesbrock F. Synthesis of poly(2-oxazoline)-based hydrogels with tailor-made swelling degrees capable of stimulitriggered compound release. Macromol Rapid Commun. 2011;32:1815–1819. doi: 10.1002/marc.201100409. [DOI] [PubMed] [Google Scholar]
- [89].Li T, Tang H, Wu P. Stuctural investigation of thermo-responsive poly(2-isopropyl-2-oxazoline) hydrogel across the volume phase transition. Soft Matter. 2015;11:1911–1918. doi: 10.1039/c4sm02812k. [DOI] [PubMed] [Google Scholar]
- [90].Dargaville TR, Forster R, Farrugia BL, Kempe K, Voorhaar L, Schubert US, Hoogenboom R. Poly(2-oxazoline) hydrogel monoliths via thiol-ene coupling. Macromol Rapid Commun. 2012;33:1695–1700. doi: 10.1002/marc.201200249. [DOI] [PubMed] [Google Scholar]
- [91].Dargaville TR, Holier BG, Shokoohmand A, Hoogenboom R. Poly(2-oxazoline) hydrogels as next generation three-dimensional cell supports. Cell Adh Migr. 2014;8(2):88–93. doi: 10.4161/cam.28205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Hartlieb M, Pretzel D, Kempe K, Fritzsche C, Paulus RM, Gottschaldt M, Schuber US. Cationic poly(2-oxazoline) hydrogels for reversible DNA binding. Soft Matter. 2013;9:4693–4704. [Google Scholar]
- [93].Cesana S, Auernheimer J, Jordan R, Kessler H, Nuyken O. First poly(2-oxazoline)s with pendant amino groups. Macromol Chem Phys. 2006;207:183–192. [Google Scholar]
- [94].Hartlieb M, Schubert S, Kempe K, Windhab N, Schubert US. Stabilization of factor VIII by poly(2-oxazoline) hydrogels. J Polym Sci Part A Polym Chem. 2015;53:10–14. [Google Scholar]
- [95].Schenk V, Rossegger E, Ebner C, Bangerl F, Reichmann K, Hoffmann B, Höpfner M, Wiesbrock F. RGD-functionalization of poly(2-oxazoline)-based networks for enhanced adhesion to cancer cells. Polymers. 2014;6:264–279. [Google Scholar]
- [96].Tauhardt L, Kempe K, Knop K, Altuntas E, Jäger M, Schubert S, Fischer D, Schubert US. Linear polyethyleneimine: Optimized synthesis and characterization – on the way to “pharmagrade” batches. Macromol Chem Phys. 2011;212:1918–1924. [Google Scholar]
- [97].De la Rosa VR, Bauwens E, Monnery BD, De Geest BG, Hoogenboom R. Fast and accurate partial hydrolysis of poly(2-ethyl-2-oxazoline) into tailored linear polyethlenimine copolymers. Polym Chem. 2014;5:4957–4964. [Google Scholar]
- [98].Van Kuringen HPC, Lenoir J, Adriaens E, Bender J, De Geest BG, Hoogenboom R. Partial hydrolysis of poly(2-ethyl-2-oxazoline) and potential implications for biomedical applications? Macromol Biosci. 2012;12:1114–1123. doi: 10.1002/mabi.201200080. [DOI] [PubMed] [Google Scholar]
- [99].Lambermont-Thijs HML, Heuts JPA, Hoeppener S, Hoogenboom R, Schubert US. Selective partial hydrolysis of amphiphilic copoly(2-oxazoline)s as basis for temperature and pH responsive micelles. Polym Chem. 2011;2:313–322. [Google Scholar]
- [100].Van Kuringen HPC, De la Rosa VR, Fijten MWM, Heuts JPA, Hoogenboom R. Enhanced selectivity for the hydrolysis of block copoly(2-oxazoline)s in ethanol-water resulting in linear poly(ethylene imine) copolymers. Macromol Rapid Commun. 2012;33:827–832. doi: 10.1002/marc.201200046. [DOI] [PubMed] [Google Scholar]
- [101].Kelly AM, Kaltenhauser V, Mühlbacher I, Rametsteiner K, Kren H, Slugovc C, Stelzer F, Wiesbrock F. Poly(2-oxazoline)-derived contact biocides: Contributions to the understanding of antimicrobial activity. Macromol Biosci. 2013;13:116–125. doi: 10.1002/mabi.201200240. [DOI] [PubMed] [Google Scholar]