Abstract
Aim
There is a large literature reporting risk factor analyses for poor neurodevelopment in children born very preterm (VPT: ≤32wks) or very low birthweight (VLBW: ≤1250g), which to date has not been formally summarized. The aim of this paper was to identify prognostic factors for cerebral palsy (CP) and motor impairment in children born VPT/VLBW.
Method
A systematic review was conducted using Medline, Embase, and Pyscinfo databases to identify studies published between 1 January 1990 and 1 June 2014 reporting multivariable prediction models for poor neurodevelopment in VPT/VLBW children (registration number CRD42014006943). Twenty-eight studies for motor outcomes were identified.
Results
There was strong evidence that intraventricular haemorrhage and periventricular leukomalacia, and some evidence that the use of postnatal steroids and non-use of antenatal steroids, were prognostic factors for CP. Male sex and gestational age were of limited use as prognostic factors for CP in cohorts restricted to ≤32 weeks gestation; however, in children older than 5 years with no major disability, there was evidence that male sex was a predictive factor for motor impairment.
Interpretation
This review has identified factors which may be of prognostic value for CP and motor impairment in VPT/VLBW children and will help to form the basis of future prognostic research.
The incidence of preterm delivery is increasing and the survival rate of preterm children has risen steadily because of advances in obstetric and neonatal intensive care.1,2 Surviving children born very preterm (VPT: ≤32wks) or with very low birthweight (VLBW: ≤1250g) are at high risk of long-term developmental problems, including cerebral palsy (CP), motor and cognitive impairment, visual and auditory deficits, and behavioural problems.3 These children take up a disproportionate amount of neonatal intensive care unit funding and overall costs; and, as they grow, are more likely to require additional health care services beyond routine care to compensate for their functional limitations.4 The early identification and management of factors that mediate long-term outcome is necessary to assist health care professionals in selecting appropriate treatment pathways and to develop, target, and evaluate interventions.
There is a large literature reporting risk factor analyses for poor neurodevelopment in the VPT/VLBW population, which to date has not been formally summarized. Few studies have advanced beyond risk factor analyses to develop and validate risk prediction models for use in routine care and to assist with the planning and provision of health care. The only known prognostic models developed and validated are based on survival and a composite outcome of neurodevelopmental impairment at 18 to 22 months.5–7 While these are of great value, it is also important to untangle the factors that are prognostic in each developmental domain, which may be very different to those predictive of a composite outcome.
This is the third paper from a systematic review of risk factor analyses for poor neurodevelopmental outcomes in the following domains: motor function, cognition, behaviour, hearing, and vision. The objective of this comprehensive review is to consolidate the evidence on risk in children born VPT or VLBW to inform future prognostic research, summarizing multivariable outcome prediction models which aim to identify the combination of factors that is most strongly associated with outcome. The focus of this third paper is on motor function, with the aim of identifying risk factors that are robust predictors for CP and motor impairment.
Method
The methods for the overall systematic review have previously been published in a review protocol (http://www.crd.york.ac.uk/PROSPERO/), registration number CRD42014006943.
Search strategy
Three electronic search strategies were devised in the Medline, Embase, and Psycinfo databases (Appendices S1–S3, online supporting information) using the National Institutes of Health Medical Subject Headings (NIH MeSH). The searches identified any journal articles published from 1 January 1990 to 1 June 2014 reporting a multivariable risk prediction model for a neurodevelopmental outcome assessed after the age of 18 months in VPT/VLBW children. No language restrictions were made. The bibliographies of all articles included for data extraction were hand-searched for further eligible articles.
Eligibility criteria
Articles were included in the review if they satisfied the following eligibility criteria: (1) contained original data; (2) study population was born after 1 January 1990; (3) study population was 32 weeks gestational age or less, or had a birthweight of 1250g or less, and not a highly select group (based on other clinical criteria); and (4) one objective was to perform a multivariable risk factor analysis (>2 variables) of a neurodevelopmental outcome assessed after 18 months of age.
All study designs were included and 1990 was chosen as a cut-off date for year of birth as this was a time of transition from the ‘pre-surfactant’ era of high mortality and morbidity to the ‘surfactant era’ of improved survival and prognosis,3,8 with improvements in the use of assisted ventilation, prophylactic infection control, and antenatal steroid therapy. The birthweight cut-off of 1250g or less was chosen to exclude the subset of more mature but extremely growth-restricted infants included in the typical 1500g or less VLBW cohort that can cause heterogeneity and lead to confounding bias when examining the relationship between risk factors and outcome.9 Explanatory prognostic factor studies which investigate the causal pathway between a single prognostic factor and an outcome (ideally adjusted for confounders) and estimate effect size are not included in the review. In these types of studies, other risk factors are included based on the change in the regression coefficient of the prognostic factor under study whereas in multivariable outcome prediction models risk factors are included in the model based on their predictive ability of the outcome. Current guidelines recommend not combining these two distinct types of study as their objectives and model-building strategies differ which, when synthesized, could lead to biased results.10,11
Data extraction
All articles identified by the search strategies were screened on title and abstract for definite exclusions and duplicates (screen 1). For the remaining articles, the full text was retrieved and the inclusion criteria were applied (screen 2). The two screens were performed by the first author (LL) in the first instance, but if there was uncertainty about the eligibility of an article it was screened independently by the second author (RM). If a decision could not be reached it was referred to the rest of the author review team (JK, NM, and JM). Non-English articles included in the review were fully translated. Multiple articles based on the same cohort of children underwent a panel review (LL, RM, and NM). Those reporting the same outcome domain (cognitive, motor, behaviour, hearing, vision) at the same age of assessment (<5y and ≥5y) were assessed on relevance to the review, and only one article was selected for data extraction.
For all articles eligible for inclusion, both reviewers (LL and RM) completed a full data extraction form and risk of bias assessment on a customized MS Access 2010 database. These were cross-validated for discrepancies, and referred to the rest of the author review team if agreement could not be reached. If critical information was missing or unclear, the corresponding author was contacted once by e-mail for clarification. The following data items were extracted: study design, participant setting, centre selection, study location, year of birth, gestational age, birthweight, age at assessment, selection criteria of study population, sample size, completeness of data at follow-up, details of outcomes assessed, number of candidate risk factors assessed, variable selection, treatment of continuous variables, adjustment for confounders, method of analysis, model assumptions checked, missing data analysis, presentation of multivariable model, details of risk factors included in final model, strength of association, statistical validation, and clinical validation.
Risk of bias assessment
Overwhelming evidence shows that the conduct and reporting of published articles describing the development or validation prediction models are poor,12 which has led to the development of quality assessment tools specific for these types of study. In this review, the quality of studies was assessed according to a modified version of the QUIPS tool, which is a standardized set of criteria recommended for use in reviews of prognosis13 (Table SI, online supporting information). The tool focuses on six areas of potential bias pertinent to studies of prognosis: study participation, study attrition, prognostic factor measurement, outcome measurement, confounding measurement and account, and statistical analysis. Studies were graded as (yes/partly/no) for each domain and classified as having a low to moderate risk of bias if they at least partly satisfied all six bias domains and moderate to high risk of bias otherwise.
Data synthesis and reporting
Results were presented in accordance with the PRISMA guidelines.14 Risk factors that were statistically significant (p<0.05) in the final model were reported for each study. Studies were grouped according to age of assessment (<5 years and >=5 years) and type of outcome studied: CP diagnosis; fine or gross motor skills measured by scales, and neurological dysfunction not diagnosed as CP. Assessments in early infancy can be unreliable and are more crude measurements of motor development, whereas assessments in later childhood measure finer motor skills and therefore risk factors may differ. Models based on fine or gross motor skills measured by scales were further divided into those that included the whole cohort representing the complete spectrum of disability and those that excluded children with major disability, typically defined as a CP diagnosis and/or severe neurosensory or cognitive impairment. A risk factor was presented graphically if it was statistically significant in the final model of at least one low to moderate risk of bias study and included in the final model of at least two other studies (including moderate to high risk of bias studies) within the same outcome domain. The plots display the number and quality of all studies that entered each risk factor into the final model and whether it was reported as a significant predictor or non-significant. Since no clear conclusions could be made about risk factors considered in the final model of only one or two studies, the graphs were truncated at this point as they become non-informative.
Studies in which multiple models were reported for the same type of motor outcome were dealt with as follows. The full/principal model presented for the latest assessment/global score was selected for inclusion and any further subgroup or sensitivity analyses were not included. If joint models were presented including exactly the same participants, for example the same outcome with a different parameterization, then risk factors were only included in the graphical summary if both models agreed. If separate models were presented for independent subgroups, for example by gestational age group, with no overall model presented, then risk factors were included in the graphical summary if significant in either model.
Results
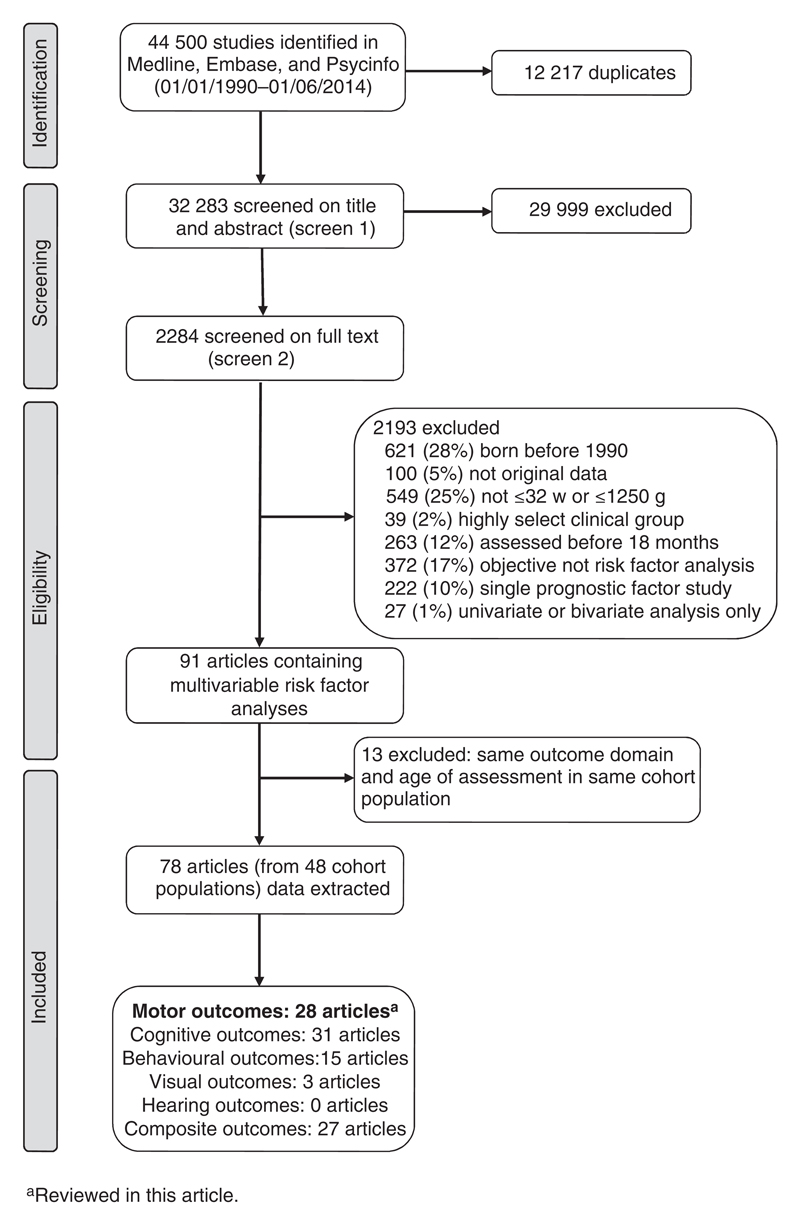
The searches retrieved 44 500 articles and, after removing duplicates, the first screen on title and abstract was performed on 32 283 articles (Fig. 1). For 29 999 articles the title or abstract clearly indicated that the topic of the article was not relevant to the review question or did not satisfy one of the inclusion criteria. The remaining 2284 articles were screened on full text, applying the full set of eligibility criteria. Eligibility was unclear in 136 articles (6%) and these were reviewed by the second independent reviewer (RM) or the author was contacted (where uncertainty was because of missing information). After applying the eligibility criteria, 91 articles (from 48 cohort populations) containing multivariable risk factor analyses were eligible for inclusion. Studies based in any centre participating in the National Institute of Child Health and Human Development Neonatal Research Network follow-up program were classified as belonging to the same cohort. Following panel review, a further 13 articles were excluded as they reported the same outcome domain in the same age group of assessment in the same cohort as another article with a more relevant objective; five of the articles excluded because of cohort overlap were based on motor outcomes.15–19 The remaining 78 articles were included in the data extraction. No further articles were identified in the hand-search of bibliographies. A total of 28 studies (from 19 cohort populations) comprising 44 risk factor analyses for motor outcome were included in this review paper.20–47
Figure 1.
Flow diagram of study search and selection.
Study characteristics
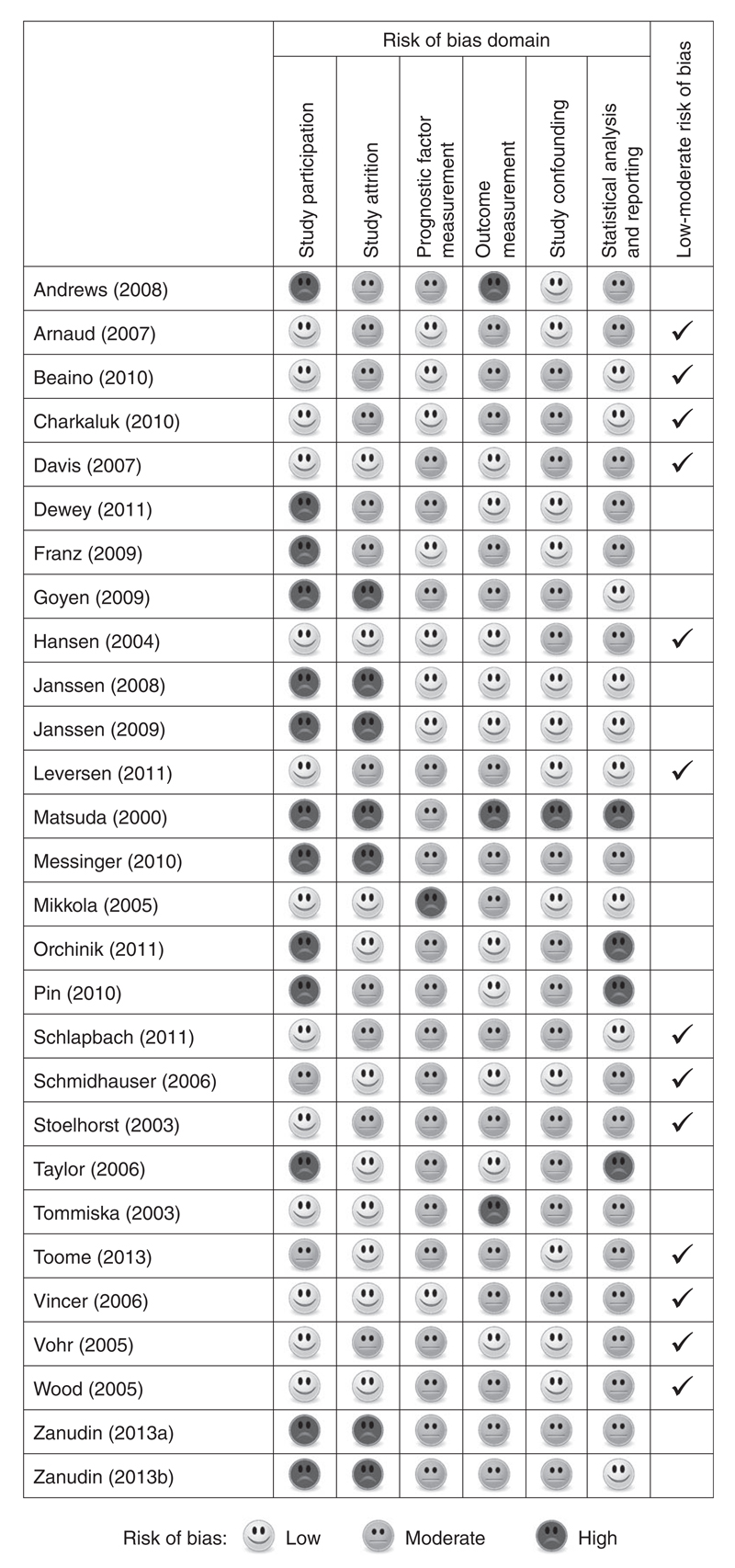
The main study design was prospective cohort (n=26); there was also one case-control study25 and one randomized controlled trial population.34 Of the 26 prospective cohorts, 12 were ascertained from all live births in a geographically defined region20,23,24,26–29,33,37,41,43,47 and nine were recruited from a single centre neonatal intensive care unit.30,35,36,38–40,42,44,46 Studies were conducted in 14 countries: United States (n=5), Australia (n=5), France (n=3), the Netherlands (n=3), Canada (n=2), Finland (n=2), and one study each from Denmark, Estonia, Germany, Japan, Norway, Sweden, Switzerland, and the UK & Republic of Ireland. The median sample size was 236 (range 48–3785). Three studies had more than 1000 participants21,29,47 and the remaining studies had 545 or less. Only two studies were restricted to extremely preterm children: less than 28 weeks22 and less than 26 weeks.26 Two studies examining risk factors for CP excluded multiple births.25,31 The risk of bias assessment classified 13 (46%) studies as low to moderate risk of bias and 15 (54%) studies as moderate to high risk of bias (Fig. 2).
Figure 2.
Risk of bias assessment of the 28 motor studies included in the review.
Risk factors for cerebral palsy
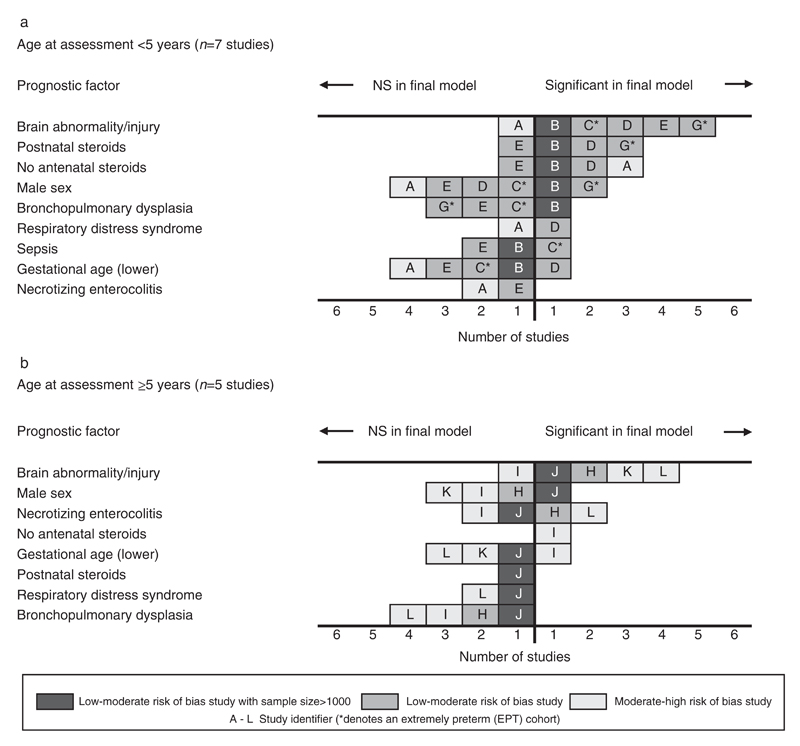
Twelve studies contained a risk factor analysis for CP (Table I), seven studies assessed outcome between 1 year 6 months to 2 years 6 months20–26 and five studies between 5 years to 8 years.27–31 Risk factors that were found to be significant in at least one low to moderate risk of bias study and examined in the final model of at least two other (independent) studies are presented in Figure 3 by age of assessment (<5y and ≥5y).
Table I.
Summary of studies reporting risk factor analyses for cerebral palsy in children born very preterm or with very low birthweight
| Study reference [Identifier in Figure 3] | Country and recruitment period | Age of assessment (y:mo) | GA (wks)/birthweight (g) | Design and participants | Number (%) of survivors assesseda | Definition and assessment of cerebral palsy as reported | Significant risk factors (p<0.05) in final model |
|---|---|---|---|---|---|---|---|
| Age of assessment <5 years | |||||||
| Tommiska et al.20 [A] | Finland 1996–1997 | 1:6 | <1000g | PC of all live births in Finland enrolled for the national routine FUP. | 208 (100) | Non-progressive motor impairment with spastic or dystonic muscle tone, brisk tendon reflexes, positive Babinski’s sign, and primitive reflexes. Clinical examination by a neurologist at local centre. | No AN steroids, hospital area, vaginal delivery. |
| Vohr et al.21 [B] | United States 1993–1998 | 1:6–1:10 | <33w and <1000g | PC of infants admitted to the NICU of 12 centres participating in the multi-centre NICHD NRN routine FUP. | 3785 (80) | Moderate-severe CP defined as non-ambulatory or requiring an assistive device for ambulation. Neurological examination based on Amiel-Tison77 by a trained, blinded developmentalist. | No AN steroids, BPD, IVH 3–4, male sex, PN steroids, PVL. |
| Schlapbach et al.22 [C] | Sweden 2000–2007 | 1:6–2:0 | <28w | PC of infants admitted to the NICU of nine Swiss perinatal centres participating in the neonatal network routine FUP. | 541 (77) | SCPE classification. Assessed by developmental paediatricians at one of the nine follow-up centres. | IVH 3–4/PVL, proven sepsis. |
| Vincer et al.23 [D] | Canada 1993–2002 | 2:0 | <31w | PC of all live births of mothers resident in the province of Nova Scotia and enrolled in the perinatal FUP. | 545 (97) | Definition compiled by Bax et al.78 Assessed in a general physical and neurodevelopmental examination. Diagnosis of infants with suspected CP confirmed by a paediatric neurologist. | No AN steroids, era of birth, GA<28w, IVH 3–4, PDA, PN steroids, RDS, resuscitation in delivery room. |
| Toome et al.24 [E] | Estonia 2007 | 2:0 | <32w | PC of all live births in Estonia enrolled in the national neonatal research routine FUP. | 155 (99) | SCPE classification. Physical assessment by a paediatrician and neurological examination by a child neurologist in one of two centres. | IVH 3-4/PVL 2-4. |
| Matsuda et al.25 b [F] | Japan 1992–1996 | 2:0–2:6 | <31w | Unmatched case-control study of all deliveries in a single centre (Kagoshima). Multiple births excluded. | 192: 22 cases, 170 controls | Chronic disability of CNS origin, characterized by aberrant control of movement or posture and non-progressive. All physical findings on presumed CP cases were reviewed retrospectively by a developmental paediatrician. | Intrauterine infection. |
| Wood et al.26 [G] | UK and Republic of Ireland 1995 | 2:6 | <26w | PC of all live births in the UK and Republic of Ireland (EPICURE Study). | 283 (92) | Non-progressive disorder of movement and posture. A structured neurological examination based on Amiel-Tison79 was performed and classified retrospectively based on Bax.80 | Enteral feeding by day 7, male sex, moved hospital <24h, parenchymal pathology and/or ventriculomegaly, PN steroids >8w. |
| Age of assessment ≥5 years | |||||||
| Hansen et al.27 [H] | Denmark 1994–1995 | 5:0 | <28w or <1000g | PC of all live births in Denmark ascertained from all 18 neonatal care units and the Danish Medical Birth Register (ETFOL Study). | 252 (94) | SCPE classification. One physician blinded to neonatal course conducted all assessments. Formal neurological assessment only performed if abnormal tone, gait, or posture. | IVH 3–4/PVL, NEC (surgery or X-ray diagnosed). |
| Mikkola et al.28 [I] | Finland 1996–1997 | 5:0 | <1000g | PC of all live births in Finland enrolled for the national routine FUP. | 193 (94) | A neurological examination using a modified partial Touwen test.81 Results were classified according to Hadders-Algra et al.82 | No AN steroids, lower GA, hospital area. |
| Beaino et al.29 [J] | France 1997 | 5:0 | <33w | PC of all live births in nine French regions comprising one-third of all births (EPIPAGE Study). | 1817 (75) | SCPE classification. Children with abnormal neurological examination underwent expert review to validate and classify diagnosis. | Cystic PVL/IPH, IVH 2–3 echodensities/VD, male sex. |
| Franz et al.30,c [K] | Germany 1996–1999 | 4:7–7:0 | <30w and <1500g | PC study of infants admitted to a single centre level-3 NICU (Ulm University). | 219 (83) | Gross Motor Function Classification Scale (GMFCS)83 ≥1. | IVH/PVH ≥3, lower maternal education, ROP ≥3, smaller HC SDS gain (birth to discharge). |
| Andrews et al.31 [L] | United States 1996–1999 | 5:0–8:0 | <32w | PC of consecutive live births in a single centre (Alabama). Multiple births excluded. | 261 (70) | Defined as abnormal muscle tone in at least one extremity and abnormal control of movement and posture. General physical and neurological examination by a certified nurse practitioner under supervision of a developmental paediatrician. | African–American race, history of seizures, IVH 3–4, NEC. |
Percentage of children surviving to the age of the intended assessment who were assessed for outcome; not all included in the final model.
Two models for cerebral palsy reported. Model using the total sample of 192 children included. Model excluding children with antenatal complications not included.
Two models for cerebral palsy reported. Same perinatal factors fitted with change in weight variables added to first model and change in head circumference variables added to second model. Perinatal factors reported as significant if p<0.05 in both models and non-significant if p≥0.05 in both models. AN, antenatal; BPD, bronchopulmonary dysplasia; CNS, central nervous system; CP, cerebral palsy; FUP, follow-up; GA, gestational age; HC SDS, head circumference standard deviation score; IPH, intraparenchymal haemorrhage; IVH, intraventricular haemorrhage; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; NICHD NRN, National Institutes of Child Health and Human Development Neonatal Research Network; PC, prospective cohort; PDA, patent ductus arteriosus; PN, postnatal; PVH, periventricular haemorrhage; PVL, periventricular leukomalacia; RDS, respiratory distress syndrome; ROP, retinopathy of prematurity; SCPE, Surveillance of Cerebral Palsy in Europe;84 VD, ventricular dilatation.
Figure 3.
Evidence synthesis of risk factors for cerebral palsy in children born very preterm or with very low birthweight.
There was strong evidence that brain injury diagnosed during the neonatal period was predictive of CP; in 11 out of the 12 studies that entered this factor in the final model, it was statistically significant in nine (odds ratio [OR] range 2.3–43).21–24,26,27,29–31 In the nine studies that found brain injury significant, four combined intraventricular haemorrhage (IVH) grade 3 to 4 and periventricular leukomalacia (PVL) into a single variable,22,24,27,30 one study used (IVH) grade 3 to 4 only,23 one study defined brain injury as parenchymal pathology and/or ventriculomegaly,26 and three studies entered IVH and PVL as separate variables.21,29,31 In the latter three studies, IVH grade 3 to 4 was significant in all models and PVL was significant in the two largest studies.21,29 The two studies with moderate to high risk of bias that did not find brain injury significant in the final model used IVH grade 2 to 420 and grade 3 to 4.28
There was some evidence that postnatal steroids administered in the neonatal period was associated with a diagnosis of CP at around 2 years of age with three low to moderate risk of bias studies reporting significance in the final model (OR range 2–5)21,23,26 and one low to moderate risk of bias study reporting non-significance.24 However, postnatal steroid use was not found to be prognostic in the single large study based on diagnosis at 5 years.29 In the two studies that reported non-significant findings, the prevalence of treatment with postnatal steroids was much lower (5% and 18% of the cohort population compared to 30% to 60% in the significant studies). Wood et al.26 examined the effect of the duration of postnatal steroid use and found that only those treated for more than 8 weeks were at substantially increased risk of CP.
The use of antenatal steroids were found to be significantly protective in two low to moderate risk of bias studies21,23 and one moderate to high risk of bias study20 based on diagnosis of CP at around 2 years of age (OR range 0.3–0.66) and not significant in one low to moderate risk of bias study.24 One moderate to high risk of bias study,28 based on the same cohort as a study included in the under 5 year age band,20 corroborated this result at 5 years. Most of these studies reported that around 80% of the study population had been exposed to antenatal steroids.
The relationship between male sex and CP was conflicting in both age groups, as three low to moderate risk of bias studies,21,26,29 including the two largest studies, found that males were significantly more likely to develop CP; however, four other low to moderate risk of bias studies22–24,27 and three moderate to high risk of bias studies20,28,30 did not support this association. The range of ORs in the significant studies was 1.4 to 2.3 and only of borderline statistical significance. Lower gestational age was found not to be predictive of CP in seven of the nine studies including this as a factor in the final model.20–22,24,29–31
Risk factors for impaired fine or gross motor skills
Six studies presented a risk factor analysis for motor impairment in children of all levels of disability21,32–36 and 10 studies in children free of major disability26,27,37–44,46 (Table II). The most common assessment used before 5 years was the Psychomotor Development Index from the Bayley Scales of Infant Development version II (BSID-II).48 This is a comprehensive, rigorously validated test which is widely used as a standardized assessment of key developmental milestones in infants up to 42 months. The Psychomotor Development Index assesses gross and fine motor development, involving some aspects of psychological functioning. All studies assessing motor development in children free of major disability after 5 years used the Movement Assessment Battery for Children (MABC).49 The MABC is a validated norm-referenced test that measures fine and gross motor skills in three key areas: manual dexterity, ball skills, and static/dynamic balance. A total score below 5th centile is indicative of definite developmental coordination disorder, and a total score below 15th centile represents a borderline motor impairment.
Table II.
Summary of studies reporting risk factor analyses for impaired fine or gross motor skills in children born very preterm or with very low birthweight
| Study reference [Identifier for Figure 4] | Country and recruitment period | Age of assessment (y:mo) | GA (wks)/birthweight (g) | Design and participants | Exclusion criteria for major disability | Number (%) of survivors assesseda | Outcome measure (continuous (cts) unless otherwise specified) | Significant risk factors for poorer outcome (p<0.05) in final model |
|---|---|---|---|---|---|---|---|---|
| Children of all levels of disability | ||||||||
| Age of assessment <5 years | ||||||||
| Pin et al.32 [M] | Australia 2006–2007 | 1:6 | <30w | PC of infants admitted to four tertiary NICUs in Melbourne. | N/A | 54 (76) | Gross motor development. Total score from Alberta Infant Motor Scale (AIMS).85 Blinded assessment. | PN steroids. |
| Vohr et al.21 [B] | United States 1993–1998 | 1:6–1:10 | <33w and <1000g | PC of infants admitted to the NICU of 12 centres participating in the multicentre NICHD NRN routine FUP. | N/A | 3785 (80) | Fine and gross motor skills. PDI score from BSID-II (<70 vs ≥70). Blinded assessment. | Adjusted age, no AN steroids, lower BW, BPD, IVH 3–4, male sex, lower maternal education, multiple pregnancy, PVL, PN steroids, sepsis (early or late). |
| Stoelhorst et al.33,b [N] | Netherlands 1996–1997 | 2:0 | <32w | PC of all live births in three Dutch health regions comprising 9% of the population. | N/A | 144 (61) | Fine and gross motor skills. PDI score from BSID-I.86 Blinded assessment. | PN steroids, SGA. |
| Messinger et al.34,c [O] | United States 1999–2001 | 2:6 | <1000g | Infants admitted to the NICU of 12 centres participating in the multi-centre NICHD NRN routine FUP and enrolled in a glutamine supplementation RCT. | N/A | 539 (47) | Fine and gross motor skills. PDI score from BSID-II. | Lower BRS at 18m, BW ≤750g, male sex, lower maternal education, lower maternal income, lower PDI at 18m. |
| Age of assessment ≥5 years | ||||||||
| Orchinik et al.35,d [R] | United States 2001–2003 | 5:0 | <28w or <1000g | PC of infants admitted to a single centre NICU (Ohio) participating in the multicentre NICHD NRN routine FUP. | N/A | 133 (67) | Fine and gross motor skills. T-score from BOT-2 short form version87 <10th centile. Blinded assessment. | Neurosensory disorder and/or MDI<70 at 20m. |
| Taylor et al.36,e [S] | United States 1992–1995 | 8:0 | <1000g | PC of infants admitted to a single centre NICU (Ohio) participating in the multicentre NICHD NRN routine FUP. | N/A | 204 (86) | Fine and gross motor skills. T-score from BOT short form version88 (cts and <1SD below mean of control group). Blinded assessment. | Model 1 (T-score<1SD): BPD, longer neonatal hospital stay. Model 2 (cts T-score): BPD, BW <750g, NEC, PN steroids, NRI>3, longer neonatal hospital stay. |
| Restricted to children free of major disability | ||||||||
| Age of assessment <5 years | ||||||||
| Charkaluk et al.37 [P] | France 1997 | 2:0 | <33w | PC of all live births in one French region (Nord-Pas-de-Calais, EPIPAGE Study). | CP or severe neurosensory impairment (n=45). | 347 (64) | Fine motor domain of Brunet–Lezine scale (revised).89 | Longer intubation, lower parental occupation. |
| Wood et al.26 [G] | UK and Republic of Ireland 1995 | 2:6 | <26w | PC of all live births in the UK and Republic of Ireland (EPICURE Study). | PDI score <55 from BSID-II or functional motor disability (n=47). | 197 (64) | Fine and gross motor skills. PDI score from BSID-II. | Breast milk in hospital, BPD, non-vaginal breech delivery, male sex, multigravida, parenchymal pathology and/or ventriculomegaly |
| Janssen et al.38 [Q] | Netherlands 1996–2001 | 2:0–3:0 | <33w | PC of infants admitted to a single centre NICU (Nijmegen) and enrolled for the Dutch neonatal routine FUP. | Under multidisciplinary treatment for disability (n=23). | 437 (56) | Fine and gross motor skills. PDI score from BSID-II (<85 vs ≥85). Blinded assessment. | BPD, male sex, neonatal convulsions, lower maternal education. |
| Age of assessment ≥5 years | ||||||||
| Hansen et al.27 [H] | Denmark 1994–1995 | 5:0 | <28w or <1000g | PC of all live births in Denmark ascertained from all 18 neonatal care units and the Danish Medical Birth Register (ETFOL Study). | CP or visual disability (n=23) | 137 (51) | Motor development. Total score from MABC. Blinded assessment. | Male sex. |
| Janssen et al.39 [T] | Netherlands1996–2001 | 5:0 | <33w | PC of infants admitted to a single centre NICU (Nijmegen) and enrolled for the Dutch Neonatal routine FUP. | CP, visual disability or under multidisciplinary treatment for disability (n=31). | 371 (47) | Motor impairment. Total score <15th centile from MABC. Blinded assessment. | Male sex, GA <30w, IVH, PDI <90 at 30m, BRS motor quality <26 at 30m. |
| Dewey et al.40 [U] | Canada 1996–2000 | 5:0 | <1000g | PC study of infants admitted to a single centre NICU (Calgary). | CP, IQ<70 or visual impairment (n=44). | 103 (52) | Motor development. Total score from MABC. | BPD, higher GA, PN steroids. |
| Leversen et al.41,f [V] | Norway 1999–2000 | 5:0 | <28w or <1000 g | PC of all live births in Norway. | CP or severe sensory deficit (n=33). | 261 (70) | Motor development. Total score from MABC. | Model 1 (GA <28w): no AN steroids, lower GA, male sex, ROP >2, SGA. Model 2 (GA ≥28w): male sex, ROP 1–2. |
| Goyen and Lui42 [W] | Australia 1992–1995 | 8:0 | <29w or <1000g | Infants admitted to a tertiary referral centre (Sydney) receiving high-risk referrals from regional centres and enrolled for the routine FUP. | CP, visual/hearing impairment, IQ<85, not attending mainstream school (n=64). | 50 (24) | Motor impairment. Total score <15th centile from MABC. | PROM, ROP. |
| Davis et al.43 [X] | Australia 1991–1992 | 8:0 | <29w or <1000 g | PC of all live births in the state of Victoria. | CP or IQ>2SD below mean (n=45). | 210 (70) | Motor impairment. Total score <5th centile from MABC. Blinded assessment. | Male sex. |
| Zanudin et al.44 [Y] | Australia 1992–1994 | 12:0 | <1000 g | Retrospective longitudinal study of infants admitted to a single centre NICU (Brisbane). | CP, neurological impairment, cognitive index >2SD below mean at 4 years (n not reported) | 48 (<50) | Motor development. Total score from MABC. | BPD, male sex. |
Percentage of children surviving to the age of the intended assessment who were assessed for outcome; not all included in the final model.
Two models for motor skills reported. Model based on 2-year outcome included. Model based on 1.5-year outcome not included.
Several models for motor skills fitted. Full model adjusting for 18-month PDI and BRS total score included.
Each risk factor was fitted separately and adjusted for sex, race, parental SES, and months in school at testing (the article did not report results for the adjustment factors).
Two models for motor skills reported; one based on dichotomous outcome and one based on continuous outcome. Risk factors reported separately for each model in the table. Each risk factor was fitted separately and adjusted for sex, race, parental SES, family stressors, and family resources (the article did not report results for the adjustment factors).
Two models for motor skills reported for each gestational age group (<28wks and ≥28wks). Risk factor considered as significant in Figure 4 if p<0.05 in either model. AN, antenatal; BOT, Bruinincks–Oseretsky Test of Motor Proficiency; BPD, bronchopulmonary dysplasia; BRS, Behaviour Rating Scale from BSID; BSID, Bayley Scales of Infant Development;48 BW, birthweight; CP, cerebral palsy; FUP, follow-up; GA, gestational age; IVH, intraventricular haemorrhage; IQ, intelligence quitient; MABC, Movement Assessment Battery for Children;49 MDI, Mental Developmental Index from BSID; N/A, not applicable; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; NICHD NRN, National Institutes of Child Health and Human Development Neonatal Research Network; NRI, neonatal risk index; PC, prospective cohort; PDI, Psychomotor Developmental Index from BSID; PN, postnatal; PROM, prolonged rupture of membranes; PVL, periventricular leukomalacia; RCT, randomized controlled trial; ROP, retinopathy of prematurity; SD, standard deviation.
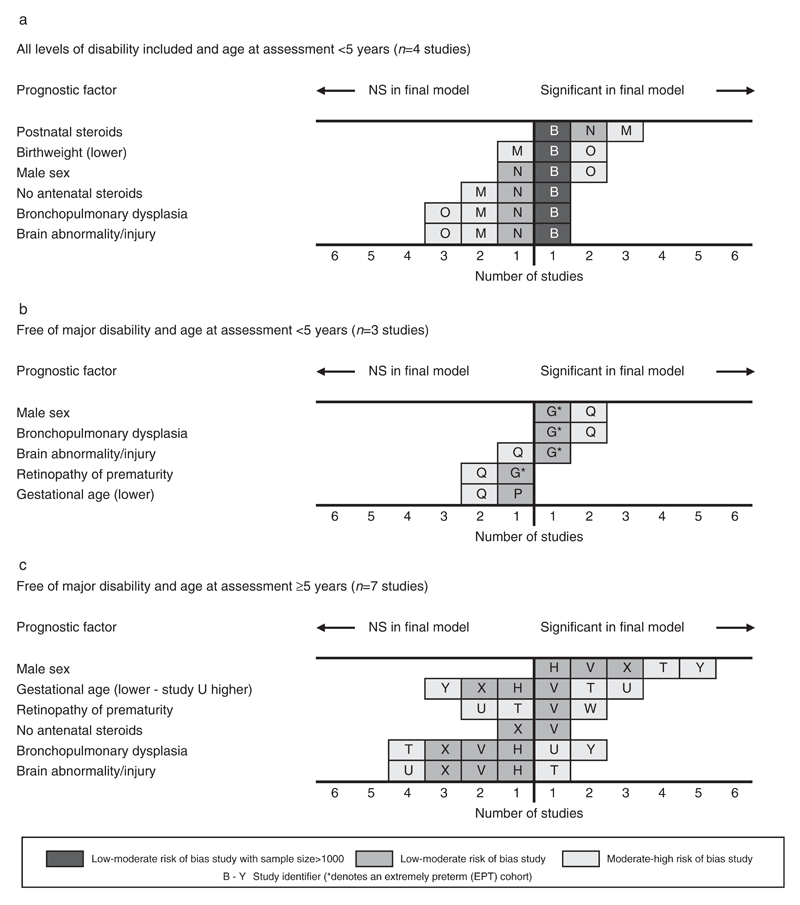
The risk factors emerging from the four studies reporting outcomes before 5 years for children of all levels of disability21,32–34 are summarized in Figure 4a. There were only two low to moderate risk of bias studies in this grouping and the results are dominated by the largest study.21 There was evidence that postnatal steroids were prognostic in three studies,21,32,33 but no other clear patterns emerged from this small sample of studies. The results from the 10 studies assessing motor development in children free of major disability21,22,32–38,41 are summarized in Figures 4b and c. In the seven studies using the total score from the MABC after 5 years of age the score was treated as continuous in four studies,27,40,41,44 dichotomized at below 15th centile in two studies,39,42 and at below 5th centile in one study.43 Male sex emerged as the only risk factor for which there was evidence of an association with motor impairment in children free of major disability in both age groups. All four low to moderate risk of bias studies26,27,41,43 and three moderate to high risk of bias studies38,39,44 that included male sex in the final model found a significant association.
Figure 4.
Evidence synthesis of risk factors for impaired fine or gross motor skills in children born very preterm or with very low birthweight.
Risk factors for neurological dysfunction not diagnosed as CP
Seven studies presented risk factor analysis for general neurological dysfunction20,26,28,30,45–47 (Table SII, online supporting information). The outcome measures were heterogeneous and there were only one or two studies within each stratum so it was not possible to synthesize the results in any meaningful way; however, the risk factors that were significant in the final models are listed for completeness. Similarly to CP, IVH and/or PVL appeared to be a prominent feature in the medical history of children developing later signs of neurological dysfunction.
Discussion
We found strong evidence that IVH grade 3 to 4 alone or in combination with PVL was predictive of CP. The importance of IVH and PVL as predictors of CP has been long established, with large well-conducted studies that have examined these factors in isolation reporting strong linear trends with grade,50–53 although the evidence for the influence of lower grade 1 to 2 IVH remains mixed.54–56 The two studies that did not find brain injury significant were based on the same cohort population and used routine data gathered from the national health care system, which may have been less standardized and more liable to bias and misclassification.20,28 PVL alone was found to be significant in the two largest21,29 of the three studies that tested this as a separate factor to IVH. The non-significant result in the smaller third study31 is likely to be explained by a lack of power with fewer cases of PVL included in the analysis. Since severe PVL is highly predictive, but also quite rare, it would seem sensible to combine it with IVH for the purposes of prognostic modelling to avoid it being dropped from models based on small samples with few cases.
In studies where postnatal steroids were commonly prescribed there was moderate evidence of an association with CP, whereas in two studies where the prevalence of treatment with postnatal steroids in the cohort population was much lower24,29 the findings were non-significant. Several Cochrane reviews of randomized controlled trials have been performed on the use of postnatal corticosteroids in the preterm population. Early use (<8d) was found to increase the incidence of CP (relative risk [RR] 1.45, 95% confidence interval [CI] 1.06–1.98),57 although late use (>7d) was found to be non-significant (RR 1.22, 95% CI 0.84–1.77).58 Studies that have focused solely on the relationship between postnatal steroids and increased risk of CP have also found a positive association.59,60 It is unclear whether postnatal steroids themselves are the causal factor or whether the reasons underlying the use of these drugs (typically in sicker, more immature infants) are responsible for the increased risk. Since the use of postnatal steroids has declined greatly since the 1990s, its utility as a prognostic factor is probably less relevant in current preterm populations.
We found moderate evidence that the use of antenatal steroids was a protective factor for CP. The one study that found it not significant24 was based on children born around a decade later than the other studies. A Cochrane review of randomized controlled trials on the use of antenatal corticosteroids among women at risk of preterm birth concluded that it was a non-significant protective factor for developing CP (RR 0.60, 95% CI 0.34–1.03).61 In four observational studies examining the effect of antenatal steroids on CP exclusively, two found a significant association62,63 and two no significant association,64,65 although the RR was less than 1 in the latter two studies. Recent evidence has emerged on the neuroprotective effect of magnesium sulphate and the subsequent reduction in the incidence of CP when prescribed as a tocolytic or to prevent preeclamptic convulsions;66,67 however, the studies included in this review predate this finding.
Male sex was found to be of limited use as a prognostic factor for CP, but there was fairly strong evidence that it was predictive of later motor impairment in children free of major disability. Other studies that have focused solely on the association of infant sex with motor function in very preterm children have reported mixed findings;68–71 however, these were not restricted to children free of major disability. One small imaging study found that at 12 years VPT males without evidence of IVH or PVL on neonatal ultrasound had widespread reductions in regional white and grey matter volumes compared to VPT females who did not differ from term-born controls.72 This implies that very preterm males continue to demonstrate abnormal brain development into childhood in the absence of any brain injury in the neonatal period.
The inverse relationship between the prevalence of CP and gestational age is well established;73–75 hence the lack of association in this review was surprising. One explanation is the restriction to cohorts 32 weeks or less – the prevalence of CP declines quite steeply after 32 weeks, hence discrimination below this threshold may become less apparent.74 Another explanation is that gestational age is associated with other critical risk factors so its power as an independent predictive factor may be reduced because of multicollinearity with factors that are more strongly causally related to CP, such as neonatal brain injury. It is also worth noting that although a strong positive relationship with gestational age is seen when survival without neurodevelopmental impairment is calculated as a function of all live births, when the denominator becomes survivors at discharge, as with all the studies included in this review, the association weakens. This is because the proportion of surviving children rises steeply with gestational age while the proportion of impaired survivors does not.
Study strengths and limitations
We used a broad search filter with no language restriction in order to capture all studies with exploratory risk factor analyses, which is recommended in this type of review.76 No further articles were identified in the hand-search of bibliographies of all studies included, so it is unlikely that there were any major omissions. The study cohorts spanned a 16-year period, hence some of the factors affecting outcome in the early 1990s, such as postnatal steroid therapy, may not be so relevant to current preterm populations. They also represent diverse international populations with differing methods of ascertainment and clinical practices which may explain the unclear pattern of results for some factors. Also, studies did not all consider the same sets of candidate factors. Some studies, including one large study, were based on diagnoses of CP younger than usually required by the Surveillance of Cerebral Palsy in Europe classification outcome and so may be unreliable. Study attrition was high in some studies and few studies reported the number of children included in the final model, making it difficult to assess how representative the children analyzed were of the original sample.
A major challenge in this review was multiplicity, arising from studies based on the same cohort population or single studies reporting more than one model. We selected studies/models for inclusion before data synthesis was conducted using standard rules, although it was difficult to apply a strict set of criteria for each case. We were unable to conduct a quantitative synthesis of the size of effect for different risk factors as this review did not include explanatory studies that investigated the causal relationship between a single risk factor and outcome. For example, to perform a meta-analysis to estimate the relative risk of CP after IVH, one would have to include all the articles that focus solely on IVH as a risk factor, which were specifically excluded in this review so as not to introduce bias, as outlined earlier.
Conclusion
In the VPT/VLBW population there was strong evidence that IVH and PVL are robust prognostic factors for CP and some evidence that the use of postnatal steroids increases the risk and the use of antenatal steroids reduces the risk of CP. There was moderate evidence that male sex is prognostic for motor impairment at school age in children free of major disability. Gestational age was found to be of limited use as a prognostic factor for CP, probably because of a reduced discriminatory power in cohorts restricted to earlier gestational ages and confounding with other important clinical events which are more strongly causally related. Future research should use the results from this review as a basis for developing and validating prognostic models that go beyond the scope of fitting risk factor models, testing their performance and robustness over time and in other independent cohort populations. The use of meta-analysis to determine the strength of association of the factors identified with outcome, including the results of single risk factor studies, would also be a valuable future direction.
Supplementary Material
What this paper adds.
Strong evidence that IVH and PVL are robust prognostic factors for CP.
Some evidence that use of postnatal steroids increases risk of CP and that use of antenatal steroids is a protective factor.
Gestational age was of limited use as a prognostic factor for CP in cohorts restricted to ≤32 weeks, likely due to reduced discriminatory power in very preterm subgroups and confounding with other important clinical events which are more strongly causally related.
Moderate evidence that male sex is prognostic for motor impairment at school age in children free of major disability.
Acknowledgements
We thank Nia Wyn Roberts (MSc Econ), Outreach Librarian, Bodleian Health Care Libraries, Knowledge Centre, ORC Research Building, Old Road Campus, Headington, Oxford OX3 7DQ for her input and expertise during the search phase of the review. National Institute for Health Research (NIHR) Doctoral Research Fellowship, NIHR-DRF-2012-05-206. The funder had no role in the study design, data collection, data analysis, manuscript preparation, and publication decisions. This article presents independent research funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The sponsor, funder, and authors have stated that they had no interests that might be perceived as posing a conflict or bias.
Abbreviations
- IVH
Intraventricular haemorrhage
- MABC
Movement Assessment Battery for Children
- PVL
Periventricular leukomalacia
- VLBW
Very low birthweight
- VPT
Very preterm
References
- 1.Tucker J, McGuire W. Epidemiology of preterm birth. BMJ. 2004;329:675–78. doi: 10.1136/bmj.329.7467.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron IS, Rey-Casserly C. Extremely preterm birth outcome: a review of four decades of cognitive research. Neuropsychol Rev. 2010;20:430–52. doi: 10.1007/s11065-010-9132-z. [DOI] [PubMed] [Google Scholar]
- 4.Mangham LJ, Petrou S, Doyle LW, Deraper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics. 2009;123:312–27. doi: 10.1542/peds.2008-1827. [DOI] [PubMed] [Google Scholar]
- 5.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. Intensive care for extreme prematurity – moving beyond gestational age. NEJM. 2008;358:1672–81. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambalavanan N, Carlo WA, Tyson JE, et al. Outcome trajectories in extremely preterm infants. Pediatrics. 2012;130:e115–25. doi: 10.1542/peds.2011-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boland RA, Davis PG, Dawson JA, Doyle LW, Victorian Infant Collaborative Study G Predicting death or major neurodevelopmental disability in extremely preterm infants born in Australia. Arch Dis Child Fetal Neonatal Ed. 2013;98:F201–14. doi: 10.1136/archdischild-2012-301628. [DOI] [PubMed] [Google Scholar]
- 8.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990’s. Early Hum Dev. 1999;53:193–218. doi: 10.1016/s0378-3782(98)00052-8. [DOI] [PubMed] [Google Scholar]
- 9.Arnold CC, Kramar MS, Hobbs CA, Mclean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;134:604–13. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]
- 10.Van den Berg T, Heymans MW, Leone SS, et al. Overview of data-synthesis in systematic reviews of studies on outcome prediction models. BMC Med Res Methodol. 2013;13:42. doi: 10.1186/1471-2288-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley RD, Hayden JA, Steyerberg EW, et al. Prognosis Research Strategy (PROGRESS) 2: Prognostic Factor Research. PLOS Medi. 2013;10:e1001380. doi: 10.1371/journal.pmed.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins G, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. J Clin Epidemiol. 2015;68:134–43. doi: 10.1016/j.jclinepi.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Hayden JA, Van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–86. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costantine MM, How HY, Coppage K, Maxwell RA, Sibai BM. Does peripartum infection increase the incidence of cerebral palsy in extremely low birthweight infants? Am J Obstet Gynecol. 2007;196:e6–8. doi: 10.1016/j.ajog.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Hack M, Wilson-Costello D, Friedman H, Taylor GH, Schluchter M, Fanaroff AA. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g 1992–1995. Arch Pediatr Adolesc Med. 2000;154:725–31. doi: 10.1001/archpedi.154.7.725. [DOI] [PubMed] [Google Scholar]
- 17.Laptook AR, O’Shea TM, Shankaran S, Bhaskar B. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115:673–80. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 18.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105:1216–26. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 19.Vohr BR, Wright LL, Dusick AM, et al. Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113:781–89. doi: 10.1542/peds.113.4.781. [DOI] [PubMed] [Google Scholar]
- 20.Tommiska V, Heinonen K, Kero P, et al. A national two year follow up study of extremely low birthweight infants born in 1996-1997. Arch Dis Child Fet Neonat Ed. 2003;88:F29–34. doi: 10.1136/fn.88.1.F29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vohr BR, Wright LL, Poole WK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks’ gestation between 1993 and 1998. Pediatrics. 2005;116:635–43. doi: 10.1542/peds.2004-2247. [DOI] [PubMed] [Google Scholar]
- 22.Schlapbach LJ, Aebischer M, Adams M, et al. Impact of sepsis on neurodevelopmental outcome in a swiss national cohort of extremely premature infants. Pediatrics. 2011;128:e348–57. doi: 10.1542/peds.2010-3338. [DOI] [PubMed] [Google Scholar]
- 23.Vincer MJ, Allen AC, Joseph KS, Stinson DA, Scott H, Wood E. Increasing prevalence of cerebral palsy among very preterm infants: a population-based study. Pediatrics. 2006;118:e1621–26. doi: 10.1542/peds.2006-1522. [DOI] [PubMed] [Google Scholar]
- 24.Toome L, Varendi H, Mannamaa M, Vals MA, Tanavsuu T, Kolk A. Follow-up study of 2-year-olds born at very low gestational age in Estonia. Acta Paediatr. 2013;102:300–07. doi: 10.1111/apa.12091. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda Y, Kouno S, Hiroyama Y, et al. Intrauterine infection, magnesium sulfate exposure and cerebral palsy in infants born between 26 and 30 weeks of gestation. Eur J Obstet Gynecol Reprod Biol. 2000;91:159–64. doi: 10.1016/s0301-2115(99)00256-0. [DOI] [PubMed] [Google Scholar]
- 26.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and entecedents of neurological and developmental disability at the 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90:F134–40. doi: 10.1136/adc.2004.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen BM, Hoff B, Uldall P, Greisen G. Perinatal risk factors of adverse outcome in very preterm children: a role of initial treatment of respiratory insufficiency? Acta Paediatr. 2004;93:185–89. doi: 10.1080/08035250310008230. [DOI] [PubMed] [Google Scholar]
- 28.Mikkola K, Ritari N, Tommiska V, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996–1997. Pediatrics. 2005;116:1391–400. doi: 10.1542/peds.2005-0171. [DOI] [PubMed] [Google Scholar]
- 29.Beaino G, Khoshnood B, Kaminski M, et al. Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Dev Med Child Neurol. 2010;52:e119–25. doi: 10.1111/j.1469-8749.2010.03612.x. [DOI] [PubMed] [Google Scholar]
- 30.Franz AR, Pohlandt F, Bode H, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009;123:e101–09. doi: 10.1542/peds.2008-1352. [DOI] [PubMed] [Google Scholar]
- 31.Andrews WW, Cliver SP, Biasini F, et al. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol. 2008;198:e461–66. doi: 10.1016/j.ajog.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pin TW, Eldridge B, Galea MP. Motor trajectories from 4 to 18months corrected age in infants born at less than 30weeks of gestation. Early Hum Dev. 2010;86:573–80. doi: 10.1016/j.earlhumdev.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Stoelhorst GMSJ, Rijken M, Martens SE, et al. Developmental outcome at 18 and 24 months of age in very preterm children: a cohort study from 1996 to 1997. Early Hum Dev. 2003;72:83–95. doi: 10.1016/s0378-3782(03)00011-2. [DOI] [PubMed] [Google Scholar]
- 34.Messinger D, Lambert B, Bauer CR, Bann CM, Hamlin-Smith K, Das A. The relationship between behavior ratings and concurrent and subsequent mental and motor performance in toddlers born at extremely low birth weight. J Early Interv. 2010;32:214–33. doi: 10.1177/1053815110380917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orchinik LJ, Taylor HG, Espy KA, et al. Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten. J Int Neuropsychol Soc. 2011;17:1067–79. doi: 10.1017/S135561771100107X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor HG, Klein N, Drotar D, Schluchter M, Hack M. Consequences and risks of <1000-g birth weight for neuropsychological skills, achievement, and adaptive functioning. J Dev Behav Pediatr. 2006;27:459–69. doi: 10.1097/00004703-200612000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Charkaluk ML, Truffert P, Fily A, Ancel PY, Pierrat V. Neurodevelopment of children born very preterm and free of severe disabilities: the Nord-Pas de Calais Epipage cohort study. Acta Paediatr. 2010;99:684–89. doi: 10.1111/j.0803-5253.2010.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janssen AJWM, Nijhuis-van Der Sanden MWG, Akkermans RP, Oostendorp RAB, Kollee LAA. Influence of behaviour and risk factors on motor performance in preterm infants at age 2 to 3 years. Dev Med Child Neurol. 2008;50:926–31. doi: 10.1111/j.1469-8749.2008.03108.x. [DOI] [PubMed] [Google Scholar]
- 39.Janssen AJWM, Nijhuis-van Der Sanden MWG, Akkermans RP, Tissingh J, Oostendorp RAB, Kollee LAA. A model to predict motor performance in preterm infants at 5 years. Early Hum Dev. 2009;85:599–604. doi: 10.1016/j.earlhumdev.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Dewey D, Creighton DE, Heath JA, et al. Assessment of developmental coordination disorder in children born with extremely low birth weights. Dev Neuropsychol. 2011;36:42–56. doi: 10.1080/87565641.2011.540535. [DOI] [PubMed] [Google Scholar]
- 41.Leversen KT, Sommerfelt K, Ronnestad A, et al. Prediction of neurodevelopmental and sensory outcome at 5 years in Norwegian children born extremely preterm. Pediatr. 2011;127:e630–38. doi: 10.1542/peds.2010-1001. [DOI] [PubMed] [Google Scholar]
- 42.Goyen TA, Lui K. Developmental coordination disorder in “apparently normal” schoolchildren born extremely preterm. Arch Dis Child. 2009;94:298–302. doi: 10.1136/adc.2007.134692. [DOI] [PubMed] [Google Scholar]
- 43.Davis N, Ford G, Anderson P, Doyle L. Developmental coordination disorder at 8 years of age in a regional cohort of extremely-lowbirthweight or very preterm infants. Dev Med Child Neurol. 2007;49:325–30. doi: 10.1111/j.1469-8749.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- 44.Zanudin A, Gray PH, Burns Y, Danks M, Watter P, Poulsen L. Perinatal factors in non-disabled ELBW school children and later performance. J Paediatr Child Health. 2013;49:E62–67. doi: 10.1111/jpc.12022. [DOI] [PubMed] [Google Scholar]
- 45.Schmidhauser J, Caflisch J, Rousson V, Bucher HU, Largo RH, Latal B. Impaired motor performance and movement quality in very-low-birthweight children at 6 years of age. Dev Med Child Neurol. 2006;48:718–22. doi: 10.1017/S001216220600154X. [DOI] [PubMed] [Google Scholar]
- 46.Zanudin A, Burns Y, Gray PH, Danks M, Poulsen L, Watter P. Perinatal events and motor performance of children born with ELBW and nondisabled. Pediatrics. 2013;25:30–35. doi: 10.1097/PEP.0b013e31827aa424. [DOI] [PubMed] [Google Scholar]
- 47.Arnaud C, Daubisse-Marliac L, White-Koning M, et al. Prevalence and associated factors of minor neuromotor dysfunctions at age 5 years in prematurely born children: the EPIPAGE study. Arch Pediatr Adolesc Med. 2007;161:1053–61. doi: 10.1001/archpedi.161.11.1053. [DOI] [PubMed] [Google Scholar]
- 48.Bayley N. Bayley Scales of Infant Development-II. 2nd edn. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 49.Henderson SE, Sugden DA. Movement Assessment Battery for Children. London, UK: Psychological Corporation; 1992. [Google Scholar]
- 50.Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121:e1167–77. doi: 10.1542/peds.2007-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherlock RL, Anderson PJ, Doyle LW, et al. Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum Dev. 2005;81:909–16. doi: 10.1016/j.earlhumdev.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Kuban KCK, Allred EN, O’Shea MT, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24:63–72. doi: 10.1177/0883073808321048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marret S, Marchand-Martin L, Picaud JC, et al. Brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: the EPIPAGE cohort study. PLoS ONE. 2013;8:e62683. doi: 10.1371/journal.pone.0062683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klebermass K, Olischar M, Waldhoer T, Fuiko R, Pollak A, Weninger M. Amplitude-integrated EEG pattern predicts further outcome in preterm infants. Pediatr Res. 2011;70:102–08. doi: 10.1203/PDR.0b013e31821ba200. [DOI] [PubMed] [Google Scholar]
- 55.Patra K, Wilson-Costello D, Taylor HG, Mercuri-Minich N, Hack M. Grades I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatri. 2006;149:169–73. doi: 10.1016/j.jpeds.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Payne AH, Hintz SR, Hibbs AM, et al. Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JJAMA Pediatr. 2013;167:451–59. doi: 10.1001/jamapediatrics.2013.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doyle LW, Ehrenkranz RA, Halliday HL. Early (<8 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2014;5:CD001146. doi: 10.1002/14651858.CD001146.pub4. [DOI] [PubMed] [Google Scholar]
- 58.Doyle LW, Ehrenkranz RA, Halliday HL. Late (>7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2009;1:CD001145. doi: 10.1002/14651858.CD001145.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Doyle LW, Bowman E, Callanan C, et al. Postnatal corticosteroids and sensorineural outcome at 5 years of age. J Paediatr Child Health. 2000;36:256–61. doi: 10.1046/j.1440-1754.2000.00493.x. [DOI] [PubMed] [Google Scholar]
- 60.Wilson-Costello D, Walsh MC, Langer JC, et al. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics. 2009;123:e430–37. doi: 10.1542/peds.2008-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 62.Carlo WA, McDonald SA, Fanaroff AA, et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011;306:2348–58. doi: 10.1001/jama.2011.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong D, Abdel-Latif M, Kent A, Network N. Antenatal steroid exposure and outcomes of very premature infants: a regional cohort study. Arch Dis Child Fetal Neonatal Ed. 2014;99:F12–20. doi: 10.1136/archdischild-2013-304705. [DOI] [PubMed] [Google Scholar]
- 64.Lee BH, Stoll BJ, McDonald SA, Higgins RD. Neurodevelopmental outcomes of extremely low birth weight infants exposed prenatally to dexamethasone versus betamethasone. Pediatrics. 2008;121:289–96. doi: 10.1542/peds.2007-1103. [DOI] [PubMed] [Google Scholar]
- 65.Amin SB, Kamaluddeen M, Sangem M. Neurodevelopmental outcome of premature infants after exposure to antenatal indomethacin. Am J Obstet Gynecol. 2008;199:e41–41. doi: 10.1016/j.ajog.2007.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doyle LW, Crowther CA, Middleton P, Marret S. Antenatal magnesium sulfate and neurologic outcome in preterm infants: a systematic review. Obstet Gynecol. 2009;113:1327–33. doi: 10.1097/AOG.0b013e3181a60495. [DOI] [PubMed] [Google Scholar]
- 67.Conde-Agudelo A, Romero R. Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks’ gestation: a systematic review and metaanalysis. Am J Obstet Gynecol. 2009;200:596–606. doi: 10.1016/j.ajog.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hintz SR, Kendrick DE, Vohr BR, Poole WK, Higgins RD. Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birth-weight infants. Acta Paediatr. 2006;95:1239–48. doi: 10.1080/08035250600599727. [DOI] [PubMed] [Google Scholar]
- 69.Kent AL, Wright IMR, Abdel-Latif ME, Bowen J, Bajuk B, Vincent T. Mortality and adverse neurologic outcomes are greater in preterm male infants. Pediatrics. 2012;129:124–31. doi: 10.1542/peds.2011-1578. [DOI] [PubMed] [Google Scholar]
- 70.Neubauer V, Griesmaier E, Ralser E, Kiechl-Kohlendorfer U. The effect of sex on outcome of preterm infants – a population-based survey. Acta Paediatr. 2012;101:906–11. doi: 10.1111/j.1651-2227.2012.02709.x. [DOI] [PubMed] [Google Scholar]
- 71.Skiold B, Alexandrou G, Padilla N, Blennow M, Vollmer B, Aden U. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr. 2014;164:1012–18. doi: 10.1016/j.jpeds.2013.12.051. [DOI] [PubMed] [Google Scholar]
- 72.Kesler SR, Reiss AL, Vohr B, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age 12. J Pediatr. 2008;152:513–20. doi: 10.1016/j.jpeds.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Himpens E, Van den Broeck C, Oostra A, Vanhaesebrouck P. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta-analytic review. Dev Med Child Neurol. 2008;50:334–40. doi: 10.1111/j.1469-8749.2008.02047.x. [DOI] [PubMed] [Google Scholar]
- 74.Oskoui M, Coutinho F, Dykeman J, Jette N, Pringsheim T. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013;55:509–19. doi: 10.1111/dmcn.12080. [DOI] [PubMed] [Google Scholar]
- 75.Himmelmann K, Hagberg G, Uvebrant P. The changing panorama of cerebral palsy in Sweden. X. Prevalence and origin in the birth-year period 1999–2002. Acta Paediatr. 2010;99:1337–43. doi: 10.1111/j.1651-2227.2010.01819.x. [DOI] [PubMed] [Google Scholar]
- 76.Geersing G-J, Bouwmeester W, Zuithoff P, Spijker R, Leeflang M, Moons K. Search filters for finding prognostic and diagnostic prediction studies in Medline to enhance systematic reviews. PLoS ONE. 2012;7:e32844. doi: 10.1371/journal.pone.0032844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amiel-Tison C. Neuromotor status. In: Taeusch H, Yogman M, editors. Follow-up Management of the High-Risk Infant. Boston, MA: Little, Brown and Company; 1987. pp. 115–26. [Google Scholar]
- 78.Bax M, Goldstein M, Rosenbaum P. Proposed definition and classification of cerebral palsy. Dev Med Child Neurol. 2005;47:571–76. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 79.Amiel-Tison C, Stewart A. Follow-up studies during the first five years of life: a pervasive assessment of a neurological function. Arch Dis Child. 1989;64:496–502. doi: 10.1136/adc.64.4_spec_no.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bax M. Terminology and classification of cerebral palsy. Dev Med Child Neurol. 1964;6:295–7. doi: 10.1111/j.1469-8749.1964.tb10791.x. [DOI] [PubMed] [Google Scholar]
- 81.Touwen BCL. Examination of the Child With Minor Neurological Dysfunction. 2nd edn. Suffolk, UK: The Lavenham Press Ltd; 1979. [Google Scholar]
- 82.Hadders-Algra M, Mavinkurve-Groothuis AM, Groen SE, Stremmelaar EF, Martijn A, Butcher PR. Quality of general movements and the development of minor neurological dysfunction at toddler and school age. Clin Rehabil. 2004;18:287–99. doi: 10.1191/0269215504cr730oa. [DOI] [PubMed] [Google Scholar]
- 83.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 84.(SCPE) SoCPiE. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816–24. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- 85.Piper MC, Darrah J. Motor Assessment of the Developing Infant. London, UK: Elsevier Health Sciences; 1994. [Google Scholar]
- 86.Bayley N. Bayley Scales of Infant Development. New York: Psychological Corporation; 1969. [Google Scholar]
- 87.Bruininks RH, Bruininks BD. BOT-2: Bruininks–Oseretsky Test of Motor Proficiency. 2nd edn. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- 88.Bruininks RH. Bruininks-Oseretsky Test of Motor Proficiency: Examiners Manual. Circle Pines, MN: American Guidance Service; 1978. [Google Scholar]
- 89.Josse D. Brunet-Lezine Revise: Echelle de Developpement Psychomoteur de la Premiere Enfance. Paris: Etablissements d’applications Psychotechniques; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.