Background and Aims
Hepatobiliary cancers, including hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (CCA), together comprise the fifth most common cancer and third leading cause of cancer death globally [1]. The majority of patients present with advanced, unresectable disease, with median survival less than a year [2–4]. Immune dysregulation may contribute to hepatobiliary carcinogenesis, though mechanisms are not well understood [5–7].
Immunotherapy approaches in oncology may be most efficacious in cancers susceptible to intrinsic immune modulation [8, 9]. Recently, increasing understanding of tumor immunogenicity spurred the development of novel classes of immune-targeted therapies, including inhibition of co-stimulatory pathways mediated by cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and the programmed death-1 (PD-1) receptor and its ligand (PD-L1). In a phase II trial in hepatitis C virus (HCV)-associated advanced HCC patients, tremelimumab-mediated CTLA-4 inhibition demonstrated a promising antitumor activity, accompanied by intriguing antiviral responses [10]. Table 1 displays selected ongoing HCC immunotherapy clinical trials.
Table 1.
Selected immunotherapies in clinical development for HCC
| Drug | Title | NCT trial number | Trial design | Mechanism |
|---|---|---|---|---|
| Nivolumab | Dose Escalation Study of Nivolumab (Anti-PD-1; BMS-936558; ONO-4538) in Patients (Pts) With Advanced Hepatocellular Carcinoma (HCC) With or Without Chronic Viral Hepatitis (Anti-PD-1 HCC) | NCT01658878 | Phase I | Anti-PD-1 antibody |
| Tremelimumab | Tremelimumab With Chemoembolization or Ablation for Liver Cancer | NCT01853618 | Phase I | Anti-CTLA-4 antibody plus TACE or ablation |
| CC-122 | Study of CC-122 to Evaluate the Safety, Tolerability, and Effectiveness for Patients With Advanced Solid Tumors, Non-Hodgkin’s Lymphoma, or Multiple Myeloma | NCT01421524 | Phase I expansion cohort | Pleiotropic pathway modulation (IMID) |
| AMP-514 | A Phase 1, Multi-Center, Open-label, Multi-Dose Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of AMP-514 in Subjects With Advanced Solid Malignancies | NCT02013804 | Phase I | Anti-PD-1 monoclonal antibody |
We present three cases of spontaneous regression of advanced hepatobiliary cancers in patients with HCV cirrhosis, one in a patient with mixed HCC and CCA tumor histology and two in patients with HCC. Our aims are to highlight the potential activity of native antitumor immune mechanisms against these malignancies and explore the implications for emerging immunotherapies in this grim family of cancers refractory to conventional therapies.
Methods
This retrospective case series was approved as an exempted category of research by the University of California, San Francisco Institutional Review Board. Cases were identified by the treating investigator (RKK). Pathology slides were reviewed by KE.
Case 1
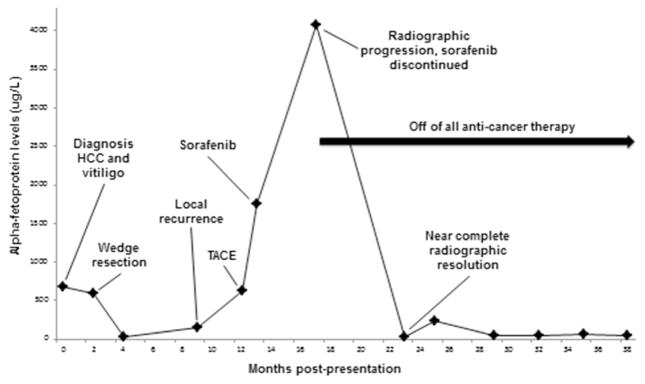
A 69-year-old man with HCC metastatic to lymph nodes presented to our hospital for medical oncology consultation after progression on first-line sorafenib therapy. HCV genotype 1a had been diagnosed 20 years earlier and treated with interferon and ribavirin without response. Two years prior to presentation, screening labs revealed new alpha-fetoprotein (AFP) elevation to 677 μg/L; this prompted an abdominal computed tomography (CT) scan, which showed a 2.5-cm hypervascular lesion with nonenhancing central hypodensity in segment 8 of the liver, radiographically consistent with HCC. Around the same time as his HCC diagnosis, he developed white patches on his skin that were diagnosed as adult-onset vitiligo. A wedge resection of the liver tumor with radiofrequency ablation of an adjacent lesion was performed, and pathology confirmed a 2.7-cm well-differentiated HCC with negative margins. Postoperative AFP levels declined to 26 μg/L. Several months later, AFP levels rose to 141 μg/L, and a CT scan showed two new 1.1- and 1.3-cm hyperenhancing lesions, which were not amenable to resection or ablation and instead were treated with transarterial chemoembolization (TACE). A post-TACE CT scan showed new, multifocal enhancing lesions not amenable to further liver-directed therapy, accompanied by an AFP rise to 1760 μg/L. The patient initiated sorafenib 400 mg two times daily for multifocal HCC, which he tolerated without complication. Four months later, CT imaging showed multifocal radiographic progression, including an increase in size of the enhancing porta hepatis mass to 5.2 cm and increasing size and enhancement of a dome lesion measuring 2.2 cm, coupled with an AFP rise to 4077 μg/L (Fig. 1). Sorafenib was discontinued due to radiographic and AFP progression.
Fig. 1.
Synchronous spontaneous radiographic tumor regression and AFP response in case 1. Timeline of spontaneous radiographic tumor regression and AFP response in case 1, a patient with HCV-associated HCC and vitiligo
Six months after discontinuing sorafenib, he presented for medical oncology evaluation to reevaluate treatment options. Unexpectedly, a repeat CT scan showed complete resolution of all previous lesions, except for one residual 7-mm enhancing liver dome lesion, and AFP had declined to 37 μg/L (Fig. 1). During this interval since cessation of sorafenib, the patient had received no treatment for HCC and took no supplements or medications with known antitumor effects. Due to the finding of spontaneous tumor regression radiographically and by AFP, systemic treatment was deferred, and a program of close surveillance was initiated. Two years later, surveillance imaging revealed multifocal hepatic enhancing lesions consistent with recurrent HCC, with a corresponding rise in AFP to 123 μg/L. The patient is now receiving treatment on a clinical trial of a novel multikinase inhibitor for advanced HCC after the failure of sorafenib. His vitiligo persists.
Case 2
A 63-year-old man with mixed HCC-cholangiocarcinoma metastatic to the lymph nodes was referred to our clinic for consideration of systemic therapy. He had been diagnosed with HCV genotype 3 20 years prior to presentation and had never received antiviral therapy. Abdominal and pelvic CT scan upon presentation revealed a 3.5-cm enhancing lesion in segment 7 of the liver, radiographically consistent with hepatocellular carcinoma. Serum AFP was elevated at 762 μg/L. He elected to defer treatment for 6 months when a repeat CT scan revealed significant progression of the mass to a size of 6.5 cm. He twice underwent TACE to the segment 7 lesion via a right hepatic artery segmental branch using cisplatin, mitomycin-C, doxorubicin, and ethiodol, with no residual enhancing tumor on follow-up imaging and an AFP decline to 23 μg/L. He again elected to delay his follow-up visit for 10 months, after which time a CT scan revealed enhancing tissue superior and medial to the treatment site, with tumor infiltration into perihepatic adipose tissue and enlarging retroperitoneal lymphadenopathy. Meanwhile, AFP rose to 91 μg/L.
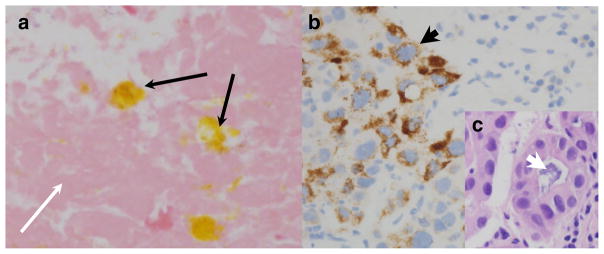
Before starting chemotherapy, a repeat CT scan to establish the extent and pace of disease progression instead showed no definitive hypervascularity in the liver lesions, along with a significant decrease in the size of retroperitoneal lymphadenopathy, 2 months after the preceding scan had showed progression. Concurrently, the serum AFP dropped spontaneously from 91 to 27 μg/L. He had not received any interval therapy including no herbal or alternative medications, with his most recent prior TACE having occurred over 1 year earlier. A fine-needle aspiration of a retroperitoneal lymph node showed bile-stained necrotic tissue with adjacent lymphocytes but no viable tumor cells (Fig. 2a). Given the appearance of spontaneous tumor regression, systemic therapy was deferred. Unfortunately, 2 months later, repeat CT imaging showed a new 2.6-cm hepatic lesion adjacent to the prior TACE site, a new 2.1-cm diaphragmatic lesion, a new adrenal gland metastasis, and increasing size of lymphadenopathy, while AFP rose to 79 μg/L. Biopsy of the adrenal gland metastasis showed mixed histology with focal areas of Hep-Par1 expression (Fig. 2b), as well as areas of mucinous gland formation consistent with mixed HCC and cholangiocarcinoma histology (Fig. 2c), prompting initiation of systemic therapy.
Fig. 2.
Spontaneous tumor necrosis in a mixed HCC and CCA tumor. a H&E staining of a lymph node biopsy at the time of spontaneous radiographic tumor regression which showed necrotic tissue (thin white arrow) with focal areas of bile pigment deposition (thin black arrows) and occasional lymphocytes (not shown), ×400 magnification. b Biopsy of adrenal metastasis in the same patient at the time of subsequent progression, with areas of focal HCC features with positive Hep-Par1 staining (thick black arrow, ×400). c An area of tumor glandular formation producing mucin (thick white arrow) on an H&E stain from the same biopsy (×400)
Case 3
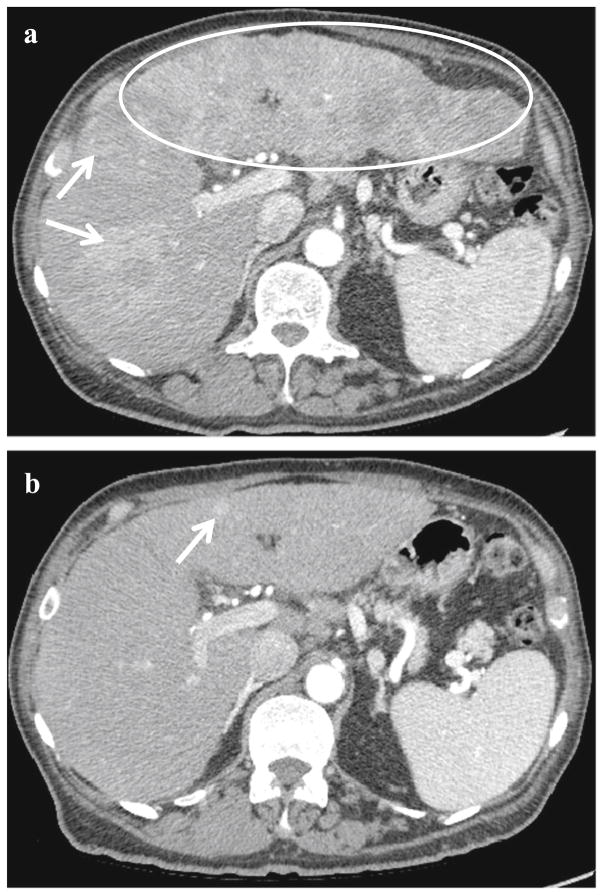
A 67-year-old male with chronic HCVand cirrhosis presented with generalized weakness, nausea, and a 20-lb weight loss. He also had eye pain diagnosed as bullous keratopathy post-cataract surgery. A CT scan showed innumerable enhancing lesions with portal venous washout consistent with multifocal HCC radiographically (Fig. 3a). An area of expansile soft tissue enhancement involving the right portal vein was consistent with tumor thrombus. AFP was 35,689 μg/L. A core biopsy of a tumor in the left lobe of the liver showed moderately differentiated HCC, along with the presence of an inflammatory infiltrate. The patient was diagnosed with advanced HCC, with recommendation for sorafenib therapy. Due to his eye pain, he elected to defer initiation of sorafenib in order to undergo vitrectomy and lens repositioning for his keratopathy. Approximately 5 months later without any interval treatment except for a cornea surgery, repeat imaging showed significant interval decrease in tumor burden, with some lesions demonstrating new fibrotic changes (Fig. 3b). Meanwhile, his AFP value had spontaneously declined to 7.8 μg/L. The patient denied any new medications including no alternative therapies, and he reported feeling much better than at presentation, with resolution of prior nausea and weakness and spontaneous weight gain. He is currently being monitored for recurrence by periodic imaging and AFP values, without any anticancer therapy.
Fig. 3.
Spontaneous radiographic tumor regression in case 3. a Innumerable confluent, hyperenhancing lesions (examples marked with white arrows) throughout both lobes of the liver at the time of diagnosis in case 3, with near-complete infiltration left lobe (white ellipse). Portal vein tumor thrombus was present. b Marked interval decrease in tumor burden with a small residual hyperenhancing focus (white arrow)
Conclusions
These three cases demonstrate that spontaneous regressions can occur in hepatobiliary cancers and may be the result of immune mechanisms. A literature review in 2009 identified 59 published case reports of spontaneous regression of HCC [11]. The clinical characteristics varied greatly among the cases, including patients with partial and complete remissions, single or multiple lesions, and with or without extrahepatic metastases. Proposed mechanisms for spontaneous regression included ischemic events such as tumor necrosis (31 %), initiation of new medications without known antiproliferative effects (28 %), and alcohol cessation (9 %). In 46 % of cases, no explanation for regression could be identified. Analogous to our first case, in which the patient’s HCC spontaneously regressed in the presence of vitiligo suggestive of active autoimmunity, 18 % had recently undergone treatments such as external beam radiation to other sites or experienced illnesses, such as infection, known to invoke an immune response. Spontaneous regression has also been reported in rare cases of cholangiocarcinoma [12, 13]. These cases further highlight the potential for an abscopal effect to occur in hepatobiliary cancers, as has been reported in other cancers susceptible to immune modulation, including melanoma [14]. Characterization of tumoral immune infiltrates, present on biopsy pathology in both cases 2 and 3, could better inform our understanding of the mechanisms of this phenomenon, though the material available in these cases was too scarce for further profiling. Tumor lymphocytic infiltration may underlie the radiographic finding of “pseudo-progression” in some cases of response to immunotherapy [15].
With the advent of novel immunotherapies in oncology, an understanding of the immunogenicity of hepatobiliary tumors and its potential link to spontaneous regression is essential to identify targets and guide the development of this new class of anticancer drugs in hepatobiliary cancers. In the phase II study of tremelimumab in 21 patients with HCV-associated HCC, Sangro et al. reported that CTLA-4 blockade with tremelimumab achieved antitumor effects, including a 76 % disease control rate and 6.4-month time to progression in patients with HCC refractory to standard therapies. Interestingly, tremelimumab therapy was also associated with antiviral activity, inducing a significant decline in HCV viral load and an increase in lymphocytes specific to HCV viral antigens [10]. In parallel, 45 % of patients experienced transient grade ≥3 transaminitis after the first tremelimumab dose, postulated to be due to the reactivation of suppressed antiviral CD4+ helper and CD8+ T cells within the HCV-infected liver. An ongoing phase I trial of nivolumab will assess the antitumor, antiviral, and immunologic effects of targeting PD-1 in HCC patients with and without viral hepatitis (NCT01658878) (Table 1). The three cases we present of spontaneous regression of hepatobiliary cancers support the existing evidence that this lethal family of cancers may be susceptible to intrinsic immune surveillance, a mechanism that could be harnessed by a new generation of immunotherapies now in development.
Acknowledgments
This work was supported by the Bili Project Foundation, Inc. KE and RKK are members of the UCSF Liver Center, supported by P30 DK026743.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
Anna L. Parks, School of Medicine, University of California, San Francisco (UCSF), 505 Parnassus Ave., San Francisco, CA 94143, USA
Ryan M. McWhirter, Helen Diller Family Comprehensive Cancer Center, UCSF, 1600 Divisadero St., Box 1770, San Francisco, CA 94143, USA
Kimberley Evason, Department of Pathology, UCSF, 505 Parnassus Ave., San Francisco, CA 94143, USA.
Robin K. Kelley, Helen Diller Family Comprehensive Cancer Center, UCSF, 1600 Divisadero St., Box 1770, San Francisco, CA 94143, USA. 550 16th St., Box 3211, San Francisco, CA 94143, USA
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Breous E, Thimme R. Potential of immunotherapy for hepatocellular carcinoma. J Hepatol. 2011;54(4):830–4. doi: 10.1016/j.jhep.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler SL, et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006;45(2):246–53. doi: 10.1016/j.jhep.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356(9232):802–7. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 8.Maio M. Melanoma as a model tumour for immuno-oncology. Ann Oncol. 2012;23(Suppl 8):viii10–4. doi: 10.1093/annonc/mds257. [DOI] [PubMed] [Google Scholar]
- 9.Scott M, Lawrance J, Dennis M. Regression of a renal cell carcinoma following allogeneic peripheral blood stem cell transplant for acute myeloid leukaemia: evidence of a graft-versus-tumour effect without significant graft-versus-host disease. Br J Haematol. 2012;159(1):1. doi: 10.1111/j.1365-2141.2012.09238.x. [DOI] [PubMed] [Google Scholar]
- 10.Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81–8. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Oquinena S, Inarrairaegui M, Vila JJ, Alegre F, Zozaya JM, Sangro B. Spontaneous regression of hepatocellular carcinoma: three case reports and a categorized review of the literature. Dig Dis Sci. 2009;54(5):1147–53. doi: 10.1007/s10620-008-0447-z. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimitsu K, Honda H, Kaneko K, Fukuya T, Irie H, Aibe H, et al. Temporary spontaneous regression of intrahepatic cholangiocarcinoma. Comput Med Imaging Graph. 1996;20(2):115–8. doi: 10.1016/0895-6111(96)00036-5. [DOI] [PubMed] [Google Scholar]
- 13.Peddu P, Huang D, Kane PA, Karani JB, Knisely AS. Vanishing liver tumours. Clin Radiol. 2008;63(3):329–39. doi: 10.1016/j.crad.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]