Abstract
Background
Task-switching deficits are common among older adults and those with insomnia. Such deficits may be driven by difficulties with sleep continuity and dampened homeostatic sleep drive.
Objective
Identify the aspects of task-switching affected by insomnia and insomnia treatment, and determine whether such effects were associated with sleep continuity and homeostatic sleep drive.
Methods
Polysomnographic sleep and task-switching were tested in healthy older adults aged 60–93 with insomnia (n=48) or normal sleeping controls (n=51). Assessments were repeated in the insomnia group after 8 weeks of cognitive behavioral treatment for insomnia. Sleep measures included wake after sleep onset (WASO) and quantitative indices of homeostatic sleep drive (delta power during non rapid-eye movement (NREM) sleep and the ratio of delta power during the first and second NREM periods). A cued task-switching paradigm instructed participants to perform one of two tasks with varying preparatory cue-target intervals, manipulating task-alternation, task-repetition, and task-preparation.
Results
The effect of preparatory cues on accuracy was diminished in the insomnia group compared to controls. Across the two groups, a stronger effect of preparatory cues was associated with a higher delta sleep ratio. Following insomnia treatment, task-repetition accuracy significantly improved. This improvement was associated with improvements in WASO. There were no group or treatment effects on response time or task alternation accuracy.
Conclusions
Effects of insomnia diagnosis and treatment apply to conditions that depend on maintenance of a task-set, rather than a domain general effect across task-switching. Such effects are associated with homeostatic sleep drive and sleep continuity.
Keywords: Task-switching, insomnia, cognitive behavioral treatment for insomnia, older adults, sleep drive, wake after sleep onset
1. Introduction
Deficits in executive functions are among the most prevalent cognitive changes observed with advancing age (Buckner, 2004; Verhaeghen, 2011; Verhaeghen & Cerella, 2002) and are also commonly found among patients with insomnia (Edinger, Means, Carney, & Krystal, 2008; Espie & Kyle, 2008; Fortier-Brochu, Beaulieu-Bonneau, Ivers, & Morin, 2012; Fulda & Schulz, 2001). For instance, a meta-analysis by Fortier-Brochu et al. (2012) found negative effects of insomnia for executive functions such as maintaining and manipulating information in working memory and problem solving, though not for verbal fluency or cognitive flexibility, suggesting that there may be domain specific effects of insomnia on executive function. Executive function deficits among older adults include difficulties selecting relevant and inhibiting irrelevant information, and maintaining and manipulating information in working memory (Gazzaley, Cooney, Rissman, & D’Esposito, 2005; Hasher & Zacks, 1988). Task-switching, a model paradigm of executive function, is often used to assess these age-related executive deficits (Verhaeghen, 2011). Older adults commonly exhibit greater difficulty with managing two tasks compared to younger adults (Mayr, 2001; Verhaeghen, 2011; Wasylyshyn, Verhaeghen, & Sliwinski, 2011; Wilckens, Woo, Kirk, Erickson, & Wheeler, 2014). However, task-switching performance may be improved when task cues are provided that allow for time to prepare on a given trial (Schapkin, Gajewski, & Freude, 2014). This “preparation effect” reflects controlled processes to maintain and reconfigure a task-set in working memory. We previously reported that in older adults not selected on the basis of sleep disorders, higher wake after sleep onset (WASO) was associated with diminished preparation effects (Wilckens, Woo, Erickson, & Wheeler, 2014). Thus, older adults with lower sleep continuity, such as those with insomnia, may be less likely to engage control processes to boost task-switching performance in the presence of preparatory cues.
Deficits in other tasks of executive function have been associated with insomnia, sleep continuity, and slow-wave sleep or homeostatic sleep drive. For instance, higher delta encephalographic (EEG) power (0.5–4.0 Hz), particularly within the first non-rapid eye movement (NREM) period, has been associated with better performance on tasks tapping onto working memory, set-shifting, and reasoning in older adults (Anderson & Horne, 2003; Wilckens, Hall, Nebes, Monk, & Buysse, 2016). Delta power is often interpreted as an indicator of homeostatic sleep drive (Fuller, Gooley, & Saper, 2006). Thus, less continuous sleep and lower homeostatic sleep drive in insomnia (Krystal, Edinger, Wohlgemuth, & Marsh, 2002; Merica, Blois, & Gaillard, 1998) may contribute to deficient executive control and in turn, affect task-switching performance.
Given the observed associations between these sleep measures and executive function, it seems plausible that improvements in sleep continuity and homeostatic sleep drive could lead to improvements in executive processes critical for task-switching in older adults. Cognitive-behavioral treatment for insomnia involves restricting time-in-bed in order to increase sleep continuity (Friedman, Bliwise, Yesavage, & Salom, 1991; Morin et al., 2006; Spielman, Saskin, & Thorpy, 1987) and sleep drive (Brunner, Dijk, Tobler, & Borbély, 1990; Espie, 2002; Pigeon & Perlis, 2006). These treatment approaches commonly lead to significant reductions in WASO, clinically meaningful improvements or remission from insomnia (Buysse, 2013; Buysse et al., 2011; Morin et al., 2006; Morin et al., 1999), and in some reports, increased NREM delta power (Cervena et al., 2004; Krystal & Edinger, 2010). However, there is limited evidence from a small number of studies to suggest that these sleep improvements are also associated with improved executive function in older adults (Altena, Van Der Werf, Strijers, & Van Someren, 2008; Miró et al., 2011).
The present report was part of a larger study assessing sleep, arousal, and circadian rhythms among older adults with and without insomnia and, in the former group, before and after Cognitive Behavioral Treatment for Insomnia (CBTI). The parent study was designed to use CBTI as a treatment probe of neurobiological model of insomnia. The first goal of the parent study was to contrast older adults with and without insomnia, and the second goal was to determine whether improved insomnia is associated with changes in neurobiological indices of insomnia. For the present report, we aimed to identify the aspects of task-switching affected by insomnia and insomnia treatment, and whether such effects are associated with sleep continuity and homeostatic sleep drive. Based on our prior finding that older adults with lower sleep continuity exhibit weaker preparation effects on accuracy during task-switching, we hypothesized that patients with insomnia would exhibit weaker preparation effects in accuracy compared with good sleeping controls. Further, we hypothesized that such deficits in preparation would be associated with reduced sleep continuity (e.g., increased WASO) and homeostatic sleep drive (NREM delta power and the ratio of delta power between the first and second NREM periods). Finally, we tested whether CBTI improved performance and whether improvements in performance were associated with improvements in sleep continuity and homeostatic sleep drive. We hypothesized that preparation effects would be strengthened following CBTI and that such improvement would be associated with decreased WASO and increased whole night delta power and delta sleep ratio.
2. Materials and Methods
2.1 Overview
Participants were adults with insomnia or normal sleeping controls recruited as part of the AgeWise program project (AG020677). The insomnia group participated in CBTI over eight weeks. Participants completed sleep and task-switching assessments at baseline (control and insomnia groups) and at follow-up at the end of the CBTI treatment (insomnia group only). Task-switching performance was assessed with a paradigm that included a block of trials in which participants were cued trial-by-trial to perform one of two tasks with a varying cue-target interval (Wilckens, Woo, Erickson, et al., 2014).
2.2 Participants
Participants were aged 60–93 and recruited from the greater Pittsburgh area. All participants provided informed consent as required by the University of Pittsburgh Institutional Review Board. Demographic and clinical characteristics for control and insomnia groups are displayed in Table 1. Participants were eligible to participate in the insomnia group if they met diagnostic criteria for general insomnia disorder according to DSM-IV/ICSD-2 and met a severity criteria including an Insomnia Severity Index (ISI) score ≥ 10, a combined mean diary-assessed sleep latency + wake after sleep onset > 40 minutes, and sleep efficiency < 90% (Levenson et al., 2013). Sleep efficiency was calculated with a denominator of “lights out” to final awakening and therefore required a higher threshold value than typically reported. This stringent definition of sleep efficiency has been shown to have greater sensitivity and specificity in distinguishing older adults with insomnia from good sleeping controls compared to sleep efficiency based on time in bed to time out of bed (Levenson et al., 2013). To ensure that current sleep problems were specific to insomnia, participants who met criteria for significant sleep apnea (apnea-hypopnea index > 20 events per hour) or restless legs syndrome (based on structured clinical interview) were excluded from the study. An apnea-hypopnea index of 20 was chosen in order to retain participants with mild to moderate sleep apnea given the distribution of apnea-hypopnea indices among older adults (Ancoli-Israel et al., 1991; Durán, Esnaola, Rubio, & Iztueta, 2001), and evidence that the mild to moderate apnea-hypopnea index range has little impact on cognitive function (Quan et al., 2014; Quan et al., 2006). Participants were eligible to participate in the control group if they did not meet criteria for a primary sleep disorder and had an ISI score < 10. Participants were not eligible for either group if they engaged in current nighttime shift work, had any untreated current severe psychiatric conditions, unstable medical conditions, or neurological disorders, including multiple sclerosis, stroke, Parkinson’s Disease, Alzheimer’s Disease, seizure disorder, delirium, dementia, or previous loss of consciousness > 24 hours. Participants with a past history of psychiatric disorders were eligible except in the case of bipolar disorder or psychosis. Participants were not taking over-the-counter sleep aids, or other medications affecting sleep such as antidepressants, antipsychotic medications, anticonvulsants, steroids, or beta blockers during the study. Participants taking hypnotics at screening were given the option to enroll if they agreed to a medically-supervised medication taper and discontinuation and were no longer taking hypnotics during the duration of the study. The complete list of medications reported at screening in each group is presented in the supplementary materials section. Participants were ineligible if they reported alcohol consumption > 14 drinks per week or > 6 at one sitting, or greater than 3 caffeine drinks per day (or 400 mg caffeine equivalent) reported in a sleep diary.
Table 1.
Demographic information and self-report and polysomnographic sleep and task-switching variables for controls at baseline (T1) and the insomnia group at baseline (T1) and at Follow-up (T2). Standard deviation or percent of sample in parentheses. Switch cost units are proportion correct for accuracy and milliseconds for response time. Polysomnographic sleep variables are presented in minutes. Sleep latency complaint and WASO complaint reflect responses on a baseline survey of sleep. For T1 in the insomnia group, standard PSG variables n = 46; delta sleep ratio n = 45.
| Control T1 (n = 51) | Insomnia T1 (n = 48) | Insomnia T2 (n =38) | |
|---|---|---|---|
| Age | 67.26 (5.16) | 69.26 (7.87) | |
| N White/Caucasian | 49 (96.1%) | 42 (87.5%) | |
| N College Degree or greater | 38 (74.5%) | 36 (75%) | |
| N Female | 33 (64.7%) | 29 (60.4%) | |
| Number of medications | 4.63 (2.75) | 6.19 (3.76) | |
| Apnea-hypopnea index | 4.87 (5.43) | 6.23 (6.29) | |
| Sleep latency complaint | 1 (2%) | 34 (70.8%) | |
| WASO complaint | 1 (2%) | 36 (75%) | |
| Insomnia Severity Index | 0.69 (1.33) | 14.67 (4.56) | 5.68 (4.48) |
| Total Sleep Time (mins) | 375.21 (46.90) | 337.12 (59.82) | 339.21 (46.934) |
| Sleep Efficiency (%) | 83.48 (8.29) | 75.76 (11.19) | 83.54 (10.61) |
| Sleep Latency (mins) | 12.59 (11.75) | 13.95 (13.96) | 10.37 (6.70) |
| WASO (mins) | 62.28 (37.65) | 95.16 (49.93) | 57.42 (44.16) |
| N1% | 7.28 (4.57) | 8.50 (5.21) | 7.28 (4.27) |
| N2% | 58.83 (9.68) | 58.60 (10.00) | 58.73 (9.92) |
| N3% | 11.47 (9.10) | 9.78 (8.41) | 10.06 (9.27) |
| Absolute Delta Power (μV2/Hz) | 24.93 (11.44) | 22.95 (10.77) | 24.60 (13.52) |
| Absolute Delta Sleep Ratio NREM 1/NREM 2 | 1.30 (0.46) | 1.17 (0.41) | 1.08 (0.38) |
Three participants (two from the control group and one from the insomnia group) with task accuracy greater than 3 standard deviations below the mean were identified as outliers. These three participants were excluded from the present analyses to ensure that all participants included understood the task. One participant was excluded due to a tremor that interfered with their ability to perform the task with both hands. The present report included fifty-one participants in the control group and forty-eight participants in the insomnia group at baseline. All of these ninety-nine participants had usable task-switch data. Two insomnia participants did not complete the baseline polysomnography study and therefore had no data for polysomnographic sleep measures. One participant’s baseline polysomnographic record had excessive artifact that precluded spectral analysis within the first two NREM periods, and therefore this participant was excluded from analyses with delta sleep ratio. Thirty-eight participants from the insomnia group returned following CBTI and completed the follow-up polysomnographic and task-switching assessments. Reasons for attrition between baseline and follow-up included withdrawal from the study due to time commitment (n = 1), changes in availability (n = 1), being no longer interested (n = 2), poor compliance with CBTI (n = 3), and hospitalization the day of the follow-up (n = 1). Two participants were present for the follow-up session, but chose not to do follow up assessments including the task-switching paradigm (n = 2). A final sample of 51 participants in the control group at baseline, 48 participants in the insomnia group at baseline, and 38 participants in the insomnia group at follow up was available for data analysis.
2.3 Intervention
CBTI was conducted by two masters’ level mental health clinicians. The treatment occurred over eight consecutive weeks with six 45–50 minute in-person sessions and two telephone sessions, which included education and instruction on healthy sleep practices, sleep restriction, stimulus control, relaxation training, cognitive restructuring and behavioral experiments regarding daytime fatigue management, and techniques to reduce worrying/thinking at night. Participants monitored their sleep with a sleep diary throughout the CBTI treatment.
2.4 Assessments and sleep measures
Laboratory-based polysomnography was recorded on a single night at the participants’ diary-assessed habitual sleep times following an adaptation night. EEG was recorded from F3, F4, C3, and C4 electrodes, referenced to A1–A2. The sleep record was visually scored in 30-second epochs using American Academy of Sleep Medicine criteria (AASM, 2007). Spectral analysis was performed on F and C EEG channels with a 512-point fast Fourier transform using epochs scored as NREM sleep (Vasko et al., 1997). Following decimation, the EEG signal was parsed into 0.5 Hz bins over 4-second epochs and weighted by a Hamming window. Four-second epochs identified as movement artifact by an automated algorithm (Brunner et al., 1996) and visual inspection were not included in the scored record. Spectral density data collapsed across the F3 and F4 channels within the 0.5–4 Hz (delta) frequency band (absolute power) was analyzed for the present report given the tendency for delta power to be highest among frontal channels. The present report focused on the following polysomnographic sleep variables: wake after sleep onset (WASO), and absolute NREM delta power assessed two ways: whole night NREM delta power, and the ratio of delta power in the first compared to the second NREM interval (delta sleep ratio). Rather than testing both absolute power and relative power across analyses, we focused on absolute power to avoid an inflated Type I error rate. This measure was chosen due to observed greater inter-individual variability of absolute power compared to relative power, which we reasoned would be better suited for relating delta power to cognitive performance. However, to confirm consistency across absolute and relative measures, for significant results involving delta power, we confirmed whether such results replicated using relative power. Absolute power was calculated by first taking the average power within each 4-second epoch, and then taking the grand average across each 4-second epoch across each 0.5 Hz bin within the 0.5–4 Hz bandwidth for each channel. Relative power was calculated by dividing the area under the curve within the 0.5–4 Hz bandwidth by the area under the curve within the 0.5–32 Hz bandwidth. The delta sleep ratio was chosen to capture homeostatic sleep drive through the time course of delta power across the first two NREM intervals which show the highest delta power and the largest change across NREM intervals (Kupfer, Frank, McEachran, & Grochocinski, 1990). WASO and whole night NREM delta power were log transformed to correct for skewed distributions. Table 1 provides means for sleep variables at baseline and follow-up.
2.5 Cognitive Task
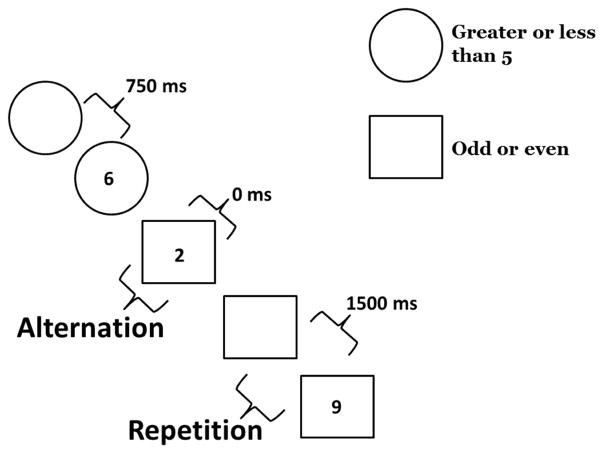
The task-switching paradigm, which has been described elsewhere (Wilckens, Woo, Erickson, et al., 2014), was performed in the afternoon between 12:00 pm and 4:00 pm. Participants performed a practice block, single task block, and switching block at baseline and follow-up. In the switching block, the cue-target interval (preparation time) varied on a trial-by-trial basis. Participants were cued on each trial to perform one of two tasks that required judgments about a single-digit number presented on the screen. For one task they judged whether the number was greater than or less than 5 (GL task). In the other task, they judged whether the number was odd or even (OE task). A circle preceding or accompanying the target number cued participants to perform the GL task. A square preceding or accompanying the target number cued participants to perform the OE task. The preparation time was either 0 ms (simultaneous cue and target), 750 ms, or 1500 ms. Participants first completed the practice block, comprised of 16 trials of each task, followed by two single-task blocks comprised of 32 trials each. Finally, participants completed the switching block. The switching block comprised a total of 96 trials: 16 trials of each cue type (GL and OE) for each of the 3 preparation conditions were presented randomly in the switching block with 8 of each correct response type (greater than, less than, odd, even). Thus, successive trials within this block could be classified as “task repetition” or “task alternation” depending on whether successive trials used the same task. An example sequence of trials within the switching block is displayed in Figure 1.
Figure 1.
Task-switching paradigm adapted from Wilckens et al. (2014): Example sequence of trials including task alternations, task repetitions, and each preparation condition (0 ms, 750 ms, and 1500 ms preparation interval)
This task-switching paradigm was used to assess accuracy and response time on task alternation and repetition trials as a function of preparation time. The 750 and 1500 ms preparation conditions were highly correlated across all conditions and time points in accuracy and response time. Thus to facilitate interpretation, we collapsed across these two conditions. This approach was further justified based on the fact that these two conditions both represent an opportunity to prepare for the current task relative to the 0 ms preparation condition and based on evidence that the greatest preparation benefits among older adults tend to occur within 750 ms of preparation time or less (Kray, 2006). Accordingly, we report performance with and without time to prepare (preparation+ and preparation−). Task-switching performance for each group and time point are displayed in Table 2.
Table 2.
Task-switching performance for control and insomnia groups
| Controls | Accuracy (proportion correct) | Response Time (ms) | ||
|---|---|---|---|---|
| Prep− | Prep+ | Prep− | Prep+ | |
| Alternation | 0.61 (0.15) | 0.80 (0.11) | 867.67 (212.20) | 616.82 (125.47) |
| Repetition | 0.73 (0.15) | 0.83 (0.12) | 782.16 (152.93) | 595.40 (108.94) |
| Insomnia T1 | Accuracy | Response Time | ||
| Prep− | Prep+ | Prep− | Prep+ | |
| Alternation | 0.61 (0.15) | 0.76 (0.11) | 814.71 (174.53) | 614.95 (130.94) |
| Repetition | 0.75 (0.13) | 0.80 (0.15) | 760.38 (144.57) | 588.59 (101.77) |
| Insomnia T2 | Accuracy | Response Time | ||
| Prep− | Prep+ | Prep− | Prep+ | |
| Alternation | 0.58 (0.16) | 0.80 (0.11) | 816.44 (237.50) | 621.49 (160.66) |
| Repetition | 0.80 (0.11) | 0.87 (0.09) | 739.00 (158.58) | 583.47 (120.16) |
2.6 Statistical analyses
Primary analyses tested effects of patient group (control versus insomnia) and time point (baseline versus follow-up) on switching and preparation for accuracy and response time. Follow-up analyses tested whether WASO, NREM delta power, and delta sleep ratio were associated with conditions found to have a significant effect of insomnia group or treatment. Ancillary analyses tested the effect of time point on each sleep variable of interest using paired t-tests to confirm which sleep variables showed significant changes after CBTI. Results are reported with an uncorrected alpha level of 0.05, as well as Bonferroni-corrected alpha levels. A corrected alpha level of 0.025 was used for each ANOVA to account for separate tests of accuracy and response time. A corrected alpha level of 0.017 was used for each follow-up correlation analysis and each ancillary paired t-test to account for separate tests with each of the three sleep variables.
Effects of patient type and time point were tested with repeated measures ANCOVA including interactions with switch condition (alternation vs repetition) and preparation condition (preparation− or preparation+). Switch condition and preparation condition were included as repeated factors. For baseline analyses, patient type was included as a between subjects factor. For time point analyses in the insomnia group, time point was included as a repeated measure.
Linear regression analyses across control and insomnia groups were used to test the association between sleep variables of interest and task-switching conditions found to be affected by insomnia. Similarly, linear regression was used to test whether improvements in WASO, delta power and the delta sleep ratio explained improvements following CBTI. Between baseline and follow-up, change scores were calculated to test the association between changes in sleep and changes in performance. Change scores were calculated for each sleep variable of interest. Change scores in performance were only calculated for conditions that showed significant effects of time point, to focus on explaining significant effects of insomnia treatment with each sleep variable of interest.
2.7 Covariates
Age was included as a covariate in patient type analyses to account for slight age differences in the two groups. Age and sex were included as covariates in time point and regression analyses, to account variation associated with these demographic factors and sleep variables as well as changes in cognitive performance. Sensitivity analyses at baseline included apnea-hypopnea index as a covariate to determine whether the effects of insomnia or relationships with sleep variables were affected by sleep apnea. Apnea-hypopnea index data were available for 44 participants in the control group and 46 participants in the insomnia group.
3. Results
3.1 Effects of patient type (control vs. insomnia)
Task switching performance in each group is displayed in Table 2. Main effects of patient type and interactions with switch condition and preparation condition for baseline performance are displayed in Table 3. There were significant main effects of preparation and switch condition on accuracy and response time, as would be expected with the current task. There was a significant patient type × preparation interaction in accuracy (Figure 2), significant at corrected α = 0.025: the control group was disproportionately more accurate in the preparation+ condition compared to the insomnia group, Cohen’s d = 0.51. This effect remained significant after controlling for apnea-hypopnea index, p = 0.027. For response time, there were no significant effects of patient type, nor did patient type interact significantly with switch condition (all p > 0.13).
Table 3.
Repeated measures analysis of main effects of patient type and interactions with switching and preparation (prep).
| F | df | p | ||
|---|---|---|---|---|
| Accuracy | Patient Type | 0.58 | 1, 96 | 0.450 |
| Prep | 176.90 | 1, 96 | <0.001* | |
| Switch | 45.26 | 1, 96 | <0.001* | |
| Patient Type × Prep | 6.19 | 1, 96 | 0.015* | |
| Patient Type × Switch | 0.21 | 1, 96 | 0.648 | |
| Patient Type × Prep × Switch | 0.06 | 1, 96 | 0.802 | |
| Response Time | Patient Type | 0.77 | 1, 96 | 0.383 |
| Prep | 347.13 | 1, 96 | <0.001* | |
| Switch | 36.22 | 1, 96 | <0.001* | |
| Patient Type × Prep | 2.30 | 1, 96 | 0.133 | |
| Patient Type × Switch | 0.70 | 1, 96 | 0.404 | |
| Patient Type × Prep × Switch | 1.85 | 1, 96 | 0.177 |
denotes significance uncorrected and corrected.
Figure 2.
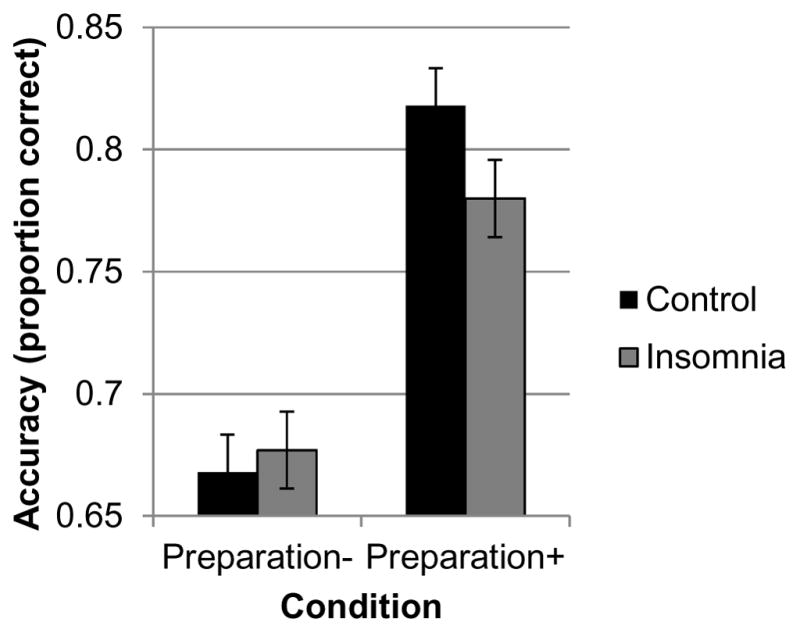
Patient type × preparation condition interaction. The preparation effect was greater in the control group compared with the insomnia group, with controls exhibiting a greater increase in accuracy in the preparation+ condition.
3.2 Baseline relationships with sleep variables
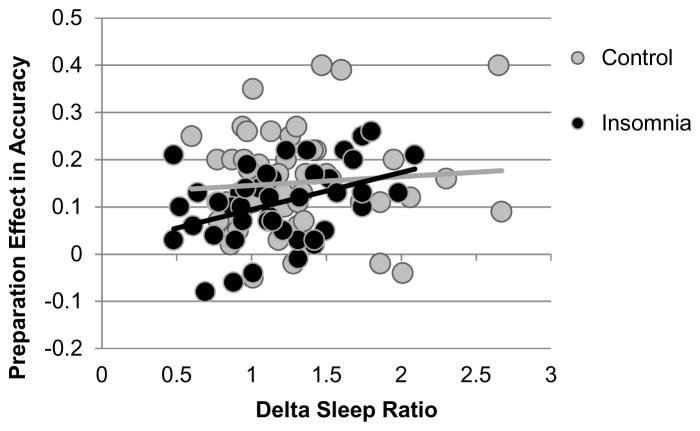
Linear regression analyses tested the associations between the preparation effect in accuracy and sleep variables across the control and insomnia groups. Delta sleep ratio was the only sleep variable significantly associated with the preparation effect, R2 change = 0.045, β = 0.22, t = 2.13, uncorrected p = 0.035 (Figure 3), not significant at corrected α = 0.017. This association replicated using relative power, R2 change = 0.044, β = 0.21, t = 2.12, p = 0.037. The significant uncorrected effect of patient type no longer reached significance after accounting for WASO (p = 0.053) or the delta sleep ratio (p = 0.076), but remained significant after accounting for delta power, p = 0.046).
Figure 3.
Across control and insomnia groups, the delta sleep ratio is associated with a preparation effect in accuracy. Preparation effect reflects the difference between preparation+ and preparation− conditions collapsed across alternation and repetition trials.
3.3 Effects of CBTI on sleep and task-switching in insomnia
WASO showed a significant main effect of time point, indicating improvement following CBTI, t(37) = 6.30, p < 0.001, significant at corrected α = 0.017. There was no treatment-related change in absolute delta power, t(37) = −0.65, p = 0.52, or delta sleep ratio, t(37) = 1.50, p = 0.14 (Table 1).
Task-switching performance at baseline and follow-up is displayed in Table 2 and effects of time point are displayed in Table 4. There was a marginally significant main effect of time point on accuracy and a significant switch condition × time point interaction, not significant at corrected α = 0.025. This interaction reflected a greater improvement in repetition trial accuracy compared to alternation trial accuracy (Figure 4) across preparation conditions. Preparation condition did not significantly interact with time point, suggesting no significant improvement in the preparation effect following CBTI.
Table 4.
Repeated measures analysis of main effects of time point on accuracy and response time and interactions with switch and preparation conditions in the insomnia group.
| F | df | p | ||
|---|---|---|---|---|
| Accuracy | Time | 3.45 | 1, 35 | 0.072† |
| Time × Prep | 1.80 | 1, 35 | 0.188 | |
| Time × Switch | 4.80 | 1, 35 | 0.035* | |
| Time × Prep × Switch | 1.66 | 1, 35 | 0.206 | |
| Response Time | Time | 0.77 | 1, 35 | 0.387 |
| Time × Prep | 1.42 | 1, 35 | 0.241 | |
| Time × Switch | 0.61 | 1, 35 | 0.440 | |
| Time × Prep × Switch | 0.14 | 1, 35 | 0.712 |
denotes uncorrected significance,
denotes marginal uncorrected significance.
Figure 4.
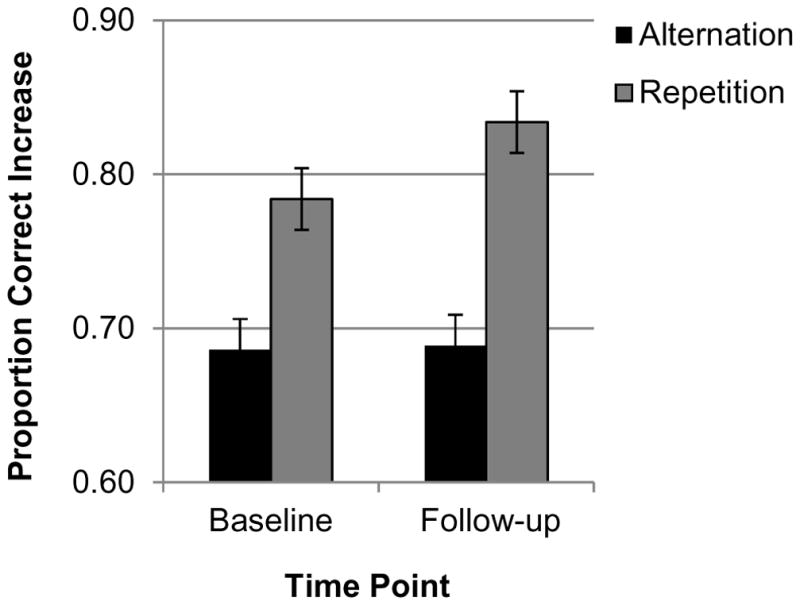
Time point × switch condition interaction (insomnia group). The improvement in accuracy was greater in the task repetition condition.
Linear regression analyses with change scores revealed that reductions in WASO were significantly associated with improvements in repetition trial accuracy, R2 change = 0.13, β = 0.372, t = 2.51, p = 0.017, marginally significant at corrected α = 0.017 as displayed in Figure 5. The association between increases in whole night delta power, the delta sleep ratio and repetition accuracy improvement did not reach significance (delta power R2 change = 0.03, β = 0.191, t = 1.14, p = 0.262; delta sleep ratio R2 change = 0.027, β = 0.17, t = 1.06, p = 0.295).
Figure 5.
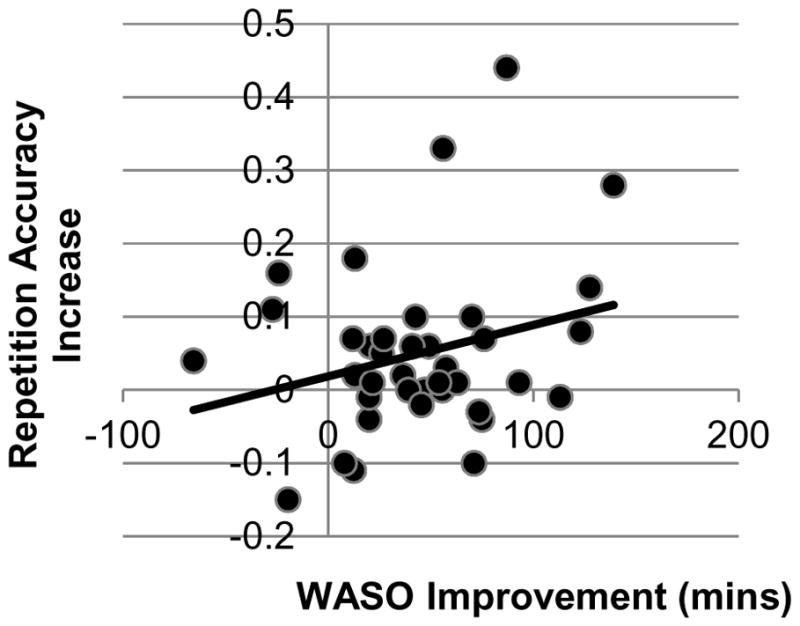
In the insomnia group, improved repetition accuracy was associated with reduced WASO.
4. Discussion
4.1 Effects of insomnia
There is growing consensus that age-related changes in sleep may contribute to deficits in goal-driven executive functions such as task-switching. Here, we used a task-switching paradigm which varied preparation time to test whether insomnia and insomnia treatment affect specific aspects of task-switching. Although overall task-switching performance was similar between control and insomnia groups, preparation effects were diminished in the insomnia group relative to good sleepers. Participants with insomnia showed less of a benefit in accuracy from time to prepare compared to controls. This finding is consistent with our previous work in a separate sample (Wilckens, Woo, Kirk, et al., 2014) showing that greater WASO was associated with a diminished preparation effect in older adults who were not selected on the basis of sleep problems. Broadly, this result suggests that normal sleepers more effectively made use of preparatory cues to adopt a task-set in light of switching demands. Effective use of preparatory cues is accomplished through the engagement of control processes during the cue-target interval to support the flexible and rapid task-set reconfiguration process (Gruber, Karch, Schlueter, Falkai, & Goschke, 2006). This effect applied across switch conditions, and switch condition did not interact with insomnia group, suggesting that it is not necessarily the “most challenging” task conditions that are most affected in insomnia.
4.2 Baseline associations with sleep continuity and homeostatic sleep drive
Partially confirming our hypotheses, across patients and control participants, a stronger preparation effect was associated with a higher homeostatic sleep drive assessed with delta sleep ratio. The delta sleep ratio represents the ratio of delta power between the first two NREM periods thus highlighting the diminishing homeostatic sleep drive between the two first NREM periods. This finding is broadly consistent with prior studies (Anderson & Horne, 2003; Scullin, 2013; Wilckens et al., 2016): Anderson and Horne found that slow-wave activity within the first NREM interval was associated with set-shifting. The current finding extends the existing literature by demonstrating that delta sleep ratio is associated with components of task-switching that depend on maintenance of a task-set in working memory. Nonetheless, whether this association is driven by working memory has yet to be determined.
Contrary to our hypotheses, WASO and whole night delta power were not significantly associated with the preparation effect. Although there are a number of existing studies demonstrating a relationship between WASO and executive functions, including switching abilities (Blackwell et al., 2006; Bonnet, 1993; Miyata et al., 2013; Nair et al., 2011; Nebes, Buysse, Halligan, Houck, & Monk, 2009; Wilckens et al., 2016; Wilckens, Woo, Erickson, et al., 2014; Wilckens, Woo, Kirk, et al., 2014), we only tested baseline associations with the preparation effect in accuracy. Thus, the present results simply demonstrate that WASO and whole night delta power are not associated with the preparation effect. Wilckens et al. (2016) found that delta power across the whole night was associated with working memory and abstract reasoning. However, that study did not examine delta sleep ratio, thus the robustness of the association between the delta sleep ratio and the preparation effect or other aspects of task-switching remain to be determined.
4.3 Effects of time point and associations with sleep continuity and homeostatic sleep drive
From baseline to follow-up within the insomnia group, a significant interaction between time point and switch condition reflected a greater improvement in repetition trial accuracy compared to alternation trial accuracy. The improvement in repetition trial accuracy was associated with improvements in WASO. This is the first demonstration that improvements in a common symptom of insomnia following insomnia treatment are associated with significantly improved cognitive performance. In contrast to alternation accuracy, which reflects the inhibition and selection processes required for trial-by-trial switching, repetition accuracy reflects the working memory processes relevant to maintaining two tasks throughout the task block. Similar to this finding, Wilckens et al. (2014) found that actigraphically-measured WASO in older adults was associated with repetition trial response time when compared to single task performance, but not alternation trial performance. These findings converge to suggest that sleep continuity may be important for working memory processes required for maintaining a task-set (Monsell, 2003; Wasylyshyn et al., 2011). Additionally, given that working memory processes are considered responsible for both preparation effects and repetition trial performance, our results broadly suggest that working memory processes involved in task-switching may drive associations with insomnia, WASO, and homeostatic sleep drive.
In contrast to our prior findings (Wilckens et al., 2016), changes in whole night delta power were not associated with changes in performance. One explanation for this discrepancy is that specific aspects of executive function not tested here are benefitted by whole night delta power. However, our prior study (Wilckens et al., 2016) involved a limited time frame with brief behavioral treatment for insomnia (a 4-week follow-up as opposed to 8 weeks here). While neither study found significant increases in delta power following behavioral insomnia treatment, delta power tends to remain stable from night to night (Israel et al., 2012), and therefore, it may be challenging to capture changes in delta power with a long follow-up interval, compared to what is observed with acute sleep restriction (Onen, Alloui, Gross, Eschallier, & Dubray, 2001). Further, single-channel EEG, as used here, may not be sensitive enough to capture important characteristics of delta power relevant to executive function. Future work using high-density EEG should examine more acute temporospatial effects of increased delta power on performance to evaluate the relevance of slow-wave sleep measures to executive deficits in older adults with sleep difficulties.
In the present study, the lack of consistency should be noted between baseline and follow-up in the cognitive conditions found to interact with patient type and time point. As a result, baseline association and change score association analyses cannot be directly compared and should be interpreted with caution. In line with our plan to test whether significant effects of patient type and time point were driven by sleep variables of interest, we did not test the association between baseline WASO and repetition trial accuracy. This approach allowed us to determine the sleep features that may explain effects of insomnia and treatment. However, it is possible that these sleep features are related to other aspects of task-switching at baseline not tested here. Indeed, our prior research demonstrates that WASO is cross-sectionally associated with multiple domains of executive function in older adults including preparation effects (Wilckens, Woo, Erickson, et al., 2014). Thus, it is important for future work to test differential effects of sleep continuity and homeostatic sleep drive on multiple domains of executive function in older adults.
4.4 Limitations and future directions
Limitations of the current study are that the control group did not complete follow-up sleep or cognitive assessments, there was no separate group of untreated participants with insomnia to serve as an intervention control, and only a subset of participants with insomnia returned for follow-up assessments (38 out of 48). These limitations make it difficult to confirm that sleep improvements, rather than practice or learning effects, caused changes in repetition trial accuracy in the insomnia group at follow-up. Learning curve effects may have influenced cognitive improvements and habituation to the sleep environment may have influenced sleep improvements. For instance, although WASO was similar between controls at baseline and insomnia at follow-up (Table 1), some of this improvement in the insomnia group may be due to habituation to the sleep laboratory environment. Thus, although participants did participate in an adaptation night before the baseline visit, we cannot be certain that participants with insomnia improved to near-control level polysomnography-assessed WASO as a result of CBTI. Future studies should assess both groups at baseline and follow-up and test randomized control trials with an insomnia intervention control group to disentangle learning effects from treatment-related cognitive improvements and habituation effects from improved sleep.
Although the current task taps multiple dimensions of executive function, including preparation, working memory, and inhibition, only one task was assessed in the present study. To better understand the domains of executive function affected by insomnia diagnosis and treatment, future work should assess cognition using multiple similarly demanding tasks. This will further address the domains of executive function that are diminished in older adults with insomnia, resulting in the effects on task-switching found here.
Finally, it should be noted that the baseline association with the delta sleep ratio and the interaction between time point and switch condition did not survive the Bonferroni correction for multiple comparisons. Thus future work using larger sample sizes are needed to confirm the robustness of these effects.
4.3 Conclusions
In a task-switching paradigm, insomnia and lower homeostatic sleep drive are associated with diminished preparation effects in accuracy. Following CBTI, improvements in sleep continuity are associated with improved accuracy on repetition trials that depend on maintenance of a task-set.
Supplementary Material
Highlights.
Task-switching and polysomnographic sleep was assessed in older adults with and without insomnia.
Older adults with insomnia showed diminished task-switching benefits from preparation time compared to controls.
The task-switching benefit from preparation time was associated with the delta sleep ratio.
Following cognitive behavioral treatment for insomnia in the insomnia group, repetition trial accuracy improved and this improvement was associated with wake time after sleep onset
Acknowledgments
The authors thank Mary Fletcher, Erica Pais, Bonnee Wettlaufer, Brian Allison, and Zachary Chakan for assistance with recruitment, data management and collection. This work was supported by the National Institutes of Health grants MH019986, AG020677, and AG049879. Infrastructure support was provided by UL1 TR000005 and UL1 RR024153.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altena E, Van Der Werf YD, Strijers RL, Van Someren EJ. Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. Journal of Sleep Research. 2008;17(3):335–343. doi: 10.1111/j.1365-2869.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C, Horne JA. Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology. 2003;40(3):349–357. doi: 10.1111/1469-8986.00038. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, … Stone KL. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. The Journals of Gerontology: Series A. 2006;61(4):405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- Bonnet M. Cognitive effects of sleep and sleep fragmentation. Sleep: Journal of Sleep Research & Sleep Medicine. 1993 doi: 10.1093/sleep/16.suppl_8.s65. [DOI] [PubMed] [Google Scholar]
- Brunner DP, Dijk DJ, Tobler I, Borbély AA. Effect of partial sleep deprivation on sleep stages and EEG power spectra: evidence for non-REM and REM sleep homeostasis. Electroencephalography and clinical neurophysiology. 1990;75(6):492–499. doi: 10.1016/0013-4694(90)90136-8. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2013;37(1):9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, … Monk TH. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Archives of Internal Medicine. 2011;171(10):887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervena K, Dauvilliers Y, Espa F, Touchon J, Matousek M, Billiard M, Besset A. Effect of cognitive behavioural therapy for insomnia on sleep architecture and sleep EEG power spectra in psychophysiological insomnia. Journal of Sleep Research. 2004;13(4):385–393. doi: 10.1111/j.1365-2869.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- Durán J, Esnaola S, Rubio R, Iztueta Á. Obstructive sleep apnea–hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. American journal of respiratory and critical care medicine. 2001;163(3):685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights’ sleep among individuals with primary insomnia. Sleep. 2008;31(5):599. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annual Review of Psychology. 2002;53(1):215–243. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- Espie CA, Kyle SD. Towards an Improved Neuropsychology of Poor Sleep? Sleep. 2008;31(5):591–592. doi: 10.1093/sleep/31.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier-Brochu É, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: A meta-analysis. Sleep Medicine Reviews. 2012;16(1):83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Friedman L, Bliwise DL, Yesavage JA, Salom SR. A preliminary study comparing sleep restriction and relaxation treatments for insomnia in older adults. Journal of gerontology. 1991;46(1):P1–P8. doi: 10.1093/geronj/46.1.p1. [DOI] [PubMed] [Google Scholar]
- Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Medicine Reviews. 2001;5(6):423–445. doi: 10.1053/smrv.2001.0157. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. Journal of biological rhythms. 2006;21(6):482–493. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8(10):1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gruber O, Karch S, Schlueter E, Falkai P, Goschke T. Neural mechanisms of advance preparation in task switching. Neuroimage. 2006;31(2):887–895. doi: 10.1016/j.neuroimage.2005.12.043. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. I. G. G. B, editor. The psychology of learning and motivation. San Diego, CA: Academic Press; 1988. Working memory, comprehension, and aging: A review and a new view. [Google Scholar]
- Israel B, Buysse DJ, Krafty RT, Begley A, Miewald J, Hall M. Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: for some measures, one night is enough. Sleep. 2012;35(9):1285. doi: 10.5665/sleep.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kray J. Task-set switching under cue-based versus memory-based switching conditions in younger and older adults. Brain Research. 2006;1105(1):83–92. doi: 10.1016/j.brainres.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD. Sleep EEG predictors and correlates of the response to cognitive behavioral therapy for insomnia. Sleep. 2010;33(5):669. doi: 10.1093/sleep/33.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25(6):630–640. [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, McEachran AB, Grochocinski VJ. Delta sleep ratio: a biological correlate of early recurrence in unipolar affective disorder. Archives of General Psychiatry. 1990;47(12):1100–1105. doi: 10.1001/archpsyc.1990.01810240020004. [DOI] [PubMed] [Google Scholar]
- Levenson JC, Troxel WM, Begley A, Hall M, Germain A, Monk TH, Buysse DJ. A quantitative approach to distinguishing older adults with insomnia from good sleeper controls. Journal of Clinical Sleep Medicine. 2013;9(2):125–131. doi: 10.5664/jcsm.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U. Age differences in the selection of mental sets: the role of inhibition, stimulus ambiguity, and response-set overlap. Psychology & Aging. 2001;16(1):96–109. doi: 10.1037/0882-7974.16.1.96. [DOI] [PubMed] [Google Scholar]
- Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. European Journal of Neuroscience. 1998;10(5):1826–1834. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Miró E, Lupiáñez J, Martínez MP, Sánchez AI, Díaz-Piedra C, Guzmán MA, Buela-Casal G. Cognitive-behavioral therapy for insomnia improves attentional function in fibromyalgia syndrome: A pilot, randomized controlled trial. Journal of Health Psychology. 2011;16(5):770–782. doi: 10.1177/1359105310390544. [DOI] [PubMed] [Google Scholar]
- Miyata S, Noda A, Iwamoto K, Kawano N, Okuda M, Ozaki N. Poor sleep quality impairs cognitive performance in older adults. Journal of Sleep Research. 2013;22(5):535–541. doi: 10.1111/jsr.12054. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Science. 2003;7(3):134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998–2004) SLEEP-NEW YORK THEN WESTCHESTER- 2006;29(11):1398. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep. 1999;22(8):1134–1156. doi: 10.1093/sleep/22.8.1134. [DOI] [PubMed] [Google Scholar]
- Nair D, Zhang SX, Ramesh V, Hakim F, Kaushal N, Wang Y, Gozal D. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase–dependent pathways in mouse. American journal of respiratory and critical care medicine. 2011;184(11):1305–1312. doi: 10.1164/rccm.201107-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2009;64(2):180–187. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. Journal of Sleep Research. 2001;10(1):35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- Pigeon WR, Perlis ML. Sleep homeostasis in primary insomnia. Sleep Medicine Reviews. 2006;10(4):247–254. doi: 10.1016/j.smrv.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Quan SF, Budhiraja R, Batool-Anwar S, Gottlieb DJ, Eichling P, Patel S, … Kushida CA. Lack of impact of mild obstructive sleep apnea on sleepiness, mood and quality of life. Southwest Journal of Pulmonary & Critical Care. 2014;9(1):44. doi: 10.13175/swjpcc082-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan SF, Wright R, Baldwin CM, Kaemingk KL, Goodwin JL, Kuo TF, … Bootzin RR. Obstructive sleep apnea–hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Medicine. 2006;7(6):498–507. doi: 10.1016/j.sleep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Schapkin SA, Gajewski PD, Freude G. Age differences in memory-based task switching with and without cues: An ERP study. Journal of Psychophysiology. 2014;28(3):187. [Google Scholar]
- Scullin MK. Sleep, memory, and aging: The link between slow-wave sleep and episodic memory changes from younger to older adults. Psychology and aging. 2013;28(1):105. doi: 10.1037/a0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep: Journal of Sleep Research & Sleep Medicine. 1987 [PubMed] [Google Scholar]
- Verhaeghen P. Aging and executive control: reports of a demise greatly exaggerated. Current Directions in Psychological Science. 2011;20(3):174–180. doi: 10.1177/0963721411408772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention: a review of meta-analyses. Neuroscience & Biobehavioral Reviews. 2002;26(7):849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Wasylyshyn C, Verhaeghen P, Sliwinski MJ. Aging and task switching: a meta-analysis. Psychology & Aging. 2011;26(1):15–20. doi: 10.1037/a0020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Hall MH, Nebes RD, Monk TH, Buysse DJ. Changes in Cognitive Performance Are Associated with Changes in Sleep in Older Adults With Insomnia. Behavioral Sleep Medicine. 2016;14:1–16. doi: 10.1080/15402002.2014.1002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Woo SG, Erickson KI, Wheeler ME. Sleep continuity and total sleep time are associated with task-switching and preparation in young and older adults. Journal of Sleep Research. 2014;23(5):508–516. doi: 10.1111/jsr.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Woo SG, Kirk AR, Erickson KI, Wheeler ME. The role of sleep continuity and total sleep time in executive function across the adult lifespan. Psychology & Aging. 2014;29(3) doi: 10.1037/a0037234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.