Abstract
Objective
To examine mental health conditions among hospitalized individuals with Parkinson disease in the United States.
Methods
Serial cross sectional study of hospitalizations of individuals ages ≥60 identified in the Nationwide Inpatient Sample dataset from 2000–2010. We identified all hospitalizations with a diagnosis of PD, alcohol abuse, anxiety, bipolar disorder, depression, impulse control disorders, mania, psychosis, substance abuse and attempted suicide/suicidal ideation. National estimates of each mental health condition were compared between hospitalized individuals with and without PD. Hierarchical logistic regression models determined which inpatient mental health diagnoses were associated with PD, adjusting for demographic, payer, geographic and hospital characteristics.
Results
We identified 3,918,703 mental health and substance abuse hospitalizations. Of these, 2.8% (n=104, 437) involved a person also diagnosed with PD. The majority of MHSA patients were white (86.9% of PD versus 83.3% of non-PD). Women were more common than men in both groups: (male: female prevalence ratio- PD: 0.78, 0.78–0.79, non-PD: 0.58, 0.57–0.58).
Depression (AOR 1.32, 1.31–1.34), psychosis (AOR: 1.25, 1.15–1.33), bipolar disorder (AOR 2.74, 2.69–2.79), impulse control disorders (AOR: 1.51, 1.31–1.75), and mania (AOR 1.43, 1.18–1.74) were more likely among PD patients, alcohol abuse was less likely (AOR: 0.26, 0.25–0.27). We found no PD-associated difference in suicide related care.
Conclusions
Parkinson disease patients have unique patterns of acute care for mental health and substance abuse. Research is needed to guide PD treatment in individuals with pre-existing psychiatric illnesses, determine cross provider reliability of psychiatric diagnoses in PD patients, and inform efforts to improve psychiatric outcomes.
Keywords: Parkinson Disease, Suicide, Psychiatry, Addiction, Epidemiology
Introduction
Parkinson disease (PD) is a common neurodegenerative disease that is diagnosed in 2% of older adults in the United States.(1) PD is associated with psychiatric disorders such as depression, anxiety and psychosis, which can occur as part of the disease processes or as a treatment side effect.(2, 3) Additionally, PD patients may be at higher risk of impulse control disorders (ICDs; compulsive gambling, buying, sexual and eating behaviors) and dopamine dysregulation syndrome (DDS; compulsive PD medication use) (4), and these disorders have been described as a side effect of all classes of dopaminergic medications used to treat the motor symptoms of PD.(5)
Current epidemiology studies of psychiatric illness in PD primarily contain data from neuropsychiatric research instruments administered to academic center or clinical trial populations. Most studies focus on the prevalence of psychiatric conditions or symptoms, draw conclusions based on populations of 10 to 200, with the largest study to date examining 423 de novo patients enrolled in a specialty center observational study. (6) (7) This approach provides valuable granular detail on psychiatric symptoms and illness in PD, at the expense of offering perspectives on psychiatric illness in female, Asian, Black or Hispanic individuals with PD, because these groups are underrepresented in specialty center and clinical trial populations. Current data on health care utilization associated with psychiatric illness have focused on the U.S. veteran population. A national VA database study found veterans diagnosed with PD and depression were more likely to have other medical diagnoses, (such as stroke, congestive heart failure, diabetes, chronic obstructive pulmonary disease) were more likely to have medical (OR = 1.34, 1.25–1.44) and psychiatric hospitalizations (OR = 2.14, 1.83–2.51), and had more outpatient visits than PD patients without a depression diagnosis.(8) Two regional VA studies also found increased outpatient health care use and greater comorbid burden among veterans with a recorded mental illness diagnosis. (9, 10)
Although there are numerous studies of mood disorders in PD, the data on suicide and PD consist largely of individual case reports of suicidal attempts (11–13) and question whether dopaminergic intoxication/withdrawal or deep brain stimulation (DBS) surgery (14, 15) are contributing factors. Two academic center based studies separately reported that suicidal ideation or attempts in PD patients correlated with measures of depression and anxiety. (16, 17) It is unclear whether the increased depression and anxiety experienced by PD patients translates into a greater burden of health care utilization for suicide attempts/ideations.
Current PD care guidelines endorse outpatient screening for symptoms and signs of depression, anxiety, impulse control disorders and psychosis. (18) Utilization data on acute care for psychiatric emergencies in the PD population would provide the necessary foundation for evaluations of the effectiveness of such guidelines and may generate hypotheses about the community level burden of highly relevant but less studied mental health conditions (e.g., substance abuse, suicide attempt).
The aim of this study was to investigate the patterns of acute care for Mental Health and Substance Abuse (MHSA) conditions among individuals diagnosed with PD. We used data from the Nationwide Inpatient Sample (NIS) Database, which contains detailed patient, clinical, hospital, and payer data from hospital discharges from 44 states to characterize and compare psychiatric hospitalizations of persons with and without PD. Knowing the patterns of severe psychiatric illness in PD would increase public and clinician understanding of disabling aspects this disease, as well as provide new potential targets for preventative strategies.
Methods
This study was approved by the institutional review board at the University of Pennsylvania.
Study Dataset
The National Inpatient Sample is the largest all-payer inpatient care health care utilization database in the United States and contains data from approximately 8 million hospital stays each year. The hospital universe that the NIS draws from consists of community hospitals, as defined by the American Hospital Association (AHA), excluding rehabilitation hospitals. The AHA defines a community hospital as a “nonfederal short term general and other specialty hospitals, excluding hospital units of institutions.” Veteran Hospitals and other federal hospitals are excluded. Hospitals are stratified by census region (Northeast, Midwest, West, South), location (rural, urban) then progressively by teaching status (teaching, non-teaching), bed size category (small, medium, large) and ownership (public, private not-for-profit, proprietary). A random sample of 20% hospitals in each stratum is drawn, and all discharges from the sampled hospitals are included in the NIS. Sample weights and statistical programs that account for the stratification and survey design are provided to allow researchers to calculate national estimates and confidence intervals, such that researchers can expect that the NIS data collection process will not impact study results.
Study Population
The study population consisted of adults ages 60 and above who were hospitalized at a NIS community hospital between January 1, 2000 and December 31, 2010. We chose to limit this study to older adults to capture the population most at risk for Parkinson Disease, and to minimize the contributions of age related variability in psychiatric illness, such as increased risk of schizophrenia diagnosis in individuals age 20–29, and distinct psychiatric comorbidity and health utilization patterns in younger PD patients.(19) We excluded individuals diagnosed with secondary or drug induced parkinsonism to limit the impact of coding error. Persons with PD were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 332 (Parkinson’s disease) or 332.0 (paralysis agitans). Individuals without a PD diagnosis were designated as controls. We extracted patient characteristics (age, sex, race, age, primary insurance/expected payer), and all recorded inpatient diagnoses. Hospital characteristics available to us included hospital size (defined in the NIS using the number of inpatient beds and categorized as small, medium, or large), teaching status and hospital location (rural/urban).
Our primary event of interest was hospital care for mental health or substance abuse conditions, identified using HCUP Mental Health Substance Abuse Clinical Classification Software (CCS-MHSA).(20) We identified hospitalizations with a primary discharge diagnosis of alcohol abuse, anxiety, bipolar disorder, depression, impulse control disorders, mania, psychosis, psychotic depression, substance abuse, and attempted suicide/suicidal ideation.
Statistical Analyses
The NIS is a 20% stratified probability sample of hospital admissions; therefore, stratification clustering and survey weights are required to calculate national estimates of particular diagnoses. The weighted proportions of each mental health/substance abuse condition were compared between inpatients with and without PD. Demographic and hospital characteristic variables were compared by PD diagnosis status using a Pearson chi-square or Mann Whitney U test. Logistic regression models were built to compare the odds of each MHSA diagnosis in PD versus the general inpatient population, and examine the associations of individual characteristics (race, age, sex) with MHSA diagnosis. Our models adjusted for payer (Medicare, Medicaid, private, Health Maintenance Organization, or self-pay), admission type (emergent, elective) and hospital teaching status (teaching, non-teaching). Several states in the NIS withhold race data (21); these states were excluded from race/sex analyses.
We performed several sensitivity analyses, considering that coding accuracy and bias can affect claims based studies. We allowed a MSHA diagnosis in any position (not only the primary diagnosis), performed analyses stratified by hospital teaching status, payer type. We also repeated our analyses in the subset of NIS hospitals which are also designated as primary stroke centers, which are more likely to have subspecialty care. All statistical analyses were performed using SAS™ version 9.3 software.
Results
Demographic characteristics
We identified 3,918,703 qualifying hospitalizations for mental health or substance abuse 2000–2010 NIS data. Of these, 2.8% (n=104,437) involved a person also diagnosed with PD. The majority of MHSA hospitalizations involved White individuals (86.9% in PD group versus 83.3% in control group). The PD group contained fewer hospitalizations of Black patients (prevalence ratio= 0.51, 0.50–0.53), and more hospitalizations of Asian patients (prevalence ratio=1.32, 1.25–1.39) (Table 1). The age distribution of MHSA hospitalizations in the PD group was left skewed-7% (n=9,817) were of individuals ages 60–64; this proportion grew to 41% (n=55,014) for PD patients ages >80. MHSA burden was more evenly distributed across age strata in the general inpatient group (Table 1). These findings agree with previous studies which demonstrate 1) PD prevalence increases with age and 2) psychosis and complicated dementia are more common in older PD patients or in later disease stages. (2, 18, 22) Several studies have reported that women are more likely to have a documented psychiatric diagnosis or utilize psychiatric care services.(23–25) We found sex differences MHSA diagnoses in both inpatient groups, but the magnitude of the difference was less in the PD population (male: female prevalence ratio PD: 0.78, 0.78–0.79 vs. general: 0.58, 0.57–0.58).
Table 1.
Population Characteristics of Older Adult MHSA Hospitalizations, Nationwide Inpatient Sample, 2000–2010.
| Characteristics (No. of non-missing data) | PD | General Population | Prevalence Ratio (95%CI) PD vs. General Population |
|---|---|---|---|
| Race * (n=3,918,703) | |||
| White | 91, 809 (86.9%) | 3,175,455 (83.3%) | 1.04 (1.04–1.05) |
| Black | 4095 (3.9%) | 289,341 (7.6%) | 0.51 (0.50–0.53) |
| Hispanic | 5985 (5.7%) | 223,101 (5.8%) | 0.93 (0.90–0.95) |
| Asian | 1397 (1.3%) | 38,201 (1.0%) | 1.32 (1.25–1.39) |
| Native American | 304 (0.3%) | 14,418 (0.4%) | 0.76 (0.68–0.85) |
| Unknown | 1947 (1.8%) | 72,650 (1.9%) | 0.97 (0.93–1.01) |
| Age group (n=4,998,604) | |||
| 60–64 | 9817 (7.4%) | 960,977 (19.7%) | 0.37 (0.37–0.38) |
| 65–69 | 15,511 (11.7%) | 881,175 (18.1%) | 0.65 (0.64–0.65) |
| 70–74 | 21,986 (16.6%) | 787,493 (16.2%) | 1.02 (1.01–1.04) |
| 75–79 | 30,410 (22.9%) | 799,649 (16.4%) | 1.39 (1.38–1.41) |
| 80+ | 55,014 (41.4%) | 1,436,572 (29.5%) | 1.40 (1.39–1.41) |
| Sex (n=4,998,604) | |||
| Male | 58,399 (44.0%) | 1,787,796 (36.7%) | 1.20 (1.19–1.20) |
| Female | 74,339 (56.0%) | 3,078,070 (63.3%) | 0.89 (0.88–0.89) |
| Expected Primary Payer (n= 4,991,268) | |||
| Medicare | 118,784 (89.6%) | 3,838,248 (79.0%) | 1.13 (1.13–1.14) |
| Medicaid | 2804 (2.1%) | 205,773 (4.2%) | 0.50 (0.48–0.52) |
| Private insurance | 9238 (7.0%) | 669,272 (13.8%) | 0.51 (0.50–0.52) |
| Self-pay | 558 (0.4%) | 63,791 (1.3%) | 0.32 (0.30–0.35) |
| No charge | 70 (0.05%) | 7035 (0.1%) | 0.36 (0.29–0.46) |
| Other | 1088 (0.8%) | 74,607 (1.5%) | 0.53 (0.50–0.57) |
| Admission Type (n=4,484,731) | |||
| Emergent | 73,576 (62.4%) | 2,618,335 (60.0%) | 0.03 (0.03–0.03) |
| Urgent | 23,912 (20.3%) | 872,784 (20.0%) | 0.03 (0.03–0.03) |
| Elective | 20,006 (17.0%) | 863,039 (19.8%) | 0.02 (0.02–0.02) |
| Trauma | 161 (0.1%) | 8006 (0.20%) | 0.02 (0.02–0.02) |
| Other | 169 (0.1%) | 4744 (0.10%) | 0.03 (0.03–0.04) |
| Hospital Teaching Status (n= 4,976,467) | |||
| Non-teaching | 83,159 (62.9%) | 2,932,836 (60.5%) | 1.17 (1.16–1.17) |
| Teaching | 49,026 (37.1%) | 1,911,446 (39.5%) | 1.05 (1.05–1.06) |
MHSA Diagnoses Associated with PD
Affective Disorders
As shown in Table 2, affective disorders (anxiety, depression, bipolar disorder or mania) accounted for 88% of hospitalizations of persons with PD, compared to 78.8% of psychiatric hospitalizations in the general population (chi square, p <0.01). Multivariable regression models that adjusted for patient, payer and hospital factors found that hospitalized PD patients had greater odds of diagnosis of bipolar disorder (AOR 2.74, 2.69–2.79), mania (AOR 1.43, 1.18–1.74), and depression (AOR 1.32, 1.31–1.34). An admitting diagnosis of anxiety disorder was less likely among PD patients (AOR 0.68, 0.67–0.69).
Table 2.
MHSA hospitalization patterns in Parkinson Disease versus General Population, Nationwide Inpatient Sample, 2000–2010.
| Diagnosis | MHSA Hospitalizations | PD vs. General Population Adjusted OR** (95%CI) | |||
|---|---|---|---|---|---|
|
|
|
||||
| PD | General Population | ||||
| n | %* | n | %* | ||
| Alcohol Abuse | 5601 | 4.2% | 682,966 | 14.0% | 0.26 (0.25–0.27) |
|
| |||||
| Anxiety | 24,349 | 18.3% | 1,232,109 | 25.3% | 0.68 (0.67–0.69) |
|
| |||||
| Bipolar | 14,708 | 11.1% | 261,327 | 5.4% | 2.74 (2.69–2.79) |
|
| |||||
| Depression | 77,697 | 58.5% | 2,469,428 | 50.8% | 1.33 (1.31–1.34) |
|
| |||||
| Impulse Control | 227 | 0.2% | 4897 | 0.1% | 1.52 (1.31–1.76) |
|
| |||||
| Mania | 118 | 0.1% | 3285 | 0.1% | 1.44 (1.18–1.74) |
|
| |||||
| Psychosis | 996 | 0.8% | 29,512 | 0.6% | 1.25 (1.17–1.33) |
|
| |||||
| Substance Abuse | 8191 | 6.2% | 301,892 | 6.2% | 1.06 (1.04–1.09) |
|
| |||||
| Suicide | 1121 | 0.8% | 49,906 | 1.0% | 0.98 (0.92–1.04) |
Total % may be greater than 100 because multiple diagnoses are allowed per hospitalization.
Adjusted for sex, age, payer, admission type, teaching status
Addiction
Only 4.2% of PD group hospitalizations were for alcohol intoxication, abuse or dependence, compared with 13.6% in the control group (p<0.001). This difference represented a 74% lower likelihood of an alcohol abuse diagnosis among PD inpatients (AOR 0.26, 0.25–0.27) (Table 2, Figure 1). Conversely, the odds of a substance abuse diagnosis were slightly more likely in the PD group (AOR 1.06, 1.04–1.09).
Figure 1.
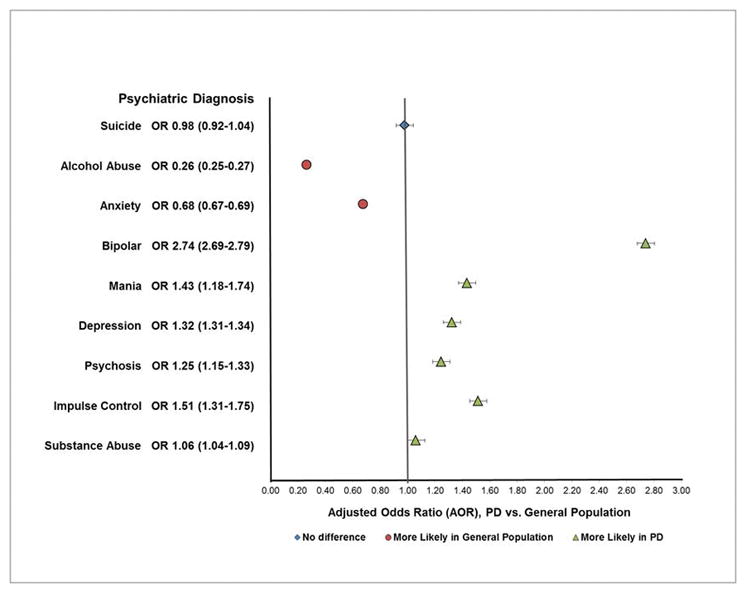
MHSA Hospitalizations in Parkinson Disease vs General Population
Suicide
Not all psychiatric behaviors or diagnoses were more common in PD. Hospitalization for suicide ideation or attempt was less common among PD patients (0.84% versus 0.99% in the general population, chi-square p<0.05). However, the adjusted odds of a suicide-related hospitalization were not statistically different between PD patients controls (AOR 0.98, 0.92–1.04).
Impulse Control Disorders
Impulse Control Disorders are associated with dopaminergic medication use in PD(26), and psychosis in PD may be primary, or may occur secondary to PD medication use, infections, metabolic derangements, or acute drug reactions. Hospitalization for both ICDs and psychosis were more common in the PD group (ICD: 0.17% in PD versus 0.10% in control group; Psychosis: 0.75% in PD versus 0.59% in controls, p value <0.001 for both comparisons).
Demographic Differences in MHSA Diagnoses
Table 3 displays the results of subgroup analyses which examined the extent to which inpatient diagnoses varied by race and gender between PD and control groups, adjusting for age, hospital and payer characteristics. In general, the associations between PD and mood disorders, bipolar disorder, psychosis, impulse disorders and alcohol abuse were preserved across race and sex subgroups. However, substance abuse was more likely in white (AOR 1.13, 1.10–1.67) and male (AOR 1.15, 1.11–1.20) PD patients, and less likely in Blacks with PD (AOR 0.69, 0.60–0.79). Hospitalization with suicide ideation or attempt was increased in the Hispanic PD group (AOR 1.42, 1.06–1.90). The sensitivity analyses (as described in the Methods section) did not produce adjusted odds ratios that differed substantially in magnitude in direction from our primary analyses (Supplementary Table 1).
Table 3.
MHSA Hospitalizations in Parkinson Disease by Race and Sex, NIS 2000–2010.
| Diagnosis | MHSA Hospitalizations | PD vs. General Population Adjusted OR** (95%CI) | |||
|---|---|---|---|---|---|
|
|
|
||||
| PD | General Population | ||||
| No. / Total No. | Prevalence % | No. / Total No. | Prevalence % | ||
| Alcohol Abuse | |||||
|
| |||||
| White | 3808 / 91,809 | 0.04 | 400,051 / 3,175,455 | 0.13 | 0.29 (0.28–0.30) |
| Black | 379 / 4095 | 0.09 | 78,065 / 289,341 | 0.27 | 0.27 (0.24–0.31) |
| Hispanic | 309 / 5985 | 0.05 | 40,113 / 223,101 | 0.18 | 0.21 (0.18–0.25) |
| Asian | 32 / 1397 | 0.02 | 4257 / 38,201 | 0.11 | 0.11 (0.06–0.21) |
| Male | 4490 / 58,399 | 0.08 | 511,377 / 1,787,796 | 0.29 | 0.27 (0.26–0.28) |
| Female | 1111 / 74,339 | 0.01 | 171,589 / 3,078,070 | 0.06 | 0.31 (0.29–0.33) |
|
| |||||
| Anxiety Disorder | |||||
|
| |||||
| White | 16,803 / 91,809 | 0.18 | 823,749 / 3,175,455 | 0.26 | 0.66 (0.65–0.67) |
| Black | 557 / 4095 | 0.14 | 52,892 / 289,341 | 0.18 | 0.67 (0.61–0.74) |
| Hispanic | 1199 / 5985 | 0.20 | 53,962 / 223,101 | 0.24 | 0.76 (0.70–0.82) |
| Asian | 229 / 1397 | 0.16 | 9555 / 38,201 | 0.25 | 0.64 (0.51–0.81) |
| Male | 8806 / 58,399 | 0.15 | 349,131 / 1,787,796 | 0.20 | 0.70 (0.68–0.71) |
| Female | 15,543 / 74,339 | 0.21 | 882,978 / 3,078,070 | 0.29 | 0.65 (0.64–0.66) |
|
| |||||
| Bipolar Disorder | |||||
|
| |||||
| White | 10,601 / 91,809 | 0.12 | 177,984 / 3,175,455 | 0.06 | 2.74 (2.68–2.8) |
| Black | 451 / 4095 | 0.11 | 14,786 / 289,341 | 0.05 | 2.91 (2.62–3.23) |
| Hispanic | 470 / 5985 | 0.08 | 9065 / 223,101 | 0.04 | 2.3 (2.05–2.59) |
| Asian | 111 / 1397 | 0.08 | 1553 / 38,201 | 0.04 | 3.07 (2.29–4.1) |
| Male | 6499 / 58,399 | 0.11 | 88,203 / 1,787,796 | 0.05 | 2.9 (2.8–2.99) |
| Female | 8209 / 74,339 | 0.11 | 173,124 / 3,078,070 | 0.06 | 2.57 (2.5–2.65) |
|
| |||||
| Depression | |||||
|
| |||||
| White | 53,935 / 91,809 | 0.59 | 1,657,607 / 3,175,455 | 0.52 | 1.28 (1.26–1.3) |
| Black | 2291 / 4095 | 0.56 | 114,700 / 289,341 | 0.40 | 1.7 (1.59–1.82) |
| Hispanic | 3326 / 5985 | 0.56 | 102,159 / 223,101 | 0.46 | 1.38 (1.3–1.47) |
| Asian | 806 / 1397 | 0.58 | 18,476 / 38,201 | 0.48 | 1.66 (1.39–1.99) |
| Male | 32844 / 58,399 | 0.56 | 746,793 / 1,787,796 | 0.42 | 1.48 (1.45–1.51) |
| Female | 44853 / 74,339 | 0.60 | 1,722,635 / 3,078,070 | 0.56 | 1.15 (1.12–1.17) |
|
| |||||
| Psychosis | |||||
|
| |||||
| White | 698 / 91,809 | 0.21 | 18,993 / 3,175,455 | 0.00 | 1.27 (1.17–1.38) |
| Black | 28 / 4095 | 0.04 | 177 / 289,341 | 0.00 | 1.11 (.76–1.63) |
| Hispanic | 223 / 5985 | 0.22 | 1319 / 223,101 | 0.00 | 1.35 (0.99–1.85) |
| Asian | 13 / 1397 | 0.16 | 229 / 38,201 | 0.00 | 1.63 (0.75–3.51) |
| Male | 505 / 58,399 | 0.01 | 11,619 / 1,787,796 | 0.01 | 1.35 (1.21–1.50) |
| Female | 491 / 74,339 | 0.01 | 17,893 / 3,078,070 | 0.01 | 1.18 (1.06–1.31) |
|
| |||||
| Substance Abuse | |||||
|
| |||||
| White | 5687 / 91,809 | 0.06 | 179,247 / 3,175,455 | 0.06 | 1.13 (1.10–1.17) |
| Black | 259 / 4095 | 0.06 | 35,611 / 289,341 | 0.12 | 0.69 (0.60–0.80) |
| Hispanic | 275 / 5985 | 0.05 | 13,087 / 223,101 | 0.06 | 0.92 (0.79–1.08) |
| Asian | 70 / 1397 | 0.05 | 1925 / 38,201 | 0.05 | 0.73 (0.47–1.14) |
| Male | 4384 / 58,399 | 0.08 | 143,014 / 1,787,796 | 0.08 | 1.15 (1.11–1.20) |
| Female | 3807 / 74,339 | 0.05 | 158,878 / 3,078,070 | 0.05 | 1.04 (1.00–1.09) |
|
| |||||
| Suicide | |||||
|
| |||||
| White | 793 / 91,809 | 0.01 | 33,467 / 3,175,455 | 0.01 | 0.95 (0.88–1.03) |
| Black | 35 / 4095 | 0.01 | 288 / 289,341 | <0.01 | 1.23 (0.87–1.73) |
| Hispanic | 61 / 5985 | 0.01 | 2129 / 223,101 | 0.01 | 1.42 (1.06–1.90) |
| Asian | 20 / 1397 | 0.01 | 662 / 38,201 | 0.02 | 0.77 (0.38–1.57) |
| Male | 621 / 58,399 | 0.01 | 23,298 / 1,787,796 | 0.01 | 0.97 (0.88–1.07) |
| Female | 500 / 74,339 | 0.01 | 26,608 / 3,078,070 | 0.01 | 0.94 (0.85–1.05) |
|
| |||||
| Mania | |||||
|
| |||||
| White | 82 / 91,809 | 0.02 | 2201 / 3,175,455 | <0.01 | 1.42 (1.23–1.80) |
| Black | 0 / 4095 | 0.04 | 165 / 289,341 | <0.01 | <0.001 (<0.001–>999.99) |
| Hispanic | 7 / 5985 | 0.02 | 98 / 223,101 | <0.01 | 2.50 (0.90–6.87) |
| Asian | 0 / 1397 | 0.03 | 35 / 38,201 | <0.01 | <0.001 (<0.001–>999.99) |
| Male | 65 / 58,399 | 0.00 | 1223 / 1,787,796 | <0.01 | 1.66 (1.27–2.18) |
| Female | 53 / 74,339 | 0.00 | 2062 / 3,078,070 | <0.01 | 1.25 (0.94–1.65) |
|
| |||||
| Impulse Control | |||||
|
| |||||
| White | 161 / 91,809 | <0.01 | 3146 / 3,175,455 | <0.01 | 1.52 (1.27–1.82) |
| Black | 12 / 4095 | <0.01 | 287 / 289,341 | <0.01 | 2.56 (1.35–4.85) |
| Hispanic | 7 / 5985 | <0.01 | 164 / 223,101 | <0.01 | 1.49 (0.60–3.66) |
| Asian | 2 / 1397 | <0.01 | 57 / 38,201 | <0.01 | <0.001 (<0.001–999.99) |
| Male | 171 / 58,399 | <0.01 | 3111 / 1,787,796 | <0.01 | 1.64 (1.35–1.98) |
| Female | 56 / 74,339 | <0.01 | 1786 / 3,078,070 | <0.01 | 1.29 (0.91–1.82) |
Adjusted for age, sex, payer, hospital type, admission type
Discussion
In this health care utilization study, we examined mental health and substance abuse hospitalizations of older adults with PD. Psychiatric disorders are common in PD, and are associated to varying degrees with disease processes (specific neurotransmitters, brain regions and neural circuits) and PD treatments (dopaminergic medications and DBS). Depression and anxiety may precede PD motor signs by several years (27, 28) lending biological plausibility for the high prevalence of these disorders in PD patients. (29, 30) Psychosis, ICDs and mania most commonly occur in the context of treatment with dopaminergic therapy or other PD treatments (e.g., DBS, amantadine or anticholinergic medications), although psychosis is also associated with the disease process itself. Our data demonstrate that PD patients have distinct acute care needs for mental illnesses, potentially providing new insights about the relationships between PD and psychiatric disorders, and informing efforts to improve outcomes in PD.
Measuring the burden of psychiatric illness using hospital discharge data provides a different perspective than psychometric studies of academic center populations. Hospitalization for a given illness not only reflects its baseline prevalence in a population, but also the effectiveness of outpatient screening, detection, action, and the relative success of outpatient treatment. The higher likelihood of a depression diagnosis in PD patients is consistent with the high prevalence (30–40%) of depression in PD, but also indicates that severe depression (requiring hospitalization) is also common in PD. In contrast, a higher risk of bipolar disorder associated with PD has not been reported previously. The direction of this finding was robust to several sensitivity analyses, although the magnitude was decreased among hospitals which likely have inpatient neurological and psychiatric care available. Greater need for inpatient care for bipolar disorder may reflect the difficulty treating emergent PD in an older adult with a history of bipolar disorder. Basic PD symptom management can precipitate a manic episode in a person with previously controlled bipolar disorder both directly (by the use of dopaminergic medications), or indirectly (by attempting to discontinue lithium or neuroleptics). Inexperienced clinicians may misdiagnose PD patients presenting with mania, ICD behaviors, sleep disturbance, hyperkinesis/dyskinesias, akathisia, or tachyphemia (as a result of disease process of PD drugs) and a history of depression as having bipolar disorder.
Anxiety disorders are very common in PD, so it might be considered surprising that PD patients were less likely to have an anxiety disorder listed as an admission diagnosis. Depression and anxiety disorders are highly co-morbid in PD (up to 80%), but research emphasizes detecting and treating depression, impulse control disorders. Patients with anxiety and depression symptoms may have been coded as having a primary depression diagnosis on admission. Alternatively, anxiety symptoms occurring in PD may require psychiatric hospitalization less often.
The prevalence of attempted or completed suicide in PD is not known, although death ideation is common.(17) Previous research has not reported higher rates of suicide in PD compared with the general population, even post DBS surgery.(31–33) Our results of relatively high burden admission for depression but no increased risk of suicide ideation or behaviors likely reflects practice patterns-PD patients are admitted for depression in the absence of suicidality, versus patients in the general population, where suicidality is more likely to prompt admission.
The association between substance use and alcohol disorders and PD appears complex. PD patients are not widely thought to be at increased risk of substance use disorders, rather, it has hypothesized that PD patients are risk aversive due to disease related personality changes.(34) Substance abuse disorders can be a manifestation of ICDs in PD. (35, 36) A Swedish registry study that found a history of admission for alcohol abuse was associated with increased risk (HR 1.38, 1.25–1.53) of future diagnosis of PD, an intriguing finding potentially linking the biological processes of the two diseases.(37) The relationship was strongest for the younger onset population, and our previous studies of disabled younger PD patients also found an increased risk of hospitalization for substance abuse(19), together suggesting an interaction between for age and substance use disorders in PD patients.
Our subpopulation analyses found that race, and to a lesser extent, sex, were moderators of psychiatric admissions when comparing PD patients with the general population. White PD patients were more likely to be hospitalized for substance abuse, while Hispanics were more vulnerable to hospitalization for suicidal ideation or attempt. These differences may reflect cultural differences in the tendency to seek hospital care for mental illness, reduced access of hospitalization, or be evidence of a greater requirement for inpatient treatment. Another possibility is that our subpopulation data reflects underservice of PD patients who are Black or Asian, groups who experience greater disparities in specialty care (38, 39) and state of the art care (40) compared with Whites and Hispanics. Of course, there may be genetic differences in phenotype, which remain understudied in spite of the known race and gender associated differences in risk for developing PD.(22, 41, 42) Future studies are needed to investigate further race disparities in suicide and substance abuse in PD.
This represents the largest study of psychiatric illness in PD, and the results indicate differences in patterns of acute psychiatric hospitalizations in PD patients compared with the general population. These differences possibly reflect the increased the risk in PD patients for certain psychiatric disorders, the association between PD medications and certain psychiatric side effects, possible disparities in quality psychiatric evaluation in when comorbid PD present, and a tempering of other disorders or behaviors consistent with overall risk averseness in PD patients. Limitations of this study include that many psychiatric illnesses can be effectively treated in the outpatient setting; therefore, one cannot draw conclusions about the epidemiology of psychiatric illness in the PD community using this data. Personal, physician, socioeconomic and local market factors can influence whether a patient receives inpatient psychiatric care. Retrospective studies, whether a consisting of a chart review at an academic center, secondary review of clinical trial data, or hospital claims data (as in this case), are subject to recognition, reporting and coding bias. Our results may be affected by misdiagnosis of PD, under or over diagnosis of psychiatric illness in PD, coding error, deliberate miscoding by physicians or coders to increase revenue. Although our findings were robust to sensitivity analyses aimed to detect bias related to hospital and payer characteristics, and we achieved similar results when we did not limit the MHSA diagnosis by position, we cannot be certain of the magnitude or direction of these biases. Future studies should consider the efficacy of coordinated care between mental health and neurological specialties, improved recognition of common movement disorders and the potential role of anti-PD treatments in older patients presenting with psychiatric disturbances.
Supplementary Material
Acknowledgments
Funding Sources for Study: This study was funded primarily by the National Institutes of Health (NIH) and the National Institute of Neurological Disease and Stroke (NINDS) via a Mentored Career Development Award K23NS081087(PI-Willis) and the University of Pennsylvania Perelman School of Medicine Department of Neurology Movement Disorders Division.
Footnotes
Financial Disclosure/Conflict of Interest
Dr. Willis has provided unpaid educational activities for the American Parkinson Disease Association, the Parkinson Disease Foundation and the National Parkinson foundation.
Mr. Thibault reports no competing disclosures.
Dr. E. Ray Dorsey: Dr. Ray Dorsey has received compensation for consulting activities from Amgen, Clintrex, Lundbeck, mc10, Medtronic, and the National Institute of Neurological Disorders and Stroke, research support from Davis Phinney Foundation, Great Lakes Neurotechnologies, Huntington Study Group, Lundbeck, Michael J. Fox Foundation, Patient-Centered Outcomes Research Institute, Prana Biotechnology, Sage Bionetworks, stock options from Grand Rounds, and compensation for expert testimony.
Dr. Schmidt has received research funding from the National Parkinson Foundation and the Patient-Centered Outcomes Research Institute; honoraria from CHDI Foundation; and license fees from the New York Society for the Relief of the Ruptured and Crippled for US Patent 6626693.
Dr. Weintraub has received research funding from Michael J. Fox Foundation for Parkinson’s Research, National Institutes of Health, Novartis Pharmaceuticals, Department of Veterans Affairs, and Alzheimer’s Disease Cooperative Study; honoraria from Teva Pharmaceuticals, Lundbeck Inc., Pfizer, Avanir Pharmaceuticals, Acadia Pharmaceuticals, Merck & Co., UCB, Bristol-Myers Squibb Company, Novartis Pharmaceuticals, Clintrex LLC, Theravance, Medivation, CHDI Foundation, Alzheimer’s Disease Cooperative Study and the Weston Foundation; license fee payments from the University of Pennsylvania for the QUIP and QUIP-RS; and fees for testifying in two court cases related to impulse controls disorders in Parkinson’s disease (March, 2013–April, 2014, payments by Eversheds and Roach, Brown, McCarthy & Gruber, P.C.).
Reference List
- 1.Wright WA, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34:143–151. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weintraub D, Burn DJ. Parkinson’s disease: the quintessential neuropsychiatric disorder. Mov Disord. 2011 May;26:1022–1031. doi: 10.1002/mds.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord. 2001 May;16:507–510. doi: 10.1002/mds.1099. [DOI] [PubMed] [Google Scholar]
- 4.Voon V, Hassan K, Zurowski M, et al. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology. 2006 Jun 13;66:1750–1752. doi: 10.1212/01.wnl.0000218206.20920.4d. [DOI] [PubMed] [Google Scholar]
- 5.Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010 May;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 6.de la Riva P, Smith K, Xie SX, Weintraub D. Course of psychiatric symptoms and global cognition in early Parkinson disease. Neurology. 2014 Aug 15; doi: 10.1212/WNL.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qureshi SU, Amspoker AB, Calleo JS, Kunik ME, Marsh L. Anxiety disorders, physical illnesses, and health care utilization in older male veterans with Parkinson disease and comorbid depression. J Geriatr Psychiatry Neurol. 2012 Dec;25:233–239. doi: 10.1177/0891988712466458. [DOI] [PubMed] [Google Scholar]
- 8.Calleo J, Amspoker AB, Marsh L, Kunik ME. Mental health diagnoses and health care utilization in persons with dementia, Parkinson’s disease, and stroke. J Neuropsychiatry Clin Neurosci. 2015;27:e117–e121. doi: 10.1176/appi.neuropsych.13110333. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi SU, Amspoker AB, Calleo JS, Kunik ME, Marsh L. Anxiety disorders, physical illnesses, and health care utilization in older male veterans with Parkinson disease and comorbid depression. J Geriatr Psychiatry Neurol. 2012 Dec;25:233–239. doi: 10.1177/0891988712466458. [DOI] [PubMed] [Google Scholar]
- 10.Chen P, Kales HC, Weintraub D, et al. Depression in veterans with Parkinson’s disease: frequency, co-morbidity, and healthcare utilization. Int J Geriatr Psychiatry. 2007 Jun;22:543–548. doi: 10.1002/gps.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flament M, Loas G, Godefroy O, Krystkowiak P. Suicide without depression after withdrawal of a dopamine agonist in a patient with Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2011;23:E32. doi: 10.1176/jnp.23.4.jnpe32. [DOI] [PubMed] [Google Scholar]
- 12.Mahgoub NA, Kotbi N. Acute depression and suicidal attempt following lowering the frequency of deep brain stimulation. J Neuropsychiatry Clin Neurosci. 2009;21:468. doi: 10.1176/jnp.2009.21.4.468. [DOI] [PubMed] [Google Scholar]
- 13.Santos-Garcia D, Macias M, Llaneza M, Aneiros A. Suicide following duodenal levodopa infusion for Parkinson’s disease. Mov Disord. 2009 Oct 15;24:2029–2030. doi: 10.1002/mds.22708. [DOI] [PubMed] [Google Scholar]
- 14.Voon V, Krack P, Lang AE, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson’s disease. Brain. 2008 Oct;131:2720–2728. doi: 10.1093/brain/awn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weintraub D, Duda JE, Carlson K, et al. Suicide ideation and behaviours after STN and GPi DBS surgery for Parkinson’s disease: results from a randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2013 Oct;84:1113–1118. doi: 10.1136/jnnp-2012-304396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kostic VS, Pekmezovic T, Tomic A, et al. Suicide and suicidal ideation in Parkinson’s disease. J Neurol Sci. 2010 Feb 15;289:40–43. doi: 10.1016/j.jns.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Nazem S, Siderowf AD, Duda JE, et al. Suicidal and death ideation in Parkinson’s disease. Mov Disord. 2008 Aug 15;23:1573–1579. doi: 10.1002/mds.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyasaki JM, Shannon K, Voon V, et al. Practice Parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006 Apr 11;66:996–1002. doi: 10.1212/01.wnl.0000215428.46057.3d. [DOI] [PubMed] [Google Scholar]
- 19.Willis AW, Schootman M, Kung N, Racette BA. Epidemiology and neuropsychiatric manifestations of Young Onset Parkinson’s Disease in the United States. Parkinsonism Relat Disord. 2013 Feb;19:202–206. doi: 10.1016/j.parkreldis.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AHRQ. HCUP Mental Health Substance Abuse Clinical Classification Software. 2014. [Google Scholar]
- 21.AHRQ. Race Reporting by State in the HCUP NIS. 2014. [Google Scholar]
- 22.Van Den Eeden SK, Tanner CM, Bernstein AL, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003 Jun 1;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 23.Nolen-Hoeksema S. Sex differences in unipolar depression: evidence and theory. Psychological bulletin. 1987;101:259. [PubMed] [Google Scholar]
- 24.Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatric Clinics of North America. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- 25.Angermeyer MC, Goldstein JM, Kuehn L. Gender differences in schizophrenia: rehospitalization and community survival. Psychological medicine. 1989;19:365–382. doi: 10.1017/s0033291700012411. [DOI] [PubMed] [Google Scholar]
- 26.Voon V, Sohr M, Lang AE, et al. Impulse control disorders in Parkinson disease: a multicenter case--control study. Ann Neurol. 2011 Jun;69:986–996. doi: 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- 27.Shiba M, Bower JH, Maraganore DM, et al. Anxiety disorders and depressive disorders preceding Parkinson’s disease: a case-control study. Mov Disord. 2000 Jul;15:669–677. doi: 10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Bower JH, Grossardt BR, Maraganore DM, et al. Anxious personality predicts an increased risk of Parkinson’s disease. Mov Disord. 2010 Oct 15;25:2105–2113. doi: 10.1002/mds.23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneier FR, Liebowitz MR, Abi-Dargham A, Zea-Ponce Y, Lin SH, Laruelle M. Low dopamine D(2) receptor binding potential in social phobia. Am J Psychiatry. 2000 Mar;157:457–459. doi: 10.1176/appi.ajp.157.3.457. [DOI] [PubMed] [Google Scholar]
- 30.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005 Jun;128:1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- 31.Myslobodsky M, Lalonde FM, Hicks L. Are patients with Parkinson’s disease suicidal? J Geriatr Psychiatry Neurol. 2001;14:120–124. doi: 10.1177/089198870101400304. [DOI] [PubMed] [Google Scholar]
- 32.Stenager EN, Wermuth L, Stenager E, Boldsen J. Suicide in patients with Parkinson’s disease. An epidemiological study. Acta Psychiatr Scand. 1994 Jul;90:70–72. doi: 10.1111/j.1600-0447.1994.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 33.Weintraub D, Duda JE, Carlson K, et al. Suicide ideation and behaviours after STN and GPi DBS surgery for Parkinson’s disease: results from a randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2013 Oct;84:1113–1118. doi: 10.1136/jnnp-2012-304396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsh L, McDonald WM, Cummings J, Ravina B. Provisional diagnostic criteria for depression in Parkinson’s disease: report of an NINDS/NIMH Work Group. Mov Disord. 2006 Feb;21:148–158. doi: 10.1002/mds.20723. [DOI] [PubMed] [Google Scholar]
- 35.Weintraub D, Nirenberg MJ. Impulse control and related disorders in Parkinson’s disease. Neurodegener Dis. 2013;11:63–71. doi: 10.1159/000341996. [DOI] [PubMed] [Google Scholar]
- 36.Giovannoni G, O’Sullivan JD, Turner K, Manson AJ, Lees AJ. Hedonistic homeostatic dysregulation in patients with Parkinson’s disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry. 2000 Apr;68:423–428. doi: 10.1136/jnnp.68.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eriksson AK, Lofving S, Callaghan RC, Allebeck P. Alcohol use disorders and risk of Parkinson’s disease: findings from a Swedish national cohort study 1972–2008. BMC Neurol. 2013;13:190. doi: 10.1186/1471-2377-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology. 2011 Aug 30;77:851–857. doi: 10.1212/WNL.0b013e31822c9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahodwala N, Xie M, Noll E, Siderowf A, Mandell DS. Treatment disparities in Parkinson’s disease. Ann Neurol. 2009 Aug;66:142–145. doi: 10.1002/ana.21774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willis AW, Schootman M, Kung N, Wang XY, Perlmutter JS, Racette BA. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology. 2014 Jan 14;82:163–171. doi: 10.1212/WNL.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayeux R, Marder K, Cote LJ, et al. The frequency of idiopathic Parkinson’s disease by age, ethnic group, and sex in northern Manhattan, 1988–1993. Am J Epidemiol. 1995 Oct 15;142:820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- 42.Wright WA, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34:143–151. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


