Abstract
Amongst the side effects of triptans, a substantial percentage of patients experience injection site pain and tenderness, the underlying mechanism of which is unknown. We found the dose range from 10 fg – 1000 ng (intradermal) sumatriptan induced a complex dose-dependent mechanical hyperalgesia in the male rat, with distinct peaks, at 1 pg and 10 ng, with no hyperalgesia at 1 ng. In female rats, there was 1 broad peak. The highest dose (1000 ng) did not produce hyperalgesia in either sex. We evaluated the receptors mediating sumatriptan hyperalgesia (1 pg, 1 and 10 ng). In male rats, while the injection of an antagonist for the serotonin (5-hydroxytryptamine, 5-HT) receptor subtype 1B (5-HT1B), but not 5-HT1D, markedly inhibited sumatriptan (1 pg hyperalgesia, at 10 ng a 5-HT1D receptor antagonist completely eliminated hyperalgesia. In contrast, in female rats, the 5-HT1D, but not 5-HT1B, receptor antagonist completely blocked sumatriptan (1 pg and 10 ng) hyperalgesia. Both 5-HT1B and 5-HT1D receptor antagonists attenuated hyperalgesia (1 ng) in females. Sumatriptan (1 ng)-induced hyperalgesia in female rats is G-protein-coupled estrogen receptor 30 dependent. While selective 5-HT1B, but not 5-HT1D, receptor agonists produces a robust hyperalgesia, when co-injected the hyperalgesia induced by 5-HT1B receptor agonist was attenuated. The mechanical hyperalgesia induced by sumatriptan (1 pg and 10 ng) is dependent on the Gi-protein α subunit and protein kinase A (PKA). Understanding the mechanisms responsible for the complex dose dependence for triptan hyperalgesia may provide useful information for the design of anti-migraine drugs with improved therapeutic profiles.
Keywords: hyperalgesia, 5-HT1B receptor, 5-HT1D receptor, triptans, migraine
Introduction
Amongst the well-described side effects of the triptan family of anti-migraine drugs is injection site pain and allodynia/hyperalgesia (i.e., local tenderness), especially problematic for a self-administered therapy. Thus, administered by the subcutaneous route triptans can induce injection site pain (Dahlof et al., 1994, Solomon et al., 1997, Duquesnoy et al., 1998, Linde et al., 2004), while sublingual/intranasal administration has been reported to induce headache, chest and abdominal pain, and pain/tenderness in the extremities (Hillis and Macintyre, 1993, Dahlof et al., 1994, Houghton et al., 1994, Ottervanger et al., 1994, Gomez-Mancilla et al., 2001, Wang et al., 2002, Coulter et al., 2003). Sumatriptan-induced injection-site reactions, the most common adverse event, is reported in 58.7% of patients with moderate to a severe migraine treated with sumatriptan (6 mg, administered subcutaneously) compared with 23.8% of placebo controls (Cady et al., 1991). Moreover, in a double-blind, placebo-controlled study by Mushet and colleagues (Mushet et al., 1996) injection-site reaction was reported in 34% of patients with moderate to a severe migraine treated with sumatriptan 6 mg (patient-administered via single-dose syringe cartridges) compared with 18% of placebo controls. Although Burstein and colleagues had already demonstrated, more than decade ago, that triptans acutely sensitize dural nociceptors (Burstein, 2001, Burstein et al., 2004, Burstein and Jakubowski, 2004), there remains a paucity of information regarding the mechanism underlying triptan-induced nociceptor sensitization, their dose-response curves and sex differences in this effect.
In the present study, we evaluated sumatriptan-induced injection-site mechanical hyperalgesia, including dose-dependence, sexual dimorphism, the 5-HT receptor subtypes and intracellular mechanism involved for sumatriptan. Sumatriptan induces a dose-dependent decrease in the mechanical nociceptive threshold at its site of injection in female rats and, an extremely complex dose-dependent effect in males. We also show that sumatriptan-induced hyperalgesia is estrogen dependent, acting through the G-protein-coupled estrogen receptor 30 (GPR30), in females. The receptors, mediating this hyperalgesia (5-HT1B, and 5-HT1D) were sex and dose-dependent. Sumatriptan-induced mechanical hyperalgesia was also G-protein αi subunit and protein kinase A (PKA) dependent.
Experimental Procedures
Animals
Experiments were performed on 230–280 g adult male and female [except for ovariectomized females (described below)] Sprague–Dawley rats (Charles River Laboratories, Hollister, CA, USA). Experimental animals were housed in a controlled environment in the animal care facility at the University of California, San Francisco, under a 12-h light/dark cycle. Food and water were available ad libitum. The experimental protocol was approved by the UCSF Institutional Animal Care and Use Committee and adhered to the guidelines for the use of animals in research of the American Association of Laboratory Animal Care, the National Institutes of Health (NIH), and the Committee for Research and Ethical Issues of the International Association for the Study of Pain (IASP). All efforts were made to minimize the number of animals used and their suffering.
Mechanical nociceptive threshold testing
Mechanical nociceptive threshold was quantified using an Ugo Basile Analgesymeter® (Randall-Selitto paw-withdrawal test; Stoelting, Chicago, IL), which applies a linearly increasing mechanical force to the dorsum of the rat’s hind paw, as described previously (Taiwo et al., 1989, Taiwo and Levine, 1989, Ferrari and Levine, 2015). Nociceptive threshold was defined as the force in grams at which the animal withdrew its paw; baseline threshold was defined as the mean of 3 readings taken just before a test agent was injected. Each paw was treated as an independent measure, and each experiment performed on a separate group of rats. Data are presented as mean change from baseline mechanical nociceptive threshold.
Ovariectomy
Ovariectomy was performed on female rats at 3–4 weeks (21–28 days) of age (i.e. before puberty). Under isoflurane (2.5%) inhalation anesthesia (Phoenix Pharmaceuticals, St. Joseph, MO, USA) in 97.5% O2, six female rats were treated with 0.5% of bupivacaine (from Henry Schein, Melville, NY, USA) at their surgical incision site and an intramuscular injection of a non-steroidal anti-inflammatory, carprofen [5 mg/kg; Sigma-Aldrich (St. Louis, MO, USA)], followed by removal of the ovaries through bilateral upper flank incisions (Joseph and Levine, 2003b, a). The ovarian bundles were tied off with 4-0 silk sutures and the ovaries excised. The fascia and skin were closed with 5-0 silk suture. Five weeks after the surgery sumatriptan (1 ng) was injected intradermally on the dorsum of the hindpaw.
Chronic administration of estrogen in male rats
Chronic administration of estrogen in male rats was performed as described previously by Smith and colleagues (Smith et al., 1977). Briefly, 17β-estradiol [from Sigma-Aldrich (St. Louis, MO, USA)] was administered by implanting estrogen filled Silastic tubes [(I.D. 1.57 mm, O.D. 3.18 mm) of 5 mm effective length; Dow Corning, Midland, MI, USA)]. The ends of the implants were sealed with silicone rods (Goodfellow, Cambridge, UK). Implants were then washed in ethanol and equilibrated in four changes of warm phosphate-buffered saline over a 24-h period before placement in the rat. Under inhalation anesthesia, isoflurane (2.5%; Phoenix Pharmaceuticals, St. Joseph, MO, USA) in 97.5% O2, six male rats were treated with 0.5% of bupivacaine at the incision site and an intramuscular injection of carprofen (5 mg/kg). Implants were placed subcutaneously on the rat’s back, at the time of surgery (Smith et al., 1977). Implants were placed 2 weeks before sumatriptan (1 ng) was injected intradermally on the dorsum of the hindpaw.
Drugs and their administration
The following drugs were used: sumatriptan succinate (prototypical 5-HT1B and 5-HT1D receptor agonist), CP-93129 dihydrochloride hydrate [a selective 5-HT1B receptor agonist; (Araldi et al., 2016b)] and pertussis toxin [PTX; Gi-protein inhibitor; (Araldi et al., 2015, 2016b, a)] from Sigma-Aldrich (St. Louis, MO, USA); H-89 dihydrochloride [inhibitor of protein kinase A (PKA); (Araldi et al., 2015, 2016a, b)] from Santa Cruz Biotechnology (Dallas, TX, USA); BRL 15572 [5-HT1D receptor antagonist; (Araldi et al., 2016b)], L-694,247 [a selective 5-HT1D receptor agonist; (Araldi et al., 2016b)] and NAS-181 [5-HT1B receptor antagonist; (Araldi et al., 2016b)] from Tocris Bioscience (Avonmouth, Bristol, UK); and G-36 [GPR30 receptor antagonist; (Alvarez et al., 2014)] from Azano Pharmaceuticals (Albuquerque, NM, USA).
Sumatriptan, CP-93129 and pertussis toxin were dissolved in saline. All other drugs were dissolved in 100% DMSO (Sigma-Aldrich) and further diluted in saline containing 2% Tween 80 (Sigma-Aldrich). The final concentration of DMSO and Tween 80 was 2%. All drugs were injected intradermally on the dorsum of the hind paw, in a volume of 5 μL, using a 30-gauge hypodermic needle adapted to a 50 μL Hamilton syringe (Reno, NV, USA). The injection of H-89 and pertussis toxin was preceded by a hypotonic shock (2 μL of distilled water, separated by a bubble of air to avoid mixing in the same syringe), to facilitate entry of these compounds into the nerve terminal (Borle and Snowdowne, 1982, Burch and Axelrod, 1987).
Oligodeoxynucleotide antisense to estrogen receptor alpha (α), beta (β) and GPR30 mRNA
To investigate the role of estrogen receptor (ER) subtypes in the mechanical hyperalgesia induced by intraplantar injection of sumatriptan (1 ng) in female rats, oligodeoxynucleotides (ODN) antisense (AS) for ER alpha (ER-α), ER beta (ER-β) or GPR30 mRNA were used (Liang et al., 2002, Edinger and Frye, 2007, Alvarez et al., 2014, Ferrari et al., 2016). The sequences for the ERα, 5′-CAT GGT CAT GGT CAG-3, the ERβ, 5′-GAA TGT CAT AGC TGA-3′, and the GPR30, 5′-ATG TTC AGA GAG GTC CCC AG-3′ ODN AS (Invitrogen Life Technologies, Carlsbad, CA, USA), were directed against a unique region of each subtype of estrogen receptor sequence, in the rat [GeneBank accession numbers NM_012689.1 (ERα), NM_012754.1 (ERβ) and NM_133573 (GPR30)]. The ODN mismatch (MM) sequences, 5′-ATC GTG GAT CGT GAC-3′, for ERα, 5′-AAG GTT ATC GCA AGT-3′, for ERβ, and 5′-AGG TCC AGA AAG ATG CCA AG-3′ for GPR30 were a scrambled version of the antisense sequence that has the same base pairs and GC ratio, but the order was randomized, with little or no homology to any mRNA sequences posted at GenBank.
Before use, the ODNs were reconstituted in nuclease-free 0.9% NaCl and then administered intrathecally at a dose of 2 μg/μL in a volume of 20 μL, for 3 consecutive days, when at the 4th day, sumatriptan (1 ng) was injected, and the presence of mechanical hyperalgesia evaluated. As described previously (Alessandri-Haber et al., 2003), female rats were anesthetized with isoflurane (2.5% in O2), and the ODN injected using a microsyringe with a 30-gauge needle, inserted into the subarachnoid space, between the L4 and L5 vertebrae. A total of 40 μg of ODN, in a volume of 20 μL, was then injected. The intrathecal site of injection was confirmed by a sudden flick of the rat’s tail, a reflex that is evoked by subarachnoid space access and bolus injection (Mestre et al., 1994). Animals regained consciousness approximately 1 minute after completion of the injection. The use of AS ODN to manipulate the expression of proteins, essential for their role in nociceptor sensitization, is well supported by previous studies by others (Song et al., 2009, Su et al., 2011, Quanhong et al., 2012, Sun et al., 2013), as well as our group (Parada et al., 2003, Bogen et al., 2012, Alvarez et al., 2014, Araldi et al., 2015, 2016a, Ferrari et al., 2016).
Intrathecal administration of IB4-saporin and SSP-saporin
IB4-saporin
Isolectin B4 (IB4)-saporin, an IB4-positive nociceptor neurotoxin (Advanced Targeting Systems, San Diego, CA), was diluted in saline, and a dose of 3.2 μg, in a volume of 20 μL administered intrathecally, 15 days prior to experiments. The dose and timing of IB4-saporin administration were chosen based on previous reports from our group and others (Vulchanova et al., 2001, Nishiguchi et al., 2004, Joseph et al., 2008, Joseph and Levine, 2010, Araldi et al., 2015, 2016b, a).
SSP-saporin
[Sar9, Met(O2)11]-substance P-saporin, a SP-positive nociceptor neurotoxin (SSP-Saporin, Advanced Targeting Systems, San Diego, CA) was diluted in saline, and a dose of 100 ng, in a volume of 20 μL administered intrathecally, 15 days before priming experiments. The addition of [Sar9, Met(O2)11] to the substance P conjugated to saporin makes the agent more stable and potent than when substance P alone is bound to saporin. The dose and pre-treatment interval were based on the studies of Wiley and colleagues (Wiley et al., 2007) and Choi and colleagues (Choi et al., 2012), who observed no loss of intrinsic lumbar dorsal horn neurons expressing the neurokinin 1 (NK1) receptor in deeper laminae and prominent loss of NK1 receptor in laminae I, and others (Khasabov et al., 2002, Vierck et al., 2003, Weisshaar and Winkelstein, 2014, Kras et al., 2015, Araldi et al., 2016b).
To administer IB4-saporin and SSP saporin, rats were briefly anesthetized. Then, a 30-gauge hypodermic needle was inserted, on the midline, into the subarachnoid space, between the L4 and L5 vertebrae. The control treatment consisted of intrathecal injection of the same volume of vehicle (saline). Animals regained consciousness approximately 1 min after stopping anesthesia. There was no effect of IB4-saporin or SSP-saporin on the mechanical nociceptive threshold per se (data not shown).
Statistics
In all experiments, the dependent variable was mechanical paw withdrawal threshold, expressed as percentage change from pre-intervention baseline. The total number of paws used in this study was 394. Group data are represented as mean ± SEM. Statistical significance was determined by unpaired Student’s t-test or by a one-way repeated-measures ANOVA, followed by Dunnett’s multiple comparison or Bonferroni post hoc test. Graph Pad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA) was used to graph data and to perform statistical analyses; a p-value less than 0.05 was considered statistically significant.
Results
Dose dependence of sumatriptan hyperalgesia in female and male rats
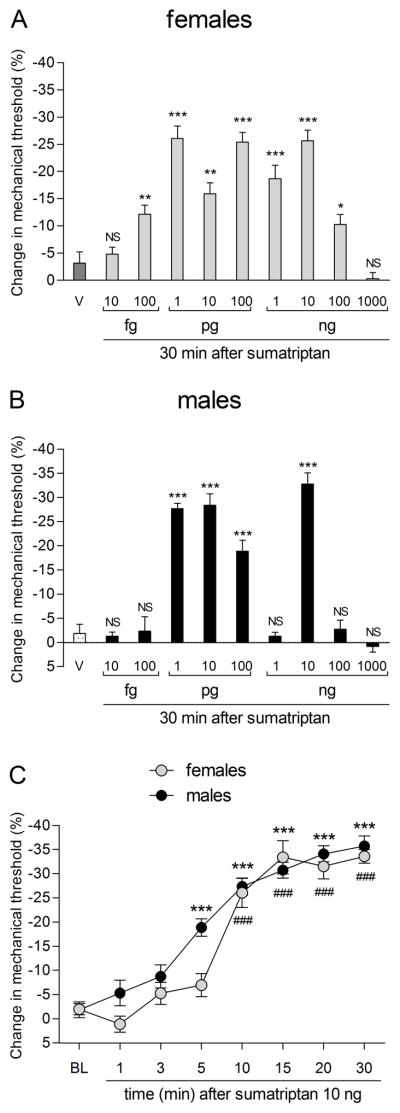
To characterize the pronociceptive effect of sumatriptan, we performed a dose-response study of the effect of intradermal sumatriptan on mechanical nociceptive threshold at the site of injection, in female and male rats. In female rats, over the dose range 10 femtograms (fg) to 100 nanograms (ng) (Fig. 1A), sumatriptan induced a dose-dependent decrease in mechanical nociceptive threshold, significant by 100 fg (Fig. 1A). In male rats we observed, using the same dose range, that sumatriptan induced a complex dose-dependent effect on mechanical nociceptive threshold; Hyperalgesia was first significant at 1 picogram (pg) (Fig. 1B) while 1 ng, had no effect on nociceptive threshold; As the dose was further increased, a second peak in hyperalgesia was observed at 10 ng (Fig. 1B). At the highest dose (1000 ng) sumatriptan did not induce hyperalgesia in either sex. For most of the remaining experiments we used 3 sumatriptan doses 1 pg, 1 ng or 10 ng, to cover the two peaks and the trough in sumatriptan hyperalgesia in the male rats.
Figure 1. Sex differences in dose dependence for sumatriptan-induced mechanical hyperalgesia.
Female (A) and male (B) rats received a single intradermal injection of vehicle (V; saline, 5 μL) or sumatriptan (10 fg at 1000 ng) on the dorsum of the hindpaw and 30 min later, the mechanical nociceptive threshold was evaluated using the Randall-Sellitto paw withdrawal test. A. Significant hyperalgesia was observed in female rats treated with sumatriptan 100 fg (** p = 0.0068), 1 pg (*** p < 0.0001), 10 pg (** p = 0.0013), 100 pg (*** p < 0.0001), 1 ng (*** p = 0.0007), 10 ng (*** p < 0.0001) and 100 ng (* p = 0.0272) when were compared to vehicle (dark gray bar; unpaired Student’s t-test). However, non-significant (NS) changes in the mechanical nociceptive threshold were observed in females that received the doses of 10 fg (NS; p = 0.5165) and 1000 ng (NS; p = 0.2457) when were compared to vehicle (dark gray bar; unpaired Student’s t-test). (N = 6 paws per dose). B. In male rats, we observed a significant hyperalgesia in the groups treated with the doses of 1 and 10 pg (*** p < 0.0001), 100 pg (*** p = 0.0002) and 10 ng (*** p < 0.0001) of sumatriptan when were compared to vehicle group (dotted bar; unpaired Student’s t-test). However, in males that received 10 fg (NS; p = 0.7816), 100 fg (NS; p = 0.8959), 1 ng (NS; p = 0.7689), 100 ng (NS; p = 0.7501) and 1000 ng (NS; p = 0.2471) non-significant (NS) changes in the mechanical nociceptive threshold were observed when compared to vehicle group (dotted bar; unpaired Student’s t-test). (N = 6 paws per dose). C. The mechanical nociceptive threshold was evaluated 1, 3, 5, 10, 15, 20 and 30 minutes after a single injection of sumatriptan (10 ng) on the dorsum of the hindpaw of male rats. Significant hyperalgesia was observed 5 min after injection of sumatriptan (F7,63 = 51.92, ***p < 0.0001; one-way repeated-measures ANOVA followed by Bonferroni post hoc test). BL: baseline. (N = 10 paws)
Sumatriptan hyperalgesia: time dependence in male rats
When the latency to onset of mechanical hyperalgesia was evaluated at the dose of 10 ng of sumatriptan, in male rats, a significant decrease in nociceptive threshold was observed with a latency of ~5 minutes; peak hyperalgesia took 30 minutes to develop (Fig. 1C).
Involvement of estrogen in sexual dimorphism
At a dose of 1 ng, sumatriptan induced mechanical hyperalgesia in female but not male rats (Fig. 1A and 1B, respectively). Male rats implanted with 17β-estradiol did show a decrease in mechanical nociceptive threshold at this dose (Fig. 2). In ovariectomized female rats, sumatriptan (1 ng) did not induce hyperalgesia, indicating that sumatriptan (1 ng)-induced hyperalgesia is estrogen dependent (Fig. 2).
Figure 2. Sumatriptan (1 ng) induces mechanical hyperalgesia in male with the implant of estrogen and not in ovariectomized female rats.
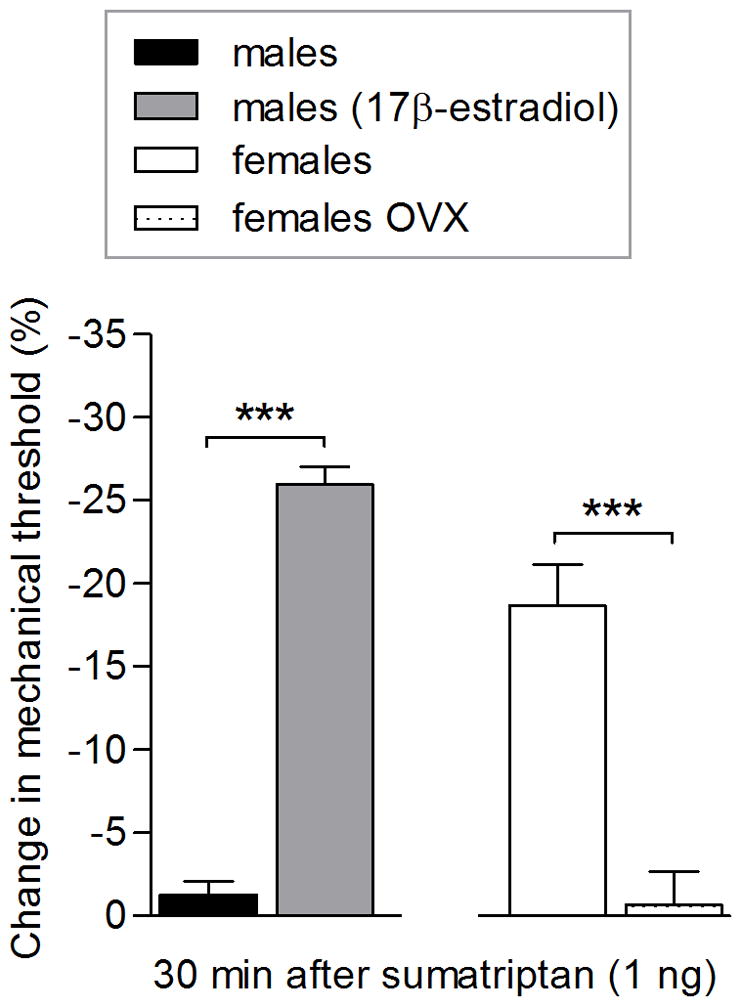
Two weeks after male rats had received a subcutaneous implant of 17β-estradiol (males (17β-estradiol); dotted bar), sumatriptan (1 ng) was injected on the dorsum of the hind paw and the mechanical threshold was evaluated 30 min after its injection. Only in male rats implanted with 17β-estradiol (gray bar) was sumatriptan (1 ng) able to induce hyperalgesia (t = 18.24, *** p < 0.0001; unpaired Student’s t-test). Female rats were ovariectomized (females OVX; dotted bar), and 5 weeks later, sumatriptan (1 ng) was injected showing that OVX females did not develop mechanical hyperalgesia (1 ng; dotted bar). However, in females that were not OVX, sumatriptan (1 ng) was able to induce hyperalgesia (white bar; t = 5.689, *** p = 0.0002; when females and females OVX were compared; unpaired Student’s t-test) indicating that the hyperalgesia induced by sumatriptan (1 ng) is dependent of estrogen. (N = 6 paws per group).
Based on this observation, we evaluated the participation of different estrogen receptors on sumatriptan (1 ng)-induced hyperalgesia in female rats using intrathecal antisense treatment, for 3 consecutive days, with ODN antisense for ER-α, ER-β or GPR30 (Fig. 3A). While treatment with ODN antisense for ER-α or ER-β did not modify sumatriptan (1 ng)-induced hyperalgesia, in female rats that received ODN antisense for GPR30, mechanical hyperalgesia induced by sumatriptan (1 ng) was attenuated (Fig. 3A). The intradermal injection of GPR30 receptor antagonist G-36, also significantly inhibited the sumatriptan (1 ng)-induced hyperalgesia in female rats (Fig. 3B).
Figure 3. Sumatriptan (1 ng)-induced hyperalgesia in female rats is GPR30 dependent.
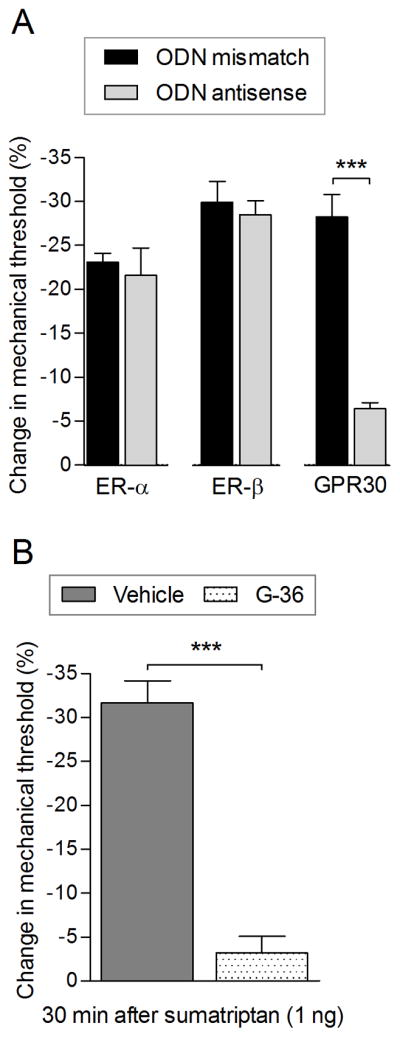
A. Female rats were treated with daily spinal intrathecal injections of ODN mismatch sequence (black bars) or ODN antisense (gray bars) for ER-α, ER-β or GPR30, for 3 consecutive days. On the fourth day, intradermal injection of sumatriptan (1 ng) on the dorsum of the hind paw was performed and, the mechanical nociceptive threshold was evaluated 30 minutes later. Treatment with ODN-antisense for ER-α and ER-β did not affect sumatriptan (1 ng)-induced hyperalgesia. However, in females treated with ODN antisense for GPR30, compared to ODN mismatch for GPR30, 30 minutes after injection of sumatriptan (1 ng) the mechanical hyperalgesia was significantly attenuated (t = 8.336, *** p < 0.0001; when ODN mismatch and ODN antisense for GPR30-treated groups were compared; unpaired Student’s t-test), indicating that the GPR30 plays a role in the sumatriptan (1 ng)-induced hyperalgesia in female rats. (N = 6 paws per group). B. A separate group of female rats received an injection on the dorsum of the hind paw of vehicle (5 μL; dark gray bar) or G-36 (1 μg; a GRP30 receptor antagonist; dotted bar) followed by the injection of sumatriptan (1 ng) at the same site. The mechanical nociceptive threshold was evaluated 30 minutes after sumatriptan injection. Treatment with G-36 (dotted bar), compared to vehicle (dark gray bar), significantly inhibited sumatriptan (1 ng)-induced hyperalgesia measured 30 min after its injection (t = 9.002, *** p < 0.0001; when vehicle and G-36-treated groups were compared; unpaired Student’s t-test), supporting a role for GPR30 in sumatriptan (1 ng)-induced hyperalgesia. (N = 6 paws per group)
Role of 5-HT1B and 5-HT1D receptors
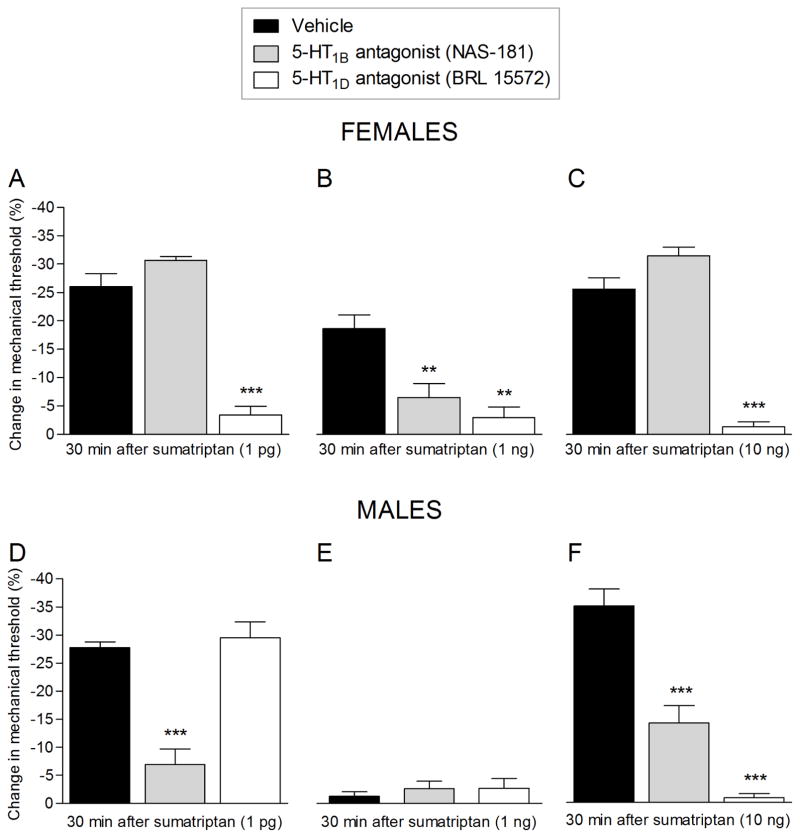
To determine the 5-HT1 receptors mediating the mechanical hyperalgesia induced by sumatriptan we co-administered 1 pg, 1 ng or 10 ng of sumatriptan with an antagonist for the 5-HT1B (NAS-181) or 5-HT1D (BRL 15572) receptors, in female (Fig. 4A, B, and C, upper panel) and male (Fig. 4D, E and F, lower panel) rats. In female rats, the decrease in mechanical nociceptive threshold induced by sumatriptan 1 pg (Fig. 4A, upper panel) and 10 ng (Fig. 4C, upper panel), was completely blocked by the co-injection of 5-HT1D receptor antagonist, but not by blocked by the 5-HT1B receptor antagonist (Fig. 4A and 4C, white bar, upper panel). In contrast, sumatriptan (1 ng)-induced hyperalgesia in the female, was attenuated by antagonists for both 5-HT1B and 5-HT1D receptor (Fig. 4B, upper panel). In male rats, the hyperalgesia induced by 1 pg of sumatriptan was markedly inhibited by the 5-HT1B receptor antagonist, but not significantly by the 5-HT1D receptor antagonist (Fig. 4D, lower panel). The co-administration of 1 ng, which alone did not induce mechanical hyperalgesia, with antagonists for the 5-HT1B or 5-HT1D receptor, also failed to uncover mechanical hyperalgesia (Fig. 4E, lower panel). In marked contrast, while co-injection of the 10 ng dose of sumatriptan, with the 5-HT1B receptor antagonist partially inhibited sumatriptan hyperalgesia, co-injection with the 5-HT1D antagonist completely inhibited mechanical hyperalgesia (Fig. 4F, lower panel).
Figure 4. Sexual dimorphism in the effect of 5-HT1B and 5-HT1D receptor antagonists.
Upper panel: Female rats received vehicle (5 μL; black bar), NAS-181 (1 μg; 5-HT1B receptor antagonist; gray bar) or BRL 15572 (1 μg; 5-HT1D receptor antagonist; white bar) co-injected with sumatriptan [1 pg (A), 1 ng (B) or 10 ng (C)] on the dorsum of the hind paw. The mechanical nociceptive threshold was evaluated 30 min after sumatriptan injection. A. In the group co-injected with BRL 15572, but not with NAS-181, mechanical hyperalgesia induced by sumatriptan (1 pg) was completely prevented (white bar; F = 89.24, *** p < 0.0001; when vehicle, NAS-181, and BRL 15572 groups were compared; one-way repeated-measures ANOVA followed Dunnett’s multiple comparison test). B. A dose of 1 ng of sumatriptan, injected with vehicle (black bar), was able to induce mechanical hyperalgesia in female rats that was significantly attenuated by NAS-181 (gray bar) and BRL 15572 (white bar; F = 12.32, ** p = 0.0020, when vehicle, NAS-181, and BRL 15572 groups were compared; one-way repeated-measures ANOVA followed Dunnett’s multiple comparison test). C. When the dose of 10 ng of sumatriptan was co-injected with vehicle (black bar) or NAS-181 (gray bar) a robust hyperalgesia was observed; however, when co-injected with BRL 15572 (white bar) the hyperalgesia was completely blocked (F = 91.70, *** p < 0.0001; when vehicle, NAS-181, and BRL 15572 groups were compared; one-way repeated-measures ANOVA followed Dunnett’s multiple comparison test). (N = 6 paws per group)
Lower panel: Male rats received vehicle (5 μL; black bar), NAS-181 (1 μg; 5-HT1B receptor antagonist; gray bar) or BRL 15572 (1 μg; 5-HT1D receptor antagonist; white bar) co-injected on the dorsum of the hind paw, with sumatriptan [1 pg (D), 1 ng (E) or 10 ng (F)]. The mechanical nociceptive threshold was evaluated 30 min after sumatriptan injection. D. Mechanical hyperalgesia induced by co-injection of vehicle and sumatriptan (1 pg; black bar) was prevented by co-injection of NAS-181 (gray bar; F = 46.67, *** p < 0.0001; when vehicle, NAS-181, and BRL 15572 groups were compared; one-way repeated-measures ANOVA followed Dunnett’s multiple comparison test) but not by BRL 15572. E. When sumatriptan (1 ng; black bar) was co-injected with vehicle, we did not observe changes in mechanical threshold, neither when sumatriptan (1 ng) was co-injected with NAS-181 (gray bar) nor BRL 15572 (white bar). F. Vehicle co-injected with sumatriptan (10 ng; black bar) induced a robust hyperalgesia that was significantly attenuated by the co-injection of NAS-181 (gray bar) and was completely inhibited by BRL 15572 (white bar; F = 62.94, *** p < 0.0001; when vehicle, NAS-181 or BRL 15572 groups were compared; one-way repeated-measures ANOVA followed Dunnett’s multiple comparison test). (N = 6 paws per group)
5-HT1B and 5-HT1D agonists in male rats
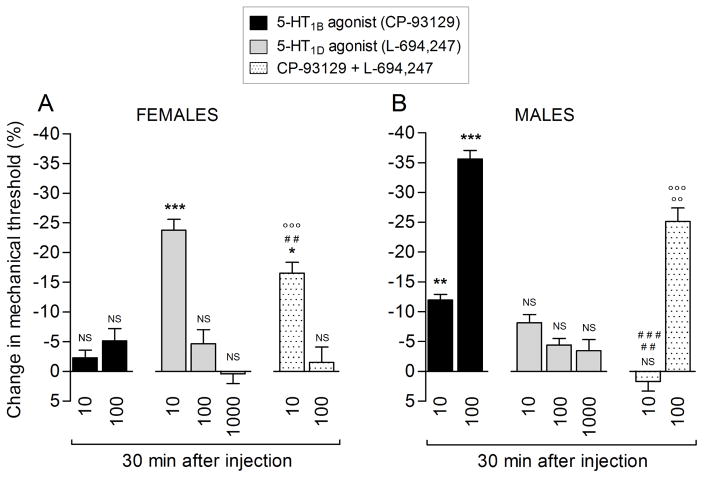
Sumatriptan (1 pg and 10 ng) induces 5-HT1B and/or 5-HT1D receptor-mediated mechanical hyperalgesia in male rats (Fig. 4D and 4F, lower panel). To confirm the pronociceptive effect of agonism at these triptan receptors, we studied the nociceptive effect of selective 5-HT1B and 5-HT1D receptor agonists. The 5-HT1B receptor agonist (CP-93129, 100 ng; Fig. 5, black bar) induced a robust decrease in mechanical nociceptive threshold, while, at the 10 ng dose (Fig. 5, black bar), the change observed was small when compared to baseline. The 5-HT1D-receptor agonist (L-694,247; Fig. 5, gray bars) did not induce a change in the mechanical nociceptive threshold, at the doses tested, 10, 100 and 1000 ng. We also tested the effect of the combination of 5-HT1B and 5-HT1D receptor agonists (Fig. 5, dotted bars; CP-93129 and L-694,247, respectively) at the dose of 10 ng and 100 ng. We observed that mechanical nociceptive threshold induced by CP-93129 (100 ng) was significantly attenuated when co-injected with 100 ng of L-694,247.
Figure 5. Agonists for the 5-HT1B, but not 5-HT1D, receptor induce hyperalgesia.
Rats were treated on the dorsum of the hind paw with a single injection of an agonist for 5-HT1B receptor (CP-93129; 10 or 100 ng; black bars), 5-HT1D receptor (L-694,247; 10 or 100 ng; gray bars) or a combination of CP-93129 + L-694,247 (10 or 100 ng; dotted bars). Thirty minutes later the mechanical nociceptive threshold was evaluated. We found that the agonist for 5-HT1B (CP-93129; black bars) at the dose of 10 and 100 ng induced a decrease in the mechanical nociceptive threshold (** p = 0.0031 and *** p < 0.0001, respectively; unpaired Student’s t-test) when compared to baseline (before the agonist injection). However, the agonist for 5-HT1D (L-694,247; gray bars) did not induce changes in mechanical nociceptive threshold, at the doses of 10 (NS; p = 0.0963), 100 (NS; p = 0.0907) or 1000 ng (NS; p = 0.0999) when compared to baseline (unpaired Student’s t-test). When co-injected on the dorsum of the hind paw CP-93129 + L-694,247 (10 ng; dotted bar) we observed non-significant (NS) change in the mechanical nociceptive threshold (NS; p = 0.4980) when compared to baseline; however, when compared to CP-93129 (10 ng, black bar; ### p = 0.0010) or L-694,247 (10 ng, gray bar; ## p = 0.0030) we observed an increase in the mechanical nociceptive threshold (unpaired Student’s t-test). At the dose of 100 ng of a combination of CP-93129 + L-694,247 (dotted bar), we observed a decrease in mechanical nociceptive threshold when was compared to baseline and L-694,247 (gray bar; ○○○ p < 0.0001; unpaired Student’s t-test); on the other hand, when the combination (100 ng; dotted bar) was compared to CP-93129 (100 ng; black bar) we observed an attenuation in the mechanical hyperalgesia induced by CP-93129 (○○ p = 0.0048; unpaired Student’s t-test). (N = 6 paws per group)
Inhibitory G-protein αi subunit and PKA dependence in male rats
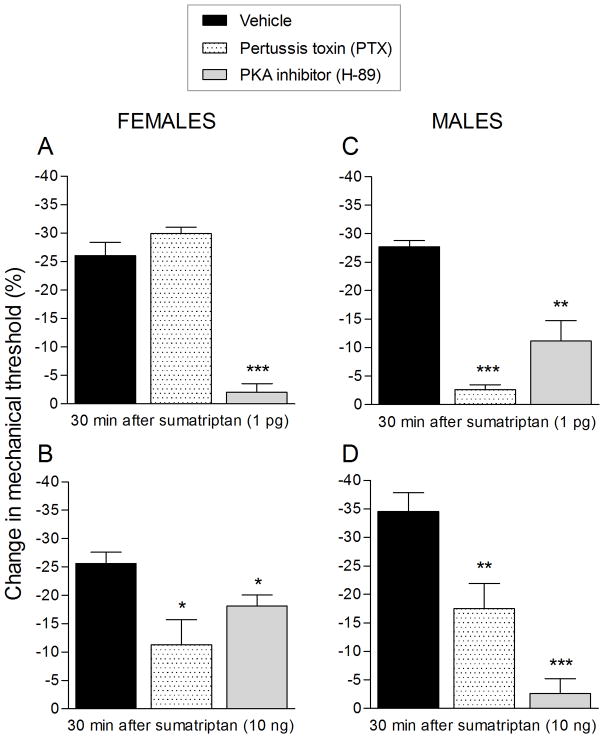
Sumatriptan (1 pg)-induced mechanical hyperalgesia was attenuated by G-protein αi subunit inhibitor (PTX, Fig. 6A; gray bar) and completely blocked in the presence of PKA inhibitor (H-89, Fig. 6A; dotted bar). On the other hand, the mechanical hyperalgesia induced by sumatriptan (10 ng) was completely blocked by PTX (Fig. 6B, gray bar) and attenuated in the presence of H-89 (Fig. 4B, white bar).
Figure 6. Mechanical hyperalgesia induced by sumatriptan (1 pg or 10 ng) depends on G-protein αi subunit and PKA.
Male rats were treated, on the dorsum of the hind paw, with vehicle (5 μL; black bar), pertussis toxin (PTX; 1 μg; gray bar) or PKA inhibitor (H-89, 1 μg; dotted bar). Ten minutes after the treatment, sumatriptan [1 pg (A) or 10 ng (B)] was injected at the same site and the mechanical hyperalgesia evaluated 30 min after its injection. A. Treatment with pertussis toxin (PTX; gray bar) significantly attenuated the sumatriptan (1 pg)-induced mechanical hyperalgesia (** p = 0.0012, when vehicle and PTX groups were compared; unpaired Student’s t-test). A PKA inhibitor (H-89; dotted bar), completely blocked the sumatriptan (1 pg)-induced hyperalgesia (*** p < 0.0001; unpaired Student’s t-test; when vehicle and PKA inhibitor groups were compared). B. In a group of rats treated with PTX (gray bar) the mechanical hyperalgesia induced by sumatriptan (10 ng) was completely blocked 30 min after its injection (*** p = 0.0003; when vehicle and PTX groups were compared; unpaired Student’s t-test) and, in the group treated with H-89 (dotted bar), sumatriptan (10 ng)-induced hyperalgesia was attenuated (** p = 0.0085; when vehicle and H-89 groups were compared; unpaired Student’s t-test). (N = 6 paws per group)
Role of IB4-positive and negative nociceptors
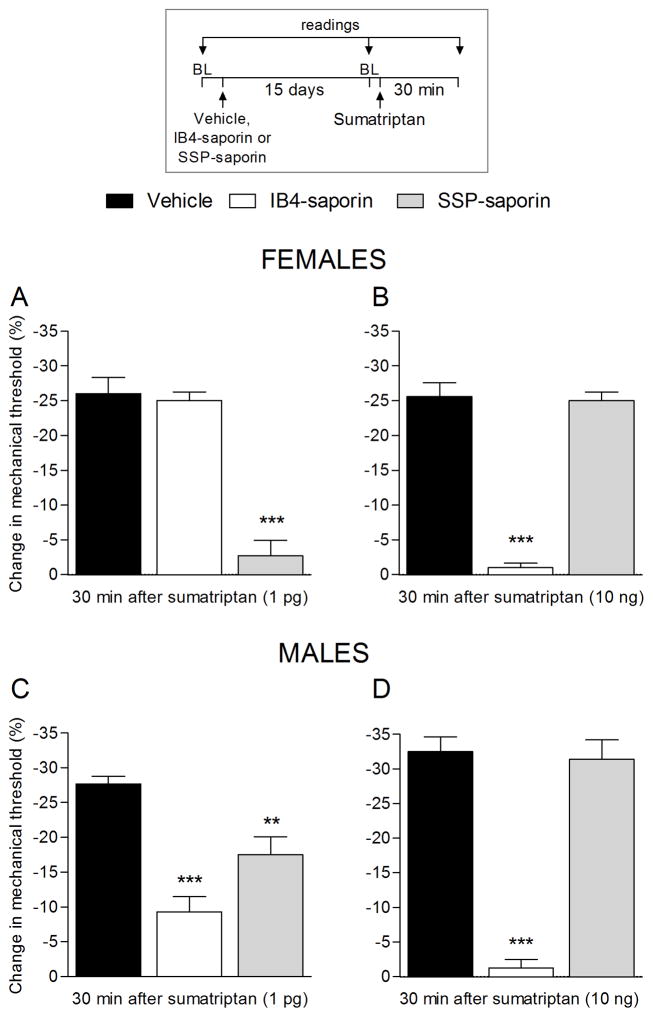
In a recent study, we demonstrated that sumatriptan induces type I hyperalgesic priming by action at 5-HT1B and 5-HT1D receptors on IB4-positive nociceptors (Araldi et al., 2016b). To determine if sumatriptan-induced hyperalgesia is mediated by action at receptors on IB4-positive or IB4-negative nociceptors we pre-treated male rats, intrathecally, with IB4-saporin or SSP-saporin, to destroy IB4-positive or IB4-negative nociceptors, respectively. Treatment with IB4-saporin or SSP-saporin, attenuated sumatriptan (1 pg)-induced hyperalgesia (Fig. 7A, white and gray bars). In contrast, the treatment with IB4-saporin, but not SSP-saporin, completely prevented sumatriptan (10 ng)-induced mechanical hyperalgesia (Fig. 7B, white bar), demonstrating dose dependence for the effect of sumatriptan on different classes of nociceptors.
Figure 7. Mechanical hyperalgesia induced by sumatriptan depends on IB4-positive and IB4-negative neurons (1 pg) or only IB4-positive neurons (10 ng).
Male rats were treated with vehicle (control, black bars), IB4-saporin (3.2 μg/20 μL; white bars) or SSP-saporin (100 ng/20 μL; gray bars) by intrathecal injection. Fifteen days later, sumatriptan [1 pg (A) or 10 ng (B)] was injected on the dorsum of the hind paw and the mechanical nociceptive threshold was evaluated 30 min later. A. One-way repeated-measures ANOVA followed Dunnett’s multiple comparison test demonstrated a significant attenuation of sumatriptan (1 pg)-induced hyperalgesia in the group previously treated with IB4-saporin (F = 32.13, *** p < 0.0001) and with SSP-saporin (** p < 0.0001; when vehicle, IB4-saporin, and SSP-saporin groups were compared; one-way repeated-measures ANOVA followed Dunnett’s multiple comparison test). B. A complete inhibition of sumatriptan (10 ng)-induced hyperalgesia was observed in the group previously treated with IB4-saporin (F = 78.92, *** p < 0.0001; when the vehicle, IB4-saporin, and SSP-saporin groups were compared; one-way repeated-measures ANOVA followed Dunnett’s multiple comparison test) but not with SSP-saporin. BL: baseline. (N = 6 paws per group)
Discussion
While triptans provide effective relief for migraine in many patients, they produce pain at the injection site, and headache, chest and abdominal pain, and pain/tenderness in the extremities (Hillis and Macintyre, 1993, Dahlof et al., 1994, Houghton et al., 1994, Ottervanger et al., 1994, Solomon et al., 1997, Duquesnoy et al., 1998, Gomez-Mancilla et al., 2001, Wang et al., 2002, Coulter et al., 2003, Linde et al., 2004). In the present experiments we observed that intradermal injection of sumatriptan (10 fg – 1000 ng) produces mechanical hyperalgesia at the injection site, as has been reported in patients being treated for a migraine (Burstein and Jakubowski, 2004, Burstein et al., 2005, Olesen et al., 2009, Tipton et al., 2015), and evaluated dose dependence, sexual dimorphism and the underlying mechanism.
In female rats, hyperalgesia was first detected at 100 fg (Fig. 1A). In male rats, a complex dose-dependence was observed (Fig. 1B). Two low doses of sumatriptan (1 and 10 pg) induced robust mechanical hyperalgesia. As the dose was increased the magnitude of the hyperalgesia decreased, to the point where 1 ng had no effect on nociceptive threshold. However, the next higher dose (10 ng) again induced robust hyperalgesia, which became undetectable at the 100 and 1000 ng doses. At the highest dose, 1000 ng, we did not observe hyperalgesia in either female or male rats. Sumatriptan has a very high affinity for 5-HT1B and 5-HT1D receptors and may achieve a large degree of receptor saturation at very low concentration. Thus, the loss of hyperalgesia at the highest dose, in both male and female rats, likely reflects action at an additional target. While a direct comparison is not possible, Kayser and colleagues also found a non-monotonic relationship for the effect of both sumatriptan and zolmitriptan, injected subcutaneously, in reducing mechanical hypersensitivity after chronic constriction injury to the infraorbital nerve in male rats (Kayser et al., 2002). While there is some data on dose dependence of the anti-migraine effect of triptans (Cady et al., 1991, Visser et al., 1992, Tfelt-Hansen, 1993, Mushet et al., 1996, Brandes et al., 2009, Derry et al., 2014), similar data for its pronociceptive effects have not been reported.
At the 1 ng dose, we observed hyperalgesia in females, but not in males, a sex difference that is estrogen dependent since, in male rats implanted with 17β-estradiol we observed hyperalgesia, while we did not in ovariectomized females. In good agreement with previous reports (Lu et al., 2009, Rossi et al., 2010) our data support the idea that estrogen desensitizes 5-HT1B and 5-HT1D receptors since when we knock down GPR30 receptor, but not ER-α or ER-β, using antisense ODN or antagonist for GPR30 (G-36), we attenuated sumatriptan (1 ng)-induced hyperalgesia in female rats.
Since the clinical effect of sumatriptan is thought to be mediated by two receptors, 5-HT1B and 5-HT1D, we evaluated the role of these receptors in sumatriptan-induced hyperalgesia. In female rats, mechanical hyperalgesia induced by the 1 pg or 10 ng doses of sumatriptan is mediated entirely by the 5-HT1D receptor while that induced by the 1 ng dose is mediated by 5-HT1B and 5-HT1D. In contrast, in male rats, the hyperalgesia induced by 1 pg is dependent predominantly on 5-HT1B while that induced by 10 ng is dependent almost entirely of 5-HT1D. The intermediate dose, 1 ng, did not have a nociceptive effect alone or in the presence of 5-HT1B or 5-HT1D receptor antagonists, in male rats. Unexpectedly, while the 5-HT1B receptor agonist (CP-93129) produced a decrease in mechanical nociceptive threshold, the 5-HT1D receptor agonist (L-694,247) had no effect on nociceptive threshold. In contrast, when we co-injected 5-HT1B and 5-HT1D receptor agonists, we found that the hyperalgesia induced by the 5-HT1B receptor agonist was attenuated.
In the present experiments, we found that sumatriptan (1 pg and 10 ng)-induced mechanical hyperalgesia is G-protein αi subunit and PKA-dependent. Of note, we have previously shown that agonists at two other Gi-protein-coupled receptors, mu-opioid and A1-adenosine, can also induce mechanical hyperalgesia; however, the hyperalgesia induced by agonists at these latter two Gi-GPCRs requires repeated administration (Araldi et al., 2015, 2016a).
Gi-protein-coupled receptor agonists, typically act to attenuate neuronal activity, prototypically by decreasing cyclic adenosine monophosphate (cAMP), constitute important targets for analgesic drugs, such as opioids (Pierre et al., 2009, Al-Hasani and Bruchas, 2011) and triptans (Pierre et al., 2009). Paradoxically, we found that sumatriptan-induced hyperalgesia is mediated by the αi subunit of the heterotrimeric Gi-protein and by PKA. Of note, the ability of pertussis toxin (a Gi-protein inhibitor) to prevent sumatriptan-induced hyperalgesia is also characteristic of the mechanical hyperalgesia induced by repeated injections of the A1-adenosine receptor agonist CPA (Araldi et al., 2016a), but not by repeated injections of the mu-opioid receptor agonist DAMGO (Araldi et al., 2015).
We found that hyperalgesia induced by 1 pg of sumatriptan is dependent on both IB4-positive and negative nociceptors, while that induced by 10 ng is dependent on the IB4-positive population. While the literature suggests that expression of 5-HT1B and 5-HT1D receptors are restricted to non-peptidergic neurons (Hou et al., 2001, Ma et al., 2001, Potrebic et al., 2003), Harriott and Gold detected 5-HT1D receptors in both peptidergic and non-peptidergic fibers in the rat dura mater (Harriott and Gold, 2008). Our data are in agreement since the sumatriptan (1 pg)-induced hyperalgesia is dependent on IB4-positive (non-peptidergic) and IB4-negative (peptidergic) nociceptors while sumatriptan (10 ng) hyperalgesia did not occur in rats in which IB4-positive nociceptors have been destroyed. In addition to trigeminal sensory neurons, it has been demonstrated by us, and others, that 5-HT1B and 5-HT1D receptors are located in lumbar dorsal root ganglion neurons (Pierce et al., 1996, Wotherspoon and Priestley, 2000, Potrebic et al., 2003). Our study does not exclude a differential action by the different doses of sumatriptan at different 5-HT receptor subtypes and populations of nociceptors since we tested neurons mediating the effect of the dose of 1 pg and 10 ng of sumatriptan.
Agonists at the mu-opioid (Gi-protein-coupled) receptor can also produce an exacerbation of pain (Mercadante and Arcuri, 2005, Chu et al., 2008, Chen et al., 2009, Hay et al., 2009, Lee et al., 2011), a phenomenon referred to as opioid-induced hyperalgesia (OIH). Recently, we have shown that repeated intradermal injections of agonists of Gi-protein-coupled receptors (GPCRs), such as mu-opioid receptor agonist DAMGO (Araldi et al., 2015) or A1-adenosine receptor agonist CPA (Araldi et al., 2016a), induce mechanical hyperalgesia, which started ~5 minutes after the fourth (DAMGO) or the third (CPA) injection. However, our present data demonstrated that a single injection of sumatriptan induced a robust decrease in mechanical nociceptive threshold, which was significant 5 minutes after its injection. Linde and colleagues have shown that sumatriptan may cause a mechanical allodynia and a reduction of heat and cold pain thresholds in humans (Linde et al., 2004).
Conclusion
In summary, our study demonstrates a marked sexual dimorphism in the pronociceptive effects of sumatriptan, by its action at 5-HT1B and/or 5-HT1D receptors, which is GPR30 dependent. Our data also demonstrate that sumatriptan-induced hyperalgesia is dependent on the G-protein αi subunit, and PKA activation in IB4-positive and negative nociceptors. The full details of the mechanisms responsible for the complex dose dependence for triptans hyperalgesia remain to be established. The elucidation of the basic features of triptan-induced hyperalgesia may provide important information needed to design novel anti-migraine drugs with improved therapeutic profiles.
Highlights.
Mechanical hyperalgesia induced by sumatriptan is markedly sexually dimorphic;
Sumatriptan produces hyperalgesia by acting on 5-HT1B and/or 5-HT1D receptors;
Sumatriptan hyperalgesia in female rats is dependent on estrogen and GPR30;
G-protein αi subunit and PKA play a role in sumatriptan hyperalgesia;
Sumatriptan hyperalgesia is mediated by IB4-positive and negative nociceptors.
Acknowledgments
This study was funded by the National Institutes of Health (NIH), United States, grant number NS084545. The authors report no conflicts of interest.
Abbreviations
- 5-HT
5-hydroxytryptamine
- 5-HT1B
5-hydroxytryptamine receptor subtype 1B
- 5-HT1D
5-hydroxytryptamine receptor subtype 1D
- PKA
protein kinase A
- GPCR
G-protein-coupled receptor
- GPR30
G-protein-coupled estrogen receptor 30 (GPR30)
- ANOVA
analysis of variance
- CPA
N6-Cyclopentyladenosine
- DAMGO
[d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
Footnotes
Author’s contribution
D.A.: designed research and performed experiments, analyzed the data and wrote the manuscript; L.F.F.: performed experiments; P.G.: performed experiments; J.D.L.: designed research, wrote the manuscript. All authors read and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115:1363–1381. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Bogen O, Levine JD. Role of nociceptor estrogen receptor GPR30 in a rat model of endometriosis pain. Pain. 2014;155:2680–2686. doi: 10.1016/j.pain.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD. Repeated Mu-Opioid Exposure Induces a Novel Form of the Hyperalgesic Priming Model for Transition to Chronic Pain. J Neurosci. 2015;35:12502–12517. doi: 10.1523/JNEUROSCI.1673-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD. Adenosine-A1 receptor agonist induced hyperalgesic priming type II. Pain. 2016a;157:698–709. doi: 10.1097/j.pain.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD. Gi-Protein Coupled 5-HT1B/D Receptor Agonist Sumatriptan Induces Type I Hyperalgesic Priming. Pain. 2016b doi: 10.1097/j.pain.0000000000000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD. Generation of a pain memory in the primary afferent nociceptor triggered by PKCepsilon activation of CPEB. J Neurosci. 2012;32:2018–2026. doi: 10.1523/JNEUROSCI.5138-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle AB, Snowdowne KW. Measurement of intracellular free calcium in monkey kidney cells with aequorin. Science. 1982;217:252–254. doi: 10.1126/science.6806904. [DOI] [PubMed] [Google Scholar]
- Brandes JL, Cady RK, Freitag FG, Smith TR, Chandler P, Fox AW, Linn L, Farr SJ. Needle-free subcutaneous sumatriptan (Sumavel DosePro): bioequivalence and ease of use. Headache. 2009;49:1435–1444. doi: 10.1111/j.1526-4610.2009.01530.x. [DOI] [PubMed] [Google Scholar]
- Burch RM, Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987;84:6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–110. doi: 10.1016/s0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- Burstein R, Levy D, Jakubowski M. Effects of sensitization of trigeminovascular neurons to triptan therapy during migraine. Rev Neurol (Paris) 2005;161:658–660. doi: 10.1016/s0035-3787(05)85109-4. [DOI] [PubMed] [Google Scholar]
- Cady RK, Wendt JK, Kirchner JR, Sargent JD, Rothrock JF, Skaggs H., Jr Treatment of acute migraine with subcutaneous sumatriptan. JAMA. 1991;265:2831–2835. [PubMed] [Google Scholar]
- Chen L, Malarick C, Seefeld L, Wang S, Houghton M, Mao J. Altered quantitative sensory testing outcome in subjects with opioid therapy. Pain. 2009;143:65–70. doi: 10.1016/j.pain.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JI, Koehrn FJ, Sorkin LS. Carrageenan induced phosphorylation of Akt is dependent on neurokinin-1 expressing neurons in the superficial dorsal horn. Mol Pain. 2012;8:4. doi: 10.1186/1744-8069-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- Coulter DM, Passier JL, Clark DW, van Puijenbroek EP. Activation of pain by sumatriptan. Headache. 2003;43:994–999. doi: 10.1046/j.1526-4610.2003.03192.x. [DOI] [PubMed] [Google Scholar]
- Dahlof C, Ekbom K, Persson L. Clinical experiences from Sweden on the use of subcutaneously administered sumatriptan in migraine and cluster headache. Arch Neurol. 1994;51:1256–1261. doi: 10.1001/archneur.1994.00540240100023. [DOI] [PubMed] [Google Scholar]
- Derry CJ, Derry S, Moore RA. Sumatriptan (all routes of administration) for acute migraine attacks in adults - overview of Cochrane reviews. Cochrane Database Syst Rev. 2014:CD009108. doi: 10.1002/14651858.CD009108.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquesnoy C, Mamet JP, Sumner D, Fuseau E. Comparative clinical pharmacokinetics of single doses of sumatriptan following subcutaneous, oral, rectal and intranasal administration. Eur J Pharm Sci. 1998;6:99–104. doi: 10.1016/s0928-0987(97)00073-0. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Androgens’ effects to enhance learning may be mediated in part through actions at estrogen receptor-beta in the hippocampus. Neurobiol Learn Mem. 2007;87:78–85. doi: 10.1016/j.nlm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Khomula EV, Araldi D, Levine JD. Marked Sexual Dimorphism in the Role of the Ryanodine Receptor in a Model of Pain Chronification in the Rat. Sci Rep. 2016:8. doi: 10.1038/srep31221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Levine JD. Plasma membrane mechanisms in a preclinical rat model of chronic pain. J Pain. 2015;16:60–66. doi: 10.1016/j.jpain.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Mancilla B, Cutler NR, Leibowitz MT, Spierings EL, Klapper JA, Diamond S, Goldstein J, Smith T, Couch JR, Fleishaker J, Azie N, Blunt DE. Safety and efficacy of PNU-142633, a selective 5-HT1D agonist, in patients with acute migraine. Cephalalgia. 2001;21:727–732. doi: 10.1046/j.1468-2982.2001.00208.x. [DOI] [PubMed] [Google Scholar]
- Harriott AM, Gold MS. Serotonin type 1D receptors (5HTR) are differentially distributed in nerve fibres innervating craniofacial tissues. Cephalalgia. 2008;28:933–944. doi: 10.1111/j.1468-2982.2008.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JL, White JM, Bochner F, Somogyi AA, Semple TJ, Rounsefell B. Hyperalgesia in opioid-managed chronic pain and opioid-dependent patients. J Pain. 2009;10:316–322. doi: 10.1016/j.jpain.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Hillis WS, Macintyre PD. Sumatriptan and chest pain. Lancet. 1993;342:683. doi: 10.1016/0140-6736(93)91792-k. [DOI] [PubMed] [Google Scholar]
- Hou M, Kanje M, Longmore J, Tajti J, Uddman R, Edvinsson L. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res. 2001;909:112–120. doi: 10.1016/s0006-8993(01)02645-2. [DOI] [PubMed] [Google Scholar]
- Houghton LA, Foster JM, Whorwell PJ, Morris J, Fowler P. Is chest pain after sumatriptan oesophageal in origin? Lancet. 1994;344:985–986. doi: 10.1016/s0140-6736(94)91642-x. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain. 2008;9:463–472. doi: 10.1016/j.jpain.2008.01.335. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Sexual dimorphism for protein kinase c epsilon signaling in a rat model of vincristine-induced painful peripheral neuropathy. Neuroscience. 2003a;119:831–838. doi: 10.1016/s0306-4522(03)00203-3. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Sexual dimorphism in the contribution of protein kinase C isoforms to nociception in the streptozotocin diabetic rat. Neuroscience. 2003b;120:907–913. doi: 10.1016/s0306-4522(03)00400-7. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169:431–435. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser V, Aubel B, Hamon M, Bourgoin S. The antimigraine 5-HT 1B/1D receptor agonists, sumatriptan, zolmitriptan and dihydroergotamine, attenuate pain-related behaviour in a rat model of trigeminal neuropathic pain. Br J Pharmacol. 2002;137:1287–1297. doi: 10.1038/sj.bjp.0704979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabov SG, Rogers SD, Ghilardi JR, Peters CM, Mantyh PW, Simone DA. Spinal neurons that possess the substance P receptor are required for the development of central sensitization. J Neurosci. 2002;22:9086–9098. doi: 10.1523/JNEUROSCI.22-20-09086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kras JV, Weisshaar CL, Pall PS, Winkelstein BA. Pain from intra-articular NGF or joint injury in the rat requires contributions from peptidergic joint afferents. Neurosci Lett. 2015;604:193–198. doi: 10.1016/j.neulet.2015.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145–161. [PubMed] [Google Scholar]
- Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord. 2002;26:1103–1109. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- Linde M, Elam M, Lundblad L, Olausson H, Dahlof CG. Sumatriptan (5-HT1B/1D-agonist) causes a transient allodynia. Cephalalgia. 2004;24:1057–1066. doi: 10.1111/j.1468-2982.2004.00782.x. [DOI] [PubMed] [Google Scholar]
- Lu CL, Hsieh JC, Dun NJ, Oprea TI, Wang PS, Luo JC, Lin HC, Chang FY, Lee SD. Estrogen rapidly modulates 5-hydroxytrytophan-induced visceral hypersensitivity via GPR30 in rats. Gastroenterology. 2009;137:1040–1050. doi: 10.1053/j.gastro.2009.03.047. [DOI] [PubMed] [Google Scholar]
- Ma QP, Hill R, Sirinathsinghji D. Colocalization of CGRP with 5-HT1B/1D receptors and substance P in trigeminal ganglion neurons in rats. Eur J Neurosci. 2001;13:2099–2104. doi: 10.1046/j.0953-816x.2001.01586.x. [DOI] [PubMed] [Google Scholar]
- Mercadante S, Arcuri E. Hyperalgesia and opioid switching. Am J Hosp Palliat Care. 2005;22:291–294. doi: 10.1177/104990910502200411. [DOI] [PubMed] [Google Scholar]
- Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Mushet GR, Cady RK, Baker CC, Clements B, Gutterman DL, Davis R. Efficacy and tolerability of subcutaneous sumatriptan administered using the IMITREX STATdose System. Clin Ther. 1996;18:687–699. doi: 10.1016/s0149-2918(96)80219-0. [DOI] [PubMed] [Google Scholar]
- Nishiguchi J, Sasaki K, Seki S, Chancellor MB, Erickson KA, de Groat WC, Kumon H, Yoshimura N. Effects of isolectin B4-conjugated saporin, a targeting cytotoxin, on bladder overactivity induced by bladder irritation. Eur J Neurosci. 2004;20:474–482. doi: 10.1111/j.1460-9568.2004.03508.x. [DOI] [PubMed] [Google Scholar]
- Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- Ottervanger JP, van Witsen TB, Valkenburg HA, Grobbee DE, Stricker BH. Adverse reactions attributed to sumatriptan. A postmarketing study in general practice. Eur J Clin Pharmacol. 1994;47:305–309. doi: 10.1007/BF00191159. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- Pierce PA, Xie GX, Levine JD, Peroutka SJ. 5-Hydroxytryptamine receptor subtype messenger RNAs in rat peripheral sensory and sympathetic ganglia: a polymerase chain reaction study. Neuroscience. 1996;70:553–559. doi: 10.1016/0306-4522(95)00329-0. [DOI] [PubMed] [Google Scholar]
- Pierre S, Eschenhagen T, Geisslinger G, Scholich K. Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov. 2009;8:321–335. doi: 10.1038/nrd2827. [DOI] [PubMed] [Google Scholar]
- Potrebic S, Ahn AH, Skinner K, Fields HL, Basbaum AI. Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: implications for the selective antimigraine action of triptans. J Neurosci. 2003;23:10988–10997. doi: 10.1523/JNEUROSCI.23-34-10988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quanhong Z, Ying X, Moxi C, Tao X, Jing W, Xin Z, Li W, Derong C, Xiaoli Z, Wei J. Intrathecal PLC(beta3) oligodeoxynucleotides antisense potentiates acute morphine efficacy and attenuates chronic morphine tolerance. Brain Res. 2012;1472:38–44. doi: 10.1016/j.brainres.2012.06.030. [DOI] [PubMed] [Google Scholar]
- Rossi DV, Dai Y, Thomas P, Carrasco GA, DonCarlos LL, Muma NA, Li Q. Estradiol-induced desensitization of 5-HT1A receptor signaling in the paraventricular nucleus of the hypothalamus is independent of estrogen receptor-beta. Psychoneuroendocrinology. 2010;35:1023–1033. doi: 10.1016/j.psyneuen.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ER, Damassa DA, Davidson JM. Hormone administration: Peripheral and intracranial implants. In: Meyer RD, editor. Methods in Psychobiology. Academic Press; New York: 1977. pp. 259–279. [Google Scholar]
- Solomon S, Lipton RB, Newman LC. The site of common side effects of sumatriptan. Headache. 1997;37:289–290. doi: 10.1046/j.1526-4610.1997.3705289.x. [DOI] [PubMed] [Google Scholar]
- Song MJ, Wang YQ, Wu GC. Additive anti-hyperalgesia of electroacupuncture and intrathecal antisense oligodeoxynucleotide to interleukin-1 receptor type I on carrageenan-induced inflammatory pain in rats. Brain Res Bull. 2009;78:335–341. doi: 10.1016/j.brainresbull.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011;12:120. doi: 10.1186/1471-2202-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JL, Xiao C, Lu B, Zhang J, Yuan XZ, Chen W, Yu LN, Zhang FJ, Chen G, Yan M. CX3CL1/CX3CR1 regulates nerve injury-induced pain hypersensitivity through the ERK5 signaling pathway. J Neurosci Res. 2013;91:545–553. doi: 10.1002/jnr.23168. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32:577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Prostaglandin effects after elimination of indirect hyperalgesic mechanisms in the skin of the rat. Brain Res. 1989;492:397–399. doi: 10.1016/0006-8993(89)90928-1. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P. Sumatriptan for the treatment of migraine attacks--a review of controlled clinical trials. Cephalalgia. 1993;13:238–244. doi: 10.1046/j.1468-2982.1993.1304238.x. [DOI] [PubMed] [Google Scholar]
- Tipton AF, Tarash I, McGuire B, Charles A, Pradhan AA. The effects of acute and preventive migraine therapies in a mouse model of chronic migraine. Cephalalgia. 2015 doi: 10.1177/0333102415623070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, Jr, Kline RH, Wiley RG. Intrathecal substance p-saporin attenuates operant escape from nociceptive thermal stimuli. Neuroscience. 2003;119:223–232. doi: 10.1016/s0306-4522(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Visser WH, Ferrari MD, Bayliss EM, Ludlow S, Pilgrim AJ. Treatment of migraine attacks with subcutaneous sumatriptan: first placebo-controlled study. The Subcutaneous Sumatriptan International Study Group. Cephalalgia. 1992;12:308–313. doi: 10.1046/j.1468-2982.1992.1205308.x. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience. 2001;108:143–155. doi: 10.1016/s0306-4522(01)00377-3. [DOI] [PubMed] [Google Scholar]
- Wang JT, Barr CE, Goldfarb SD. Impact of chest pain on cost of migraine treatment with almotriptan and sumatriptan. Headache. 2002;42(Suppl 1):38–43. doi: 10.1046/j.1526-4610.2002.0420s1038.x. [DOI] [PubMed] [Google Scholar]
- Weisshaar CL, Winkelstein BA. Ablating spinal NK1-bearing neurons eliminates the development of pain and reduces spinal neuronal hyperexcitability and inflammation from mechanical joint injury in the rat. J Pain. 2014;15:378–386. doi: 10.1016/j.jpain.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley RG, Kline RHt, Vierck CJ., Jr Anti-nociceptive effects of selectively destroying substance P receptor-expressing dorsal horn neurons using [Sar9,Met(O2)11]-substance P-saporin: behavioral and anatomical analyses. Neuroscience. 2007;146:1333–1345. doi: 10.1016/j.neuroscience.2007.01.066. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Priestley JV. Expression of the 5-HT1B receptor by subtypes of rat trigeminal ganglion cells. Neuroscience. 2000;95:465–471. doi: 10.1016/s0306-4522(99)00465-0. [DOI] [PubMed] [Google Scholar]