Introduction
Endothelin 1 (ET1, see table 1 for gene and protein abbreviations) signaling has been recognized as a driver of osteoblastic metastasis for more than a decade and recent work points to its having a broader role in bone biology. This review will first outline the ET signaling pathway and ET metabolism. It will next summarize the role of ET1 signaling in craniofacial development. Then, it will discuss observations relating ET signaling to osteoblastic and other osteosclerotic processes in cancer. Finally, it will describe recent work in our laboratory that points to endothelin signaling as the role of as an upstream mediator of WNT signaling, promoting bone matrix synthesis and mineralization. It will conclude with a statement of some remaining gaps in knowledge and proposals for future research. These will be informed by insights gained from study of ET signaling in the development and physiology of the cardiovascular system.
Table 1.
Genes and Protein Abbreviations
| Protein | Human Gene | Mouse Gene | |
|---|---|---|---|
| Endothelin 1 | ET1 | EDN1 | Edn1 |
| Endothelin 2 | ET2 | EDN2 | Edn2 |
| Endothelin 3 | ET3 | EDN3 | Edn3 |
| Endothelin A Type Receptor | EDNRA | EDNRA | Ednra |
| Endothelin B Type Receptor | EDNRB | EDNRB | Ednrb |
| Endothelin Converting Enzyme 1 | ECE1 | ECE1 | Ece1 |
| Endothelin Converting Enzyme 2 | ECE2 | ECE2 | Ece2 |
Overview of the ET Signaling Pathway
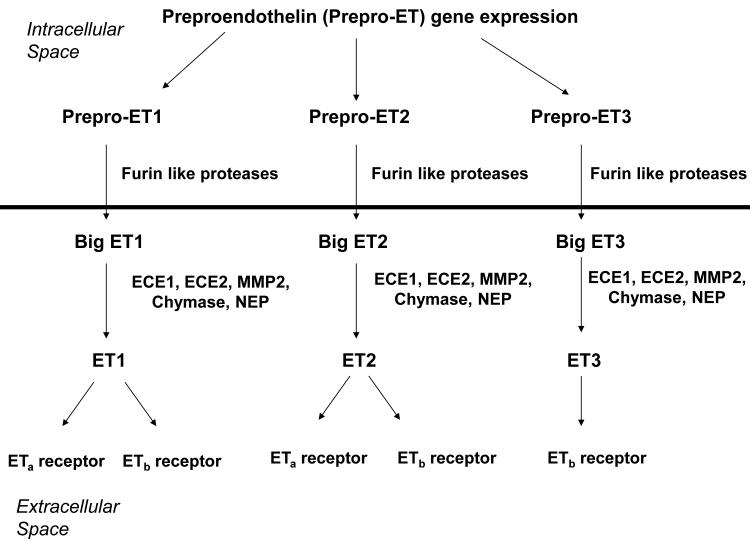
The ET system includes 3 small peptide hormones1-3, ET1, ET2, and ET3, 2 G-protein coupled receptors4,5, EDNRA and EDNRB, and 2 specific converting enzymes6,7, ECE1 and ECE2. The ETs are synthesized as prepropeptides that are first processed to biologically inactive, 37-41 amino acid propeptides, commonly known as “big ET’s,” by furin-like proteases prior to secretion8,9. Following secretion, the big ETs must be converted to their active forms by proteolytic cleavage in the extracellular space. ECE1 and ECE2, which have different pH optima (neutral pH optimal for ECE1, acidi pH optimal for ECE2), catalyze ET activation by cleaving the big ET’s to 21 amino acid active ETs. In addition, big ET’s can be converted by a variety of other proteases (figures 1 and 2)10-12.
Figure 1. Schematic representation of endothelin synthesis, secretion, and receptor binding.
The horizontal line represents the cell membrane, with events above the line occurring intracellularly and those below the line occurring extracellularly. Both the ET receptors and the ECEs are membrane bound, but their ligand binding/catalytic sites are extracellular. ETA represents the A type endothelin receptor, encoded by EDNRA in humans and Ednra in mice. ETb represents the B type endothelin receptor, encoded by EDNRB in humans and Ednrb in mice. All 3 endothelins are initially synthesized as pre-propeptides, encoded by EDN1, EDN2, and EDN3 in humans and Edn1, Edn2, and Edn3 in mice. They are processed to the respective big ETs by furin-like proteases prior to secretion. The big ETs are further processed to the mature, biologically active ETs by the ECEs or other extracellular proteases. B type receptors in endothelial cells promote NO synthesis, cell survival, and ET clearance. Both A type and B type receptors promote smooth muscle cell contraction and collagen synthesis by fibroblasts.
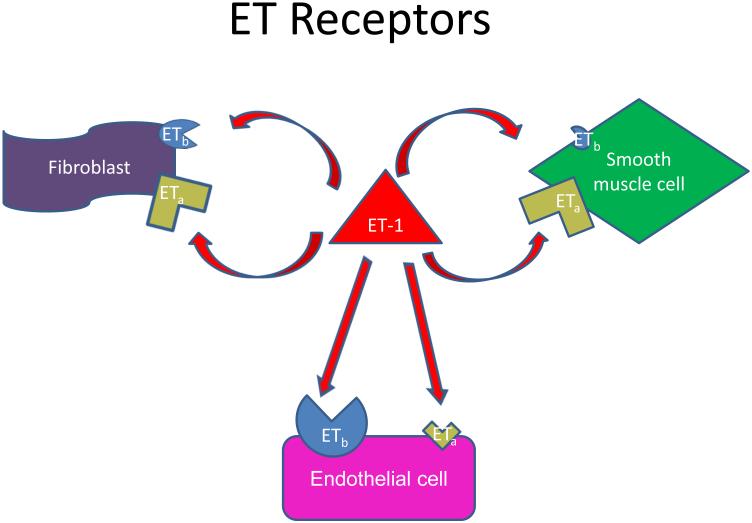
Figure 2. Schematic representation of autocrine and paracrine ET1 signaling.
In blood vessels, the balance of autocrine and paracrine signaling is important in determining the biological response to ET signaling. Big ET1 is secreted by endothelial cells. It can be processed to mature ET1 by membrane-bound ECE1 by the endothelial cells and signal in an autocrine fashion via either A type or B type receptors on the endothelial cells. These cells express predominantly B type receptor, thus favoring vasodilatory responses. Alternatively, mature ET1 (21 amino acids) or big ET1 (38 amino acids) can diffuse in the extracellular space. Big ET1 can be activated by other tissue proteases and can signal via ET receptors located on smooth muscle cells, fibroblasts, or other cells present in the vessel wall or perivascular space. Activation of these cell types by ET1 promotes vasoconstriction and thickening/stiffening of the media. The activity of ECE1 can alter the balance of autocrine v paracrine ET1 signaling.
The ET system was discovered in arteries, and it has since been shown that various elements of the system are expressed in a wide variety of tissues, but expression is not ubiquitous. Immortalized osteoblasts in culture express ET1, EDNRA, and ECE1, thus having the capacity for autocrine ET signaling within the lineage13. Conversely, ET2, ET3, EDNRB and ECE2 are either not detected or expressed at very low levels in these cells13.
ET1 Signaling in Development
Knockout mice lacking either ET1 or EDNRA have very similar, lethal phenotypes that result from malformations of the craniofacial bones14,15. Mice die shortly after birth due to asphyxia, which can be overcome by tracheostomy. They have hypoplastic mandibles, homeotic transformation of the mandible to a maxillary morphology16,17. There are multiple defects in other facial and basilar skull bones, and the hyoid bones. Together, these defects obstruct the airway, leading to the observed lethality. Identical craniofacial defects are observed in ECE1 knockout mice18.
In addition to the craniofacial abnormalities, ET1 and EDNRA knockout mice share defects of the cardiac outflow tract and great vessels15,19. These defects are not fully penetrant, and include tubular hypoplasia of the aortic arch, absent right subclavian artery and perimembranous ventricular septal defect. In ECE1 knockout mice, these defects are both more severe and more penetrant than in the ET1 and EDNRA knockouts18. Box 1 provides further information about ET signaling in cardiovascular physiology.
BOX 1. Physiological ET Signaling in the Cardiovascular System.
The ET system was first discovered in the vasculature and its biology is best understood in that setting. ET signaling via EDNRB on endothelial cells causes vasodilation and clearance of ETs from the circulation 48-50. In contrast, signaling via both EDNRA and EDNRB in smooth muscle cells has potent vasoconstrictive effects 1,51. Endothelial cells are the primary source of ET1 and have a high density of EDNRB, so autocrine signaling favors vasodilation and hypotensive responses, while paracrine signaling leads to hypertensive responses. In experiments we performed using the same mice in which we identified Ece1 as a candidate gene for bone biomechanical performance, we found that mice harboring a high-expressing Ece1 allele had larger femoral and arterial cross-sections, greater arterial compliance, and lower BP than mice harboring a low-expressing Ece1 allele 52.
Shear stress plays a critical role in vascular remodeling, both in developmental 53 and physiological54-56 settings. NO is known to play a central role in mediating vascular remodeling in pregnancy and its induction by shear stress is well established57-59. In this setting, NO is produced by NOS3 and the Nos3 gene is induced by EDNRB activation in endothelial cells49. Insufficient EDNRB function exacerbates inward hypertophic vascular remodeling by low flow60. Taken together, these findings suggest that ET signaling via EDNRB could contribute to outward remodeling in response to high shear stress.
While osteoblasts and endothelial cells express all the genes necessary for autocrine ET1 signaling, in blood vessels there is a necessary balance between autocrine and paracrine ET1 signaling. Paracrine signaling contributes to the greater thickness and higher smooth muscle content of arterial v venous walls. Even though arterial and venous identities are established prior to the onset of circulation61, mechanical signals reinforce and amplify those differences 53. It is presently unknown whether specific mechanical environments lead to differential endothelial cell expression of Edn1 and of Ece1. Should this prove to be the case, differential regulation of these genes by distinct mechanical environments could provide a mechanism by which wall stress and shear stress might lead to distinct adaptive responses.
The common element linking the craniofacial and cardiovascular anomalies is that the affected structures are derived from the neural crest. Cranial and cardiac neural crest cells migrate early in development and express EDNRA15. It is worth noting that knockouts of ET3 and EDNRB, which are also lethal, affect a different population of neural crest derivatives. Phenotypes of these mutations include colonic aganglionosis (Hirschsprung’s disease) and white spotting20,21. As in the case of EDNRA/ET1, EDNRB/ET3 knockouts have very similar phenotypes. ECE1 knockout animals have all the defects characteristic of both the EDNRA/ET1 and EDNRB/ET3 knockouts18.
ET Signaling in Osteoblastic Metastasis
ET signaling occurs in both mammary and prostate glands, and when it is expressed in neoplasms arising from those tissues, it promotes osteoblastic metastasis22-26. Osteoblastic metastasis is very common in prostate cancer and uncommon in breast cancer. In both cases, lesions feature synthesis of sclerotic, woven bone that is mechanically deficient. We recently found that ET1 is highly expressed in the setting of osteosclerosis associated with myelofibrosis27, with very similar features to those encountered in breast and prostate cancer. Promotion of osteogenesis by ET1 signaling is mediated at least in part by modulation of WNT signaling. In cultured mouse calvarial osteoblasts, ET1 reduces transcription and secretion of DKK1, and concomitantly increases bone formation28. In men with metastatic prostate cancer, EDNRA blockade suppressed progression of bone disease as measured by bone turnover markers29.
Human breast cancer cells can convert big ET1 to active ET124,30, suggesting the potential role of ET1 in bone metastasis in breast cancer. EDNRA blockade reduced osteoblastic lesions in mice inoculated with ZR-75-1 human breast cancer cells24. Collectively, these results show that ET1 action via EDNRA mediate bone formation in both breast and prostate cancer. The bone cells, in turn, secrete factors that can support proliferation of the tumor cells, such as IGF1, resulting in a vicious cycle of concurrent bone and tumor growth supported by reciprocal paracrine signaling between tumor cells and osteoblasts.
ET Signaling in Bone Physiology
In the course of pursuing our long-standing interest in the genetic basis of bone biomechanical performance, we identified Ece1 as a candidate gene for a pleiotropic quantitative trait locus (QTL) for bone size, shape, and strength31-35. The QTL increases the cross-sectional size and ellipticity of the femoral diaphysis, leading to an increase in the whole bone strength as measured by 3-point bending. This constellation of phenotypes suggested that the primary process affected by the QTL was bone modeling in response to mechanical loading (see box 2). Other investigators had determined that our QTL lies within a genomic region that contributes to load-induced bone modeling36, leading us to hypothesize that both sets of phenotypes were mediated by the same genes. It is worth noting that the mouse QTL corresponds to a confirmed human BMD QTL37.
BOX 2. The Skeletal Mechanostat and the WNT Pathway.
The ability of bone to alter its size and shape in response to its habitual mechanical environment is well established. Overloading leads to an increase in long bone cross-sectional size, as was demonstrated in elite racquet sport athletes 62. Conversely, underloading, as occurs in spaceflight, prolonged bed rest, or spinal cord injury leads to loss of skeletal mass 63-65. The notion that bone mass is physiologically regulated has been formalized in the mechanostat model of bone modeling 66,67. Briefly stated, the model holds that bone modeling (change in diameter and/or cross-sectional geometry) occurs to maintain mechanical strain (fractional change in length) within a narrow physiological range. Once such physiological equilibrium is reached, bone size and shape remains stable unless disturbed.
Widely accepted experimental interventions to allow study of defined skeletal loading conditions in experimental animals have been developed. Ulnar loading coupled with dynamic histomorphometry allows the in vivo modeling response to loading to be measured, using the contralateral, unloaded limb as a control 12. Tail suspension 68 and sciatic neurectomy 69 both allow study of in vivo unloading. These powerful investigative tools have been used in genetically engineered mice to identify critical molecules mediating mechanotransduction.
The canonical WNT signaling pathway is one of the principal mechanisms by which bone responds to its mechanical environment. Recognition of its central role in bone biology emerged from the recognition that inactivating and activating mutations of LRP5, a WNT co-receptor, caused two rare Mendelian conditions, the osteoporosis pseudoglioma syndrome and hereditary high bone mass, respectively 70-72. Mendelian high bone mass high bone mass disorders, sclerosteosis and Van Buchem’s disease, arise as a consequence of mutations in SOST, the gene encoding the WNT antagonist sclerostin 73,74. Overexpression of the WNT inhibitor DKK1 drives bone resorption in multiple myeloma75.
While human disease provided the first clues that WNT signaling is critical in bone physiology, mechanistic understanding of bone mass regulation has been achieved through study of mouse models. Experiments featuring ulnar loading demonstrated that loss of function Lrp5 (mouse homolog of LRP5) mutations lead to decreased load-induced modeling76,77, while mutations that mimic human high bone mass variants display increased modeling in response to mechanical loading77-79. These modeling responses demonstrate that disruption of the WNT pathway affects physiology as well as development.
SOST is a WNT inhibitor and is produced constitutively by mature osteocytes, but its expression is decreased in the presence of mechanical loading47. Mice in which Sost (the mouse gene encoding SOST) has been knocked out display increased bone mass, mimicking the human sclerosteosis phenotype80,81.
Furthermore, SNPs within or near genes involved in the WNT pathway are associated with mass and fractures in humans37,82-85. In the case of LRP5, there is evidence from the Framingham cohort that the association with BMD is exercise dependent86. Anti-SOST antibodies are presently being tested as possible drugs to increase bone mass and prevent fracture87,88.
In subsequent experiments, we found that murine osteoblasts grown in tissue culture engage in autocrine ET1 signaling via EDNRA13 (and Johnson et al., unpublished data). They express Edn1, Ednra, and Ece1, but not Ednrb. These cells recapitulate maturation of the osteoblast lineage when placed in medium supplemented with vitamin C and phosphate, forming mineralized nodules after 2 weeks in mineralization medium. Supplementation of the medium with big ET1 increases mineralization, which is blocked by pharmacological inhibition of either ECE1 or EDNRA, treatment with SOST, or by transfection of siRNA targeting Ece1 message. In addition to promoting mineralization, ET1 treatment reduces secretion of SOST and DKK1, in spite of increasing transcription of their mRNAs. These divergent effects on transcription and protein secretion are mediated in part by miR 126-3p, which is increased by ~120-fold via ET signaling, which targets Sost message. The effects of ET1 signaling on mineralization and SOST secretion are mimicked by transfection of a miR 126-3p expressing lentivirus, while the effects of ET1 signaling blockade are mimicked by transfection of a lentivirus expressing a miR 126-3p antagonist.
MiR 126-3p is a critical molecule in angiogenesis38. MiR 126-3p ablated mice have a high rate of embryonic lethality with impaired blood vessel formation, while the surviving mice are deficient in the angiogenic response to experimental ischemia39. Important endothelial cell targets of miR-126-3p include, but are not limited to, mRNAs encoding a pair of VEGF inhibitors, Pik3r2 and Spred1. It is interesting to note that both in osteoblasts and in endothelial cells, miR 126-3p acts by releasing repression of critical signaling pathways.
Future Considerations
Current understanding of ET biology is very uneven. The developmental roles of ET signaling are relatively well characterized, as is ET signaling within arterial walls. It is also clear that ET drives osteoblastic metastasis in breast and prostate cancer, but little is known regarding the role of ET signaling in the normal function of each of these glands, or of the contribution ET signaling to normal bone physiology. Greater understanding of the ET signaling pathway’s role in the normal biology of tissues outside the vasculature is therefore of great importance.
It is important to recall that ET1 signaling is essential in development14,15,18,19, and that the phenotypes resulting from knockout of Edn1, Ednra, and Ece1 overlap some of those arising from mutants affecting WNT signaling. Ablation of Ece1, unlike that of either Edn1 or Ednra, leads to significant mid-gestational in utero lethality due to heart failure, reflecting a greater severity of cardiac outflow tract abnormalities18. In the chick, ET1 signaling mediates essential mechanotransductive signals in Purkinje system development40-43.
However, the situation is more complex. WNT signaling has an unequivocal trophic impact on cells already committed to the osteoblast lineage; indeed, canonical WNT signaling has been shown by some investigators to inhibit commitment of mesenchymal stem cells to the osteoblast lineage44,45. In addition, mice constitutively expressing β-catenin in late stage osteoblasts and osteocytes have both increased bone volume and osteomalacia46. These findings demonstrate that normal bone mineralization requires down-regulation of canonical WNT signaling at late stages of osteoblast maturation, even while elaboration of bone matrix by less mature cells is promoted.
Down-regulation of SOST, leading to derepression of the WNT pathway has been demonstrated in an in vivo experimental mechanical loading protocol47. It is unknown at this point whether ET signaling mediates the SOST response in this setting, thus functioning upstream of the WNT pathway in mediating mechanotransduction in bone. Our laboratory’s findings that ET1 signaling reduces SOST expression via miR-126-3p provide a mechanistic basis for pursuing this line of investigation13 (and Johnson et al., unpublished data). Other investigators have identified DKK1 as another WNT-related target of ET1 signaling in cancer28, further supporting the idea that ET signaling is upstream of the WNT pathway in bone.
More broadly, little attention has been devoted to identifying common mechanisms of mechanotransduction in bones and arteries. Both are tubular organs whose function requires adaptation to highly variable mechanical environments. Bone and vascular biology might both benefit by further work in this area.
Finally, osteoblastic metastases arise in other tumors in addition to prostate and breast cancer. It is worth studying other tumor types to determine whether they share ET1 signaling as the underlying mechanism.
Key Points.
The endothelin system includes 3 small peptide hormones that are secreted as inactive precursors, a pair of G-protein coupled receptors, and a pair of membrane bound, extracellular converting enzymes.
Endothelin 1/endothelin receptor A signaling is essential for the development of the craniofacial skeleton. Knockouts of the Edn1, Ednra, and Ece1 genes have lethal phenotypes.
Endothelin signaling is osteogenic in the setting of prostate and breast cancer.
Genetic evidence points to allelic variation of Ece1 as a mediator of bone biomechanical performance.
In vitro experiments indicate that ET signaling derepresses WNT signaling, and thus may function upstream of WNT in mediating mechanical homeostasis of skeletal mass.
Synopsis.
The endothelin (ET) system includes 3 small peptide hormones and a pair of G-protein coupled receptors. All 3 ETs are secreted as biologically inert precursors that must be activated by proteolytic cleavage after secretion. This reaction can be catalyzed by a pair of specific, membrane bound extracellular endothelin converting enzymes or by nonspecific tissue proteases. The ET1/EDNRA axis is essential in development, with knockout mice for either ET1 or EDNRA displaying a similar phenotype featuring multiple defects of the craniofacial skeleton and cardiac outflow tract. Prostate and breast cancers sometimes display osteoblastic metastases driven by high tumor cell expression of ET1. ET1-driven osteosclerosis may also occur in the setting of myelofibrosis. Osteoblasts express ET1, EDNRA, and ECE1, and therefore are capable of autocrine ET signaling. Searches for genes that mediate individual differences in bone biomechanical performance have identified the gene encoding ECE1 as a candidate. Mechanistic studies in vitro show that ET signaling in osteoblasts increases matrix synthesis and mineralization and derepresses WNT signaling, acting in part via the micro-RNA miR 126-3p.
Acknowledgments
This work was supported in part by SPiRe Award #1I21 RX1440 from the United States Department of Veterans Affairs Rehabilitation Research and Development Service to Robert D. Blank. In addition, this material is the result of work supported with resources and the use of facilities at the Geriatrics Research, Education, and Clinical Center at the William S. Middleton Veterans Hospital in Madison, WI. The opinions expressed herein are those of the authors, and do not represent the views of the US government.
This work was supported in part by American Heart Association grant 15GRNT25700126 to Robert D. Blank.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. Blank is an investigator in a clinical trial sponsored by Novo-Nordisk, a consultant for Bristol-Myers Squibb, and a contributor to UpToDate and receives royalties for this work. Drs Kristianto, Johnson and Afzal have nothing to disclose.
References
- 1.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 2.Yanagisawa M, Inoue A, Ishikawa T, et al. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci U S A. 1988;85(18):6964–6967. doi: 10.1073/pnas.85.18.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue A, Yanagisawa M, Kimura S, et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai T, Yanagisawa M, Takuwa Y, et al. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 6.Xu D, Emoto N, Giaid A, et al. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78(3):473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- 7.Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J Biol Chem. 1995;270(25):15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- 8.Denault JB, Claing A, D'Orleans-Juste P, et al. Processing of proendothelin-1 by human furin convertase. FEBS letters. 1995;362(3):276–280. doi: 10.1016/0014-5793(95)00249-9. [DOI] [PubMed] [Google Scholar]
- 9.Inoue A, Yanagisawa M, Takuwa Y, Mitsui Y, Kobayashi M, Masaki T. The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. The Journal of biological chemistry. 1989;264(25):14954–14959. [PubMed] [Google Scholar]
- 10.Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85(10):906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- 11.Wypij DM, Nichols JS, Novak PJ, Stacy DL, Berman J, Wiseman JS. Role of mast cell chymase in the extracellular processing of big-endothelin-1 to endothelin-1 in the perfused rat lung. Biochem Pharmacol. 1992;43(4):845–853. doi: 10.1016/0006-2952(92)90252-e. [DOI] [PubMed] [Google Scholar]
- 12.Abassi ZA, Tate JE, Golomb E, Keiser HR. Role of neutral endopeptidase in the metabolism of endothelin. Hypertension. 1992;20(1):89–95. doi: 10.1161/01.hyp.20.1.89. [DOI] [PubMed] [Google Scholar]
- 13.Johnson MG, Kristianto J, Yuan B, Konicke K, Blank R. Big endothelin changes the cellular miRNA environment in TMOb osteoblasts and increases mineralization. Connect Tissue Res. 2014;55(Suppl 1):113–116. doi: 10.3109/03008207.2014.923866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurihara Y, Kurihara H, Suzuki H, et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368(6473):703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- 15.Clouthier DE, Hosoda K, Richardson JA, et al. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125(5):813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- 16.Ozeki H, Kurihara Y, Tonami K, Watatani S, Kurihara H. Endothelin-1 regulates the dorsoventral branchial arch patterning in mice. Mech Dev. 2004;121(4):387–395. doi: 10.1016/j.mod.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Ruest LB, Clouthier DE. Elucidating timing and function of endothelin-A receptor signaling during craniofacial development using neural crest cell-specific gene deletion and receptor antagonism. Dev Biol. 2009;328(1):94–108. doi: 10.1016/j.ydbio.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanagisawa H, Yanagisawa M, Kapur RP, et al. Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development. 1998;125(5):825–836. doi: 10.1242/dev.125.5.825. [DOI] [PubMed] [Google Scholar]
- 19.Kurihara Y, Kurihara H, Oda H, et al. Aortic arch malformations and ventricular septal defect in mice deficient in endothelin-1. J Clin Invest. 1995;96(1):293–300. doi: 10.1172/JCI118033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baynash AG, Hosoda K, Giaid A, et al. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79(7):1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 21.Hosoda K, Hammer RE, Richardson JA, et al. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79(7):1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 22.Nelson JB, Hedican SP, George DJ, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nature medicine. 1995;1(9):944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 23.Berruti A, Dogliotti L, Gorzegno G, et al. Differential patterns of bone turnover in relation to bone pain and disease extent in bone in cancer patients with skeletal metastases. Clin Chem. 1999;45:1240–1247. 8 Pt 1. [PubMed] [Google Scholar]
- 24.Yin JJ, Mohammad KS, Kakonen SM, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci U S A. 2003;100(19):10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohammad KS, Guise TA. Mechanisms of osteoblastic metastases: role of endothelin-1. Clin Orthop Relat Res. 2003;(415 Suppl):S67–74. doi: 10.1097/01.blo.0000093047.96273.4e. [DOI] [PubMed] [Google Scholar]
- 26.Guise TA, Yin JJ, Mohammad KS. Role of endothelin-1 in osteoblastic bone metastases. Cancer. 2003;97(3 Suppl):779–784. doi: 10.1002/cncr.11129. [DOI] [PubMed] [Google Scholar]
- 27.Yachoui R, Kristianto J, Sitwala K, Blank RD. Role of Endothelin-1 in a Syndrome of Myelofibrosis and Osteosclerosis. J Clin Endocrinol Metab. 2015;100(11):3971–3974. doi: 10.1210/jc.2015-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clines GA, Mohammad KS, Bao Y, et al. Dickkopf homolog 1 mediates endothelin-1-stimulated new bone formation. Mol Endocrinol. 2007;21(2):486–498. doi: 10.1210/me.2006-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson JB, Nabulsi AA, Vogelzang NJ, et al. Suppression of prostate cancer induced bone remodeling by the endothelin receptor A antagonist atrasentan. J Urol. 2003;169(3):1143–1149. doi: 10.1097/01.ju.0000042162.08938.27. [DOI] [PubMed] [Google Scholar]
- 30.Patel KV, Sheth HG, Schrey MP. Stimulation or endothelin-1 secretion by human breast cancer cells through protein kinase A activation: a possible novel paracrine loop involving breast fibroblast-derived prostaglandin E2. Mol Cell Endocrinol. 1997;126(2):143–151. doi: 10.1016/s0303-7207(96)03983-4. [DOI] [PubMed] [Google Scholar]
- 31.Saless N, Litscher SJ, Vanderby R, Demant P, Blank RD. Linkage mapping of principal components for femoral biomechanical performance in a reciprocal HCB-8 x HCB-23 intercross. Bone. 2011;48(3):647–653. doi: 10.1016/j.bone.2010.10.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saless N, Litscher SJ, Houlihan MJ, et al. Comprehensive skeletal phenotyping and linkage mapping in an intercross of recombinant congenic mouse strains HcB-8 and HcB-23. Cells Tissues Organs. 2011;194(2-4):244–248. doi: 10.1159/000324774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saless N, Lopez Franco GE, Litscher S, et al. Linkage mapping of femoral material properties in a reciprocal intercross of HcB-8 and HcB-23 recombinant mouse strains. Bone. 2010;46(5):1251–1259. doi: 10.1016/j.bone.2010.01.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saless N, Litscher SJ, Lopez Franco GE, et al. Quantitative trait loci for biomechanical performance and femoral geometry in an intercross of recombinant congenic mice: restriction of the Bmd7 candidate interval. FASEB J. 2009;23(7):2142–2154. doi: 10.1096/fj.08-118679. [DOI] [PubMed] [Google Scholar]
- 35.Kristianto J, Litscher SJ, Johnson MG, et al. Congenic Strains Confirm the Pleiotropic Effect of Chromosome 4 QTL on Mouse Femoral Geometry and Biomechanical Performance. PloS one. 2016;11(2):e0148571. doi: 10.1371/journal.pone.0148571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robling AG, Li J, Shultz KL, Beamer WG, Turner CH. Evidence for a skeletal mechanosensitivity gene on mouse chromosome 4. FASEB J. 2003;17(2):324–326. doi: 10.1096/fj.02-0393fje. [DOI] [PubMed] [Google Scholar]
- 37.Rivadeneira F, Styrkarsdottir U, Estrada K, et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41(11):1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gourdie RG, Wei Y, Kim D, Klatt SC, Mikawa T. Endothelin-induced conversion of embryonic heart muscle cells into impulse-conducting Purkinje fibers. Proc Natl Acad Sci U S A. 1998;95(12):6815–6818. doi: 10.1073/pnas.95.12.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall CE, Hurtado R, Hewett KW, et al. Hemodynamic-dependent patterning of endothelin converting enzyme 1 expression and differentiation of impulse-conducting Purkinje fibers in the embryonic heart. Development. 2004;131(3):581–592. doi: 10.1242/dev.00947. [DOI] [PubMed] [Google Scholar]
- 42.Sedmera D, Harris BS, Grant E, et al. Cardiac expression patterns of endothelin-converting enzyme (ECE): implications for conduction system development. Dev Dyn. doi: 10.1002/dvdy.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takebayashi-Suzuki K, Yanagisawa M, Gourdie RG, Kanzawa N, Mikawa T. In vivo induction of cardiac Purkinje fiber differentiation by coexpression of preproendothelin-1 and endothelin converting enzyme-1. Development. 2000;127(16):3523–3532. doi: 10.1242/dev.127.16.3523. [DOI] [PubMed] [Google Scholar]
- 44.Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93(6):1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- 45.Liu G, Vijayakumar S, Grumolato L, et al. Canonical Wnts function as potent regulators of osteogenesis by human mesenchymal stem cells. J Cell Biol. 2009;185(1):67–75. doi: 10.1083/jcb.200810137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen S, Feng J, Bao Q, et al. Adverse Effects of Osteocytic Constitutive Activation of ss-Catenin on Bone Strength and Bone Growth. J Bone Miner Res. 2015;30(7):1184–1194. doi: 10.1002/jbmr.2453. [DOI] [PubMed] [Google Scholar]
- 47.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283(9):5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 48.Masaki T, Kimura S, Yanagisawa M, Goto K. Molecular and cellular mechanism of endothelin regulation. Implications for vascular function. Circulation. 1991;84(4):1457–1468. doi: 10.1161/01.cir.84.4.1457. [DOI] [PubMed] [Google Scholar]
- 49.Tsukahara H, Ende H, Magazine HI, Bahou WF, Goligorsky MS. Molecular and functional characterization of the non-isopeptide-selective ETB receptor in endothelial cells. Receptor coupling to nitric oxide synthase. The Journal of biological chemistry. 1994;269(34):21778–21785. [PubMed] [Google Scholar]
- 50.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227(4693):1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 51.Sumner MJ, Cannon TR, Mundin JW, White DG, Watts IS. Endothelin ETA and ETB receptors mediate vascular smooth muscle contraction. Br J Pharmacol. doi: 10.1111/j.1476-5381.1992.tb14537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Kristianto J, Yen Ooi C, et al. Blood pressure, artery size, and artery compliance parallel bone size and strength in mice with differing ece1 expression. Journal of biomechanical engineering. 2013;135(6):61003–61009. doi: 10.1115/1.4024161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134(18):3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231(4736):405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 55.Tuttle JL, Nachreiner RD, Bhuller AS, et al. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol. 2001;281(3):H1380–1389. doi: 10.1152/ajpheart.2001.281.3.H1380. [DOI] [PubMed] [Google Scholar]
- 56.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101(4):731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Zheng J, Bird IM, Magness RR. Effects of pulsatile shear stress on nitric oxide production and endothelial cell nitric oxide synthase expression by ovine fetoplacental artery endothelial cells. Biology of reproduction. 2003;69(3):1053–1059. doi: 10.1095/biolreprod.102.013474. [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Zheng J, Bird IM, Magness RR. Effects of pulsatile shear stress on signaling mechanisms controlling nitric oxide production, endothelial nitric oxide synthase phosphorylation, and expression in ovine fetoplacental artery endothelial cells. Endothelium : journal of endothelial cell research. 2005;12(1-2):21–39. doi: 10.1080/10623320590933743. [DOI] [PubMed] [Google Scholar]
- 59.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. The American journal of physiology. 1997;272:R441–463. doi: 10.1152/ajpregu.1997.272.2.R441. 2 Pt 2. [DOI] [PubMed] [Google Scholar]
- 60.Murakoshi N, Miyauchi T, Kakinuma Y, et al. Vascular endothelin-B receptor system in vivo plays a favorable inhibitory role in vascular remodeling after injury revealed by endothelin-B receptor-knockout mice. Circulation. 2002;106(15):1991–1998. doi: 10.1161/01.cir.0000032004.56585.2a. [DOI] [PubMed] [Google Scholar]
- 61.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93(5):741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 62.Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204–208. [PubMed] [Google Scholar]
- 63.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19(6):1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 64.Donaldson CL, Hulley SB, Vogel JM, Hattner RS, Bayers JH, McMillan DE. Effect of prolonged bed rest on bone mineral. Metabolism. 1970;19(12):1071–1084. doi: 10.1016/0026-0495(70)90032-6. [DOI] [PubMed] [Google Scholar]
- 65.Griffiths HJ, Zimmerman RE. The use of photon densitometry to evaluate bone mineral in a group of patients with spinal cord injury. Paraplegia. 1973;10(4):279–284. doi: 10.1038/sc.1973.51. [DOI] [PubMed] [Google Scholar]
- 66.Frost HM. The Utah paradigm of skeletal physiology: an overview of its insights for bone, cartilage and collagenous tissue organs. J Bone Miner Metab. 2000;18(6):305–316. doi: 10.1007/s007740070001. [DOI] [PubMed] [Google Scholar]
- 67.Frost HM. From Wolff's law to the Utah paradigm: insights about bone physiology and its clinical applications. Anat Rec. 2001;262(4):398–419. doi: 10.1002/ar.1049. [DOI] [PubMed] [Google Scholar]
- 68.Morey-Holton ER, Globus RK. Hindlimb unloading of growing rats: a model for predicting skeletal changes during space flight. Bone. 1998;22(5 Suppl):83S–88S. doi: 10.1016/s8756-3282(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 69.Hert J, Sklenska A, Liskova M. Reaction of bone to mechanical stimuli. 5. Effect of intermittent stress on the rabbit tibia after resection of the peripheral nerves. Folia Morphol (Praha) 1971;19(4):378–387. [PubMed] [Google Scholar]
- 70.Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 71.Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 72.Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brunkow ME, Gardner JC, Van Ness J, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68(3):577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balemans W, Patel N, Ebeling M, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39(2):91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 76.Sawakami K, Robling AG, Ai M, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281(33):23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 77.Cui Y, Niziolek PJ, MacDonald BT, et al. Lrp5 functions in bone to regulate bone mass. Nature medicine. 2011;17(6):684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akhter MP, Wells DJ, Short SJ, et al. Bone biomechanical properties in LRP5 mutant mice. Bone. 2004;35(1):162–169. doi: 10.1016/j.bone.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 79.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281(42):31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 80.Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 81.Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10(5):537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 82.Sims AM, Shephard N, Carter K, et al. Genetic analyses in a sample of individuals with high or low BMD shows association with multiple Wnt pathway genes. J Bone Miner Res. 2008;23(4):499–506. doi: 10.1359/jbmr.071113. [DOI] [PubMed] [Google Scholar]
- 83.Richards JB, Rivadeneira F, Inouye M, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371(9623):1505–1512. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uitterlinden AG, Arp PP, Paeper BW, et al. Polymorphisms in the sclerosteosis/van Buchem disease gene (SOST) region are associated with bone-mineral density in elderly whites. Am J Hum Genet. 2004;75(6):1032–1045. doi: 10.1086/426458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Estrada K, Styrkarsdottir U, Evangelou E, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kiel DP, Ferrari SL, Cupples LA, et al. Genetic variation at the low-density lipoprotein receptor-related protein 5 (LRP5) locus modulates Wnt signaling and the relationship of physical activity with bone mineral density in men. Bone. 2007;40(3):587–596. doi: 10.1016/j.bone.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McClung MR, Grauer A. Romosozumab in postmenopausal women with osteopenia. N Engl J Med. 2014;370(17):1664–1665. doi: 10.1056/NEJMc1402396. [DOI] [PubMed] [Google Scholar]
- 88.Recker RR, Benson CT, Matsumoto T, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res. 2015;30(2):216–224. doi: 10.1002/jbmr.2351. [DOI] [PubMed] [Google Scholar]