Abstract
IMPORTANCE
Zoledronic acid, a third-generation aminobisphosphonate, reduces the incidence of skeletal-related events and pain in patients with bone metastases. The optimal dosing interval for zoledronic acid is uncertain.
OBJECTIVE
To determine whether zoledronic acid administered every 12 weeks is noninferior to zoledronic acid administered every 4 weeks.
DESIGN, SETTING, PARTICIPANTS
Randomized, open-label clinical trial conducted at 269 academic and community sites in the United States. Patients (n = 1822) with metastatic breast cancer, metastatic prostate cancer, or multiple myeloma who had at least 1 site of bone involvement were enrolled between May 2009 and April 2012; follow-up concluded in April 2014.
INTERVENTIONS;
Patients were randomized to receive zoledronic acid administered intravenously every 4 weeks (n = 911) vs every 12 weeks (n = 911) for 2 years.
MAIN OUTCOMES AND MEASURES;
The primary end point was the proportion of patients having at least 1 skeletal-related event (defined as clinical fracture, spinal cord compression, radiation to bone, or surgery involving bone) within 2 years after randomization and a between-group absolute difference of 7%as the noninferiority margin. Secondary end points included the proportion of patients with at least 1 skeletal-related event by disease type, pain as assessed by the Brief Pain Inventory (range, 0–10; higher scores indicate worse pain), Eastern Cooperative Oncology Group performance status (range, 0–4; higher scores indicate worse disability), incidence of osteonecrosis of the jaw, kidney dysfunction, skeletal morbidity rate (mean number of skeletal-related events per year), and, in a subset of 553 patients, suppression of bone turnover (assessed by C-terminal telopeptide levels).
RESULTS
Among 1822 patients who were randomized (median age, 65 years; 980 [53.8%] women; 855 with breast cancer, 689 with prostate cancer, and 278 with multiplemyeloma), 795 completed the study at 2 years. A total of 260 patients (29.5%) in the zoledronic acid every 4-week dosing group and 253 patients (28.6%) in the every 12-week dosing group experienced at least 1 skeletal-related event within 2 years of randomization (risk difference of −0.3%[1-sided 95%CI, −4% to ∞]; P < .001 for noninferiority). The proportions of skeletal-related events did not differ significantly between the every 4-week dosing group vs the every 12-week dosing group for patients with breast cancer, prostate cancer, or multiple myeloma. Pain scores, performance status scores, incidence of jaw osteonecrosis, and kidney dysfunction did not differ significantly between the treatment groups. Skeletal morbidity rates were numerically identical in both groups, but bone turnover was greater (C-terminal telopeptide levels were higher) among patients who received zoledronic acid every 12 weeks.
CONCLUSIONS AND RELEVANCE
Among patients with bone metastases due to breast cancer, prostate cancer, or multiplemyeloma, the use of zoledronic acid every 12 weeks compared with the standard dosing interval of every 4 weeks did not result in an increased risk of skeletal events over 2 years. This longer interval may be an acceptable treatment option.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00869206
Bone involvement in metastatic cancer is a common clinical problem. The US Food and Drug Administration approved zoledronic acid, a third-generation aminobisphosphonate, for the treatment of patients with multiple myeloma and bone metastases from solid tumors. Zoledronic acid administered intravenously every 3 to 4 weeks reduces pain and the incidence of skeletal-related events, including clinical fracture, spinal cord compression, radiation to bone, and surgery to bone by 25% to 40%.1–3
Bisphosphonates are generally well tolerated, but are associated with toxic effects, including osteonecrosis of the jaw, nephrotoxicity, and hypocalcemia. The incidence of osteonecrosis of the jaw increases with cumulative drug exposure from 1.5%for patients treated for 4months to 12months to 7.7%for patients treated for 37 months to 48 months.4 Bisphosphonates are nephrotoxic, manifesting as elevated serum creatinine levels. Hypocalcemia is rarely clinically symptomatic.5
The optimal dosing interval for zoledronic acid has not been determined. The standard dosing interval of every 4 weeks was derived empirically rather than from comparative studies or compelling pharmacodynamic data. Several studies have addressed the dosing interval. In the ZOOM6 and OPTIMIZE-27 trials, patients with breast cancer and skeletal metastases were pretreated with zoledronic acid every 4 weeks for9months to15monthsandthen randomized to receive zoledronic acid every4weeks or every 12weeks for 12months. Neither study showed significant differences in efficacy or toxicity for the 2 dosing regimens.
The hypothesis of this study was that zoledronic acid administered every 12weeks for 2 years would be noninferior to zoledronic acid administered at the standard interval of every 4weeks for 2 years among patients with metastatic breast cancer, metastatic prostate cancer, or multiplemyeloma.
Methods
The human protection committee at each participating institution approved the trial protocol (CALGB 70604 [Alliance]) and the patient consent form (Supplement 1). Written informed consent was obtained from each study participant. Separate written consent was obtained for the companion study assessing suppression of bone turnover (CALGB150804 [Alliance]). No stipends were given to patients for participating in the study.
Patient Eligibility
Patients were required to have histologically proven breast cancer, prostate cancer, or multiple myeloma with at least 1 site of bone involvement as documented by plain radiograph, computed tomographic scan, positron emission tomographic scan, combination computed tomographic and positron emission tomographic scan, magnetic resonance imaging, bone scan, or skeletal survey. Indeterminate lesions were confirmed using a second imaging method.
Other eligibility requirements included age of 18 years or older, Eastern Cooperative Oncology Group (ECOG) performance status8 score of 0 to 2, calculated creatinine clearance of 30mL/minor greater using the Cockroft and Gault formula,9 andcorrectedserumcalciumlevelbetween8.0mg/dLor greater (≥2.00 mmol/L) and less than 11.6mg/dL (<2.90 mmol/L).
Key Points.
Question Is zoledronic acid administered every 12 weeks for 2 years noninferior to zoledronic acid administered at the standard dosing interval of every 4 weeks for 2 years?
Findings In this randomized, open-label, noninferiority clinical trial of 1822 patients with metastatic breast cancer, metastatic prostate cancer, or multiplemyeloma, 795 completed the study, among whom 29.5%of patients receiving zoledronic acid every 4 weeks and 28.6%of patients receiving zoledronic acid every 12 weeks experienced at least 1 skeletal-related event within 2 years of randomization, meeting criteria for noninferiority.
Meaning Among patients with bone metastases due to breast cancer, prostate cancer, or multiplemyeloma, the use of zoledronic acid every 12 weeks compared with every 4 weeks did not result in an increased risk of skeletal events over 2 years.
Patients were ineligible if they had brain metastases or had received prior intravenous bisphosphonates (prior use of oral bisphosphonates was allowed if discontinued prior to randomization), denosumab, or bone-targeting radiopharmaceuticals. Women who were pregnant or nursing were excluded. Prior radiation to bone was permitted if completed by the time of randomization and at least 1 site of bone involvement had not been irradiated.
Trial Design and Treatment
This was a noninferiority trial with patient stratification by cancer type, baseline serum creatinine level, prior skeletal-related events, and prior use of oral bisphosphonates. The CALGB registrar assigned patients to receive zoledronic acid every4weeks or every 12weeks for 2 years at a 1:1 ratio using a stratified permuted block randomization scheme10 that was performed on a central computer. Allocations were concealed until patients were registered and enrolled. Commercially available zoledronic acid was intravenously administered for a duration of 15 minutes or longer. Each zoledronic acid dose was adjusted for calculated creatinine clearance using actual body weight. Patients were instructed to take approximately500mg of elemental calcium and 400 IU to 800 IU of vitamin D per day. Adverse events and toxic effects were graded using version 3.0 of the Common Terminology Criteria for Adverse Events.11 Protocol treatment was discontinued in patients with grade 3 or 4 hypersensitivity, creatinine clearance of less than 30 mL/min, or development of osteonecrosis of the jaw.
Patients who agreed to participate in the C-terminal telopeptide companion study were accrued sequentially as they enrolled in the primary study. At baseline and at 12-week intervals for 2 years, 10 mL of peripheral venous blood was collected from each participant. Serum was separated and refrigerated immediately. Serum samples were submitted to a central repository where they were stored at −70°C until analysis. C-terminal telopeptide levels were assayed using the Serum Crosslaps enzyme-linked immunosorbent assay kit (Immunodiagnostics Systems Holding PLC).
Outcomes
The primary outcome was the proportion of patients having at least 1 skeletal-related event within 2 years after randomization. The end point for skeletal-related events was defined as clinical fracture, spinal cord compression, radiation to bone, and surgery involving bone. Clinical fractures were defined as those identified during the evaluation of symptomatic patients and confirmed by written report of radiographic testing. Fractures identified incidentally or involving the hands, feet, face, or skull were not included. Spinal cord compression, manifesting as neurological impairment, back pain, or both, required radiographic confirmation. Radiation to bone was defined as radiation to palliate a painful bone lesion, radiation to treat or prevent fractures, radiation to treat or prevent spinal cord compression, or the use of bone-targeted radio pharmaceuticals. Surgery involving bone was defined as surgical procedures to prevent imminent fractures or to treat pathological fractures or spinal cord compression.
Prespecified secondary end points included (1) the proportion of patients having at least 1 skeletal-related event within 2 years after randomization for the subgroups of patients with breast cancer, prostate cancer, and multiple myeloma; (2) pain as assessed by the Brief Pain Inventory (scores ranged from 0 [no pain] to 10 [pain as bad as you can imagine])12; (3) ECOG performance status (scores ranged from 0 [fully active] to 4 [completely disabled]); and (4) incidences of osteonecrosis of the jaw and kidney dysfunction using version 3.0 of the Common Terminology Criteria for Adverse Events criteria (and defined as grade 1, serum creatinine levels greater than the upper limit of normal to 1.5 times the upper limit of normal; grade 2, serum creatinine levels >1.5–3.0 times the upper limit of normal; grade 3, serum creatinine levels >3.0–6.0 times the upper limit of normal; grade 4, serum creatinine levels >6 times the upper limit of normal). The skeletal morbidity rate was assessed and defined as the mean number of skeletal-related events per year. Suppression of the bone turnover marker C-terminal telopeptide was measured in a subset of patients. Additional prespecified secondary outcomes from the companion pharmacogenetic and cost-effectiveness studies will be reported elsewhere.
End points were not modified during the course of the study and outcome assessments were not blinded. Skeletal related event forms detailing if and when a clinical fracture, spinal cord compression, radiation to bone, or surgery involving bone had occurred were collected every 4 weeks for all patients. Imaging reports documenting skeletal-related events, Brief Pain Inventory scores, ECOG performance status scores, serum creatinine levels, calculated creatinine clearance, and adverse event data were also collected at 4-week intervals for all patients. The zoledronic acid dose was obtained following administration of each treatment. Trial participants were queried about dental problems, visits, and procedures they had within the past 1 month to 12 months; the information was recorded in a monthly case report form. Osteonecrosis of the jaw was documented by reports from patients’ dentists.
Statistical Methods
Noninferiority Margin
Based on published rates of skeletal-related events among patients treated with zoledronic acid, the noninferiority margin was prespecified at a 7% absolute difference. Specifically, 4 previous studies1,2,13,14 comparing zoledronic acid with placebo reported absolute differences (in favor of zoledronic acid) ranging from 5.7% to 37.0% for the percentages of skeletal-related events. All observed differences of 9% or greater were significant at the .05 level, whereas differences below 6% were not. Statistical significance does not inherently indicate clinical importance. However, based on these study results and the experience of the clinicians who developed the protocol, there was a consensus that 7% was a reasonable and clinically meaningful noninferiority margin.
Sample Size Determination
The target accrual was 1538 patients in the original study protocol, which was selected under an assumption that approximately 20% of patients would drop out, resulting in 1230 evaluable patients. However, after more than 20% of patients were noted during an interim analysis to have dropped out, the assumed dropout rate was increased to 30%, and thus the target accrual was increased from 1538 to 1758 patients to yield 1230 evaluable patients. With 1230 patients remaining after an anticipated 30% dropout rate, the power to reject a null hypothesis of inferiority using a 1-sided test at a .05 significance level was estimated to be 82% under the alternative hypothesis that the rates for skeletal-related events were equal in the 2 treatment groups. The null hypothesis was that zoledronic acid administered every 12 weeks was inferior to zoledronic acid administered every 4 weeks. Therefore, a 1-sided Cochran-Mantel-Haenszel15 P ≤ .05 was required to demonstrate noninferiority for the primary analysis.
Analytic Plan
Prespecified Analyses of the Primary Outcome
To determine whether zoledronic acid administered every 12 weeks was noninferior to zoledronic acid administered every 4 weeks, a stratified Cochran-Mantel-Haenszel test was used to compare the proportion of patients with at least 1 skeletal-related event at 2 years between the 2 treatment groups. Stratification factors were cancer type (breast, prostate, or multiple myeloma), baseline serum creatinine level (≤1.4 mg/dL or >1.4 mg/dL), prior skeletal-related events (yes or no), and prior use of oral bisphosphonates (yes or no). Following the intent-to-treat (ITT) principle, all randomized patients were included in this analysis. Per the study protocol, the analyses were performed by assuming that among patients with less than 2 years of follow-up, those without at least 1 skeletal-related event at the time of dropout had (ITT analysis) or did not have (sensitivity analysis) at least 1 skeletal-related event.
The 2-year rates for skeletal-related events were also compared across treatment groups using a logistic regression model and a binary (logit link) mixed-effects model that included a random, study site–specific intercept to account for potential clustering within site. Both models included main effects for disease type, prior use of oral bisphosphonates, prior skeletal related events, and baseline serum creatinine level.
Prespecified Analyses of Secondary Outcomes
To assess disease-specific treatment differences, the stratified Cochran-Mantel-Haenszel test was reimplemented within each of the 3 disease groups. Changes over time from baseline in the Brief Pain Inventory composite score were compared using a linear mixed-effects model16 adjusted for disease type, baseline creatinine level, prior skeletal-related events, prior use of oral bisphosphonates, age, sex, body surface area, and race. The ECOG performance status score was compared using a linear mixed-effects model adjusted for disease type, baseline creatinine level, prior skeletal related events, prior use of oral bisphosphonates, age, sex, body surface area, race, and baseline ECOG performance status score.
Both the Brief Pain Inventory and the ECOG performance status models included a time × treatment interaction term and a random patient-specific time slope. The incidences of osteonecrosis of the jaw and kidney dysfunction were compared between the 2 treatment groups using the Cochran- Mantel-Haenszel test stratified by cancer type, baseline serum creatinine level, prior skeletal-related events, and prior use of oral bisphosphonates. The skeletal morbidity rate was defined as the mean number of skeletal-related events per year and was estimated for each treatment group. The median observed skeletal morbidity rate and the interquartile range (IQR) were determined for each group.
To help reduce the risk of false-positive findings, a 2-sided significance level of .001 was used for all secondary outcome analyses. All secondary analyses were performed to assess differences between groups rather than noninferiority.
Post Hoc and Exploratory Analyses
To determine whether the incidence curves for skeletal related events were equal, a Wald test with a 2-sided alternative was used. A Wald 95%CI was calculated for the hazard ratio specific for skeletal-related events. A Kruskal-Wallis test was used to compare the distribution of skeletal-related events by disease type.
To compare incidence trajectories for skeletal-related events, the cumulative incidences for skeletal-related events were estimated over 2 years for both treatment groups using response data as observed (patients were censored at the time of dropout and assumed not to have had at least 1 skeletal related event unless 1 event was observed during the study). This was performed using a Cox proportional hazards model, in which death was treated as a competing risk. This model included treatment group, age, body surface area, disease type, performance status, prior skeletal-related events, prior use of oral bisphosphonates, baseline serum creatinine level, and race as predictors.
Because a higher dropout rate might bias results toward noninferiority, an additional sensitivity analysis (commonly referred to as a tipping point analysis) was performed.17,18 Specifically, the assumption was made that 29.5%(the observed rate for skeletal-related events in the every 4-week dosing group, which would also be the expected rate among dropouts in the 4-week group) of dropouts in both treatment groups who did not experience at least 1 skeletal-related event would have experienced at least 1 skeletal-related event if they had remained in the study until 2 years after randomization. The groups were compared by a noninferiority test and the P value for noninferiority was calculated.
The assumed rate for skeletal-related events was then slowly increased among dropouts in the every 12-week dosing group (while holding the rate for skeletal-related events in the every 4-week dosing group fixed at 29.5%), each time rerunning the Cochran-Mantel-Haenszel test and noting the P value until the significant (at the .05 level) noninferiority result was no longer significant (ie, until the null hypothesis that the every 12-week dosing group was inferior to the every 4-week dosing group at the .05 significance level was no longer able to be rejected). This was performed using a stratified Cochran-Mantel-Haenszel testandanunadjusted2-sample test of proportions.
In addition to the evaluation of kidney dysfunction using version 3.0 of the Common Terminology Criteria for Adverse Events criteria, an additional analysis of kidney dysfunction was performed using the Cochran-Mantel-Haenszel test, stratified by cancer type, baseline serum creatinine level, prior skeletal- related events, and prior use of oral bisphosphonates. For this analysis, kidney dysfunction was defined (as in previous reports1,2,5,13,14) as an increase in serum creatinine level of 0.5mg/dL or greater if the baseline level was 1.4mg/dL or less, or an increase in serum creatinine level of 1mg/dL or greater if the baseline level was greater than 1.4mg/dL.
For patients in the companion C-terminal telopeptide study, a linear mixed-effects model was used to compare C-terminal telopeptide levels over time between the 2 treatment groups. Variables included in this model were treatment, linear time, quadratic time (time^2), and a linear time × treatment interaction term. In addition, this model allowed for random, patient-specific intercepts and linear time slopes.
The distribution of the cumulative dose of zoledronic acid was compared across the 2 study groups using a Kruskal- Wallis test. Treatment delays were compared using the χ2 test. The incidence of hypocalcemia was compared between the 2 treatment groups using the Cochran-Mantel-Haenszel test, stratified by cancer type, baseline serum creatinine level, prior skeletal-related events, and prior use of oral bisphosphonates.
Interim Analyses
Five interim analyses were performed at 6-month intervals from November 2012 until November 2014. A 1-sided Fisher exact test at significance levels of P = .15, P = .15, P = .20, P = .20, and P = .25 for the first through fifth interim analyses, respectively, was conducted for futility, and a Cochran- Mantel-Haenszel test was performed for noninferiority. Significance levels for the interim noninferiority tests were determined based on the Haybittle-Peto method, and thus for each interim noninferiority test, the significance level was set at .001. In addition, because of differential dropout early in the trial (which eventually became more balanced), time-to-event analyses (Kaplan-Meier) for the last 3 interim analyses also were conducted to compare the 2 treatment groups (log-rank test).
The Alliance Statistics and Data Center conducted data collection and statistical analyses. Data quality was ensured by review of the data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. The Alliance data and safety monitoring board reviewed the results of each interim analysis at each of its semiannual meetings, including the primary and secondary end points. All analyses were based on the study database, which was frozen on December 8, 2014. Statistical analyses were performed using R version 3.2.3 (R Project for Statistical Computing) and SAS versions 9.3 and 9.4 (SAS Institute Inc).
Results
Baseline Patient Characteristics
Between May 2009 and April 2012, 1822 patients were enrolled in the study with 911 randomized to receive zoledronic acid every 4 weeks and 911 randomized to receive zoledronic acid every 12 weeks (equal sample size being a result of permuted block randomization); follow-up concluded in April 2014. The baseline characteristics of the patients by treatment group appear in Table 1. Among 1822 patients who were randomized (median age, 65 years; 980 [53.8%] women; 855 with breast cancer,689with prostate cancer, and 278 with multiple myeloma), 795 completed the study at 2 years. A total of 553 patients were enrolled in the companion C-terminal telopeptide study. These patients were similar to the entire trial population by baseline characteristics (eTable 1 in Supplement 2). Because of administrative stopping point errors, enrollments for the primary and C-terminal telopeptide studies exceeded target accruals by 64 and 357 patients, respectively. All available samples were assayed.
Table 1.
Baseline Characteristics of Patients
| Characteristic | Zoledronic Acid Dose Group | |
|---|---|---|
| Every 4 wk (n = 911) | Every 12 wk (n = 911) | |
| Age, median (range), y | 65 (26–93) | 65 (33–94) |
| Sex, No. (%) | ||
| Male | 414 (45.4) | 428 (47.0) |
| Female | 497 (54.6) | 483 (53.0) |
| Race | ||
| White | 772 (84.7) | 760 (83.4) |
| Black | 111 (12.2) | 118 (13.0) |
| Other or unknown | 28 (3.1) | 33 (3.6) |
| Body surface area, mean (SD), m2 | 1.9 (0.3) | 2.0 (0.3) |
| ECOG performance status, No. (%) | ||
| 0 | 432 (47.4) | 423 (46.4) |
| 1 | 379 (41.6) | 381 (41.8) |
| 2 | 78 (8.6) | 81 (8.9) |
| Unspecified | 22 (2.4) | 26 (2.9) |
| Diagnosis, No. (%) | ||
| Breast cancer | 427 (46.9) | 428 (47.0) |
| Prostate cancer | 345 (37.9) | 344 (37.8) |
| Multiple myeloma | 139 (15.3) | 139 (15.3) |
| Serum creatinine, median (IQR), mg/dL | 0.9 (0.7–1.0) | 0.9 (0.7–1.1) |
| Prior skeletal-related events, No. (%) | 239 (26.2) | 234 (25.7) |
| Prior use of oral bisphosphonates, No. (%) | 77 (8.5) | 72 (7.9) |
| Brief Pain Inventory score | ||
| Worst pain, mean (SD) | 3.41 (3.10) | 3.40 (3.19) |
| Median (IQR) | 3.0 (0–6.0) | 3.0 (0–6.0) |
| Least pain, mean (SD) | 1.54 (1.95) | 1.62 (2.25) |
| Median (IQR) | 1.0 (0–3.0) | 0 (0–3.0) |
| Average pain, mean (SD) | 2.57 (2.36) | 2.65 (2.56) |
| Median (IQR) | 2.0 (0–4.0) | 2.0 (0–5.0) |
| Current pain, mean (SD) | 1.92 (2.43) | 1.94 (2.48) |
| Median (IQR) | 1.0 (0–3.0) | 1.0 (0–3.0) |
| Composite pain, mean (SD) | 2.36 (2.23) | 2.41 (2.39) |
| Median (IQR) | 2.0 (0–4.0) | 2.0 (0–4.0) |
| Relief from pain, mean (SD) | 5.63 (3.65) | 5.47 (3.78) |
| Median (IQR) | 7.0 (2.0–9.0) | 6.0 (2.0–9.0) |
| Interference, mean (SD) | 2.17 (2.54) | 2.07 (2.53) |
| Median (IQR) | 1.1 (0–3.9) | 0.9 (0–3.7) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range.
SI conversion factors: To convert creatinine to μmol/L, multiply by 88.4.
Primary Outcomes
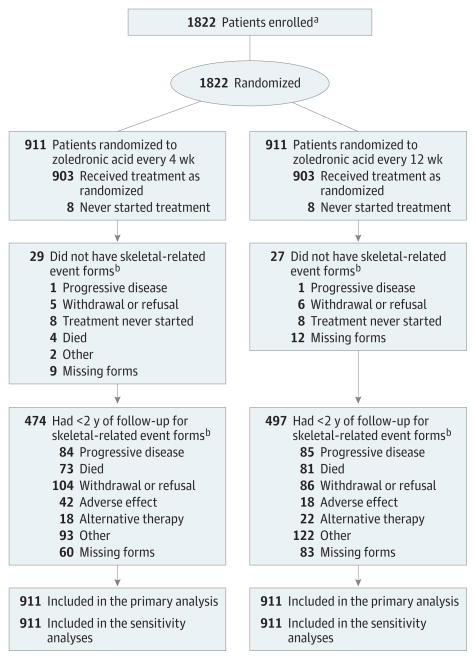
All 1822 patients were included in the ITT and sensitivity analyses (Figure 1). The most common reasons for dropping out of the trial in both groups were withdrawal or refusal, disease progression, and death; however, neither disease progression nor death were specified in the protocol as reasons to stop participation in the study. The median length of patient follow-up was 425 days (1.2 years).
Figure 1. Flow Diagram of Progress Through Phases of a Randomized Trial Comparing Standard Dosing vs Longer Dosing of Zoledronic Acid Among Patients with Metastatic Cancer.
aData on the number of patients screened for the study and excluded prior to enrollment were not collected.
bSkeletal-related event forms detailing if and when a clinical fracture, spinal cord compression, radiation to bone, or surgery involving bone had occurred were collected every 4 weeks for all patients.
A complete breakdown of the observed outcome data for skeletal-related events by study group, disease type, and dropout status appears in Table 2. Two hundred sixty patients (29.5%) who received zoledronic acid every 4 weeks and 253 patients (28.6%)who received zoledronic acid every 12weeks experienced at least 1 skeletal-related event within 2 years of study randomization. The type of skeletal-related event was due to radiation to bone in 185 patients in the zoledronic acid every 4-week dosing group and in 163 patients in the zoledronic acid every 12-week dosing group; clinical fractures, 62 and 79 patients, respectively; spinal cord compression, 23 and 30 patients; and surgery involving bone, 22 and 42 patients (eTable 2 in Supplement 2).
Table 2.
Skeletal-Related Events (SRE) by Disease Sitea
| Disease Site by Zoledronic Acid Dose Group | No. of Patients | Follow-up, No. (%) | Intent-to-Treat Analysisc | Sensitivity Analysisd | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Completed 2 y | Dropped Out Before 2 yb | ||||||||||
|
|
|
|
|
||||||||
| SRE | No SRE | SRE | No SRE | No. (%) With Aggregated SRE | Proportion Difference (4-wk Dose Group Minus 12-wk Dose Group) in SRE | P Valuee | No. (%) With Aggregated SRE | Proportion Difference (4-wk Dose Group Minus 12-wk Dose Group) in SRE | P Valuee | ||
| Total | |||||||||||
|
| |||||||||||
| Every 4 wk | 911 | 113 (6) | 295 (16) | 147 (8) | 356 (20) | 616 (68) | 0 (1-sided 95% CI, −0.04 to ∞) | <.001f | 260 (29) | 0.01 (1-sided 95% CI, −0.03 to ∞) | <.001f |
|
|
|
||||||||||
| Every 12 wk | 911 | 95 (5) | 292 (16) | 158 (9) | 366 (20) | 619 (68) | 253 (28) | ||||
|
| |||||||||||
| Breast Cancer | |||||||||||
|
| |||||||||||
| Every 4 wk | 427 | 57 (7) | 155 (18) | 57 (7) | 158 (18) | 272 (64) | −0.05 (99.9% CI, −0.16 to 0.05) | .50 | 114 (27) | −0.02 (99.9% CI, −0.13 to 0.09) | .58 |
|
|
|
||||||||||
| Every 12 wk | 428 | 46 (5) | 132 (16) | 76 (9) | 174 (20) | 296 (69) | 122 (29) | ||||
|
| |||||||||||
| Prostate Cancer | |||||||||||
|
| |||||||||||
| Every 4 wk | 345 | 36 (5) | 85 (12) | 74 (11) | 150 (22) | 260 (75) | 0.03 (99.9% CI, −0.08 to 0.15) | .59 | 110 (32) | 0.02 (99.9% CI, −0.10 to 0.14) | .58 |
|
|
|
||||||||||
| Every 12 wk | 344 | 35 (5) | 98 (14) | 67 (10) | 144 (21) | 246 (72) | 102 (30) | ||||
|
| |||||||||||
| Multiple Myeloma | |||||||||||
|
| |||||||||||
| Every 4 wk | 139 | 20 (7) | 55 (20) | 16 (6) | 48 (17) | 84 (60) | 0.05 (99.9% CI, −0.15 to 0.25) | .14 | 36 (26) | 0.06 (99.9% CI, −0.12 to 0.24) | .35 |
|
|
|
||||||||||
| Every 12 wk | 139 | 14 (5) | 62 (22) | 15 (6) | 48 (17) | 77 (55) | 29 (21) | ||||
Skeletal-related events were defined as clinical fracture, spinal cord compression, radiation to bone, and surgery involving bone.
Includes participants without SRE forms as indicated in Figure 1.
Analysis assumes dropouts without at least 1 SRE had SRE.
Analysis assumes dropouts without at least 1 SRE did not have SRE.
Cochran-Mantel-Haenszel test for any between-group difference adjusted for cancer type, baseline serum creatinine level, prior SRE, and prior use of oral bisphosphonates.
Indicates noninferiority.
The stratified ITT Cochran-Mantel-Haenszel test to compare these proportions under the assumption that dropouts (those individuals who did not complete 2 years of treatment) experienced at least 1 skeletal-related event indicated that zoledronic acid administered every 12 weeks was noninferior with regard to skeletal-related events (risk difference, −0.3% [1-sided 95% CI, −4% to ∞]; P < .001 for noninferiority). The prespecified sensitivity analysis, using the assumption that dropouts without at least 1 skeletal-related event at the time of dropout did not have at least 1 skeletal-related event, also demonstrated noninferiority (risk difference, 1.0% [1-sided 95% CI, −3% to ∞]; Cochran-Mantel-Haenszel P < .001) (Table 2).
The 1-sided 95% CI for the difference in these proportions (ITT analysis based on an assumption of normality) between the zoledronic acid every 4-week group and the every 12-week group was −4%to ∞. The lower bound of this interval was greater than the noninferiority margin of −7%, indicating that zoledronic acid administered every 12weeks was noninferior to zoledronic acid administered every 4 weeks.
The 2-year rate analyses for skeletal-related events from logistic regression and binary mixed-effects models were nearly identical, and were consistent with the primary analyses (no significant difference was found between the zoledronic acid 12-week dosing group and the 4-week dosing group). Specifically, under the assumption that dropouts experienced at least 1 skeletal-related event, the logistic regression model odds ratio (OR) for zoledronic acid administered every 12weeks vs every 4weeks was 1.02 (95%CI, 0.84–1.24; P = .85 for superiority) and the mixed-model OR was 1.02 (95% CI, 0.83–1.25; P = .88 for superiority). Using the assumption that dropouts without at least 1 skeletal-related event at the time of dropout did not have at least 1 skeletal-related event, the OR for both modelswas0.96(95%CI,0.78–1.17; superiority P = .68for zoledronic acid 12-week dosing group vs 4-week dosing group).
Secondary Outcomes
The probability of experiencing at least 1 skeletal-related event within 2 years of randomization was not significantly different between the zoledronic acid 4-week group and the 12-week group for patients with breast cancer (between-group difference, −0.02 [99.9%CI, −0.13 to 0.09]; P = .50), prostate cancer (between-group difference,0.02 [99.9%CI, −0.10to0.14]; P = .59), or multiplemyeloma (between-group difference,0.06 [99.9%CI, −0.12 to 0.24]; P = .14) (Table 2). Prespecified sensitivity analyses performed within each disease group under the assumption that dropouts without at least 1 skeletal related event did not have at least 1 skeletal-related event showed no significant differences between the 2 zoledronic acid treatment groups (Cochran-Mantel-Haenszel P = .58 for breast cancer, P = .58 for prostate cancer, and P = .35 for multiplemyeloma).
No significant differences between the zoledronic acid every 4-week group and the every 12-week group were seen in the mean pain scores at any individual time point (Cochran- Mantel-Haenszel P > .001at all time points for mean worst pain within the past 24 hours, mean least pain within the past 24 hours, mean average pain, mean current pain, composite pain [mean of the 4 pain items: worst, least, average, and current], mean relief from pain with treatments or medications, and mean interference score). In addition, trajectories of the mean pain scores were not found to differ significantly between the 2 groups (time × treatment interaction P = .96 for mean worst pain within the past24hours;P = .38formeanleast pain within the past 24 hours; P = .75 for mean average pain; P = .82 for mean current pain; P = .88 for mean composite pain; P = .59 for mean relief from pain with treatments or medications; and P = .68 for mean interference score). No significant differences in ECOG performance status over time were seen (P = .64 for time × treatment interaction) or at each time point (Cochran-Mantel-Haenszel P > .001for all26time points) (eFigures 1–8 in Supplement 2).
Osteonecrosis of the jaw occurred in 18 patients (2.0%) in the zoledronic acid every 4-week group and 9 patients (1.0%) in the every 12-week group (2-sided Cochran-Mantel- Haenszel P = .08). Kidney dysfunction, defined as grade 3 or 4 increased creatinine levels, occurred in 10 patients (1.2%) receiving zoledronic acid every 4 weeks and 4 patients (0.5%) receiving zoledronic acid every 12 weeks (2-sided Cochran-Mantel-Haenszel P = .10). The mean number of skeletal-related events per year (skeletal morbidity rate) was 0.4 (median, 0 [IQR, 0–0.5]) for the zoledronic acid every 4-week group and 0.4 (median, 0 [IQR, 0–0.5]) for the zoledronic acid every 12-week group.
Among the 9 covariates included in these analytic models (disease type, baseline creatinine level, prior skeletal related events, prior use of oral bisphosphonates, age, sex, body surface area, race, and baseline ECOG performance status), only baseline ECOG performance status had any missing data for 48 patients (2.6%).
Post Hoc and Exploratory Analyses
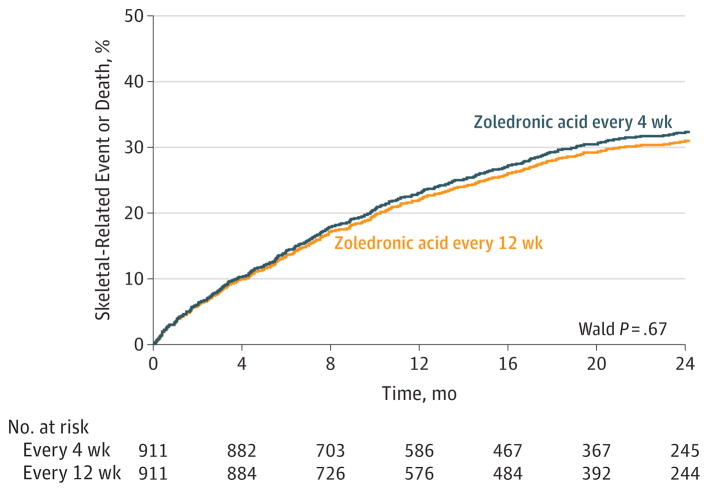
The cumulative incidence of skeletal-related events for both treatment groups appears in Figure 2. A total of 56 randomized patients were excluded because they dropped out immediately, and thus did not have any evaluations for skeletal related events (so their time to at least 1 skeletal-related event was missing). These curves were similar and were not found to differ significantly, suggesting that throughout the course of the study, skeletal-related events occurred at a similar rate in both groups (hazard ratio for skeletal-related events, 0.96 [Wald 95% CI, 0.81–1.15];Wald P = .67 for zoledronic acid every 12-week group vs every 4-week group).
Figure 2. Cause-Specific Cumulative Incidence of Skeletal-Related Events.
There were 256 patients with skeletal-related events in the zoledronic acid every 4-week dose group and 246 patients in the every 12-week dose group (hazard ratio, 0.96 [95%CI, 0.81–1.15]). The median follow-up was 15.7 months (interquartile range, 6.4–24.1 months) in the zoledronic acid every 4-week dose group and 16.8 months (interquartile range, 6.4–24.0 months) in the every 12-week dose group. There were 122 patients who died in the zoledronic acid every 4-week dose group and 118 in the every 12-week dose group.
In the additional sensitivity (tipping point) analyses, the necessary increase in the rate of the skeletal-related events among dropouts (relative to the assumed rate of 29.5%in the zoledronic acid every 4-week dosing group) in the zoledronic acid every 12-week dosing group was found to be 10.2%vs9.2% for the every 4-week group. These results suggested that the rate of skeletal-related events among dropouts in the every 12-week dosing group would have to be approximately 31%to 35% higher (depending on which test was used) than that among dropouts in the every 4-week dosing group for the significant noninferiority result to become nonsignificant.
Of the patients receiving zoledronic acid every 4 weeks, 174 (19.9%) experienced a significant creatinine level increase of 0.5mg/dL or greater with a baseline creatinine level of 1.4mg/dL or less and an increase of 1mg/dL or greater with a baseline creatinine level of greater than 1.4 mg/dL. For patients receiving zoledronic acid every 12weeks, 137 (15.5%)had a significant creatinine level increase of 0.5mg/dL or greater with a baseline creatinine level of 1.4mg/dL or less and an increase of 1mg/dL or greater with a baseline creatinine level of greater than 1.4 mg/dL (Cochran-Mantel-Haenszel P = .02) (Table 3).
Table 3.
Selected Secondary End Points
| Secondary End Points | Zoledronic Acid Dose Group | Zoledronic Acid 4-wk Dose Group Minus 12-wk Dose Group (95% CI) | P Value | |
|---|---|---|---|---|
| Every 4 wk | Every 12 wk | |||
| Brief Pain Inventory scorea | ||||
| Worst pain | 0.021 | 0.022 | −0.001 (−0.022 to 0.021) | .96 |
| Least pain | 0.013 | 0.007 | 0.006 (−0.008 to 0.021) | .38 |
| Average pain | 0.011 | 0.008 | 0.003 (−0.014 to 0.02) | .75 |
| Current pain | 0.018 | 0.016 | 0.002 (−0.014 to 0.018) | .82 |
| Composite pain | 0.022 | 0.021 | 0.001 (−0.017 to 0.019) | .88 |
| Relief from pain | 0.016 | 0.009 | 0.007 (−0.018 to 0.032) | .59 |
| Interference | 0.019 | 0.023 | −0.004 (−0.023 to 0.015) | .68 |
| ECOG performance statusa | 0.025 | 0.024 | 0.001 (−0.005 to 0.008) | .64 |
| Osteonecrosis of the jaw, No./total available for analysis (%) | 18/911 (2.0) | 9/911 (1.0) | 1.0 (−0.2 to 2.2) | .08b |
| Kidney dysfunction | ||||
| Increased creatinine level, No./total available for analysis (%)c | 10/852 (1.2) | 4/837 (0.5) | 0.7 (−0.3 to 1.7) | .10b |
| Increased creatinine level vs baseline level, No./total available for analysis (%)d | 174/875 (19.9) | 137/882 (15.5) | 4.4 (0.7 to 8.0) | .02b |
| Skeletal morbidity rate, mean (median) [IQR]e | 0.4 (0) [0–0.5] | 0.4 (0) [0–0.5] | ||
| Total available for analysis | 882 | 884 | ||
| Total person-years of follow-up | 1397.5 | 1367.8 | ||
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range.
Estimated time slopes from longitudinal models. These represent the estimated score change that is associated with a 1-unit (in this case 1 visit) increase in time.
Cochran-Mantel-Haenszel test for any between-group difference adjusted for cancer type, baseline serum creatinine level, prior skeletal-related events, and prior use of oral bisphosphonates.
Patients had grade 3 or 4 Common Terminology Criteria for Adverse Events.
Increased by0.5mg/dL or greater if the baseline level was 1.4mg/dL or less or increased by 1mg/dL or greater if the baseline level was greater than 1.4mg/dL.
Skeletal morbidity rate is the number of skeletal-related events per year.
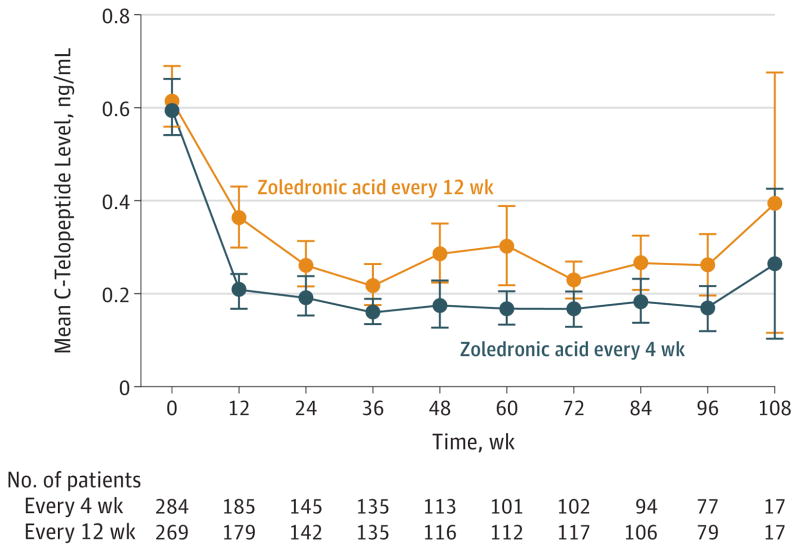
For the analysis of C-terminal telopeptide (the bone turnover marker), 2530 samples were tested from553 patients, 284 (51%)in the zoledronic acid every 4-week group and 269 (49%) in the every 12-week group. The mean C-terminal telopeptide levels (with 95% CI) are displayed graphically over time for each treatment group in Figure 3. This Figure is meant to be descriptive, and involves no statistical test; however, observed C-terminal telopeptide levels were higher at each time point among patients receiving zoledronic acid every 12weeks. Similar results were seen in the longitudinal C-terminal telopeptide model, which found C-terminal telopeptide levels to be significantly lower in the zoledronic acid every4-week group than in the every 12-week group (P = .05). In addition, the linear time × treatment interaction in this model was not significant (P = .14), suggesting that the C-terminal telopeptide trajectories for the 2 treatment groups were parallel with the zoledronic acid every 4-week group always lower than the every 12-week group.
Figure 3. Mean C-Telopeptide Levels by Treatment Group at Each Week.
Error bars indicate 95%confidence intervals.
Patients randomized to the zoledronic acid every 4-week group received a mean cumulative dose 2.5 times that of patients in the every 12-week group (mean [SD], 54.7 mg [33.6 mg] vs 21.6mg [12.4mg]; Kruskal-Wallis P < .001). Treatment delays were found to be more common among patients taking zoledronic acid every 4 weeks (62% vs 37% for the every 12-week group; 2-sided χ2 P < .001).
Any grade of hypocalcemia was reported in 329 patients (38%) in the zoledronic acid every 4-week group and in 298 patients (35%) in the every 12-week group (2-sided χ2 P = .25). Grade4hypocalcemia (<6mg/dL) occurred in8patients (0.9%) in the zoledronic acid every 4-week group and in 5 patients (0.6%) in the every 12-week group (2-sided χ2 P = .61).
Interim Analyses
In each of the interim analyses, the Fisher exact P values (for futility) were above the futility significance level (ie, the criteria for stopping the trial early for futility were not met), and the Cochran-Mantel-Haenszel noninferiority P values were above the early stopping threshold of .001 (ie, the criteria for stopping the trial early for noninferiority were not met). In the last 3 interim analyses, the log-rank P-values were .42 or higher (ie, not meeting the early stopping threshold); thus, it could not be concluded that the hazard ratio for skeletal-related events was different from1.
Discussion
In this randomized clinical trial involving patients with bone metastases due to breast cancer, prostate cancer, or multiple myeloma, zoledronic acid administered every 12 weeks was noninferior to zoledronic acid administered every 4weeks for reducing the occurrence of skeletal events. The proportion of patients experiencing at least 1 skeletal-related event within 2 years of randomization was not significantly different for the zoledronic acid every 12-week dose group compared with the every 4-week dose group within each disease type.
The rates of skeletal-related events (29.5% for the zoledronic acid every 4-week dosing group and 28.6% for the every 12-week dosing group) observed in this study were fairly consistent with previously reported rates of skeletal-related events, which ranged between 29.8% and 38.5%1,2,13,14; however, the duration of treatment with zoledronic acid was less than 2 years in these previous studies, ranging from 9 months to 15 months. Although some previous studies were too short to allow for calculation of median time to first skeletal related event,1,2,14 the 2-year treatment duration of this study allowed estimation of the median times to first skeletal related event or death (treated as a competing risk), which were 15.7 months in the zoledronic acid every 4-week dosing group and 16.8 months in the every 12-week dosing group (Figure 2).
Adherence to the treatment schedule was better among patients who received zoledronic acid every 12 weeks (63% had no treatment delays) compared with patients who received zoledronic acid every 4 weeks (38% had no treatment delays) (eTable 3 in Supplement 2). Bone turnover as measured by changes in C-terminal telopeptide levels was suppressed to a lesser degree when zoledronic acid was administered every 12 weeks, although this difference most likely was not clinically significant because incidence of skeletal-related events for the 12-week group was noninferior to that in the 4-week group, and the secondary end points between the 2 treatment groups were not statistically significantly different.
Despite receiving a lower cumulative dose of zoledronic acid, patients treated every 12 weeks had similar rates of toxic effects compared with those treated every 4 weeks, although osteonecrosis of the jaw occurred in 2.0% of patients in the 4-week group vs 1.0% in the 12-week group (Cochran-Mantel-Haenszel P = .08). The incidence of osteonecrosis of the jaw was lower than the incidence reported in earlier studies of zoledronic acid (7.7%),4 possibly reflecting the shorter duration of treatment in this study (24 months vs 37–48 months), greater awareness of this complication among oncologists and dentists, and the practice of sending patients for dental treatment and clearance prior to initiating bisphosphonate therapy. The occurrence of kidney dysfunction as measured by an increase in creatinine levels over baseline was higher in the zoledronic acid every 4-week group (19.9%) than the occurrence reported in other studies of zoledronic acid (11%–17%),1,2,5,13,14 perhaps because zoledronic acid doses were administered even if creatinine levels had not decreased to levels within 10% of baseline.
This study had several strengths. First, in addition to enrolling patients with metastatic breast cancer, patients with metastatic prostate cancer and with multiple myeloma were also included, broadening the applicability of the results to these populations. Second, randomization started with the first dose of zoledronic acid without the wash-in period of monthly zoledronic acid used in prior dosing-interval studies. Third, more than 800 patients with metastatic breast cancer were enrolled in this study, which is more than twice the number of patients enrolled in previous dosing-interval trials. Fourth, this was a cooperative group trial involving 269 academic and community centers, whereas the prior dosing-interval studies were each supported and funded by industry.
This study also has several limitations. First, the percentage of patients who did not complete 2 years of treatment was higher than the expected 30%, in part because of patient withdrawal. Even though 40% of patients dropped out without experiencing at least 1 skeletal-related event, the findings from the ITT analysis and the sensitivity analyses were consistent: zoledronic acid administered every 12 weeks was noninferior to zoledronic acid administered every 4 weeks. Second, the study was open-label, which could potentially bias reporting of skeletal-related events and toxic effects and could affect patient and physician decision making regarding continued study participation if the patient experienced a skeletal-related event. Sham infusions administered during the off months in the zoledronic acid every 12-week dosing group would have addressed these issues, but there was concern that accrual would be hindered if sham infusions were included, and funding was not available for placebo infusions or central review. Third, the noninferiority design as opposed to a superiority design could be considered a limitation; however, noninferiority trials are well accepted and this design was ideally suited to test the trial hypothesis. Fourth, the study did not address survival or the risks and benefits of administering zoledronic acid for more than 2 years.
Denosumab, a monoclonal antibody, was approved by the US Food and Drug Administration for treatment of bone metastases from solid tumors (but not multiple myeloma) in November 2010,19 about 1 ½ years after our study opened. In a study examining the use of this drug for the prevention of skeletal-related events in 2862 patients with breast cancer, prostate cancer, other solid tumors, or multiple myeloma, Lipton et al20 reported the median time to first skeletal related event was 27.7 months for denosumab administered every 4 weeks vs 19.5 months for zoledronic acid administered every 4 weeks and 934 first skeletal-related events. An expert opinion panel sponsored by the American Society of Clinical Oncology and the National Comprehensive Cancer Center Network, based on a review of the available evidence, did not recommend denosumab over zoledronic acid.21 A study comparing denosumab administered every 4 weeks vs every 12 weeks in patients with metastatic breast cancer and metastatic prostate cancer is currently under way in Switzerland with an expected completion date of 2022.22
Conclusions
Among patients with bone metastases due to breast cancer, prostate cancer, or multiple myeloma, the use of zoledronic acid every 12 weeks compared with the standard dosing interval of every 4 weeks did not result in an increased risk of skeletal events over 2 years. This longer interval may be an acceptable treatment option.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by grants UG1CA189823 (awarded to the Alliance for Clinical Trials in Oncology), U10CA016359, U10CA037404, U10CA037447, U10CA059518, U10CA180790, U10CA045418, U10CA180866, and UG1CA189819 from the National Cancer Institute, National Institutes of Health. The C-terminal telopeptide assays were supported by grant UL1TR001070 from the National Center for Advancing Translational Sciences, National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Qin reported owning stock in Regeneron Pharmaceuticals, United Health Group, and Gilead Sciences. Dr O’Mara reported owning stock in Pfizer. No other disclosures were reported.
Role of the Funder/Sponsor: The National Cancer Institute participated in and approved the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Cancer Institute, or the National Center for Advancing Translational Sciences.
Meeting Presentation: Presented in part at the 51st annual meeting of the American Society of Clinical Oncology; May 29–June 2, 2015; Chicago, Illinois.
Author Contributions: Drs Himelstein and Shapiro had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Khatcheressian, Roberts, Grubbs, O’Mara, Shapiro.
Acquisition, analysis, or interpretation of data: Himelstein, Foster, Seisler, Novotny, Qin, Go, Grubbs, O’Connor, Velasco, Weckstein, O’Mara, Loprinzi, Shapiro.
Drafting of the manuscript: Himelstein, Foster, Roberts, Novotny, Go, Velasco, Loprinzi, Shapiro.
Critical revision of the manuscript for important intellectual content: Himelstein, Foster, Khatcheressian, Seisler, Novotny, Qin, Go, Grubbs, O’Connor, Velasco, Weckstein, O’Mara, Loprinzi, Shapiro.
Statistical analysis: Foster, Seisler, Novotny, Qin.
Administrative, technical, or material support: Himelstein, Weckstein, O’Mara, Loprinzi, Shapiro.
Study supervision: Himelstein, O’Connor, Velasco, Weckstein, Loprinzi, Shapiro.
Additional Contributions: We thank Trina Wemlinger, BS, and William Malarkey, MD, at the Analytic and Development Lab of the Center for Clinical Translation and Science at Ohio State University for their work on the C-terminal telopeptide assays. No compensation was provided for this work. In addition, we especially thank all patients, with support from families and caregivers, for enrolling in this trial. A list of participating institutions can be found in the eAppendix in Supplement 2.
References
- 1.Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23(15):3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 2.Saad F, Gleason DM, Murray R, et al. Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 3.Pavlakis N, Schmidt R, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev. 2005;(3):CD003474. doi: 10.1002/14651858.CD003474.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 5.Novartis Inc. [Accessed December 6, 2016];Zometa: prescribing information. 2005 Dec; http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021223s012lbl.pdf.
- 6.Amadori D, Aglietta M, Alessi B, et al. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14(7):663–670. doi: 10.1016/S1470-2045(13)70174-8. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN, Lipton A, Chew HK, et al. Efficacy and safety of continued zoledronic acid every 4 weeks vs every 12 weeks in women with bone metastases from breast cancer: results of the OPTIMIZE-2 trial. J Clin Oncol. 2014;32(5 suppl):LBA9500^. [Google Scholar]
- 8.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 9.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 10.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27(7–8):365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Therapy Evaluation Program. [Accessed December 6, 2016];Common Terminology Criteria for Adverse Events, Version 3.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 12.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 13.Rosen LS, Gordon D, Tchekmedyian NS, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 14.Lipton A, Zheng M, Seaman J. Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinoma. Cancer. 2003;98(5):962–969. doi: 10.1002/cncr.11571. [DOI] [PubMed] [Google Scholar]
- 15.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 16.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford, England: Clarendon Press; 1994. [Google Scholar]
- 17.National Research Council Panel on Handling Missing Data in Clinical Trials. The Prevention and Treatment of Missing Data in Clinical Trials. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 18.Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355–1360. doi: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration. [Accessed October 26, 2015];Denosumab. http://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cder/ucm248277.htm.
- 20.Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48(16):3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Van Poznak CH, Temin S, Yee GC, et al. American Society of Clinical Oncology. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011;29(9):1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 22. [Accessed July 19, 2016];Prevention of symptomatic skeletal events with denosumab administered every 4 weeks vs every 12 weeks. https://clinicaltrials.gov/ct2/show/NCT02051218?term=denosumab+metastatic&rank=2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.