Abstract
Identifying the role of axonal injury in the development of permanent, irreversible neurologic disability is important to the study of central nervous system (CNS) demyelinating diseases. Our understanding of neurologic dysfunction in demyelinating diseases and the ability to assess therapeutic interventions depends on the development of objective functional assays that can non-invasively measure axonal loss. In this study, we demonstrate in a murine model of progressive CNS demyelination that assessment of the hindlimb width of stride provides a powerful indicator of axonal loss and can dissociate between deficits induced by demyelination versus axonal loss.
Keywords: Multiple sclerosis, Neurodegeneration, Oligodendrocyte, Footprint, Theiler’s virus, Motor function
Central nervous system (CNS) demyelinating diseases can result in axonal damage that may contribute to irreversible neurologic dysfunction [1,2]. The role of this axonal damage in the development of neurologic dysfunction is not completely understood. Theiler’s murine encephalomyelitis virus (TMEV) induces a chronic inflammatory CNS demyelinating disease in spinal cords of susceptible mice that is pathologically similar to human multiple sclerosis [3–6]. Intracerebral infection with TMEV results in a biphasic disease that is characterized by an acute infection of the gray matter followed by demyelination of the spinal cord [7]. The virus is cleared from the gray matter within 20 days and establishes chronic persistence in the brainstem and spinal cord white matter. We have demonstrated previously that medium and large axons are lost during the late, chronic stage (~180 days post-infection) of this demyelinating disease [8], and we have hypothesized that this fiber loss contributes significantly to disruptions in motor coordination at this time point.
Because both demyelination and axonal loss can contribute independently to neurologic dysfunction during the course of a progressive CNS demyelinating disease, it is important to develop objective functional assays that can distinguish between these two pathologic variables. Analysis of footprints in rodents has provided a useful functional assay for determining the effects of peripheral nerve injury / repair [9–12], experimental allergic neuritis [13], spinal cord injury / repair [14–16], drug-induced motor impairment [17], and x-irradiation [18]. We have demonstrated previously that reductions in forelimb and hindlimb stride length are progressive through the first 100 days of TMEV infection in susceptible mice [19], which coincides with the demyelinating phase of the disease [8]. However, no reductions in forelimb or hindlimb stride width were observed during this time period [19]. The present study describes a reduction in the hindlimb width of stride during the late, chronic stage of TMEV-induced demyelinating disease and addresses whether or not this functional abnormality is an indicator of the severity of spinal cord axonal loss observed at this time point.
For gait analyses, male / female SJL/ J (prototypic susceptible strain; n=34) and C57BL/6J (prototypic resistant strain; n=20) mice were purchased from The Jackson Laboratories (Bar Harbor, ME, USA) and injected intracerebrally at 8 weeks of age with 2×106 PFU of TMEV in a 10-μl volume (n=10 mice per strain) or 10 μl PBS (n=10 mice per strain) to serve as sham-infected controls. Forelimb and hindlimb width of stride was measured, as described previously [19]. Forelimb and hindlimb paws were painted with red and blue nontoxic paint (RoseArt Industries; Livingston, NJ). The mice were then expected to walk along a strip of white paper. A minimum of five prints per mouse were digitized with a color scanner and measured using a program written for the KS400 image analysis software (Kontron Elektronik, Munich, Germany). The program automatically calculated the length and width of stride for each measured step. Forelimb and hindlimb width measurements were quantified at the baseline, 25, 50, 94, and 171–184 days post-infection (d.p.i.). Ratios of infected / sham-infected forelimb and hindlimb width measurements were used to control for intracerebral injection, age, and environmental stimuli at each time point post-infection. Ratios were calculated by dividing infected distances by the mean sham-infected distance at each time point. The change in hindlimb width from baseline (Δ) at 171 d.p.i. was calculated by subtracting the baseline width measurement from the 171 d.p.i. width measurement for each infected mouse.
Following the last gait measurement, all mice were anesthetized and perfused via intracardiac puncture with 50 ml of Trump’s fixative (phosphate-buffered 4% formaldehyde with 1% glutaraldehyde, pH 7.2). The spinal cords were removed, sectioned into 1-mm transverse blocks, post-fixed with 1% osmium tetroxide (in 0.2 M phosphate buffer), and embedded in Araldite (Polysciences; Warrington, PA, USA). Quantification of axonal loss in the normal-appearing white matter was performed on randomly selected sham-infected (n=7) and infected (n=6) SJL/ J mice, as described previously [8,20]. Sections (1 μm) were cut from the 1-mm block corresponding to T6 in each mouse. Sections were stained with 4% paraphenyl-enediamine to visualize myelin. An Olympus AX70 microscope fitted with a 60× oil objective and a SPOT color digital camera was used to digitize seven representative fields (in the lateral, anterolateral, and anterior columns) from the normal-appearing white matter of each T6 cross-section. A program written for the KS400 image analysis software was then used to automatically calculate the axonal areas for all normally myelinated axons in each field. To facilitate comparisons between sham-infected and infected SJL/ J mice, relative axonal frequency distributions were calculated. Relative frequency distributions for each mouse were calculated by dividing the total number of axons in any given size category by the total number of axons sampled. Axonal data for infected SJL/ J mice were represented as a percentage loss of medium and large axons (≥4 μm2) compared to age-matched, shaminfected controls. The percentage of loss of medium and large axons was calculated by dividing the frequency of medium and large fibers for each infected SJL/ J mouse by the mean frequency of medium and large fibers for all shaminfected mice. For electron microscopy, selected areas from the anterolateral columns of the mid-thoracic spinal cord were trimmed, cut at 0.1 μm, and counterstained with uranyl acetate and lead citrate. Photographs were captured at 3000× using the JEOL® 1200 electron microscope.
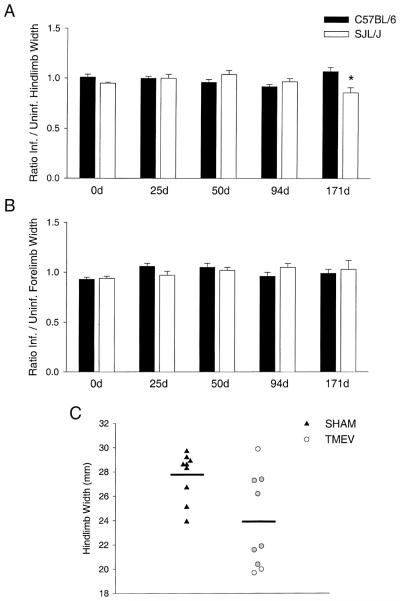
C57BL/6 and SJL/ J mice have no alterations in hindlimb or forelimb stride width by 94 d.p.i. (Fig. 1A,B) [19]. Data for the hindlimb (Fig. 1A) and forelimb (Fig. 1B) width of stride are shown for C57BL/6 and SJL/ J mice at various time points post-infection. Data are represented as a ratio of infected / sham-infected hindlimb widths. Ratios approximately equal to one signify no significant differences between infected and sham-infected mice for a given strain at any time point. Resistant C57BL/6 mice clear the virus following the acute phase of the disease, have no spinal cord demyelination / axonal loss, and show no significant alterations in gait at any time point post-infection. Interestingly, when compared to C57BL/6 mice, infected SJL/ J mice only showed a significant decrease in the hindlimb width of stride at 171 d.p.i. (Fig. 1A). This is a time point characterized by a substantial loss of medium and large normally myelinated axons in infected SJL/ J mice [8]. Reductions in the width of stride were not observed in the forelimbs of SJL/ J mice (Fig. 1B).
Fig. 1.
Assessment of hindlimb and forelimb stride width. Hindlimb (A) and forelimb (B) stride width were assessed in sham-infected and TMEV-infected C57BL/6 and SJL/ J mice at baseline (0d), 25, 50, 94, and 171 d.p.i. Data are represented as a ratio of infected / sham-infected hindlimb widths (mean±S.E.M.) at each time point. Statistical differences (denoted by an asterisk) were assessed by two-way repeated measures ANOVA. Pairwise comparisons were made using the Student–Newman–Keuls method (P<0.05). (C) Actual hindlimb widths (in mm) are shown for sham-infected (triangles) and TMEV-infected (circles) SJL/ J mice at 171 d.p.i. Horizontal lines represent the means for each group. The gray circles represent the six TMEV-infected mice that were randomly selected for quantification of axonal loss in the normal-appearing spinal cord white matter. (One TMEV-infected SJL/ J mouse could not be included on this graph because it was severely impaired and unable to walk at 171 d.p.i.).
To confirm that alterations in hindlimb width are only observed during the late, chronic phase of TMEV-induced demyelinating disease, a second set of sham-infected (n=9) and infected (n=5) SJL/ J mice were assessed at baseline, 94, and 184 d.p.i. No statistically significant differences in forelimb stride width were observed between sham-infected and infected mice at any time point post-infection. However, it is of interest that a statistically significant reduction in hindlimb stride width was only observed for infected SJL/ J mice at 184 d.p.i. (mean stride width=22.4±2.0) compared to age-matched, sham-infected controls (mean stride width=29.7±1.1; P<0.05). No significant differences were observed at baseline (sham=26.0±0.7 vs. TMEV=26.2±0.9) or at 94 d.p.i. (sham=26.1±0.9 vs. TMEV=26.0±1.5). These results support the hypothesis that alterations in hindlimb stride width are only observed during the late, chronic phase of disease in susceptible SJL/ J mice.
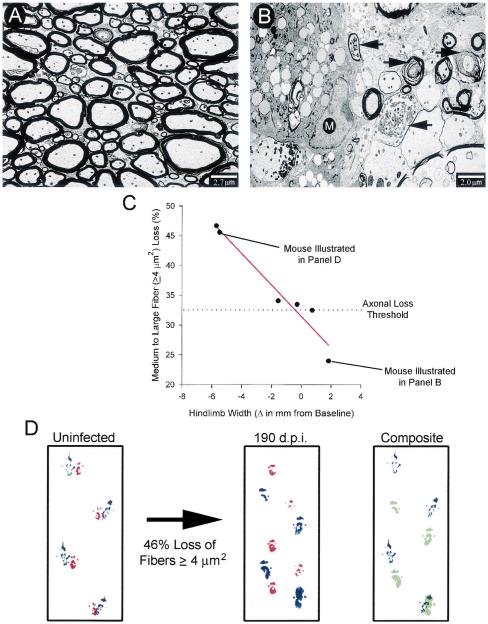
The total spinal cord demyelinating lesion load reaches a plateau by 100 d.p.i. [8]. This is followed by a significant loss of medium and large normally myelinated fibers. The fact that reductions in hindlimb width coincided temporally with this loss of medium and large normally myelinated fibers in the same mice led to the hypothesis that changes in hindlimb width serve as an indicator of axonal loss rather than demyelination. Comparison of hindlimb widths between sham-infected and TMEV-infected SJL/ J mice at 171 d.p.i. revealed heterogeneity amongst the infected mice (Fig. 1C). Four infected mice had hindlimb widths comparable to sham-infected controls, whereas five mice had significantly reduced hindlimb widths. When compared to sham-infected controls (Fig. 2A), electron micrographs of the spinal cord white matter in chronically infected SJL/ J mice (Fig. 2B) revealed evidence of axonal degeneration, including the accumulation of numerous floccular dense bodies, granular disintegration of neurofilaments/vesicles, and dark amorphous axoplasm. To determine if the heterogeneity in hindlimb width changes was related to axonal loss, we assessed correlative relationships between the percentage of medium and large fiber loss and the change (Δ) in hindlimb width from baseline in six randomly selected TMEV-infected SJL/J mice; three mice had hindlimb stride widths comparable to those of sham-infected controls, and three mice had significantly reduced widths of stride (Fig. 1C). Interestingly, a near perfect negative correlation (r=−0.97, P=0.001) was obtained between the percentage of medium and large normally myelinated fiber loss and the change in hindlimb width from baseline (Fig. 2C). In contrast, the change in hindlimb width from baseline did not correlate as well with the previously reported [8] percentage of total spinal cord demyelination (a measure of total spinal cord lesion load) (r=−0.65, P=0.166), demonstrating that hindlimb width is a better indicator of axonal loss at this time point. From the linear relationship illustrated in Fig. 2C, a threshold of axonal loss was established. A reduction in medium and large normally myelinated axons greater than 33% resulted in a decrease in hindlimb stride width. Hindlimb stride alterations are illustrated for a chronically infected mouse following a 46% loss of medium and large axons (Fig. 2D).
Fig. 2.
Alterations in hindlimb width as an indicator of spinal cord axonal loss. (A) An electron micrograph from the anterolateral columns of a sham-infected mouse illustrates normal myelin (thick black sheaths) surrounding intact axons. (B) In contrast, an electron micrograph from the lesion of a 190-day-infected mouse shows macrophage infiltration (M) and numerous degenerating axons (black arrows). (C) A strong negative correlation was found between the percentage of medium and large normally myelinated axon loss and the change in hindlimb width from baseline in chronically infected SJL/ J mice. Correlation coefficients were calculated using the Pearson product moment correlation (P<0.05). (D) A forelimb (red) and hindlimb (blue) stride pattern is shown for the same mouse before and after a 46% loss of medium and large axons. The composite image shows an overlay of hindlimb steps for this mouse at the baseline (blue) and 190 d.p.i. (green). Note that, after significant medium and large fiber loss, the infected mouse is one stride length short and there is a narrowing in the width of the stride.
The results from this study demonstrate that analysis of hindlimb width of stride can serve as a powerful indicator of spinal cord axonal loss in mice with a progressive CNS demyelinating disease. The fact that a near perfect correlation coefficient (r=−0.97) and statistical significance (P=0.001) was obtained using only six infected SJL/ J mice demonstrates the power of this functional assay. Additionally, of greater importance is that this functional measurement only changed when a threshold of axonal loss was reached, thus allowing the design of future studies that distinguish between neurologic deficits induced by axonal loss rather than demyelination. That alterations in hindlimb stride width were not observed during the progressive demyelinating phase of disease (within 100 d.p.i.) in two independent time courses with susceptible SJL/ J mice demonstrates the specificity of this functional assay in detecting axonal loss rather than demyelination (or total spinal cord lesion load).
Following a demyelinating insult, it is known that CNS axons can be remyelinated [21–23]. This remyelination can completely restore CNS conduction [24,25]. However, if a threshold of axons is lost as a consequence of a demyelinating event, this has the potential to induce permanent, irreversible neurologic disability. Therefore, the design of therapeutic interventions that prevent axonal loss during the course of demyelinating diseases is of the utmost importance. To this end, the analysis of hindlimb width of stride described in this study can be used as a powerful non-invasive surrogate measure of axonal loss.
Acknowledgements
This work was supported by the National Institutes of Health grants: RO1 NS24180, RO1 NS32129, and the generous contributions of Mr. and Mrs. Eugene Applebaum and Ms. Kathryn Peterson. D.B.M. is supported by a predoctoral NRSA from the National Institute of Mental Health (Grant#1F31ME12120). S.S. is supported by the Faculty of Medicine, Siriraj Hospital, Mahidol University, Thailand.
Footnotes
Theme: Disorders of the nervous system
Topic: Degenerative disease, other
References
- [1].Trapp BD, Ransohoff RM, Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr. Opin. Neurol. 1999;12:295–302. doi: 10.1097/00019052-199906000-00008. [DOI] [PubMed] [Google Scholar]
- [2].Bjartmar C, Yin XH, Trapp BD. Axonal pathology in myelin disorders. J. Neurocytol. 1999;28:383–395. doi: 10.1023/a:1007010205037. [DOI] [PubMed] [Google Scholar]
- [3].Dal Canto MC, Lipton HL. Multiple sclerosis. Animal model: Theiler’s virus infection in mice. Am. J. Pathol. 1977;88:497–500. [PMC free article] [PubMed] [Google Scholar]
- [4].Dal Canto MC, Lipton HL. Recurrent demyelination in chronic central nervous system infection produced by Theiler’s murine encephalomyelitis virus. J. Neurol. Sci. 1979;42:391–405. doi: 10.1016/0022-510x(79)90172-2. [DOI] [PubMed] [Google Scholar]
- [5].Lipton HL, Dal Canto MC. Chronic neurologic disease in Theiler’s virus infection of SJL / J mice. J. Neurol. Sci. 1976;30:201–207. doi: 10.1016/0022-510x(76)90267-7. [DOI] [PubMed] [Google Scholar]
- [6].Rodriguez M, Oleszak E, Leibowitz J. Theiler’s murine encephalomyelitis: A model of demyelination and persistence of virus. Crit. Rev. Immunol. 1987;7:325–365. [PubMed] [Google Scholar]
- [7].Lipton HL. Theiler’s virus infection in mice: an unusual biphasic disease leading to demyelination. Infect. Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McGavern DB, Murray PD, Rivera-Quinones C, Schmelzer JD, Low PA, Rodriguez M. Axonal loss results in spinal cord atrophy, electrophysiologic abnormalities, and neurologic deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain. 2000;123:519–531. doi: 10.1093/brain/123.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol. 1982;77:634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- [10].Bisby MA. Enhancement of the conditioning lesion effect in rat sciatic motor axons after superimposition of conditioning and test lesions. Exp. Neurol. 1985;90:385–394. doi: 10.1016/0014-4886(85)90027-5. [DOI] [PubMed] [Google Scholar]
- [11].Wikholm RP, Swett JE, Torigoe Y, Blanks RH. Repair of severed peripheral nerve: a superior anatomic and functional recovery with a new ‘reconnection’ technique. Otolaryngol. Head Neck Surg. 1988;99:353–361. doi: 10.1177/019459988809900401. [DOI] [PubMed] [Google Scholar]
- [12].Walker JL, Evans JM, Meade P, Resig P, Sisken BF. Gait–stance duration as a measure of injury and recovery in the rat sciatic nerve model. J. Neurosci. Methods. 1994;52:47–52. doi: 10.1016/0165-0270(94)90054-x. [DOI] [PubMed] [Google Scholar]
- [13].Wietholter H, Eckert S, Stevens A. Measurement of atactic and paretic gait in neuropathies of rats based on analysis of walking tracks. J. Neurosci. Methods. 1990;32:199–205. doi: 10.1016/0165-0270(90)90141-2. [DOI] [PubMed] [Google Scholar]
- [14].Kunkel-Bagden E, Dai HN, Bregman BS. Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Exp. Neurol. 1993;119:153–164. doi: 10.1006/exnr.1993.1017. [DOI] [PubMed] [Google Scholar]
- [15].Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- [16].Cheng H, Almstrom S, Gimenez-Llort L, Chang R, Ove OS, Hoffer B, Olson L. Gait analysis of adult paraplegic rats after spinal cord repair. Exp. Neurol. 1997;148:544–557. doi: 10.1006/exnr.1997.6708. [DOI] [PubMed] [Google Scholar]
- [17].Sykes EA. Mescaline-induced motor impairment in rats, assessed by two different methods. Life. Sci. 1986;39:1051–1058. doi: 10.1016/0024-3205(86)90196-7. [DOI] [PubMed] [Google Scholar]
- [18].Mullenix P, Norton S, Culver B. Locomotor damage in rats after x-irradiation in utero. Exp. Neurol. 1975;48:310–324. doi: 10.1016/0014-4886(75)90159-4. [DOI] [PubMed] [Google Scholar]
- [19].McGavern DB, Zoecklein L, Drescher KM, Rodriguez M. Quantitative assessment of neurologic deficits in a chronic progressive murine model of CNS demyelination. Exp. Neurol. 1999;158:171–181. doi: 10.1006/exnr.1999.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McGavern DB, Murray PD, Rodriguez M. Quantitation of spinal cord demyelination, remyelination, atrophy, and axonal loss in a model of progressive neurologic injury. J. Neurosci. Res. 1999;58:492–504. doi: 10.1002/(sici)1097-4547(19991115)58:4<492::aid-jnr3>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gledhill RF, Harrison BM, McDonald WI. Pattern of remyelination in the CNS. Nature. 1973;244:443–444. doi: 10.1038/244443a0. [DOI] [PubMed] [Google Scholar]
- [22].Yajima K, Suzuki K. Demyelination and remyelination in the rat central nervous system following ethidium bromide injection. Lab. Invest. 1979;41:385–392. [PubMed] [Google Scholar]
- [23].Miller DJ, Rivera-Quinones C, Njenga MK, Leibowitz J, Rodriguez M. Spontaneous CNS remyelination in β2 microglobulin-deficient mice following virus-induced demyelination. J. Neurosci. 1995;15:8345–8352. doi: 10.1523/JNEUROSCI.15-12-08345.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Smith KJ, Blakemore WF, McDonald WI. Central remyelination restores secure conduction. Nature. 1979;280:395–396. doi: 10.1038/280395a0. [DOI] [PubMed] [Google Scholar]
- [25].Smith KJ, Blakemore WF, McDonald WI. The restoration of conduction by central remyelination. Brain. 1981;104:383–404. doi: 10.1093/brain/104.2.383. [DOI] [PubMed] [Google Scholar]