Abstract
Dendritic cells (DCs) comprise the major antigen-presenting cells (APCs) of the host, uniquely programmed to stimulate immunologically naïve T lymphocytes. Viruses that can target and disorder the function of these cells enjoy a selective advantage. The cellular receptor for lymphocytic choriomeningitis virus (LCMV), Lassa fever virus (LFV), and several other arenaviruses is α-dystroglycan (α-DG). Among cells of the immune system, CD11c+ and DEC-205+ DCs primarily and preferentially express α-DG. By selection, strains and variants of LCMV generated as quasi-species that bind α-DG with high affinity replicate in the majority of CD11c+ and DEC-205+ (>75%) DCs, causing a generalized immunosuppression, and establish a persistent infection. In contrast, viral strains and variants that bind with low affinity to α-DG display minimal replication in CD11c+ and DEC-205+ DCs (<10%), rarely replicate in the white pulp, and generate a robust anti-LCMV CTL response that clears the virus infection. Hence, receptor-virus interaction on DCs in vivo is an essential step in the initiation of virus-induced immunosuppression and viral persistence. Investigation into the mechanism of how virus-infected DCs cause immunosuppression reveals loss of MHC class II surface expression and costimulatory molecules on surface of such DCs. As a consequence DCs are unable to act as APCs, initiate immune responses, and have a defect in migration into the T cell area. These data indicate that LCMV infection influences DC maturation and migration, leading to decreased T cell stimulatory capacity of DCs, events essential for the initiation of immune responses. Because several other viruses known to cause immunosuppression (HIV, measles) interact with DCs, the observations noted here are likely a common selective mechanism by which viruses also are able to evade the host s immune system.
Introduction
Viruses use a number of strategies, including escape from immune recognition or induction of immunosuppression, to avoid immunological surveillance and thereby persist in their host (reviewed in Borrow and Shaw 1998; Tortorella et al. 2000). The downregulation of immune responses provides the infecting pathogens with the opportunity to maximize their chances of survival, replication, and transmission. The generalized immunosuppression caused by viral infection is often associated with secondary infections with unrelated viral and/or bacterial pathogens and, as such, represents a serious clinical problem. Understanding the mechanisms involved in the induction of immunosuppression is therefore a crucial step for controlling and eliminating infections caused by several viruses including important human pathogens such as hepatitis B and C and human immunodeficiency virus (HIV). To better understand how viruses may cause immunosuppression, we have studied this phenomenon in an animal model system in which the noncytopathic lymphocytic choriomeningitis virus (LCMV) infects its natural host, the mouse, and causes transient to long-lasting immunosuppression (Oldstone 1989). In some ways, LCMV infection resembles HIV and HIV-triggered AIDS in humans (reviewed in Hazenberg et al. 2000; Lieberman et al. 2001; McMichael and Rowland-Jones 2001). In this review, we focus on one of the main strategies used by LCMV to subvert normal defense mechanisms and cause a generalized immunosuppression: infection and interference with the functions of dendritic cells (DCs).
Initiation of an adaptive immune response to infectious agents is mediated by a class of sentinel phagocytic leukocytes termed DCs, the primary function of which is to capture, process, and present antigens to unprimed T cells (Ibrahim et al. 1995; Steinman 1991). DCs are present in nonlymphoid organs such as the liver and skin in an immature state. During the course of an infection, DCs take up and process antigen, mature, and migrate to the local draining lymph node, where they efficiently activate both T and B cells (Banchereau and Steinman 1998). A reduced antigen-processing capacity and an increased cell surface expression of MHC class II and costimulatory molecules such as B7-1 and B7-2 occurs during the migration of DCs to lymph organs, thus making the DCs the most potent antigen-presenting cells (APCs) in the immune repertoire (Banchereau and Steinman 1998). Activated DCs are essential for the subsequent activation of both naïve and activated T cells. In addition, with the release of specific cytokines and chemokines, activated DCs either directly or indirectly play a crucial role in the recruitment and/or activation of other effector cells, including NK and NKT cells, macrophages, and B cells (Rescigno et al. 1999). In this review, we describe the infection of DCs by LCMV in vivo and the consequence of this infection on DC functionality.
LCMV and Immunosuppressive Variants
LCMV is the prototypic member of the family Arenaviridae, which includes important human pathogens such as Lassa fever and Machupo, Sabia, and Junin viruses (Buckley et al. 1970; Peters et al. 1996; Southern et al., 1987). Its genome consists of two negative-strand RNA segments, large (L) and short (S) fragments (Riviere et al. 1985; Singh et al. 1987; Southern 1996). Each RNA segment has an ambisense coding strategy, encoding two proteins in opposite orientations separated by an intergenic region. The L RNA encodes the viral RNA-dependent RNA polymerase (L) (Singh et al. 1987) and a small RING finger protein (Z) (Salvato and Shimomaye 1989). The S RNA encodes the three major structural proteins: the nucleoprotein (NP) and the precursor polypeptide, GP-C (Wright et al. 1990), which is processed into two products, the peripheral glycoprotein GP1 and the transmembrane glycoprotein GP2 (Buchmeier and Oldstone 1979). These mature forms of the glycoprotein form 5- to 10-nm-long surface projections (spikes) that are speculated to be composed of tetramers of GP2 that serve as platforms for tetramers of GP1 (Burns and Buchmeier 1993). The GP1 portion of the glycoprotein mediates the virus-receptor interaction based on the fact that neutralizing antibodies are formed exclusively against GP1 (Borrow and Oldstone 1992; Parekh and Buchmeier 1986). The main cellular receptor for LCMV has been identified as α-dystroglycan (α-DG) (Cao et al. 1998), which is a highly versatile cellular receptor for proteins of the extracellular matrix (ECM) (Ervasti and Campbell 1993; Ibraghimov-Beskrovnaya et al. 1992; Winder 2001). Analysis of the interaction of LCMV with α-DG is discussed later in this chapter.
LCMV, on congenital, neonatal, or in utero infection, can cause a persistent infection in its natural host, the mouse (reviewed in Borrow and Oldstone 1997). During this persistent infection, we (Ahmed and Oldstone 1988; Ahmed et al. 1984; Dockter et al. 1996; Evans et al. 1994; Sevilla et al. 2000) and others (Ahmed et al. 1991; Matloubian et al. 1993; Villarete et al. 1994) have described the emergence of LCMV variants. Viral variants were found with different biological properties: One variant predominated in the central nervous system (CNS) in neurons (Dockter et al. 1996; Evans et al. 1994; Rodriguez et al. 1983; Villarete et al. 1994) and another predominated in cells of the immune system such as lymphocytes and macrophages (Ahmed et al. 1991; Borrow et al. 1995; Dockter et al. 1996; Matloubian et al. 1993; Sevilla et al. 2000; Villarete et al. 1994). The parental virus, Armstrong 53b (ARM), and the vast majority of viruses and variants isolated from the CNS, when inoculated intravenously into adult mice, caused an acute infection characterized by profound CD8+-T cell expansion and IFN-γ production that efficiently terminated the viral infection by 7–10 days after infection. The prototype variant that has tropism for cells of the immune system is called LCMV clone 13 (Cl 13) (Ahmed et al. 1991). Inoculation of Cl 13 intravenously into adult immunocompetent mice aborted cytotoxic T lymphocyte responses (CTL) and led to viral persistence (Matloubian et al. 1993; Salvato et al. 1991; Tishon et al. 1988). In contrast to the persistent infection initiated early in life that is due to thymic deletion of antiviral immune cells (Borrow and Oldstone 1997), persistent infection of adult mice with Cl 13 is due to exhaustion of virus-specific T cells and is associated with a generalized immunosuppression (Borrow et al. 1995; Tishon et al. 1993).
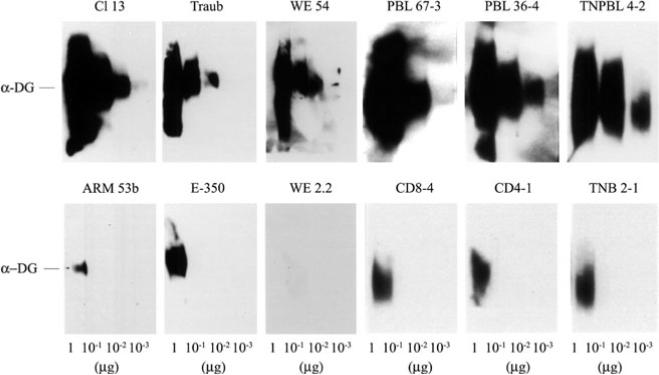
Sequence comparison between ARM and Cl 13 showed five nucleotide changes in the entire genome, and only two of them caused amino acid (aa) substitutions (Salvato et al. 1991; Salvato and Shimomaye 1989). One of these aa substitutions is in position 260 of GP-1, with ARM having phenylalanine (F) and Cl 13 leucine (L); the other aa change was in position 1079 of the L protein from lysine (K) (ARM) to glutamine (Q) (Cl 13). Recent studies of numerous LCMV strains and viral variants generated in vivo demonstrated a correlation between a F(260)→L/I mutation in the viral GP-1, the component that binds the cellular receptor, and the immunosuppressive phenotype (Sevilla et al. 2000; Smelt et al. 2001). Furthermore, all LCMV variants and strains with a L or I in position 260 of GP-1 showed high affinity binding to α-DG, with binding of at least 1 log and often 2–3 logs greater than that observed with viruses with F at position 260 (Fig. 1; Sevilla et al. 2000). These findings indicated that an aliphatic aa-like L or I in position 260 of GP-1 of LCMV viruses is associated with high-affinity binding to the cellular receptor α-DG and with the ability to cause immunosuppression in adult mice, whereas those viruses containing an aromatic (F) aa at this position bound with a lower affinity to α-DG and did not cause immunosuppression on inoculation of adult mice.
Fig. 1.
LCMV binding affinity to α-DG. Virus overlay protein binding assay (VOPBA) was done with purified α-DG and LCMV variants and strains as described by Sevilla et al. (2000). Decreasing amounts of purified α-DG were loaded on an SDS-PAGE, electrophoresed, and transferred onto nitrocellulose. The nitrocellulose was incubated with 107 PFUs of each viral isolate, and viral proteins were detected with monoclonal antibodies specific for LCMV GP. The top panel shows the VOPBA of immunosuppressive LCMV strains (Traub and WE 54) and variants (Cl 13, PBL 67–3, PBL 36–4, and TNPBL 4–2); the bottom panel shows the VOPBA of nonimmunosuppressive LCMV strains (ARM 53b and E-350) and variants (WE 2.2, CD8–4, CD4–1, and TNB 2–1)
Competition Between LCMV and the Extracellular Matrix Molecules for a-DG
The LCMV receptor on dendritic cells, dystroglycan (DG), is initially encoded as a single protein and undergoes post-translational cleavage to form α-DG, a peripheral protein, and β-DG, a membrane protein. DG is expressed in most developing and adult tissues, typically in cell types that adjoin basement membranes (Durbeej et al. 1998). At those sites, DG plays a critical role in cell-mediated assembly of basement membranes (Ervasti and Campbell 1991, 1993; Gee et al. 1994; Henry and Campbell 1998; Talts et al. 1999; Williamson et al. 1997). α-DG undergoes high-affinity interactions with the ECM proteins laminin-1, laminin-2, agrin, and perlecan. In muscle tissue, DG is found associated with two additional types of membrane proteins, the sarcoglycans and sarcospan (Henry and Campbell 1999). α-DG is noncovalently associated with the membrane-spanning β-DG, which binds to the cytoskeletal proteins dystrophin and utrophin, as well as signal transduction molecules such as grb2 and the focal adhesion kinase FAK (Cavaldesi et al. 1999; Jung et al. 1995; Yang et al. 1995).
The complex binding pattern of α-DG results in the formation of tissue-specific α-DG complexes in the cell membrane. Because specific DG-associated proteins may enhance or block virus binding, their coexpression with DG may modulate the susceptibility of a specific cell for virus infection and represent an important determinant for viral tissue tropism in vivo. Given their expression in lymphoid organs, high binding affinity, and large size, ECM molecules may interfere with virus binding to α-DG and, therefore, provide a barrier for virus infection of specific DG+ cell types such as CD11c+ DCs. In vitro studies demonstrated that high-affinity binding LCMV variants like Cl 13 compete successfully with laminin-1 and laminin-2 for α-DG binding. In contrast, the low-affinity binding variant ARM was blocked from its receptor binding site by laminin (Kunz et al. 2001). Because recent binding studies suggest overlapping binding sites on α-DG for the ECM components laminin-1, laminin-2, perlecan, and agrin (Talts et al. 1999), these molecules likely provide a barrier for infection of cells by low-affinity binding LCMV variants.
The expression of α-DG-associated ECM in lymphoid tissues such as the spleen may be an important determinant for the differential anatomic distribution of LCMV variants with different receptor binding affinities and the ability to cause distinct pathologies. Immunohistochemical analysis of the expression of laminin in spleen tissue (Fig. 2) revealed particularly strong staining in the marginal zone at the periphery of the white pulp (Kunz et al. 2001; van den Berg et al. 1993), where large numbers of CD11c+ DC are found (Kunz et al. 2001; Steinman et al. 1997). The overlap between laminin expression and infection with Cl 13 suggests that this virus efficiently infects cells surrounded by laminin. By contrast, the lack of association between laminin expression and the distribution of ARM suggests that laminin interferes with ARM infection. High receptor binding affinity likely enables variants like Cl 13 to displace α-DG-associated ECM (Fig. 3) molecules from the surface of CD11c+ DCs in the marginal zone, resulting in infection of these cells. Infected DCs subsequently migrate into the white pulp and disseminate the virus into the T cell area (Lewicki et al., unpublished observation). In contrast to Cl 13 and the high-affinity binders, low-affinity binders like ARM may be unable to displace α-DG-associated ECM components (Fig. 3) and thus unable to effectively infect CD11c+ DC. This may explain why LCMV variants like ARM are excluded from the white pulp and are found predominantly in the red pulp. The more efficient infection of cells in the red pulp exhibited by low-affinity binding variants may reflect their ability to use alternative cellular receptors for infection. The biochemical nature of this alternate receptor is currently unknown.
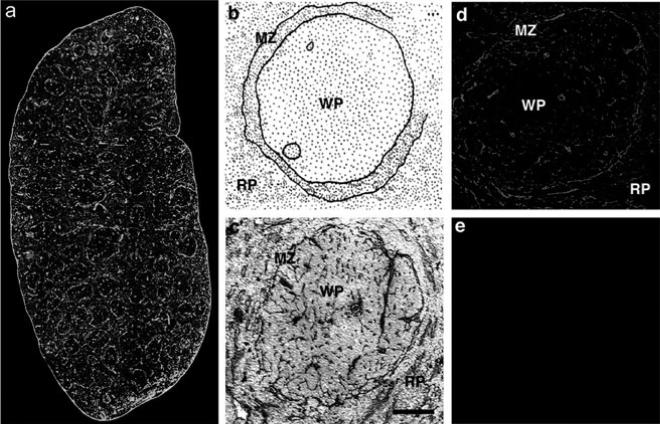
Fig. 2a-e.
Expression pattern of laminin and CD11c in the spleen. Detection of laminin and CD11c in whole spleen sections (a): Laminin was detected with a rabbit anti-mouse laminin-1 antibody and a FITC-labeled secondary antibody (green). CD11c was detected with a hamster anti CD11c antibody and a rhodamine-X-conjugated secondary antibody (red). Overlapping fluorescence appears in yellow as a rhodamine-X/FITC double-filter device was used. Localization of laminin in the marginal zone of the white pulp (b): Schematic representation of spleen histology. In c, laminin was stained with rabbit anti-laminin-1 antibody with a HRP-conjugated secondary antibody and DAB as a chromophore. Sections were counterstained with hematoxylin and eosin. Laminin staining of a single white pulp area (d, e): Laminin was detected with a rabbit anti-laminin-1 antibody and a Texas-red conjugated secondary antibody (d); secondary antibody only is shown in e. MZ, marginal zone; RP, red pulp; WP, white pulp. Bars: a, 200 μm; b–e, 50 μm
Fig. 3.
Targeting DCs by LCMV: role of the ECM molecules in the interaction of LCMV with α-DG. α-DG undergoes high-affinity interactions with ECM such as laminin. These ECM components may interfere with virus binding to the cell and, therefore, provide a barrier for virus infection of specific cells such as CD11c+ DCs. High-affinity binding LCMV variants such as Cl 13 (upper panel) may compete successfully with laminin for α-DG, whereas low-affinity binders such as ARM (lower panel) are unable to displace laminin. The lack of association between laminin expression (Fig. 2) and ARM distribution (Fig. 5) suggests that laminin may interfere with ARM infection
The scenario in the spleen may reflect the situation in other lymphoid organs, where immunohistochemical studies revealed expression of laminin and other ECM components that bind to α-DG. In lymph nodes, laminin is found in vessel walls, in a layer underlying the subcapsular sinus, and in an extensive array of ECM fibers (Jaspars et al. 1995; Kramer et al. 1988; van den Berg et al. 1993). In bone marrow, widespread laminin expression was detected in vascular basement membranes and intersinusoidal interstitial tissue (Gu et al. 1999; Nilsson et al. 1999). The laminin-containing ECM structures in lymphoid organs are thought to form a scaffold for localization and/or migration of immune cells (Kramer et al. 1988). However, despite a large number of studies describing laminin expression in lymphoid organs, the detailed distribution of laminin iso-forms in these tissues is poorly understood. The identification of specific laminin isoforms that may be expressed in a specific temporal and spatial pattern in resting lymphoid tissue and during acute infection processes is clearly of great importance for an understanding of the molecular basis of LCMV-mediated immunosuppression. Furthermore, a similar in vivo selection of LCMV variants with high affinity for α-DG is also observed in nonlymphoid organs such as kidney, liver, and lung (Evans et al. 1994), suggesting that the selection of variants with high receptor binding affinity is a more general phenomenon and may represent a selective advantage during the natural infectious process. Because α-DG binding laminin isoforms are widely expressed in these organs (Miner et al. 1997), it is likely that competition between virus and ECM components for α-DG may also play a role in the in vivo selection of viral variants in these tissues.
Replication of LCMV in DCs
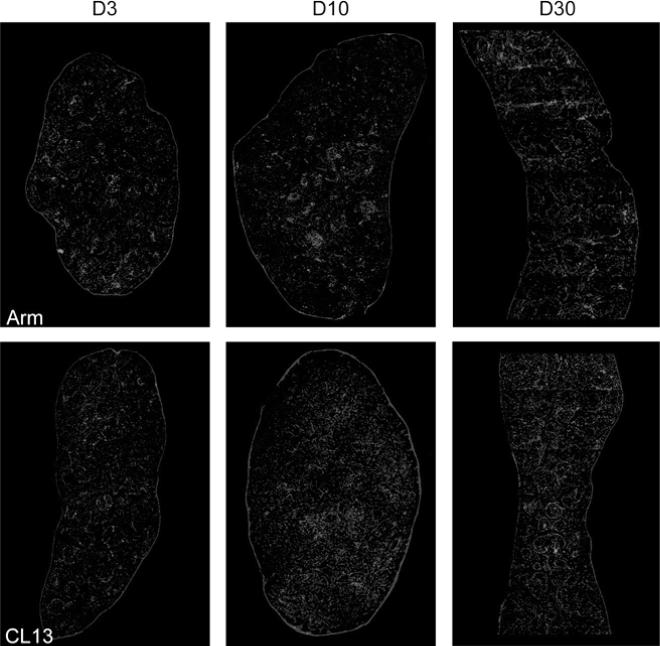
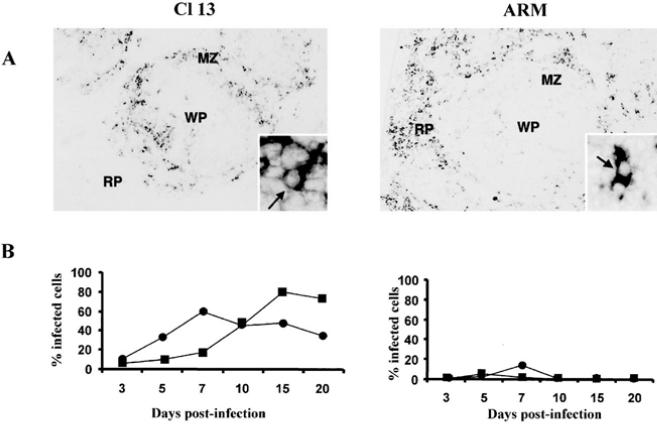
The defect underlying the generalized immunosuppression in mice infected with immunosuppressive LCMV variants maps primarily to antigen presentation rather than T or B cell function per se (Althage et al. 1992; Odermatt et al. 1991) and would be predicted by the observation that these immunosuppressive viruses infect lymphocytes inefficiently both in vivo and in vitro (Borrow et al. 1991; Matloubian et al. 1993; Sevilla et al. 2000). The analysis of the anatomic distribution of immunosuppressive and nonimmunosuppressive variants and strains in the spleens of adult immunocompetent mice 3 days after infection showed a different tropism for both groups of viruses (Borrow et al. 1995; Sevilla et al. 2000; Smelt et al. 2001). Cl 13-like viruses localized exclusively in cells of the marginal zone and white pulp of spleen, whereas ARM-like viruses localized primarily in cells within the red pulp (Fig. 4). By days 3–5 after infection, mice infected with ARM or Cl 13 showed some loss of follicular structure as evidenced by laminin stains (Fig. 4). By day 7, all follicular organization was absent and CD11c+ DCs had distributed throughout the entirely of the spleen. Interestingly, at day 10, CD11c+ DCs in ARM-infected mice had returned to small nests suggestive of splenic reorganization, whereas Cl 13-infected mice maintained disorganized DCs and also showed elevated laminin staining (Fig. 4). However, by day 30 mice infected with either strain showed a complete restoration of spleen architecture, yet CD11c+ staining appeared to be less intense in Cl 13-infected spleens (Fig. 4).
Fig. 4.
Comparison of splenic architecture in Cl 13- and ARM-infected mice. The distribution of laminin and CD11c in whole spleen section from ARM- or Cl 13-infected mice at days 3, 10, and 30 after infection. Laminin was detected with a rabbit anti-mouse laminin-1 antibody and a FITC-labeled secondary antibody (blue). CD11c was detected with a hamster anti-CD11c antibody and a rhodamine-X-conjugated secondary antibody (red). Overlapping fluorescence appears in pink
This distinct distribution of virus in spleen indicated that different subsets of cells were infected by immunosuppressive and nonimmunosuppressive viruses. The identification of cells in spleen infected by LCMV revealed two subsets mainly infected by immunosuppressive viruses, CD11c- and DEC-205 positive DCs (Fig. 5; Sevilla et al. 2000). CD11c+ cells localized in the central white pulp, T cell areas (interdigitating cells), and “nests” of cells that interrupt the marginal zone and extend into the T cell but not the B cell areas. The latter have been termed “marginal zone DC.” The DEC-205 antigen is expressed on a subset of DCs in spleen, and labeling appears to be confined largely to interdigitating DCs, localized into the T cell area (Steinman et al. 1997). In contrast, nonimmunosuppressive viruses infected mainly F4/80 positive macrophages and minimal number of DCs (Fig. 5). The in vivo replication kinetics over the first 20 days after infection showed that immunosuppressive viruses infected more than 80% of DCs (Fig. 5), indicating that DCs are the primary cell infected in vivo by immunosuppressive LCMV variants (Sevilla et al. 2000). The fact that LCMV immunosuppressive and nonimmunosuppressive variants bound with different affinities to the viral receptor α-DG suggested a crucial role for virus-receptor interaction in the infection of DCs and the initiation of immunosuppression. Only those viruses binding with a high affinity to α-DG have a preferential tropism for DCs leading to a generalized suppression of the host immune system, including the anti-LCMV CTL response required for clearing the infection. Moreover, a recent study in which we evaluated the selective pressures involved in the emergence of immunosuppressive variants during a persistent infection indicated that only variants with high-affinity binding to the receptor were selected from the viral quasi-species to cause immunosuppression (Sevilla et al. 2000). All immunosuppressive variants had L or I in aa 260 of GP-1, instead of F found in nonimmunosuppressive viruses, implying the importance of this aa position in the selection of the immunosuppressive variants. This study suggested that those viral variants with increased binding receptor affinity might be selected within the replicating quasi-species to bind to CD11c+ and DEC-205+ cells. The selective pressure is likely based on the interaction of the virus with its cellular receptor, thereby offering a marked advantage to the virus in initiating the dysfunction of the host APCs, aborting the necessary host immune responses required to terminate the viral infection. In addition, the major cell population expressing α-DG in spleen is CD11c+ cells (Sevilla et al. 2000), which further confirms the role of virus-receptor interaction in the pathogenesis of LCMV.
Fig. 5a, b.
Infection of DCs by LCMV. a The comparison of the anatomic localization of viral nucleic acids in spleen between Cl 13 and ARM at day 3 after infection shows ARM nucleic acid localized predominantly in the red pulp (RP) and Cl 13 nucleic acid localized with the white pulp (WP) or its marginal zone (MZ). A higher-power view is shown of both panels. The arrows indicate viral nucleic detected in individual cells. b Replication of Cl 13 and ARM in DCs in vivo over a 20-day observation period. Four adult BALB/c ByJ mice were infected with ARM or Cl 13. CD11c+ and DEC-205+ cells obtained from spleens made into single splenocyte suspensions were labeled with specific antibodies for these cell subsets. (■) CD11c+ DCs; (●) DEC-205+ DCs
Impairment of LCMV-Infected DCs
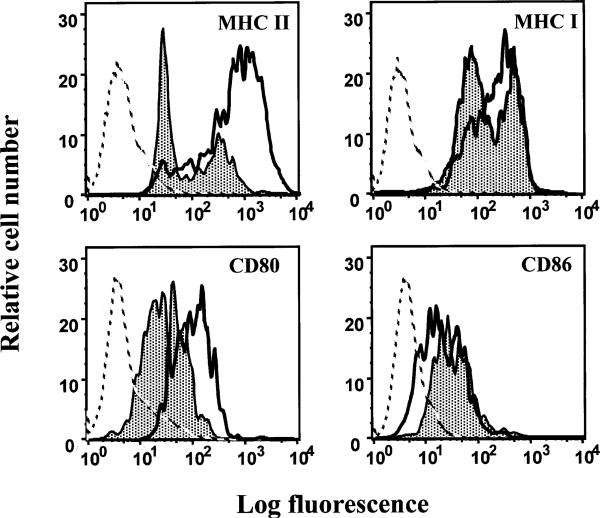
The observation that LCMV is able to infect more than 80% of DCs in vivo suggested the possibility that LCMV may functionally impair DCs. Indeed, DCs isolated from Cl 13-infected mice showed a functional deficit in antigen presentation as evidenced by the inability to stimulate the proliferation of naïve T cells in a primary mixed lymphocyte reaction (MLR; Borrow et al. 1995; Sevilla et al., manuscript in preparation). The prolonged interaction between naïve T cells and APCs establishes that APCs are highly adhesive and costimulatory. In fact, adhesion molecules allow formation of stable APC-T cell conjugates that are essential to deliver the sustained TCR triggering (Sykulev et al. 1996). Additionally, costimulation increases the possibility that this scheduled threshold is reached by lowering the time required for commitment. Consequently, mature DCs that express high levels of adhesion and costimulatory molecules can form stable clusters (Austyn et al. 1988). Because DCs from Cl 13-infected mice were impaired in MLR, it was important to map out any phenotypic changes that could potentially contribute to this dysfunction. Analysis of in vivo-isolated DCs resulted in the identification of two phenotypic populations. In ARM-infected mice a CD11c+high MHC class IIhigh population of DCs were isolated, whereas CD11c+high MHC class IIlow DCs were found in Cl 13-infected mice (Fig. 6). In addition, phenotypic analysis of infected DCs showed severely reduced expression of CD80 but normal expression of CD86 during the course of the persistent infection (Fig. 6; Sevilla et al., manuscript in preparation). The engagement of CD80 with CD28 on T cells can direct naïve T cells to differentiate along a TH1 pathway whereas the ligation of CD28 with CD86 directs the differentiation of TH precursors to a TH2 pathway (Kuchroo et al. 1995; Webb and Feldmann 1995). The consequences of downregulation of CD80 on DCs could impair the development of a TH1 immune response that mediates the elimination of LCMV. Moreover, CD80 acts as a triggering signal for NK cell-mediated cytolysis (Chambers et al. 1996). The downregulation of CD80 molecules on LCMV-infected DCs could also render DCs less susceptible to NK cell-mediated lysis. In comparison to the viral effect on MHC class II expression, we found a less pronounced albeit significant downregulation of MHC class I molecules by LCMV infected DCs (Fig. 6). This downregulation could blunt the generation of antiviral CD8+ T lymphocytes by infected DC, thereby weakening the host defense.
Fig. 6.
LCMV modulates expression of functionally important surface molecules on mature DCs. At day 15 after infection, spleens from ARM- or Cl 13-infected mice were collected and individual splenocyte suspensions were prepared from each mouse. Splenocytes were stained with specific monoclonal antibodies for CD11c, MHC II, MHC I, CD80, and CD86. The figure shows FACS histograms of surface expression of MHC I, MHC II, CD80, and CD86 on CD11c+ cells. The gray filled-in curves represent expression of the corresponding marker molecules on DCs from Cl 13-infected mice, whereas the black curves show surface expression on DCs from ARM-infected mice. Broken lines depict staining of DCs with an irrelevant antibody (isotype control). In the X-axis, the fluorescence intensity (log scale) is given, whereas the Y-axis depicts the relative cell number
Virus infection also inhibited production of cytokines such as IL-12. IL-12 is a critical cytokine produced by DCs that directs naïve T cells to differentiate toward a TH1 phenotype (Macatonia et al. 1995). However, LCMV infection does not depend on the presence of IL-12 for the induction of protective type 1 T cell responses (Oxenius et al. 1999). A TH2 immunodeviation, as suggested with measles virus (reviewed in Patterson et al. 2001), has not been observed in the LCMV system. The expression of IL-4, as the more potent polarizing signal for TH2 differentiation (Ohshima and Delespesse 1997), was not found to be increased in LCMV Cl 13 infected mice (Sevilla et al., manuscript in preparation). On the basis of these findings, the infection of DCs allows LCMV to evade T cell attack by disrupting antigen presentation and, hence, the initiation of an efficient antiviral immune response. However, the role of other mechanisms is currently under investigation. Elucidation of these mechanisms will help to further understand LCMV infection and other immunosuppressive viral infections.
Concluding Remarks
This chapter has considered the more recent studies regarding the interaction of LCMV with DCs. This interaction, as in many other viral systems, leads to immunosuppression in the host. In fact, infection plus impairment of DC function provides a selective advantage to the virus. The aforementioned studies clearly demonstrate that it is beneficial for the virus to directly interact with DCs in vivo. Variants and strains of LCMV showing high-affinity binding with the cellular receptor α-DG infect DCs and cause immunosuppression in mice. This high receptor binding affinity may allow immunosuppressive variants to displace α-DG-associated ECM molecules and infect DCs. The infection of DCs by LCMV leads to function impairment. Phenotypic analysis of infected DCs revealed that MHC class II and costimulatory molecules were expressed at reduced levels. Although further experiments are required to elucidate the specific mechanisms involved in the impairment of infected DC function, the model system described in this review should allow dissection of the molecules involved with virus-DC interactions.
References
- Ahmed R, Hahn CS, Somasundaram T, Villarete L, Matloubian M, Strauss JH. Molecular basis of organ-specific selection of viral variants during chronic infection. J Virol. 1991;65:4242–4247. doi: 10.1128/jvi.65.8.4242-4247.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R, Oldstone MB. Organ-specific selection of viral variants during chronic infection. J Exp Med. 1988;167:1719–1724. doi: 10.1084/jem.167.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althage A, Odermatt B, Moskophidis D, Kundig T, Hoffman-Rohrer U, Hengartner H, Zinkernagel RM. Immunosuppression by lymphocytic choriomeningitis virus infection: competent effector T and B cells but impaired antigen presentation. Eur J Immunol. 1992;22:1803–1812. doi: 10.1002/eji.1830220720. [DOI] [PubMed] [Google Scholar]
- Austyn JM, Weinstein DE, Steinman RM. Clustering with dendritic cells precedes and is essential for T-cell proliferation in a mitogenesis model. Immunology. 1988;63:691–696. [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Oldstone MB. Characterization of lymphocytic choriomeningitis virus-binding protein(s): a candidate cellular receptor for the virus. J Virol. 1992;66:7270–7281. doi: 10.1128/jvi.66.12.7270-7281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Oldstone MB. Lymphocytic choriomeningitis virus. Viral pathogenesis N Nathanson et al Eds Lippincott-Raven Publishers Philadelphia. 1997:593–627. [Google Scholar]
- Borrow P, Shaw GM. Cytotoxic T-lymphocyte escape viral variants: how important are they in viral evasion of immune clearance in vivo? Immunol Rev. 1998;164:37–51. doi: 10.1111/j.1600-065X.1998.tb01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Tishon A, Oldstone MB. Infection of lymphocytes by a virus that aborts cytotoxic T lymphocyte activity and establishes persistent infection. J Exp Med. 1991;174:203–212. doi: 10.1084/jem.174.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier MJ, Oldstone MB. Protein structure of lymphocytic choriomeningitis virus: evidence for a cell-associated precursor of the virion glycopep-tides. Virology. 1979;99:111–120. doi: 10.1016/0042-6822(79)90042-4. [DOI] [PubMed] [Google Scholar]
- Buckley SM, Casals J, Downs WG. Isolation and antigenic characterization of Lassa virus. Nature. 1970;227:174. doi: 10.1038/227174a0. [DOI] [PubMed] [Google Scholar]
- Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- Cavaldesi M, Macchia G, Barca S, Defilippi P, Tarone G, Petrucci TC. Association of the dystroglycan complex isolated from bovine brain synaptosomes with proteins involved in signal transduction. J Neurochem. 1999;72:1648–1655. doi: 10.1046/j.1471-4159.1999.721648.x. [DOI] [PubMed] [Google Scholar]
- Chambers BJ, Salcedo M, Ljunggren HG. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1). Immunity. 1996;5:311–317. doi: 10.1016/s1074-7613(00)80257-5. [DOI] [PubMed] [Google Scholar]
- Dockter J, Evans CF, Tishon A, Oldstone MB. Competitive selection in vivo by a cell for one variant over another: implications for RNA virus quasispecies in vivo. J Virol. 1996;70:1799–1803. doi: 10.1128/jvi.70.3.1799-1803.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P. Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem. 1998;46:449–457. doi: 10.1177/002215549804600404. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophinglycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Dystrophin and the membrane skeleton. Curr Opin Cell Biol. 1993;5:82–87. doi: 10.1016/s0955-0674(05)80012-2. [DOI] [PubMed] [Google Scholar]
- Evans CF, Borrow P, de la Torre JC, Oldstone MB. Virus-induced immunosuppression: kinetic analysis of the selection of a mutation associated with viral persistence. J Virol. 1994;68:7367–7373. doi: 10.1128/jvi.68.11.7367-7373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-alpha a dystrophin-associated glycoprotein is a functional agrin receptor. Cell. 1994;77:675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Hazenberg MD, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat Immunol. 2000;1:285–289. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP. A role for dystroglycan in basement membrane assembly. Cell. 1998;95:859–870. doi: 10.1016/s0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP. Dystroglycan inside and out. Curr Opin Cell Biol. 1999;11:602–607. doi: 10.1016/s0955-0674(99)00024-1. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Ibrahim MA, Chain BM, Katz DR. The injured cell: the role of the dendritic cell system as a sentinel receptor pathway. Immunol Today. 1995;16:181–186. doi: 10.1016/0167-5699(95)80118-9. [DOI] [PubMed] [Google Scholar]
- Jaspars LH, Bloemena E, Bonnet P, Van der Valk P, Meijer CJ. Distribution of extracellular matrix components and their receptors in human lymphoid tissue and B-cell non-Hodgkin lymphomas. Histopathology. 1995;26:113–121. doi: 10.1111/j.1365-2559.1995.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Jung D, Yang B, Meyer J, Chamberlain JS, Campbell KP. Identification and characterization of the dystrophin anchoring site on beta-dystroglycan. J Biol Chem. 1995;270:27305–27310. doi: 10.1074/jbc.270.45.27305. [DOI] [PubMed] [Google Scholar]
- Kramer RH, Rosen SD, McDonald KA. Basement-membrane components associated with the extracellular matrix of the lymph node. Cell Tissue Res. 1988;252:367–375. doi: 10.1007/BF00214379. [DOI] [PubMed] [Google Scholar]
- Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7–1 and B7–2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- Kunz S, Sevilla N, McGavern DB, Campbell KP, Oldstone MB. Molecular analysis of the interaction of LCMV with its cellular receptor [alpha]-dystroglycan. J Cell Biol. 2001;155:301–310. doi: 10.1083/jcb.200104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J, Shankar P, Manjunath N, Andersson J. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immuno-deficiency in HIV-1 infection. Blood. 2001;98:1667–1677. doi: 10.1182/blood.v98.6.1667. [DOI] [PubMed] [Google Scholar]
- Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- Matloubian M, Kolhekar SR, Somasundaram T, Ahmed R. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1993;67:7340–7349. doi: 10.1128/jvi.67.12.7340-7349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–987. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- Odermatt B, Eppler M, Leist TP, Hengartner H, Zinkernagel RM. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc Natl Acad Sci USA 88. 1991:8252–8256. doi: 10.1073/pnas.88.18.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y, Delespesse G. T cell-derived IL-4 and dendritic cell-derived IL-12 regulate the lymphokine-producing phenotype of alloantigen-primed naive human CD4 T cells. J Immunol. 1997;158:629–636. [PubMed] [Google Scholar]
- Oldstone MB. Viral persistence. Cell. 1989;56:517–520. doi: 10.1016/0092-8674(89)90573-4. [DOI] [PubMed] [Google Scholar]
- Oxenius A, Karrer U, Zinkernagel RM, Hengartner H. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J Immunol. 1999;162:965–973. [PubMed] [Google Scholar]
- Parekh BS, Buchmeier MJ. Proteins of lymphocytic choriomeningitis virus: antigenic topography of the viral glycoproteins. Virology. 1986;153:168–178. doi: 10.1016/0042-6822(86)90020-6. [DOI] [PubMed] [Google Scholar]
- Patterson JB, Manchester M, Oldstone MB. Disease model: dissecting the pathogenesis of the measles virus. Trends Mol Med. 2001;7:85–88. doi: 10.1016/s1471-4914(01)01918-9. [DOI] [PubMed] [Google Scholar]
- Peters CJ, Buchmeier MJ, Rollin PE, Kisiazek TG. Arenavirus. In: Knipe DM, Fields BN, Howlley PM, editors. Fields Virology. 3rd edition Vol. 2. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 1521–1551. [Google Scholar]
- Rescigno M, Granucci F, Ricciardi-Castagnoli P. Dendritic cells at the end of the millennium. Immunol Cell Biol. 1999;77:404–410. doi: 10.1046/j.1440-1711.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Riviere Y, Ahmed R, Southern PJ, Buchmeier MJ, Dutko FJ, Oldstone MB. The S RNA segment of lymphocytic choriomeningitis virus codes for the nucleoprotein and glycoproteins 1 and 2. J Virol. 1985;53:966–968. doi: 10.1128/jvi.53.3.966-968.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Buchmeier MJ, Oldstone MB, Lampert PW. Ultrastructural localization of viral antigens in the CNS of mice persistently infected with lymphocytic choriomeningitis virus (LCMV). Am J Pathol. 1983;110:95–100. [PMC free article] [PubMed] [Google Scholar]
- Salvato M, Borrow P, Shimomaye E, Oldstone MB. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J Virol. 1991;65:1863–1869. doi: 10.1128/jvi.65.4.1863-1869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvato MS, Shimomaye EM. The completed sequence of lymphocytic choriomeningitis virus reveals a unique RNA structure and a gene for a zinc finger protein. Virology. 1989;173:1–10. doi: 10.1016/0042-6822(89)90216-x. [DOI] [PubMed] [Google Scholar]
- Sevilla N, Kunz S, Holz A, Lewicki H, Homann D, Yamada H, Campbell KP, de La Torre JC, Oldstone MB. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Fuller-Pace FV, Buchmeier MJ, Southern PJ. Analysis of the genomic L RNA segment from lymphocytic choriomeningitis virus. Virology. 1987;161:448–456. doi: 10.1016/0042-6822(87)90138-3. [DOI] [PubMed] [Google Scholar]
- Smelt SC, Borrow P, Kunz S, Cao W, Tishon A, Lewicki H, Campbell KP, Old-stone MB. Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor alpha-dystroglycan correlate with viral tropism and disease kinetics. J Virol. 2001;75:448–457. doi: 10.1128/JVI.75.1.448-457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern PJ. Arenaviridae: the viruses and their replication. In: Knipe DM, Fields BN, Howlley PM, editors. Fields Virology. 3rd edition Vol. 2. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 1505–1551. [Google Scholar]
- Southern PJ, Singh MK, Riviere Y, Jacoby DR, Buchmeier MJ, Oldstone MB. Molecular characterization of the genomic S RNA segment from lymphocytic choriomeningitis virus. Virology. 1987;157:145–155. doi: 10.1016/0042-6822(87)90323-0. [DOI] [PubMed] [Google Scholar]
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- Talts JF, Andac Z, Gohring W, Brancaccio A, Timpl R. Binding of the G domains of laminin alpha1 and alpha2 chains and perlecan to heparin sulfatides alpha-dystroglycan and several extracellular matrix proteins. EMBO J. 1999;18:863–870. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishon A, Borrow P, Evans C, Oldstone MB. Virus-induced immuno-suppression. 1. Age at infection relates to a selective or generalized defect. Virology. 1993;195:397–405. doi: 10.1006/viro.1993.1389. [DOI] [PubMed] [Google Scholar]
- Tishon A, Southern PJ, Oldstone MB. Virus-lymphocyte interactions. II. Expression of viral sequences during the course of persistent lymphocytic choriomeningitis virus infection and their localization to the L3T4 lymphocyte subset. J Immunol. 1988;140:1280–1284. [PubMed] [Google Scholar]
- Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- van den Berg TK, van der Ende M, Dopp EA, Kraal G, Dijkstra CD. Localization of beta 1 integrins and their extracellular ligands in human lymphoid tissues. Am J Pathol. 1993;143:1098–1110. [PMC free article] [PubMed] [Google Scholar]
- Villarete L, Somasundaram T, Ahmed R. Tissue-mediated selection of viral variants: correlation between glycoprotein mutation and growth in neuronal cells. J Virol. 1994;68:7490–7496. doi: 10.1128/jvi.68.11.7490-7496.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb LM, Feldmann M. Critical role of CD28/B7 costimulation in the development of human Th2 cytokine-producing cells. Blood. 1995;86:3479–3486. [PubMed] [Google Scholar]
- Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP. Dystroglycan is essential for early embryonic development: disruption of Reichert s membrane in Dag1-null mice. Hum Mol Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- Winder SJ. The complexities of dystroglycan. Trends Biochem Sci. 2001;26:118–124. doi: 10.1016/s0968-0004(00)01731-x. [DOI] [PubMed] [Google Scholar]
- Wright KE, Spiro RC, Burns JW, Buchmeier MJ. Post-translational processing of the glycoproteins of lymphocytic choriomeningitis virus. Virology. 1990;177:175–183. doi: 10.1016/0042-6822(90)90471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Jung D, Motto D, Meyer J, Koretzky G, Campbell KP. SH3 domain-mediated interaction of dystroglycan and Grb2. J Biol Chem. 1995;270:11711–11714. doi: 10.1074/jbc.270.20.11711. [DOI] [PubMed] [Google Scholar]