Abstract
Recurrent deletions and duplications at chromosomal region 16p11.2 are variably associated with speech delay, autism spectrum disorder, developmental delay, schizophrenia, and cognitive impairments. Social communication deficits are a primary diagnostic symptom of autism. Here we investigated ultrasonic vocalizations (USVs) in young adult male 16p11.2 deletion mice during a novel three-phase male–female social interaction test that detects vocalizations emitted by a male in the presence of an estrous female, how the male changes its calling when the female is suddenly absent, and the extent to which calls resume when the female returns. Strikingly fewer vocalizations were detected in two independent cohorts of 16p11.2 heterozygous deletion males (+/−) during the first exposure to an unfamiliar estrous female, as compared to wildtype littermates (+/+). When the female was removed, +/+ emitted calls, but at a much lower level, whereas +/− males called minimally. Sensory and motor abnormalities were detected in +/−, including higher nociceptive thresholds, a complete absence of acoustic startle responses, and hearing loss in all +/− as confirmed by lack of auditory brainstem responses to frequencies between 8 and 100 kHz. Stereotyped circling and backflipping appeared in a small percentage of individuals, as previously reported. However, these sensory and motor phenotypes could not directly explain the low vocalizations in 16p11.2 deletion mice, since (a) +/− males displayed normal abilities to emit vocalizations when the female was subsequently reintroduced, and (b) +/− vocalized less than +/+ to social odor cues delivered on an inanimate cotton swab. Our findings support the concept that mouse USVs in social settings represent a response to social cues, and that 16p11.2 deletion mice are deficient in their initial USVs responses to novel social cues.
Keywords: mouse model of autism, 16p11.2 deletion, ultrasonic vocalization, social Interaction, autism
Introduction
Recurrent heterozygous deletions and duplications of a ~600kb segment on human chromosomal region 16p11.2 appear in up to 1% of individuals with autism [Christian et al., 2008; Hanson et al., 2010; Kumar et al., 2008; Marshall et al., 2008; McCarthy et al., 2009; Levy et al., 2011; Pinto et al., 2010; Rosenfeld, Coe, Eichler, Cuckle, & Shaffer, 2013; Rosenfeld et al., 2010; Sanders et al., 2011; Sebat et al., 2007; Shen et al., 2010; Weiss et al., 2008]. 16p11.2 deletions and duplications are additionally associated with speech delay, intellectual impairment, and developmental delays [Bassuk et al., 2013; Bijlsma et al., 2009; Cooper et al., 2011; Fernandez et al., 2010; Guilmatre et al., 2009; Hanson et al., 2010; Owen et al., 2014; Rosenfeld et al., 2010; Shinawi et al., 2010; Weiss et al., 2008; Zufferey et al., 2012], schizophrenia [Guilmatre et al., 2009; McCarthy et al., 2009; Rosenfeld et al., 2010; Steinberg et al., 2014; Szatkiewicz et al., 2014], bipolar disorder [Fernandez et al., 2010; McCarthy et al., 2009], depression [Degenhardt et al., 2012; Rosenfeld et al., 2010], and anxiety [Fernandez et al., 2010; Pinto et al., 2010; Rosenfeld et al., 2010]. Physiological abnormalities associated with 16p11.2 copy number variants are diverse, including motor hypotonia, seizures, feeding difficulty, obesity (deletion), low body weight (duplication), immune deficiency, syringomyelia, hearing loss, and cardiac defects [Bassuk et al., 2013; Bijlsma et al., 2009; Ciuladaite et al., 2011; Fernandez et al., 2010; Ghebranious, Giampietro, Wesbrook, & Rezkalla, 2007; Hanson et al., 2010; Jacquemont et al., 2011; Puvabanditsin et al., 2010; Rosenberg et al., 2006; Reinthaler et al. 2014; Sanders et al., 2011; Schaaf et al., 2011; Shinawi et al., 2010; Shiow et al., 2009; Steinberg et al., 2014; Walters et al., 2010]. 16p11.2 deletions and duplications are not fully penetrant and some individuals are largely asymptomatic, though 16p11.2 deletions appear to be more deleterious than duplications [Bijlsma et al., 2009; Cooper et al., 2011; Fernandez et al., 2010; Glessner et al., 2009; Hanson et al., 2010; Kumar et al., 2008; Rosenfeld et al., 2010; Rosenfeld et al., 2013]. Several genes in the 16p11.2 region have been implicated in autism, including SEZ6L, MAPK3, and KCTD13 [Blumenthal et al., 2014; Golzio et al., 2012; Konyukh et al., 2011; Kumar et al., 2009; Schaaf et al., 2011].
Two mouse models of 16p11.2 heterozygous deletions (+/ −) were independently generated [Horev et al., 2011; Tian et al., 2015; Portmann et al., 2014].+/− of both lines exhibited low body weight, perinatal mortality, increased spontaneous locomotor activity in a novel home cage environment, and sporadic motor stereotypies. In addition, we observed that juvenile and adult +/− males and females exhibited normal general health, neurological reflexes, responses to social and nonsocial odors, motor learning, normal social approach, and normal juvenile reciprocal social interaction [Portmann et al., 2014]. We further detected an inability to swim, hypoactivity during the first 5 min in an open field, and a deficit in novel object recognition in multiple cohorts across two testing sites [Portmann et al., 2014].
Mice emit vocalizations in the ultrasonic range in response to olfactory and other sensory cues from a social partner [Whitney & Nyby, 1979; Byatt & Nyby, 1986; Malkesman et al., 2010; Roullet, Wohr & Crawley, 2011]. Here, we investigated another phenotype of potential relevance to autism, ultrasonic vocalizations (USVs) in social settings, in 16p11.2 deletion mice, as well as sensory phenotypes and anxiety-related behaviors, to better understand the consequences of 16p11.2 deletions in mice. We recorded USVs from male +/− and their +/+ littermates when exposed to a novel estrous female, when the female was removed, and subsequently when the same female returned, to evaluate responses to the presence and absence of a social partner [Hanson & Hurley, 2012; Yang, Loureiro, Kalikhman, & Crawley, 2013]. Considerable evidence indicates that the USVs detected during male–female interaction are emitted by the male rather than the female [Wang, Liang, Burgdorf, Wess, & Yeomans, 2008; White, Prasad, Barfield, & Nyby, 1998; Whitney, Coble, Stockton, & Tilson, 1973; Sugimoto et al., 2011]. A dramatic reduction in vocalizations by young adult +/− males first presented with a female, and fewer vocalizations in response to social pheromones in female urine on an inanimate cotton swab may indicate deficits in perceiving the presence of, or responding to, salient social cues.
Materials and Methods
All procedures were approved by the Institutional Animal Care and Use Committees of the National Institute of Mental Health Intramural Research Program, University of California Davis, and Washington State University Vancouver. Generation of the 16p11.2 deletion mice (contributed to The Jackson Laboratory, stock number 025100) was previously described [Portmann et al., 2014]. Breeding pairs were imported from Stanford University to NIMH to generate Cohort 1, and subsequently rederived at UC Davis to generate Cohorts 2 and 3. 16p11.2 deletion and wildtype littermate control mice were tested on a battery of behavioral assays. Behavioral findings in the +/− mice, described in a recent publication [Portmann et al., 2014], included low pup survival, low body weights, absence of acoustic startle response, and impaired novel object recognition, along with normal scores on juvenile reciprocal social interactions, three-chambered social approach, self-grooming, grip strength, general health and neurological reflexes, rotarod motor learning, novel home cage activity, and olfactory habituation/dishabituation. Here, we evaluate USVs emitted by 16p11.2 mice in response to social cues in three paradigms: (1) our novel three-phase male-female social interaction task, (2) the female urine sniff test, and (3) separation-induced USVs in pups. To evaluate sensory, motor, and anxiety-like phenotypes that could contribute to low vocalization responses to social cues, we evaluated anxiety-like behaviors, 60-min open field activity, nociceptive responses, olfactory habituation/dishabituation, functional hearing, and conducted an in-depth analysis of motor stereotypies including circling and back-flipping behaviors. Only male subjects were tested for adult USVs in response to female cues, because adult mice rarely vocalize during same-sex interaction without a prolonged period of isolation (3–7 days) before the experiment [Nakagawa, Matsunaga, & Okanoya, 2012; Scattoni, Ricceri, & Crawley, 2011], and our subjects were group-housed by sex. All other tests used animals of both sexes. Supporting Information describes detailed methods on breeding strategy, genotyping methods, testing procedures for each behavioral assay, and statistical analyses. Behavioral methods used in this study were comprehensively described in previous publications [Chadman et al., 2008; Yang & Crawley, 2009; Silverman et al., 2011; Yang, Silverman & Crawley, 2011; Brielmaier et al., 2012; Ey et al., 2012; Yang et al., 2012, 2013; Portmann et al., 2014]. The auditory brainstem response (ABR) assay to measure hearing sensitivity to pure tones across a wide frequency range was conducted in a similar manner as previously described [Mahrt, Perkel, Tong, Rubel, & Portfors, 2013].
Results
USV call numbers and behaviors in the three-phase male– female social interaction test
The present experiments used male subjects for adult USVs in response to female cues, a well-established standard paradigm to elicit large numbers of calls [Ey et al., 2012; Holy & Guo, 2005; Scattoni et al., 2011].
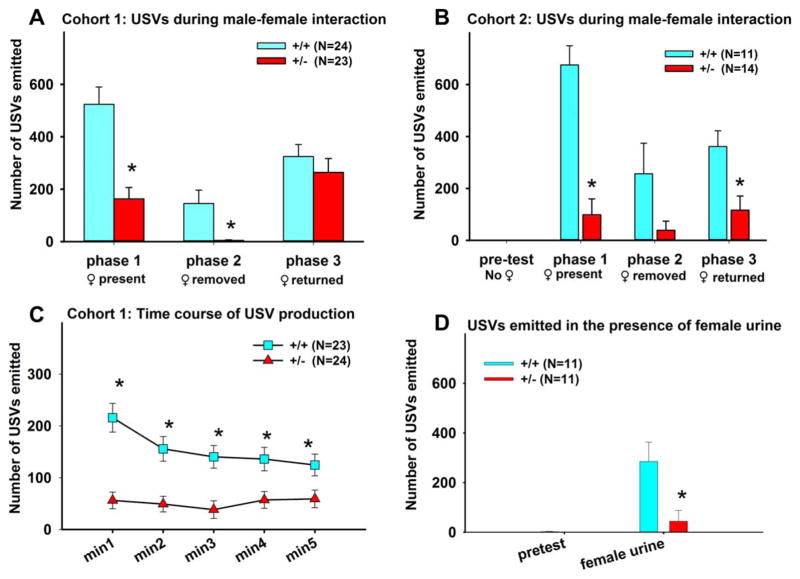
We examined USVs in +/− mice and control +/+ littermates to determine if the deletion alters this form of response to social cues. Our novel three-phase male– female interaction paradigm consists of a 5-min Phase 1 in which the male subject freely interacted with an unfamiliar female, a 3-min Phase 2 in which the female was absent, and a 3-min Phase 3 in which the same female returned [Yang et al., 2013]. Figure 1 illustrates genotype differences in call numbers in the three-phase male-female social interaction test. Cohort 1 +/− males emitted significantly fewer calls than +/+ males in Phase 1 (Mann–Whitney U test, U=135, P < 0.01) and Phase 2 (U=168, P < 0.05), but not in Phase 3 (U=236, NS) (Fig. 1A). Similar results were seen in Cohort 2 (Fig. 1B). +/− emitted significantly fewer calls than +/+ in Phase 1 (U=10, P < 0.01) and Phase 3 (U=2, P < 0.01). A similar but nonsignificant trend was detected in Phase 2 (U=44, P < 0.075). Figure 1C illustrates the time course of call emission in Cohort 1. +/− males produced fewer USVs than +/− males in min 1 (U=98, P < 0.01), min 2 (U=140, P < 0.01), min 3 (U=143, P < 0.01), min 4 (U=165, P < 0.05), and min 5 (U=167, P < 0.05). A significant effect of time was detected in +/+ (Repeated Measures ANOVA on ranks, P < 0.01), but not in +/− (NS). The observed reduction in call rate across minutes 3–5 justified the use of minutes 1–3 for subsequent scoring of call categories. Figure 1D illustrated a robust genotype difference in number of USVs during the female urine sniff test in which the male subject was presented with a cotton swab saturated with fresh female urine. +/+ emitted high numbers of calls when exposed to a cotton swab containing fresh urine from a +/+ female in estrus, whereas +/− emitted significantly fewer USVs than +/+ (F1,20=7.38, P < 0.01). Almost no calls were emitted by either genotype in the 3-min pretest when the male was alone in the cage, indicating that environmental novelty is not a stronger trigger of vocalizations. These results from the female urine sniff test support an interpretation that impaired responsivity to social cues in +/− males is not limited to their response to live moving mice. See Figure 1 legend for statistical results.
Figure 1.
Reduced numbers of ultrasonic vocalizations in +/− males in the three-phase male–female social interaction test and the female urine sniff test. (A) In Cohort 1, male heterozygotes (+/−) emitted fewer USVs than wildtype littermates (+/+) during the initial male–female social interaction session (Phase 1) and after the female was removed (Phase 2). USV numbers in +/− and +/+ were similar during the second interaction session when the female was reintroduced (Phase 3). (B) In Cohort 2, no calls were emitted by any mice of either genotype during a 3-min pretest period when the subject was alone in the test cage, confirming the specificity of USVs to the presence of a social partner in our paradigm. +/− males again emitted fewer USVs in Phase 1, with a nonsignificant trend for fewer calls in Phase 2, and fewer calls in Phase 3. (C) Time course of call emission in Cohort 1. +/− produced fewer USVs than +/+ in minutes 1–5. A significant effect of time was detected in +/+, but not in +/−. Data are presented as mean ± standard error of the mean. *, P < 0.05 +/+ vs. +/−. (D) +/− males emitted fewer USVs than +/+ males in the presence of fresh urine from a +/+ non-littermate female in estrus, provided on a cotton swab, in a 3-min test session. *, P < 0.05 +/+ vs. +/−.
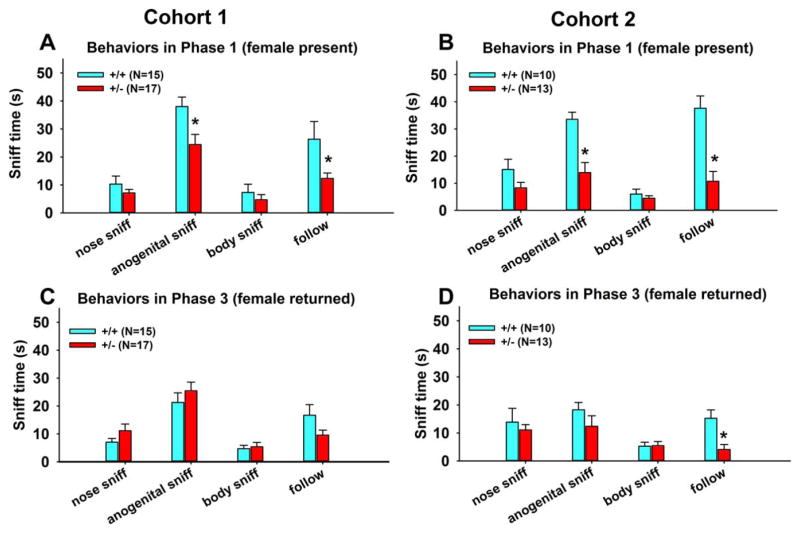
Figure 2 illustrates genotype differences in social behaviors in the three-phase male–female social interaction test. In Cohort 1, +/− males exhibited less anogenital sniffing and less following than +/+ males during Phase 1 (Fig. 2A). When the female was returned after a 3-min absence (Phase 3), no genotype differences were found on any behavioral parameter (Fig. 2C). Similarly, Cohort 2 +/− males exhibited less anogenital sniffing and following than +/+ males in Phase 1 (Fig. 2B). In Phase 3, +/− males exhibited less following than +/+ males (Fig. 2D). Mounting attempts rarely occurred in this short test, and were not statistically analyzed. See Supporting Information, Table S1 for statistical results of Figure 2. Correlation analyses between behavioral and USV data were conducted in both cohorts (Cohort 1: 10 +/+ and 12 +/−; Cohort 2: 11 +/+ and 13 +/−). In two cohorts of +/+ and +/− tested, no significant correlations (data not shown) were found between number of USVs and any behavioral parameter, indicating that social sniff and vocalizations are probably independent measures of response to social cues.
Figure 2.
Reduced social sniffing in 16p11.2 +/− males in the three-phase male-female social interaction test. (A) Cohort 1 +/− males exhibited less anogenital sniffing and following, as compared to +/+ males, during the first male-female interaction session (Phase 1). (C) No genotype differences were detected during the second exposure to the female (Phase 3). (B and D) Cohort 2 +/− males exhibited less anogenital sniffing and less following than +/+ males in Phase 1, and less following in Phase 3. None of the individual social interaction parameter scores was significantly correlated with total numbers of USVs, indicating that social sniffing and vocalization are probably independent measures of response to social cues. Data are presented as mean ± standard error of the mean. *, P < 0.05 +/+ vs. +/−. See Supporting Information, Table S1 for statistical results of Figure 2.
USV call categories
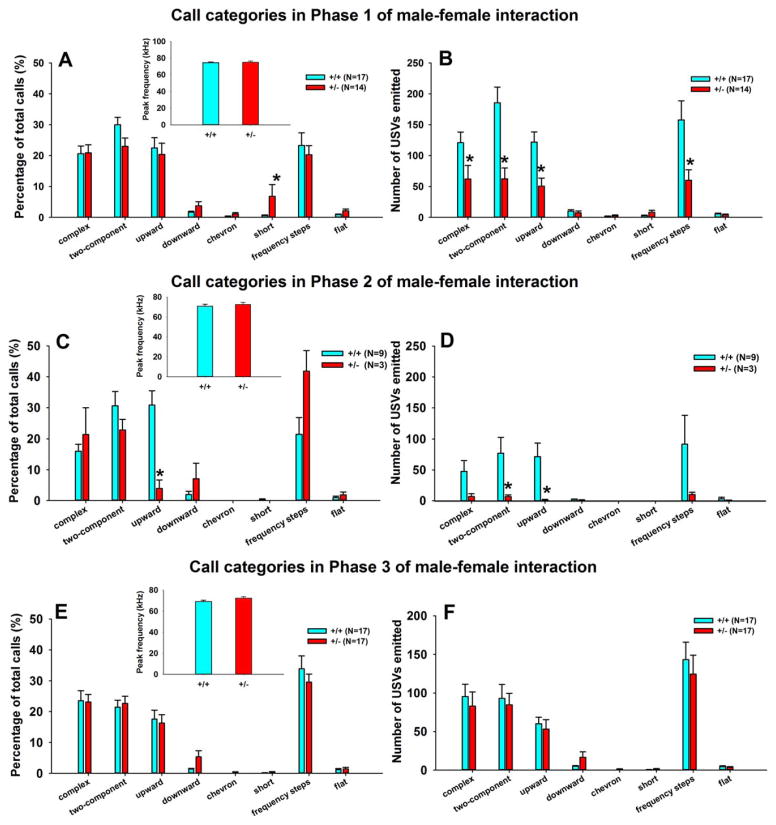
Figure 3 illustrates USV call categories and peak call frequencies during the initial male–female social interaction (Phase 1), after the female was removed (Phase 2), and when the same female was reintroduced (Phase 3), respectively. No significant genotype differences were found for peak call frequency in Phase 1 (Fig. 3A inset, F1,21=0.07, NS), Phase 2 (Fig. 3C inset, F1,11=0.55, NS), or Phase 3 (Fig. 3E inset, F1,23=2.37, NS). Although +/− differed from +/+ in call numbers on some categories, males of the two genotypes displayed mostly similar call repertoires on relative percentages of calls in each category in the presence of the female, as illustrated by relative percentages (Fig. 3A, C, E). Relative percentages provide a more relevant quantification of call categories, since low numbers of total calls by +/− was responsible for the low of number of calls in each category. Physical ability of +/− to emit USVs of normal quantities and qualities is confirmed by the absence of genotype differences in call numbers in Phase 3. See Supporting Information, Table S2 for statistical results of call categories illustrated in Figure 3.
Figure 3.
Call categories of USVs emitted during Phase 1 (the first exposure to a novel estrous female), Phase 2 (after the female was removed from the interaction cage), and Phase 3 (when the female returned). In all three phases, +/+ and +/− differed in call numbers but displayed generally similar repertoires. (A, C, E) Analysis of percentages of call types indicated that +/− had a higher percentage of Short than +/+ in Phase 1, a lower percentage of upward in Phase 2, and did not differ from +/+ in Phase 3. (B, D, E) Analysis of number of calls in each category indicated that +/− emitted fewer Complex, Two-component, Upward, and Frequency steps as compared to +/+ in Phase 1, fewer Two-component and Upward in Phase 2, and did not differ from +/+ in Phase 3. Only Cohort 1 male subjects that emitted USVs were included in call category analysis. Out of a total of 23 +/− in Cohort 1, 14 called in Phase 1, 3 in Phase 2, and 17 in Phase 3. Out of a total of 24 +/+ in Cohort 1, 17 called in Phase 1, 9 in Phase 2, and 17 in Phase 3 (see Supporting Information, Table S8). No significant genotype differences were found for peak call frequency in Phase 1 (A inset), Phase 2 (C inset), or Phase 3 (E inset). Data are presented as mean ± standard error of the mean. *, P <.05 +/ + vs. +/−. See Supporting Information, Table S2 for statistical results of Figure 3.
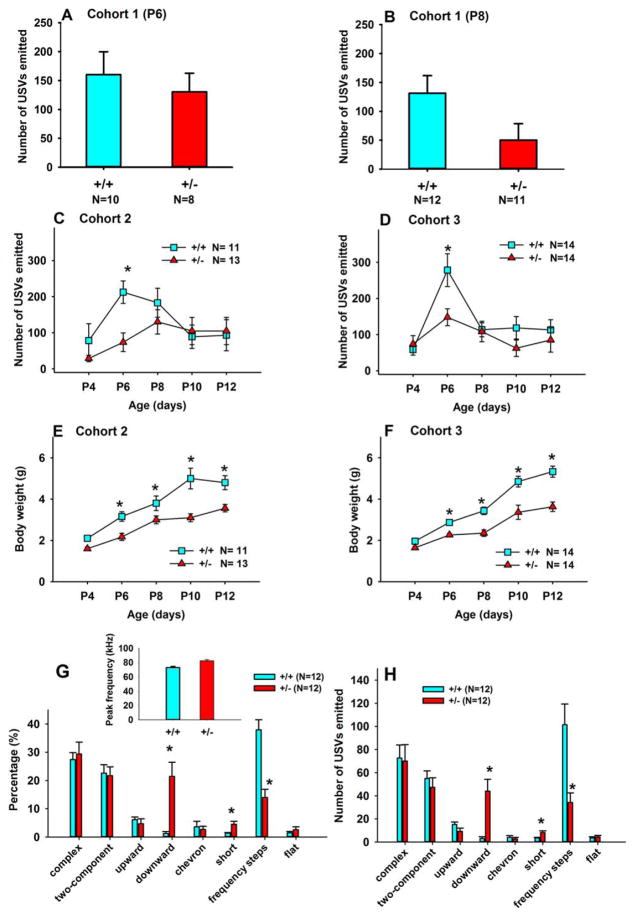
Pup USVs and body weights
Separation calls by mouse pups represent a different type of vocalization in a social setting. Pup calls may be more analogous to human infant crying than to adult social communication. We evaluated numbers and categories of vocalizations in +/+ and +/− pups separated from the nest for the 3-min test. To determine whether body weight affects calling, we weighed each animal after each separation session. In Cohort 1, no significant genotype differences were found on number of USVs emitted by pups tested on postnatal day 6 (P6) (Fig. 4A). A trend was seen for +/− pups to emit fewer USVs than +/+ littermates on P8 (Fig. 4B). In this cohort, +/− pups had significantly lower body weights than +/+ pups on P6, as previously reported [Portmann et al., 2014] and on P8 (data not shown). In Cohort 2, a significant main effect of genotype was detected across days, with +/− pups emitting significantly fewer USVs than +/+ pups on P6. A significant main effect of genotype was found for body weight (Fig. 4E), with +/− pups having lower body weights than +/+ pups on P6, 8, 10, and 12. Similarly, in Cohort 3, a significant main effect of genotype was found for number of USVs across days (Fig. 4D). Posthoc comparisons indicated that +/− pups emitted fewer calls than +/+ pups on P6. A significant main effect of genotype was again found for body weight (Fig. 4F). +/− pups weighed less than +/+ pups on P6, 8, 10, and 12. These results indicate that the 16p11.2 deletion affected number of calls in separated mouse pups on P6 in two of three cohorts. As previously reported [Portmann et al., 2014], +/− pups displayed reduced body weights. Figure G and H illustrate call repertoires and peak call frequencies in 8-day old pups. Peak call frequency was significantly higher in +/− pups than in +/+ pups (Fig. 4G inset, F1,21=15.4, P < 0.01). Genotype differences were found in three call categories: Downward, Short, and Frequency steps. Call category differences in pups, at a developmental time point when the pinna of the ear remains closed and presumably mouse pups are not yet able to hear [Ehret 2005; Grimsley, Monaghan, & Wenstrup, 2011], will be interesting to explore further. See Supporting Information, Table S3 for statistical results of Figure 4.
Figure 4.
Pup vocalizations and body weights. (A, B) Pups from Cohort 1 were tested on postnatal days 6 and 8. A trend was detected for +/− pups to emit fewer USVs as compared to +/+ littermates on P8. (C) In Cohort 2, a significant genotype effect was found across P4, 6, 8, 10, and 12, with +/− emitting fewer USVs than +/+ littermates on P6. (D) Similarly, in Cohort 3, a significant genotype effect was found across days, with +/− emitting fewer USVs than +/+ on P6. (E–F) Consistent results from multiple cohorts indicated that +/− weighed less than +/+ across days, with genotype differences being significant on P6, 8, 10, and 12. (G, H) +/− and +/+ pups differed in three call categories: Downward, Short, and Frequency steps. Peak call frequency was higher in +/− than +/+ (Panel G inset). Data are presented as mean ± standard error of the mean. *, P < 0.05 +/+ vs. +/−. See Supporting Information, Tables S3 and S4 for statistical results of Figure 4.
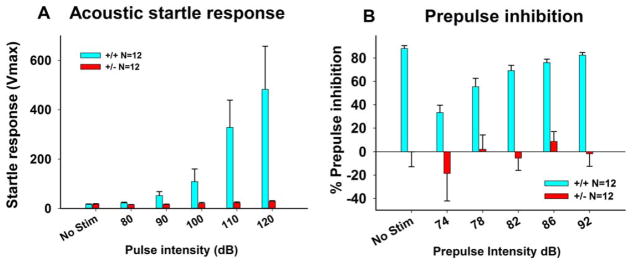
Acoustic startle and prepulse inhibition
The ability to hear sounds in the human hearing range was assayed in the acoustic startle response test that measures flinching responses following a loud sound. As in our previous study [Portmann et al., 2014], +/− exhibited essentially no startle response at any decibel level, while +/+ responded to acoustic startle tones normally (Fig. 5A, F1,22=22.06, P < 0.01). Results of prepulse inhibition indicated normal sensory gating in +/+ mice (Fig. 5B). Because +/− mice are deaf (see below), prepulse inhibition data of +/− do not reflect sensorimotor gating ability and are included for illustrative purposes only.
Figure 5.
Acoustic startle response and sensorimotor gating in 16p11.2 mice. (A) +/+ showed normal acoustic startle responses, whereas a complete absence of startle response to all decibel levels was seen in +/−, replicating our previous findings on deafness in +/− [Portmann et al. 2014]. (B) +/+ exhibited normal prepulse inhibition. Because the +/− mice are deaf, prepulse inhibition data of +/− do not reflect sensory gating ability, and are included for illustrative purposes only. Data are presented as mean ± standard error of the mean.
16p11.2 +/− have no ABRs across a wide range of frequencies
The absence of acoustic startle responses in +/− mice indicated the possibility of deafness, and prompted us to conduct comprehensive analysis using more broad test of hearing sensitivity based on auditory brain stem responses (ABR) to pure tones across a wide frequency range. Figure 6 illustrates ABR thresholds to pure tones between 8 and 100 kHz in +/+ and +/− tested at 6–7 weeks (young) and 27–32 weeks (middle-aged). In young +/+, the minimum threshold was 12 kHz (21 ± 6 dB SPL) and the maximum threshold was 32 kHz (71 ± 18 dB SPL). These mice did not have any evoked brainstem responses above 32 kHz. Similar results were found in the middle-aged +/+ mice with the exception that the thresholds were significantly higher in the middle-aged mice than in the young mice (12 kHz; 27 ± 2 dB SPL and 32 kHz; 93 ± 2 dB SPL, Kruskal–Wallis, P < 0.01). These higher thresholds are consistent with documented age-related hearing loss in C57BL/6N mice [Kane et al., 2012], the predominant genetic background in the 16p11.2 line. The ABR thresholds of +/+ were similar to previously reported ABR thresholds of C57B6/6J and other normal hearing strains [Kane et al., 2012; Zheng, Johnson, & Erway 1999]. None of the young or middle-aged +/− displayed any evoked ABR to any frequencies presented at the maximum intensity, indicating complete deafness.
Figure 6.
Deafness was confirmed by auditory brainstem response (ABR) test. ABR thresholds to pure tones between 8 and 100 kHz in +/+ and +/− tested at 6−7 weeks (young) and 27−32 weeks (middle-aged). The dashed lines indicate that no brainstem response was detected at maximum output intensity. Differences between dashed lines are due to differences in maximum output of the two speakers used in experiments with the different aged mice. In both young and middle-aged +/+, the minimum threshold was detected at 12 kHz and the maximum threshold at 32 kHz. Tones 50 kHz and higher did not elicit any response in +/+ of any age, even at the maximum intensity (dashed lines). Middle-aged +/+ had significantly higher thresholds than young +/+ to tones of all frequencies, indicating age-related hearing loss in +/+, consistent with the B6 genetic background. No young or middle-aged +/− displayed evoked responses at any frequencies presented at maximum intensity (dashed line), indicating complete deafness in all +/. Error bars indicate standard deviations.
Pain sensitivity and olfaction
In the hot plate test, latency to respond to a 55°C heat stimulus was significantly longer in +/− than +/+, indicating reduced sensitivity to thermal pain. Consistent results were found in Cohort 1 (F1,27=9.4, P < 0.01) and Cohort 2 (F1,23=5.5, P < 0.05) (Fig. 7A,B). No genotype differences were found on response latency in the tail flick test in Cohort 1 (F1,25=0.41, NS) or Cohort 2 (F1,21=0.36, NS) (Fig. 7C,D). Since tail flick is primarily a spinal reflex, while hot plate responses involve cortical regions [Pastoriza, Morrow, & Casey, 1996; South & Smith, 1998], the 16p11.2 deletion may affect central processing of pain perception, of relevance to unusual sensory responsiveness in some cases of autism [Elwin, Ek, Schroder, & Kjellin, 2012; Moore 2014]. As in our previous study [Portmann et al., 2014], olfactory habituation/dishabituation responses to sequentially presented nonsocial (water, banana, and vanilla) and social odors (from two cages of sex-matched novel mice) were normal in 16p11.2 deletion mice (Fig. 7E). See Supporting Information, Table S5 and Figure 7 legend statistical results.
Figure 7.
Reduced pain sensitivity and normal olfaction in +/− mice. (A–D) +/− mice exhibited increased latency to respond in the hot plate test, which is dependent on higher brain function, but not in the tail flick test, which is a spinal reflex, suggesting a small reduction in pain sensitivity. Consistent results were detected in two cohorts. (E) Both +/− and +/+ exhibited significant habituation and dishabituation responses to sequentially presented non-social and social odors, indicating a normal ability to differentiate odors in both genotypes. This result was consistent with our previous finding [Portmann et al. 2014]. Data are presented as mean ± standard error of the mean. *, P < 0.05 +/+ versus +/−. See Supporting Information, Table S5 for statistical results of Figure 7E.
Home cage behaviors
No unusual sleeping, huddling, nesting, feeding, or aggressive behaviors were seen during observations of home cages in the vivarium room at three different times of the day (data not shown), indicating that the 16p11.2 deletion did not affect easily observable home cage behaviors.
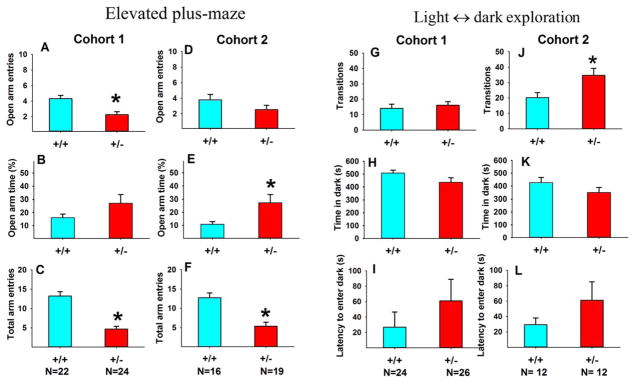
Anxiety-like behaviors
Figure 8 illustrates results of the elevated plus-maze and light ↔ dark exploration tests of anxiety-like behaviors in two cohorts of 16p11.2 mice. In the 5-min elevated plus-maze test (Fig. 8A–F), +/− exhibited low levels of overall exploratory activity and normal or lower levels of anxiety-like behaviors. In the 10-min light ↔ dark exploration test (Fig. 8G–L), +/− exhibited normal exploratory activity and normal or lower levels of anxiety-like behaviors. See Supporting Information, Table S6 for statistical results of Figure 8.
Figure 8.
No consistent anxiety-like phenotypes in 16p11.2 mice. In the elevated plus-maze test, +/− had normal open arm entries (A and D), normal or higher % open arm time (B and E), and markedly lower total arm entries than +/+ (C and F). Lower total arm entries indicated reduced exploratory activity which confounds the interpretation of an anxiety-like phenotype. In the light ↔ dark exploration test, +/− made normal or more transitions between chambers (G and J), and were not different from +/ + on time spent in the dark chamber (H and K) and latency to enter the dark chamber (I and L), indicating normal anxiety-like behaviors. Results on several measures from two anxiety-related tasks indicate a generally normal anxiety profile in 16p11.2 deletion mice. Data are presented as mean ± standard error of the mean. *, P < 0.05 +/+ versus +/−. See Supporting Information, Table S6 for statistical results of Figure 8.
Open field exploration
Two cohorts were tested for locomotor activity in a novel open field across a 60-min session. As shown in Figure 9, In Cohort 1, one out of 15 +/− exhibited high levels of repetitive circling. None of the +/+ exhibited any circling or other stereotypic behaviors. With data from the +/− circler included, there were no genotype differences on any measures across 60 min. Similarly, in Cohort 2, one out of 15 +/− exhibited circling. See Supporting Information, Table S7 for statistical results of Figure 9. Unlike in cohort 1, +/− in Cohort 2 had increased center time as compared to +/ +. The combined results of the two cohorts indicate normal open field exploratory activity across 60 min in +/−, although in both cohorts there was a trend for +/ − to travel less during the first 5 min, similar to our previous findings [Portmann et al., 2014].
Figure 9.
Open field activity across a 60-min session revealed no genotype differences in locomotor activity. (A–D) In Cohort 1, no significant genotype differences were found overall on total distance traveled, horizontal activity, vertical activity, and center time. A trend was seen for +/− mice to be more active than +/+ mice, but only when the one circling +/− mouse was included (data not shown). (E–H) In Cohort 2, no significant genotype differences were found overall on total distance traveled, horizontal activity, vertical activity. Center time was higher in +/− than in +/+. A trend was found for +/− to move less than +/+ in the first 5 min, consistent with our previous report [Portmann et al. 2014]. Data are presented as mean ± standard error of the mean. See Supporting Information, Table S7 for statistical results of Figure 9.
Motor stereotypies
In agreement with our previous report [Portmann et al., 2014], when placed in a novel empty cage for 60 min, a small number of +/− displayed stereotypic circling and backflipping (Table 1).
Table 1.
Repetitive Circling and Backflipping were Detected in a Small Number of +/− at 6, 8, and 10−12 Weeks of Age
| Age | Cohort 1
|
Cohort 2
|
||
|---|---|---|---|---|
| High circling | High backflipping | High circling | High backflipping | |
| 6 weeks | 0/33 | 0/33 | 1/40 | 1/40 |
| 8 weeks | 1/33 | 2/33 | 0/36 | 2/36 |
| 10–12 weeks | 1/30 | 3/30 | 0/27 | 1/27 |
The same individuals generally exhibited motor stereotypies consistently across ages. No +/+ exhibited repetitive circling and backflipping at any age. Data are presented as the number of +/− exhibiting repetitive circling/backflipping out of total number of +/− tested.
The same individual +/− consistently displayed the same stereotypies across ages in most but not all cases. No +/+ exhibited circling or backflipping at any age (data not shown). These quantitative results indicate that motor stereotypies are a consequence of the 16p11.2 deletion, but represent a trait with low penetrance.
Adult body weights
+/− weighed significantly less than +/+ littermates at 6 weeks (+/+, 22.1 ± 0.70 g; +/−, 16.4 ± 0.61 g; F1,62=36.7, P < 0.01), 8 weeks (+/+, 24.3 ± 0.76 g; +/ −, 17.1 ± 0.86 g; F1,58=35.7, P < 0.01), and 10−12 weeks (+/+, 26.9 ± 0.91 g; +/−, 19.4 ± 0.94 g; F1,45=31.1, P < 0.01). Low body weights in 16p11.2 deletion mice were seen in previous cohorts [Portmann et al., 2014], consistently from birth throughout development. No significant correlations were detected between body weight and USV numbers in +/− (r=0.24, NS), supporting an interpretation that decreased vocalization number was not a direct result of lower body weight.
Discussion
Individuals with 16p11.2 deletions have a high incidence of language impairment, commonly manifested as delayed onset of expressive language, complete lack of spoken language, low verbal skills, and difficulties in articulation [Bijlsma et al., 2009; Ciuladaite et al., 2011; Hanson et al., 2010; Raca et al., 2013; Schaaf et al., 2011; Shinawi et al., 2010; Weiss et al., 2008]. We investigated vocalizations in 16p11.2 heterozygous deletion mice, to test the hypothesis that vocal emissions would similarly be unusual in the mouse model of 16p11.2 deletion syndrome. Employing a novel paradigm of social interaction, separation, and reintroduction [Yang et al., 2013], we discovered that young adult male heterozygous 16p11.2 deletion mice displayed moderately reduced social interactions and strikingly reduced USVs during the initial interaction session with an unfamiliar estrous female. These results suggest that 16p11.2 deletion males are less responsive to social cues when first encountering female conspecifics. Other major findings are sensory deficits (deafness and increased pain threshold) in +/− mice. Anxiety-like behaviors and open field exploration appear largely normal in +/− mice.
One possible explanation for the low number of emitted USVs in adult +/− males is their inability to hear the female. However, existing literature and current data do not support this interpretation. First, females generally do not vocalize during male–female interactions [Sugimoto et al., 2011; Wang et al., 2008; White et al., 1998; Whitney et al., 1973]. Second, genotype differences in call numbers (+/− males emitted significantly fewer USVs than +/+ males) were found during both male–female interaction and the female urine sniff test, suggesting that social vocalization deficits in +/− males are not solely due to their inability to hear USVs or movements of a live female partner. Males of both genotypes emitted markedly more calls when interacting with a live female than when sniffing female urine alone, suggesting that motion, visual, and tactile cues could contribute to the salience of social cues in mice. Further, low USV numbers in +/− males are not due to an inability to vocalize, because both genotypes in Cohort 1 emitted similar numbers of USVs when the female returned in Phase 3, indicating that the mutation did not disable the vocal apparatus, nor directly impair other physical abilities required to emit USVs. Normal levels of vocalizations during Phase 3 may, therefore, reflect improved social cue detection, interest, and/or responsiveness after the olfactory and visual cues of the female mouse became familiar. Alternatively, the lack of genotype difference in Phase 3 may be due to a lack of habituation to familiar social cues in +/−, which could indicate a social memory deficit.
A genotype difference in call number was detected in Cohort 2 during Phase 3. The slight discrepancy between Phase 3 data of the two cohorts may be attributable to the fact that Cohort 2 males had a brief 5-min pretest experience with females, whereas Cohort 1 did not. No correlations were detected between social sniffing and call numbers, in either genotype, indicating that USV emission and social sniffing are independent response traits evoked by social stimuli. Our previous study [Portmann et al., 2014] presents findings indicating that +/− have normal olfactory habituation/dishabituation to nonsocial and social odors and have normal vision, suggesting that social vocalization deficits in +/− males are not attributable to impaired olfaction or vision. Body weight was lower in +/−, but there were no correlations between body weights and call numbers in either genotype, indicating that low body weight does not impair the ability to emit USVs.
Analysis of qualitatively different call types revealed few category differences between genotypes. Focusing on percentages of call categories, a more accurate representation than number of calls when the totals are low, we discovered that the deaf +/− and hearing +/+ males differed in only one category, Short, in Phase 1, only in the Upward category in Phase 2, and none in Phase 3. These results indicate that the deaf +/− and hearing +/+ had similar call repertoires and lend support to the suggestion that auditory experience may not have a crucial role in regulating USV in mice [Mahrt et al., 2013]. In pups tested at an age before hearing onset [Ehret 2005; Sonntag, Englitz, Kopp- Scheinpflug, & Rubsamen, 2009], genotype differences were found in both percentage and absolute number of Downward, Short, and Frequency Steps calls, an intriguing finding that may be worth further investigation.
One of the most striking findings in this study is the complete deafness in our line of +/− mice. We replicated our previous finding that adult +/− are deaf to low frequency sounds [Portmann et al., 2014] and further demonstrated that the +/− mice are deaf to high frequency tones as well. In contrast, the Mills line of 16p11.2 deletion mice [Horev et al., 2011] appear to display normal hearing in an operant task with an auditory contingency (personal communication, Anne Churchland, Cold Spring Harbor Laboratory, Ted Abel, University of Pennsylvania). Differences between the present Portmann/Dolmetsch line and the Mills line may be related to differences in background strain and to the precise length of the deletion.
To fully understand the extent of hearing loss, and because the B6 background strain is known to display an age-related hearing loss, we evaluated 16p11.2 mice at two ages. At both young and middle ages, +/− mice failed to display an ABR to stimulus tones in the 8−100 kHz frequency range, consistent with their lack of acoustic startle responses. In contrast, young +/+ mice displayed ABR thresholds up to 32 kHz at levels comparable to young B6 mice [Kane et al., 2012; Zheng et al., 1999]. It may prove fruitful to explore interactions between genes in the 16p11.2 deletion sequence and B6 background genes that may regulate hearing at young ages in our line, and in comparison to the genetic background of the Mills 16p11.2 deletion line. Since deafness is rare in human 16p11.2 deletion syndrome, with only six cases of hearing loss reported [Schaaf et al., 2011; Shinawi et al., 2010; Rosenfeld et al., 2010], species differences are likely to affect the consequences of the 16p11.2 deletion on hearing.
Absence of ABR responses to high frequency tones in +/+ mice does not imply that they cannot detect USVs. Minimal or no ABR responses in +/+ mice to frequencies within the range of social USVs is comparable to single neuron electrophysiological results in the auditory midbrain [Portfors & Roberts, 2014]. Very few neurons in the mouse auditory system respond to pure tones greater than 60 kHz, yet neurons that respond to lower frequency tones do respond to the USVs that contain energy in the 60 kHz and higher frequency range [Holmstrom Eeuwes, Roberts, & Portfors, 2010 ; Portfors, Roberts, & Jonson, 2009; Portfors & Roberts, 2014]. Electrophysiological and modeling experiments suggest that the low frequency neurons respond to combinations of frequencies in the USVs that create cochlear distortions [Portfors & Roberts, 2014; Portfors et al., 2009]. Thus, the finding that the mice in this study did not have ABRs to frequencies greater than 32 kHz was expected, but it does not imply that the +/+ mice cannot detect the USVs emitted during social interactions. In addition, it has been shown that normal hearing CBA/CaJ mice can discriminate different USVs [Neilans, Holfoth, Radziwon, Portfors, & Dent, 2014].
Multiple behavioral phenotypes were evaluated in this study, to identify potential artifacts that could conceivably influence social behaviors and USVs. Motor stereotypies were observed in a small number of adult +/− mice, consistent with previous reports on our Portmann–Dolmetsch line and in the Mills line of 16p11.2 deletion mice [Horev et al., 2011; Portmann et al., 2014]. A higher threshold for a thermal nociceptive response in +/− mice may reflect unusual responsiveness to pain. Motor stereotypies and unusual pain response are additional phenotypes with potential face validity to symptoms of 16p11.2 deletion syndrome, particularly in cases diagnosed with autism [Lord et al., 2006]. No consistent genotype differences were detected across parameters on two anxiety-like tasks or on summed open field parameters.
Generally normal scores on anxiety-related, exploration and olfactory tests reported here, and previously [Portmann et al., 2014] indicate that responses to novelty, conflict, and sensory detection of social and nonsocial odors are similar across genotypes, and therefore, unlikely to be responsible for the vocalization differences during social interactions. Moderately reduced adult reciprocal social interactions in the present two cohorts of adult 16p11.2 mice, in contrast to the lack of genotype differences we previously reported for juvenile reciprocal social interactions and 3-chambered social approach in adult 16p11.2 mice [Portmann et al., 2014], suggest that the 16p11.2 deletion may have selective effects on responsiveness to highly salient social cues such as female pheromones. Reduced vocalization responses to social cues in 16p11.2 deletion mice, along with their sensory abnormalities, well-replicated sporadic motor stereotypies, and a previously reported cognitive deficit [Portmann et al., 2014], thus recapitulate features of several symptoms of the 16p11.2 deletion syndrome.
Supplementary Material
Acknowledgments
This work was supported by the Simons Foundation, SFARI grant #204340 to RD and JC, the National Institute of Mental Health Intramural Research Program and the University of California Davis MIND Institute to JC and MY, National Institutes of Health Pioneer Award to RD, The Swiss National Science Foundation #PBSKP3-123434 and #PA00P3_134196 to TP, and National Science Foundation IOS 1257768 to CP.
Footnotes
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- Bassuk AG, Geraghty E, Wu S, Mullen SA, Berkovic SF, Scheffer IE, et al. Deletions of 16p11.2 and 19p13.2 in a family with intellectual disability and generalized epilepsy. American Journal of Medical Genetics Part A. 2013;161A:1722–1725. doi: 10.1002/ajmg.a.35946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlsma EK, Gijsbers AC, Schuurs-Hoeijmakers JH, van Haeringen A, Fransen van de Putte DE, Anderlid BM, et al. Extending the phenotype of recurrent rearrangements of 16p11.2: deletions in mentally retarded patients without autism and in normal individuals. European Journal of Medical Genetics. 2009;52:77–87. doi: 10.1016/j.ejmg.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Blumenthal I, Ragavendran A, Erdin S, Klei L, Sugathan A, Guide JR, et al. Transcriptional consequences of 16p11.2 deletion and duplication in mouse cortex and multiplex autism families. American Journal of Human Genetics. 2014;94:870–883. doi: 10.1016/j.ajhg.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier J, Matteson PG, Silverman JL, Senerth JM, Kelly S, Genestine M, et al. Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLos One. 2012;7:e40914. doi: 10.1371/journal.pone.0040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byatt S, Nyby J. Hormonal regulation of chemosignals of female mice that elicit ultrasonic vocalizations from males. Hormones and Behavior. 1986;20:60–72. doi: 10.1016/0018-506x(86)90029-2. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Research. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian SL, Brune CW, Sudi J, Kumar RA, Liu S, Karamohamed S, et al. Novel submicroscopic chromosomal abnormalities detected in autism spectrum disorder. Biological Psychiatry. 2008;63:1111–1117. doi: 10.1016/j.biopsych.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuladaite Z, Kasnauskiene J, Cimbalistiene L, Preiksaitiene E, Patsalis PC, Kucinskas V. Mental retardation and autism associated with recurrent 16p11.2 microdeletion: incomplete penetrance and variable expressivity. Journal of Applied Genetics. 2011;52:443–449. doi: 10.1007/s13353-011-0063-z. [DOI] [PubMed] [Google Scholar]
- Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C, et al. A copy number variation morbidity map of developmental delay. Nature Genetics. 2011;43:838–846. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt F, Priebe L, Herms S, Mattheisen M, Muhleisen TW, Meier S, et al. Association between copy number variants in 16p11.2 and major depressive disorder in a German case-control sample. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2012;159B:263–273. doi: 10.1002/ajmg.b.32034. [DOI] [PubMed] [Google Scholar]
- Ehret G. Infant rodent ultrasounds -- a gate to the understanding of sound communication. Behavior Genetics. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- Elwin M, Ek L, Schroder A, Kjellin L. Autobiographical accounts of sensing in Asperger syndrome and high-functioning autism. Archives of Psychiatry Nursing. 2012;26:420–429. doi: 10.1016/j.apnu.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Ey E, Yang M, Katz AM, Woldeyohannes L, Silverman JL, Leblond CS, et al. Absence of deficits in social behaviors and ultrasonic vocalizations in later generations of mice lacking neuroligin4. Genes Brain and Behavior. 2012;11:928–941. doi: 10.1111/j.1601-183X.2012.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez BA, Roberts W, Chung B, Weksberg R, Meyn S, Szatmari P, et al. Phenotypic spectrum associated with de novo and inherited deletions and duplications at 16p11.2 in individuals ascertained for diagnosis of autism spectrum disorder. Journal of Medical Genetics. 2010;47:195–203. doi: 10.1136/jmg.2009.069369. [DOI] [PubMed] [Google Scholar]
- Ghebranious N, Giampietro PF, Wesbrook FP, Rezkalla SH. A novel microdeletion at 16p11.2 harbors candidate genes for aortic valve development, seizure disorder, and mild mental retardation. American Journal of Medical Genetics part A. 2007;143A:1462–1471. doi: 10.1002/ajmg.a.31837. [DOI] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio C, Willer J, Talkowski ME, Oh EC, Taniguchi Y, Jacquemont S, et al. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature. 2012;485:363–367. doi: 10.1038/nature11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley JM, Monaghan JJ, Wenstrup JJ. Development of social vocalizations in mice. Plos One. 2011;6:e17460. doi: 10.1371/journal.pone.0017460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmatre A, Dubourg C, Mosca AL, Legallic S, Goldenberg A, Drouin-Garraud V, et al. Recurrent rearrangements in synaptic and neurodevelopmental genes and shared biologic pathways in schizophrenia, autism, and mental retardation. Archives of General Psychiatry. 2009;66:947–956. doi: 10.1001/archgenpsychiatry.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hurley LM. Female presence and estrous state influence mouse ultrasonic courtship vocalizations. PLoS One. 2012;7:e40782. doi: 10.1371/journal.pone.0040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E, Nasir RH, Fong A, Lian A, Hundley R, Shen Y, et al. Cognitive and behavioral characterization of 16p11.2 deletion syndrome. Journal of Developmental and Behavioral Pediatrics. 2010;31:649–657. doi: 10.1097/DBP.0b013e3181ea50ed. [DOI] [PubMed] [Google Scholar]
- Holmstrom LA, Eeuwes LB, Roberts PD, Portfors CV. Efficient encoding of vocalizations in the auditory midbrain. Journal of Neuroscience. 2010;30:802–819. doi: 10.1523/JNEUROSCI.1964-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biology. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horev G, Ellegood J, Lerch JP, Son YE, Muthuswamy L, Vogel H, et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17076–17081. doi: 10.1073/pnas.1114042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Reymond A, Zufferey F, Harewood L, Walters RG, Kutalik Z, et al. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11.2 locus. Nature. 2011;478:97–102. doi: 10.1038/nature10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane KL, Longo-Guess CM, Gagnon LH, Ding D, Salvi RJ, Johnson KR. Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice. Hearing Research. 2012;283:80–88. doi: 10.1016/j.heares.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konyukh M, Delorme R, Chaste P, Leblond C, Lemiere N, Nygren G, et al. Variations of the candidate SEZ6L2 gene on Chromosome 16p11.2 in patients with autism spectrum disorders and in human populations. PLoS One. 2011;6:e17289. doi: 10.1371/journal.pone.0017289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, Badner JA, et al. Recurrent 16p11.2 microdeletions in autism. Human Molecular Genetics. 2008;17:628–638. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- Kumar RA, Marshall CR, Badner JA, Babatz TD, Mukamel Z, Aldinger KA, et al. Association and mutation analyses of 16p11.2 autism candidate genes. PLoS One. 2009;4:e4582. doi: 10.1371/journal.pone.0004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Ronemus M, Yamrom B, Lee YH, Leotta A, Kendall J, et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of General Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Mahrt EJ, Perkel DJ, Tong L, Rubel EW, Portfors CV. Engineered deafness reveals that mouse courtship vocalizations do not require auditory experience. Journal of Neuroscience. 2013;33:5573–5583. doi: 10.1523/JNEUROSCI.5054-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, et al. The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biological Psychiatry. 2010;67:864–871. doi: 10.1016/j.biopsych.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, et al. Structural variation of chromosomes in autism spectrum disorder. American Journal of Human Genetics. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, et al. Microduplications of 16p11.2 are associated with schizophrenia. Nature Genetics. 2009;41:1223–1227. doi: 10.1038/ng.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ. Acute pain experience in individuals with autism spectrum disorders: A review. Autism. 2014 doi: 10.1177/1362361314527839. [DOI] [PubMed] [Google Scholar]
- Nakagawa R, Matsunaga E, Okanoya K. Defects in ultrasonic vocalization of cadherin-6 knockout mice. PLoS One. 2012;7:e49233. doi: 10.1371/journal.pone.0049233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilans EG, Holfoth DP, Radziwon KE, Portfors CV, Dent ML. Discrimination of ultrasonic vocalizations by CBA/CaJ mice (Mus musculus) is related to spectrotemporal dissimilarity of vocalizations. PLoS One. 2014;9:e85405. doi: 10.1371/journal.pone.0085405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JP, Chang YS, Pojman NJ, Bukshpun P, Wakahiro ML, Marco EJ, et al. Aberrant white matter microstructure in children with 16p11.2 deletions. Journal of Neuroscience. 2014;34:6214–6223. doi: 10.1523/JNEUROSCI.4495-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoriza LN, Morrow TJ, Casey KL. Medial frontal cortex lesions selectively attenuate the hot plate response: possible nocifensive apraxia in the rat. Pain. 1996;64:11–17. doi: 10.1016/0304-3959(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV, Roberts PD. Mismatch of structural and functional tonotopy for natural sounds in the auditory midbrain. Neuroscience. 2014;258:192–203. doi: 10.1016/j.neuroscience.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Roberts PD, Jonson K. Over-representation of species-specific vocalizations in the awake mouse inferior colliculus. Neuroscience. 2009;162:486–500. doi: 10.1016/j.neuroscience.2009.04.056. [DOI] [PubMed] [Google Scholar]
- Portmann T, Yang M, Mao R, Panagiotakos G, Ellegood J, Bader P, et al. Behavioral abnormalities and circuit defect in basal ganglia in a mouse model of 16p11.2 microdeletion syndrome. Cell Reports. 2014;7:1077–1092. doi: 10.1016/j.celrep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvabanditsin S, Nagar MS, Joshi M, Lambert G, Garrow E, Brandsma E. Microdeletion of 16p11.2 associated with endocardial fibroelastosis. American Journal of Medical Genetics. Part A. 2010;152A:2383–2386. doi: 10.1002/ajmg.a.33562. [DOI] [PubMed] [Google Scholar]
- Raca G, Baas BS, Kirmani S, Laffin JJ, Jackson CA, Strand EA, et al. Childhood Apraxia of Speech (CAS) in two patients with 16p11.2 microdeletion syndrome. European Journal of Human Genetics. 2013;21:455–459. doi: 10.1038/ejhg.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinthaler EM, Lal D, Lebon S, Hildebrand MS, Dahl HH, Regan BM, et al. 16p11.2 600 kb Duplications Confer Risk for Typical and Atypical Rolandic Epilepsy. Human Molecular Genetics. 2014;23:6069–6080. doi: 10.1093/hmg/ddu306. [DOI] [PubMed] [Google Scholar]
- Rosenberg C, Knijnenburg J, Bakker E, Vianna-Morgante AM, Sloos W, Otto PA, et al. Array-CGH detection of micro rearrangements in mentally retarded individuals: clinical significance of imbalances present both in affected children and normal parents. Journal of Medical Genetics. 2006;43:180–186. doi: 10.1136/jmg.2005.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JA, Coe BP, Eichler EE, Cuckle H, Shaffer LG. Estimates of penetrance for recurrent pathogenic copy-number variations. Genetic Medicine. 2013;15:478–481. doi: 10.1038/gim.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JA, Coppinger J, Bejjani BA, Girirajan S, Eichler EE, Shaffer LG, et al. Speech delays and behavioral problems are the predominant features in individuals with developmental delays and 16p11.2 microdeletions and microduplications. Journal of Neurodevelpomental Disorders. 2010;2:26–38. doi: 10.1007/s11689-009-9037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet FI, Wohr M, Crawley JN. Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behavior Brain and Research. 2011;216:19–28. doi: 10.1016/j.bbr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain, and Behavior. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf CP, Goin-Kochel RP, Nowell KP, Hunter JV, Aleck KA, Cox S, et al. Expanding the clinical spectrum of the 16p11.2 chromosomal rearrangements: three patients with syringomyelia. European Journal of Human Genetics. 2011;19:152–156. doi: 10.1038/ejhg.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Dies KA, Holm IA, Bridgemohan C, Sobeih MM, Caronna EB, et al. Clinical genetic testing for patients with autism spectrum disorders. Pediatrics. 2010;125:e727–735. doi: 10.1542/peds.2009-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinawi M, Liu P, Kang SH, Shen J, Belmont JW, Scott DA, et al. Recurrent reciprocal 16p11.2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head size. Journal of Medical Genetics. 2010;47:332–341. doi: 10.1136/jmg.2009.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Paris K, Akana MC, Cyster JG, Sorensen RU, Puck JM. Severe combined immunodeficiency (SCID) and attention deficit hyperactivity disorder (ADHD) associated with a Coronin-1A mutation and a chromosome 16p11.2 deletion. Clinical Immunology. 2009;131:24–30. doi: 10.1016/j.clim.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, et al. Sociability and motor functions in Shank1 mutant mice. Brain Research. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag M, Englitz B, Kopp-Scheinpflug C, Rubsamen R. Early postnatal development of spontaneous and acoustically evoked discharge activity of principal cells of the medial nucleus of the trapezoid body: an in vivo study in mice. Journal of Neuroscience. 2009;29:9510–9520. doi: 10.1523/JNEUROSCI.1377-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South SM, Smith MT. Apparent insensitivity of the hotplate latency test for detection of antinociception following intraperitoneal, intravenous or intracerebroventricular M6G administration to rats. The Journal of Pharmacology and Experimental Therapeutics. 1998;286:1326–1332. [PubMed] [Google Scholar]
- Steinberg S, de Jong S, Mattheisen M, Costas J, Demontis D, Jamain S, et al. Common variant at 16p11.2 conferring risk of psychosis. Molecular Psychiatry. 2014;19:108–114. doi: 10.1038/mp.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Okabe S, Kato M, Koshida N, Shiroishi T, Mogi K, et al. A role for strain differences in waveforms of ultrasonic vocalizations during male-female interaction. PLoS One. 2011;6:e22093. doi: 10.1371/journal.pone.0022093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkiewicz JP, O’Dushlaine C, Chen G, Chambert K, Moran JL, Neale BM, et al. Copy number variation in schizophrenia in Sweden. Molecular Psychiatry. 2014;19:762–773. doi: 10.1038/mp.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D, Stoppel LJ, Heynen AJ, Lindemann L, Jaeschke G, Mills AA, Bear MF. Contribution of mGluR5 to pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion. Nat Neurosci. 2015 doi: 10.1038/nn.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RG, Jacquemont S, Valsesia A, de Smith AJ, Martinet D, Andersson J, et al. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463:671–675. doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, et al. Association between microdeletion and microduplication at 16p11.2 and autism. New England Journal of Medicine. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- White NR, Prasad M, Barfield RJ, Nyby JG. 40- and 70-kHz vocalizations of mice (Mus musculus) during copulation. Physiology & Behavior. 1998;63:467–473. doi: 10.1016/s0031-9384(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Whitney G, Coble JR, Stockton MD, Tilson EF. Ultrasonic emissions: do they facilitate courtship of mice. Journal of Comparative and Physiological Psychology. 1973;84:445–452. doi: 10.1037/h0034899. [DOI] [PubMed] [Google Scholar]
- Whitney G, Nyby JG. Cues that elicit ultrasounds from adult male mice. American Zoologist. 1979;19:457–464. [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wohr M, Roullet FI, Katz AM, et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. Journal of Neuroscience. 2012;32:6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;Chapter 8(Unit 8):24. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Loureiro D, Kalikhman D, Crawley JN. Male mice emit distinct ultrasonic vocalizations when the female leaves the social interaction arena. Frontiers in Behavioral Neuroscience. 2013;7:159. doi: 10.3389/fnbeh.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Current Protocols in Neuroscience. 2011;Chapter 8(Unit 8):26. doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hearing Research. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey F, Sherr EH, Beckmann ND, Hanson E, Maillard AM, Hippolyte L, et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. Journal of Medical Genetics. 2012;49:660–668. doi: 10.1136/jmedgenet-2012-101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.