Abstract
Background
While Syk has been shown to associate with TLR4, the immune consequences of Syk-TLR interactions and related molecular mechanisms are unclear.
Methods
Gain- and loss- of function approaches were utilized to determine the regulatory function of Syk and elucidate the related molecular mechanisms in TLR4-mediated inflammatory responses. Cytokine production was measured by ELISA and phosphorylation of signaling molecules determined by western blotting.
Results
Syk deficiency in murine dendritic cells resulted in the enhancement of LPS-induced IFNβ and IL-10 but suppression of pro-inflammatory cytokines (TNFα, IL-6). Deficiency of Syk enhanced activity of PI3K and elevated the phosphorylation of PI3K and Akt, which in turn, lead to the phospho-inactivation of the downstream, central gatekeeper of the innate response, GSK3β. Inhibition of PI3K or Akt abrogated the ability of Syk deficiency to enhance IFNβ and IL-10 in Syk deficient cells, confirmed by the overexpression of Akt (Myr-Akt) or constitutively active GSK3β (GSK3 S9A). Moreover, neither inhibition of PI3K-Akt signaling nor neutralization of de novo synthesized IFNβ could rescue TNFα and IL-6 production in LPS-stimulated Syk deficient cells. Syk deficiency resulted in decreased phosphorylation of IKKβ and the NF-κB p65 subunit, further suggesting a divergent influence of Syk of pro- and anti-inflammatory TLR responses.
Conclusions
Syk negatively regulates TLR4-mediated production of IFNβ and IL-10 and promotes inflammatory responses in dendritic cells through divergent regulation of downstream PI3K-Akt and NF-κB signaling pathways.
General Significance
Syk may represent a novel target for manipulating the direction or intensity of the innate response, depending on clinical necessity.
Keywords: Syk, Toll like receptor 4, Interferon beta, IL-10, PI3K, GSK3β
Introduction
Toll-like receptor (TLR)-mediated production of inflammatory cytokines and type I interferon (IFN) plays a critical role in the host response to microbial pathogens. Inflammatory cytokines promote the control of infection by elevating body temperature, activating and recruiting innate cells, enhancing phagocytosis, and boosting the adaptive arm of the immune system. Type I IFNs are particularly important in the control of viruses and cancers [1–3]. However, in order to prevent collateral tissue damage during inflammatory responses, the magnitude and quality of inflammatory cytokines and type I IFN must be tightly controlled [4–6]. TLR4-engagement results in the propagation of signals that will induce both inflammatory cytokines and type I IFNs in a manner mediated by the recruitment of different adaptor molecules. Whereas activation of TIRAP-MyD88 controls the induction of pro- and anti- inflammatory cytokines through downstream NF-κB-mediated transcription[7], the TRAM-TRIF-mediated signaling cascade controls the production of type I IFN, including IFNβ, through two noncanonical IκB kinases, TBK-1 and IKKε, as well as NF-κB, ATF-2/c-Jun, and IRF-3[8–10]. However, the regulatory signaling events involved in the control of TLR4-mediated inflammatory cytokines and type I IFN remain less well defined.
Stimulation of TLR4 has been demonstrated to activate the PI3K pathway and restrain the production of pro-inflammatory cytokines through downstream phospho-inactivation of GSK3β in innate cells [11]. Our previous studies have identified the pivotal role of GSK3β in negative regulation of TLR4-mediated IFNβ and IL-10 release [12, 13]. Cao et al. demonstrated that PI3K-mediated mTOR-p70S6K signaling is required for the induction of type I IFN production [14]. Since we have previously demonstrated that mTOR-p70S6K relays the TLR4-activated PI3K signal to the downstream GSK3β in innate immune cells [15], we hypothesized that PI3K-mTOR-p70S6K-GSK3β may be a major pathway responsible for regulation of IFNβ. Although above studies established the influence of PI3K on TLR-mediated inflammatory cytokine and IFN production, the molecular events upstream of the PI3K responsible for regulation of IFNβ and inflammatory cytokine production are still poorly understood.
Spleen tyrosine kinase (Syk) is a 72kDa non-receptor tyrosine kinase primarily expressed by haematopoietic cells and typically considered as a crucial regulator in adaptive immunity. Syk deficiency leads to a complete absence of mature B cells and to severe defects in T cell development [16–18]. Recent studies suggest that Syk may also be important in pattern recognition receptor (PRR)-mediated signaling in innate cells [19–21]. Binding of the SH2 domain of Syk to phosphorylated immunoreceptor tyrosine-based activation motifs (ITAMs) of various PRRs and adaptors, such as C-type lectin receptors and DAP12 (DNAX activation protein of 12 kDa), enables Syk to relay the upstream signals initiated by these receptors [22–24]. While the interaction of Syk with multiple ITAMs including MyD88, TRIF and TRAF6, is well established, the consequences of such interactions remain unclear. In this regard, using pharmacological inhibitors of Syk, several studies reported Syk is essential for TLR-mediated inflammatory responses, and inhibition of Syk suppresses the production of inflammatory mediators, including pro-inflammatory cytokines and IFNβ [25–27]. In contrast, Syk was also reported to be involved in the CD11b-mediated inhibition of TLR signaling, by which it suppresses TLR-mediated pro-inflammatory cytokines and IFNβ [28]. These studies are further complicated by the finding of Lin et al. [29], who showed that deficiency of Syk enhances production of pro-inflammatory cytokines and reduces type I IFN levels in LPS-stimulated macrophages, which occurs through differentially regulating ubiquitination of TRAF3 and TRAF6. Thus, the functional role and related molecular mechanism of Syk in the regulation TLR-mediated pro-inflammatory cytokine and IFNβ production is yet to be resolved. Therefore, we set out to elucidate the function of Syk in the context of TLR4-mediated IFNβ and IL-10 production.
Material and Methods
Mice and reagents
CD11c-Cre/syk1 fl/fl mice were generated by crossing CD11c-drived Cre C57BL/6 (from Jackson Laboratory) to loxP flanked Syk1 mice (gifted by Dr. Alexander; Dartmouth Medical School). Control mice were negative littermates from this breeding. All the mice were housed in a specific pathogen-free facility at the University of Louisville, and with the ethical approval of the University of Louisville Institutional Animal Care and Use Committee (IACUC No. 130490). Ultra-pure LPS from E. coli 0111:B4, Poly (I:C) LMW, and ODN1826 were from Invivogen (San Diego, CA). Phosphorylated Syk1 antibody was from Assay Biotech. Total Syk antibodies were from Proteintech (Chicago, IL). IFNβ neutralizing antibody was from R&D (Minneapolis, MN). IFNβ ELISA kits were from R & D Systems (Minneapolis, MN). IL-10 neutralizing antibody and cytokine ELISA kits were from eBioscience (San Diego, CA). Isotype control antibody was from Santa Cruz. All other antibodies were from Cell Signaling Technology. PI3 kinase activity assays ELISA kit was from Echelon Biosciences (Salt Lake City, UT). Protease inhibitor cocktail was from Sigma Aldrich. Transfection reagents Lipofectamine LTX (and Plus) were from Invitrogen (Carlsbad, CA). PI3K inhibitors LY294002 and ZSTK474 were from LC Laboratories (Woburn, MA), Akti was from Sigma-Aldrich (St. Louis, MO), and SB216763 was from Tocris (Bristol, United Kingdom). pBSFI-Myr-Akt (Cat. No. #49186), pcDNA3-GSK3β (S9A), and empty control vectors were obtained from Addgene (Cambridge, MA). EZview Red Protein G Affinity Gel Beads were from Sigma Aldrich (St. Louis, MO). RNeasy Mini Kit and SYBR green master mix were from Qiagen (Hilden, German). RPMI1640, Penicillin, FBS, and SuperScript III Platinum Two-Step QRT-PCR kits were from Invitrogen Life Technologies (Carlsbad, CA). GM-CSF and M-CSF were from Peprotech (Rocky Hill, NJ). Low-attachment six-well plates were from Corning Life Sciences (MA, US)
Cell culture, viability and flow cytometry
Bone marrows were isolated from control or Syk1 Cre-loxP mice, and then bone marrow cells were isolated via flushing the tibia and fibula. After RBC lysis, cells were plated at 1.5 × 106 cells/ml and cultured in RPMI 1640 medium supplemented with 10% FBS (R10), 50μM 2-mercaptoethanol, 1mM sodium pyruvate, 2mM L-glutamine, 20mM HEPES, 50 U/ml penicillin, and 50 μg/ml streptomycin (RPMI-complete). GM-CSF (10 ng/ml) was added to facilitate the differentiation of bone marrow cells for 6 days as previously described [30, 31], and bone marrow-derived dendritic cells (BMDCs) were harvested and the expression of CD80 and CD86 examined using flow cytometry. Briefly, cells were harvested, washed twice with 2 ml of fluorescence-activated cell sorting (FACS) buffer (PBS containing 2% fetal bovine serum and 0.01% sodium azide), and fixed with formaldehyde at a final concentration of 4% in PBS for 10 min at room temperature. Cells were washed twice in PBS containing 2% FBS and analyzed immediately by flow cytometry. RPMI-complete medium containing 10 ng/ml M-CSF and 30% L929 were used to generate bone marrow-derived macrophages (BMDMs) following the procedure described previously [13]. In brief, bone marrow cells were cultured overnight in standard tissue culture plates in the presence of 10 ng/ml M-CSF. Non-adherent cells from this initial culture were then transferred to low-attachment six-well plates in 4 ml R5F (RPMI with 5% FBS) containing 30% L929 conditioned medium and 10 ng/ml M-CSF for 7 d, adding 1.5 ml fresh medium on days 3 and 5. Cells were verified to be at least 90–98% CD11b+/CD11c−/MHC-II. Cell viability was determined with the trypan blue exclusion assaying, and the cell number is expressed relative to the initial cell number.
RT-PCR, Transfection, cytokine detection and western blot
Total RNA was isolated using the RNeasy Mini Kit and the cDNA was synthesized using the SuperScript III Platinum Two-Step QRT-PCR kit. RT-PCR was performed using an ABI 7500 system. GAPDH was used as the endogenous control and fold increases were calculated according to the ΔΔCT method. BMDCs were transfected with Myr-Akt plasmids, pcDNA3-GSK3 (S9A), or empty vector controls using lipofectamine LTX, and BMDM were transfected with non-targeting siRNA and syk siRNA using lipofectamine RNAiMAX, following the manufacturer’s protocol. The levels of total Akt and GSK3β protein were assessed by Western blot on day 3. In 96-well plates, 2 × 105 BMDCs were cultured and pretreated for 2 h with 0.01% DMSO (organic solvent control for inhibitor) or the inhibitor of PI3K (LY294002), Akt (Akti ), or GSK3β ( SB216763), prior to stimulation with LPS (1 μg/ml). Cell lysates were prepared as previously described [15]. Images were acquired using the ImageQuant LAS 4100 and densitometry performed and analyzed using ImageQuant TL software (GE Healthcare Life Sciences, Pittsburg, PA). Cell-free supernatants were assayed for cytokine levels by ELISA 24 h after the addition of LPS.
Measurement of PI3K kinase activity
PI3 kinase activity was determined using a ELISA kit to measure the enzymatic production of phosphatidylinositol-3,4,5 trisphosphate (PIP3) from phosphatidylinositol 4,5-bisphosphate (PIP2) substrate, as previously described [32]. Briefly, wild type and Syk deficient BMDCs (4 ×106) were plated in six-well plates and stimulated with LPS (1 μg/ml) for 30 or 60 min. Cell lysates were prepared using immunoprecipitation compatible lysis buffer (20 mM Tris; pH 7.4), 10 mM EDTA, 100 mM NaCl, 1% polyethoxyethanol, 1 mM Na3VO4, 50 mM NaF, and protease inhibitor cocktail. Protein concentrations were determined using the bicinchoninic acid assay (Pierce). PI3K in cell lysates (300 μg protein per sample) was immunoprecipitated using p85–specific Ab and EZview Red Protein G Affinity Gel Beads for 24 h at 4°C. The immunoprecipitates were subsequently incubated with PIP2 substrate in kinase reaction buffer for 2 h at room temperature, then the generation of PIP3 product was determined by competitive ELISA.
Statistical analysis
A Shapiro-Wilk test was used to confirm the normal distribution of our data before we start statistical analysis. Statistical significance between groups was evaluated by one-way ANOVA and the Tukey multiple-comparison test using the InStat program (GraphPad, San Diego, CA). Differences between groups were considered significant at p values < 0.05.
Results
Syk knockdown does not influence maturation of dendritic cells
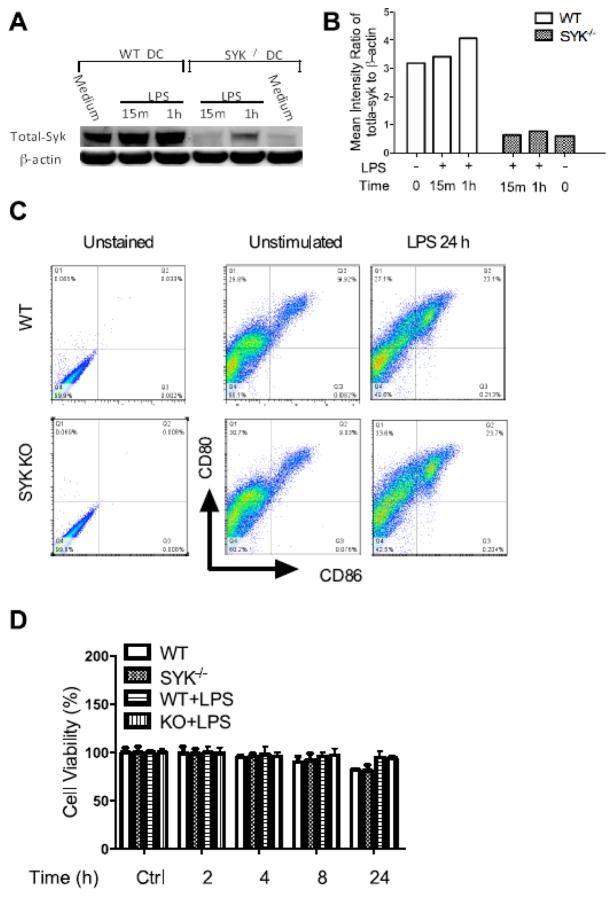
To examine the Cre-loxP-mediated knock out efficiency of Syk and determine if Syk deficiency affects the maturation of dendritic cells, we first detected the expression of Syk and the maturation markers CD80 and CD86 in wild type and Syk-deficient LPS-stimulated BMDCs. As shown in Figure 1 A and B, only trace amounts of Syk could be detected in Syk deficient BDMCs, as compared with wild type, suggesting efficient Cre-loxP system-mediated Syk knockdown. Moreover, Syk knockdown did not influence the expression of dendritic cell maturation markers (CD80; CD86) at rest or following 24 h stimulation with LPS (1 μg/ml), as shown by flow cytometry (Fig. 1C). In addition, trypan blue exclusion was performed to determine the cell viability, with no statistically significant difference between the wild type and Syk deficient cells noted (Fig. 1D).
Figure 1. Expression of Syk and its influences on the maturation of dendritic cells.
(A)Western blot of the whole cell lysates from wild type and Cre-loxP-mediated Syk deficient dendritic cells. Blots were probed with antibodies to total-Syk and GAPDH as a loading control; (B) Densitometric quantification of the ratio of total-Syk to total-GAPDH; (C) WT and Syk deficient cells were stimulated with LPS (1 μg/ml) for 24 hours, and the expression of CD80 and CD86 was detected by flow cytometry; (D) Cell viability was determined by trypan blue exclusion.
Syk deficiency enhances LPS-induced IFNβ and IL-10 production, but reduces TNFα and IL-6 levels in LPS-stimulated BMDCs
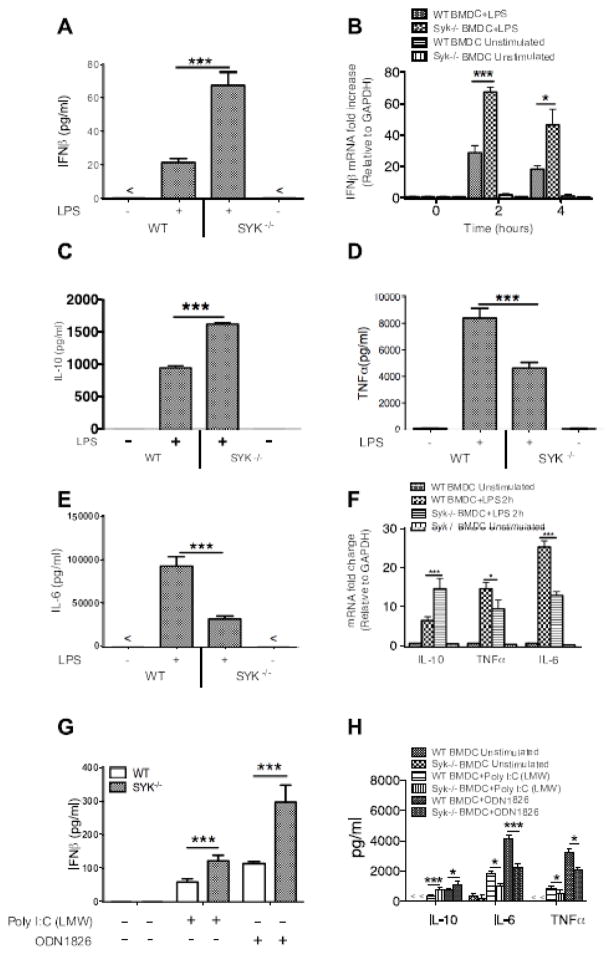
To determine the regulatory effects of Syk on LPS-induced inflammatory cytokines, BMDCs were stimulated with LPS (1 μg/ml) for 24 h. The IFNβ protein and transcript levels were examined by ELISA and RT-PCR, respectively (A, B). Moreover, the production of IL-10, TNFα, and IL-6 was detected by ELISA. As shown in Figure 2, Syk deficiency resulted in a robust increase in IFNβ (A, B) and IL-10 (C) produced by LPS-stimulated BDMCs, but a diminished TNFα (D) and IL-6 (E) production at protein and mRNA levels (F), compared to wild type BMDCs. Moreover, we examined the production of IFNβ upon the activation of other TLRs (TLR3, TLR9) and found that Syk deficiency significantly enhanced the production of IFNβ and IL-10, while concurrently decreased TNFα and IL-6 levels in poly I:C and ODN stimulated BMDCs (Fig. 2G, H). Considering the ability of IFNβ to promote the production of IL-10 [12, 33], these results demonstrate that Syk differentially regulates the production of pro- and anti-inflammatory cytokines, indicating the pro-inflammatory bias of Syk upon LPS stimulation.
Figure 2. Deficiency of Syk elevates the production of IFNβ and IL-10, but suppresses the levels of TNFα and IL-6 in LPS-stimulated dendritic cells.
Cell-free supernatants were collected from wild type and Syk deficient dendritic cells stimulated with LPS for 24 h, and the production of (A) IFNβ, (C) IL-10, (D) TNFα, and (E) IL-6 was determined by ELISA. The mRNA levels of IFNβ (B) and other cytokines (F) from wild type and Syk deficient cells stimulated with LPS (1 μg/ml) for the time indicated were examined by RT- PCR; TLR3 (Poly I:C LMW; 1 μg/ml) and TLR9 (ODN1826; 10μg/ml) agonists were used to stimulate wild type and Syk deficient BMDCs for 24 h, and the production of IFNβ (G) and other cytokines (H) measured by ELISA, “*” and “***” indicates P<0.05 and P<0.001, respectively. Data represents the mean ± S.D. of three biological replicates.
Deficiency of Syk elevated IFNβ production leads to the enhancement of STATs phosphorylation
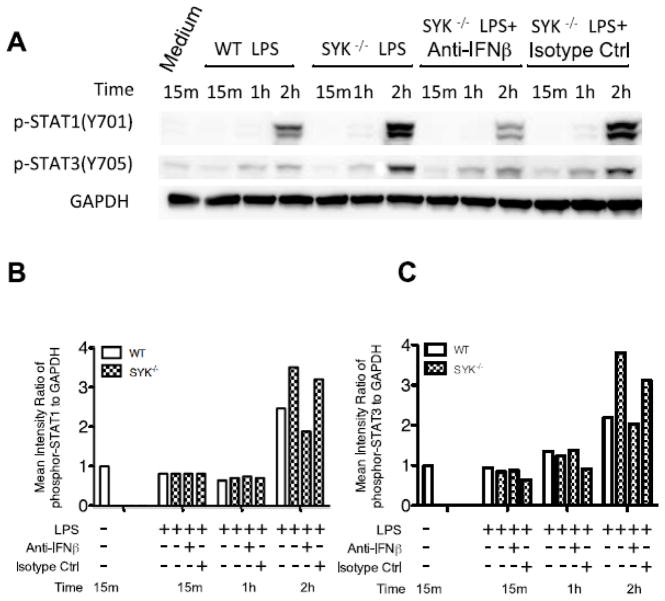
Binding of type I IFNs to the interferon-alpha/beta receptor (IFNAR) complex results in the activation of two receptor associated tyrosine kinases, Tyk2 and JAK1, which, in turn, phospho-activate downstream STATs [6, 34]. Therefore, we next determined if Syk deficiency influenced the phosphorylation state of STAT1 and STAT3 in LPS-stimulated BMDCs. Since a late (2 h), but profound increase in STAT phosphorylation, particularly STAT1, was observed in LPS-stimulated Syk-deficient BMDCs (Fig. 3), we next determined if STAT1 and STAT3 phosphorylation was due to enhanced de novo synthesis of IFNβ. IFNβ neutralizing antibody, but not isotype control antibody, efficiently blocked STAT activation following LPS stimulation, which corroborated Syk deficiency indeed enhances the production of IFNβ in LPS stimulated BMDCs.
Figure 3. Deficiency of Syk elevated IFNβ production and leads to the enhancement of STATs phosphorylation.
Wild type and Syk deficiency cells were pretreated with IFNβ neutralizing antibody or isotype control for 2 h and then stimulated with LPS. Cell lysates were prepared at the indicated time points and then analyzed by Western blot. (A) Blots were probed with antibodies to phospho-STATs and GAPDH as a loading control; (B) Densitometric quantification of the ratio of phospho-STATs to total-GAPDH.
Syk deficiency enhances the activity of PI3 kinase and phosphorylation of Akt, and GSK3β upon LPS stimulation
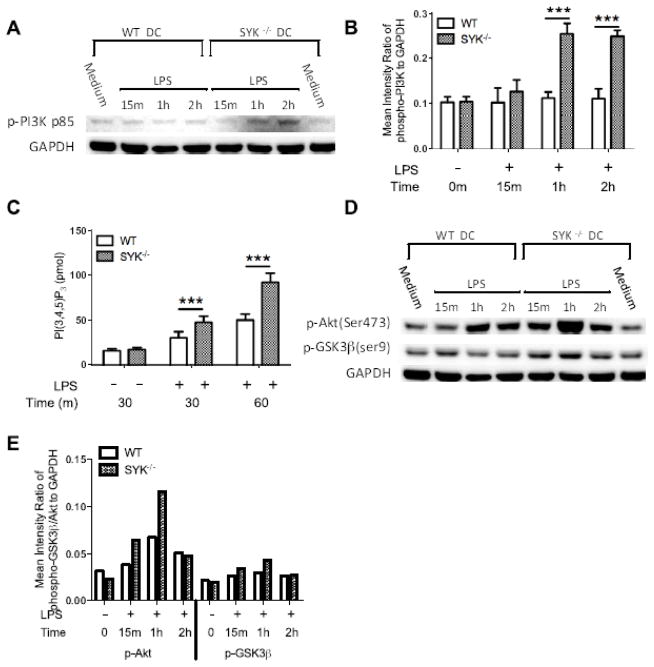
TLR4-engagement is known to lead to the activation of PI3K, the restraint of pro-inflammatory cytokine production and the enhancement of IFNβ and IL-10 in innate immune cells [13, 14, 35]. To determine if Syk deficiency regulates cytokine production through the PI3K signaling axis, we first examined if Syk deficiency affects LPS-induced PI3K activation. As shown in Figure 4A, B, phosphorylation of the regulatory subunit of PI3K (p85) was significantly increased in LPS-stimulated Syk deficient BMDCs, compared with wild type control. This phenomenon was confirmed upon monitoring PI3K activity in LPS-stimulated BMDCs. As shown in Figure 4C, production of PIP3, which directly represents PI3K activity, was significantly higher in LPS-stimulated Syk deficient cells, as compared with that of wild type. Moreover, the downstream molecules in the PI3K pathway, Akt and GSK3β, also exhibited enhanced phosphorylation in LPS-stimulated Syk deficient BMDCs, as compared with wild type control (Fig. 4D, E). These results demonstrate that Syk deficiency enhances activity of TLR4-mediated PI3K and its downstream signaling partners in LPS-stimulated BMDCs.
Figure 4. Syk deficiency enhances PI3-kinase activity and phosphorylation of downstream molecules, Akt and GSK3β in LPS-stimulated cells.
(A) Western blots of whole cell lysates from LPS-stimulated wild type and Syk deficient cells. Blots were probed with antibodies to phospho-p85 and total GAPDH as a loading control; (B) Densitometric quantification of the ratio of phospho-p85 to total-GAPDH; (C) Wild type and Syk deficient cells were stimulated for 30 min and 1 h with 1 μg/ml LPS. Subsequently, PI3K was immunoprecipitated from cell lysates and enzymatic activity was assessed. Data are presented as the mean ±SD from three biological replicates. Syk deficiency resulted in the significant (***, p<0.001) enhancement of PIP3 generation where indicated. (D) Blots in (A) were also probed with antibodies to phospho-Akt, GSK3β, and total GAPDH as a loading control; (E) Densitometric quantification of the ratio of phospho- Akt, -GSK3β to total-GAPDH.
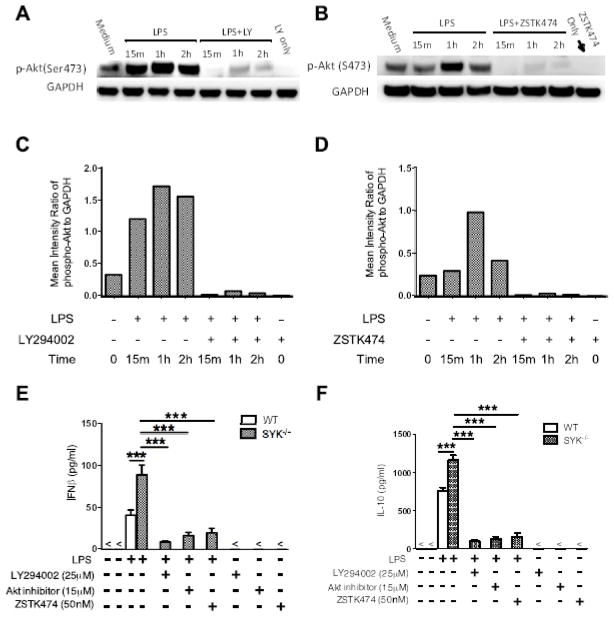
Inhibition of the PI3K/Akt axis normalizes the anti-inflammatory response in Syk deficient cells
As Syk deficiency enhances PI3K activity and anti-inflammatory mediator production upon TLR4 engagement, we next investigated if the regulatory effects of Syk on IFNβ and IL-10 production are similarly dependent on PI3K activity. Specific PI3K inhibitors LY294002 and ZSTK474 were employed to determine the regulatory function of PI3K in Syk inhibition-enhanced IFNβ and IL-10. As shown in Figure 5, either LY294002 or ZSTK474 abrogated phosphorylation of Akt (Fig. 5A–D) and significantly decreased the enhancement of IFNβ and IL-10 produced by LPS-stimulated BMDCs from Syk-deficient mice (Fig. 5E, F). The Akt inhibitor, Akti, had similar effects in LPS-stimulated, Syk deficient BMDCs (Fig. 5E, F). These results suggest that Syk deficiency enhances production of IFNβ and IL-10 in a PI3K-dependent manner.
Figure 5. Inhibition of PI3K or Akt abrogates the regulatory effects of Syk deficiency on TLR4-mediated IFNβ and IL-10 production.
(A, B) Syk deficiency cells were pre-treated with LY294002 (25 μM) or ZSTK474 (50 nM) and stimulated with LPS (1 μg/ml). Cell lysates were collected at the time points indicated and phosphorylation of Akt were detected by Western blot. (C, D) Densitometric quantification of the ratio of phospho-Akt to total-GAPDH. (E, F) Wild type and Syk deficient cells were pretreated with PI3K inhibitor, LY294002 or ZSTK474, or Akt inhibitor, for 2 h and then stimulated with LPS for 24 h. Cell free supernatants were collected and LPS-induced production of IFNβ (E) and IL-10 (F) was determined by ELISA. “***” indicates P<0.001. Data represent the mean ± S.D. of three biological replicates.
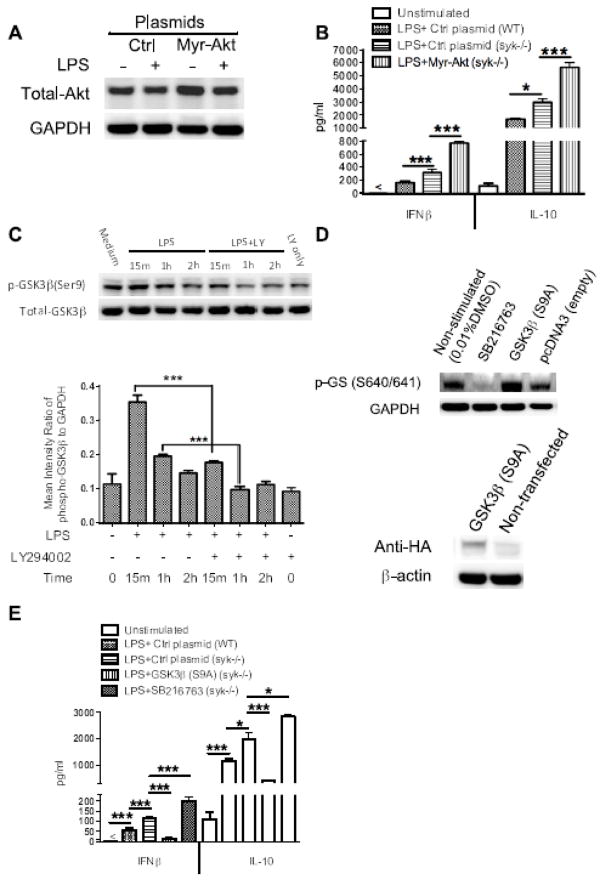
Enhanced IFNβ and IL-10 production in Syk deficient cells is Akt-and GSK3β-dependent
To control for possible non-specific effects of pharmacological inhibitors, we next utilized a gain-of-function approach to confirm the role of PI3K-Akt in the regulation of IFNβ and IL-10 during Syk deficiency. Stable transfection of Syk deficient cells with a plasmid expression exogenous Akt (Myr-Akt) (Fig. 6A) led to enhanced IFNβ and IL-10 production following LPS stimulation, compared to Syk-deficient cells expressing an empty control vector (p<0.001) (Fig. 6B). Ourselves and others have previously demonstrated that PI3K-Akt signaling restrains TLR4-mediated production of pro-inflammatory cytokines through phosphorylation (inactivation) of downstream GSK3β [35, 36]. Thus, we next determined the importance of GSK3β in the control of IFNβ and IL-10 during Syk-deficiency. Initially, we monitored the influences of PI3K activity on the level of phospho-GSK3β in Syk deficient cells upon LPS stimulation. As compared with wild type, inhibition of PI3K in LPS stimulated-Syk deficient cells resulted in a significant decrease in phosphorylation of GSK3β (Fig. 6C). Moreover, the specific GSK3 inhibitor, SB216763, and the successful over-expression of a constitutively active form of GSK3β (S9A), verified by the expression of HA (Human influenza hemagglutinin) epitope tag (Fig. 6D) influenced IFNβ and IL-10 production. SB216763 significantly enhanced production of IFNβ and IL-10 in LPS-stimulated Syk-deficient BMDCs (p<0.001) (Fig. 6E). In contrast, LPS stimulation of Syk deficient cells expressing constitutively active GSK3β produced significantly less IFNβ and IL-10, as compared with cells expressing an empty control vector (p<0.001) (Fig. 6E). Taken together, these findings demonstrate that enhanced TLR4-mediated production of IFNβ and IL-10 in Syk-deficient BMDCs is dependent on the activity of downstream targets of PI3K signaling, Akt and GSK3β.
Figure 6. Syk deficiency-mediated elevated IFNβ and IL-10 production is dependent on the activity of Akt-GSK3β signaling.
Syk-deficient cells were transfected with Myr-Akt or pcDNA3-GSK3β (S9A) plasmid which encode constitutively active Akt and GSK3β, respectively. Empty vectors were also transfected into wild type and Syk-deficient cells as a control. After 48 h transfection, cells were stimulated with LPS (1 μg/ml) for 6 h and cells lysates were collected for Western blot analysis. (A) Blot was probed with antibodies to total Akt and GAPDH to ensure equivalent sample loading. (B) After 48 h, transfected cells were stimulated with LPS for 24 h, and then the cell free supernatants were harvested to detect the production of IFNβ and IL-10. (C) Western blot of cell lysates from Syk deficient BMDCs pretreated with PI3K inhibitor, LY294002, for 2 h, then stimulated with LPS to 2 h, the blot was probed with antibodies to phospho-and total GSK3β, densitometric quantification of the ratio of phospho- to total-GSK3β was also calculated as shown. (D) For GSK3β transfection, phosphorylation levels of glycogen synthase (GS), a specific substrate of GSK3β, and the expression level of Hemagglutinin (HA) tag protein, encoded by the HA DNA sequence inserted at the C terminal of GSK3β mutant, were detected by Western blot to assess the transfection efficiency. (E) ELISA of LPS-induced IFNβ and IL-10 in cell-free supernatant in the absence and presence of SB216763 and constitutively active GSK3β. For B and E, data are representative of at least three separate experiments. “*” and “***” indicate p<0.05, and p<0.001 respectively. Data represent the mean ± S.D. of three biological replicates.
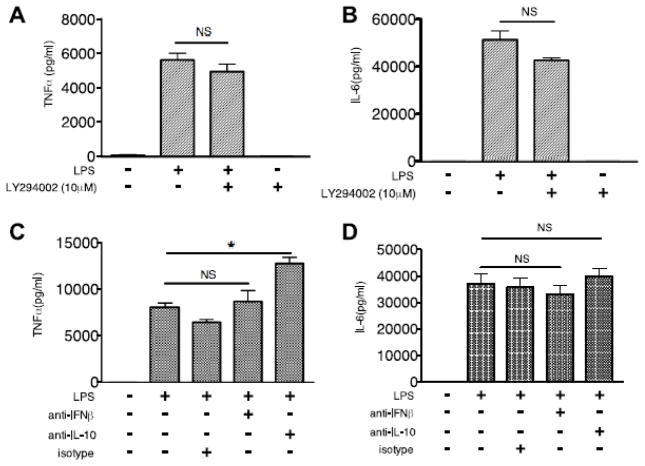
Suppression of TNFα and IL-6 production during Syk deficiency is independent of PI3K
We and others have previously demonstrated that TLR-mediated activation of the PI3K-Akt-GSK3β pathway differentially regulates the production of pro- and anti- inflammatory cytokines in human innate immune cells, with inhibition of PI3K or Akt resulting in a significant increase in pro-inflammatory cytokines, including TNFα and IL-6 [11, 35]. Since we have now demonstrated that enhanced IFNβ and IL-10 production in LPS-stimulated Syk-deficient BMDCs is dependent on PI3K-Akt-GSK3β signaling, we next determined if Syk deficiency-mediated alternation of TNFα and IL-6 are also regulated by the PI3K pathway. Surprisingly, inhibition of PI3K with LY294002 did not influence the production of TNFα and IL-6 in LPS-stimulated Syk-deficient BMDCs (Fig. 7A, B), suggesting that the decrease of TNFα and IL-6 was independent of PI3K-Akt-GSK3 signaling. IFNβ can induce IL-10, and act as an anti-inflammatory signal [12, 37, 38]. Therefore, to determine if de novo synthesis of IFNβ and IL-10 may contribute to the decrease of TNFα and IL-6 in TLR4-engaged Syk deficient BMDCs, we employed neutralizing antibodies. As shown in Figure 7 C and D, pretreatment with IL-10 neutralizing antibody led to a significant increase of TNFα, but only a slight increase of IL-6, while IFNβ neutralization did not significant alter TNFα or IL-6 production in LPS-stimulated Syk deficient BMDCs, as compared with isotype controls. Taken together, these results suggest Syk deficiency-decreased TNFα and IL-6 was independent of the activity of PI3K in BMDCs stimulated with LPS.
Figure 7. Syk regulation of TNFα and IL-6 was not affected by the activity of PI3K signaling and the de novo production of IFNβ.
Syk-deficient BMDCs were pre-treated with LY294002, IFNβ or IL-10 neutralizing antibody, or isotype control for 2 h, then stimulated with LPS (1 μg/ml) for 24 h. Cell free supernatants were collected to determine the production of TNFα (A, C) and IL-6 (B, D) by ELISA. “*” represents p<0.05. Data are representative of at least three separate experiments.
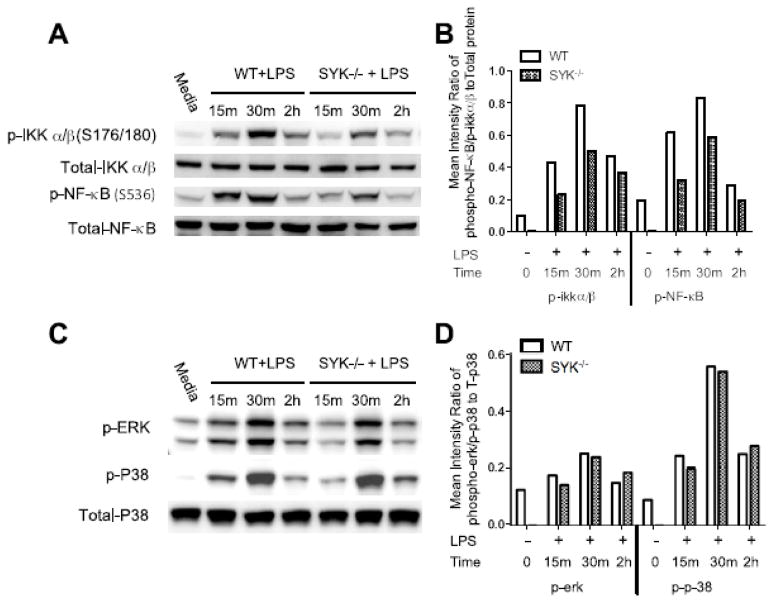
Syk deficiency leads to the decreased phosphorylation of NF-kB
NF-κB and MAPK are prototypic inflammatory signaling molecules in TLR-initiated responses. As shown in Figure 8 A and B, Syk deficiency robustly decreases phosphorylation of IKKβ and NF-κB p65, as compared with wild type control. Interestingly, Syk deficiency did not alter the phosphorylation of MAPK signaling components (Fig. 8 C, D). These results suggest that deficiency in Syk selectively alters the activity of downstream signaling pathways. Considering the critical role of NF-κB in the production of TNFα and IL-6 [39–41], Syk-deficiency mediated decreases of TNFα and IL-6 might be attributed to diminished phosphorylation of NF-κB in LPS-stimulated BMDCs.
Figure 8. Syk deficiency reduces phosphorylation of IKKβ and NF-κB p65 but not phosphorylation of MAPK signaling.
Cell lysates were collected from LPS-stimulated wild type and Syk-deficient BMDCs at the time points indicated for Western blot analysis. Blots were probed with the antibodies to phospho-and total IKKβ, NF-κBp65 (A, B), P38, ERK (C, D), and GAPDH as a loading control. (B, D) Densitometric quantification of the ratio of phospho- to total proteins.
Discussion
The function of Syk in the regulation of TLR4-mediated production of IFNβ and inflammatory cytokines is controversial. Here, we demonstrate that deficiency of Syk enhances the production of TLR4-mediated IFNβ and IL-10, and concurrently suppresses pro-inflammatory cytokine levels including TNFα and IL-6 in LPS-stimulated dendritic cells. Moreover, we found deficiency of Syk enhances activity of the PI3K-Akt signaling pathway and in turn phospho-inactivates GSK3β, which leads to an increase of IFNβ and IL-10 production but has no notable effect on the production of TNFα and IL-6 in BMDCs stimulated with LPS. In addition, deficiency of Syk attenuated phosphorylation of IKKβ and NF-κB, but not the MAPK pathway, suggesting Syk deficiency selectively alters activity of downstream signaling pathways, and results in the decrease of TNFα and IL-6 possibly through suppressing the activity of NF-κB. These findings clarify the negative regulatory role of Syk in IFNβ and IL-10 production, highlighting the pro-inflammatory property of Syk, and elucidating the molecular mechanism responsible for this regulation in TLR4-mediated inflammatory responses.
Our previous studies have demonstrated stimulation of TLR4 can activate PI3K-Akt and in turn phospho-inactivate GSK3β, which resulted in the decrease of TLR4-mediated pro-inflammatory cytokine production and the increase of IFNβ and anti-inflammatory cytokines such as IL-10 [11, 13, 35]. Although the downstream transcription factors such as NF-κB, CREB, ATF2/c-Jun have been well defined, the upstream signaling events of PI3K remain unclear. In this current study, we found Syk deficiency leads to the increased phosphorylation of P85, Akt, and GSK3β in LPS stimulated dendritic cells. This result differs from previous reports that Syk is required for activation of PI3K signaling through binding with ITAM-containing immunoreceptors or adaptor molecules [19, 42] and suggests context-dependent Syk function. The mechanism by which Syk deficiency enhances PI3K activity remains to be established, however one possible explanation is TLR4-activation of PI3K mediated by other molecules that are negatively regulated by Syk. Support for this is provided by a recent study showing that Syk is required for Cbl-mediated degradation of MyD88, which was reported to associate with P85 through its YXXM domain and thus activate PI3K upon LPS stimulation [28, 43]. Another possibility is the existence of “inhibitor ITAM” pathways, which lead to the association of Syk with P85 and in turn suppress PI3K activity [43, 44]. Nevertheless, accumulating studies on the regulatory effects of Syk-mediated PI3K signaling highlight its key role in regulating TLR4-mediated inflammatory responses [28, 43, 44], and the various effects of Syk also suggest pleiotropic functions and versatility of this pathway in different contexts.
Syk contains tandem SH2 and C-terminal kinase domains [45]. The tandem SH2 domains of Syk selectively bind to the ITAM of the cytoplasmic region of immune receptors, propagate intracellular signaling, and, therefore, play critical roles in lymphocyte development and activation of immune cells [19]. Deletion of Syk has been reported to impair B cell differentiation, suppress the natural cytotoxicity of NK cells and abrogate the phagocytic ability of macrophages [19, 46]. Moreover, Syk is critical for tyrosine phosphorylation of various proteins which regulate multiple ITAM-mediated important physical processes, such as mobilization of Ca2+, release of reactive oxygen species, activation of platelets, metabolism of arachidonic acid and production of cytokines [47–51]. In addition, studies have revealed that Syk is also essential for the as generation of osteoclasts and suppression of tumor growth [52, 53]. Despite numerous studies reporting that Syk involvement in regulating the production of inflammatory mediators through binding with ITAM-containing immunoreceptors, the regulatory effects and related molecular mechanism remains poorly understood. Whereas some studies suggest Syk was required for TLR- or TNF-mediated inflammatory responses, other groups obtained opposite findings. A recent study reported inhibition of Syk enhances pro-inflammatory cytokine production and reduces type I IFN levels upon LPS stimulation [29], in contrast to another study of the effect of Syk on type I IFN production [28]. Of interest is the reported molecular mechanisms responsible for the regulatory function of Syk were totally different. In our current study, using Syk deficient dendritic cells, we found that loss of Syk resulted in an increase of IFNβ and IL-10 production through enhancing activation of PI3K-Akt signaling and thus phospho-inactivating GSK3β. Moreover, Syk deficiency suppression of TLR4-mediated pro-inflammatory cytokine production may be attributed to a decrease in NF-κB signaling. Considering multiple ITAM containing adaptor molecules such as MyD88, Cbl, are all interact with Syk, the regulatory effects and related molecular mechanisms of Syk are likely dependent on a number of, rather than an individual, spatial and temporal factors, such as the cell type, adaptor molecules associated, the property of stimulus, and duration of stimulation.
Activation of PI3K-Akt-GSK3β pathway has been demonstrated to restrain the duration and intensity of TLR-mediated inflammation responses [11, 54]. Inhibition of PI3K or Akt enhances the production of pro-inflammatory cytokine such as IL-12, TNFα, IL-6, and concurrently decreases the level of anti-inflammatory cytokines such as IL-10 in human innate immune cells stimulated with TLR agonists [55, 56]. In our current study, we utilized specific inhibitors of PI3K and gain-of-function of Akt to examine the influences of PI3K signaling on the production of IL-12, TNFα and IL-6 in LPS-stimulated BMDCs from Syk-deficient mice. Our data showed inhibition of PI3K/Akt slightly decreased LPS-induced TNFα and IL-6, which differs from the role of PI3K/Akt in human innate immune cells. Although there is strong evidence suggesting IL-12 is regulated by the PI3K pathway [11, 36], we also didn’t observe a significant increase of IL-12 in Syk deficient BMDCs (Supplemental Fig 1A, B). In this regard, other studies have also reported different effects of PI3K/Akt signaling on TLR-mediated pro-inflammatory cytokine production. For example, inhibition of PI3K/Akt suppressed LPS-induced expression of TNFα in macrophage [57], and inhibition of PI3K-Akt or overexpression its downstream kinase GSK3β attenuated production of TNFα in LPS stimulated cardiomyocytes [58]. These studies substantiate our finding and provide evidence for varying effects of PI3K on TLR-mediated pro-inflammatory cytokine production, suggesting contribution of PI3K/Akt pathway to expression of pro-inflammatory cytokines may be cell or tissue specific.
In contrast to the effect of Syk deficiency in BMDCs, we found that Syk deficiency decreases LPS-induced production of IFNβ in BMDMs (Supplemental Fig 1C), which is consistent with several previous studies [25, 29]. Additionally, we found the influence of Syk deficiency on the production of TNFα and IL-6 is different in macrophages and dendritic cells. In contrast to dendritic cells, in LPS-stimulated macrophages Syk deficiency results in a significant decrease of IL-10, as reported previously by others [29], but has no significant effect on the production of TNFα and IL-6 (Supplemental Fig 1D). We next utilized neutralizing IL-10 antibodies to evaluate the possible influence of de novo synthesized IL-10 on the production of IFNβ. The inability of IL-10 antibodies to modulate IFNβ levels (Supplemental Fig 1F) suggests that the effect of Syk on LPS-induced IFNβ was not through paracrine signaling in macrophages. Interestingly, the changes of the IFNβ and IL-10 were synchronous in BMDMs and BMDCs. Since our previous studies have demonstrated GSK3β negatively regulates IFNβ and IL-10 [12, 13], and GSK3β is also critical for the development and maturation of BMDCs [59], we postulate that the expression level of GSK3β could be responsible for the distinct effects of Syk on IFNβ production in BMDCs and BMDMs. Silencing of Syk in BMDMs, unlike in BMDCs, decreased phosphorylation of GSK3β upon LPS stimulation (Supplemental Figure 1E), suggesting altered levels of GSK3β in Syk deficient macrophages, resulting from signaling possibly independent of PI3K, could be the reason for the distinct effects of Syk on the production of IFNβ in BMDMs. Collectively, these results indicate that TLR-mediated activation of Syk impinges on the production of IFNβ and other inflammatory cytokines through distinct signaling pathways, and the level of GSK3β is critical for the production of IFNβ and IL-10.
In this study, we found neutralization of IFNβ was unable to enhance production of TNFα and IL-6, and neutralization of IL-10 only elevated TNFα in Syk deficient BMDCs stimulated with LPS, indicating Syk deficiency impacts other signaling pathways which contribute to the decrease of TLR4-mediated pro-inflammatory cytokine production. Our data showed Syk deficiency attenuated phosphorylation of IKKβ and NF-κB rather than MAPK-P38 in LPS-stimulated BMDCs. Due to the well-established function of the NF-κB signaling pathway in the control of the production of pro-inflammatory cytokines including TNFα and IL-6 in innate immune cells, these data strongly suggest decreased TNFα and IL-6 in Syk deficient BMDCs may be attributed to this pathway. Moreover, differential effects of Syk deficiency on the NF-κB and MAPK pathways, in combination with enhanced activity of PI3K, demonstrated the ability of Syk to modulate multiple TLR-mediated signaling pathways and suggest Syk selectively activated pathway(s) may control the production of inflammatory cytokines and consequently play an immunomodulatory role in immune responses.
In summary, our study demonstrated Syk negatively regulates production of TLR4-mediated IFNβ and IL-10 through modifying the activity of PI3K-Akt and the downstream GSK3β. We also established the pro-inflammatory nature of Syk, which is essential for the production of TNFα and IL-6 in BMDCs stimulated with LPS. Moreover, we found Syk deficiency reduced pro-inflammatory cytokine levels were not directly dependent on the activity of PI3K in LPS-stimulated dendritic cells. These findings demonstrated the negative regulatory effects of Syk on IFNβ and IL-10, and the essential role of Syk in TLR4-mediated immune responses, suggesting the immunomodulatory potential of Syk could be a novel target for intervening in the process of inflammatory immune responses.
Supplementary Material
(A, B) The production of IL-12p70 and IL-12p40 from the supernatants of LPS-stimulated WT and Syk deficient BMDCs for 24 h was determined by ELISA; BMDMs were transfected with control or syk siRNA for 48 h followed by a 24 h LPS (1 μg/ml) stimulation. The production of IFNβ (C) and other cytokines (D) was determined by ELISA, (E) Western blots of whole cell lysates of LPS-stimulated cells were probed with antibodies to phospho-GSK3β and GAPDH as a loading control. (F) Syk deficient BMDMs were pre-treated with IL-10 neutralizing antibody or isotype control for 2 h, then stimulated with LPS (1 μg/ml) for 24 h. Cell free supernatants were collected to determine the production of IFNβ by ELISA. “*” and “***” represents p<0.05 and p<0.001, respectively. Data are representative of at least three separate experiments.
Highlights.
Syk deficiency enhances the production of IFNβ and IL-10 through promoting the activity of PI3K-Akt-GSK3β signaling.
Syk deficiency decreases the production of pro-inflammatory cytokines through suppressing the activity of NF-κB.
Syk is a novel innate regulator and potential intervention target for the control of inflammatory responses.
Acknowledgments
This research was supported by grants DE023633 (HW), DE017921, DE011111 (RJL), and DE017680 (DAS) from National Institute of Dental and Craniofacial Research, NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horvath CM. Weapons of STAT destruction. Interferon evasion by paramyxovirus V protein. Eur J Biochem. 2004;271:4621–4628. doi: 10.1111/j.1432-1033.2004.04425.x. [DOI] [PubMed] [Google Scholar]
- 2.Takaoka A, Yanai H. Interferon signalling network in innate defence. Cell Microbiol. 2006;8:907–922. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi T, Takaoka A. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr Opin Immunol. 2002;14:111–116. doi: 10.1016/s0952-7915(01)00305-3. [DOI] [PubMed] [Google Scholar]
- 4.Garlanda C, Di Liberto D, Vecchi A, La Manna MP, Buracchi C, Caccamo N, Salerno A, Dieli F, Mantovani A. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J Immunol. 2007;179:3119–3125. doi: 10.4049/jimmunol.179.5.3119. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill LA. ‘Fine tuning’ TLR signaling. Nat Immunol. 2008;9:459–461. doi: 10.1038/ni0508-459. [DOI] [PubMed] [Google Scholar]
- 6.Hertzog PJ, Williams BR. Fine tuning type I interferon responses. Cytokine Growth Factor Rev. 2013;24:217–225. doi: 10.1016/j.cytogfr.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 8.Balachandran S, Beg AA. Defining emerging roles for NF-kappaB in antivirus responses: revisiting the interferon-beta enhanceosome paradigm. PLoS Pathog. 2011;7:e1002165. doi: 10.1371/journal.ppat.1002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panne D, Maniatis T, Harrison SC. An atomic model of the interferon-beta enhanceosome. Cell. 2007;129:1111–1123. doi: 10.1016/j.cell.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thanos D, Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- 11.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Brown J, Garcia CA, Tang Y, Benakanakere MR, Greenway T, Alard P, Kinane DF, Martin M. The role of glycogen synthase kinase 3 in regulating IFN-beta-mediated IL-10 production. J Immunol. 2011;186:675–684. doi: 10.4049/jimmunol.1001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Garcia CA, Rehani K, Cekic C, Alard P, Kinane DF, Mitchell T, Martin M. IFN-beta production by TLR4-stimulated innate immune cells is negatively regulated by GSK3-beta. J Immunol. 2008;181:6797–6802. doi: 10.4049/jimmunol.181.10.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Brown J, Gu Z, Garcia CA, Liang R, Alard P, Beurel E, Jope RS, Greenway T, Martin M. Convergence of the mammalian target of rapamycin complex 1- and glycogen synthase kinase 3-beta-signaling pathways regulates the innate inflammatory response. J Immunol. 2011;186:5217–5226. doi: 10.4049/jimmunol.1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 17.Elder ME, Lin D, Clever J, Chan AC, Hope TJ, Weiss A, Parslow TG. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–1599. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 18.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 19.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abtahian F, Bezman N, Clemens R, Sebzda E, Cheng L, Shattil SJ, Kahn ML, Koretzky GA. Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells. Mol Cell Biol. 2006;26:6936–6949. doi: 10.1128/MCB.01040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham DB, Stephenson LM, Lam SK, Brim K, Lee HM, Bautista J, Gilfillan S, Akilesh S, Fujikawa K, Swat W. An ITAM-signaling pathway controls cross-presentation of particulate but not soluble antigens in dendritic cells. J Exp Med. 2007;204:2889–2897. doi: 10.1084/jem.20071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhary A, Fresquez TM, Naranjo MJ. Tyrosine kinase Syk associates with toll-like receptor 4 and regulates signaling in human monocytic cells. Immunol Cell Biol. 2007;85:249–256. doi: 10.1038/sj.icb7100030. [DOI] [PubMed] [Google Scholar]
- 23.Yamada T, Lizhong S, Takahashi N, Kubo S, Narita N, Suzuki D, Takabayashi T, Kimura Y, Fujieda S. Poly(I:C) induces BLyS-expression of airway fibroblasts through phosphatidylinositol 3-kinase. Cytokine. 2010;50:163–169. doi: 10.1016/j.cyto.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis e Sousa C. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Weiss G, Maaetoft-Udsen K, Stifter SA, Hertzog P, Goriely S, Thomsen AR, Paludan SR, Frokiaer H. MyD88 drives the IFN-beta response to Lactobacillus acidophilus in dendritic cells through a mechanism involving IRF1, IRF3, and IRF7. J Immunol. 2012;189:2860–2868. doi: 10.4049/jimmunol.1103491. [DOI] [PubMed] [Google Scholar]
- 26.Arndt PG, Suzuki N, Avdi NJ, Malcolm KC, Worthen GS. Lipopolysaccharide-induced c-Jun NH2-terminal kinase activation in human neutrophils: role of phosphatidylinositol 3-Kinase and Syk-mediated pathways. J Biol Chem. 2004;279:10883–10891. doi: 10.1074/jbc.M309901200. [DOI] [PubMed] [Google Scholar]
- 27.Lin YC, Huang DY, Chu CL, Lin WW. Anti-inflammatory actions of Syk inhibitors in macrophages involve non-specific inhibition of toll-like receptors-mediated JNK signaling pathway. Mol Immunol. 2010;47:1569–1578. doi: 10.1016/j.molimm.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11:734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- 29.Lin YC, Huang DY, Chu CL, Lin YL, Lin WW. The tyrosine kinase Syk differentially regulates Toll-like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3. Sci Signal. 2013;6:ra71. doi: 10.1126/scisignal.2003973. [DOI] [PubMed] [Google Scholar]
- 30.Singer K, Morris DL, Oatmen KE, Wang T, DelProposto J, Mergian T, Cho KW, Lumeng CN. Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLoS One. 2013;8:e57929. doi: 10.1371/journal.pone.0057929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 32.Hajishengallis G, Ratti P, Harokopakis E. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. J Biol Chem. 2005;280:38902–38913. doi: 10.1074/jbc.M507326200. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Pelfrey CM, Cotleur A, Lee JC, Rudick RA. Immunomodulatory effects of interferon beta-1a in multiple sclerosis. J Neuroimmunol. 2001;112:153–162. doi: 10.1016/s0165-5728(00)00403-3. [DOI] [PubMed] [Google Scholar]
- 34.van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Brown J, Gao S, Liang S, Jotwani R, Zhou H, Suttles J, Scott DA, Lamont RJ. The role of JAK-3 in regulating TLR-mediated inflammatory cytokine production in innate immune cells. J Immunol. 2013;191:1164–1174. doi: 10.4049/jimmunol.1203084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 37.Chang EY, Guo B, Doyle SE, Cheng G. Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J Immunol. 2007;178:6705–6709. doi: 10.4049/jimmunol.178.11.6705. [DOI] [PubMed] [Google Scholar]
- 38.Kuwata H, Watanabe Y, Miyoshi H, Yamamoto M, Kaisho T, Takeda K, Akira S. IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-alpha production in macrophages. Blood. 2003;102:4123–4129. doi: 10.1182/blood-2003-04-1228. [DOI] [PubMed] [Google Scholar]
- 39.Onnureddy K, Ravinder, Onteru SK, Singh D. IGF-1 attenuates LPS induced pro-inflammatory cytokines expression in buffalo (Bubalus bubalis) granulosa cells. Mol Immunol. 2015;64:136–143. doi: 10.1016/j.molimm.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Schow SR, Joly A. N-acetyl-leucinyl-leucinyl-norleucinal inhibits lipopolysaccharide-induced NF-kappaB activation and prevents TNF and IL-6 synthesis in vivo. Cell Immunol. 1997;175:199–202. doi: 10.1006/cimm.1996.1061. [DOI] [PubMed] [Google Scholar]
- 41.Xie S, Liu B, Fu S, Wang W, Yin Y, Li N, Chen W, Liu J, Liu D. GLP-2 suppresses LPS-induced inflammation in macrophages by inhibiting ERK phosphorylation and NF-kappaB activation. Cell Physiol Biochem. 2014;34:590–602. doi: 10.1159/000363025. [DOI] [PubMed] [Google Scholar]
- 42.Jiang K, Zhong B, Gilvary DL, Corliss BC, Vivier E, Hong-Geller E, Wei S, Djeu JY. Syk regulation of phosphoinositide 3-kinase-dependent NK cell function. J Immunol. 2002;168:3155–3164. doi: 10.4049/jimmunol.168.7.3155. [DOI] [PubMed] [Google Scholar]
- 43.Laird MH, Rhee SH, Perkins DJ, Medvedev AE, Piao W, Fenton MJ, Vogel SN. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol. 2009;85:966–977. doi: 10.1189/jlb.1208763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sada K, Takano T, Yanagi S, Yamamura H. Structure and function of Syk protein-tyrosine kinase. J Biochem. 2001;130:177–186. doi: 10.1093/oxfordjournals.jbchem.a002970. [DOI] [PubMed] [Google Scholar]
- 46.Brumbaugh KM, Binstadt BA, Billadeau DD, Schoon RA, Dick CJ, Ten RM, Leibson PJ. Functional role for Syk tyrosine kinase in natural killer cell-mediated natural cytotoxicity. J Exp Med. 1997;186:1965–1974. doi: 10.1084/jem.186.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beaven MA, Baumgartner RA. Downstream signals initiated in mast cells by Fc epsilon RI and other receptors. Curr Opin Immunol. 1996;8:766–772. doi: 10.1016/s0952-7915(96)80002-1. [DOI] [PubMed] [Google Scholar]
- 48.Blanco-Menendez N, Del Fresno C, Fernandes S, Calvo E, Conde-Garrosa R, Kerr WG, Sancho D. SHIP-1 Couples to the Dectin-1 hemITAM and Selectively Modulates Reactive Oxygen Species Production in Dendritic Cells in Response to Candida albicans. J Immunol. 2015 doi: 10.4049/jimmunol.1402874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 50.Kurosaki T, Tsukada S. BLNK: connecting Syk and Btk to calcium signals. Immunity. 2000;12:1–5. doi: 10.1016/s1074-7613(00)80153-3. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki-Inoue K. Essential in vivo roles of the platelet activation receptor CLEC-2 in tumour metastasis, lymphangiogenesis and thrombus formation. J Biochem. 2011;150:127–132. doi: 10.1093/jb/mvr079. [DOI] [PubMed] [Google Scholar]
- 52.Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci U S A. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coopman PJ, Mueller SC. The Syk tyrosine kinase: a new negative regulator in tumor growth and progression. Cancer Lett. 2006;241:159–173. doi: 10.1016/j.canlet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Kumar A, Lamont RJ, Scott DA. GSK3beta and the control of infectious bacterial diseases. Trends Microbiol. 2014;22:208–217. doi: 10.1016/j.tim.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harokopakis E, Albzreh MH, Martin MH, Hajishengallis G. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J Immunol. 2006;176:7645–7656. doi: 10.4049/jimmunol.176.12.7645. [DOI] [PubMed] [Google Scholar]
- 56.Brown J, Wang H, Suttles J, Graves DT, Martin M. Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1. J Biol Chem. 2011;286:44295–44305. doi: 10.1074/jbc.M111.258053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, Han SK, Park SM, Park JH, Park HI, Sung JM, Jang Y, Chung N, Hwang KC, Kim TW. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol. 2006;545:192–199. doi: 10.1016/j.ejphar.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 58.Shen E, Fan J, Peng T. Glycogen synthase kinase-3beta suppresses tumor necrosis factor-alpha expression in cardiomyocytes during lipopolysaccharide stimulation. J Cell Biochem. 2008;104:329–338. doi: 10.1002/jcb.21629. [DOI] [PubMed] [Google Scholar]
- 59.Rodionova E, Conzelmann M, Maraskovsky E, Hess M, Kirsch M, Giese T, Ho AD, Zoller M, Dreger P, Luft T. GSK-3 mediates differentiation and activation of proinflammatory dendritic cells. Blood. 2007;109:1584–1592. doi: 10.1182/blood-2006-06-028951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A, B) The production of IL-12p70 and IL-12p40 from the supernatants of LPS-stimulated WT and Syk deficient BMDCs for 24 h was determined by ELISA; BMDMs were transfected with control or syk siRNA for 48 h followed by a 24 h LPS (1 μg/ml) stimulation. The production of IFNβ (C) and other cytokines (D) was determined by ELISA, (E) Western blots of whole cell lysates of LPS-stimulated cells were probed with antibodies to phospho-GSK3β and GAPDH as a loading control. (F) Syk deficient BMDMs were pre-treated with IL-10 neutralizing antibody or isotype control for 2 h, then stimulated with LPS (1 μg/ml) for 24 h. Cell free supernatants were collected to determine the production of IFNβ by ELISA. “*” and “***” represents p<0.05 and p<0.001, respectively. Data are representative of at least three separate experiments.