Abstract
DNA topoisomerases are important cellular enzymes found in almost all types of living cells (eukaryotic and prokaryotic). These enzymes are essential for various DNA metabolic processes e.g. replication, transcription, recombination, chromosomal decatenation etc. These enzymes are important molecular drug targets and inhibitors of these enzymes are widely used as effective anticancer and antibacterial drugs. However, topoisomerase inhibitors have some therapeutic limitations and they exert serious side effects during cancer chemotherapy. Thus, development of novel anticancer topoisomerase inhibitors is necessary for improving cancer chemotherapy. Nature serves as a repertoire of structurally and chemically diverse molecules and in the recent years many DNA topoisomerase inhibitors have been identified from natural sources. The present review discusses anticancer properties and therapeutic importance of eighteen recently identified natural topoisomerase inhibitors (from the year 2009 to 2015). Structural characteristics of these novel inhibitors provide backbones for designing and developing new anticancer drugs.
Keywords: DNA topoisomerases, Topoisomerase inhibitors, Anticancer agents, Natural products
1. INTRODUCTION
Since ancient times nature has been a prime source of various therapeutic molecules including anticancer agents [1, 2]. Natural compounds have diverse chemical structures and important anticancer chemotherapeutic agents such as etoposide, anthracyclines like doxorubicin (adriamycin or ADR) and daunorubicin, camptothecin derivatives like topotecan and irinotecan, paclitaxel, docetaxel, vincristine, vinblastine, mitomycin C, actinomycin D, bleomycin, and many more have been developed or derived from natural sources [1-3] camptothecin derivatives like topotecan and irinotecan, paclitaxel, docetaxel, vincristine, vinblastine, mitomycin C, actinomycin D, bleomycin, and many more have been developed or derived from natural sources [1-3]. Original natural compounds may not always serve as effective anticancer agents in therapy but they provide the skeleton to develop therapeutically effective anticancer drugs. Approximately 60% of the anticancer drugs currently used in the cancer chemotherapy have natural origin [1, 2]. Thus, nature serves as a repertoire for anticancer molecules and identification of new anticancer molecules from natural sources will help to improve cancer chemotherapy [1-3].
DNA topoisomerases are essential enzymes that control the topological state of DNA during DNA replication, transcription, recombination and chromosomal decatenation to facilitate chromosomal segregation during mitosis, as reviewed in [4-11]. Topoisomerases introduce transient single or double strand breaks in DNA and thus solve the topological problems associated with DNA metabolic processes and chromosomal decatenation during cell division, as depicted in (Figs. 1A and B), and reviewed in [4-8], single strand breaks in DNA and thus solve the topological problems associated with DNA metabolic processes and chromosomal decatenation during cell division, as depicted in (Fig. 1 and 1B), and reviewed in [4-8]. In 1971, James C. Wang first discovered DNA topoisomerase enzyme in Escherichia coli and designated it as ω protein (topA) [10]. The ω protein was found to reduce superhelical turns in the covalently closed negatively supercoiled DNA duplex. In 1972, James J. Champoux and Renato Dulbecco discovered first eukaryotic topoisomerase enzyme and found that nuclear extracts from secondary mouse embryo cells possess an enzymatic activity of untwisting closed circular DNAs containing negative or positive superhelical turns [11]. The prokaryotic and eukaryotic enzymes were found to possess distinct mechanisms of action. They were designated as prokaryotic DNA topoisomerase I (topA) and III (topB), and eukaryotic DNA
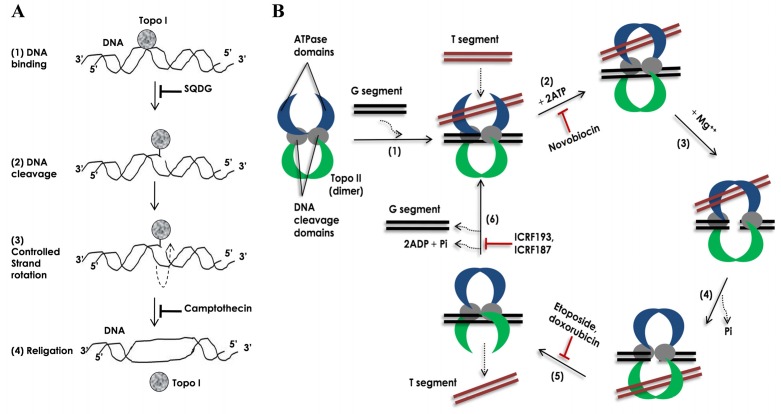
Fig. (1).
Mechanisms of action for human TOP1 and TOP2 enzymes. (A) Mechanism of action of TOP1. (1) DNA binding: first the enzyme binds to its preferential binding site on dsDNA. (2) DNA cleavage: the enzyme then cleaves one strand of double-stranded (ds) DNA by making a nucleophilic attack and forming a transient 3’-phosphotyrosyl bond. (3) Controlled strand rotation: through the single strand break generated by the enzyme other strand of the DNA (uncleaved strand) is passed in a controlled manner. (4) Religation: the cleaved strand is religated and the enzyme is released away. (B) Mechanism of action of TOP2 [5, 13]. (1) DNA binding: homo-dimer of the enzyme preferentially binds to catenated, knotted and supercoiled DNA segments. Segment of dsDNA, which is cleaved during the enzymatic reaction cycle, is termed as ‘G segment’ (‘G’ for gate) and segment of dsDNA, which is passed through the cleaved G segment, is termed as ‘T segment’ (‘T’ for transported). The enzyme first binds to G segment and then to T segment. (2) ATP binding: binding of 2 ATP molecules in the ATPpase domains changes conformations of the ATP-ase domains from open to close. Novobiocin inhibits the ATP binding. (3) DNA cleavage: in the presence of Mg++ ions, enzyme transiently cleaves G segment DNA by making nucleophilic attack and forming two 5’-phosphotyrosyl bonds with DNA backbone. (4) Strand passage: the T segment is then passed through the cleaved G segment. (5) T segment release and religation: the T segment is then released away from the enzyme and the cleaved G segment is religated back. The religation is inhibited by etoposide and doxorubicin. (6) ATP-ase domain opening and G segment release: after release of the T segment the enzyme remains in a closed clamp form. Hydrolysis of ATP drives opening of the closed clamp releasing the G segment away and making the enzyme ready for next enzymatic reaction cycle. ATPase activity of the enzyme is inhibited by bisdioxopiperazines e.g. ICRF193 and ICRF187.
topoisomerase 1 (TOP1) and 2 (TOP2), as indicated in [4, 11]. In 1976, Martin Gellert and colleagues have purified DNA gyrase, an enzyme capable of introducing superhelical turns in the DNA, from Escherichia coli [12].
Human DNA topoisomerases are now classified into two different categories: TOP1 and TOP2, as reviewed in [4]. TOP1 transiently cleave single strand of a duplex DNA while TOP2 transiently cleave both the strand of a duplex DNA, as depicted in (Fig. 1 and B). Topoisomerases of both types are further sub-divided into four different subfamilies: TOP1A, -1B, -2A and -2B. Different enzymes in a subfamily have mechanical and structural similarities, whereas enzymes from different subfamilies are mechanically and structurally different. These enzymes have several functions: to remove DNA supercoils during transcription and DNA replication; for strand breakage during recombination; for chromosome condensation; and to disentangle intertwined DNA during mitosis [4]. Two types of topoisomerases, TOP1B and TOP2A, are present in the nucleus of mammalian cells, as depicted in (Fig. 2 ), and indicated in [4, 5]. TOP2 protein has two isoforms: TOP2A and TOP2B. Enzymatic reaction cycle of topoisomerases have four common steps: (a) DNA binding: the enzyme binds to its preferential binding site on DNA, (b) DNA cleavage: nucleophilic attack on phosphodiester backbone of DNA and formation of transient 3’ or 5’ phosphotyrosyl bond(s), (c) controlled strand rotation or strand passage and (d) religation, as depicted in (Fig. 1 and B), and reviewed in [4, 5, 13, 14].
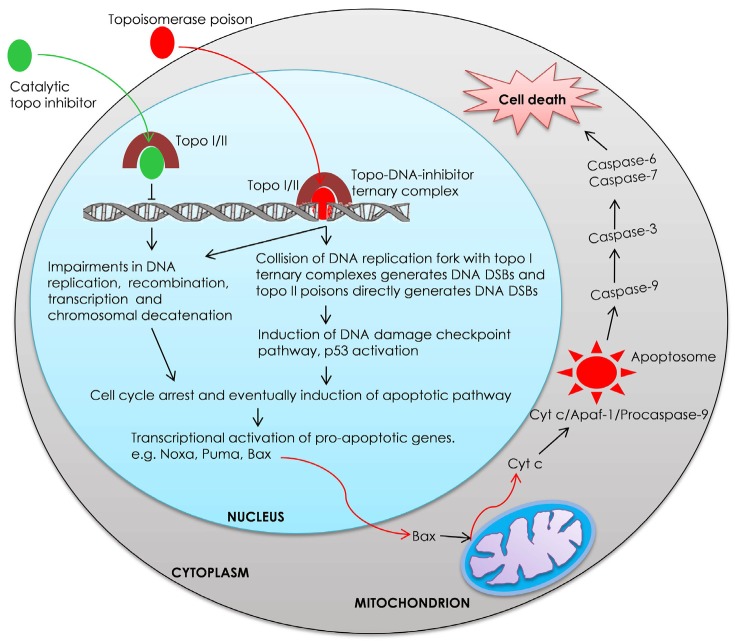
Fig. (2).
Proposed model for topoisomerase inhibitor mediated cellular death. Inhibition of DNA topoisomerases by catalytic topoisomerase inhibitors or topoisomerase poisons interferes with DNA replication, recombination, transcription and decatenation, and causes DNA damage. These generate cellular stress, lead to cell cycle arrest and ultimately induce apoptotic cell death pathway.
To facilitate rapid cell division, cancer cells require higher topoisomerase activity and indeed these enzymes have been overexpressed in numerous types of cancers [15-18]. Hence, targeting topoisomerases by small molecule inhibitors in different cancers becomes an interesting area of investigation [14, 19-23]. Till date many DNA topoisomerase inhibitors have been identified from natural sources, as reviewed in many reports regarding topoisomerase inhibitors as anticancer agents [14, 19-23]. Topoisomerase inhibitors are classified into two categories: (a) topoisomerase poisons, which inhibit either controlled strand rotation or religation, and thus stabilized covalent enzyme-DNA complexes; and (b) catalytic topoisomerase inhibitors, which inhibit DNA binding or DNA cleavage, as depicted in (Fig. 2 ), and reviewed in [13, 19, 20]. For example camptothecin, an alkaloid isolated from the bark of Chinese tree Camptotheca acuminate, acts as DNA topoisomerase I poison, and stabilizes TOP1/DNA covalent complexes by inhibiting the religation step [19-21, 24]. Stabilization of covalent enzyme-DNA complexes by topoisomerase poisons generates DNA lesions and ultimately induces apoptosis as depicted in (Fig. 2 ). Etoposide is another important topoisomerase inhibitor, which acts as a DNA topoisomerase II poison [22]. Etoposide-like fluoroquinolones, targeting both prokaryotic DNA gyrase and DNA topoisomerase 2a (top-IV, also known as parC and parE), inhibit enzymatic religation of broken DNA ends [5, 25, 26].
On the other hand, the catalytic topoisomerase inhibitors do not generate DNA lesions e.g. betulinic acid, a naturally occurring pentacyclic triterpenoid, catalytically inhibits TOP1 and TOP2 enzymes [27-29]. Catalytic inhibitors of TOP2A are known to induce G2 cell cycle arrest by the activation of decatenation checkpoint pathway [30]. Cells with defective decatenation checkpoint pathway fail to arrest the cell cycle in G2 phase and enter into M phase with catenated and under-condensed chromosomes resulting into impaired mitosis and eventually cell death [31]. Hence, catalytic inhibitors of TOP2A are also promising agents for targeting decatenation checkpoint defective cancer cells during cancer chemotherapy [32]. Since camptothecin derivatives and other DNA topoisomerase inhibitors have been extensively discussed in several review articles for their anticancer activities, therefore in this review we will discuss recently identified (from the year 2009 to 2015) natural topoisomerase inhibitors with anticancer properties.
2. DNA TOPOISOMERASES IN NORMAL AND CANCER CELLS
Since the human total genomic DNA is over 3 billion base pairs, which amount to ~ 3 meters (nearly 10 ft.) and is far greater than the ability of cell to accommodate it, the multiple mechanisms leading to DNA compaction into chromatin architecture were evolved, as reviewed in [5]. DNA topoisomerases play critical roles in chromosome compaction by working with the adenosine triphosphate (ATP)-binding-cassette ATP-hydrolases (ABC ATP-ases, also known as structural maintenance of chromosomes [SMC] proteins), as reviewed [5, 33]. SMC proteins and topoisomerases co-localize as part of the protein network that helps stabilizing long-range contacts between chromosomal segments. During DNA strand synthesis, topoisomerases are required to relieve the positive supercoiling that arises from DNA unwinding mediated by replicative helicases [34, 35]. Similarly to DNA replication, RNA transcription induces changes in DNA topology since a moving RNA polymerase produces a localized positive supercoiling ahead of the transcription [9, 36-43].
Topoisomerases have been linked to specific transcriptional events, such as the activation or repression of particular promoters, and nucleosome remodeling [38-45]. For example, inactivation of TOP1B in Saccharomyces cerevisiae leads to a specific acetylation and methylation of histones thereby increasing the transcription of telomere-proximal genes [5, 45]. The presence or absence of DNA topoisomerase Iβ (top1B) activity can influence nucleosome disassembly and assembly at the certain gene promoter regions in Schizosaccharomyces pombe [4, 5, 9, 38, 46]. Eukaryotic TOP1B has been implicated in the control of gene expression through an associated kinase activity, which is reported to phosphorylate splicing factors (e.g. serine and/or arginine-rich proteins), thus regulating their localization and enhancing their activity [5, 35, 47-49]. TOP1B -mediated phosphorylation of splicing proteins can be negatively regulated by the presence of poly (ADP-ribose), a substrate for poly (ADP-ribose) polymerase (PARP), as indicated in [5, 50]. In addition, DNA cleavage and the subsequent binding of PARP1 appear to be necessary to recruit high mobility group (HMG) factors (e.g. HMGB1 or HMGB2), leading to a stimulated transcription, as reviewed in [5, 50]. The mechanism of transcription coactivation by TOP1 was found to be dependent on basal transcription factor II (TFII) members (TFIIA and TFIID), which trigger formation of the RNA polymerase II pre-initiation complex at the specific promoters [37]. A camptothecin-mediated DNA cleavage assay demonstrated the recruitment of TOP1 to the template by TFIID [50]. Inhibition of TOP1 can cause the induction of c-JUN expression in leukemia cells, suggesting its additional role in the control of transcription [51]. TOP2B associates with the promoter regions of genes that are regulated by retinoic acid receptor elements in human acute promyelocytic leukemia cells [52]. Furthermore, TOP1 interacts with the splicing factor ASF/SF2 by which it promotes the maturation of RNA through suppressing the formation of R-loops (RNA-DNA hybrids) and prevents collision between transcription bubble and replication fork [46-48]. DNA topoisomerases are also essential for embryonic development in mammals [53]. In humans, TOP1 and TOP2 proteins bind to the regions of the pre-replicative complex in cells during the M, early G1, and G1/S phases of the cell cycle to control the firing of replication origins [37, 54, 55].
Genome instability is one of the hallmarks of cancer cells [28]. Chromosomal decatenation (the unlinking of the components of a ring or chain structure of DNA) checkpoint is critical for chromatin untangling, and is defective in cancer cells. DNA topoisomerase IIα (TOP2A) plays an important role during chromosomal decatenation [4]. As a consequence of cellular DNA replication in the S phase, catenated sister chromatids are formed [4, 30]. In order to allow proper chromosomal condensation and segregation chromatid catenations must be removed prior to onset of M phase [30]. In the G2 phase of cell cycle TOP2A decatenates chromosomal DNA [4, 30]. The process of chromosomal decatenation is monitored by a G2 phase checkpoint, named as decatenation checkpoint [30, 56]. Decatenation checkpoint delays entry into mitosis until all the chromosomes have been decatenated [30, 56]. Recent studies have shown that TOP2A is essential for the signaling of decatenation checkpoint [57, 58]. Luo and coworkers showed that TOP2A undergoes phosphorylation at the Serine-1524 position during the checkpoint activation [58]. The phosphorylated form of TOP2A interacts with and recruits MDC1 (mediator of DNA damage checkpoint protein-1) at the chromatin. This interaction between TOP2A and MDC1 is essential for the efficient activation of decatenation checkpoint [58]. Accumulating evidence shows that the decatenation checkpoint is defective in different types of cancer cells including lung cancer, leukemia, melanoma, colon cancer and bladder cancer, as described elsewhere [31, 59-62].
The gene encoding DNA topoisomerase IIα, TOP2A, is commonly amplified or deleted in human cancer cells. Abnormal alterations in the expression levels of TOP2A, its copy number variations, and its epigenetic modifications may have a critical role for genome instability in human cancers [63-68]. Eukaryotic DNA topoisomerase proteins are subjects to numerous post-translational modifications (PTMs) that can alter their localization and activities at various stages in the cell cycle [69-74]. Thus far, four types of the DNA topoisomerase-specific PTMs have been observed: phosphorylation, acetylation, sumoylation and ubiquitylation [69-74]. In the case of poisoned topoisomerases that are trapped on DNA, modification by sumoylation and ubiquitylation in response to DNA damage appears to target these enzymes for processing and degradation [75, 76].
3. TOPOISOMERASE INHIBITORS IN CURRENT CHEMOTHERAPY OF CANCER
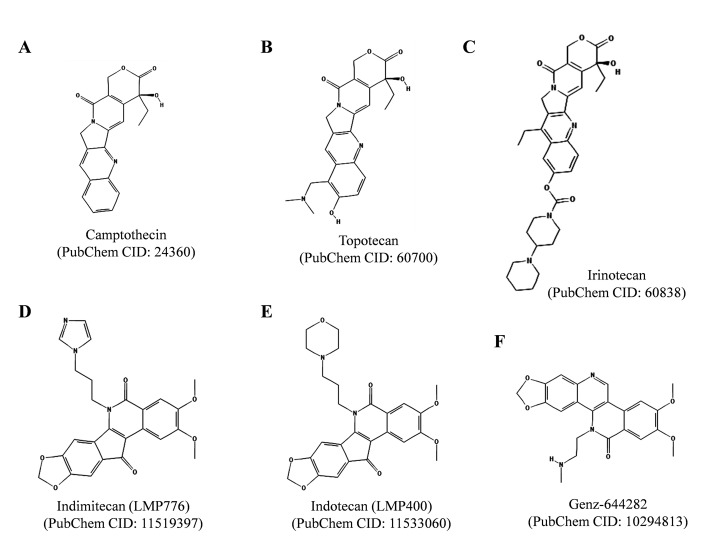
So far, the semisynthetic derivatives of plant alkaloid camptothecin, topotecan (Hycamtin) and irinotecan (Camptosar, Campto) (Fig. 3A , 3B and 3C), are only drugs targeting TOP1 approved by US Federal Drug Administration [19]. Topotecan and irinotecan are used in the chemotherapy of glioblastoma, sarcomas, ovarian, recurrent small cell lung, colorectal, and cervical cancers. But the uses of topotecan and irinotecan are limited due to their associated side effects and opening of α-hydroxylactone E-ring at physiological pH. Several modified camptothecin derivatives have been prepared but the α-hydroxylactone E-ring instability still remains a major drawback for their clinical use [19, 21].
Fig. (3).
Chemical structures of clinically important TOP1 inhibitors. Camptothecin (CPT) derivatives topotecan and irinotecan are clinically approved anticancer agents while indimitecan, indotecan and Genz-644282 are under clinical development. The structures were adopted from PubChem database. Respective PubChem CIDs are provided within the parentheses.
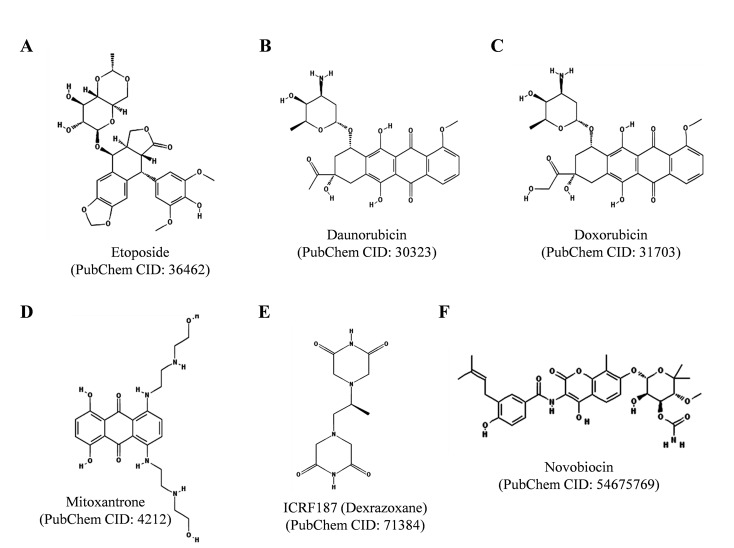
On the other hand, many TOP2 targeting drugs are currently used in the cancer chemotherapy e.g. etoposide (VP-16), daunorubicin, doxorubicin (adriamycin), mitoxantrone and ICRF187 (Dexrazoxane), as depicted in (Fig. 4). TOP2 poisons are effective anticancer agents, however their use is limited due to the associated side effects, e.g. etoposide treatment often induces therapy related secondary malignancies and anthracyclins, such as daunorubicin and doxorubicin, show severe cardiotoxicity, as indicated in [77-79]. Daunorubicin, discovered from the Streptomyces sp. soil bacteria, is used primarily for the treatment of acute leukemia and neuroblastoma in human patients [19]. Whereas doxorubicin is used for the treatment of breast cancer, sarcomas, some types of leukemia, lymphomas, multiple myeloma and thyroid cancer [19]. Etoposide is extensively used for the treatment of variety of cancers including lung cancer, testicular cancer, lymphoma, some types of leukemia, glioblastoma multiforme, Kaposi’s and Ewing’s sarcomas [19]. Mitoxantrone is used for the treatment of prostate cancer, acute leukemia and breast cancer [79]. ICRF187 (Dexrazoxane), which is a catalytic TOP2 inhibitor, is administered with anthracyclins to reduce their cardiotoxicity [19]. Novobiocin is another clinically important TOP2 catalytic inhibitor, which inhibits ATP binding to the enzyme (Fig. 4 and B), as described in [5].
Fig. (4).
Chemical structures of clinically important TOP2 inhibitors. The structures were adopted from PubChem database.
4. NOVEL TOPOISOMERASE INHIBITORS, WHICH ARE UNDER CLINICAL DEVELOPMENT
The chemical instability of camptothecin and its derivatives has been a major problem for their clinical use; therefore many non-camptothecin TOP1 inhibitors have been developed, as reviewed in [19]. Two families of such non-camptothecin inhibitors of TOP1 are under clinical development [19]. One family of such compounds is indenoisoquinolines and two of the indenoisoquinoline derivatives, indotecan (LMP400) and indimitecan (LMP776) are currently in clinical trials (Fig. 3D and 3E ) [19]. These derivatives were developed by the National Cancer Institute (National Institute of Health, MD, USA) and Purdue University (West Lafayette, Indiana, USA) and are licensed to Linus Oncology (Miami, FL, USA). The indenoisoquinolines
induced protein-linked DNA breaks, which persisted longer after drug removal than those produced by camptothecin and its derivatives; therefore they may assist in overcoming multidrug drug resistance [19, 80]. Another TOP1 inhibitor of non-camptothecin nature is 8, 9-dimethoxy-5- (2-N-methylaminoethyl)-2, 3-methylenedioxy-5H-dibenzo[c, h] [1, 6] naphthyridin-6-one (also known as Genz-644282; SAR402674), as depicted in (Fig. 3F ), and described in [19, 81]. Genz-644282 was found to be more potent than camptothecins against cancer cells in cell proliferation and colony forming assays in vitro and showed equal or superior anti-tumor activity in human tumor xenorafts in mice in vivo compared with standard drugs [19, 81]. Genz-644282 is currently under phase I clinical trial.
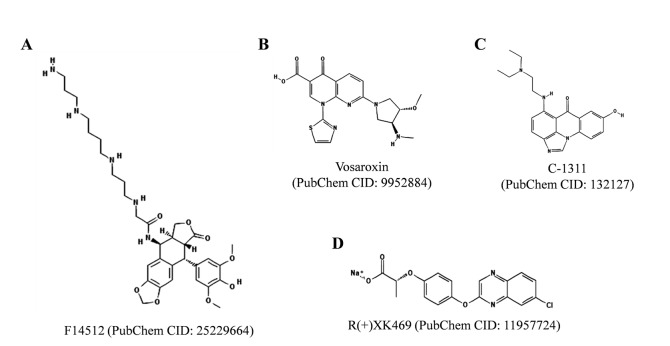
On the other hand, four TOP2 poisons, F14512, vosaroxin, C-1311, and R(+)XK469, are under clinical development (Fig. 5 ). F14512 is currently in phase I clinical trials on adult acute myeloid leukemia patients [19, 22, 82]. F14512 contains an epipodophyllotoxin moiety linked with a spermine side chain by a glycine linker [19, 22, 82]. The spermine side chain facilitates cellular uptake and accumulation of F14512 in the cancer cells expressing polyamine transport system. F14512 forms more persistent ternary TOP2-drug-DNA covalent complex than etoposide and exhibits a more superior antitumor activity of F14512 in vivo than etoposide. F14512 has been reported to exhibit in vivo synergistic anti-leukemic effects in combination with cytosine arabinoside (Ara-C), doxorubicin, gemcitabine, bortezomib, or suberoylanilide hydroxamic acid [82]. F14512 has been reported to exert synergistic effects with cisplatin and to enhance effects of ionizing radiation in head and neck squamous cell carcinomas [83]. F14512 was also found to be more effective than etoposide on pediatric glioma and neuroblastoma cell lines [84].
Fig. (5).
Chemical structures of TOP2 inhibitors under clinical development. The structures were adopted from PubChem database.
Vosaroxin (also known as voreloxin) is another TOP2 inhibitor under clinical development [22]. Vosaroxin intercalates into DNA, inhibits TOP2 and generates DNA single-stranded breaks (DSBs), as described in [22, 85-87]. Vosaroxin in combination with cytarabine exerts synergy on primary acute myeloid leukemia blasts. Vosaroxin has shown promising anticancer effects and a phase III clinical trial study for vosaroxin in combination with cytarabine (VALOR) for the treatment of relapsed and refractory acute myeloid leukemia has been completed by Sunesis Pharmaceuticals, Inc. (http://www.sunesis.com/patients_and_caregivers/clinical_trials/Vosaroxin.php), as indicated in [86, 87]. C-1311 (Symadex) is water soluble, DNA intercalating iminodazoacridinone derivatve [88-91]. C-1311 poisons TOP2 and exerts potent anticancer activity. C-1311 has undergone phase I and II clinical trials, as indicated in [22, 89, 90]. Another TOP2 inhibitor in clinical development is R(+)XK469, which was developed by the National Cancer Institute (Bethesda, MD), as indicated in [92]. It was initially reported as a selective TOP2B inhibitor, however the TOP2 targeting does not solely contribute to its cytotoxic activity and the other mechanisms have also been reported [19, 22, 92].
5. RECENTLY IDENTIFIED TOPOISOMERASE INHIBITORS FROM NATURAL SOURCES
(Table 1) lists recently identified (from the year 2009 to 2015) anti-cancer topoisomerase inhibitors from natural sources. In the following sections we shall discuss these inhibitors one-by-one.
Table 1.
Recently identified topoisomerase inhibitors from natural sources.
| Compound Name | Source | Target | Type of Inhibitor | References |
|---|---|---|---|---|
| Lamellarin D | Lamellaria sp. | TOP1 (nuclear and mitochondrial) | Poison | [93-98] |
| Erybraedin C | Bituminaria bituminosa | TOP1 | Catalytic inhibitor | [99, 100] |
| Bis(2,3-dibromo-4,5-dihydroxybenzyl) ether (BDDE) | Leathesia nana, Rhodomela confervoides, Rhodomela confervoides | TOP1 | Catalytic inhibitor | [101-103] |
| Thaspine | Croton lechleri | TOP1, TOP2 | Catalytic and poison for TOP1, not studied for TOP2 | [104, 105] |
| Evodiamine | Evodia rutaecarpa | TOP1, TOP2 | Catalytic inhibitor | [106, 107] |
| Albanol A | Morus alba | TOP1, TOP2 | Not studied | [108] |
| Alternariol | Alternaria alternate | TOP1, TOP2A, TOP2B | Poison | [110-116] |
| 4-Hydroxyderricin | Angelica keiskei | TOP2 | Not studied | [117, 118] |
| (−)-Xanthatin | Xanthium strumarium | TOP2A | Catalytic inhibitor | [120, 121] |
| Echinoside A | Holothuria nobilis | TOP2 | Interfere with DNA binding and catalytic cycle | [122, 123] |
| Riccardin D | Dumortiera hirsute | TOP2 | Not studied | [125] |
| Wedelolactone | Wedelia chinensis, Eclipta prostrata | TOP2A | Not studied | [133] |
| Tricitrinol B | Penicillium citrinum | TOP2A | Poison | [136] |
| Daurinol | Haplophyllum dauricum | TOP2A | Catalytic inhibitor | [137] |
| Fisetin | Found in many fruits and vegetables | TOP1, TOP2 | Catalytic inhibitor | [142, 143, 145] |
| Myricetin | Found in many fruits and vegetables | TOP1, TOP2 | Poison | [143, 145] |
| SQDG | Azadirachta indica | TOP1 | Catalytic inhibitor | [151] |
| Plumbagin | Plumbago Zeylanica | TOP1 | Not studied | [155] |
5.1. Lamellarin D
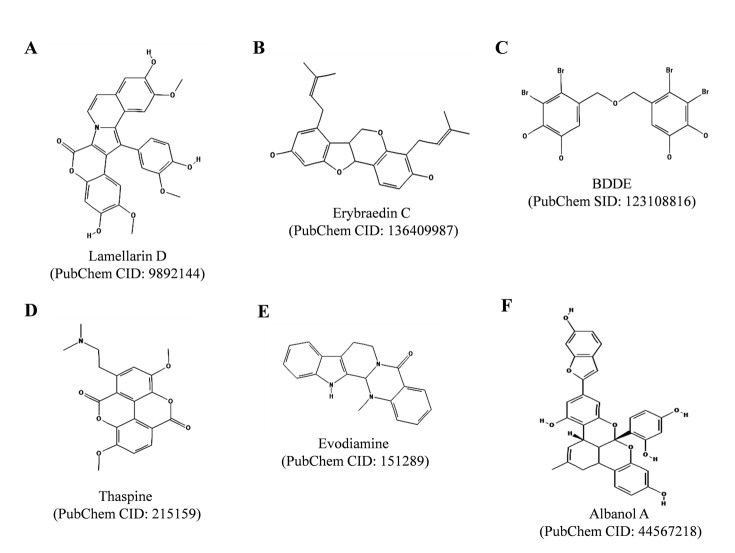
Lamellarin D (Fig. 6A ), a hexacyclic marine alkaloid found in marine mollusk of the genus Lamellaria and in ascidians, was initially reported as a nuclear TOP1 poison [93, 94]. Lamellarin D was also found to directly target mitochondria and mitochondria was reported to play essential role in the lamellarin D mediated apoptosis of cancer cells [95-98]. Khiati and co-authors have recently reported that lamellarin D also inhibits mitochondrial DNA (mtDNA) topoisomerase I (TOP1MT) and stabilizes TOP1MT/mtDNA covalent complexes, supporting the role of mitochondria in lamellarin D-mediated apoptosis in cancer cells [97]. In the same study, lamellarin D was found to rapidly accumulate inside mitochondria. Lamellarin D also inhibits protein kinases and induces mitochondrial perturbations. Thus, lamellarin D inhibits growth of cancer cells in a pleiotropic mode of action, which includes: inhibition of nuclear TOP1 and TOP1MT enzymes, inhibition of protein kinases and induction of mitochondrial perturbations. These studies suggest that lamellarin D is a potent and promising anticancer agent.
Fig. (6).
Chemical structures of Lamellarin D, Erybraedin C, BDDE, Thaspine, Evodiamine and Albanol A. The structures were adopted from PubChem database.
5.2. Erybraedin C
Erybraedin C (Fig. 6B ) is a pterocarpan isolated from the plant Bituminaria bituminosa, as described elsewhere [99]. Tesauro and co-authors have reported that erybraedin C can inhibit human TOP1 enzyme by suppressing both cleavage and religation steps of the enzyme reaction [99]. The compound has been shown to induce apoptosis in two human adenocarcinoma cell lines HT29 (proficient in mismatch repair system, MMR +/+, p53 −/− and BCL-2 +/+, a cell line) and LoVo (deficient in mismatch repair system, MMR −/−, p53 +/+ and BCL-2 −/−), as described in [100]. However, in both the cases to achieve complete enzyme inhibition pre-incubation of the enzyme with the compound was required. The study suggested that erybraedin C act as a catalytic TOP1 inhibitor in vitro.
5.3. Bis (2, 3-dibromo-4, 5-dihydroxybenzyl) Ether (BDDE)
BDDE (Fig. 6C ) is a marine bromophenol compound found in the marine algae Leathesia nana, Rhodomela confervoides and Rhodomela confervoides, as described in [101-103]. BDDE binds to DNA minor groove and act as a catalytic inhibitor of TOP1 enzyme without stabilizing covalent TOP1/DNA complexes [102]. BDDE exerts broad-spectrum in vitro anticancer activities and induces apoptosis in K562 cells via mitochondrial pathway. BDDE has also been reported to inhibit α-glucosidase activity [103].
5.4. Thaspine
Thaspine (Fig. 6D ), an alkaloid found in the cortex of South American tree Croton lechleri, was reported to inhibit human TOP1 and TOP2 enzymes in vitro, as described [104]. Thaspine was found to be effective proliferation and survival inhibitor against certain tumor cells overexpressing P-glycoprotein (PgP) or multiple drug resistance (MDR) drug efflux transporters and also shown to induce apoptosis in colon carcinoma multicellular spheroids in vitro, and in xenograft mouse models in vivo. Recently, Castelli and co-authors have reported that thaspine inhibits cleavage and religation steps of the enzymatic reaction [105]. The inhibition was found to be reversible and was enhanced upon pre-incubation. In the same study, molecular docking analysis predicted that thaspine binds in proximity of active site residues preventing the cleavage reaction while docking of with TOP1/DNA cleavable complex. These studies suggest that thaspine is a promising topoisomerase inhibitor and further chemical modifications may increase its selectivity for the inhibition type and may also increase its cytotoxic potential [105].
5.5. Evodiamine
Evodiamine is a quinazolinocarboline alkaloid (Fig. 6E ) found in Chinese medicinal plant, Evodia rutaecarpa. Evodiamine is known for its vaso-relaxation, anti-obesity, anticancer and anti-inflammatory activities, as described in [106]. Evodiamine was initially reported as a TOP1 poison. However, recently Pan and co-authors reported that evodiamine functions as dual catalytic inhibitor for human TOP1 and TOP2 enzymes with IC50 values of 60.74 and 78.81 µM, respectively [107]. Evodiamine was found to arrest cell cycle of K562 cells in G2/M phase and exerted anti-proliferative activities against human leukemia cell lines (K562, THP-1, CCRF-CEM and camptothecin-resistant cell line CCRF-CEM/C1), as indicated in [107].
5.6. Albanol A
Albanol A (also known as mulberrofuran G, Fig. 6F ), a polycyclic compound found in the root bark of Morus alba (mulberry), was reported to exhibit potent antileukemic activity on human leukemia cell line, HL60 with IC50 value of 1.7 µM and inhibitory activity on human topoisomerase II enzyme, as described elsewhere [108]. At higher concentration (100 µM) the compound was also found to inhibit relaxation activity of TOP1 enzyme. In the same study, Albanol A was also reported to induce apoptosis in HL60 cells via stimulation of cell death receptor pathway and mitochondrial pathway through caspase-2 activation and TOP2 inhibition. Recently, Geng and co-authors reported that Albanol A also inhibits DNA replication of hepatitis B virus in HepG 2.2.15 cell line in vitro [109].
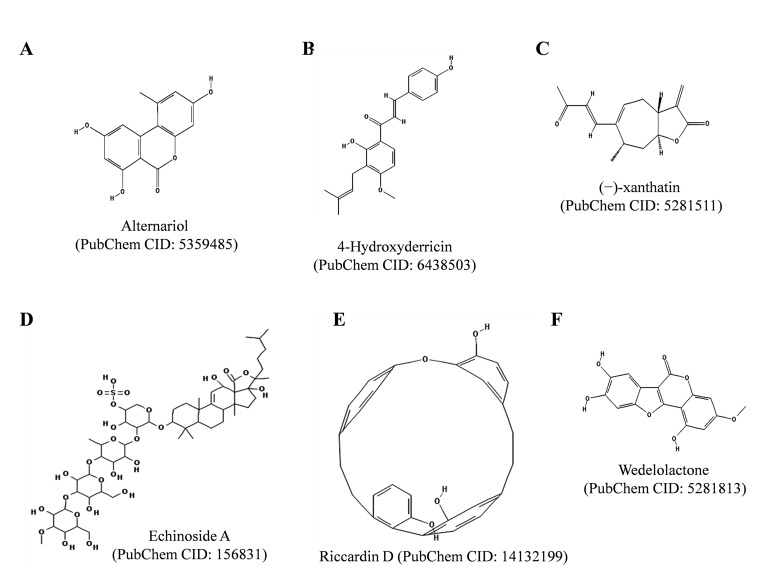
5.7. Alternariol
Alternariol (Fig. 7A ), a mycotoxin produced by Alternaria alternate, has been reported to intercalate DNA, to exert genotoxic effects and to increase rate of DNA double-strand breaks in human carcinoma cell lines, HT29 and A431, as described in [110, 111]. Alternariol inhibited in vitro DNA relaxation activities of TOP1, TOP2A and TOP2B enzymes and increased the DNA cleavage activity of these enzymes, suggesting that the toxin is a topoisomerase poison [111]. Alternariol treatment also stabilized covalent TOP1A/DNA complexes in cultured cells. The toxin was found to bind DNA minor groove and to preferentially target TOP2A enzyme. Fehr and co-authors have found that tyrosyl DNA phsphodiesterase 1 is involved in the repair of DNA damage caused by alternariol [112]. Solhaug and collaborators have shown that alternariol induces autophagy and senescence in RAW264.7 macrophage cells [113]. Alternariol has also been reported to induce G2 phase cell cycle arrest in RAW264.7 macrophage cells, chinese hamster V79 lung fibroblast cells and mouse lymphoma cells [114, 115]. However, in primary porcine endometrial cells, alternariol was found to induce G0/G1 phase cell cycle arrest [115, 116].
Fig. (7).
Chemical structures of Alternariol, 4-Hydroxyderricin, (−)-Xanthatin, Echinoside A, Riccardin D and Wedelolactone. The structures were adopted from PubChem database.
5.8. 4-Hydroxyderricin
4-Hydroxyderricin (Fig. 7B ) is a prenylated chalcone found in the roots of Angelica keiskei. 4-Hydroxyderricin has been reported to exert cytotoxic activity against cancer cell lines and antitumor and antimetastatic activities in vivo, as described elsewhere [117]. Recently, Akihisa and co-authors have reported that 4-hydroxyderricin inhibits DNA relaxation activity mediated by TOP2 with IC50 value 21.9 µM, while failed to affect DNA relaxation activity of TOP1 enzyme [118]. In a recent study, Yasuda and co-authors have shown that 4-hydroxyderricin reduces lipopolysaccharide (LPS)-mediated production of nitric oxide (NO), inhibits LPS-induced secretion of tumor necrosis factor-alpha (TNF- α) and downregulates the expression of inducible nitric oxide synthase (iNOS, also known as NOS2) and cyclooxygenase-2 (COX-2), suggesting that 4-hydroxyderricin is a promising agent for prevention of inflammatory diseases, as well [119].
5.9. (−)-Xanthatin
(−)-Xanthatin (Fig. 7C ), a major xanthanolide present in the Cocklebur plant Xanthium strumarium has been reported to stimulate caspase-independent apoptosis in human breast cancer MDA-MB-231 cells, as described elsewhere [120]. (−)-Xanthatin also inhibits catalytic activity of TOP2A enzyme, promotes DNA damage and elevates reactive oxygen species (ROS) levels, as indicated in [120]. Takeda and co-authors have concluded that inhibition of TOP2A by (−)-xanthatin triggers expression of the growth arrest and DNA-damage-inducible protein (GADD45)-γ mRNA and ROS production further enhances the GADD45γ mRNA/ GADD45γ protein induction process leading to death of breast cancer cells [121]. Thus, (−)-xanthatin is a promising anti-breast cancer agent targeting TOP2A enzyme.
5.10. Echinoside A
Echinoside A (Fig. 7D ) is a saponin isolated from the sea cucumber Holothuria nobilis Selenka, as described in [122]. Li and co-authors have reported that echinoside A is a non-intercalative TOP2A inhibitor, which inhibits the non-covalent binding of TOP2A to DNA rather than inhibiting ATP-ase activity of TOP2A. Echinoside A was found to generate TOP2A-dependent DSBs. The study indicated that echinoside A is a first marine-derived TOP2A inhibitor with the saponin skeleton. Recently, Zhao and co-authors have shown that echinoside A isolated from the sea cucumber Pearsonothuria graeffei exerts anticancer activity on HepG2 cells by blocking cell cycle progression in G0/G1 phase and inducing apoptosis via mitochondrial pathway [123]. In the same study treatment of the mice bearing H22 hepatocarcinoma tumors with echinoside A reduced tumor weight, suggesting that echinoside A exerts antitumor activities in vivo. Echinoside A was also shown to decrease pancreatic lipase activity, increased fatty acid excretion in the feces and decreased the adipose tissue accumulation in mice A, suggesting that echinoside A exert anti-obesity activity [124].
5.11. Riccardin D
Riccardin D (Fig. 7E ), a macrocyclic bisbibenzyl compound isolated from Chinese liverwort plant Dumortiera hirsute, was reported to show anti-proliferative activities and to induce apoptosis in human leukemia cell lines, HL-60, K562 and multidrug resistant (MDR) counterpart K562/A02, as described elsewhere [125]. Riccardin D was found to inhibit in vitro DNA relaxation activity of TOP2 at the greater extent than etoposide. Treatment of K562 and K562/A02 cell lines with riccardin D decreased relaxation activities of the nuclear extracts from these cell lines. The compound induced apoptosis in the leukemic cell lines in a TOP2-dependent manner, as it had no effect on the growth of TOP2-deficient HL-60/MX2 cells. Riccardin D also decreased the expression of P-glycoprotein (P-gp) in multidrug-resistant K562/A02 cells. Besides that, riccardin D has also been reported to inhibit growth of other types of cancer cell lines via different modes of action [126-128]. Riccardin D was shown to inhibit angiogenesis in lung carcinoma cells, induce apoptosis and autophagy in osteosarcoma cells and induce DNA damage in PC-3 prostate cancer cells [126-128]. Riccardin D widely affected growth of different types of cancer cells including multidrug-resistant leukemic cells, thus the compound has marked therapeutic potential.
5.12. Wedelolactone
Wedelolactone (Fig. 7F ), a coumestan found in the plants of the Asteraceae family (Wedelia chinensis, Eclipta prostrata), as described else where [129]. Wedelolactone has been reported to block androgen receptor function, to block the inhibitor of kappa B kinase (IKK), to induce caspase dependent apoptosis in prostate cancer cells and to inhibit RNA polymerase activity of hepatitis C virus [129-132]. Benes and co-authors have found that wedelolactone inhibits DNA relaxation and decatenation activities of TOP2A enzyme [133]. They also showed that wedelolactone arrests cell cycle in S and G2/M phases and induces DNA damage signalling in androgen receptor negative human breast cancer MDA-MB-231 cells. Wedelolactone has shown to suppresses osteoclastogenesis induced by breast cancer via decreasing AKT/mTOR signalling, thus preventing bone metastasis in breast cancer [134]. Wedelolactone also disrupts interaction between histone-lysine N-methyltransferase enzyme Enhancer of Zeste Homolog 2 (EZH2) and Polycomb protein EED, suggesting its role in pigenetic regulation of gene expression and making it a potential epigenetic drug candidate for the treatment of Polycomb Repressive Complex (PRC)-2-dependent cancer [135]. In recent years, several studies regarding the effects of wedelolactone on different cancers have been performed and the compound serves as a potential therapeutic agent [129-135].
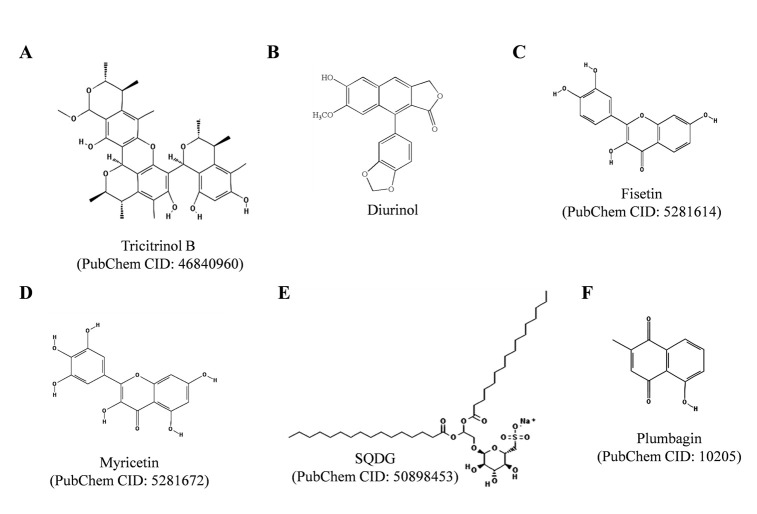
5.13. Tricitrinol B
Tricitrinol B (Fig. 8A), an unprecedented citrinin trimer, isolated from the volcano ash extract of the fungus Penicillium citrinum HGY1-5, was found to induce apoptosis in human promyelocytic leukemia HL-60 cells and human colon colorectal cancer HCT116 cells via extrinsic pathways and G2/M cell cycle arrest, as depicted in and described in [136]. In the same study tricitrinol B was reported as intercalating TOP2A poison inducing DNA damage. Tricitrinol B mainly interferes with TOP2A mediated poststrand-passage cleavage/religation equilibrium. The compound also exerted a broad-spectrum anticancer activity in vitro, in seventeen different cancer cell lines.
Fig. (8).
Chemical structures of Tricitrinol B, Diurinol, Fisetin, Myricetin, SQDG and Plumbagin. The structures were adopted from PubChem database.
5.14. Daurinol
Daurinol (Fig. 8B), an arylnaphthalene lignin isolated from traditional ethnopharmacological plant Haplophyllum dauricum has recently reported as catalytic inhibitor of TOP2A [137]. Daurinol induced cell cycle arrest at the S-phase in HCT116 cells by enhancing the expression of cyclins E and A and by activating ATM/CHK/CDC25A pathway in vitro. Daurinol displayed anti-tumor effects in vivo in nude mice xenograft models. Daurinol treatment did not lead to significant loss in body weight and haematological parameters, while etoposide treatment led to loss in body weight and changed haematological parameters i.e. decreased white and red blood cell counts and haemoglobin concentration. Daurinol did not induce nuclear enlargement in HCT116 cells, whereas etoposide induced nuclear enlargement in HCT116 cells. Recently, Kang and co-authors reported that daurinol inhibits the ATP hydrolysis activity of TOP2A enzyme and the compound has no effect on TOP1 enzyme activity [138]. In this study anti-proliferative activity of daurinol was also studied in ovarian, small cell lung and testicular cancer cell lines. In SNU-840 human ovarian cancer cell line daurinol induced S-phase arrest, increased expression of cyclins E, A and E2F-1 and did not affect the size of nucleus while etoposide increased nuclear size. In another recent study, gene expression profile in daurinol treated HCT116 cells was studied by gene expression microarray analysis [139]. The study showed that daurinol treatment decreased expression of 18 genes involved in the mitotic spindle assembly checkpoint, including aurora kinase A (AURKA) and aurora kinase B (AURKB). Daurinol was found to exert antitumor and radio-sensitizing activities in xenograft mouse models through decreased expressions of AURKA and AURKB genes [139].
5.15. Fisetin
Fisetin (Fig.8C), a polyphenolic flavonoid found in many fruits and vegetables has been shown to exert antioxidant, anticancer and anti-inflammatory activities, as depicted in (Fig. 5), and described elsewhere [140]. Fisetin has also been reported to prevent migration and invasion of human cervical cancer cells, and micronucleus induction in human lymphoblastoid TK6 cells and promyelocytic HL60 cells in vitro [141, 142]. Fisetin was reported as dual catalytic inhibitor for both TOP1 and TOP2 enzymes [143]. Recently, Sahu and co-authors have reported that fisetin exerts renoprotective effects and protects kidneys from cisplatin induced nephrotoxicity in rats [140]. Fisetin pre-treatment ameliorated cisplatin induced renal impairments, histopathological alterations, and restored antioxidant and mitochondrial respiratory activities in kidney tissues. When used along with cisplatin, fisetin increases cytotoxic potential of cisplatin in human embryonic carcinoma cells, as well as the therapeutic doses of cisplatin could be minimized during cancer chemotherapy and thus toxicity caused by cisplatin could be controlled [144].
5.16. Myricetin
Myricetin (Fig. 8D), a polyphenolic flavonoid found in many fruits and vegetables, exerts antioxidant, anticancer and anti-inflammatory properties. Myricetin acts as a dual topoisomerase inhibitor and stabilizes DNA covalent complexes with both TOP1 and TOP2 enzymes in human leukemia K562 cells [145]. Murine embryo fibroblasts lacking TOP2B were found to be resistant to myricetin induced cell death, suggesting that myricetin targets TOP2B enzyme. Besides, myricetin has also been reported to inhibit mammalian DNA polymerase and to induce G2/M cell cycle arrest and apoptosis in human colon cancer HCT116 cells [145].
5.17. Sulfonoquinovosyl Diacylglyceride (SQDG)
SQDG (Fig. 8E) is a member of plant sulfolipids. SQDG has been reported for its anti-leukemic, anti-bacterial and anti-viral activities [146-152]. Recently, Jain and co-authors have shown that SQDG purified from the leaves of Azadirachta indica catalytically inhibits human TOP1 enzyme by inhibiting DNA binding to TOP1 protein [153]. SQDG docked in the DNA binding region of TOP1 and thereby inhibited DNA binding activity of the enzyme. SQDG selectively killed acute lymphoblastic leukemia cell lines in TOP1- and tumor protein (TP) 53-dependent manner [150, 153]. In the study, SQDG was reported to generate DNA replication stress and to induce TP53-dependent apoptotic pathway in human acute lymphoblastic leukemia MOLT-4 cells [153]. SQDG treatment induced recruitment of ATR protein to the chromatin sites associated with DNA damage and arrested cell cycle at the S-phase. Downregulation of TOP1 or TP53 renders tumor cells less sensitive to SQDG, while ectopic expression of wild type TP53 protein in TP53-deficient human bone marrow chronic myelogenous leukemia (CML) K562 cells resulted in chemosensitization of tumor cells to SQDG exposure. Constant ratio combinations of SQDG with etoposide and doxorubicin were found to exert synergy on MOLT-4 cell killing. SQDG treatment delayed tumor- doubling time and reduced the expression of cell proliferation marker, Ki67, indicating in vivo anti-tumorigenic activity of SQDG. The doses of etoposide/ doxorubicin can be substantially reduced if the combination with SQDG was during acute lymphoid leukemia chemotherapy and side effects caused by these agents can also be minimized. The study also indicated that dual targeting of TOP1 and TOP2 enzymes is a promising strategy for improving chemotherapy against ALL [153].
5.18. Plumbagin
Plumbagin (Fig. 8F) is a naphthoquinone isolated from the roots of Plumbago zeylanica, Juglans regia, Juglans cinerea, and Juglans nigra [154-160]. Plumbagin exerts anti-cancer, anti-bacterial, anti-fungal, anti-inflammatory and anti-oxidant properties, as reviewed in [154, 155]. In 1992, plumbagin was reported to intercalate into DNA and induce TOP2-mediated DNA cleavage in vitro [155]. In 2009, Chen and co-workers have reported that plumbagin and its copper complexes possess TOP1 inhibitory activities in vitro [156]. Copper complexes of plumbagin showed enhanced cytotoxicity and were found to be more potent TOP1 inhibitors than plumbagin. Plumbagin displays cytotoxicity against variety of cancer cell lines including breast cancer, prostate cancer, ovarian cancer, pancreatic cancer, myeloma, lung cancer, melanoma, leukemia, renal and cervical cancers [154-160]. Plumbagin was also found to display radio-sensitizing effects and to inhibit NF-ĸB activity in cancer cells [157, 158]. Plumbagin treatment was found to induce G2/M cell cycle arrest and promotes apoptosis and autophagy in human tongue squamous cell carcinoma (TSCC) cells via p38 MAPK- and PI3K/AKT/mTOR-mediated pathways [159]. In another study, plumbagin was recently found to suppress epithelial to mesenchymal transition and stemness via inhibiting NRF2-mediated oxidative stress signalling pathway in TSCC cells [160].
5.19. Other Polyphenolic Compounds
Flavonoids and other polyphenolic compounds have been shown to inhibit human TOP1B through both inhibition of relaxation activity and through stabilization of the cleavable complex (poisoning), as reviewed in [19, 161, 162]. The most potent TOP1 poisons are the flavones and flavonols, whose activity is generally associated with DNA intercalation. Aqueous and methanolic extracts from Vitis vinifera enriched in polyphenols (caffeic acid, ferulic acid, gallic acid, protocatechuic acid and rutin) were found to act as potent inhibitors of TOP1 activity, indicating the potential mechanism for their anticancer activity [163]. These compounds also exert prooxidant activity leading to enhancement of DNA damage, which may explain their cytotoxic and apoptosis-inducing properties against cancer cells.
A flavonoid-enriched fraction isolated from the peels of Northern Spy apples was found to inhibit proliferation of human hepatocellular carcinoma HepG2 cells via a marked poisoning TOP2 catalytic activity [164]. Ellagic acid, a natural polyphenol abundant in fruits, inhibits the TOP2A and TOP2B isoforms via binding to the ATP pocket of the human DNA topoisomerase enzymes [165]. Various organic and aqueous extracts from the mulberry leaves of Morus alba L. (Moraceae) contained rutin, isoquercetin, and various derivatives of kaempferol and quercetin glycosides as their major constituents, as well as chlorogenic acid and caffeoylquinic acid derivatives [166]. These compounds were found to inhibit the growth of human hepatocellular carcinoma HepG2 cells by inducing cell cycle arrest in the G2/M phase and caspase cascade-dependent apoptosis in vitro. Furthermore, methanolic extracts reduced the level of TOP2A, but increased the level of cyclin-dependent kinase inhibitor CDKN1B (p27Kip1).
(-)-Epigallocatechin gallate, and (-)-epigallocatechin were shown to act as redox-dependent TOP2 poisons, while quercetin, and kaempferol were traditional DNA topoisomerase poisons suggesting that structural variations might account for their mechanisms [167-171]. Epicatechin gallate (IC50 = 0.029 microM), procyanidin B2 (PB2, 4.5 microM), and resveratrol (65.7 microM), constituents of the grape cell culture (TP-4), showed the marked ability to inhibit human TOP2 catalytic activity [171, 172]. Tea phenolic compounds from Ardisia compressa (e.g. gallic acid, epicatechin gallate, several proanthocyanidin dimers, kaempferol, naringenin and ardisin) were shown to inhibit proliferation of human colorectal adenocarcinoma cell lines, HT-29 and Caco-2, as described in [173]. Their cytostatic effect was found to be associated with the ability to inhibit TOP2 catalytic activity.
6. THERAPEUTIC IMPORTANCE OF RECENTLY IDENTIFIED TOPOISOMERASE INHIBITORS
The novel natural topoisomerase inhibitors discussed in this review display fascinating pre-clinical features. Chemical structures of these inhibitors offer the novel skeletons to develop modified derivatives with enhanced therapeutic efficacy and target selectivity. MtDNA is essential for mitochondria, and its mutations are known to increase the risk of cancer development [174, 175]. Thus, compound like lamellarin D, which targets both TOP1MT and TOP1, may serve as important therapeutic agent to confront a mitochondrial dysfunction shown to be associated with cancers. Erybraedin C, which is equally cytotoxic for DNA mismatch repair (MMR) system-deficient and proficient cells, may be clinically important with respect to colon cancers, as MMR deficiency is well appreciated contributor to genetic instability in human colon cancers [176].
Thaspine, which was found to be effective against cells overexpressing drug efflux transporters and induced apoptosis in multicellular spheroids in vivo, has therapeutic importance for drug resistant solid tumors [105]. Evodiamine, albanol A and riccardin D exhibited anti-leukemic activities, and thus may serve as potential anti-leukemic chemotherapeutic agents [107, 108, 125]. Riccardin D also exerted a wide spectrum of anticancer activities [126-128]. Riccardin D exerted a cytotoxic activity on multidrug resistant (MDR) K562/A02 cells, inhibited angiogenesis in lung carcinoma cells, induced apoptosis and autophagy in osteosarcoma cells and caused DNA damage in prostate cancer cells [126-128]. (−)-Xanthatin induced GADD45γ expression an ROS production in breast cancer cell line leading to cell death [120, 121].
Wedelolactone, another TOP2 inhibitor, kills androgen receptor negative breast cancer cells, suppresses osteoclastogenesis induced in breast cancer and disrupts EZH2-EED interaction and thus it is a potential drug candidate for the treatment of breast cancers and PRC2-dependent cancers [129-134]. Daurinol displayed antitumor activity and was found superior than etoposide (with respect to body weight and the haematological parameters) in xenografted tumor models [137-139]. Daurinol exhibited a wide spectrum of anticancer activities and in vivo radio-sensitizing activity [139]. Fisetin is important agent to use along with cisplatin in cancer chemotherapy as it exerts renoprotective effects, ameliorates cisplatin-induced renal impairments, protects kidneys from cisplatin-induced nephrotoxicity, and increases cytotoxic potential of cisplatin on tumor cells [141-144]. SQDG is another promising anti-leukemic agent as it exerts anti-leukemic activity in vivo and synergies with etoposide and doxorubicin [153]. Echinoside A might serve as a potential anticancer agent since it exerts antitumor activity against hepatocellular carcinoma in vivo [123].
CONCLUSION
Topoisomerase inhibitors are effective anticancer agents [19, 98]. However, they affect rapidly growing normal cells by damaging cellular DNA (e.g. etoposide and doxorubicin) and also become inactive at physiological pH (e.g. camptothecins) and thus their clinical use is limited. The novel naturally occurring topoisomerase inhibitors discussed in this review demonstrate the chemical and structural diversities of topoisomerase inhibitors with distinct mechanisms of inhibition. Structures of these inhibitors offer novel skeletons for development of more effective and targeted chemotherapeutic agents. Among these inhibitors some possesses excellent anticancer activities. However, merely identifying anticancer activities does not make them therapeutically useful anticancer drugs and more sophisticated studies on the effects of these molecules are necessary for their use in the cancer chemotherapy. Using genomics and proteomics approaches study of global cellular changes in the gene and protein expressions in response to these agents will certainly reveal their mechanisms and effects on cells and will help to better understand their chemotherapeutic potentials [177, 178]. In addition, novel drug delivery strategies have been developed in recent years, which may be utilized to enhance the anticancer efficacies of chemotherapeutic drugs and to deliver them at targeted sites with reduced side effects and increased drug availability at desired sites [179-183].
ACKNOWLEDGEMENT
We thank Dr. Samit Chattopadhyay, the Director of our Institute, for his interest in this work.
LIST OF ABBREVIATIONS
- ALL
Acute lymphoid leukemia
- ATM
Ataxia telangiectasia mutated
- ATR
Ataxia telangiectasia and Rad3-related
- BDDE
bis (2,3-dibromo-4, 5-dihydroxybenzyl) ether
- BRCA1
Breast cancer 1
- CDC25A
Cell division cycle 25 homolog A
- CHK
Checkpoint-like protein kinase
- CDK
Cyclin-dependent kinase
- COX-2
Cyclooxygenase-2
- EZH2
Enhancer of Zeste Homolog 2
- GADD
Growth arrest and DNA damage, DNA double-stranded braks, DSBs
- HMG
High mobility group
- IKK
Inhibitor of kappa B kinase
- iNOS
Inducible nitric oxide synthase
- LPS
Lipopolysaccharide
- MMR
Mismatch repair
- mtDNA
Mitochondrial DNA
- TOP1MT*
Mitochondrial TOP1
- MDR
Multidrug resistant
- NO
Nitric oxide
- PARP
Poly (ADP-ribose) polymerase
- top*
Prokaryotic DNA topoisomerase
- PTMs
Post-translational modifications
- ROS
Reactive oxygen species
- top1*
Shizosaccharomyces pombe DNA topoisomerase I
- SMC
Structural maintenance of chromosomes
- SQDG
Sulfonoquinovosyl diacylglyceride
- TOP*
Topoisomerase
- TNF-α
Tumor necrosis factor-alpha
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Cragg G.M., Newman D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005;100(1-2):72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Cragg G.M., Newman D.J. Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta. 2013;1830(6):3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson L.R., Chen H., Collins A.R., Connell M., Damia G., Dasgupta S., Malhotra M., Meeker A.K., Amedei A., Amin A., Ashraf S.S., Aquilano K., Azmi A.S., Bhakta D., Bilsland A., Boosani C.S., Chen S., Ciriolo M.R., Fujii H., Guha G., Halicka D., Helferich W.G., Keith W.N., Mohammed S.I., Niccolai E., Yang X., Honoki K., Parslow V.R., Prakash S., Rezazadeh S., Shackelford R.E., Sidransky D., Tran P.T., Yang E.S., Maxwell C.A. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin. Cancer Biol. 2015 doi: 10.1016/j.semcancer.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J.C. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 2002;3(6):430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 5.Vos S.M., Tretter E.M., Schmidt B.H., Berger J.M. All tangled up: how cells direct, manage and exploit topoisomerase function. Nat. Rev. Mol. Cell Biol. 2011;12(12):827–841. doi: 10.1038/nrm3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanagida M. Basic mechanism of eukaryotic chromosome segregation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360(1455):609–621. doi: 10.1098/rstb.2004.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belmont A.S. Mitotic chromosome structure and condensation. Curr. Opin. Cell Biol. 2006;18(6):632–638. doi: 10.1016/j.ceb.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Nitiss J.L. DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer. 2009;9(5):327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durand-Dubief M., Svensson J.P., Persson J., Ekwall K. Topoisomerases, chromatin and transcription termination. Transcription. 2011;2(2):66–70. doi: 10.4161/trns.2.2.14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J.C. Interaction between DNA and an Escherichia coli protein omega. J. Mol. Biol. 1971;55(3):523–533. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]
- 11.Champoux J.J., Dulbecco R. An activity from mammalian cells that untwists superhelical DNA - a possible swivel for DNA replication (polyoma-ethidium bromide-mouse-embryo cells-dye binding assay). Proc. Natl. Acad. Sci. USA. 1972;69(1):143–146. doi: 10.1073/pnas.69.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gellert M., Mizuuchi K., O'Dea M.H., Nash H.A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc. Natl. Acad. Sci. USA. 1976;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pommier Y., Pourquier P., Fan Y., Strumberg D. Mechanism of action of eukaryotic DNA topoisomerase I and drugs targeted to the enzyme. Biochim. Biophys. Acta. 1998;1400(1-3):83–105. doi: 10.1016/s0167-4781(98)00129-8. [DOI] [PubMed] [Google Scholar]
- 14.Pommier Y., Leo E., Zhang H., Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010;17(5):421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfister T.D., Reinhold W.C., Agama K., Gupta S., Khin S.A., Kinders R.J., Parchment R.E., Tomaszewski J.E., Doroshow J.H., Pommier Y. Topoisomerase I levels in the NCI-60 cancer cell line panel determined by validated ELISA and microarray analysis and correlation with indenoisoquinoline sensitivity. Mol. Cancer Ther. 2009;8(7):1878–1884. doi: 10.1158/1535-7163.MCT-09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fry A.M., Chresta C.M., Davies S.M., Walker M.C., Harris A.L., Hartley J.A., Masters J.R., Hickson I.D. Relationship between topoisomerase II level and chemosensitivity in human tumor cell lines. Cancer Res. 1991;51(24):6592–6595. [PubMed] [Google Scholar]
- 17.Ashour M.E., Atteya R., El-Khamisy S.F. Topoisomerase-mediated chromosomal break repair: an emerging player in many games. Nat. Rev. Cancer. 2015;15(3):137–151. doi: 10.1038/nrc3892. [DOI] [PubMed] [Google Scholar]
- 18.Chen T., Sun Y., Ji P., Kopetz S., Zhang W. Topoisomerase IIalpha in chromosome instability and personalized cancer therapy. Oncogene. 2015;34(31):4019–4031. doi: 10.1038/onc.2014.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pommier Y. Drugging topoisomerases: lessons and challenges. ACS Chem. Biol. 2013;8(1):82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer. 2006;6(10):789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 21.Pommier Y. DNA topoisomerase I inhibitors: chemistry, biology, and interfacial inhibition. Chem. Rev. 2009;109(7):2894–2902. doi: 10.1021/cr900097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailly C. Contemporary challenges in the design of topoisomerase II inhibitors for cancer chemotherapy. Chem. Rev. 2012;112(7):3611–3640. doi: 10.1021/cr200325f. [DOI] [PubMed] [Google Scholar]
- 23.Baikar S., Malpathak N. Secondary metabolites as DNA topoisomerase inhibitors: A new era towards designing of anticancer drugs. Pharmacogn. Rev. 2010;4(7):12–26. doi: 10.4103/0973-7847.65320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiang Y.H., Hertzberg R., Hecht S., Liu L.F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 1985;260(27):14873–14878. [PubMed] [Google Scholar]
- 25.Anderson V.E., Zaniewski R.P., Kaczmarek F.S., Gootz T.D., Osheroff N. Quinolones inhibit DNA religation mediated by Staphylococcus aureus topoisomerase IV. Changes in drug mechanism across evolutionary boundaries. J. Biol. Chem. 1999;274(50):35927–35932. doi: 10.1074/jbc.274.50.35927. [DOI] [PubMed] [Google Scholar]
- 26.Fisher L.M., Pan X.S. Methods to assay inhibitors of DNA gyrase and topoisomerase IV activities. Methods Mol. Med. 2008;142:11–23. doi: 10.1007/978-1-59745-246-5_2. [DOI] [PubMed] [Google Scholar]
- 27.Chowdhury A.R., Mandal S., Mittra B., Sharma S., Mukhopadhyay S., Majumder H.K. Betulinic acid, a potent inhibitor of eukaryotic topoisomerase I: identification of the inhibitory step, the major functional group responsible and development of more potent derivatives. Med. Sci. Monit. 2002;8(7):BR254–BR265. [PubMed] [Google Scholar]
- 28.Ganguly A., Das B., Roy A., Sen N., Dasgupta S.B., Mukhopadhayay S., Majumder H.K. Betulinic acid, a catalytic inhibitor of topoisomerase I, inhibits reactive oxygen species-mediated apoptotic topoisomerase I-DNA cleavable complex formation in prostate cancer cells but does not affect the process of cell death. Cancer Res. 2007;67(24):11848–11858. doi: 10.1158/0008-5472.CAN-07-1615. [DOI] [PubMed] [Google Scholar]
- 29.Wada S., Tanaka R. Betulinic acid and its derivatives, potent DNA topoisomerase II inhibitors, from the bark of Bischofia javanica. Chem. Biodivers. 2005;2(5):689–694. doi: 10.1002/cbdv.200590045. [DOI] [PubMed] [Google Scholar]
- 30.Deming P.B., Cistulli C.A., Zhao H., Graves P.R., Piwnica-Worms H., Paules R.S., Downes C.S., Kaufmann W.K. The human decatenation checkpoint. Proc. Natl. Acad. Sci. USA. 2001;98(21):12044–12049. doi: 10.1073/pnas.221430898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain C.K., Roychoudhury S., Majumder H.K. Selective killing of G2 decatenation checkpoint defective colon cancer cells by catalytic topoisomerase II inhibitor. Biochim. Biophys. Acta. 2015;1853(5):1195–1204. doi: 10.1016/j.bbamcr.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 32.Lyu Y.L., Kerrigan J.E., Lin C.P., Azarova A.M., Tsai Y.C., Ban Y., Liu L.F. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67(18):8839–8846. doi: 10.1158/0008-5472.CAN-07-1649. [DOI] [PubMed] [Google Scholar]
- 33.Hirano T. At the heart of the chromosome: SMC proteins in action. Nat. Rev. Mol. Cell Biol. 2006;7:311–322. doi: 10.1038/nrm1909. [DOI] [PubMed] [Google Scholar]
- 34.Sarbajna S., West S.C. Holliday junction processing enzymes as guardians of genome stability. Trends Biochem. Sci. 2014;39(9):409–419. doi: 10.1016/j.tibs.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Gospodinov A., Herceg Z. Chromatin structure in double strand break repair. DNA Repair (Amst.) 2013;12(10):800–810. doi: 10.1016/j.dnarep.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Tuduri S., Crabbé L., Conti C., Tourrière H., Holtgreve-Grez H., Jauch A., Pantesco V., DeVos J., Thomas A., Theillet C., Pommier Y., Tazi J., Coquelle A., Pasero P. Topoisomerase I suppresses genomic instability by preventing interference between replication and transcription. Nat. Cell Biol. 2009;11(11):1315–1324. doi: 10.1038/ncb1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoeffler A.J., Berger J.M. DNA topoisomerases: harnessing and constraining energy to govern chromosome topology. Q. Rev. Biophys. 2008;41:41–101. doi: 10.1017/S003358350800468X. [DOI] [PubMed] [Google Scholar]
- 38.Durand-Dubief M., Persson J., Norman U., Hartsuiker E., Ekwall K. Topoisomerase I regulates open chromatin and controls gene expression in vivo. EMBO J. 2010;29:2126–2134. doi: 10.1038/emboj.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merino A., Madden K.R., Lane W.S., Champoux J.J., Reinberg D. DNA topoisomerase I is involved in both repression and activation of transcription. Nature. 1993;365:227–232. doi: 10.1038/365227a0. [DOI] [PubMed] [Google Scholar]
- 40.Mondal N., Zhang Y., Jonsson Z., Dhar S.K., Kannapiran M., Parvin J.D. Elongation by RNA polymerase II on chromatin templates requires topoisomerase activity. Nucleic Acids Res. 2003;31:5016–5024. doi: 10.1093/nar/gkg705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L.F., Wang J.C. Supercoiling of the DNA template during transcription. Proc. Natl. Acad. Sci. USA. 1987;84:7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyu Y.L., Lin C.P., Azarova A.M., Cai L., Wang J.C., Liu L.F. Role of topoisomerase IIβ in the expression of developmentally regulated genes. Mol. Cell. Biol. 2006;26:7929–7941. doi: 10.1128/MCB.00617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brill S.J., DiNardo S., Voelkel-Meiman K., Sternglanz R. Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA. Nature. 1987;326:414–416. doi: 10.1038/326414a0. [DOI] [PubMed] [Google Scholar]
- 44.Ju B.G., Lunyak V.V., Perissi V., Garcia-Bassets I., Rose D.W., Glass C.K., Rosenfeld M.G. A topoisomerase IIβ-mediated dsDNA break required for regulated transcription. Science. 2006;312:1798–1802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- 45.Lotito L., Russo A., Chillemi G., Bueno S., Cavalieri D., Capranico G. Global transcription regulation by DNA topoisomerase I in exponentially growing Saccharomyces cerevisiae cells: activation of telomere-proximal genes by TOP1 deletion. J. Mol. Biol. 2008;377:311–322. doi: 10.1016/j.jmb.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 46.Baxter J., Diffley J.F. Topoisomerase II inactivation prevents the completion of DNA replication in budding yeast. Mol. Cell. 2008;30:790–802. doi: 10.1016/j.molcel.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Coelho P.A., Queiroz-Machado J., Carmo A.M., Moutinho-Pereira S., Maiato H., Sunkel C.E. Dual role of topoisomerase II in centromere resolution and aurora B activity. PLoS Biol. 2008;6:e207. doi: 10.1371/journal.pbio.0060207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossi F., Labourier E., Forné T., Divita G., Derancourt J., Riou J.F., Antoine E., Cathala G., Brunel C., Tazi J. Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature. 1996;381:80–82. doi: 10.1038/381080a0. [DOI] [PubMed] [Google Scholar]
- 49.Juge F., Fernando C., Fic W., Tazi J. The SR protein B52/SRp55 is required for DNA topoisomerase I recruitment to chromatin, mRNA release and transcription shutdown. PLoS Genet. 2010;6:e1001124. doi: 10.1371/journal.pgen.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malanga M., Czubaty A., Girstun A., Staron K., Althaus F.R. Poly (ADP-ribose) binds to the splicing factor ASF/SF2 and regulates its phosphorylation by DNA topoisomerase I. J. Biol. Chem. 2008;283:19991–19998. doi: 10.1074/jbc.M709495200. [DOI] [PubMed] [Google Scholar]
- 51.Iyama T., Wilson D.M., III DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst.) 2013;12(8):620–636. doi: 10.1016/j.dnarep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shykind B.M., Kim J., Stewart L., Champoux J.J., Sharp P.A. Topoisomerase I enhances TFIID-TFIIA complex assembly during activation of transcription. Genes Dev. 1997;11(3):397–407. doi: 10.1101/gad.11.3.397. [DOI] [PubMed] [Google Scholar]
- 53.Mialon A., Sankinen M., Söderström H., Junttila T.T., Holmström T., Koivusalo R., Papageorgiou A.C., Johnson R.S., Hietanen S., Elenius K., Westermarck J. DNA topoisomerase I is a cofactor for c-Jun in the regulation of epidermal growth factor receptor expression and cancer cell proliferation. Mol. Cell. Biol. 2005;25(12):5040–5051. doi: 10.1128/MCB.25.12.5040-5051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mcnamara S., Wang H., Hanna N., Miller W. Topoisomerase IIβ negatively modulates retinoic acid receptor a function: a novel mechanism of retinoic acid resistance. Mol. Cell. Biol. 2008;28:2066–2077. doi: 10.1128/MCB.01576-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liani-Leibson K., Har-Vardi I., Priel E. Inhibition of topoisomerase I by anti-cancer drug altered the endometrial cyclicity and receptivity. Curr. Mol. Med. 2014;14(1):141–150. doi: 10.2174/1566524013666131118111241. [DOI] [PubMed] [Google Scholar]
- 56.Abdurashidova G., Radulescu S., Sandoval O., Zahariev S., Danailov M.B., Demidovich A., Santamaria L., Biamonti G., Riva S., Falaschi A. Functional interactions of DNA topoisomerases with a human replication origin. EMBO J. 2007;26(4):998–1009. doi: 10.1038/sj.emboj.7601578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rampakakis E., Zannis-Hadjopoulos M. Transient dsDNA breaks during pre-replication complex assembly. Nucleic Acids Res. 2009;37(17):5714–5724. doi: 10.1093/nar/gkp617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Damelin M., Bestor T.H. The decatenation checkpoint. Br. J. Cancer. 2007;96(2):201–205. doi: 10.1038/sj.bjc.6603537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bower J.J., Karaca G.F., Zhou Y., Simpson D.A., Cordeiro-Stone M., Kaufmann W.K. Topoisomerase IIalpha maintains genomic stability through decatenation G(2) checkpoint signaling. Oncogene. 2010;29(34):4787–4799. doi: 10.1038/onc.2010.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo K., Yuan J., Chen J., Lou Z. Topoisomerase IIalpha controls the decatenation checkpoint. Nat. Cell Biol. 2009;11(2):204–210. doi: 10.1038/ncb1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brooks K., Chia K.M., Spoerri L., Mukhopadhyay P., Wigan M., Stark M., Pavey S., Gabrielli B. Defective Decatenation Checkpoint Function Is a Common Feature of Melanoma. J. Invest. Dermatol. 2013;134(1):150–158. doi: 10.1038/jid.2013.264. [DOI] [PubMed] [Google Scholar]
- 62.Nakagawa T., Hayashita Y., Maeno K., Masuda A., Sugito N., Osada H., Yanagisawa K., Ebi H., Shimokata K., Takahashi T. Identification of decatenation G2 checkpoint impairment independently of DNA damage G2 checkpoint in human lung cancer cell lines. Cancer Res. 2004;64(14):4826–4832. doi: 10.1158/0008-5472.CAN-04-0871. [DOI] [PubMed] [Google Scholar]
- 63.Doherty S.C., McKeown S.R., McKelvey-Martin V., Downes C.S., Atala A., Yoo J.J., Simpson D.A., Kaufmann W.K. Cell cycle checkpoint function in bladder cancer. J. Natl. Cancer Inst. 2003;95(24):1859–1868. doi: 10.1093/jnci/djg120. [DOI] [PubMed] [Google Scholar]
- 64.Wray J., Williamson E.A., Sheema S., Lee S.H., Libby E., Willman C.L., Nickoloff J.A., Hromas R. Metnase mediates chromosome decatenation in acute leukemia cells. Blood. 2009;114(9):1852–1858. doi: 10.1182/blood-2008-08-175760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engstrøm M.J., Ytterhus B., Vatten L.J., Opdahl S., Bofin A.M. TOP2A gene copy number change in breast cancer. J. Clin. Pathol. 2014;67(5):420–425. doi: 10.1136/jclinpath-2013-202052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glynn R.W., Miller N., Whelan M.C., Kerin M.J. Topoisomerase 2 alpha and the case for individualized breast cancer therapy. Ann. Surg. Oncol. 2010;17(5):1392–1397. doi: 10.1245/s10434-009-0855-0. [DOI] [PubMed] [Google Scholar]
- 67.Järvinen T.A., Tanner M., Bärlund M., Borg A., Isola J. Characterization of topoisomerase II alpha gene amplification and deletion in breast cancer. Genes Chromosomes Cancer. 1999;26(2):142–150. [PubMed] [Google Scholar]
- 68.Olsen K.E., Knudsen H., Rasmussen B.B., Balslev E., Knoop A., Ejlertsen B., Nielsen K.V., Schönau A., Overgaard J., Danish Breast Cancer Co-operative Group Amplification of HER2 and TOP2A and deletion of TOP2A genes in breast cancer investigated by new FISH probes. Acta Oncol. 2004;43(1):35–42. doi: 10.1080/02841860310019007. [DOI] [PubMed] [Google Scholar]
- 69.Park K., Han S., Gwak G.H., Kim H.J., Kim J., Kim K.M. Topoisomerase II-alpha gene deletion is not frequent as its amplification in breast cancer. Breast Cancer Res. Treat. 2006;98(3):337–342. doi: 10.1007/s10549-006-9170-7. [DOI] [PubMed] [Google Scholar]
- 70.Varga Z., Moelans C.B., Zuerrer-Hardi U., Ramach C., Behnke S., Kristiansen G., Moch H. Topoisomerase 2A gene amplification in breast cancer. Critical evaluation of different FISH probes. Breast Cancer Res. Treat. 2012;133(3):929–935. doi: 10.1007/s10549-011-1873-8. [DOI] [PubMed] [Google Scholar]
- 71.Li H., Wang Y., Liu X. Plk1-dependent phosphorylation regulates functions of DNA topoisomerase IIα in cell cycle progression. J. Biol. Chem. 2008;283:6209–6221. doi: 10.1074/jbc.M709007200. [DOI] [PubMed] [Google Scholar]
- 72.Choudhary C., Kumar C., Gnad F., Nielsen M.L., Rehman M., Walther T.C., Olsen J.V., Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 73.Mao Y., Sun M., Desai S.D., Liu L.F. SUMO-1 conjugation to topoisomerase I: a possible repair response to topoisomerase-mediated DNA damage. Proc. Natl. Acad. Sci. USA. 2000;97:4046–4051. doi: 10.1073/pnas.080536597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mao Y., Desai S.D., Ting C.Y., Hwang J., Liu L.F. 26 S proteasome-mediated degradation of topoisomerase II cleavable complexes. J. Biol. Chem. 2001;276:40652–40658. doi: 10.1074/jbc.M104009200. [DOI] [PubMed] [Google Scholar]
- 75.Mao Y., Desai S.D., Liu L.F. SUMO-1 conjugation to human DNA topoisomerase II isozymes. J. Biol. Chem. 2000;275:26066–26073. doi: 10.1074/jbc.M001831200. [DOI] [PubMed] [Google Scholar]
- 76.Desai S.D., Liu L.F., Vazquez-Abad D., D'Arpa P. Ubiquitin-dependent destruction of topoisomerase I is stimulated by the antitumor drug camptothecin. J. Biol. Chem. 1997;272:24159–24164. doi: 10.1074/jbc.272.39.24159. [DOI] [PubMed] [Google Scholar]
- 77.Desai S.D., Zhang H., Rodriguez-Bauman A., Yang J.M., Wu X., Gounder M.K., Rubin E.H., Liu L.F. Transcription-dependent degradation of topoisomerase I-DNA covalent complexes. Mol. Cell. Biol. 2003;23:2341–2350. doi: 10.1128/MCB.23.7.2341-2350.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao H., Mao Y., Desai S.D., Zhou N., Ting C.Y., Hwang J., Liu L.F. The topoisomerase IIβ circular clamp arrests transcription and signals a 26S proteasome pathway. Proc. Natl. Acad. Sci. USA. 2003;100:3239–3244. doi: 10.1073/pnas.0736401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fisher L.M., Pan X.S. Methods to assay inhibitors of DNA gyrase and topoisomerase IV activities. Methods Mol. Med. 2008;142:11–23. doi: 10.1007/978-1-59745-246-5_2. [DOI] [PubMed] [Google Scholar]
- 80.Pui C.H., Relling M.V. Topoisomerase II inhibitor-related acute myeloid leukaemia. Br. J. Haematol. 2000;109(1):13–23. doi: 10.1046/j.1365-2141.2000.01843.x. [DOI] [PubMed] [Google Scholar]
- 81.Felix C.A. Leukemias related to treatment with DNA topoisomerase II inhibitors. Med. Pediatr. Oncol. 2001;36(5):525–535. doi: 10.1002/mpo.1125. [DOI] [PubMed] [Google Scholar]
- 82.Antony S., Agama K.K., Miao Z.H., Takagi K., Wright M.H., Robles A.I., Varticovski L., Nagarajan M., Morrell A., Cushman M., Pommier Y. Novel indenoisoquinolines NSC 725776 and NSC 724998 produce persistent topoisomerase I cleavage complexes and overcome multidrug resistance. Cancer Res. 2007;67(21):10397–10405. doi: 10.1158/0008-5472.CAN-07-0938. [DOI] [PubMed] [Google Scholar]
- 83.Kurtzberg L.S., Roth S., Krumbholz R., Crawford J., Bormann C., Dunham S., Yao M., Rouleau C., Bagley R.G., Yu X.J., Wang F., Schmid S.M., Lavoie E.J., Teicher B.A. Genz-644282, a novel non-camptothecin topoisomerase I inhibitor for cancer treatment. Clin. Cancer Res. 2011;17(9):2777–2787. doi: 10.1158/1078-0432.CCR-10-0542. [DOI] [PubMed] [Google Scholar]
- 84.Kruczynski A., Pillon A., Creancier L., Vandenberghe I., Gomes B., Brel V., Fournier E., Annereau J.P., Currie E., Guminski Y., Bonnet D., Bailly C., Guilbaud N. F14512, a polyamine-vectorized anti-cancer drug, currently in clinical trials exhibits a marked preclinical anti-leukemic activity. Leukemia. 2013;27(11):2139–2148. doi: 10.1038/leu.2013.108. [DOI] [PubMed] [Google Scholar]
- 85.Mouawad F., Gros A., Rysman B., Bal-Mahieu C., Bertheau C., Horn S., Sarrazin T., Lartigau E., Chevalier D., Bailly C., Lansiaux A., Meignan S. The antitumor drug F14512 enhances cisplatin and ionizing radiation effects in head and neck squamous carcinoma cell lines. Oral Oncol. 2014;50(2):113–119. doi: 10.1016/j.oraloncology.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 86.Leblond P., Boulet E., Bal-Mahieu C., Pillon A., Kruczynski A., Guilbaud N., Bailly C., Sarrazin T., Lartigau E., Lansiaux A., Meignan S. Activity of the polyamine-vectorized anti-cancer drug F14512 against pediatric glioma and neuroblastoma cell lines. Invest. New Drugs. 2014;32(5):883–892. doi: 10.1007/s10637-014-0132-3. [DOI] [PubMed] [Google Scholar]
- 87.Hawtin R.E., Stockett D.E., Byl J.A., McDowell R.S., Nguyen T., Arkin M.R., Conroy A., Yang W., Osheroff N., Fox J.A. Voreloxin is an anticancer quinolone derivative that intercalates DNA and poisons topoisomerase II. PLoS One. 2010;5(4):e10186. doi: 10.1371/journal.pone.0010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ravandi F., Ritchie E.K., Sayar H., Lancet J.E., Craig M.D., Vey N., Strickland S.A., Schiller G.J., Jabbour E., Erba H.P., Pigneux A., Horst H.A., Recher C., Klimek V.M., Cortes J., Roboz G.J., Odenike O., Thomas X., Havelange V., Maertens J., Derigs H.G., Heuser M., Damon L., Powell B.L., Gaidano G., Carella A.M., Wei A., Hogge D., Craig A.R., Fox J.A., Ward R., Smith J.A., Acton G., Mehta C., Stuart R.K., Kantarjian H.M. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2015;16(9):1025–1036. doi: 10.1016/S1470-2045(15)00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bornhäuser M. Vosaroxin in acute myeloid leukaemia. Lancet Oncol. 2015;16(9):1000–1001. doi: 10.1016/S1470-2045(15)00165-5. [DOI] [PubMed] [Google Scholar]
- 90.Skladanowski A., Plisov S.Y., Konopa J., Larsen A.K. Inhibition of DNA topoisomerase II by imidazoacridinones, new antineoplastic agents with strong activity against solid tumors. Mol. Pharmacol. 1996;49(5):772–780. [PubMed] [Google Scholar]
- 91.Isambert N., Campone M., Bourbouloux E., Drouin M., Major A., Yin W., Loadman P., Capizzi R., Grieshaber C., Fumoleau P. Evaluation of the safety of C-1311 (SYMADEX) administered in a phase 1 dose escalation trial as a weekly infusion for 3 consecutive weeks in patients with advanced solid tumours. Eur. J. Cancer. 2010;46(4):729–734. doi: 10.1016/j.ejca.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Mazerska Z., Sowiński P., Konopa J. Molecular mechanism of the enzymatic oxidation investigated for imidazoacridinone antitumor drug, C-1311. Biochem. Pharmacol. 2003;66(9):1727–1736. doi: 10.1016/s0006-2952(03)00477-5. [DOI] [PubMed] [Google Scholar]
- 93.Mazerska Z., Dziegielewski J., Konopa J. Enzymatic activation of a new antitumour drug, 5-diethylaminoethylamino-8-hydroxyimidazoacridinone, C-1311, observed after its intercalation into DNA. Biochem. Pharmacol. 2001;61(6):685–694. doi: 10.1016/s0006-2952(01)00527-5. [DOI] [PubMed] [Google Scholar]
- 94.Gao H., Huang K.C., Yamasaki E.F., Chan K.K., Chohan L., Snapka R.M. XK469, a selective topoisomerase IIbeta poison. Proc. Natl. Acad. Sci. USA. 1999;96(21):12168–12173. doi: 10.1073/pnas.96.21.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Facompre M., Tardy C., Bal-Mahieu C., Colson P., Perez C., Manzanares I., Cuevas C., Bailly C. Lamellarin D: a novel potent inhibitor of topoisomerase I. Cancer Res. 2003;63(21):7392–7399. [PubMed] [Google Scholar]
- 96.Kluza J., Gallego M.A., Loyens A., Beauvillain J.C., Sousa-Faro J.M., Cuevas C., Marchetti P., Bailly C. Cancer cell mitochondria are direct proapoptotic targets for the marine antitumor drug lamellarin D. Cancer Res. 2006;66(6):3177–3187. doi: 10.1158/0008-5472.CAN-05-1929. [DOI] [PubMed] [Google Scholar]
- 97.Ballot C., Kluza J., Martoriati A., Nyman U., Formstecher P., Joseph B., Bailly C., Marchetti P. Essential role of mitochondria in apoptosis of cancer cells induced by the marine alkaloid Lamellarin D. Mol. Cancer Ther. 2009;8(12):3307–3317. doi: 10.1158/1535-7163.MCT-09-0639. [DOI] [PubMed] [Google Scholar]
- 98.Ballot C., Kluza J., Lancel S., Martoriati A., Hassoun S.M., Mortier L., Vienne J.C., Briand G., Formstecher P., Bailly C., Neviere R., Marchetti P. Inhibition of mitochondrial respiration mediates apoptosis induced by the anti-tumoral alkaloid lamellarin D. Apoptosis. 2010;15(7):769–781. doi: 10.1007/s10495-010-0471-2. [DOI] [PubMed] [Google Scholar]
- 99.Khiati S., Seol Y., Agama K., Dalla Rosa I., Agrawal S., Fesen K., Zhang H., Neuman K.C., Pommier Y. Poisoning of mitochondrial topoisomerase I by lamellarin D. Mol. Pharmacol. 2014;86(2):193–199. doi: 10.1124/mol.114.092833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bailly C. Anticancer properties of lamellarins. Mar. Drugs. 2015;13(3):1105–1123. doi: 10.3390/md13031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tesauro C., Fiorani P., D'Annessa I., Chillemi G., Turchi G., Desideri A. Erybraedin C, a natural compound from the plant Bituminaria bituminosa, inhibits both the cleavage and religation activities of human topoisomerase I. Biochem. J. 2010;425(3):531–539. doi: 10.1042/BJ20091127. [DOI] [PubMed] [Google Scholar]
- 102.Maurich T., Iorio M., Chimenti D., Turchi G. Erybraedin C and bitucarpin A, two structurally related pterocarpans purified from Bituminaria bituminosa, induced apoptosis in human colon adenocarcinoma cell lines MMR- and p53-proficient and -deficient in a dose-, time-, and structure-dependent fashion. Chem. Biol. Interact. 2006;159(2):104–116. doi: 10.1016/j.cbi.2005.10.103. [DOI] [PubMed] [Google Scholar]
- 103.Liu M., Zhang W., Wei J., Lin X. Synthesis and alpha-glucosidase inhibitory mechanisms of bis (2, 3-dibromo-4, 5-dihydroxybenzyl) ether, a potential marine bromophenol alpha-glucosidase inhibitor. Mar. Drugs. 2011;9(9):1554–1565. doi: 10.3390/md9091554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu M., Zhang W., Wei J., Qiu L., Lin X. Marine bromophenol bis (2, 3-dibromo-4, 5-dihydroxybenzyl) ether, induces mitochondrial apoptosis in K562 cells and inhibits topoisomerase I in vitro. Toxicol. Lett. 2012;211(2):126–134. doi: 10.1016/j.toxlet.2012.03.771. [DOI] [PubMed] [Google Scholar]
- 105.Liu M., Wang G., Xiao L., Xu X., Liu X., Xu P., Lin X. Bis (2, 3-dibromo-4, 5-dihydroxybenzyl) ether, a marine algae derived bromophenol, inhibits the growth of Botrytis cinerea and interacts with DNA molecules. Mar. Drugs. 2014;12(7):3838–3851. doi: 10.3390/md12073838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fayad W., Fryknas M., Brnjic S., Olofsson M.H., Larsson R., Linder S. Identification of a novel topoisomerase inhibitor effective in cells overexpressing drug efflux transporters. PLoS One. 2009;4(10):e7238. doi: 10.1371/journal.pone.0007238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Castelli S., Katkar P., Vassallo O., Falconi M., Linder S., Desideri A. A natural anticancer agent thaspine targets human topoisomerase IB. Anticancer. Agents Med. Chem. 2013;13(2):356–363. doi: 10.2174/1871520611313020021. [DOI] [PubMed] [Google Scholar]
- 108.Chan A.L., Chang W.S., Chen L.M., Lee C.M., Chen C.E., Lin C.M., Hwang J.L. Evodiamine stabilizes topoisomerase I-DNA cleavable complex to inhibit topoisomerase I activity. Molecules. 2009;14(4):1342–1352. doi: 10.3390/molecules14041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pan X., Hartley J.M., Hartley J.A., White K.N., Wang Z., Bligh S.W. Evodiamine, a dual catalytic inhibitor of type I and II topoisomerases, exhibits enhanced inhibition against camptothecin resistant cells. Phytomedicine. 2012;19(7):618–624. doi: 10.1016/j.phymed.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 110.Kikuchi T., Nihei M., Nagai H., Fukushi H., Tabata K., Suzuki T., Akihisa T. Albanol A from the root bark of Morus alba L. induces apoptotic cell death in HL60 human leukemia cell line. Chem. Pharm. Bull. (Tokyo) 2010;58(4):568–571. doi: 10.1248/cpb.58.568. [DOI] [PubMed] [Google Scholar]
- 111.Geng C.A., Ma Y.B., Zhang X.M., Yao S.Y., Xue D.Q., Zhang R.P., Chen J.J. Mulberrofuran G and isomulberrofuran G from Morus alba L.: anti-hepatitis B virus activity and mass spectrometric fragmentation. J. Agric. Food Chem. 2012;60(33):8197–8202. doi: 10.1021/jf302639b. [DOI] [PubMed] [Google Scholar]
- 112.DiCosmo F., Straus N.A. Alternariol, a dibenzopyrone mycotoxin of Alternaria spp., is a new photosensitizing and DNA cross-linking agent. Experientia. 1985;41(9):1188–1190. doi: 10.1007/BF01951722. [DOI] [PubMed] [Google Scholar]
- 113.Fehr M., Pahlke G., Fritz J., Christensen M.O., Boege F., Altemoller M., Podlech J., Marko D. Alternariol acts as a topoisomerase poison, preferentially affecting the IIalpha isoform. Mol. Nutr. Food Res. 2009;53(4):441–451. doi: 10.1002/mnfr.200700379. [DOI] [PubMed] [Google Scholar]
- 114.Fehr M., Baechler S., Kropat C., Mielke C., Boege F., Pahlke G., Marko D. Repair of DNA damage induced by the mycotoxin alternariol involves tyrosyl-DNA phosphodiesterase 1. Mycotoxin Res. 2010;26(4):247–256. doi: 10.1007/s12550-010-0063-6. [DOI] [PubMed] [Google Scholar]
- 115.Solhaug A., Torgersen M.L., Holme J.A., Lagadic-Gossmann D., Eriksen G.S. Autophagy and senescence, stress responses induced by the DNA-damaging mycotoxin alternariol. Toxicology. 2014;326:119–129. doi: 10.1016/j.tox.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 116.Brugger E.M., Wagner J., Schumacher D.M., Koch K., Podlech J., Metzler M., Lehmann L. Mutagenicity of the mycotoxin alternariol in cultured mammalian cells. Toxicol. Lett. 2006;164(3):221–230. doi: 10.1016/j.toxlet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 117.Solhaug A., Vines L.L., Ivanova L., Spilsberg B., Holme J.A., Pestka J., Collins A., Eriksen G.S. Mechanisms involved in alternariol-induced cell cycle arrest. Mutat. Res. 2012;738-739:1–11. doi: 10.1016/j.mrfmmm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 118.Wollenhaupt K., Schneider F., Tiemann U. Influence of alternariol (AOH) on regulator proteins of cap-dependent translation in porcine endometrial cells. Toxicol. Lett. 2008;182(1-3):57–62. doi: 10.1016/j.toxlet.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 119.Kimura Y., Taniguchi M., Baba K. Antitumor and antimetastatic activities of 4-hydroxyderricin isolated from Angelica keiskei roots. Planta Med. 2004;70(3):211–219. doi: 10.1055/s-2004-815537. [DOI] [PubMed] [Google Scholar]
- 120.Akihisa T., Kikuchi T., Nagai H., Ishii K., Tabata K., Suzuki T. 4-Hydroxyderricin from Angelica keiskei roots induces caspase-dependent apoptotic cell death in HL60 human leukemia cells. J. Oleo Sci. 2011;60(2):71–77. doi: 10.5650/jos.60.71. [DOI] [PubMed] [Google Scholar]
- 121.Yasuda M., Kawabata K., Miyashita M., Okumura M., Yamamoto N., Takahashi M., Ashida H., Ohigashi H. Inhibitory effects of 4-hydroxyderricin and xanthoangelol on lipopolysaccharide-induced inflammatory responses in RAW264 macrophages. J. Agric. Food Chem. 2014;62(2):462–467. doi: 10.1021/jf404175t. [DOI] [PubMed] [Google Scholar]
- 122.Takeda S., Matsuo K., Yaji K., Okajima-Miyazaki S., Harada M., Miyoshi H., Okamoto Y., Amamoto T., Shindo M., Omiecinski C.J., Aramaki H. (-)-Xanthatin selectively induces GADD45gamma and stimulates caspase-independent cell death in human breast cancer MDA-MB-231 cells. Chem. Res. Toxicol. 2011;24(6):855–865. doi: 10.1021/tx200046s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takeda S., Noguchi M., Matsuo K., Yamaguchi Y., Kudo T., Nishimura H., Okamoto Y., Amamoto T., Shindo M., Omiecinski C.J., Aramaki H. (-)-Xanthatin up-regulation of the GADD45gamma tumor suppressor gene in MDA-MB-231 breast cancer cells: role of topoisomerase IIalpha inhibition and reactive oxygen species. Toxicology. 2013;305:1–9. doi: 10.1016/j.tox.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li M., Miao Z.H., Chen Z., Chen Q., Gui M., Lin L.P., Sun P., Yi Y.H., Ding J. Echinoside A, a new marine-derived anticancer saponin, targets topoisomerase2alpha by unique interference with its DNA binding and catalytic cycle. Ann. Oncol. 2010;21(3):597–607. doi: 10.1093/annonc/mdp335. [DOI] [PubMed] [Google Scholar]
- 125.Zhao Q., Xue Y., Wang J.F., Li H., Long T.T., Li Z., Wang Y.M., Dong P., Xue C.H. In vitro and in vivo anti-tumour activities of echinoside A and ds-echinoside A from Pearsonothuria graeffei. J. Sci. Food Agric. 2012;92(4):965–974. doi: 10.1002/jsfa.4678. [DOI] [PubMed] [Google Scholar]
- 126.Wang Y., Wang J., Yanagita R.C., Liu C., Hu X., Dong P., Xue C., Xue Y. Effects of two sulfated triterpene saponins echinoside A and holothurin A on the inhibition of dietary fat absorption and obesity reduction. Biosci. Biotechnol. Biochem. 2014;78(1):139–146. doi: 10.1080/09168451.2014.877830. [DOI] [PubMed] [Google Scholar]
- 127.Xue X., Qu X.J., Gao Z.H., Sun C.C., Liu H.P., Zhao C.R., Cheng Y.N., Lou H.X. Riccardin D, a novel macrocyclic bisbibenzyl, induces apoptosis of human leukemia cells by targeting DNA topoisomerase II. Invest. New Drugs. 2012;30(1):212–222. doi: 10.1007/s10637-010-9554-8. [DOI] [PubMed] [Google Scholar]
- 128.Sun C.C., Zhang Y.S., Xue X., Cheng Y.N., Liu H.P., Zhao C.R., Lou H.X., Qu X.J. Inhibition of angiogenesis involves in anticancer activity of riccardin D, a macrocyclic bisbibenzyl, in human lung carcinoma. Eur. J. Pharmacol. 2011;667(1-3):136–143. doi: 10.1016/j.ejphar.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 129.Wang Y., Ji Y., Hu Z., Jiang H., Zhu F., Yuan H., Lou H. Riccardin D induces cell death by activation of apoptosis and autophagy in osteosarcoma cells. Toxicol. In Vitro. 2013;27(6):1928–1936. doi: 10.1016/j.tiv.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 130.Hu Z., Kong F., Si M., Tian K., Yu L.X., Young C.Y., Yuan H., Lou H., Riccardin D. Exerts Its Antitumor Activity by Inducing DNA Damage in PC-3 Prostate Cancer Cells In Vitro and In Vivo. PLoS One. 2013;8(9):e74387. doi: 10.1371/journal.pone.0074387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sarveswaran S., Gautam S.C., Ghosh J. Wedelolactone, a medicinal plant-derived coumestan, induces caspase-dependent apoptosis in prostate cancer cells via downregulation of PKCepsilon without inhibiting Akt. Int. J. Oncol. 2012;41(6):2191–2199. doi: 10.3892/ijo.2012.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin F.M., Chen L.R., Lin E.H., Ke F.C., Chen H.Y., Tsai M.J., Hsiao P.W. Compounds from Wedelia chinensis synergistically suppress androgen activity and growth in prostate cancer cells. Carcinogenesis. 2007;28(12):2521–2529. doi: 10.1093/carcin/bgm137. [DOI] [PubMed] [Google Scholar]
- 133.Kaushik-Basu N., Bopda-Waffo A., Talele T.T., Basu A., Costa P.R., da Silva A.J., Sarafianos S.G., Noel F. Identification and characterization of coumestans as novel HCV NS5B polymerase inhibitors. Nucleic Acids Res. 2008;36(5):1482–1496. doi: 10.1093/nar/gkm1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kobori M., Yang Z., Gong D., Heissmeyer V., Zhu H., Jung Y.K., Gakidis M.A., Rao A., Sekine T., Ikegami F., Yuan C., Yuan J. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ. 2004;11(1):123–130. doi: 10.1038/sj.cdd.4401325. [DOI] [PubMed] [Google Scholar]
- 135.Benes P., Knopfova L., Trcka F., Nemajerova A., Pinheiro D., Soucek K., Fojta M., Smarda J. Inhibition of topoisomerase IIalpha: novel function of wedelolactone. Cancer Lett. 2011;303(1):29–38. doi: 10.1016/j.canlet.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 136.Hsieh C.J., Kuo P.L., Hou M.F., Hung J.Y., Chang F.R., Hsu Y.C., Huang Y.F., Tsai E.M., Hsu Y.L. Wedelolactone inhibits breast cancer-induced osteoclastogenesis by decreasing Akt/mTOR signaling. Int. J. Oncol. 2015;46(2):555–562. doi: 10.3892/ijo.2014.2769. [DOI] [PubMed] [Google Scholar]
- 137.Chen H., Gao S., Li J., Liu D., Sheng C., Yao C., Jiang W., Wu J., Chen S., Huang W. Wedelolactone disrupts the interaction of EZH2-EED complex and inhibits PRC2-dependent cancer. Oncotarget. 2015;6(30):13049–13059. doi: 10.18632/oncotarget.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Du L., Liu H.C., Fu W., Li D.H., Pan Q.M., Zhu T.J., Geng M.Y., Gu Q.Q. Unprecedented citrinin trimer tricitinol B functions as a novel topoisomerase IIalpha inhibitor. J. Med. Chem. 2011;54(16):5796–5810. doi: 10.1021/jm200511x. [DOI] [PubMed] [Google Scholar]
- 139.Kang K., Oh S.H., Yun J.H., Jho E.H., Kang J.H., Batsuren D., Tunsag J., Park K.H., Kim M., Nho C.W. A novel topoisomerase inhibitor, daurinol, suppresses growth of HCT116 cells with low hematological toxicity compared to etoposide. Neoplasia. 2011;13(11):1043–1057. doi: 10.1593/neo.11972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kang K., Nho C.W., Kim N.D., Song D.G., Park Y.G., Kim M., Pan C.H., Shin D., Oh S.H., Oh H.S. Daurinol, a catalytic inhibitor of topoisomerase IIalpha, suppresses SNU-840 ovarian cancer cell proliferation through cell cycle arrest in S phase. Int. J. Oncol. 2014;45(2):558–566. doi: 10.3892/ijo.2014.2442. [DOI] [PubMed] [Google Scholar]
- 141.Woo J.K., Kang J.H., Shin D., Park S.H., Kang K., Nho C.W., Seong J.K., Lee S.J., Oh S.H. Daurinol enhances the efficacy of radiation therapy in lung cancer via suppression of aurora kinase A/B expression. Mol. Cancer Ther. 2015;14(7):1693–1704. doi: 10.1158/1535-7163.MCT-14-0960. [DOI] [PubMed] [Google Scholar]
- 142.Sahu B.D., Kalvala A.K., Koneru M., Mahesh Kumar J., Kuncha M., Rachamalla S.S., Sistla R. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-kappaB activation and antioxidant defence. PLoS One. 2014;9(9):e105070. doi: 10.1371/journal.pone.0105070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chou R.H., Hsieh S.C., Yu Y.L., Huang M.H., Huang Y.C., Hsieh Y.H. Fisetin inhibits migration and invasion of human cervical cancer cells by down-regulating urokinase plasminogen activator expression through suppressing the p38 MAPK-dependent NF-kappaB signaling pathway. PLoS One. 2013;8(8):e71983. doi: 10.1371/journal.pone.0071983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Olaharski A.J., Mondrala S.T., Eastmond D.A. Chromosomal malsegregation and micronucleus induction in vitro by the DNA topoisomerase II inhibitor fisetin. Mutat. Res. 2005;582(1-2):79–86. doi: 10.1016/j.mrgentox.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 145.Lopez-Lazaro M., Willmore E., Austin C.A. The dietary flavonoids myricetin and fisetin act as dual inhibitors of DNA topoisomerases I and II in cells. Mutat. Res. 2010;696(1):41–47. doi: 10.1016/j.mrgentox.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 146.Tripathi R., Samadder T., Gupta S., Surolia A., Shaha C. Anticancer activity of a combination of cisplatin and fisetin in embryonal carcinoma cells and xenograft tumors. Mol. Cancer Ther. 2011;10(2):255–268. doi: 10.1158/1535-7163.MCT-10-0606. [DOI] [PubMed] [Google Scholar]
- 147.Shiomi K., Kuriyama I., Yoshida H., Mizushina Y. Inhibitory effects of myricetin on mammalian DNA polymerase, topoisomerase and human cancer cell proliferation. Food Chem. 2013;139(1-4):910–918. doi: 10.1016/j.foodchem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 148.Maeda N., Kokai Y., Ohtani S., Sahara H., Hada T., Ishimaru C., Kuriyama I., Yonezawa Y., Iijima H., Yoshida H., Sato N., Mizushina Y. Anti-tumor effects of the glycolipids fraction from spinach which inhibited DNA polymerase activity. Nutr. Cancer. 2007;57(2):216–223. doi: 10.1080/01635580701277908. [DOI] [PubMed] [Google Scholar]
- 149.Maeda N., Hada T., Yoshida H., Mizushina Y. Inhibitory effect on replicative DNA polymerases, human cancer cell proliferation, and in vivo anti-tumor activity by glycolipids from spinach. Curr. Med. Chem. 2007;14(9):955–967. doi: 10.2174/092986707780362952. [DOI] [PubMed] [Google Scholar]
- 150.Maeda N., Matsubara K., Yoshida H., Mizushina Y. Anti-cancer effect of spinach glycoglycerolipids as angiogenesis inhibitors based on the selective inhibition of DNA polymerase activity. Mini Rev. Med. Chem. 2011;11(1):32–38. doi: 10.2174/138955711793564042. [DOI] [PubMed] [Google Scholar]
- 151.Kuriyama I., Musumi K., Yonezawa Y., Takemura M., Maeda N., Iijima H., Hada T., Yoshida H., Mizushina Y. Inhibitory effects of glycolipids fraction from spinach on mammalian DNA polymerase activity and human cancer cell proliferation. J. Nutr. Biochem. 2005;16(10):594–601. doi: 10.1016/j.jnutbio.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 152.Iijima H., Kasai N., Chiku H., Takeuchi T., Kuramochi K., Hanashima S., Kobayashi S., Sugawara F., Sakaguchi K., Yoshida H., Mizushina Y. Structure-activity relationship of a glycolipid, sulfoquinovosyl diacylglycerol, with the DNA binding activity of p53. Int. J. Mol. Med. 2007;19(1):41–48. [PubMed] [Google Scholar]
- 153.Chatterjee R., Singh O., Pachuau L., Malik S.P., Paul M., Bhadra K., Paul S., Kumar G.S., Mondal N.B., Banerjee S. Identification of a sulfonoquinovosyl-diacylglyceride from Azadirachta indica and studies on its cytotoxic activity and DNA binding properties. Bioorg. Med. Chem. Lett. 2010;20(22):6699–6702. doi: 10.1016/j.bmcl.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 154.Bharitkar Y.P., Bathini S., Ojha D., Ghosh S., Mukherjee H., Kuotsu K., Chattopadhyay D., Mondal N.B. Antibacterial and antiviral evaluation of sulfonoquinovosyldiacylglyceride: a glycolipid isolated from Azadirachta indica leaves. Lett. Appl. Microbiol. 2013;58(2):184–189. doi: 10.1111/lam.12174. [DOI] [PubMed] [Google Scholar]
- 155.Jain C.K., Pradhan B.S., Banerjee S., Mondal N.B., Majumder S.S., Bhattacharyya M., Chakrabarti S., Roychoudhury S., Majumder H.K. Sulfonoquinovosyl diacyl-glyceride selectively targets acute lymphoblastic leukemia cells and exerts potent anti-leukemic effects in vivo. Sci. Rep. 2015;5:12082. doi: 10.1038/srep12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Padhye S., Dandawate P., Yusufi M., Ahmad A., Sarkar F.H. Perspectives on medicinal properties of plumbagin and its analogs. Med. Res. Rev. 2012;32(6):1131–1158. doi: 10.1002/med.20235. [DOI] [PubMed] [Google Scholar]
- 157.Fujii N., Yamashita Y., Arima Y., Nagashima M., Nakano H. Induction of topoisomerase II-mediated DNA cleavage by the plant naphthoquinones plumbagin and shikonin. Antimicrob. Agents Chemother. 1992;36(12):2589–2594. doi: 10.1128/aac.36.12.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen Z.F., Tan M.X., Liu L.M., Liu Y.C., Wang H.S., Yang B., Peng Y., Liu H.G., Liang H., Orvig C. Cytotoxicity of the traditional chinese medicine (TCM) plumbagin in its copper chemistry. Dalton Trans. 2009;(48):10824–10833. doi: 10.1039/b910133k. [DOI] [PubMed] [Google Scholar]
- 159.Nair S., Nair R.R., Srinivas P., Srinivas G., Pillai M.R. Radiosensitizing effects of plumbagin in cervical cancer cells is through modulation of apoptotic pathway. Mol. Carcinog. 2008;47(1):22–33. doi: 10.1002/mc.20359. [DOI] [PubMed] [Google Scholar]
- 160.Sandur S.K., Ichikawa H., Sethi G., Ahn K.S., Aggarwal B.B. Plumbagin (5-hydroxy-2-methyl-1, 4-naphthoquinone) suppresses NF-kappaB activation and NF-kappaB-regulated gene products through modulation of p65 and IkappaBalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J. Biol. Chem. 2006;281(25):17023–17033. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- 161.Pan S.T., Qin Y., Zhou Z.W., He Z.X., Zhang X., Yang T., Yang Y.X., Wang D., Qiu J.X., Zhou S.F. Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK- and PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells. Drug Des. Devel. Ther. 2015;9:1601–1626. doi: 10.2147/DDDT.S76057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pan S.T., Qin Y., Zhou Z.W., He Z.X., Zhang X., Yang T., Yang Y.X., Wang D., Zhou S.F., Qiu J.X. Plumbagin suppresses epithelial to mesenchymal transition and stemness via inhibiting Nrf2-mediated signaling pathway in human tongue squamous cell carcinoma cells. Drug Des. Devel. Ther. 2015;9:5511–5551. doi: 10.2147/DDDT.S89621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Webb M.R., Ebeler S.E. Comparative analysis of topoisomerase IB inhibition and DNA intercalation by flavonoids and similar compounds: structural determinates of activity. Biochem. J. 2004;384(Pt 3):527–541. doi: 10.1042/BJ20040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu Y., Her C. Inhibition of Topoisomerase (DNA) I (TOP1): DNA Damage Repair and Anticancer Therapy. Biomolecules. 2015;5(3):1652–16570. doi: 10.3390/biom5031652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stagos D., Kazantzoglou G., Magiatis P., Mitaku S., Anagnostopoulos K., Kouretas D. Effects of plant phenolics and grape extracts from Greek varieties of Vitis vinifera on Mitomycin C and topoisomerase I-induced nicking of DNA. Int. J. Mol. Med. 2005;15(6):1013–1022. [PubMed] [Google Scholar]
- 166.Sudan S., Rupasinghe H.P. Flavonoid-enriched apple fraction AF4 induces cell cycle arrest, DNA topoisomerase II inhibition, and apoptosis in human liver cancer HepG2 cells. Nutr. Cancer. 2014;66(7):1237–1246. doi: 10.1080/01635581.2014.951733. [DOI] [PubMed] [Google Scholar]
- 167.Furlanetto V., Zagotto G., Pasquale R., Moro S., Gatto B. Ellagic acid and polyhydroxylated urolithins are potent catalytic inhibitors of human topoisomerase II: an in vitro study. J. Agric. Food Chem. 2012;60(36):9162–9170. doi: 10.1021/jf302600q. [DOI] [PubMed] [Google Scholar]
- 168.Naowaratwattana W., De-Eknamkul W., De Mejia E.G. Phenolic-containing organic extracts of mulberry (Morus alba L.) leaves inhibit HepG2 hepatoma cells through G2/M phase arrest, induction of apoptosis, and inhibition of topoisomerase IIα activity. J. Med. Food. 2010;13(5):1045–1056. doi: 10.1089/jmf.2010.1021. [DOI] [PubMed] [Google Scholar]
- 169.Bandele O.J., Osheroff N. Bioflavonoids as Poisons of Human Topoisomerase IIα and IIβ. Biochemistry. 2007;46(20):6097–6108. doi: 10.1021/bi7000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bandele O.J., Clawson S.J., Osheroff N. Dietary polyphenols as topoisomerase II poisons: B ring and C ring substituents determine the mechanism of enzyme-mediated DNA cleavage enhancement. Chem. Res. Toxicol. 2008;21(6):1253–1260. doi: 10.1021/tx8000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bandele O.J., Osheroff N. (−)-Epigallocatechin Gallate, A Major Constituent of Green Tea, Poisons Human Type II Topoisomerases. Chem. Res. Toxicol. 2008;21(4):936–943. doi: 10.1021/tx700434v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Berger S., Gupta S., Belfi C.A., Gosky D.M., Mukhtar H. Green Tea Constituent (−)-Epigallocatechin-3-gallate Inhibits Topoisomerase I Activity in Human Colon Carcinoma Cells. Biochem. Biophys. Res. Commun. 2001;288(1):101–105. doi: 10.1006/bbrc.2001.5736. [DOI] [PubMed] [Google Scholar]
- 173.Suzuki K., Yahara S., Hashimoto F., Uyeda M. Inhibitory activities of (−)-epigallocatechin-3-O-gallate against topoisomerases I and II. Biol. Pharm. Bull. 2001;24(9):1088–1090. doi: 10.1248/bpb.24.1088. [DOI] [PubMed] [Google Scholar]
- 174.Jo J.Y., Gonzalez de Mejia E., Lila M.A. Catalytic inhibition of human DNA topoisomerase II by interactions of grape cell culture polyphenols. J. Agric. Food Chem. 2006;54(6):2083–2087. doi: 10.1021/jf052700z. [DOI] [PubMed] [Google Scholar]
- 175.de Mejía E.G., Chandra S., Ramírez-Mares M., Wang W. Catalytic inhibition of human DNA topoisomerase by phenolic compounds in Ardisia compressa extracts and their effect on human colon cancer cells. Food Chem. Toxicol. 2006;44(8):1191–1203. doi: 10.1016/j.fct.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 176.Singh K.K., Kulawiec M. Mitochondrial DNA Polymorphism and Risk of Cancer. Methods Mol. Biol. 2009;471:291–303. doi: 10.1007/978-1-59745-416-2_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Weigl S., Paradiso A., Tommasi S. Mitochondria and Familial Predisposition to Breast Cancer. Curr. Genomics. 2013;14(3):195–203. doi: 10.2174/1389202911314030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Poulogiannis G., Frayling I.M., Arends M.J. DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology. 2010;56(2):167–179. doi: 10.1111/j.1365-2559.2009.03392.x. [DOI] [PubMed] [Google Scholar]
- 179.Ma Y., Ding Z., Qian Y., Wan Y.W., Tosun K., Shi X., Castranova V., Harner E.J., Guo N.L. An integrative genomic and proteomic approach to chemosensitivity prediction. Int. J. Oncol. 2009;34(1):107–115. doi: 10.3892/ijo_00000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Roti G., Stegmaier K. Genetic and proteomic approaches to identify cancer drug targets. Br. J. Cancer. 2012;106(2):254–261. doi: 10.1038/bjc.2011.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 182.Brannon-Peppas L., Blanchette J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2012;64:206–212. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 183.Bertrand N., Wu J., Xu X., Kamaly N., Farokhzad O.C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]