Abstract
The buttercup family, Ranunculaceae, comprising more than 2,200 species in at least 62 genera, mostly herbs, has long been used in folk medicine and worldwide ethnomedicine since the beginning of human civilization. Various medicinal phytometabolites have been found in Ranunculaceae plants, many of which, such as alkaloids, terpenoids, saponins, and polysaccharides, have shown anti-cancer activities in vitro and in vivo. Most concerns have been raised for two epiphany molecules, the monoterpene thymoquinone and the isoquinoline alkaloid berberine. At least 17 genera have been enriched with anti-cancer phytometabolites. Some Ranunculaceae phytometabolites induce the cell cycle arrest and apoptosis of cancer cells or enhance immune activities, while others inhibit the proliferation, invasion, angiogenesis, and metastasis, or reverse the multi-drug resistance of cancer cells thereby regulating all known hallmarks of cancer. These phytometabolites could exert their anti-cancer activities via multiple signaling pathways. In addition, absorption, distribution, metabolism, and excretion/toxicity properties and structure/activity relationships of some phytometabolites have been revealed assisting in the early drug discovery and development pipelines. However, a comprehensive review of the molecular mechanisms and functions of Ranunculaceae anti-cancer phytometabolites is lacking. Here, we summarize the recent progress of the anti-cancer chemo- and pharmacological diversity of Ranunculaceae medicinal plants, focusing on the emerging molecular machineries and functions of anti-cancer phytometabolites. Gene expression profiling and relevant omics platforms (e.g. genomics, transcriptomics, proteomics, and metabolomics) could reveal differential effects of phytometabolites on the phenotypically heterogeneous cancer cells.
Keywords: Ranunculaceae phytometabolites, Anticancer activity, Molecular mechanism, Genomics
1. INTRODUCTION
The bioactive natural products (plant secondary metabolites) are well known to possess therapeutic value for the prevention and treatment of various types and stages of cancer [1, 2]. Ranunculaceae phytometabolites exhibit promising effects against cancer, many of which modulate signaling pathways that are key to cancer initiation and progression, and enhance the anticancer potential of clinical drugs while reducing their toxic side effects. Although some Ranunculaceae phytometabolites were isolated decades ago, this review focuses on pharmacological properties and the latest advances in molecular mechanisms and functions. We discuss our current state of knowledge for adjuvant potential, and anti-cancer activity of Ranunculaceae phytometabolites in vitro and in vivo, and highlight their abilities to modulate the hallmarks of cancer. In this article, “anti-cancer” refers to the ability of the compound to inhibit tumor cell proliferation or induce cell death of tumor cells. The indicated activity in cultured cells is usually regarded as the “in vitro” result, while the indicated activity in experimental animals, tumor xenogradts, or human tumor samples is usually regarded as the “in vivo” result. The phytometabolites found to exhibit the cytotoxic or anti-proliferative activities considered to be promising candidates for screening of antitumor phytometabolites.
The Ranunculaceae family (eudicot Ranunculales) consists of at least 62 genera and 2 200 species, and 42 genera and about 720 species are distributed throughout Mainland China, most of which are found in the southwest mountainous region [2, 3]. In traditional Chinese medicine (TCM), at least 13 Ranunculaceae genera are used in heat-clearing and detoxification (Qing Re Jie Du in TCM), 13 genera used in ulcer disease and sore (Yong Ju Chuang Du in TCM), and seven genera used in swell-reducing and detoxification (Xiao Zhong Jie Du in TCM) [2, 4]. These genera may contain useful phytometabolites that can be used to combat against cancer. Extracts and/or isolated phytometabolites of at least 17 genera have shown anti-cancer/cytotoxic activities toward various tumor cells [1, 2, 5-7]. The distribution of anti-cancer phytometabolites within Ranunculaceae is not random but phylogeny-related [8]. For instance, Ranunculus, Clematis, Pulsatilla, Anemone, and Nigella are rich in pentacyclic triterpene saponins (e.g. Fig. 1, structures 1-6); Actaea and Cimicifuga are rich in tetracyclic triterpene saponins, diterpenoids, triterpenoids, and monoterpenes (e.g. Fig. 2, structures 7-13), which are also found in Thalictrum and Anemone; isoquinoline alkaloids (e.g. Fig. 3, structures 14-17) are abundant in Asteropyrum, Caltha, Nigella, Delphinieae, Adonideae, Thalictroideae, and Coptidoideae; diterpenoid alkaloids (e.g. Fig. 3, structures 18-20) are abundant in Delphinium, Consolida, and Aconitum, but are also found in Nigella and Thalictrum. The same class of phytometabolite, e.g., saponins, could exert the anti-cancer activity via multiple molecular pathways, as schematically depicted below in (Fig. 5), while the same signaling pathway could be the target of distinct phytometabolites, including thymoquinone (TQ, Fig. 2, structure 13), and berberine (Fig. 3, structure 14), as reviewed in [9-13].
Fig. (1).
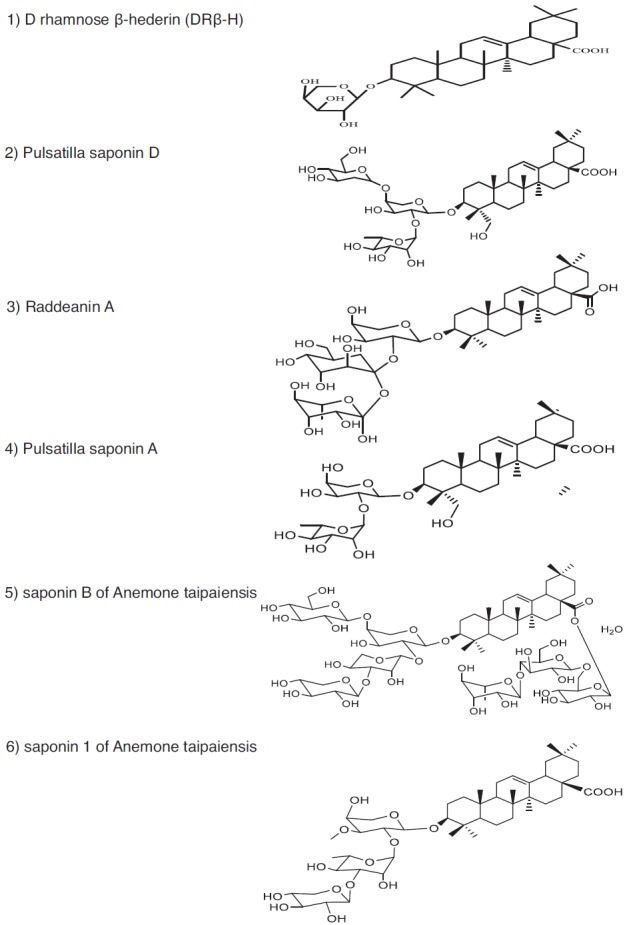
Representative anticancer phytometabolites found in the family Ranunculaceae. Chemical Structures. Pentacyclic triterpene saponins. 1) D rhamnose β-hederin (DRβ-H); 2) Pulsatilla saponin D; 3) Raddeanin A; 4) Pulsatilla saponin A; 5) saponin B of Anemone taipaiensis; 6) saponin 1 of Anemone taipaiensis.
Fig. (2).
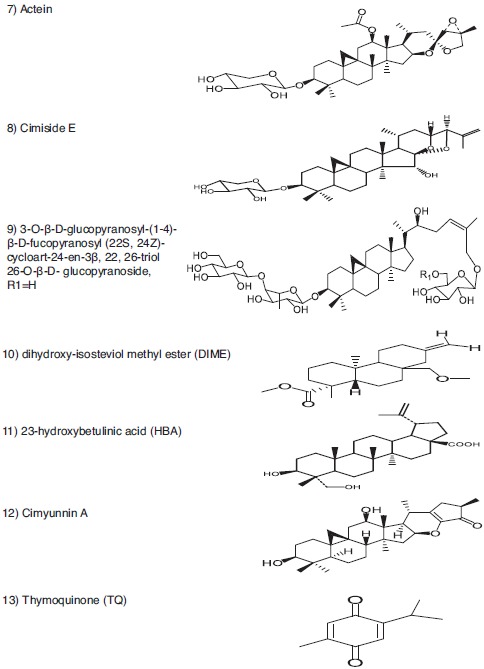
Representative anticancer phytometabolites found in the family Ranunculaceae. Chemical Structures. Tetracyclic triterpene saponin (7-9). Diterpenoid (10). Triterpenoid (11, 12). Monoterpene (13).
Fig. (3).
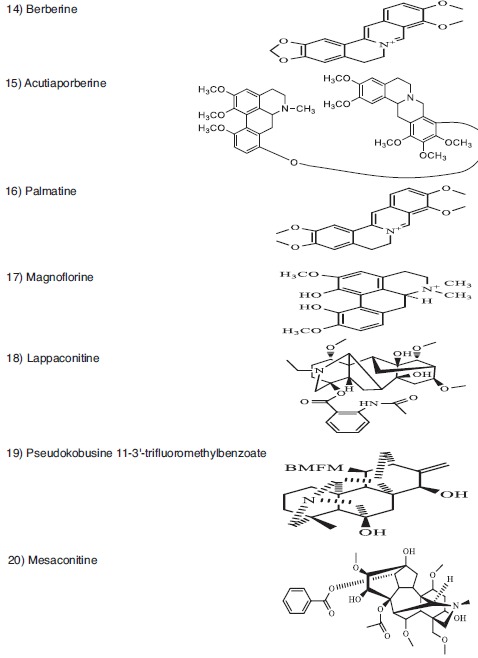
Representative anticancer phytometabolites found in the family Ranunculaceae. Chemical Structures. Isoquinoline alkaloid (14-17). Diterpenoid alkaloid (18-20).
Fig. (5).

The cancer hallmarks modulated by Ranunculaceae phytometabolites. Ranunculaceae phytometabolites regulate all hallmarks of cancer, as defined by Hanahan and Weinberg [134]. The putative PARP inhibitor has not been identified from Ranunculaceae plants. Instead, various antioxidants found in Ranunculaceae might play important roles in fighting against genome instability and mutation of cancer cells.
2. CELL DEATH PATHWAYS
As cancer cells exhibit multiple mechanisms to resist the induction of programmed cell death (apoptosis), the modulation of apoptotic signaling pathways by Ranunculaceae phytometabolites has been shown to constitute a key event in their anticancer activities, as reviewed elsewhere [10, 12, 13]. In addition, cell cycle arrest, autophagy modulation, cell senescence and other pathways are also involved in anti-cancer molecular mechanisms induced by various Ranunculaceae phytometabolites, as reviewed in [10, 12, 13].
2.1. Saponins
2.1.1. Clematis
Saponins, which are abundant in Ranunculaceae, especially in Clematis, Pulsatilla, Anemone, and Cimicifugeae, usually exert their anti-cancer activities via induction of cell cycle arrest and apoptosis [1, 2, 6, 7]. The aglycones of Clematis pentacyclic triterpene saponins mainly belong to oleanolic type (A), olean-3β, 28-diol type (B), hederagenin type (C) or hederagenin-11, 13-dien type (D), where types A and C are predominant [1, 7]. Many Clematis saponins have cytotoxic activity against human glioblastoma [14], hepatoma [15], cervical cancer [16], leukemia [15, 17], gastric cancer [15, 17], colon cancer [18], and prostate cancer [19]. However, the mechanistic study is scarce. For instance, D-Rhamnose β-hederin (DRβ-H, 1 of Fig. 1), an oleanane-type triterpenoid saponin from TCM plant Clematis ganpiniana, inhibited phosphoinositide 3-kinase (PI3K)/ protein kinase B (PKB, also known as AKT) pathway and activated an extracellular signal-regulated kinase (ERK) signaling in human breast cancer cells [20]. PI3K inhibitor LY294002 synergistically enhanced DRβ-H-induced apoptosis while MEK inhibitor U0126 reduced the apoptosis rate [20]. DRβ-H regulated the ratio of pro-apoptotic and anti-apoptotic BCL-2 family proteins. DRβ-H induced depolarization of mitochondrial membrane potential to release Apoptotic Peptidase Activating Factor 1 (APAF-1) and cytochrome C from the intermembrane space into the cytosol, where they promoted caspase-9 and caspase-3 activation [20].
2.1.2. Pulsatilla
Pulsatilla, Anemone, and Clematis belong to the tribe Anemoneae, and Pulsatilla is evolutionarily more close to Anemone than to Clematis [2]. Saponins exhibit cytostatic and cytotoxic activity against various cancer cells, but the mechanism is not fully understood. Pulsatilla koreana saponin D (SB365) strongly suppressed the growth of hepatocellular carcinoma (HCC) cells in a dose-dependent manner and induced apoptosis by increasing the proportion of sub G1 apoptotic cells from 8% to 21% through induction of BAX expression and caspase-3 cleavage [21]. SB365 effectively suppressed the phosphorylation of PI3K downstream factors, e.g., AKT, mammalian target of rapamycin (mTOR), and p70S6 kinase (p70S6K) serine/threonine kinase in vitro and in vivo [22, 23]. SB365 suppresses the proliferation of human colon cancer and pancreatic cancer cells and induces apoptosis by modulating the AKT/mTOR signaling pathway [22, 23].
The Ka (absorption rate constant) and Papp (apparent permeability coefficient) values of Pulsatilla saponin D (Fig. 1, structure 2) are highest in the colon, followed by ileum, jejunum, and duodenum [24]. The Pulsatilla saponins were not transported in a concentration dependent manner, and the transporter protein might be involved in their transport. The Pulsatilla saponins exhibited rapid absorption, quick elimination, and significant double peak on the plasma concentration-time curve [25]. However, the oral bioavailability is quite low (0.55-2.5%), due to the unfavorable molecular size (≥ 733.5 Da) of pentacyclic triterpene saponins, poor gut absorption, and/or extensive metabolism after absorption [26]. Nanoformulations have to be developed to maximize the anticancer effects of Pulsatilla saponins.
With the aid of the array of the 42 phosphorylated receptor tyrosine kinases (RTKs), SB365 was found to dock at an allosteric site on the epithelial-mesenchymal transition (c-MET) factor and strongly inhibited its expression in gastric cancer cells [27]. The activation of the c-MET signal cascade components, including AKT and mTOR, was inhibited by SB365 in a dose-dependent manner [27, 28]. SB365 inhibited the phosphorylation of c-MET proto-oncogene and the downstream signaling pathway required for growth and survival in the c-MET-amplified HCC827GR non-small cell lung cancer (NSCLC) cells [28]. SB365 suppressed the anchorage-independent growth, migration and invasion and induced apoptosis in HCC827GR cells [28]. Pulsatilla saponin A (4 of Fig. 1) of P. chinensis may exert its antitumor effect by inducing DNA damage and causing G2/M arrest and apoptosis in multiple cancer cells [29]. Pulsatilla saponin A increased p53 and cyclin B protein levels, and decreased BCL-2 protein level [29].
2.1.3. Anemone
The genus Anemone, evolutionarily closely related to Pulsatilla, is also rich in therapeutic saponins [2]. Raddeanin A (Fig. 1, structure 3), a pentacyclic triterpene saponin from Anemone raddeana (Liang Tou Jian in TCM), inhibits
proliferation and induces apoptosis of multiple cancer cells [30, 31]. Raddeanin A increased BAX expression, reduced BCL-2, BCL-xL and survivin protein levels, and significantly activated caspase-3, -8, -9 and poly-ADP ribose polymerase (PARP) [31]. Saponins B (Fig. 1, structure 5), 1 (Fig. 1, structure 6), and 6 of Anemone taipaiensis exhibit significant anticancer activity against human leukemia, glioblastoma multiforme (GBM), and HCC [32-34]. Saponin 1 caused characteristic apoptotic morphological changes in GBM cells, which was confirmed by DNA ladder electrophoresis and flow cytometry [32]. Saponin 1 also caused a time-dependent decrease in the expression and nuclear location of nuclear factor-kappa B (NF-κB) transcription factor, as indicated in [32]. The expression of inhibitors of apoptosis (IAP) family members, e.g., survivin (also known as baculoviral IAP [inhibitor of apoptosis protein] repeat-containing protein [BIRC5]) and X-linked inhibitor of apoptosis protein (XIAP, also known as BIRC4) was significantly decreased by saponin 1 [32]. Moreover, saponin 1 caused a decrease in the BCL-2/BAX ratio and initiated apoptosis by activating caspase-9 and caspase-3 in the GBM cell lines [32, 33]. Thus, saponin 1 inhibits cell growth of GBM cells at least partially by inducing apoptosis and inhibiting survival signaling mediated by NF-κB [32, 33]. Saponin B blocked the cell cycle at the S phase [34]. Saponin B induced chromatin condensation of U87MG GBM cells and led to the formation of apoptotic bodies [34]. Data obtained from the Annexin V/propidium iodide assay showed that phosphatidylserine externalization was apparent at higher drug concentrations [34]. Saponin B activated the receptor-mediated pathway of apoptosis via the activation of FAS ligand (FASL), as indicated in [34]. Saponin B increased the BAX and caspase-3
protein levels and decreased the protein expression of BCL-2 [34]. Triterpenoid saponins of Anemone flaccida induce apoptosis in human HepG2 hepatoma BEL-7402 cells, and lipopolysaccharide- stimulated human cervical cancer HeLa cells via cyclooxygenase-2 (COX-2, also known as prostaglandin-endoperoxide synthase 2)/prostaglandin E2 (PGE2) pathway [35].
2.1.4. Cimicifugeae
Cycloartane triterpenoids and their saponins are mainly distributed in Astragalus (Leguminosae), Cimicifugeae and Thalictrum (Ranunculaceae), and possess various bioactivities [36]. Actaea and Cimicifuga are closely related genera of the tribe Cimicifugeae, which are closer to Souliea than to Eranthis [1, 2, 6]. Beesia is closer to Anemonopsis than to other four genera [1, 2, 6].
Our research group found that 9, 19-cycloartane triterpene glycosides of Actaea asiatica had notable cytotoxicity against human hepatocellular carcinoma HepG2 cells and breast cancer MCF-7 cells [37]. The tetracyclic triterpene saponins and their aglycones displayed the cytotoxic activity against HCC, lung adenocarcinoma [38], gastric cancer [39], leukemia, colon cancer, and breast cancer [40]. Cycloartane glycosides from the roots of Cimicifuga foetida (Sheng Ma in TCM) showed significant WNT signaling pathway inhibitory activity, with IC50 of 3.33 and 13.34 μM, respectively [41]. Our research group found that 23-O-acetylcimigenol-3-O-β-D-xylopyranoside could inhibit the proliferation of HepG2 cells with IC50 16 μM, and could induce apoptosis and G2/M cell cycle arrest [42]. This tetracyclic triterpene saponinis able to cleave PARP, regulate protein expression of BCL-2 family and decrease the expression of cell division cycle protein 2 homolog (CDC2, also known as cyclin-dependent kinase 1, CDK1) and cyclin B (CCNB). 25-Acetyl-7, 8-didehydrocimigenol 3-O-β-D-xylopyranoside is more potent than the parent compound 7, 8-didehydrocimigenol 3-O-β-D-xylopyranoside in inhibiting ER-/HER2 overexpressing human breast cancer MDA-MB-453 cells [43]. HER2 is a receptor tyrosine-protein kinase ERB-2 (also known as CD340 [cluster of differentiation
340], proto-oncogene NEU, or ERBB2), and a member of the human epidermal growth factor (EGF) receptor (EGFR) family [44-48]. Amplification or overexpression of this oncogene has been shown to play an important role in the development and progression of certain aggressive types of breast cancer [44-48].
Cell cycle arrest in human gastric cancer cells was induced by cimiside E (Fig. 2, structure 8) of C. heracleifolia (a source plant of Sheng Ma) in S phase at a lower concentration (30 μM) and G2/M phase at higher concentrations (60 and 90 μM) [39]. Cimiside E mediated apoptosis through the induction of the caspase cascade for both the extrinsic and intrinsic pathways. Our research group found that 25-anhydrocimigenol-3-O-β-D-xylopyranoside of Souliea vaginata (Huang San Qi in TCM) caused apoptosis and G0/G1 cell cycle arrest in HepG2 cells, and exhibited a dose-dependent inhibition of tumor growth on mice implanted with H22 in vivo [43].
Actein (Fig. 2, structure 7), a triterpene glycoside from black cohosh (Actaea racemosa, Cimicifuga racemosa), strongly inhibited the growth of human breast cancer cells and induced a dose-dependent release of calcium from endoplasmic reticulum (ER) and mitochondria into the cytoplasm [49]. Heparin, the antagonist for the ER inositol 1,4,5-trisphosphate receptor (IP3), blocked this release [49]. Heparin partially blocked the growth inhibitory effect of actein, while the MEK inhibitor U0126 enhanced it [49]. Actein synergized with the ER mobilizer thapsigargin and preferentially inhibited the growth of human embryonic kidney 293T cells [49]. Nanoparticle liposomes increased the growth inhibitory activity of actein [49]. Actein alters the activity of the ER IP3 receptor and Na, K-ATP-ase, induces calcium release, modulates the NF-κB and mitogen-activated protein kinase kinase (also known as MAP2K, MEK, MAPKK) pathways, and causes cytochrome C release from mitochondria, which may partially explain the anti-cancer effect of actein [49].
Extracts (enriched for triterpene glycosides) and phytometabolites from the North American perennial black cohosh (Actaea racemosa L; Ranunculaceae) and related Cimicifuga species were shown to inhibit proliferation of human breast cancer cells (MDA-MB-453 cells and MCF7 [ER+/Her2 low] cells), as indicated in [50]. Forced expression of exogenous HER2 in human breast cancer MCF7 (ER+/Her2 low) cells made these cells more sensitive than the parental MCF7 cells to the growth inhibitory effects of actein, indicating that HER2 protein plays a role in the actein ability to inhibit tumor cell proliferation [50]. Actein was also found to alter the distribution of actin filaments and induced apoptosis in these cells, suggesting that actein might have both cytostatic and cytotoxic activity [50].
3-O-β-D-glucopyranosyl- (1→4)-β-D-fucopyranosyl (22S, 24Z)-cycloart-24-en-3β, 22, 26-triol 26-O-β-D-glucopyranoside (Fig. 2, structure 9), isolated from Thalictrum fortunei, caused apoptosis and mitochondrial membrane potential loss in human hepatoma Bel-7402 cells [46]. The intracellular reactive oxygen species (ROS) were markedly upregulated by compound 1, as indicated in [51]. Compound 1 significantly increased the expression levels of cleaved caspase-3, p53 and BAX protein, and decreased the expression of BCL-2 protein [51].
2.2. Terpenoid
Dihydroxy-isosteviol methyl ester (DIME, Fig. 2, structure 10), a diterpene isolated from Pulsatilla nigricans, caused a significant decrease in cell viability, induced nuclear condensation and inter-nucleosomal DNA fragmentation [52]. DIME interacted with calf thymus DNA, bringing apparent changes in structure and conformation [52]. 23-Hydroxy-betulinic acid (Fig. 2, structure 11) of Pulsatilla chinensis could promote cell cycle arrest in S phase and induce apoptosis of human chronic myelogenous leukemia K562 cells via intrinsic pathway [53]. HBA disrupts mitochondrial membrane potential significantly and selectively downregulates the levels of BCL-2, survivin and upregulates BAX, cytochrome C, cleaved caspase-9 and -3 [53].
Thymoquinone (TQ, 2-methyl-5-isopropyl-1, 4-benzoquinone, Fig. 2, structure 13), a monoterpene abundant in Nigella sativa, down-regulates E2F-1 protein and androgen receptors, and causes apoptosis in cancer cells [54]. Thymoquinone downregulates the expression of BCL-2 and other anti-apoptotic proteins, and upregulates the expression of pro-apoptotic proteins (caspase-3, -8, -9 & BAX) expression in cancer cells, as indicated in [55, 56]. Thymoquinone induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2- and SRC- mediated phosphorylation of EGFR tyrosine kinase [10]. Recently, a serine/threonine-protein kinase PAK1 was identified as a novel target for thymoquinone [57]. Thymoquinone-induced conformational changes in PAK1 protein interrupt prosurvival MEK-ERK signaling in human colorectal cancer and enhance apoptosis [57]. Thymoquinone targets various components of intracellular signaling pathways, particularly upstream kinases and transcription factors, which are aberrantly activated during carcinogenesis [11]. The above mechanisms exemplify how thymoquinone causes apoptosis in cancer cells.
Thymoquinone also displays anti-proliferative and cytostatic effects [58]. For instance, thymoquinone inhibits NF-κB activation induced by various carcinogens, and controls oncogenic expression [58]. In N-nitrosodiethylamine-induced rat HCC, thymoquinone strongly induced G1/S arrest in cell cycle transition [59]. Thymoquinone suppresses the activation of AKT and ERK, and activates c-Jun NH2-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways, as described in [60, 61]. Thymoquinone pretreatment overcomes the pancreatic cancer cell resistance potentiating the anticancer effect of gemcitabine through abrogation of NOTCH1, and modulation of PI3K/AKT/mTOR signaling pathways in pancreatic cancer [62]. Thymoquinone inhibited topoisomerase IIα activity when incubated with the enzyme prior to the addition of DNA, but enhanced enzyme-mediated DNA cleavage ~5-fold, which is similar to the increase seen with the anticancer drug etoposide [63]. Thymoquinone inhibits thymidine incorporation during DNA synthesis, and inhibits cancer cell growth [63, 64].
The polo-box domain (PBD), a unique functional domain of polo-like kinase (PLK), is being targeted to develop PLK1-specific inhibitors [65]. Thymoquinone and its derivative Poloxin are known PBD inhibitors and were shown to increase cell population in S phase and in G2/M, in a p53-independent manner [65]. Poloxin and Thymoquinone did not increase population of cells staining positively for phosphorylated (p)-histone H3 and M-phase phosphoprotein-2 (MPM2, also known as MPHOSPH, the protein involved in The transition of G2 to M phase), as indicated in [65]. However, both PBD inhibitors caused an increase in p21WAF1 and cell cycle arrest at the S-phase, indicating that they affected interphase before mitotic entry [65].
Cell death may occur in several mechanisms, including apoptosis, necrosis (see below, anti-inflammatory activity), and autophagy. Autophagy is a catabolic process that maintains cellular homeostasis in response to various cellular stress factors. Thymoquinone-treated head and neck squamous cell carcinoma cells showed increased levels of autophagic vacuoles and specific autophagy markers LC3-II proteins [66]. Thymoquinone treatment also increased the accumulation of autophagosomes [66]. A BALB/c nude mouse xenograft model showed that thymoquinone administered by oral gavage reduced tumor growth via induced autophagy and apoptosis in vivo [66]. Bafilomycin A1, an autophagy inhibitor, enhanced thymoquinone cytotoxicity but did not promote apoptosis [66]. Cell viability was eradicated in autophagy-defective cells [66]. These results imply that inhibition of autophagy is an emerging strategy in cancer therapy [66]. In human colon cancer CPT-11-R LoVo cells, thymoquinone caused mitochondrial outer membrane permeability and activated autophagic cell death [67]. JNK and p38 inhibitors (SP600125 and SB203580, respectively) reversed TQ autophagy [67]. Thymoquinone activated apoptosis before autophagy, and the direction of cell death was switched toward autophagy at initiation of autophagosome formation [67].
GBM cells may be dependent on the autophagic pathway for survival [68]. Thymoquinone inhibits autophagy and induces cathepsin-mediated, caspase- independent cell death in GBM cells [68]. Thymoquinone induces lysosome membrane permeabilization, which results in a leakage of cathepsin B into the cytosol and mediates caspase-independent cell death that can be prevented by pre-treatment with a cathepsin B inhibitor [68]. Thymoquinone induced apoptosis appears to be caspase- independent due to failure of the caspase inhibitor z-VAD-FMK to prevent cell death and absence of the typical apoptosis signature DNA fragmentation [68].
2.3. Alkaloid
Thalictrum, Coptis, and Hydrastis are rich in isoquinoline alkaloids [2]. The anticancer effects of Coptis Chinensis (Huang Lian in TCM) can be ascribed to its TCM trait of removing damp-heat, fire and toxicity [69]. Berberine, a major isoquinoline alkaloid of Huang Lian and other related species (Fig. 3, structure 14), is accumulated inside prostate cancer cells that were in G1 phase of the cell cycle and enhanced apoptosis [70]. Berberine inhibited the expression of prostate-specific antigen, and attenuated EGFR activation following EGF treatment in vitro [70]. Berberine induces apoptosis via the mitochondrial pathway in liver cancer cells [71]. Berberine increased the expression of BAX, followed by the activation of the caspase cascade. Procaspase-9 and its effectors, procaspase-3 and -7, were activated by berberine. Berberine activates the transcription factor Egr-1 and consequently induces the expression of non-steroidal anti-inflammatory drug (NSAID)-activated gene (NAG-1), which mediates the drug-induced pro-apoptotic action in HCC HepG2 cells [72]. Other protoberberine-type alkaloids in Huang Lian might exhibit synergistic effects, while showing the anti-cancer effects [69, 70].
Berberine significantly induced the mRNA expression of Forkhead box family members, (FOX)-O1 and O3A [73]. Their phosphorylation-mediated cytoplasmic sequestration followed by degradation was prevented by berberine-induced downmodulation of the PI3K/AKT/mTOR pathway, which promoted a nuclear retention of FOXO proteins [73]. Phosphatase and tensin homolog (PTEN), a tumor suppressor protein and negative regulator of the PI3K/AKT axis, was upregulated while phosphorylation of its Ser380 residue (possible mechanism of PTEN degradation) was significantly decreased in berberine-treated HCC HepG2 cells [73]. Berberine induced a significant increase in transcriptional activity of FoxO, which effectively enhanced BH3-only protein BIM expression, followed by the direct activation of pro-apoptotic protein BAX, increased BAX/BCL-2 ratio, mitochondrial dysfunction, caspases activation, and DNA fragmentation [73]. Silencing of BIM expression partially restored HCC HepG2 cell viability during berberine exposure, implying the pivotal role of BIM in berberine-mediated cytotoxicity [73]. Acutiaporberine (Fig. 3, structure 15), a bis-alkaloid isolated from Thalictrum acutifolium, may be a natural potential apoptosis-inducing agent for highly metastatic lung cancer [74]. Palmatine (16 of Fig. 3, structure 16), but not berberine, is a cell cycle blocker in G1 of A549 adenocarcinoma cells [75]. Berberine induced cell cycle arrest at the G2/M phase in human promyelocytic leukemia HL-60 cells and murine myelomonocytic leukemia WEHI-3 cells, which was accompanied by increased levels of WEE1 and 14-3-3σ, and decreased levels of CDC25C, CDK1, and cyclin B1 (CCNB1), while CDK2 expression was not affected by berberine [76].
In melanoma cell lines, berberine at low doses (12.5-50 μM) is concentrated in mitochondria and promotes cell cycle arrest at the G1 phase [77]. Higher doses (> 50 μM) result in cytoplasmic and nuclear accumulation of berberine, and G2 phase arrest [77]. DNA synthesis is not markedly affected by low doses of berberine, but 100 μM is strongly inhibitory [77]. Notably, 100 μM of berberine inhibits cell growth with relatively little induction of apoptosis [77]. Berberine displays multiphasic effects in malignant cell lines, which are correlated with its concentration and intracellular distribution [13, 78]. These results help explain some of the conflicting information regarding the effects of berberine, which may be more as a cytostatic agent than a cytotoxic compound [13, 78].
Berberine may inhibit protein synthesis, histone deacetylase (HDAC), or AKT/mTOR pathways [78]. Berberine induced endoplasmic reticulum (ER) stress and autophagy, which was associated with activation of AMP-activated protein kinase (AMPK), as reviewed in [13, 78]. However, berberine did not alter mTOR or HDAC activities in MDA-MB-231 human breast cancer cells [78]. Berberine induced the acetylation of α-tubulin, a substrate of HDAC6 [78]. In addition, the combination of berberine and suberoylanilide hydroxamine (SAHA), a pan-HDAC inhibitor, synergistically inhibited cell proliferation and induced cell cycle arrest. Berberine induced both autophagy and apoptosis in HCC cells, and the expression of basigin (also known as extracellular matrix metalloproteinase inducer [EMMPRIN] or cluster of differentiation 147 [CD147]) was downregulated by berberine [79].
Berberine primarily exerts its anti-cancer effect by inducing cell cycle arrest, apoptosis, and autophagy [13]. However, berberine inhibited GBM cells through induction of cellular senescence [80]. Berberine drastically reduced the level of EGFR, and the downstream c-RAF-MEK-ERK signaling pathway was remarkably inhibited, whereas AKT phosphorylation was not altered in GBM cells [80]. Pharmacologic inhibition or RNA interference of EGFR similarly induced cellular senescence of GBM cells, which was rescued by introduction of a constitutive active mitogen-activated protein kinase kinase [80]. Berberine potently inhibited the growth of tumor xenografts, which was accompanied by downregulation of EGFR and induction of senescence [80].
Diterpenoid alkaloids are abundant in Aconitum and Delphinium, and are known to exhibit anti-cancer activities, as reviewed in [1, 2, 5]. For instance, lappaconitine (Fig. 3, structure 18) caused G0/G1 cell cycle arrest, apoptosis and downregulation of cyclin E1 (CCNE) gene expression of non-small cell lung cancer [81]. Taipeinine A, a C19-diterpenoid alkaloid from the roots of Aconitum taipeicum, upregulated the protein expression of BAX and caspase-3 and downregulated the expression of BCL-2 and cyclin D1 (CCND1) [82]. The Delphinium diterpenoid alkaloids were found to be cytotoxic against human adenocarcinoma A549 cells with the IC50 values ranged from 12.03 to 52.79 μM, as indicated in [83].
2.4. Cardioactive Steroid
Hellebrigenin (Fig. 4, structure 21) of Helleborus, one of bufadienolides belonging to cardioactive steroids, potently reduced the viability and colony formation of human HCC cells [84]. Hellebrigenin triggered DNA damage through DNA double-strand breaks and subsequently induced cell cycle G2/M arrest associated with up-regulation of phosphorylated (p)-ataxia telangiectasia mutated (ATM) kinase, p-checkpoint kinase (CHK)-2, p-CDK1 and cyclin B1 (CCNB1), and down-regulation of p-CDC25C, as described in [84]. Hellebrigenin induced mitochondrial apoptosis, characterized by BAX translocation to mitochondria, disruption of mitochondrial membrane potential, release of cytochrome C into cytosol, and sequential activation of caspases and PARP, as indicated in [84]. AKT expression and phosphorylation were inhibited by hellebrigenin, whereas AKT silencing with siRNA abolished cell cycle arrest but enhanced apoptosis induced by hellebrigenin [84]. Activation of AKT by human insulin-like growth factor (IGF)-1 could obviously attenuate hellebrigenin-induced cell death [84].
Fig. (4).

Representative anticancer phytometabolites found in the family Ranunculaceae. Chemical Structures. Cardioactive steroid (21). Phenolic acid (22-25). Lactone (24, 25).
2.5. Plant Extract
The plant extracts are traditionally obtained from the whole plant, root, rhizome, stem, leaf, flower, and other uncultured/cultured tissues/cells, which may contain multiple therapeutic phytometabolites. For instance, Pulsatilla chinensis (Bai Tou Weng in TCM), rich in pentacyclic triterpene saponins, is among the top 10 potent anti-mitotic inhibitors (independent of toxicity) in a recent study that screens 897 aqueous extracts of commonly used natural products (0.00015- 0.5 mg/mL) relative to paclitaxel for anti-mitotic effects on human breast cancer MDA- MB-231 cells [85]. Pulsatilla koreana extract strongly suppressed the growth of HCC and anaplastic thyroid cancer cells in a dose-dependent manner [86, 87]. Apoptosis induced by Pulsatilla koreana extract was observed using DAPI and TUNEL staining assays and the cleaved PARP and caspase-3 were increased in HCC Huh-7 cells [86].
In MDA-MB-453 human breast cancer cells, a methanolic extract of Cimicifuga racemosa caused a significant increase in expression of ER stress (e.g., GRP78), apoptotic (GDF15), lipid biosynthetic (INSIG1 and HSD17B7) and phase I (CYP1A1) genes, and decrease in expression of cell cycle regulators, such as HELLS and PLK4, as described elsewhere [88]. A lipophilic C. racemosa rhizome extract significantly regulated 431 genes in human breast cancer MCF-7 cells, many of which are involved in anti-proliferation and pro-apoptotic networks [9]. The expression pattern differed from those induced by 17β-estradiol or the estrogen receptor antagonist tamoxifen [88]. Our group found that the ethyl acetate fraction (EAF) of C. foetida aerial part induced G0/G1 cell cycle arrest at lower concentration (25 μg/mL) in human HCC HepG2 cells, and triggered G2/M arrest and apoptosis at higher concentrations (50 and 100 μg/mL) [89]. An increase in the ratio of BAX/BCL-2, activation of downstream effector caspase 3, and cleavage of PARP were implicated in EAF-induced apoptosis [89]. EAF inhibited the growth of the implanted mouse H22 tumor in a dose-dependent manner with the growth inhibitory rate of 63.32% at 200 mg/kg, as described in [89]. Total triterpenoid glycosides of C. dahurica (a source plant of Sheng Ma) aerial part showed the similar effects as EAF [90]. An isopropanolic extract of C. racemosa induced apoptosis of human prostate androgen- dependent and -independent carcinoma cells, accompanied by the increased degradation of cytokeratin 18 (a known caspase substrate) [91].
The extract from Coptis chinensis has a wide effect on cell signaling, including cell cycle regulation (CDK6, CDK4, cyclin B1, cyclin E, cyclin D1, p27), cell adhesion (E-cadherin, osteopontin), differentiation, apoptosis (p-STAT3, p53, BRCA1), cytoskeleton (p-PKC α/β II, Vimentin, p-PKCα), MAPK signaling (RAF-1, p-p38 MAPK, p-ERK1/2), and the PI3K signaling pathway (p-AKT, p-PTEN), as described in [92]. The phytometabolites from C. chinensisis are suggested to be novel therapeutic drugs for squamous cell carcinoma [92].
3. MICRORNAS, DNA DAMAGE, EPIGENETIC REGULATION
3.1. MICRORNAs
MicroRNAs (miRs) are small 18-24-nucleotide non-coding RNAs, which repress target gene expression largely by modulating translation and mRNA stability [93]. MicroRNA expression is deregulated in many types of cancer [93]. Drug-induced microRNAs have emerged as key regulators in guiding their pharmacological effects [93, 94]. For instance, PEG4000-TQ- nanoparticles could expressively increase the expression of miR-34a through p53 [94]. MiR-34a up-regulation directly downregulated RAC1 expression followed by actin depolymerization and disrupted the actin cytoskeleton, which leads to significant reduction in the lamellipodia and filopodia formation on cell surfaces [94]. PEG4000-TQ-nanoparticles circumvent TQ's poor aqueous solubility, thermal and light volatility, and consequently minimal systemic bioavailability, and thus might be more powerful in retarding cancer cell migration [94].
A berberine-induced miR-21-3p directly down-regulates methionine adenosyltransferases 2A and 2B inhibiting the growth of human HCC Hep2B cells [95]. Overexpression of miR-21-3p increased intracellular S-adenosylmethionine content, which is disadvantageous for HCC cell growth [95]. In NVP-AUY922 (second generation Hsp90 inhibitor)-insensitive colorectal cancer cells, the combination of NVP-AUY922 and berberine caused cell growth arrest through inhibiting CDK4 expression and induction of miR-296-5p- mediated suppression of Pin1-β-catenin-cyclin D1 signaling pathway [96]. The miR-93 levels in cisplatin-resistant cells were higher than that in cisplatin-sensitive cells, as reviewed elsewhere [93, 97]. Berberine could inhibit miR-93 expression and increase its target tumor suppressor PTEN [97]. Berberine modulated the sensitivity of cisplatin through miR-93/PTEN/AKT signaling pathway in the ovarian cancer cells [97]. In multiple myeloma cells, berberine significantly down-regulated miRNA clusters miR-99a, miR-125b, miR-17-92, and miR-106 -25 [98]. RAC1, NF-κB, c-MYC, c-JUN, and CCND1, the top five differentially regulated genes, might play key roles in the progression of multiple myeloma [98]. Three common signaling pathways (TP53, ERB, and MAPK) link the three miRNA clusters and the five key mRNAs [98].
3.2. DNA Damage and Epigenetic Regulation
3.2.1. Thymoquinone
Human telomere DNA regulates gene transcriptions and folds up into G-quadruplex structures that inhibit telomerase over-expression in cancer cells, as reviewed in [99]. Thymoquinone interacts with G-quadruplex on two binding sites adjacent to the TTA sequence loop [99]. Thymoquinone is preferentially binding to G-quadruplex over duplex, which is explained by an intercalation-binding mode based on π-π stacking [99]. Thymoquinone might act as a G-quadruplex DNA stabilizer and subsequently inhibit telomerase and cancer proliferation [99].
Thymoquinone induced DNA damage, cell cycle arrest and apoptosis in GBM cells [100]. Thymoquinone facilitated telomere attrition by inhibiting the activity of telomerase. DNA-dependent protein kinase (DNA-PK) is a nuclear, serine/threonine protein kinase consisting of a 470-kDa catalytic subunit of DNA-PK and a heterodimeric regulatory complex KU70/80, as reviewed elsewhere [100]. This enzyme is essential for the repair of DNA double-strand breaks and mediates DNA repair via phosphorylation of downstream DNA binding proteins such as p53, as reviewed in [100]. Telomeres in GBM cells with DNA-PK were more sensitive to thymoquinone-mediated effects as compared to those cells deficient in PRKDC/DNA-PK gene [100].
Plant-derived antioxidants can switch to prooxidants even at low concentrations in the presence of transition metal ions such as copper, as reviewed in [101]. It is noted that tissue, cellular and serum copper levels are considerably elevated in various malignancies [101]. Thymoquinone is able to cause an oxidative cellular DNA breakage, which can be inhibited by copper-chelating agents and scavengers of ROS [101]. Thymoquinone targets cellular copper in human prostate cancer cells (PC3 and LNCaP) causing a prooxidant cell death [101]. Such a prooxidant cytotoxic mechanism could explain the anticancer activity of plant-derived antioxidants [101]. The in silico target identification analysis suggests that HDAC2 proteins could act as the potential targets of thymoquinone [102]. In cancer cells, thymoquinone treatment resulted in a significant HDAC2 inhibition and histone hyperacetylation, providing the first evidence of thymoquinone as a potential epigenetic modulator [103].
3.2.2. Bereberine and Other Alkaloids
Berberine was shown to induce DNA repair in a panel of DNA repair-deficient chicken B lymphocyte (DT40) clones, as described in [104]. For example, DT40 cells (Rev3-/-), deficient in Reversionless-3 (Rev3) gene, were found to be hypersensitive to berberine, as described in [104]. Rev3 protein (also known as DNA polymerase zeta catalytic subunit) is an enzyme that interacts with Rev7 to form Pol ζ, a B family polymerase [105]. Since, Pol ζ lacks 3' to 5' exonuclease activity, it requires Rev3 protein to contribute into translesion synthesis, a DNA damage tolerance process allowing the DNA replication machinery to replicate past DNA lesions [105]. Following berberine treatment, cell cycle analysis identified that G2/M arrest was increased in Rev3-/- cells [104]. Furthermore, berberine also induced a significant increase in double-strand breaks in Rev3-/- cells compared to wild-type cells, as revealed by chromosomal aberration analysis [104]. These results suggest that berberine is able to induce DNA damage, and that the Rev3-associated DNA repair pathway participates in the DNA repair process [104].
Histone H2AX is phosphorylated at the position S139 (also known as γH2AX) upon DNA damage, thereby its foci formation recognized by specific antibosies could be often used to detect DNA damage in many cells subjected to stress. For example, berberine was found to induce significant concentration- and time-dependent increases in DNA double-strand breaks in human osteosarcoma MG-63 cells, confirmed by the immundetecion of γH2AX foci, as indicated in [106]. Berberine and palmatine appeared to be the most potent DNA damage inducers in HCC HepG2 cells [107]. Berberine and palmatine suppressed the activities of both topoisomerase (TOP)-1, and II as described in [107]. In berberine-treated cells, DNA damage was shown to be directly associated with the inhibitory effect of TOP2, but not TOP1. DNA damage was also observed in cells treated with Hydrastis (also known as golden seal) extracts and the extents of DNA damage were positively correlated with the berberine contents [107]. The TOP2 inhibitory effect may contribute to berberine- and golden seal-induced genotoxicity [107].
Berberine increased the radio-sensitivity of the human breast cancer MCF-7 cells and MDA-MB-468 cells [108]. The radiation-induced G2/M cell cycle delay was reduced in MCF-7 cells pre-treated with berberine, as berberine caused cell cycle arrest at the G1/S phase [108]. Berberine pre-treatment prolonged the persistence of DNA double-stranded breaks in the human breast cancer MCF-7 cells [108]. The protein levels of the DNA repair protein Rad51 homolog 1 (RAD51) were decreased by berberine, and in the cells pre-treated with 15 µM berberine for 24 h, RAD51 protein decreased significantly at 0, 2, 6 and 24 h after X-ray exposure [108]. Berberine sensitizes human breast cancer cells to ionizing radiation by inducing cell cycle arrest and the downregulation of the homologous recombination repair protein, RAD51, as indicated in [108]. This emerging evidence shows that berberine may be a promising radio-sensitizer for the treatment of breast cancer.
Palmatine and berberine can induce the formation of G-quadruplex, as well as increase its stability [109]. The unsaturated ring C, N+ positively charged centers, and conjugated aromatic rings are key factors to increase the stabilization ability of palmatine and berberine [109]. Interactions between berberine and the pBR322 plasmid DNA suggest biologically significant DNA-binding abilities of berberine [109]. The possible binding sites of berberine on histone proteins were determined by molecular docking [110]. In human cervical cancer HeLa cells, berberine might modulate p53 and viral oncoproteins HPV-18 E6-E7 via epigenetic modifications [110]. Berberine can repress the expression of DNA methyltransferase (DNMT)-1 and DNMT3B, which triggers hypomethylation of tumor protein (TP)-p53 by changing the DNA methylation level in human multiple melanoma cell U266 [111].
3.2.3. Phenolic Acid
Among C. racemosa phenylpropanoids tested for protecting against menadione-induced DNA damage, methyl caffeate (Fig. 4, structure 22) is most potent, followed by caffeic acid (Fig. 4, structure 23), ferulic acid, cimiracemate A, cimiracemate B, and fukinolic acid, all of which hasantioxidant activity [112]. The methanol extracts of C. racemosa showed dose-dependent decreases in DNA single-strand breaks and oxidized bases induced by the quinone menadione.
4. OXIDATIVE PROCESS AND METABOLISM
4.1. Antioxidant vs. Prooxidan
Plant-derived dietary antioxidants have attracted considerable interest in recent decades for their chemopreventive and cancer therapeutic abilities. Pulsatilla chinensis polysaccharides enhance superoxide dismutase and catalase enzyme activities and lower MDA levels in the plasma of glioma bearing mice [113]. Polysaccharides could relieve the liver and kidney damage of glioma bearing mice, and decrease plasma levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and urea [113].
α-Hederin, a pentacyclic triterpene saponin abundant in Nigella sativa and Clematis, increases the production of ROS in cancer cells, altering mitochondrial functions and causing apoptosis [114, 115]. Inducers of phaseIIenzyme play an important role in cancer chemoprevention [116]. Quinone reductase (QR), a typical phaseIIenzyme, can convert toxic quinones to hydroquinones and reduce oxidative cycling. The oleanane saponins of P. chinensis exhibited more potent quinone reductase inducing activities than the lupine saponins, and the CD value (concentration required to double the quinone reductase-inducing activity of the control) of the compound with the most potent quinone reductase-inducing activity was 1.1 μM, as indicated in [117].
Thymoquinone inhibits carcinogen-metabolizing enzyme activity and oxidative damage of cellular macromolecules, and attenuates inflammation [11]. The anti-proliferative and pro-apoptotic effects of thymoquinone in breast cancer are mediated through p38 MAPK phosphorylation via ROS generation [118]. N. sativa extract increases the activities of antioxidant enzymes, such as superoxide dismutase, chloramphenicol transferase, and glutathione peroxidase, and protects cells against cancer [119, 120].
Berberine induced ROS production for up to six hours of incubation with human gastric cancer SNU-5 cells [121]. In PANC-1 and MIA-PaCa2 pancreatic cancer cells, apoptosis was induced by berberine via the production of ROS rather than caspase-3 and -7 activation [122]. In HepG2 cells, berberine treatment led to a pronounced increase in JNK phosphorylation and enhanced ROS generation, lipid peroxidation, decreased activities of superoxide dismutase (SOD) and catalase (CAT), and diminished glutathione levels, as reviewed in [13, 71, 73]. Berberine and d-limonene (a monoterpene) at a ratio of 1:4 exhibited a synergistic anticancer effect on gastric cancer MGC803 cells [123]. They distinctly induced intracellular ROS generation, reduced the mitochondrial transmembrane potential (ΔΨm), enhanced the expression of caspase-3, and decreased the expression of BCL-2. As TQ has strong anticancer activity, whether the combination of berberine and d-limonene exerted synergistic anticancer effects merits study [123].
4.2. Metabolism
Rabbit carcinogen metabolizing enzymes CYP1A2, 3A4, but not CYP2E1, were significantly diminished by dietary doses of thymoquinone [124]. Thymoquinone displays a significant inhibition of induced phase I CYP1A1 enzyme, and increases the content of glutathione and activity of phase II enzyme glutathione-S-transferase, in HCC HepG2 cells, providing support for the beneficial use of thymoquinone as a therapeutic and chemopreventive agent against liver cancer, as reviewed in [125]. The phase II enzyme glutathione peroxidase was also significantly induced by the high thymoquinone dose in rabbit, while the total glutathione levels were unaffected [124]. Glutathione reductase was significantly induced, which may explain the benign effect of N. sativa seeds in inhibiting the generation of bioactive metabolites known to promote carcinogenesis and oxidative cell damage [124, 125].
In human P-glycoprotein (MDR1, ABCB1) gene-transfected KB/MDR1 cells, TQ had no effect on the accumulation of daunorubicin or rhodamine-123, two fluorescent substrates of MDR1 [126]. Acerinol, a cyclolanstane triterpenoid from Cimicifuga acerina, could increase the chemosensitivity of MDR1-overexpressing HepG2/ADM and MCF-7/ADR cells to chemotherapeutic drugs doxorubicin, vincristine, and paclitaxel [127]. It could also increase the retention of MDR1 substrates doxorubicin and rhodamine-123 in the above cells [127]. Acerinol significantly stimulated the activity of MDR1 ATPase without affecting the expression of MDR1 on mRNA or protein level [127]. Acerinol reversed the resistance of MCF-7/ADR cells to vincristine [127]. Acerinol may be a competitive inhibitor of MDR1, and docking analysis data indicated that acerinol would bind to the sites on MDR1 that partially overlapped with that of verapamil [127].
The ethanolic Cimicifuga racemosa extract BNO-1055, rich in saponin, dose-dependently attenuated a cellular uptake and incorporation of thymidine and bromodeoxyuridine (BrdU) and significantly inhibited cell growth after long-time exposure [128]. These inhibitory effects of BNO-1055 could be mimicked using pharmacological inhibitors and isoform- specific siRNAs targeting the equilibrative nucleoside transporters, ENT1 and 2, as indicated in [128]. BNO-1055 also attenuated the uptake of clinically relevant nucleoside analogs, e.g. the anticancer drugs gemcitabine and fludarabine [128]. By inhibiting the salvage nucleoside uptake pathway, BNO-1055 potentiated the cytotoxicity of the de novo nucleotide synthesis inhibitor 5-fluoruracil without significantly altering its uptake [128]. In human breast cancer MCF-7 cells, both C. racemosa extract and purified cycloartane saponins upregulated the expression of CYP1A1 and CYP1B1, two carcinogen-metabolizing enzymes, as described elsewhere [9].
Aconite root could improve the energy metabolism in rats, by influencing the metabolic process of sugar, lipid and amino acid, which may be the main molecular mechanism of warming yang and dispelling cold for the treatment of the cold syndrome, common in cancer patients, according to TCM theory, as reviewed in [129]. A. napellus extract administered around the clock induced hyperthermia overall and in a time-dependent manner, with greatest effects during the resting span [130]. Thus, time of day may significantly affect the outcome of A. napellus and other homeopathic treatments and should be considered in determining optimal dosing and treatment time in order to increase the desired outcome and decrease undesired effects [130]. Fu Zi (lateral roots of A. carmichaelii) can increase and maintain the dogs' body heat for at least 6 h [131]. The body weight gain in aconitine- administered mice was less than that of the control group until day 22, as described in [132]. Transient rectal hypothermia occurred within 30 min after the last administration of aconitine [132]. Then the rectal temperature gradually increased to normal [132]. The drug metabolism of aconitine increased and the toxicity of aconitine decreased due to long-term administrations of aconitine [132].
The crude extract of secondary roots of Aconitum carmichaelii Debeaux (Fu Zi), together with its processed products, including Yanfuzi, Heishunpian and Paofupian, are commonly applied in clinic for various biomedical purposes [133]. Fingerprints of Fu Zi, Yan Fu Zi (salted prepared aconite root), Heishunpian (processed Fu Zi) and Paofupian were obtained by ultra-high performance liquid chromatography (UPLC) and their effects on mitochondrial metabolism were studied by microcalorimetry [133]. Due to inherent differences in chemical compositions, the energy metabolism of mitochondria was variably influenced by selected Fu Zi and its processed products [133]. The bioactivity sequence of the tested products was Fu Zi > Heishunpian > Paofupian > Yan Fu Zi, as indicated in [133]. The phytometabolites mesaconitine (Fig. 3, structure 20), benzoylaconitine, and benzoylhypaconitine might be the principal active components that modulate energy metabolism [133].
Reprogramming energy metabolism is an emerging cancer hallmark [134]. The cancer cells rely on aerobic glycolysis, instead of mitochondrial oxidative phosphorylation, to yield ATP [134]. The enhanced glycolysis allows the diversion of glycolytic intermediates into various biosynthetic pathways, including those generating nucleosides and amino acids, which facilitates the biosynthesis of the macromolecules and organelles required for assembling new cells [134]. Can Aconitum reverse the deregulated cellular energetics of cancer cells? The TCM formula Sini decoction (SND), consisting of Fu Zi, Glycyrrhizae Preparata Radix and Zingiberis Rhizoma, significantly inhibit LDH and glycolysis in the myocardial ischemia reperfusion injury model of rat H9c2 cardiomyocytes [135]. SND also modulates lipid metabolism, tricarboxylic acid cycle and nitrogen metabolism [135]. It is intriguing to study whether Aconitum and diterpenoid alkaloids thereof inhibit the aerobic glycolysis of cancer cells [135]. To date there is also no direct evidence that Aconitum enhances mitochondrial oxidative phosphorylation in human cancer cells [135].
5. ANTI-ANGIOGENIC AND ANTI-METASTATIC EFFECTS
5.1. Saponins
SB365 exhibited potent anti-angiogenic activity and decreased the expression of hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF), a key molecule for angiogenesis [21]. SB365 suppressed the tube formation and migration of human umbilical vein endothelial cells (HUVECs), as well as in vivo neovascularization in a mouse Matrigel plug assay [21]. SB365 treatment decreased the expressions of VEGF and CD34 in the tumor tissue of HCC xenograft models [21]. Angiogenesis of human colon cancer and pancreatic cancer cells was also suppressed by SB365 [22, 23]. SB365 inhibited tube formation in hepatocyte growth factor (HGF)-induced HUVECs and suppressed micro vessel sprouting from the rat aortic ring, ex vivo, and blood vessel formation in the Matrigel plug assay in mice [27].
Raddeanin A significantly inhibited HUVEC proliferation, motility, migration, and tube formation [30]. Raddeanin A dramatically reduced angiogenesis in chick embryo chorioallantoic membrane, restrained the trunk angiogenesis in zebrafish, and suppressed angiogenesis and growth of human colorectal cancer HCT-15 cell xenografted into mice [30]. Raddeanin A suppressed VEGF-induced phosphorylation of VEGF receptor-2 and its downstream protein kinases including PLCγ1, JAK2, FAK, SRC, and AKT, as indicated in [30]. In molecular docking simulation, Raddeanin A formed hydrogen bonds and hydrophobic interactions within the ATP-binding pocket of VEGF receptor-2 protein kinase domain [30]. Raddeanin A significantly inhibited the invasion, migration and adhesion of the BGC-823human gastric cancer cells [31]. Raddeanin A could upregulate the expression of REversion inducing Cysteine rich protein with Kazal motifs (RECK) and E-cadherin proteins, and down-regulate the expression of matrix metalloproteinase-2 (MMP-2), MMP-9, MMP-14 and RhoC protiens, as described in [31].
5.2. Terpenoid
Thymoquinone inhibits cancer angiogenesis, cell invasion, and metastasis [11, 136]. TQ treatment decreased the transcriptional activity of the TWIST1 promoter and the mRNA expression of TWIST1, a transcription factor promoting epithelial-to-mesenchymal transition (EMT), as indicated in [137]. Thymoquinone also decreased the expression of TWIST1- upregulated genes, such as N-cadherin and increased the expression of TWIST1-repressed genes such as E-cadherin, thus reducing cell migration and invasion [137]. Thymoquinone inhibited the growth and metastasis of cancer cell-derived xenografted tumors in mice and partially attenuated the migration and invasion of TWIST1-overexpressed cell lines [137]. Furthermore, thymoquinone enhanced the promoter DNA methylation of the TWIST1 gene in human breast cancer BT-459 cells [137].
Cimyunnin A (12 of Fig. 1), a triterpene isolated from the fruit of Cimicifuga yunnanensis and characterized by an unusual fused cyclopentenone ring G, exhibited comparable anti-angiogenic activities to those of sunitinib, a clinically-used first-line angiogenesis inhibitor, in the in vitro and ex vivo studies [138]. The phosphorylation of VEGF receptor-2 protein was suppressed by cimyunnin A in a dose-dependent manner [138]. Dramatic downregulation of p-AKT (Ser473) and p-ERK (Thr202/Tyr204), the known downstream targets of VEGF receptor 2, were observed at 5.0 and 10.0 μM of cimyunnin A, while total VEGF receptor 2, ERK, and AKT remain unchanged [138].
5.3. Alkaloid
Isoquinoline alkaloids such as berberine, palmatine, jatrorrhizine, and columbamine are abundant in Coptis, Thalictrum, and Hydrastis, and exert their anti-cancer activity via multiple molecular mechanisms [139]. Berberine inhibited colorectal cancer invasion and metastasis via downregulation of COX-2/PGE2-JAK2/STAT3 signaling pathway [140]. The MMP-1, -2, and -9 expressions were also decreased by berberine, while MMP-7 was not affected [121, 140]. Berberine inhibits the metastatic ability of prostate cancer cells by suppressing EMT-associated genes, e.g., BMP7, NODAL, and SNAIL, which, if highly expressed, are associated with shorter survival of prostate cancer patients. Berberine inhibited the expression of Id-1 (inhibitor of differentiation/DNA binding), a key regulator of HCC development and metastasis [141, 142]. Berberine could inhibit the ID-1 gene promoter activity [142]. The ID-1 protein overexpression in HCC models partially rescued anti-proliferative and anti-invasive activities of berberine. Purified palmatine, or in the extract, exhibits invasion inhibitory properties, as reviewed elsewhere [139]. Synergistic inhibition of the ribosomal protein S6 (RPS6)/NF-κB/FLIP axis with palmatine may have therapeutic potential for cancer with their constitutive activation, as reviewed in [139].
Anoikis, or detachment-induced apoptosis, may prevent cancer progression and metastasis by blocking signals necessary for survival of localized cancer cells, as reviewed in [143]. Resistance to anoikis is regarded as a precondition for metastasis [143]. Berberine promoted the growth inhibition of anoikis-resistant cells to a greater extent than doxorubicin treatment [143]. Berberine induced cell cycle arrest at G0/G1 in the anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cells [143].
Jatrorrhizine induced C8161 metastatic melanoma cell cycle arrest at the G0/G1 transition, which was accompanied by overexpression of the cell cycle inhibitor proteins, CDKN1A (p21) and CDKN2B (p27), at higher doses [144]. Jatrorrhizine failed to induce a significant cellular apoptosis at doses up to 320 μmol/l, as indicated [144]. Moreover, jatrorrhizine reduced C8161 cell-mediated neovascularization in vitro and in vivo and downregulated the expression of cadherin, a key protein in tumor vasculogenic mimicry and angiogenesis [144].
Columbamine induces metastatic osteosarcoma U2OS cell cycle arrest at the G2/M transition, which is associated with attenuating CDK6 gene expression and diminishing STAT3 phosphorylation, as described in [145]. Columbamine did not significantly promote cell apoptosis at any dosages tested [145]. Columbamine inhibited U2OS cell-mediated neovascularization, which was accompanied by the down-regulation of MMP-2 expression and reduction of cell migration, adhesion, and invasion [145].
5.4. Plant Extract
Pulsatilla koreana extract decreased the expression of HIF-1α and VEGF in HCC cells, and inhibited tube formation and migration of HUVEC, as described elsewhere [86]. Pulsatilla koreana extract potently suppressed in vivo neovascularization in a mouse Matrigel plug assay. In a mouse xenograft model, the expressions of Ki-67, VEGF, and CD31 in the tumor tissue were decreased by Pulsatilla koreana extract [86]. Angiogenesis of anaplastic thyroid cancer was also suppressed by Pulsatilla koreana extract [86]. The water extract of Pulsatilla koreana, Panax ginseng (Ren Shen in TCM) and Glycyrrhiza uralensis (Gan Cao in TCM) (WEPPG) significantly inhibited fibroblast growth factor-induced HUVEC proliferation, adhesion, migration, and capillary tube formation [146]. The protein levels of cyclin A (CCNA), TP63 and CDKN1C (also known as p27KIP2) were upregulated, while nibrin and focal adhesion kinase (FAK) were downregulated [146]. The blood vessel formation in a CAM treated with WEPPG was markedly reduced. WEPPG might exert its anticancer effects via the inhibition of angiogenesis [146].
Aconite from Aconiti Kusnezoffii Radix in TCM could induce cell differentiation, inhibit cell proliferation and migration, increase succinate dehydrogenase (SDH) activity and promote gap-junction intercellular communication in Lewis lung cancer cells [147]. Extract from Coptidis Rhizoma increases cell adhesion and decreases SDH activity and GJIC without cell differentiation while it also suppressed cell proliferation [147]. Aconiti Kusnezoffii Radix water decoction could maintain body temperature, blood oxygen saturation, red cell ATP-ase and blood rheology, and decrease intratumor hypoxia and capillary permeability in tumor- bearing mice, which retarded cancer growth and metastasis [147]. Extract from Aconitum vaginatum can inhibit the proliferation, invasion and metastasis of A549 cells, and MMP-2 and -9 activities were decreased [148]. Coptidis Rhizoma water decoction decreased body temperature, blood oxygen saturation, red cell ATPase, blood rheology and gap-junction intercellular communication, and promoted intratumor hypoxia and capillary permeability, which led to more cancer metastasis although it also inhibited cancer growth [147, 149]. The hot Chinese medicine (e.g., aconite) could induce cancer cell differentiation and prevent tumor poison invagination, which might be better for lung cancer treatment than cold Chinese medicine (e.g., Coptidis Rhizoma), as indicated in [147, 149].
Coptidis rhizome aqueous extract (CRAE), containing magnoflorine (17 of Fig. 1) 2.2%, jatrorrhizine 1.68%, palmatine 4.4%, and berberine 13.8%, exhibited significant inhibition on VEGF secretion from MHCC97L and HepG2 cells at nontoxic doses [149]. CRAE intervention increased the phosphorylation of eukaryotic elongation factor 2 (eEF2) in HCC cells, which blocked eEF2 activity for proceeding nascent protein synthesis [149]. Reduction of tumor size and neovascularization were observed in mice xenograft model [149].
6. IMMUNOMODULATORY ACTIVITY
The immune destruction of cancer cells is an essential anticancer mechanism, as defined in [134]. Ranunculus ternatus polysaccharides enhance the proliferation of mouse thymocytes, spleen lymphocytes, and peritoneal macrophages [150]. R. ternatus polysaccharides enhance NK cell activity and induce apoptosis in breast cancer MCF-7 cells [151, 152]. However, the anti-proliferative effects of R. ternatus polysaccharides might be less pronounced than saponins in human gastric cancer BGC823 cells [151, 152]. Pulsatilla chinensis polysaccharides (PCPS) treatment to glioma-bearing mice could elevate the thymus and spleen indices [106]. A neutral polysaccharide fraction (ARP) from the rhizome of Anemone raddeana extraordinarily promotes splenocyte proliferation, NK cell and CTL activity, as well as serum IL-2 and TNF-α production in HCC-bearing mice [153]. ARP had no toxicity to body weight, liver, and kidney [153]. Moreover it could reverse the hematological parameters induced by 5-fluorouracil to near normal [153]. A polysaccharide (ACP-a1) of 3.2×105 Da, was isolated from the roots of Aconitum coreanum, and is mainly composed of β-D-mannose and β-D-glucose in a molar ratio of 1.2:3.5, as indicated in [154]. ACP-a1 significantly inhibited the growth of hepatoma H22 transplanted in mice and prolonged the survival time of H22 tumor-bearing mice [154]. The body weight, peripheral white blood cells, thymus index and spleen index of H22 tumor- bearing mice were improved after ACP-a1 treatment [154]. ACP-a1 could promote the secretion of serum cytokines, such as IL-2, TNF-α and IFN-γ, as described in [154]. Nigella sativa is highlighted as a natural immune booster [155]. Modes of its actions include boosting and functioning of immune system, activation and suppression of immune specialized cells, interfering in several pathways that eventually lead to improved immune responses and defense system [155]. For instance, thymoquinone induces the phosphorylation of proteins involved in NK cell signaling, CD28 signaling of TH cells, and B cell receptor signaling [156, 157].
7. ANTI-INFLAMMATORY ACTIVITY
Inflammation has been identified as a significant factor in the development of solid tumors [103, 134]. For instance, both hereditary and sporadic forms of chronic pancreatitis are associated with an increased risk of developing pancreatic ductal adenocarcinoma (PDA), as indicated in [103]. Thymoquinone, the major constituent of Nigella sativa oil extract, induced cell cycle arrest and apoptosis in PDA cells via an increased CDKN1A (p21WAF1) expression, decreased HDAC activity, and induced histone hyperacetylation [103]. Thymoquinone was also shown to reduce expression of monocyte chemoattractant protein (MCP)-1, tumor necrosis factor (TNF)-α, interleukin-1β and COX-2 in PDA cells in a dose- and time- dependent fashion [103]. Thymoquinone also inhibited the constitutive and TNF-α mediated activation of NF-κB and reduced its the transport from the cytosol to the nucleus in PDA cells [103]. Similar to berberine, the anti-inflammatory activity of thymoquinone might contribute to its overall anti-cancer effects [155, 158].
Ranunculaceae tribes and genera, such as Ranunculus, Anemoneae, Cimicifuga, Helleborus, Nigella, Delphinieae, Semiaquilegia, Coptis, and Hydrastis, are rich in both anti-inflammatory and anticancer phytometabolites [1, 2]. Anemonin (24 of Fig. 1) and ranunculin (25 of Fig. 1), the potent anti-inflammatory and anti-cancer phytometabolites, are abundant in tribes Ranunculeae and Anemoneae [2, 159]. The chronic inflammation is associated with tumor formation, and tumors could be portrayed as wounds that never heal [160]. Ranunculus pedatus and R. constantinapolitanus were found to have wound healing and anti-inflammatory properties [161]. The fatty-acid fractions of R. constantinopolitanus exerted theanti-inflammatory effects via downregulation of interleukin-6 and COX-2, as indicated in [162]. The triterpene saponins, phenolic acid, lignans, and flavonoids of Clematis display various anti-inflammatory activities [1, 2, 7]. C. mandshurica extract inhibited interleukin-1β-induced MMP gene expression, implicating its use in combating against cancer invasion [163]. The inflammatory cells can release chemicals, notably ROS, which are actively mutagenic for nearby cancer cells, accelerating their genetic evolution toward states of intensified malignancy [164]. The Ranunculaceae anti-inflammatory compound remarkably inhibited ROS generation and downregulated the ROS-dependent NF-κB signaling pathway [165].
Cycloartane-type triterpene saponins (Cimicifuga and Actaea), diterpenoid alkaloids (Aconitum and Delphinium), flavonoid glycosides (Ranunculus and Trollius), and isoquinoline alkaloids (subfamily Thalictroideae, Coptis, and Hydrastis) also display various anti-inflammatory activities [2, 166]. The cytokines (e.g. IL-6 and IFN-γ) and chemokines (e.g. MIP-1β) were decreased in serum of MCS-18 (from Helleborus)-treated mice [167]. Dichocarpum (firstly identified as an independent genus by Xiao and Wang in 1964), Adonis, Beesia, Caltha, and Halerpestes are less studied in modern pharmacology, although in folk medicine and TCM they are frequently used in the inflammatory diseases [2, 168]. Taken together, Ranunculaceae plants contribute notable chemodiversity, from which promising anticancer phytometabolites and novel anticancer mechanisms could be found, as reviewed elsewhere [2].
8. STRUCTURE-ACTIVITY RELATIONSHIP
Saponins 1-14 of P. chinensis showed considerable cytotoxic activity, whereas saponins 15-36 had no significant activity, suggesting that a free carboxylic group at C-28 of aglycon is essential for their cytotoxic activity [169]. The oleanane-type saponins showed better cytotoxic activity than lupane-type saponins, and the length and linkage of glycolic chain attached to C-3 of aglycon displayed an important effect to the cytotoxic potency [169]. Oleanolic acid 3-O-α-l-rhamnopyranosyl- (1 → 2)-[β-d-glucopyranosyl- (1 →4)]-α-l-arabinopyranoside (saponin 5) exhibited the most significant cytotoxic activity [169].
Saponins 5-17 of P. koreana, with a free acidic functional group at C-28 of aglycon, exhibited moderate to considerable cytotoxic activity, while saponins 1-4, esterified with a trisaccharide at C-28 of aglycon, did not exhibit cytotoxic activity (ED50 > 300 μM). Oleanolic acid 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→4)]-α-L-arabinopyranoside (saponin 10) exhibited the most potent cytotoxic activity [170]. During in vivo studies, hederagenin 3-O-α-L-rhamnopyranosyl- (1→2)-[β-D-glucopyranosyl- (1→4)]-α-L-arabinopyranoside (saponin 6) exhibited more potent anticancer activity than paclitaxel and doxorubicin [170]. Hedragenin 3-O-β-D-glucopyranosyl- (1→4)-O-β-D-glucopyranosyl- (1→3)-O-α-L-rhamnopyranosyl- (1→2)-α-L-arabinopyranoside (saponin 17) exhibited potent anticancer activity [170]. These two saponins were similarly comprised of a hederageninaglycon and a sugar sequence O-α-L-rhamnopyranosyl- (1→2)-α-L-arabinopyranoside at C-3 of the hederagenin, suggesting that the two elements are essential for the anticancer activity [170].
The natural and derivatized C19-diterpenoid alkaloids of Aconitum tested by Hazawa et al. [171] and Wada et al. [172] showed only a slight or no effect against cancer cells. Most anticancer phytometabolites were hetisine-type C20-diterpenoid alkaloids, specifically kobusine and pseudokobusine analogs with two different substitution patterns, C-11 and C-11, 15, as indicated in [173]. Particularly, several C20-diterpenoid alkaloids were more potent against multidrug-resistant KB subline KB-VIN cells [173]. Pseudokobusine 11-3'-trifluoro-methylbenzoate (19 of Fig. 1) is a possible promising new lead meriting additional evaluation [173].
9. GENOMICS, TRANSCRIPTOMICS, PROTEOMIC, AND METABOLOMICS
It is becoming increasingly apparent that a precise and truly useful understanding of the behavior of individual phytometabolite and Ranunculaceae extracts would only come if we are able to integrate genomic, proteomic, and other omics datasets, and then distill these data. The use of omics platforms in Ranunculaceae anti-cancer research is still in its infancy. However, initial omics information is schematically summarized in (Fig. 5). For instance, a PCR expression array was used to quantify thymoquinone-mediated transcriptional regulation of 84 apoptosis-related genes [174]. At low dose (12.5 μM), thymoquinone induced expression of four pro-apoptotic genes: BIK (~22.7-fold), FASL (~2.9-fold), BCL2L10 (~2.1-fold), and CASP1 (~2-fold), as described elsewhere [174]. Thymoquinone reduced the expression of an anti-apoptotic gene implicated in NF-κB signaling and cancer: RELA (~-8-fold), as described in [174]. At high dose (100 μM), thymoquinone mediated the expression of 21 genes directly implicated in apoptosis (6 genes), TNF signaling (10 genes), and NF-κB signaling (3 genes), as indicated in [174]. They include: BIK, BID, TNFRSF10A, TNFRSF10B, TNF, TRAF3, RELA, and RELB [174]. Thus, these data support the notion that thymoquinone intervenes with TNF and NF-κB signaling during thymoquinone-mediated induction of apoptosis in cancer cells [174].
DNA microarray data for 12,600 genes were used to examine the anti-proliferative activity of Coptidis rhizoma and eight constituent molecules against eight human pancreatic cancer cell lines [175]. Twenty-seven genes showed a strong correlation with the 50% inhibitory dose (ID50) of Coptidis rhizoma after 72-h exposure [175]. The test molecules were classified into two clusters, one consisting of Coptidis rhizoma and berberine, and the other consisting of the remaining seven molecules [175]. Berberine can account for the majority of the anti- proliferative activity of Coptidis rhizoma and DNA microarray analyses can be used to improve our understanding of the actions of an intact herbal compound [175].
Identifying human or mammalian metabolites is an integral part of the molecular mechanism investigations of anti-cancer phytometabolites. Eighteen metabolites of Pulsatilla saponin D were identified in rat plasma, urine and feces samples by ESI-Q-TOF-mass spectrometry (MS/MS), and eight of them (M11-M18) were novel ones [26]. Deglycosylation, dehydrogenation, hydroxylation, and sulfation were the major metabolic transformations of Pulsatilla saponin D in vivo [26]. The metabolic information gained from metabolomic studies is relevant to the pharmacological activity of Pulsatilla saponin D, as reviewed in [26].
Bioinformatics and chemoinformatics are the indispensable part of omics platforms that contribute prolific information of molecular mechanisms of anti-cancer phytometabolites. Three different in silico reverse screening approaches proved useful for identifying the putative molecular targets of the anti-cancer phytometabolites in Nigella sativa (thymoquinone, α-hederin, dithymoquinone, and thymohydroquinone), as indicated in [176]. Novel kinase targets influenced by thymoquinone were revealed by in silico analysis of peptide array data obtained from thymoquinone-treated HCT116 colon cancer cells [57]. Thymoquinone induces the phosphorylation of a multitude of proteins that are involved in one or more cancer related biological functions: apoptosis, proliferation and inflammation [57]. Of the 104 proteins identified, 50 were upregulated ≥ 2 fold by thymoquinone and included molecules in the AKT-MEK-ERK1/2 pathway [57]. Oncogenic PAK1 emerged as an interesting thymoquinone target [57]. Thymoquinone induced an increase of pERK1/2 and triggered the early formation of an ERK1/2-PAK1 complex [57]. Modeling confirmed that thymoquinone binds in the vicinity of Thr212 accompanied by conformational changes in ERK2-PAK1 binding [57]. Structural modeling analysis suggests that thymoquinone interferes also with the kinase domain and disturbs its interaction with p-PAK (Thr423), finally inhibiting MEK-ERK1/2 signaling and disrupting its prosurvival function [57].
Using cDNA microarray technology, thymoquinone was found to alter expression of 577 genes (that showed >2-fold change in expression) in human breast cancer MCF7 cells, as described elsewhere [177]. Of these genes, 45.2% showed an upregulation and 54.7% showed a downregulation in expression [177]. The cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1) and UDP glucuronosyltransferase 1 family, polypeptide A8 (UGT1A8) genes were downregulated significantly by 43- and 11-fold, respectively [177]. The solute carrier family 7, member-11 (SLC7A11) gene was downregulated by 15-fold [177]. The interferon-induced protein with tetratricopeptide repeats (IFIT)-1, IFIT2, IFIT3, interferon, α-inducible protein (IFI)-6 (also known as G1P3), interferon regulatory factor 9 (IRF9, ISGF3), 2'-5'-oligoadenylate synthetase 1 (OAS1) and signal transducer and activator of transcription-1 (STAT1) genes showed changes in expression following treatment with thymoquinone [177]. The caspase-10 (CASP10) gene was activated and the protein tyrosine phosphatase, receptor type R (PTPRR) and myocyte enhancer factor 2C (MEF2C) genes were upregulated [177]. These findings indicate that thymquinone targets specific genes in the estrogen metabolic and interferon pathways [177].
10. CONCLUSION AND FUTURE PERSPECTIVE
This review focuses on the various Ranunculaceae chemical phytometabolites that have shown promise as anticancer agents and outlines their potential mechanism of action. Deeper insights into molecular mechanisms and functions of Ranunculaceae anti-cancer phytometabolites are the premise of designing analog and nanoformulation with improved absorption, distribution, metabolism, and excretion/toxicity properties and therapeutic efficacy. Studies of the representative Ranunculaceae phytometabolites, e.g., thymoquinone and berberine, are enlightening, since other phytometabolites of the same plant family could share many anti-cancer mechanisms and pathways. To date, little information has been reported on the anticancer effects and the underlying mechanisms of the diterpenoid alkaloids of Aconitum and Delphinium plants. Many Ranunculaceae phytometabolites have shown very promising anti-cancer properties in vitro, but have yet to be evaluated in humans. The potential role for Ranunculaceae phytometabolites in epigenetic regulation of gene expression remains largely unknown so far. Gaps are present in the extensive use of high-throughput transcriptome sequencing and proteome profiling for characterizing the interaction between Ranunculaceae phytometabolites and cancer cells, as well as the relevant regulatory network. Further studies are warranted to determine the molecular mechanisms and efficacy of Ranunculaceae phytometabolites in treating human cancers.
Many Ranunculaceae plants contain not only anti-cancer phytometabolites but also phytometabolites with free radical scavenging, immunomodulatory, and anti-inflammatory activities that are helpful against cancer insurgence. However, interaction between western drugs and herbs/botanicals should be well investigated before safe combined use, and such information must be disseminated to the allied stakeholders. As anti-cancer candidates with low toxicity, Ranunculaceae phytometabolites, such as thymoquinone and berberine, and their altered structure, as well as source plants such as N. sativa, C. chinensis and their closely related species, will attract researchers to pursue the potential anticancer effects and the mechanisms by using innovative technologies of genomics, proteomics and other advanced approaches. Current and future studies would consistently suggest the feasibility of mining anticancer pharmacological diversity from Ranunculaceae chemodiversity.
ACKNOWLEDGEMENTS
This work is supported by Natural science fund of Liaoning province (2015020663).
list of ABBREVIATIONS
- ALT
Alanine aminotransferase
- AMPK
AMP-activated protein kinase
- APAF-1
Apoptotic peptidase activating Factor 1
- AST
Aspartate aminotransferase
- ATM
Ataxia telangiectasia mutated
- BIRC5
Baculoviral inhibitor of apoptosis protein repeat-containing protein
- CAT
Catalase
- CDC2
Cell division cycle protein 2 homolog
- CHK
Checkpoint kinase
- CDK1
Cyclin-dependent kinase 1
- CRAE
Coptidis rhizome aqueous extract
- COX-2
Cyclooxygenase-2
- CYP1A1
Cytochrome P450, family 1, subfamily A, polypeptide 1
- DIME
Dihydroxy-isosteviol methyl ester
- DNA-PK
DNA-dependent protein kinase
- DNMT
DNA methyltransferase
- DRβ-H
D-Rhamnose β-hederin
- ER
Endoplasmic reticulum
- EGF
Epidermal growth factor
- EMT
Epithelial-to-mesenchymal transition
- EMMPRIN
Extracellular matrix metalloproteinase inducer
- eEF2
Eukaryotic elongation factor 2
- FAK
Focal adhesion kinase
- FOXO
Forkhead box
- GBM
Glioblastoma multiforme
- HCC
Hepatocellular carcinoma
- HGF
Hepatocyte growth factor
- HDAC
Histone deacetylase
- HUVEC
Human umbilical vein endothelial cells
- HIF-1α
Hypoxia-inducible factor-1α
- IAP
Inhibitors of apoptosis
- IFIT
Interferon-induced protein with tetratricopeptide repeats
- IFI
Interferon, α-inducible protein
- IRF
Interferon regulatory factor
- IP3
Inositol 1,4,5-trisphosphate receptor
- IGF
Insulin-like growth factor
- JNK
c-Jun NH2-terminal kinase
- mTOR
Mammalian target of rapamycin
- miR
microrna
- MAPK
mitogen-activated protein kinase
- MCP
monocyte chemoattractant protein
- MPM2
M-phase phosphoprotein-2
- MEF2C
Myocyte enhancer factor 2C
- NF-κB
Nuclear factor kappa B
- OAS1
2'-5'-oligoadenylate synthetase 1
- p70S6K
p70S6 kinase
- PDA
Pancreatic ductal adenocarcinoma
- PTEN
Phosphatase and tensin homolog
- PI3K
Phosphoinositide 3-kinase
- PBD
Polo-box domain
- PLK
Polo-like kinase
- PARP
Poly-ADP ribose polymerase
- PTPRR
Protein tyrosine phosphatase, receptor type R
- PCPS
Pulsatilla chinensis polysaccharides
- RPS6
Ribosomal protein S6
- QR
Quinone reductase
- ROS
Reactive oxygen species
- RTK
Receptor tyrosine kinase
- RECK
REversion inducing Cysteine rich protein with Kazal motifs
- STAT1
Signal transducer and activator of transcription-1
- SLC7A11
Solute carrier family 7, member-11
- SAHA
Suberoylanilide hydroxamine
- SDH
Succinate dehydrogenase
- SOD
Superoxide dismutase
- TQ
Thymoquinone
- TCM
Traditional Chinese medicine
- TOP
Topoisomerase
- TNF
Tumor necrosis factor
- TP
Tumor protein
- XIAP
X-linked inhibitor of apoptosis protein
- VEGF
Vascular endothelial growth factor
- UGT1A8
UDP glucuronosyltransferase 1 family, polypeptide A8
- CDK
Cyclin dependent kinase
- DSB
Double-strand break
- eEF
Eukaryotic elongation factor
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial to mesenchymal transition
- ER
Estrogen receptor
- ERK
Extracellular signal-regulated kinase
- HCC
Hepatocellular carcinoma
- HGF
Hepatocyte growth factor
- HIF-1α
Hypoxia-inducible factor 1α
- HUVEC
Human umbilical vein endothelial cell
- JNK
c-Jun NH2-terminal kinase
- MAPK
Mitogen-activated protein kinase
- mTOR
Mammalian target of rapamycin
- PARP
Poly-ADP ribose polymerase
- SND
Sini decoction
- TCM
Traditional Chinese medicine
- UPLC
Ultra-high performance liquid chromatography
- VEGF
Vascular endothelial growth factor
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Hao D.C., Gu X.J., Xiao P.G. Medicinal plants: Chemistry, biology and omics. 1st ed. Oxford: Elsevier-Woodhead; 2015. ISBN 9780081000854. [Google Scholar]
- 2.Hao D.C., Xiao P.G., Ma H., Peng Y., He C.N. Mining chemodiversity from biodiversity: pharmacophylogeny of medicinal plants of the Ranunculaceae. Chin. J. Nat. Med. 2015;13(7):507–520. doi: 10.1016/S1875-5364(15)30045-5. [DOI] [PubMed] [Google Scholar]
- 3.Xiao P.G. A preliminary study of the correlation between phylogeny, chemical constituents and pharmaceutical aspects in the taxa of Chinese Ranunculaceae. Acta Phytotax. Sin. 1980;18(2):142–153. [Google Scholar]
- 4.Xiao P.G., Wang L.W., Lv S.J., Qiu G.S., Sun J. Statistical analysis of the ethnopharmacologic data based on Chinese medicinal plants by electronic computer I. Magnoliidae. Chin. J. Integ. Trad. West Med. 1986;6(4):253–256. [PubMed] [Google Scholar]
- 5.Hao D.C., Gu X.J., Xiao P.G., Peng Y. Recent advances in the chemical and biological studies of Aconitum pharmaceutical resources. J. Chin. Pharm. Sci. 2013;22(3):209–221. [Google Scholar]
- 6.Hao D.C., Gu X.J., Xiao P.G., Peng Y. Recent advances in chemical and biological studies on Cimicifugeae pharmaceutical resources. Chin. Herb. Med. 2013;5(2):81–95. [Google Scholar]
- 7.Hao D.C., Gu X.J., Xiao P.G., Peng Y. Chemical and biological research of Clematis medicinal resources. Chin. Sci. Bull. 2013;58(10):1120–1129. [Google Scholar]
- 8.Hao D.C., Xiao P.G., Liu M., Peng Y., He C.N. Pharmaphylogeny vs. pharmacophylogenomics: molecular phylogeny, evolution and drug discovery. Yao Xue Xue Bao. 2014;49(10):1387–1394. [PubMed] [Google Scholar]
- 9.Gaube F., Wolfl S., Pusch L., Kroll T.C., Hamburger M. Gene expression profiling reveals effects of Cimicifuga racemosa (L.) NUTT. (black cohosh) on the estrogen receptor positive human breast cancer cell line MCF-7. BMC Pharmacol. 2007;7:11. doi: 10.1186/1471-2210-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kundu J., Choi B.Y., Jeong C.H., Kundu J.K., Chun K.S. Thymoquinone induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2- and Src-mediated phosphorylation of EGF receptor tyrosine kinase. Oncol. Rep. 2014;32(2):821–828. doi: 10.3892/or.2014.3223. [DOI] [PubMed] [Google Scholar]
- 11.Kundu J., Chun K.S., Aruoma O.I., Kundu J.K. Mechanistic perspectives on cancer chemoprevention/chemotherapeutic effects of thymoquinone. Mutat. Res. 2014;768:22–34. doi: 10.1016/j.mrfmmm.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Schneider-Stock R., Fakhoury I.H., Zaki A.M., El-Baba C.O., Gali-Muhtasib H.U. Thymoquinone: fifty years of success in the battle against cancer models. Drug Discov. Today. 2014;19(1):18–30. doi: 10.1016/j.drudis.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz L.M., Lombardi P., Tillhon M., Scovassi A.I. Berberine, an epiphany against cancer. Molecules. 2014;19(8):12349–12367. doi: 10.3390/molecules190812349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao M., Ma N., Qiu F., Tian X., Zhang Y., Tang H., Liu X. Triterpenoid saponins from the roots of Clematis argentilucida. Fitoterapia. 2014;97:234–240. doi: 10.1016/j.fitote.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M., Ma N., Qiu F., Hai W.L., Tang H.F., Zhang Y., Wen A.D. Triterpenoid saponins from the roots of Clematis argentilucida and their cytotoxic activity. Planta Med. 2014;80(11):942–948. doi: 10.1055/s-0034-1382838. [DOI] [PubMed] [Google Scholar]
- 16.Li S.G., Huang X.J., Li M.M., Wang M., Feng R.B., Zhang W., Li Y.L., Wang Y., Ye W.C. Triterpenoid saponins from the roots of Clematis uncinata. Chem. Pharm. Bull. (Tokyo) 2014;62(1):35–44. doi: 10.1248/cpb.c13-00269. [DOI] [PubMed] [Google Scholar]
- 17.Tian X., Feng J., Tang H., Zhao M., Li Y., Hai W., Zhang X. New cytotoxic triterpenoid saponins from the whole plant of Clematis lasiandra Maxim. Fitoterapia. 2013;90:233–239. doi: 10.1016/j.fitote.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 18.He Y.X., Li L., Zhang K., Liu Z.R. Cytotoxic triterpene saponins from Clematis mandshurica. J. Asian Nat. Prod. Res. 2011;13(12):1104–1109. doi: 10.1080/10286020.2011.618453. [DOI] [PubMed] [Google Scholar]
- 19.Gong Y.X., Hua H.M., Xu Y.N., Liu J.Y., Yu Z.G., Ma J., Zhang H., Jing Y.K. Triterpene saponins from Clematis mandshurica and their antiproliferative activity. Planta Med. 2013;79(11):987–994. doi: 10.1055/s-0032-1328657. [DOI] [PubMed] [Google Scholar]
- 20.Cheng L., Xia T.S., Wang Y.F., Zhou W., Liang X.Q., Xue J.Q., Shi L., Wang Y., Ding Q. The apoptotic effect of D Rhamnose β-hederin, a novel oleanane-type triterpenoid saponin on breast cancer cells. PLoS One. 2014;9(6):e90848. doi: 10.1371/journal.pone.0090848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong S.W., Jung K.H., Lee H.S., Choi M.J., Son M.K., Zheng H.M., Hong S.S. SB365 inhibits angiogenesis and induces apoptosis of hepatocellular carcinoma through modulation of PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2012;103(11):1929–1937. doi: 10.1111/j.1349-7006.2012.02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Son M.K., Jung K.H., Hong S.W., Lee H.S., Zheng H.M., Choi M.J., Seo J.H., Suh J.K., Hong S.S. SB365, Pulsatillasaponin D suppresses the proliferation of human colon cancer cells and induces apoptosis by modulating the AKT/mTORsignallingpathway. Food Chem. 2013;136(1):26–33. doi: 10.1016/j.foodchem.2012.07.096. [DOI] [PubMed] [Google Scholar]
- 23.Son M.K., Jung K.H., Lee H.S., Lee H., Kim S.J., Yan H.H., Ryu Y.L., Hong S.S. SB365, Pulsatillasaponin D suppresses proliferation and induces apoptosis of pancreatic cancer cells. Oncol. Rep. 2013;30(2):801–808. doi: 10.3892/or.2013.2517. [DOI] [PubMed] [Google Scholar]
- 24.Rao X., Gong M., Yin S., Luo X., Jian H., Feng Y., Yang S.L. Study on in situ intestinal absorption of Pulsatilla saponin D in rats. Chin. Tradit. Herbal Drugs. 2013;44:3515–3520. [Google Scholar]
- 25.Liu Y., Song Y., Xu Q., Su D., Feng Y., Li X., Khan I.A., Zhang L., Chen L., Yang S. Validated rapid resolution LC-ESI-MS/MS method for simultaneous determination of five pulchinenosides from Pulsatilla chinensis (Bunge) Regel in rat plasma: application to pharmacokinetics and bioavailability studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013;942-943:141–150. doi: 10.1016/j.jchromb.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang H., Zhou M., Guo Y., He M., Huang H., Ye X., Feng Y., Zhou X., Yang S. Metabolites profiling of Pulsatillasaponin D in rat by ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF-MS/MS). Fitoterapia. 2014;96:152–158. doi: 10.1016/j.fitote.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Hong S.W., Jung K.H., Lee H.S., Son M.K., Yan H.H., Kang N.S., Lee J., Hong S.S. SB365, Pulsatillasaponin D, targets c-Met and exerts antiangiogenic and antitumor activities. Carcinogenesis. 2013;34(9):2156–2169. doi: 10.1093/carcin/bgt159. [DOI] [PubMed] [Google Scholar]
- 28.Jang W.J., Park B., Jeong G.S., Hong S.S., Jeong C.H. SB365, Pulsatilla saponin D, suppresses the growth of gefitinib-resistant NSCLC cells with Met amplification. Oncol. Rep. 2014;32(6):2612–2618. doi: 10.3892/or.2014.3528. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q., Chen W., Jiao Y., Hou J., Wu Q., Liu Y., Qi X. Pulsatillasaponin A, an active molecule from Pulsatilla chinensis, induces cancer cell death and inhibits tumor growth in mouse xenograft models. J. Surg. Res. 2014;188(2):387–395. doi: 10.1016/j.jss.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Guan Y.Y., Liu H.J., Luan X., Xu J.R., Lu Q., Liu Y.R., Gao Y.G., Zhao M., Chen H.Z., Fang C. Raddeanin A, a triterpenoidsaponin isolated from Anemone raddeana, suppresses the angiogenesis and growth of human colorectal tumor by inhibiting VEGFR2 signaling. Phytomedicine. 2015;22(1):103–110. doi: 10.1016/j.phymed.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Xue G., Zou X., Zhou J.Y., Sun W., Wu J., Xu J.L., Wang R.P. Raddeanin A induces human gastric cancer cells apoptosis and inhibits their invasion in vitro. Biochem. Biophys. Res. Commun. 2013;439(2):196–202. doi: 10.1016/j.bbrc.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Tang H., Zhang Y., Tang C., Li B., Wang Y., Gao Z., Luo P., Yin A., Wang X., Cheng G., Fei Z. Saponin 1 induces apoptosis and suppresses NF-κB-mediated survival signaling in glioblastoma multiforme (GBM). PLoS One. 2013;8(11):e81258. doi: 10.1371/journal.pone.0081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji C., Cheng G., Tang H., Zhang Y., Hu Y., Zheng M., Fei Z. Saponin 6 of Anemone Taipaiensis inhibits proliferation and induces apoptosis of U87 MG cells. Xibao Yu Fenzi Mianyixue Zazhi. 2015;31(4):484–486. [PubMed] [Google Scholar]
- 34.Wang Y., Tang H., Zhang Y., Li J., Li B., Gao Z., Wang X., Cheng G., Fei Z. Saponin B, a novel cytostatic compound purified from Anemone taipaiensis, induces apoptosis in a human glioblastoma cell line. Int. J. Mol. Med. 2013;32(5):1077–1084. doi: 10.3892/ijmm.2013.1500. [DOI] [PubMed] [Google Scholar]
- 35.Han L.T., Fang Y., Li M.M., Yang H.B., Huang F. The antitumor effects of triterpenoid saponins from the Anemone flaccida and the underlying mechanism. Evid. Based Complement. Alternat.Med., 2013. 517931. [DOI] [PMC free article] [PubMed]
- 36.Tian Z., Xiao P.G., Wen J., Huang F., Yang M.S., Chen S.L. Review of bioactivities of natural cycloartane triterpenoids. Zhongguo Zhong Yao Za Zhi. 2006;31(8):625–629. [PubMed] [Google Scholar]
- 37.Gao J., Huang F., Zhang J., Zhu G., Yang M., Xiao P.G. Cytotoxic cycloartane triterpene saponins from Actaea asiatica. J. Nat. Prod. 2006;69(10):1500–1502. doi: 10.1021/np060113h. [DOI] [PubMed] [Google Scholar]
- 38.Lu L., Chen J.C., Li Y., Qing C., Wang Y.Y., Nian Y., Qiu M.H. Studies on the constituents of Cimicifuga foetida collected in Guizhou Province and their cytotoxic activities. Chem. Pharm. Bull. (Tokyo) 2012;60(5):571–577. doi: 10.1248/cpb.60.571. [DOI] [PubMed] [Google Scholar]
- 39.Guo L.Y., Joo E.J., Son K.H., Jeon S.J., Jang S., Shin E.M., Zhou H.Y., Kim Y.S. Cimiside E arrests cell cycle and induces cell apoptosis in gastric cancer cells. Arch. Pharm. Res. 2009;32(10):1385–1392. doi: 10.1007/s12272-009-2007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bao N.M., Nian Y., Zhu G.L., Wang W.H., Zhou L., Qiu M.H. Cytotoxic 9,19-cycloartane triterpenes from the aerial parts of Cimicifuga yunnanensis. Fitoterapia. 2014;99:191–197. doi: 10.1016/j.fitote.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Zhu D.F., Zhu G.L., Kong L.M., Bao N.M., Zhou L., Nian Y., Qiu M.H. Cycloartane Glycosides from the roots of Cimicifuga foetida with Wnt signaling pathway inhibitory activity. Nat. Prod. Bioprospect. 2015;5(2):61–67. doi: 10.1007/s13659-015-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian Z., Si J.Y., Chen S.B., Yang M.S., Xiao P.G., Wu E.X. Cytotoxicity and mechanism of 23-O-acetylcimigenol-3-O-beta-D-xylopyranoside on HepG2 cells. Zhongguo Zhong Yao Za Zhi. 2006;31(21):1818–1821. [PubMed] [Google Scholar]
- 43.Tian Z., Zhou L., Huang F., Chen S., Yang J., Wu E., Xiao P.G., Yang M. Anti-cancer activity and mechanisms of 25-anhydrocimigenol-3-O-beta-D-xylopyranoside isolated from Souliea vaginata on hepatomas. Anticancer Drugs. 2006;17(5):545–551. doi: 10.1097/00001813-200606000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Mitri Z., Constantine T., O'Regan R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother. Res. Pract., 2012. 743193. [DOI] [PMC free article] [PubMed]
- 45.Coussens L., Yang-Feng T.L., Liao Y.C., Chen E., Gray A., McGrath J., Seeburg P.H., Libermann T.A., Schlessinger J., Francke U. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230(4730):1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- 46.Olayioye M.A. Update on HER-2 as a target for cancer therapy: intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res. 2001;3(6):385–389. doi: 10.1186/bcr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy V., Perez E.A. Beyond trastuzumab: small molecule tyrosine kinase inhibitors in HER-2-positive breast cancer. Oncologist. 2009;14(11):1061–1069. doi: 10.1634/theoncologist.2009-0142. [DOI] [PubMed] [Google Scholar]
- 48.Burstein H.J. The distinctive nature of HER2-positive breast cancers. N. Engl. J. Med. 2005;353(16):1652–1654. doi: 10.1056/NEJMp058197. [DOI] [PubMed] [Google Scholar]
- 49.Einbond L.S., Mighty J., Redenti S., Wu H.A. Actein induces calcium release in human breast cancer cells. Fitoterapia. 2013;91:28–38. doi: 10.1016/j.fitote.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 50.Einbond L.S., Wen-Cai Y., He K., Wu H.A., Cruz E., Roller M., Kronenberg F. Growth inhibitory activity of extracts and phytometabolites from Cimicifuga species on human breast cancer cells. Phytomedicine. 2008;15(6-7):504–511. doi: 10.1016/j.phymed.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X., Zhao M., Chen L., Jiao H., Liu H., Wang L., Ma S. A triterpenoid from Thalictrum fortunei induces apoptosis in BEL-7402 cells through the P53- induced apoptosis pathway. Molecules. 2011;16(11):9505–9519. doi: 10.3390/molecules16119505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das S., Das J., Samadder A., Khuda-Bukhsh A.R. Dihydroxy-isosteviol methyl ester from Pulsatilla nigricans induces apoptosis in HeLa cells: its cytoxicity and interaction with calf thymus DNA. Phytother. Res. 2013;27(5):664–671. doi: 10.1002/ptr.4768. [DOI] [PubMed] [Google Scholar]
- 53.Liu M., Zhao X., Xiao L., Liu G., Liu H., Wang X., Feng X., Lin X. Cytotoxicity of the phytometabolites isolated from Pulsatilla chinensis saponins and apoptosis induced by 23-hydroxybetulinic acid. Pharm. Biol. 2015;53(1):1–9. doi: 10.3109/13880209.2014.907323. [DOI] [PubMed] [Google Scholar]
- 54.Kaseb A.O., Chinnakannu K., Chen D., Sivanandam A., Tejwani S., Menon M., et al. Androgen receptor–and E2F-1–targeted thymoquinone therapy for hormone- refractory prostate cancer. Cancer Res. 2007;67:7782–7788. doi: 10.1158/0008-5472.CAN-07-1483. [DOI] [PubMed] [Google Scholar]
- 55.Koka P.S., Mondal D., Schultz M., Abdel-Mageed A.B., Agrawal K.C. Studies on molecular mechanisms of growth inhibitory effects of thymoquinone against prostate cancer cells: role of reactive oxygen species. Exp. Biol. Med. 2010;235:751–760. doi: 10.1258/ebm.2010.009369. [DOI] [PubMed] [Google Scholar]
- 56.El-Mahdy M.A., Zhu Q., Wang Q.E., Wani G., Wani A.A. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int. J. Cancer. 2005;117:409–417. doi: 10.1002/ijc.21205. [DOI] [PubMed] [Google Scholar]
- 57.El-Baba C., Mahadevan V., Fahlbusch F.B. S, S.M.; Rau, T.T.; Gali-Muhtasib, H.; Schneider-Stock, R. Thymoquinone-induced conformational changes of PAK1 interrupt pro-survival MEK-ERK signaling in colorectal cancer. Mol. Cancer. 2014;13:201. doi: 10.1186/1476-4598-13-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sethi G., Ahn K.S., Aggarwal B.B. Targeting nuclear factor-κB activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol. Cancer Res. 2008;6:1059–1070. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- 59.Raghunandhakumar S., Paramasivam A., Senthilraja S., Naveenkumar C., Asokkumar S., Binuclara J., Jagan S., Anandakumar P., Devaki T. Thymoquinone inhibits cell proliferation through regulation of G1/S phase cell cycle transition in N-nitrosodiethylamine-induced experimental rat hepatocellular carcinoma. Toxicol. Lett. 2013;223(1):60–72. doi: 10.1016/j.toxlet.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 60.Yi T., Cho S.G., Yi Z., Pang X., Rodriguez M., Wang Y., Sethi G., Aggarwal B.B., Liu M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Ther. 2008;7:1789–1796. doi: 10.1158/1535-7163.MCT-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torres M.P., Ponnusamy M.P., Chakraborty S., Smith L.M., Das S., Arafat H.A., Batra S.K. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol. Cancer Ther. 2010;9:1419–1431. doi: 10.1158/1535-7163.MCT-10-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mu G.G., Zhang L.L., Li H.Y., Liao Y., Yu H.G. Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Dig. Dis. Sci. 2015;60(4):1067–1080. doi: 10.1007/s10620-014-3394-x. [DOI] [PubMed] [Google Scholar]
- 63.Ashley R.E., Osheroff N. Natural products as topoisomerase II poisons: effects of thymoquinone on DNA cleavage mediated by human topoisomerase IIα. Chem. Res. Toxicol. 2014;27(5):787–793. doi: 10.1021/tx400453v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badary O.A., El-Din A.M. Inhibitory effects of thymoquinone against 20-methylcholanthrene-induced fibrosarcoma tumorigenesis. Cancer Detect. Prev. 2001;25:362–368. [PubMed] [Google Scholar]
- 65.Shin S.B., Woo S.U., Yim H. Differential cellular effects of Plk1 inhibitors targeting the ATP-binding domain or Polo-box domain. J. Cell. Physiol. 2015;••• doi: 10.1002/jcp.25042. [DOI] [PubMed] [Google Scholar]
- 66.Chu S.C., Hsieh Y.S., Yu C.C., Lai Y.Y., Chen P.N. Thymoquinone induces cell death in human squamous carcinoma cells via caspase activation-dependent apoptosis and LC3-II activation-dependent autophagy. PLoS One. 2014;9(7):e101579. doi: 10.1371/journal.pone.0101579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen M.C., Lee N.H., Hsu H.H., Ho T.J., Tu C.C., Hsieh D.J., Lin Y.M., Chen L.M., Kuo W.W., Huang C.Y. Thymoquinone induces caspase-independent, autophagic cell death in CPT-11-resistant lovo colon cancer via mitochondrial dysfunction and activation of JNK and p38. J. Agric. Food Chem. 2015;63(5):1540–1546. doi: 10.1021/jf5054063. [DOI] [PubMed] [Google Scholar]
- 68.Racoma I.O., Meisen W.H., Wang Q.E., Kaur B., Wani A.A. Thymoquinone inhibits autophagy and induces cathepsin-mediated, caspase-independent cell death in glioblastoma cells. PLoS One. 2013;8(9):e72882. doi: 10.1371/journal.pone.0072882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang J., Feng Y., Tsao S., Wang N., Curtain R., Wang Y. Berberine and Coptidis rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J. Ethnopharmacol. 2009;126(1):5–17. doi: 10.1016/j.jep.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 70.Huang Z.H., Zheng H.F., Wang W.L., Wang Y., Zhong L.F., Wu J.L., Li Q.X. Berberine targets epidermal growth factor receptor signaling to suppress prostate cancer proliferation in vitro. Mol. Med. Rep. 2015;11(3):2125–2128. doi: 10.3892/mmr.2014.2929. [DOI] [PubMed] [Google Scholar]
- 71.Yip N.K., Ho W.S. Berberine induces apoptosis via the mitochondrial pathway in liver cancer cells. Oncol. Rep. 2013;30(3):1107–1112. doi: 10.3892/or.2013.2543. [DOI] [PubMed] [Google Scholar]
- 72.Auyeung K.K., Ko J.K. Coptis chinensis inhibits hepatocellular carcinoma cell growth through non-steroidal anti-inflammatory drug-activated gene activation. Int. J. Mol. Med. 2009;24(4):571–577. doi: 10.3892/ijmm_00000267. [DOI] [PubMed] [Google Scholar]
- 73.Shukla S., Rizvi F., Raisuddin S., Kakkar P. FoxO proteins' nuclear retention and BH3-only protein Bim induction evoke mitochondrial dysfunction-mediated apoptosis in berberine-treated HepG2 cells. Free Radic. Biol. Med. 2014;76:185–199. doi: 10.1016/j.freeradbiomed.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 74.Chen Q., Peng W., Qi S., Xu A. Apoptosis of human highly metastatic lung cancer cell line 95-D induced by acutiaporberine, a novel bis-alkaloid derived from Thalictrum acutifolium. Planta Med. 2002;68(6):550–553. doi: 10.1055/s-2002-32546. [DOI] [PubMed] [Google Scholar]
- 75.Ji X., Sun H., Zhou H., Xiang J., Tang Y., Zhao C. The interaction of telomeric DNA and C-myc22 G-quadruplex with 11 natural alkaloids. Nucleic Acid Ther. 2012;22(2):127–136. doi: 10.1089/nat.2012.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin C.C., Lin S.Y., Chung J.G., Lin J.P., Chen G.W., Kao S.T. Down-regulation of cyclin B1 and up-regulation of Wee1 by berberine promotes entry of leukemia cells into the G2/M-phase of the cell cycle. Anticancer Res. 2006;26(2A):1097–1104. [PubMed] [Google Scholar]
- 77.Serafim T.L., Oliveira P.J., Sardao V.A., Perkins E., Parke D., Holy J. Different concentrations of berberine result in distinct cellular localization patterns and cell cycle effects in a melanoma cell line. Cancer Chemother. Pharmacol. 2008;61(6):1007–1018. doi: 10.1007/s00280-007-0558-9. [DOI] [PubMed] [Google Scholar]
- 78.Lee K.H., Lo H.L., Tang W.C., Hsiao H.H., Yang P.M. A gene expression signature-based approach reveals the mechanisms of action of the Chinese herbal medicine berberine. Sci. Rep. 2014;4:6394. doi: 10.1038/srep06394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hou Q. Study on the autophagy and apoptosis induced by berberine in human hepatoma cells with the molecular mechanism research. 2011. [Google Scholar]
- 80.Liu Q., Xu X., Zhao M., Wei Z., Li X., Zhang X., Liu Z., Gong Y., Shao C. Berberine induces senescence of human glioblastoma cells by downregulating the EGFR-MEK-ERK signaling pathway. Mol. Cancer Ther. 2015;14(2):355–363. doi: 10.1158/1535-7163.MCT-14-0634. [DOI] [PubMed] [Google Scholar]
- 81.Sheng L.H., Xu M., Xu L.Q., Xiong F. Cytotoxic effect of lappaconitine on non-small cell lung cancer in vitro and its molecular mechanism. Zhong Yao Cai. 2014;37(5):840–843. [PubMed] [Google Scholar]
- 82.Zhang H., Guo Z., Han L., You X., Xu Y. The antitumor effect and mechanism of taipeinine A, a new C19-diterpenoid alkaloid from Aconitum taipeicum, on the HepG2 human hepatocellular carcinoma cell line. J. BUON. 2014;19(3):705–712. [PubMed] [Google Scholar]
- 83.Lin C.Z., Zhao Z.X., Xie S.M., Mao J.H., Zhu C.C., Li X.H., Zeren-dawa B., Suolang-qimei K., Zhu D., Xiong T.Q., Wu A.Z. Diterpenoid alkaloids and flavonoids from Delphinium trichophorum. Phytochemistry. 2014;97:88–95. doi: 10.1016/j.phytochem.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 84.Deng L.J., Hu L.P., Peng Q.L., Yang X.L., Bai L.L., Yiu A., Li Y., Tian H.Y., Ye W.C., Zhang D.M. Hellebrigenin induces cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells through inhibition of Akt. Chem. Biol. Interact. 2014;219:184–194. doi: 10.1016/j.cbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 85.Mazzio E., Badisa R., Mack N., Deiab S., Soliman K.F. High throughput screening of natural products for anti-mitotic effects inMDA-MB-231 human breast carcinoma cells. Phytother. Res. 2014;28(6):856–867. doi: 10.1002/ptr.5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hong S.W., Jung K.H., Lee H.S., Choi M.J., Zheng H.M., Son M.K., Lee G.Y., Hong S.S. Apoptotic and anti-angiogenic effects of Pulsatilla koreana extract on hepatocellular carcinoma. Int. J. Oncol. 2012;40(2):452–460. doi: 10.3892/ijo.2011.1204. [DOI] [PubMed] [Google Scholar]
- 87.Park B.H., Jung K.H., Son M.K., Seo J.H., Lee H.S., Lee J.H., Hong S.S. Antitumor activity of Pulsatillakoreana extract in anaplastic thyroid cancer via apoptosis and anti-angiogenesis. Mol. Med. Rep. 2013;7(1):26–30. doi: 10.3892/mmr.2012.1166. [DOI] [PubMed] [Google Scholar]
- 88.Einbond L.S., Su T., Wu H.A., Friedman R., Wang X., Jiang B., Hagan T., Kennelly E.J., Kronenberg F., Weinstein I.B. Gene expression analysis of the mechanisms whereby black cohosh inhibits human breast cancer cell growth. Anticancer Res. 2007;27(2):697–712. [PubMed] [Google Scholar]
- 89.Tian Z., Pan R., Chang Q., Si J., Xiao P.G., Wu E. Cimicifuga foetida extract inhibits proliferation of hepatocellular cells via induction of cell cycle arrest and apoptosis. J. Ethnopharmacol. 2007;114(2):227–233. doi: 10.1016/j.jep.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 90.Tian Z., Si J., Chang Q., Zhou L., Chen S., Xiao P.G., Wu E. Antitumor activity and mechanisms of action of total glycosides from aerial part of Cimicifuga dahurica targeted against hepatoma. BMC Cancer. 2007;7:237. doi: 10.1186/1471-2407-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hostanska K., Nisslein T., Freudenstein J., Reichling J., Saller R. Apoptosis of human prostate androgen-dependent and -independent carcinoma cells induced by an isopropanolic extract of black cohosh involves degradation of cytokeratin (CK) 18. Anticancer Res. 2005;25(1A):139–147. [PubMed] [Google Scholar]
- 92.Wang H., Zhang F., Ye F., Ma Y., Zhang D.Y. The effect of coptis chinensis on the signaling network in the squamous carcinoma cells. Front. Biosci. 2011;3:326–340. doi: 10.2741/e248. [DOI] [PubMed] [Google Scholar]
- 93.Ratovitski E.A. Tumor protein p63/microRNA network in epithelial cancer cells. Curr. Genomics. 2013;14(7):441–452. doi: 10.2174/13892029113146660011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhattacharya S., Ahir M., Patra P., Mukherjee S., Ghosh S., Mazumdar M., Chattopadhyay S., Das T., Chattopadhyay D., Adhikary A. PEGylated- thymoquinone-nanoparticle mediated retardation of breast cancer cell migration by deregulation of cytoskeletal actin polymerization through miR-34a. Biomaterials. 2015;51:91–107. doi: 10.1016/j.biomaterials.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 95.Lo T.F., Tsai W.C., Chen S.T. MicroRNA-21-3p, a berberine-induced miRNA, directly down-regulates human methionine adenosyltransferases 2A and 2B and inhibits hepatoma cell growth. PLoS One. 2013;8(9):e75628. doi: 10.1371/journal.pone.0075628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Su Y.H., Tang W.C., Cheng Y.W., Sia P., Huang C.C., Lee Y.C., Jiang H.Y., Wu M.H., Lai I.L., Lee J.W., Lee K.H. Targeting of multiple oncogenic signaling pathways by Hsp90 inhibitor alone or in combination with berberine for treatment of colorectal cancer. Biochim. Biophys. Acta. 2015;1853(10 Pt A):2261–2272. doi: 10.1016/j.bbamcr.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 97.Chen Q., Qin R., Fang Y., Li H. Berberine sensitizes human ovarian cancer cells to cisplatin through miR-93/PTEN/Akt signaling pathway. Cell. Physiol. Biochem. 2015;36(3):956–965. doi: 10.1159/000430270. [DOI] [PubMed] [Google Scholar]
- 98.Feng M., Luo X., Gu C., Li Y., Zhu X., Fei J. Systematic analysis of berberine-induced signaling pathway between miRNA clusters and mRNAs and identification of mir-99a ∼ 125b cluster function by seed-targeting inhibitors in multiple myeloma cells. RNA Biol. 2015;12(1):82–91. doi: 10.1080/15476286.2015.1017219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salem A.A., El Haty I.A., Abdou I.M., Mu Y. Interaction of human telomeric G-quadruplex DNA with thymoquinone: a possible mechanism for thymoquinone anticancer effect. Biochim. Biophys. Acta. 2015;1850(2):329–342. doi: 10.1016/j.bbagen.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 100.Gurung R.L., Lim S.N., Khaw A.K., Soon J.F., Shenoy K., Mohamed Ali S., Jayapal M., Sethu S., Baskar R., Hande M.P. Thymoquinone induces telomere shortening, DNA damage and apoptosis in human glioblastoma cells. PLoS One. 2010;5(8):e12124. doi: 10.1371/journal.pone.0012124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zubair H., Khan H.Y., Sohail A., Azim S., Ullah M.F., Ahmad A., Sarkar F.H., Hadi S.M. Redox cycling of endogenous copper by thymoquinone leads to ROS- mediated DNA breakage and consequent cell death: putative anticancer mechanism of antioxidants. Cell Death Dis. 2013;4:e660. doi: 10.1038/cddis.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Attoub S., Sperandio O., Raza H., Arafat K., Al-Salam S., Al Sultan M.A., Al Safi M., Takahashi T., Adem A. Thymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam. Clin. Pharmacol. 2013;27(5):557–569. doi: 10.1111/j.1472-8206.2012.01056.x. [DOI] [PubMed] [Google Scholar]
- 103.Chehl N., Chipitsyna G., Gong Q., Yeo C.J., Arafat H.A. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB. 2009;11(5):373–381. doi: 10.1111/j.1477-2574.2009.00059.x. [Oxford]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu X., Wu X., Huang Y., Tong Q., Takeda S., Qing Y. Berberine induces double-strand DNA breaks in Rev3 deficient cells. Mol. Med. Rep. 2014;9(5):1883–1888. doi: 10.3892/mmr.2014.1999. [DOI] [PubMed] [Google Scholar]
- 105.Waters L.S., Minesinger B.K., Wiltrout M.E., D'Souza S., Woodruff R.V., Walker G.C. Eukaryotic Translesion Polymerases and Their Roles and Regulation in DNA Damage Tolerance. Microbiol. Mol. Biol. Rev. 2009;73(1):134–154. doi: 10.1128/MMBR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu Y., Ma N., Li H.X., Tian L., Ba Y.F., Hao B. Berberine induces apoptosis and DNA damage in MG-63 human osteosarcoma cells. Mol. Med. Rep. 2014;10(4):1734–1738. doi: 10.3892/mmr.2014.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen S., Wan L., Couch L., Lin H., Li Y., Dobrovolsky V.N., Mei N., Guo L. Mechanism study of goldenseal-associated DNA damage. Toxicol. Lett. 2013;221(1):64–72. doi: 10.1016/j.toxlet.2013.05.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J., Liu Q., Yang Q. Radiosensitization effects of berberine on human breast cancer cells. Int. J. Mol. Med. 2012;30(5):1166–1172. doi: 10.3892/ijmm.2012.1095. [DOI] [PubMed] [Google Scholar]
- 109.Ji X., Sun H., Zhou H., Xiang J., Tang Y., Zhao C. The interaction of telomeric DNA and C-myc22 G-quadruplex with 11 natural alkaloids. Nucleic Acid Ther. 2012;22(2):127–136. doi: 10.1089/nat.2012.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saha S.K., Khuda-Bukhsh A.R. Berberine alters epigenetic modifications, disrupts microtubule network, and modulates HPV-18 E6-E7 oncoproteins by targeting p53 in cervical cancer cell HeLa: a mechanistic study including molecular docking. Eur. J. Pharmacol. 2014;744:132–146. doi: 10.1016/j.ejphar.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 111.Qing Y., Hu H., Liu Y., Feng T., Meng W., Jiang L., Sun Y., Yao Y. Berberine induces apoptosis in human multiple myeloma cell line U266 through hypomethylation of p53 promoter. Cell Biol. Int. 2014;38(5):563–570. doi: 10.1002/cbin.10206. [DOI] [PubMed] [Google Scholar]
- 112.Burdette J.E., Chen S.N., Lu Z.Z., Xu H., White B.E., Fabricant D.S., Liu J., Fong H.H., Farnsworth N.R., Constantinou A.I., Van Breemen R.B., Pezzuto J.M., Bolton J.L. Black cohosh (Cimicifuga racemosa L.) protects against menadione-induced DNA damage through scavenging of reactive oxygen species: bioassay-directed isolation and characterization of active principles. J. Agric. Food Chem. 2002;50(24):7022–7028. doi: 10.1021/jf020725h. [DOI] [PubMed] [Google Scholar]
- 113.Zhou F., Lv O., Zheng Y., Wang J., Hu P., Wang Z., Yang L. Inhibitory effect of Pulsatilla chinensis polysaccharides on glioma. Int. J. Biol. Macromol. 2012;50(5):1322–1326. doi: 10.1016/j.ijbiomac.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 114.Shafiq H., Ahmad A., Masud T., Kaleem M. Cardio-protective and anti-cancer therapeutic potential of Nigella sativa. Iran. J. Basic Med. Sci. 2014;17(12):967–979. [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng L., Xia T.S., Wang Y.F., Zhou W., Liang X.Q., Xue J.Q., Shi L., Wang Y., Ding Q., Wang M. The anticancer effect and mechanism of α-hederin on breast cancer cells. Int. J. Oncol. 2014;45(2):757–763. doi: 10.3892/ijo.2014.2449. [DOI] [PubMed] [Google Scholar]
- 116.Hao D.C., Xiao P.G., Chen S.L. Phenotype prediction of nonsynonymous single nucleotide polymorphisms in human phase II drug/xenobiotic metabolizing enzymes: perspectives on molecular evolution. Sci. China Life Sci. 2010;53(10):1252–1262. doi: 10.1007/s11427-010-4062-9. [DOI] [PubMed] [Google Scholar]
- 117.Wang D., Han L., Guo Z. Quinone reductase inducing activity of the dichloromethane /ethanol extract of the roots of Pulsatillachinensis. Nat. Prod. Commun. 2011;6(6):799–802. [PubMed] [Google Scholar]
- 118.Woo C.C., Hsu A., Kumar A.P., Sethi G., Tan K.H. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: the role of p38 MAPK and ROS. PLoS One. 2013;8(10):e75356. doi: 10.1371/journal.pone.0075356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ismail M., Al-Naqeep G., Chan K.W. Nigella sativathymoquinone-rich fraction greatly improves plasmaantioxidant capacity and expression of antioxidantgenes in hypercholesterolemic rats. Free Radic. Biol. Med. 2010;48:664–672. doi: 10.1016/j.freeradbiomed.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 120.Ebru U., Burak U., Yusuf S., Reyhan B., Arif K., Faruk T.H., Emin M., Aydin K. Atilla, II.; Semsettin, S.; Kemal, E. Cardioprotective effects of Nigella sativa oilon cyclosporine A‐induced cardiotoxicity in rats. Basic Clin. Pharmacol. Toxicol. 2008;103:574–580. doi: 10.1111/j.1742-7843.2008.00313.x. [DOI] [PubMed] [Google Scholar]
- 121.Lin J.P., Yang J.S., Wu C.C., Lin S.S., Hsieh W.T., Lin M.L., Yu F.S., Yu C.S., Chen G.W., Chang Y.H., Chung J.G. Berberine induced down-regulation of matrix metalloproteinase-1, -2 and -9 in human gastric cancer cells (SNU-5) in vitro. In Vivo. 2008;22(2):223–230. [PubMed] [Google Scholar]
- 122.Park S.H., Sung J.H., Kim E.J., Chung N. Berberine induces apoptosis via ROS generation in PANC-1 and MIA-PaCa2 pancreatic cell lines. Braz. J. Med. Biol. Res. 2015;48(2):111–119. doi: 10.1590/1414-431X20144293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang X.Z., Wang L., Liu D.W., Tang G.Y., Zhang H.Y. Synergistic inhibitory effect of berberine and d-limonene on human gastric carcinoma cell line MGC803. J. Med. Food. 2014;17(9):955–962. doi: 10.1089/jmf.2013.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Elbarbry F., Ragheb A., Marfleet T., Shoker A. Modulation of hepatic drug metabolizing enzymes by dietary doses of thymoquinone in female New Zealand White rabbits. Phytother. Res. 2012;26(11):1726–1730. doi: 10.1002/ptr.4628. [DOI] [PubMed] [Google Scholar]
- 125.ElKhoely A., Hafez H.F., Ashmawy A.M., Badary O., Abdelaziz A., Mostafa A., Shouman S.A. Chemopreventive and therapeutic potentials of thymoquinone in HepG2 cells: mechanistic perspectives. J. Nat. Med. 2015;69(3):313–323. doi: 10.1007/s11418-015-0895-7. [DOI] [PubMed] [Google Scholar]
- 126.Nabekura T., Hiroi T., Kawasaki T., Uwai Y. Effects of natural nuclear factor-kappa B inhibitors on anticancer drug efflux transporter human P-glycoprotein. Biomed. Pharmacother. 2015;70:140–145. doi: 10.1016/j.biopha.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 127.Liu D.L., Li Y.J., Yao N., Xu J., Chen Z.S., Yiu A., Zhang C.X., Ye W.C., Zhang D.M. Acerinol, a cyclolanstane triterpenoid from Cimicifuga acerina, reverses ABCB1-mediated multidrug resistance in HepG2/ADM and MCF-7/ADR cells. Eur. J. Pharmacol. 2014;733:34–44. doi: 10.1016/j.ejphar.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 128.Dueregger A., Guggenberger F., Barthelmes J., Stecher G., Schuh M., Intelmann D., Abel G., Haunschild J., Klocker H., Ramoner R., Sampson N. Attenuation of nucleoside and anti-cancer nucleoside analog drug uptake in prostate cancer cells by Cimicifuga racemosa extract BNO-1055. Phytomedicine. 2013;20(14):1306–1314. doi: 10.1016/j.phymed.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 129.Yu H., Ji X., Wu Z., Wang S. Effects of aconite root on energy metabolism and expression of related genes in rats. Zhongguo Zhong Yao Za Zhi. 2011;36(18):2535–2538. [PubMed] [Google Scholar]
- 130.de la Peña S.S., Sothern R.B., López F.S., Lujambio I.M., Waizel-Bucay J., Sánchez C.O., Monroy C.P., Betancourt E.T. Circadian aspects of hyperthermia in mice induced by Aconitum napellus. Pharmacogn. Mag. 2011;7(27):234–242. doi: 10.4103/0973-1296.84238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen T.T., Qi C., Guo H., Cheng Z., Zhou D., Liu H., Liu J. The effects of Fu Zi on changes in the body heat of dogs. J. Acupunct. Meridian Stud. 2009;2(1):71–74. doi: 10.1016/S2005-2901(09)60018-2. [DOI] [PubMed] [Google Scholar]
- 132.Wada K., Nihira M., Ohno Y. Effects of chronic administrations of aconitine on body weight and rectal temperature in mice. J. Ethnopharmacol. 2006;105(1-2):89–94. doi: 10.1016/j.jep.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 133.Zheng Q., Zhao Y., Wang J., Liu T., Zhang B., Gong M., Li J., Liu H., Han B., Zhang Y., Song X., Li Y., Xiao X. Spectrum-effect relationships between UPLC fingerprints and bioactivities of crude secondary roots of Aconitum carmichaelii Debeaux (Fuzi) and its three processed products on mitochondrial growth coupled with canonical correlation analysis. J. Ethnopharmacol. 2014;153(3):615–623. doi: 10.1016/j.jep.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 134.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 135.Li Y., Fu C.M., Ren B., Liu Y., Gao F., Yang H., Peng W., Gan Y.X. Study on attenuate and synergistic mechanism between aconiti lateralis praeparata radix and glycyrrhizae radix for toxicity reduction based on metabonomic of MI-RI mouse cardiomyocytes. Zhongguo Zhong Yao Za Zhi. 2014;39(16):3166–3171. [PubMed] [Google Scholar]
- 136.Jafri S.H., Glass J., Shi R., Zhang S., Prince M., Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J. Exp. Clin. Cancer Res. 2010;29:87. doi: 10.1186/1756-9966-29-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khan M.A., Tania M., Wei C., Mei Z., Fu S., Cheng J., Xu J., Fu J. Thymoquinone inhibits cancer metastasis by downregulating TWIST1 expression to reduce epithelial to mesenchymal transition. Oncotarget. 2015 doi: 10.18632/oncotarget.3973. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nian Y., Yang J., Liu T.Y., Luo Y., Zhang J.H., Qiu M.H. New anti-angiogenic leading structure discovered in the fruit of Cimicifuga yunnanensis. Sci. Rep. 2015;5:9026. doi: 10.1038/srep09026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hambright H.G., Batth I.S., Xie J., Ghosh R., Kumar A.P. Palmatine inhibits growth and invasion in prostate cancer cell: Potential role for RPS6/NF-κB/FLIP. Mol. Carcinog. 2014;••• doi: 10.1002/mc.22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu X., Ji Q., Ye N., Sui H., Zhou L., Zhu H., Fan Z., Cai J., Li Q. Berberine inhibits invasion and metastasis of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3 signaling pathway. PLoS One. 2015;10(5):e123478. doi: 10.1371/journal.pone.0123478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu C.H., Tang W.C., Sia P., Huang C.C., Yang P.M., Wu M.H., Lai I.L., Lee K.H. Berberine inhibits the metastatic ability of prostate cancer cells by suppressing epithelial-to-mesenchymal transition (EMT)-associated genes with predictive and prognostic relevance. Int. J. Med. Sci. 2015;12(1):63–71. doi: 10.7150/ijms.9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tsang C.M., Cheung K.C., Cheung Y.C., Man K., Lui V.W., Tsao S.W., Feng Y. Berberine suppresses Id-1 expression and inhibits the growth and development of lung metastases in hepatocellular carcinoma. Biochim. Biophys. Acta. 2015;1852(3):541–551. doi: 10.1016/j.bbadis.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 143.Kim J.B., Yu J.H., Ko E., Lee K.W., Song A.K., Park S.Y., Shin I., Han W., Noh D.Y. The alkaloid Berberine inhibits the growth of Anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell lines by inducing cell cycle arrest. Phytomedicine. 2010;17(6):436–440. doi: 10.1016/j.phymed.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 144.Liu R., Cao Z., Pan Y., Zhang G., Yang P., Guo P., Zhou Q. Jatrorrhizine hydrochloride inhibits the proliferation and neovascularization of C8161 metastatic melanoma cells. Anticancer Drugs. 2013;24(7):667–676. doi: 10.1097/CAD.0b013e328361ab28. [DOI] [PubMed] [Google Scholar]
- 145.Bao M., Cao Z., Yu D., Fu S., Zhang G., Yang P., Pan Y., Yang B., Han H., Zhou Q. Columbamine suppresses the proliferation and neovascularization of metastatic osteosarcoma U2OS cells with low cytotoxicity. Toxicol. Lett. 2012;215(3):174–180. doi: 10.1016/j.toxlet.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 146.Kim J.M., Kim K.S., Lee Y.W., Cho C.K., Yoo H.S., Bang J.Y., Kim E.Y., Kang I.C. Anti-angiogenic effects of water extract of a formula consisting of Pulsatilla koreana, Panax ginseng and Glycyrrhiza uralensis. Zhong Xi Yi Jie He Xue Bao. 2011;9(9):1005–1013. doi: 10.3736/jcim20110912. [DOI] [PubMed] [Google Scholar]
- 147.Zhao B., Hou X.D., Li H., Qi X.X., Li G.G., Liu L.X., Wang P., Du G.J. Comparative study of Coptidis Rhizoma and Aconiti Kusnezoffii Radix on cell differentiation in lewis lung cancer. Zhongguo Zhong Yao Za Zhi. 2014;39(14):2732–2738. [PubMed] [Google Scholar]
- 148.Xu X.F., Chen Y.H., Wang J., Liao J.J., Wu J.H. Effect and mechanism of Aconitum vaginatum on the proliferation, invasion and metastasis in human A549 lung carcinoma cells. Zhong Yao Cai. 2010;33(12):1909–1912. [PubMed] [Google Scholar]
- 149.Tan H.Y., Wang N., Tsao S.W., Zhang Z., Feng Y. Suppression of vascular endothelial growth factor via inactivation of eukaryotic elongation factor 2 by alkaloids in Coptidis rhizome in hepatocellular carcinoma. Integr. Cancer Ther. 2014;13(5):425–434. doi: 10.1177/1534735413513635. [DOI] [PubMed] [Google Scholar]
- 150.Lv X., Wang H., Han H., Lv S., Qin D. Effects of polysaccharide of radix ranunculi ternati on immunomodulation and anti-oxidation. Zhongguo Zhong Yao Za Zhi. 2010;35(14):1862–1865. doi: 10.4268/cjcmm20101421. [DOI] [PubMed] [Google Scholar]
- 151.Sun D.L., Xie H.B., Xia Y.Z. A study on the inhibitory effect of polysaccharides from Radix ranunculus ternati on human breast cancer MCF-7 cell lines. Afr. J. Tradit. Complement. Altern. Medicines. 2013;10(6):439–443. doi: 10.4314/ajtcam.v10i6.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Niu L., Zhou Y., Sun B., Hu J., Kong L., Duan S. Inhibitory effect of saponins and polysaccharides from Radix ranunculi ternati on human gastric cancer BGC823 cells. Afr. J. Tradit. Complement. Altern. Medicines. 2013;10(3):561–566. doi: 10.4314/ajtcam.v10i3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Y., Li Y., Yang W., Zhang L., Cao G. Anti-hepatoma activity in mice of a polysaccharide from the rhizome of Anemone raddeana. Int. J. Biol. Macromol. 2012;50(3):632–636. doi: 10.1016/j.ijbiomac.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 154.Li H., Sun M., Xu J., Li H., Zang M., Cui Y. Immunological response in H22 transplanted mice undergoing Aconitum coreanum polysaccharide treatment. Int. J. Biol. Macromol. 2013;55:295–300. doi: 10.1016/j.ijbiomac.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 155.Sultan M.T., Butt M.S., Qayyum M.M., Suleria H.A. Immunity: plants as effective mediators. Crit. Rev. Food Sci. Nutr. 2014;54(10):1298–1308. doi: 10.1080/10408398.2011.633249. [DOI] [PubMed] [Google Scholar]
- 156.Majdalawieh A.F., Fayyad M.W. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: A comprehensive review. Int. Immunopharmacol. 2015;28(1):295–304. doi: 10.1016/j.intimp.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 157.Salem M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005;5(13-14):1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 158.Li W., Hua B., Saud S.M., Lin H., Hou W., Matter M.S., Jia L., Colburn N.H., Young M.R. Berberine regulates AMP-activated protein kinase signaling pathways and inhibits colon tumorigenesis in mice. Mol. Carcinog. 2014 doi: 10.1002/mc.22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee T.H., Huang N.K., Lai T.C., Yang A.T., Wang G.J. Anemonin, from Clematis crassifolia, potent and selective inducible nitric oxide synthase inhibitor. J. Ethnopharmacol. 2008;116(3):518–527. doi: 10.1016/j.jep.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 160.Schafer M., Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 161.Akkol E.K., Süntar I., Erdoğan T.F., Keleş H., Gonenç T.M., Kıvçak B. Wound healing and anti-inflammatory properties of Ranunculus pedatus and Ranunculus constantinapolitanus: a comparative study. J. Ethnopharmacol. 2012;139(2):478–484. doi: 10.1016/j.jep.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 162.Fostok S.F., Ezzeddine R.A., Homaidan F.R., Al-Saghir J.A., Salloum R.G., Saliba N.A., Talhouk R.S. Interleukin-6 and cyclooxygenase-2 downregulation by fatty-acid fractions of Ranunculus constantinopolitanus. BMC Complement. Altern. Med. 2009;9:44. doi: 10.1186/1472-6882-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Choi C.H., Kim T.H., Sung Y.K., et al. SKI306X inhibition of glycosaminoglycan degradation in human cartilage involves down-regulation of cytokine-induced catabolic genes. Korean J. Intern. Med. 2014;29(5):647–655. doi: 10.3904/kjim.2014.29.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dilshara M.G., Lee K.T., Lee C.M., Choi Y.H., Lee H.J., Choi I.W., Kim G.Y. New compound, 5-O-isoferuloyl-2-deoxy-D-ribono-γ-lacton from Clematis mandshurica: Anti-inflammatory effects in lipopolysaccharide-stimulated BV2 microglial cells. Int. Immunopharmacol. 2015;24(1):14–23. doi: 10.1016/j.intimp.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 166.Zhou X., Gan P., Hao L., Tao L., Jia J., Gao B., Liu J.Y., Zheng L.T., Zhen X. Antiinflammatory effects of orientin-2”-O-galactopyranoside on lipopolysaccharide-stimulated microglia. Biol. Pharm. Bull. 2014;37(8):1282–1294. doi: 10.1248/bpb.b14-00083. [DOI] [PubMed] [Google Scholar]
- 167.Dietel B., Muench R., Kuehn C., Kerek F., Steinkasserer A., Achenbach S., Garlichs C.D., Zinser E. MCS-18, a natural product isolated from Helleborus purpurascens, inhibits maturation of dendritic cells in ApoE-deficient mice and prevents early atherosclerosis progression. Atherosclerosis. 2014;235(2):263–272. doi: 10.1016/j.atherosclerosis.2014.05.915. [DOI] [PubMed] [Google Scholar]
- 168.Xiao P.G., Wang W.C. A new genus of Ranunculaceae---Dichocarpum W. T. Wang Et Hsiao. Acta Phytotaxonomica Sin. 1964;9(4):315–334. [Google Scholar]
- 169.Xu K., Shu Z., Xu Q.M., Liu Y.L., Li X.R., Wang Y.L., Yang S.L. Cytotoxic activity of Pulsatilla chinensis saponins and their structure-activity relationship. J. Asian Nat. Prod. Res. 2013;15(6):680–686. doi: 10.1080/10286020.2013.790901. [DOI] [PubMed] [Google Scholar]
- 170.Bang S.C., Lee J.H., Song G.Y., Kim D.H., Yoon M.Y., Ahn B.Z. Antitumor activity of Pulsatilla koreana saponins and their structure-activity relationship. Chem. Pharm. Bull. (Tokyo) 2005;53(11):1451–1454. doi: 10.1248/cpb.53.1451. [DOI] [PubMed] [Google Scholar]
- 171.Hazawa M., Wada K., Takahashi K., Mori T., Kawahara N., Kashiwakura I. Suppressive effects of novel derivatives prepared from Aconitum alkaloids on tumor growth. Invest. New Drugs. 2009;27(2):111–119. doi: 10.1007/s10637-008-9141-4. [DOI] [PubMed] [Google Scholar]
- 172.Wada K., Ohkoshi E., Zhao Y., Goto M., Morris-Natschke S.L., Lee K.H. Evaluation of Aconitum diterpenoid alkaloids as antiproliferative agents. Bioorg. Med. Chem. Lett. 2015;25(7):1525–1531. doi: 10.1016/j.bmcl.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hazawa M., Takahashi K., Wada K., Mori T., Kawahara N., Kashiwakura I. Structure-activity relationships between the Aconitum C20-diterpenoid alkaloid derivatives and the growth suppressive activities of Non-Hodgkin's lymphoma Raji cells and human hematopoietic stem/progenitor cells. Invest. New Drugs. 2011;29(1):1–8. doi: 10.1007/s10637-009-9327-4. [DOI] [PubMed] [Google Scholar]
- 174.Sakalar C., Yuruk M., Kaya T., Aytekin M., Kuk S., Canatan H. Pronounced transcriptional regulation of apoptotic and TNF-NF-kappa-B signaling genes during the course of thymoquinone mediated apoptosis in HeLa cells. Mol. Cell. Biochem. 2013;383(1-2):243–251. doi: 10.1007/s11010-013-1772-x. [DOI] [PubMed] [Google Scholar]
- 175.Hara A., Iizuka N., Hamamoto Y., Uchimura S., Miyamoto T., Tsunedomi R., Miyamoto K., Hazama S., Okita K., Oka M. Molecular dissection of a medicinal herb with anti-tumor activity by oligonucleotide microarray. Life Sci. 2005;77(9):991–1002. doi: 10.1016/j.lfs.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 176.Sridhar A., Saremy S., Bhattacharjee B. Elucidation of molecular targets of bioactive principles of black cumin relevant to its anti-tumour functionality - An In silico target fishing approach. Bioinformation. 2014;10(11):684–688. doi: 10.6026/97320630010684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Motaghed M., Al-Hassan F.M., Hamid S.S. Thymoquinone regulates gene expression levels in the estrogen metabolic and interferon pathways in MCF7 breast cancer cells. Int. J. Mol. Med. 2014;33(1):8–16. doi: 10.3892/ijmm.2013.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]


