Abstract
Objective
Benign paroxysmal positional vertigo (BPPV) is the most common vestibular disorder with an incidence between 10.7 and 17.3 per 100,000 persons per year. The mechanism for BPPV has been postulated to involve displaced otoconia resulting in canalithiasis. While particulate matter has been observed in the endolymph of affected patients undergoing posterior canal occlusion surgery, an otoconial origin for the disease is still questioned.
Study Design
In this study, particulate matter was extracted from the posterior semicircular canal of two patients and examined with the scanning electron microscopy.
Methods
The samples were obtained from two patients intraoperatively during posterior semicircular canal occlusion. The particles were fixed, stored in ethanol and chemically dehydrated. The samples were and sputter coated and viewed under a scanning electron microscope. Digital images were obtained.
Results
Intact and degenerating otoconia with and without linking filaments were found attached to amorphous particulate matter. Many otoconia appeared to be partially embedded in a gel matrix, presumably that which encases and anchors the otoconia within the otolith membrane, while others stood alone with no attached filaments and matrix. The otoconia measured roughly 2 to 8 μm in length and displayed a uniform outer shape with a cylindrical bulbous body and a 3+3 rhombohedral plane at each end.
Conclusion
These findings suggest that the source of the particulate matter in the semicircular canals of patients with BPPV is broken off fragments of the utricular otolithic membrane with attached and detached otoconia.
Level of Evidence
NA
Keywords: Otoconia, Otolithic Membrane, Benign Positional Vertigo, Scanning Electron Microscopy
INTRODUCTION
Benign paroxysmal positional vertigo (BPPV) is the most common cause of dizziness due to inner ear disorders.1 In a national epidemiological survey conducted by Mizukoshi and colleagues, BPPV was estimated to have an incidence between 10.7 and 17.3 per 100,000 persons per year.2 Women are more likely affected than men.2,3 In the elderly, an estimated 9% of patients have unrecognized BPPV 4 which may be a significant factor in the morbidity and mortality of falls. The etiology of BPPV can be classified as either primary (idiopathic) or secondary. Primary (idiopathic) BPPV accounts for 50%–70% of cases5 while secondary causes can be divided based on the specific preceding cause. Trauma is the most common cause of secondary BPPV, accounting for 7–17% of all cases;6,7 other secondary causes of BPPV include vestibular neuritis, Meniere’s disease, migraine and inner ear surgery.
The mechanism of BPPV has been hypothesized to involve the presence of free floating particulate matter in the semicircular canal endolymph, rendering it responsive to gravitation forces. One source of such matter could be the small crystalline utricular otoconia. Otoconia range in size from 1 to 30 μm in humans8–10 and are made up of calcite crystals composed of calcium carbonate. They contain an internal organic core of glycosylated proteins and a meshwork of organized filaments. 8,10,11 Deep etching of the guinea pig otoconial complexes have shown otoconia contained in a dense network of meandering and interconnected, beaded filament matrix with each filament measuring 22 nm in diameter; these filaments are in turn cross-linked by shorter filaments 11 nm in diameter.11 The surface of the otoconium is characterized by ordered crystallites and macromolecular aggregates.11,12 Otoconia are adherent to the surface of the otolithic membrane. Within the otolithic membrane, the otoconia are interconnected by linking filaments of 50–100nm in diameter.8
Formation of otoconia involves interactions between various proteins which help to sequester calcium for otoconia formation and help define otoconia crystalline morphology, stability and growth.13–16 These proteins include otoconin-90 (OC90), collagenous scaffold protein otolin-1175, otopetrin14, fetuin-A18 and cerebellin-1. 13 Otoconin-90 accounts for 90% of the total protein in the organic core of the otoconia12–14,16,18 and together with otolin, forms iso-oriented columns of nano-crystals which then results in the complex mosaic of native otoconia.16, 19 Fetuin A and osteopontin are also believed to regulate nucleation and growth of calcite20. Otopetrin 1 has been demonstrated to regulate cytosolic concentrations of calcium during otoconial mineralization. Otoconia have been shown to exhibit degenerative changes with age. These degenerative changes are characterized by fragmentation, surface fissures and compromised linking of otoconial filaments.8,10
The pathophysiological mechanism of BPPV has been attributed to cupulolithiasis (debris of particles adherent to the cupula) or canalithiasis (debris or particles floating within a semicircular canal) 21–23 which can affect each of the three semicircular canals, although most commonly the posterior semicircular canal.24 Canalithiasis was first demonstrated in vivo by Parnes and McClure in 1992 25 when they described free floating particulate matter in the endolymph of the posterior semicircular canal. Welling and colleagues isolated particles from the posterior semicircular canal of one patient undergoing posterior canal occlusion for intractable BPPV and concluded that the particulate matter was most likely composed of degenerating otoconia based on scanning electron micrographs (SEM).26 In spite of this finding, the otoconial origin and composition of these particles is still debated because this was a single case report. Further evidence is required to support this conclusion. Current hypotheses point either to displaced otoconia or highly dense mineralized particles as the source of floating particulate matter within the semicircular canals.12 Direct evidence for an otoconial origin of these particles is based on a single case report26 and thus, the otoconial origin of pathologic material in the mechanism of BPPV is still debated.10 In this study, we have used scanning electron microscopy (SEM) to evaluate the particulate matter isolated from the posterior semicircular canal of two patients with intractable BPPV.
MATERIALS AND METHODS
Case Reports and Isolation of Posterior Canal Specimens
Free-floating particulate matter from the posterior semicircular canal was isolated from two patients with intractable BPPV undergoing posterior semicircular canal occlusion. Case 1 was a 59 year old woman with left posterior canal BPPV of 23 years duration. An attempted singular neurectomy one year after initial onset failed. Case 2 was a 70 year old woman with right vestibular neuronitis resulting in secondary severe posterior canal BPPV of 6 months duration. Both patients had multiple unsuccessful particle repositioning maneuvers.
Both patients underwent transmastoid posterior semicircular canal occlusion by author LP. Normally when identified, the particulate matter is not extracted as part of the canal occlusion procedure. In these two cases, once the matter was identified under high magnification within the 4mm long canal fenestration (Figure 1), the membranous duct containing the matter was isolated by sharply cutting the two visible ends of the membranous duct with a micro Beaver® blade. The membranous duct containing the matter was then extracted with a fine curved pick and placed in 10% buffered formalin which was later placed into 70% ethanol. Then the usual bone dust/fibrinogen sealant “plug” was used to occlude the opening in the canal from where the specimen was extracted. Both patients were permanently cured of their BPPV without any resulting hearing loss or other complications. The specimens were stored in fixative at room temperature and forgotten. In a strange coincidence, the specimens were rediscovered almost 10 years later following which they underwent scanning electron microscopy.
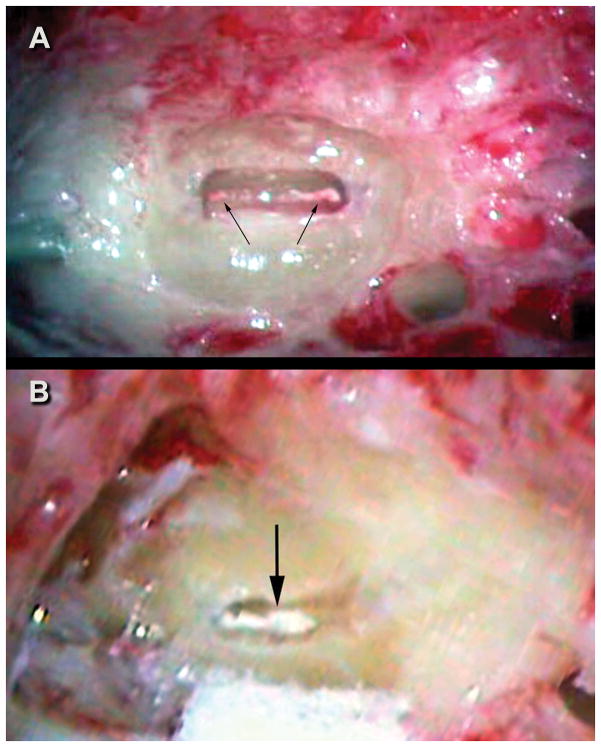
Figure 1.
Figure 1A. Case 1 intraoperative image of fenestrated left posterior semicircular canal. Arrow points to the white particulate matter within the membranous duct. Figure 1B. Case 2 intraoperative image of fenestrated right posterior canal. Arrows point to the diffuse layer of particulate matter within the membranous duct.
Scanning Electron Microscopy
Fixed particulate matter was dehydrated in ethanol. Once in 100% ethanol they were chemically dried with hexamethyldisilazane. The specimens were then mounted on studs with double-stick carbon adhesive dots and sputter coated with gold/palladium in a vacuum chamber (Tousimis Samsputter-2A, Tousimis, Rockville, MD, USA). Images of the specimens were taken using light microscopy prior to and after sputter coating. Both specimens were viewed using a scanning electron microscope (Hitachi S2600, Tokyo, Japan) at 15 kV accelerating voltage with a beam current of 50 amp and a working distance of approximately 10 mm. Digital photographs of images were obtained.
RESULTS
Particle Isolation
Both samples consisted of the membranous semicircular canal with contained particulate matter. The two samples remained whole and measured 742 and 630 μm in the longest dimension.
Scanning Electron Microscopy
Using SEM, most otoconia were seen embedded within the particulate matter of both specimens (Figure 2A Case 1).. Once imaging was complete on this specimen, the covering, presumably endolymphatic duct, was removed and the specimen re-sputter coated and imaged again with the SEM (Figure 2B). At a magnification of 2.0k, the characteristic details of an accumulation of otoconia can be seen (Figure 3). Although differing in size, these otoconia appeared bulbous with a cylindrical body and 3+3 rhombohedral planes on both ends typical of normal human otoconia. Most of the otoconia seemed to be adherent to an underlying matrix, however, some appeared to be free of the underlying substrate. The otoconia seen in this study appear to be anatomically similar to those seen in the normal utricle.10 (Figure 4) in some cases smaller particles and debris appeared to be adherent to the surface of the otoconia. (Figure 4 and 5) Degenerating otoconia embedded within the particulate matter were also seen. In specimen one (idiopathic BPPV) the specimen appeared to have fewer degenerating otoconia compared to specimen 2 (BPPV secondary to vestibular neuronitis) in which most otoconia showed signs of degeneration and surface debris. In both specimens, the otoconia-like particles seemed to be associated with underlying matrix consistent with the otolithic membrane. The characteristic shape of these degenerating otoconia was seen to be less distinct and the surface of the otoconia showed degenerative changes but did not appear to be fractured into fragments. Linking filaments (Figure 6) and remnants of linking filaments of the organic gel matrix attached to the otoconia were also seen. These filaments appeared to be 50 to 100nm in diameter. Some of the particles appeared to be partially embedded within an amorphous material similar to the gelatinous portions of the otolithic membrane of the utricle. (Figure 5) The majority of the matrix of these particles contained filaments within an amorphous gel matrix.
Figure 2.
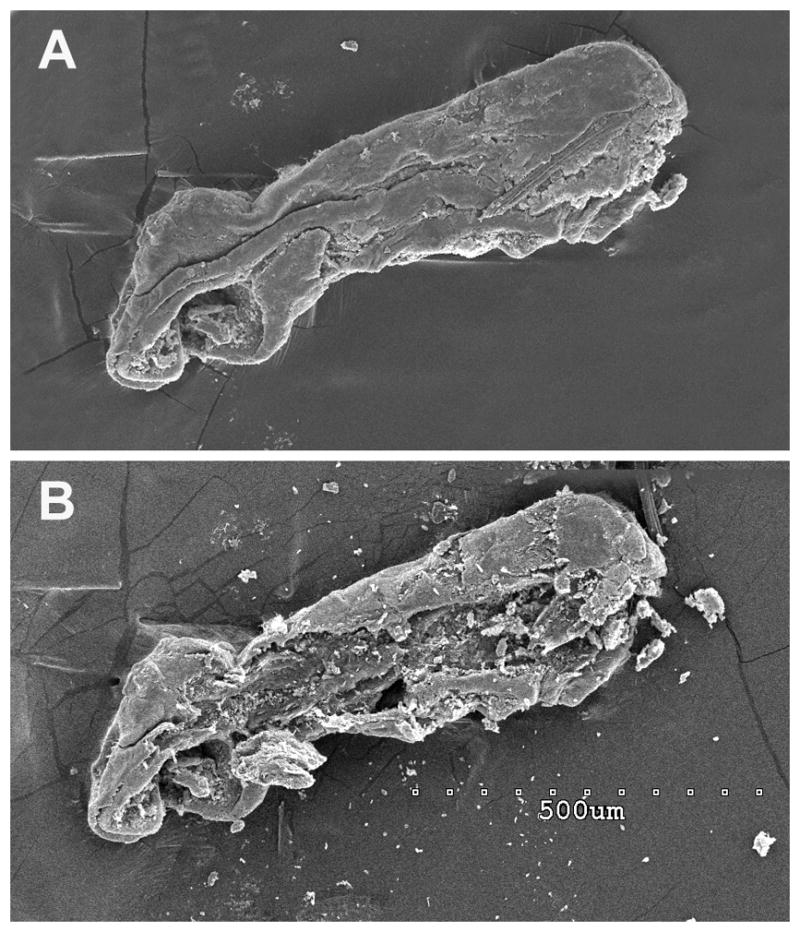
Figure 2 A. A scanning electron micrograph of the specimen from case 1 demonstrating the endolymphatic duct with contained particulate matter. Figure 2 B. The same specimen after the exposed surface layer (presumably the endolymphatic duct wall) was mechanically removed exposing the contents of the canal (particulate matter). Scale bar is shown in panel B 500μm
Figure 3.
Otoconia embedded within particulate matter of the specimen from case 1 (shown in Figure 1B): SEM image shown at 20k magnification. The otoconia exhibit characteristic features of 3+3 rhombohedral planes with bulbous cylindrical bodies embedded on the surface of an amorphous matrix. Scale bar = 20μm.
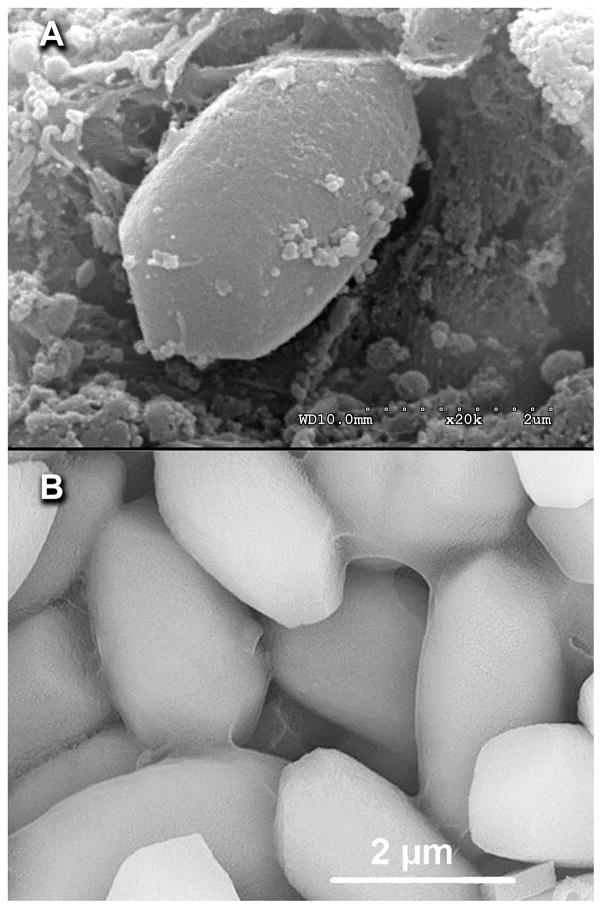
Figure 4.
Figure 4 A. SEM image of an otoconium-like crystal from specimen A demonstrating the typical rhombohedral appearance of an otoconium. Figure 4 B. Otoconia from a normal human utricle at the same magnification. (printed by permission of the author)10 Scale bar 2.0μm.
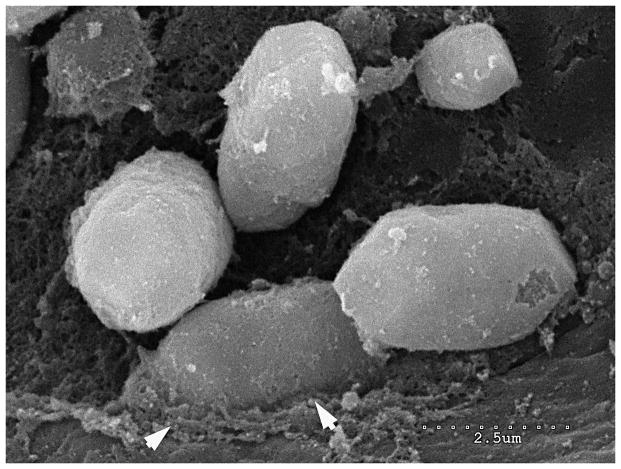
Figure 5.
This higher power view of particulate matter demonstrates that some otoconia appear to be embedded in the underlying gelatinous matrix suggesting that these particles are part of the otolithic membrane with attached otoconia. Scale bar 2.5μm
Figure 6.
Linking fibrils, measuring 50 to 100nm are present in some regions of the retrieved particles indicating that clusters of particles, dissociated from the utricle, become displaced into the semicircular canal. Scale bar 2.0μm
DISCUSSION
BPPV was first described by Adler in 1897 and then later by Barany in 1921 27. Since then, many theories have been proposed regarding the etiology and pathophysiology of BPPV. To date, canalithiasis composed from displaced utricular otoconia is the most widely accepted theory. However, irrefutable evidence that demonstrates the otoconial origin 25,26 has been lacking. 8,10,12 One prior case report demonstrated isolated single otoconia-like particles in a patient with BPPV.26 The present study demonstrates that in two patients with intractable posterior canal BPPV, the particulate matter is composed of a conglomeration of typical human otoconia, most of which are attached to a gel matrix. The otoconia are similar in size and configuration to those observed in the normal human utricle.10 (Figure 4) These structural details include a uniform outer shape with cylindrical bulbous body and a 3+3 rhombohedral plane at each end of the otoconium. The presence of otoconia adherent to amorphous particulate matter (Figure 5) suggests that the displaced particles in cases of BPPV are fragments of the utricular otolithic membrane with attached otoconia. Furthermore, some of the otoconia appear to have linking filaments (Figure 6) that are similar in location and size to those observed in the normal mammalian utricle.8
Although we observed that there appeared to be more degeneration of otoconia in the specimen from a patient with vestibular neuronitis compared to the specimen from the patient with primary BPPV, definite conclusions about the significance of these differences would be speculation since they were obtained at different times and fixation and handling of the specimens may have been different.
Otoconia are composed of a core of glycosylated proteins and are formed as the result of complex interactions between these proteins: otoconin 90, otopetrin, osteopontin, and more. Knowledge of these molecular mechanisms in otoconial formation and maintenance is crucial to understanding the otoconial role in vestibular disease processes. These proteins are critical for proper assembly and formation of otoconia, which exhibit significant degenerative changes due to age and other factors. It may be that age-related changes lead to fragmentation and degeneration of the otolithic membrane, and subsequent mobilization of sections of the otolithic membrane with attached otoconia. This in turn can lead to clinical manifestations of disease28 as this free-floating debris with negative buoyancy migrates into the dependent portions of semicircular canals under the influence of gravity.
Two theories have been posed to explain the presence of otoconia within the semicircular canals in cases of BPPV. The first has been that of dislodged otoconia in which, trauma or age-related changes weaken the interconnecting fibers between the otoconia8 resulting in displacement of intact, otoconia from the otolithic membrane into the semicircular canals.12 A variation of this theory is that displaced otoconia degenerate secondary to demineralization12,29 or age related changes8 and dissociate from the gelatinous matrix before migrating into the semicircular canals. The second theory is that proteinaceous deposits form in the canals de novo.12 Otoconia-like particles then form on these deposits. 16
Our findings, however, demonstrate the presence of otoconia and degenerating otoconia, most of which are interconnected with linking fibrils, associated with amorphous particulate matter within the posterior semicircular canal endolymph of patients with BPPV. The presence of whole otoconia within the particulate matter in our samples associated with attached and detached interconnecting fibrils suggests that fragments of the otolithic membrane with attached otoconia may become dislodged and displaced into the semicircular canal. The presence of intact otoconial filaments connecting an intact otoconium to surrounding amorphous material supports our theory that the debris is actually displaced fragments of the otolithic membrane and adherent otoconia. Additionally, we observed unattached otoconia free from the underlying matrix. In an animal model, Parker, and colleagues showed that head trauma could dislodge sections of the otolithic membrane containing otoconia into the semicircular canals of guinea pigs.30 Our second case developed BPPV secondary to vestibular neuronitis. We propose that a neuronitis could lead to degeneration of the otolith organ resulting in fragmentation and mobilization of the otolithic membrane and its attached otoconia.
There may be alternative explanations for our observations. The otoconia seen in this study appear to be embedded and enveloped by amorphous material that could have been the result of de novo calcification within the semicircular canal on the surface of acellular debris left from a prior inflammatory process. In order to form otoconia within the semicircular canal, the proteinaceous debris would have to express OC90 and Otolin to induce the crystalline structure of otoconia. 16
Two unique and novel findings in this study are the presence of discrete non-degenerated otoconia and the presence of linking filaments within the particulate matter removed from the posterior semicircular canal endolymph of patients with BPPV. To our knowledge, this is the first description of stranding, filamentous appearing organic matter attached to otoconia within the semicircular canal in patients with BPPV. Additionally, these findings support the previous observation that intact and degenerating otoconia are present in this condition.26
We propose that in BPPV, fragments of the otolithic membrane become displaced from the utricle en bloc. That is, otoconia embedded within the gel matrix and interconnected with linking fibrils dislodge together as a unit from the utricular otolith organ (Figures 2, 3, 5, 6). This could result from age related weakening of the gel matrix interconnecting filaments,12 trauma or end-organ degeneration following an infectious or ischemic process.
We are mindful that our two cases are relatively unique. The vast majority of BPPV cases can be easily corrected by particle repositioning. These two cases did not respond to multiple repositioning maneuvers, ultimately leading to the surgical interventions. It could very well be that the composition of the particulate matter in ‘intractable” BPPV is somehow different from that in the “run of the mill” type. The debris examined in this report and from the report of Welling et al 26 was all from “intractable” cases.
It has never been clear why the particles/debris in the intractable cases cannot be repositioned. We now wonder if it could have something to do with the ratio between free otoconia and gel matrix that breaks away from the utricular otoith organ. Could it be that in those that are “clumpier” with a higher ratio of matrix, some of the matrix and fibrils end up adhering to the membranous duct? This might narrow the duct enough to block the transgression of the larger clumps of debris out of the canal during the maneuver,, but not enough to occlude the canal such that the moving debris stuck in the canal behind the stuck clump still gives rise to an abnormal endolymph current with head movement. Conversely, those with more of the free otoconia and less matrix have wide-open ducts making them more easily repositioned.
CONCLUSION
This study demonstrates scanning electron micrographic evidence of displaced otoconia within the particulate matter of the posterior semicircular canal endolymph in two patients with intractable BPPV. Our images also demonstrate the presence of linking filaments between the otoconia and amorphous material within the particulate matter, suggesting that the matter derives from the utricular otolith organ. Conclusive evidence for the source of the particles in canalithiasis has long been debated. As our results suggest, these particles do indeed originate from the otolith organ, and must be displaced fragments of the utricular otolithic membrane.
Acknowledgments
Source of funding: Supported by Grants from the National Institutes of Health. DC R01 000263-28(RAC) and DC P30 004665-13(RAC)
Footnotes
Conflict of Interest: none
References
- 1.Nedzelski J, HOB, LM Diagnoses in a dizziness unit. Journal of Otolaryngology. 1986;151:101–104. [PubMed] [Google Scholar]
- 2.Mizukoshi K, Watanabe Y, Shojaku H, Okubo J, Watanabe I. Epidemiological studies on benign paroxysmal positional vertigo in Japan. Acta Otolaryngol Suppl. 1988;447:67–72. doi: 10.3109/00016488809102859. [DOI] [PubMed] [Google Scholar]
- 3.Bourgeois PM, Dehaene I. Benign paroxysmal positional vertigo (BPPV). Clinical features in 34 cases and review of literature. Acta Neurol Belg. 1988;88:65–74. [PubMed] [Google Scholar]
- 4.Oghalai JS, Manolidis S, Barth JL, Stewart MG, Jenkins HA. Unrecognized benign paroxysmal positional vertigo in elderly patients. Otolaryngol Head Neck Surg. 2000;122:630–634. doi: 10.1016/S0194-5998(00)70187-2. [DOI] [PubMed] [Google Scholar]
- 5.Gianoli GJ. Benign Paroxysmal Positional Vertigo. In: Babu S, editor. Practical Otology for the Otolaryngologist. Plural Publishing; 2013. p. 279. [Google Scholar]
- 6.Baloh RW, Honrubia V, Jacobson K. Benign positional vertigo: clinical and oculographic features in 240 cases. Neurology. 1987;37:371–378. doi: 10.1212/wnl.37.3.371. [DOI] [PubMed] [Google Scholar]
- 7.Katsarkas A. Benign paroxysmal positional vertigo (BPPV): idiopathic versus post-traumatic. Acta oto-laryngologica. 1999;119:745–749. doi: 10.1080/00016489950180360. [DOI] [PubMed] [Google Scholar]
- 8.Jang YS, Hwang CH, Shin JY, Bae WY, Kim LS. Age-related changes on the morphology of the otoconia. Laryngoscope. 2006;116:996–1001. doi: 10.1097/01.mlg.0000217238.84401.03. [DOI] [PubMed] [Google Scholar]
- 9.Ross MD, Peacor D, Johnsson LG, Allard LF. Observations on normal and degenerating human otoconia. The Annals of otology, rhinology, and laryngology. 1976;85:310–326. doi: 10.1177/000348947608500302. [DOI] [PubMed] [Google Scholar]
- 10.Walther LE, Wenzel A, Buder J, Bloching MB, Kniep R, Blodow A. Detection of human utricular otoconia degeneration in vital specimen and implications for benign paroxysmal positional vertigo. Eur Arch Otorhinolaryngol. 2014;271:3133–3138. doi: 10.1007/s00405-013-2784-6. [DOI] [PubMed] [Google Scholar]
- 11.Lins U, Farina M, Kurc M, et al. The otoconia of the guinea pig utricle: internal structure, surface exposure, and interactions with the filament matrix. J Struct Biol. 2000;131:67–78. doi: 10.1006/jsbi.2000.4260. [DOI] [PubMed] [Google Scholar]
- 12.Thalmann R, Ignatova E, Kachar B, Ornitz DM, Thalmann I. Development and maintenance of otoconia: biochemical considerations. Ann N Y Acad Sci. 2001;942:162–178. doi: 10.1111/j.1749-6632.2001.tb03743.x. [DOI] [PubMed] [Google Scholar]
- 13.Deans MR, Peterson JM, Wong GW. Mammalian Otolin: a multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein Otoconin-90 and Cerebellin-1. PLoS One. 2010;5:e12765. doi: 10.1371/journal.pone.0012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes I, Blasiole B, Huss D, et al. Otopetrin 1 is required for otolith formation in the zebrafish Danio rerio. Dev Biol. 2004;276:391–402. doi: 10.1016/j.ydbio.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundberg YW, Zhao X, Yamoah EN. Assembly of the otoconia complex to the macular sensory epithelium of the vestibule. Brain Res. 2006;1091:47–57. doi: 10.1016/j.brainres.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 16.Thalmann R, Thalmann I, Lu W. Significance of tertiary conformation of otoconial matrix proteins - clinical implications. Acta oto-laryngologica. 2011;131:382–390. doi: 10.3109/00016489.2010.548401. [DOI] [PubMed] [Google Scholar]
- 17.Murayama E, Takagi Y, Ohira T, Davis JG, Greene MI, Nagasawa H. Fish otolith contains a unique structural protein, otolin-1. Eur J Biochem. 2002;269:688–696. doi: 10.1046/j.0014-2956.2001.02701.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Kowalski PE, Thalmann I, Ornitz DM, Mager DL, Thalmann R. Otoconin-90, the mammalian otoconial matrix protein, contains two domains of homology to secretory phospholipase A2. Proc Natl Acad Sci U S A. 1998;95:15345–15350. doi: 10.1073/pnas.95.26.15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreland KT, Hong M, Lu W, et al. In vitro calcite crystal morphology is modulated by otoconial proteins otolin-1 and otoconin-90. PLoS One. 2014;9:e95333. doi: 10.1371/journal.pone.0095333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong M, Moreland KT, Chen J, Teng HH, Thalmann R, De Yoreo JJ. Effect of Otoconial Proteins Fetuin A, Osteopontin, and Otoconin 90 on the Nucleation and Growth of Calcite. Cryst Growth Des. 2015;15:129–136. doi: 10.1021/cg501001r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall SF, Ruby RR, McClure JA. The mechanics of benign paroxysmal vertigo. J Otolaryngol. 1979;8:151–158. [PubMed] [Google Scholar]
- 22.Schuknecht HF. Cupulolithiasis. Arch Otolaryngol. 1969;90:765–778. doi: 10.1001/archotol.1969.00770030767020. [DOI] [PubMed] [Google Scholar]
- 23.Schuknecht HF, Ruby RR. Cupulolithiasis. Adv Otorhinolaryngol. 1973;20:434–443. doi: 10.1159/000393114. [DOI] [PubMed] [Google Scholar]
- 24.Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV) CMAJ. 2003;169:681–693. [PMC free article] [PubMed] [Google Scholar]
- 25.Parnes LS, McClure JA. Free-floating endolymph particles: a new operative finding during posterior semicircular canal occlusion. Laryngoscope. 1992;102:988–992. doi: 10.1288/00005537-199209000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Welling DB, Parnes LS, O’Brien B, Bakaletz LO, Brackmann DE, Hinojosa R. Particulate matter in the posterior semicircular canal. Laryngoscope. 1997;107:90–94. doi: 10.1097/00005537-199701000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Hornibrook J. Benign Paroxysmal Positional Vertigo (BPPV): History, Pathophysiology, Office Treatment and Future Directions. Int J Otolaryngol. 2011;2011:835671. doi: 10.1155/2011/835671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim E, Hyrc KL, Speck J, et al. Regulation of cellular calcium in vestibular supporting cells by otopetrin 1. J Neurophysiol. 2010;104:3439–3450. doi: 10.1152/jn.00525.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oas JG. Benign paroxysmal positional vertigo: a clinician’s perspective. Ann N Y Acad Sci. 2001;942:201–209. doi: 10.1111/j.1749-6632.2001.tb03746.x. [DOI] [PubMed] [Google Scholar]
- 30.Parker DE, Covell WP, von Gierke HE. Exploration of vestibular damage in guinea pigs following mechanical stimulation. Acta oto-laryngologica. 1968;(Suppl 239):237. [PubMed] [Google Scholar]