Abstract
Objective
To establish the effects on shoulder biomechanics from a peripheral nerve stimulation (PNS) treatment compared to Physical Therapy (PT) in stroke survivors with chronic hemiplegic shoulder pain.
Design
Single-site, pilot, randomized controlled trial for adults with chronic shoulder pain after stroke. Participants were randomized to receive a 3-week treatment of single-lead PNS or physical therapy (PT). The outcomes included isometric shoulder abduction strength, pain-free shoulder external rotation range of motion (ROM), delay in initiation and termination of shoulder abduction electromyogram (EMG) activity, and the Fugl-Meyer Motor Assessment (upper extremity section). Outcomes were measured at baseline, and at weeks 1, 4, 12, and 16.
Results
Twenty-five participants were recruited, 13 to PNS and 12 to PT. There were significant improvements for both PNS and PT in maximum isometric shoulder abduction strength, pain-free external rotation ROM, and Fugl-Meyer Motor Assessment. There were no significant changes in delay of initiation or termination of deltoid EMG with either treatment.
Conclusions
Both PNS and PT are capable of improving shoulder biomechanics in those with HSP, though changes in biomechanics alone do not account for the greater pain relief associated with PNS than PT.
Keywords: Electric Stimulation Therapy, Impairment, Peripheral Nerve Stimulation, Hemiplegia, Stroke
INTRODUCTION
Hemiplegic shoulder pain (HSP) is a common complication following stroke and is associated with poor functional outcomes and poor quality of life (QoL). The prevalence of shoulder pain is 17% one week after stroke, and up to 24% from one to 16 months after stroke. The cause of HSP remains unclear, though many etiologies have been reported. The potential causes include changes that affect biomechanics (e.g., impingement, rotator cuff dysfunction, contractures, spasticity, and hemiplegia) as well as tendinopathy, bursitis, adhesive capsulitis, peripheral nerve injuries, complex regional pain syndrome, spasticity, and central hypersensitivity. Due to the lack of a clear understanding of HSP many different treatment modalities have been proposed; however, there seems to be consensus that the most appropriate approach to treatment is one that corrects the biomechanical abnormalities that increase the likelihood of injury.1
A biomechanical approach to treat HSP is appropriate due to the anatomy of the shoulder and the potential effects of a stroke. Unlike most joints in the human body, the stability of the glenohumeral joint is maintained primarily by muscle. Thus, with the hemiparesis and hypertonia following stroke, the mechanical stability of the glenohumeral joint may be compromised, increasing the risk for micro and macrotrauma of the capsular and extra-capsular tissue and subsequent development of HSP. Accordingly, the prevalence of HSP is substantially higher in those with higher severity weakness. Thus, treatments that increase the strength of shoulder muscles may increase shoulder stability, increase active range of motion, reduce disability, and reduce pain.
Previous studies have suggested that electrical stimulation of paretic muscles improves biomechanics and function of the shoulder.2, 3 Thus, percutaneous peripheral nerve stimulation (PNS), a treatment which stimulates the axillary nerve via a percutaneous intramuscular electrode that causes the middle and posterior deltoid muscles to contract4, was originally developed to reduce shoulder dysfunction, increase glenohumeral stability, and thereby reduce HSP. Two randomized controlled trials (RCTs) have demonstrated that a short-term PNS treatment (i.e., 3 or 6 weeks), the first demonstrating efficacy in long-term pain relief in more than 60% of participants for greater than 12 months.5 The second trial, in which the data for this paper were also collected, corroborated the finding that more than 60% of participants receiving PNS achieved long-term pain reduction, and also showed that PNS reduces pain more than that achieved with physical therapy.6
The purpose of this article is to report on the effects of PNS on shoulder strength, range of motion, and motor control and determine if there is a correlation between pain reduction and these biomechanical outcomes in order to test the hypothesis that PNS mediated reduction in motor impairment (i.e., improvement in biomechanics) is the mechanism responsible for the reduction of HSP. Specifically, we hypothesized that the PNS group, compared to best-practices physical therapy, will exhibit greater improvements in the following measures: 1) isometric shoulder abduction strength; 2) active pain-free shoulder external rotation range of motion (ROM); 3) motor control as measured by a) the Fugl-Meyer Motor Assessment (upper extremity section), and b) the ability to initiate and terminate shoulder abduction electromyogram (EMG) activity.
METHODS
Subjects
This paper represents analyses of secondary outcomes from a single center, assessor-blinded RCT of percutaneous, intramuscular PNS compared to physical therapy (PT) for pain reduction for those with HSP.6 Full inclusion and exclusion criteria were described previously.6 In brief, participants included in the study were: at least 21 years old; more than 3 months after stroke with new or worsened shoulder pain on their affected side; severity of pain rated at least 4 out of 10 on the 11-point numeric rating scale (NRS) of the Brief Pain inventory Short Form, question 3 (BPI-SF3); and duration of HSP of more than 3 months. Subjects with any of the following were excluded: evidence of infection of the shoulder joint or overlying skin; complete hemisensory deficit on the affected side; use of more than 1 opioid or nonopioid analgesic medication daily for shoulder pain; daily use of pain medications for any other chronic pain; corticosteroid or botulinum toxin injections to the affected shoulder in the previous 3 months; currently receiving physical or occupational therapies for HSP; other confounding neurological conditions affecting the upper limb; or any conditions deemed by the investigators as increasing the risk of infections.
The study was conducted at an urban, academic rehabilitation center, and the project was approved by an authorized institutional human research review board and written informed consent from human participants was obtained as required. The trial was registered at clinicaltrials.gov (NCT01123382). Subjects were randomized to PNS or PT by an adaptive randomization algorithm which ensured equal distribution of three key characteristics: time from stroke onset (less than 18 months vs. greater than 18 months)7, shoulder subluxation (absence vs. presence)8, and sensation of the affected shoulder (normal vs. abnormal)9. The unblinded study coordinator entered the variables into a computer algorithm and received the group assignment. The research therapist completing outcomes was blinded to treatment assignment.
Interventions
The interventions were previously described in detail,6 but a brief description is provided here. Participants randomized to PNS had a single percutaneous electrode placed between the motor points of the middle and posterior deltoids of the affected shoulder. The electrode was placed via a hypodermic needle which was withdrawn, leaving the electrode in the muscle. After one week the lead was connected to an external stimulator (SMARTPATCH® PNS System, SPR Therapeutics, LLC., Cleveland, Ohio) and parameters were set to stimulate with 12 pulses per second at 20 mA. Pulse duration (40–200μs) was adjusted to produce the strongest muscle contraction that was comfortable for the participant. Subjects were prescribed 6 hours of stimulation per day for 3 weeks for a total of 126 hours of stimulation, following the same regimen as in previous studies.4, 6 The stimulator completed a cycle every 30 seconds to produce a comfortable muscle contraction that lasted 20 seconds (including 5-second ramps up and down) followed by a 10 second rest period. At the conclusion of the 3-week stimulation period, the electrode was removed by gently pulling on its external lead. Subjects in the PNS group did not receive formal PT, nor were they instructed on a home exercise program.
Subjects randomized to the PT treatment received 8 hours of outpatient PT over a 4-week period from a licensed therapist, and were advised on a home exercise program. The treatment program was based on published best practice guidelines and recommendations.10–12 The treatments were individualized to be consistent with the needs of the participant and included: (1) proper positioning and handling, and the use of slings and supports to reduce the risk of trauma to the hemiparetic upper limb; (2) range of motion (ROM) and strengthening exercises within pain-free range and loads, respectively; (3) task-specific therapy to reduce impairments and to improve basic and instrumental activities of daily living (ADLs); and, (4) a home exercise program on days participants do not receive PT and during follow-up.
Outcomes were measured during 5 blinded assessment sessions. The outcome assessor was blinded during the treatment phase by having PT participants wear a bandage covering a sham electrode exit site. Outcome assessments were completed before randomization, at the beginning and end of the 4-week treatment period, and at 6 and 12 weeks after treatment ended.
Outcome measures
Isometric shoulder abduction strength
Isometric shoulder abduction torque was measured with a Biodex Biomechanical Measurement System (Biodex Medical Systems, Shirley, NY) as a measure of abduction strength. Subjects were seated and fastened securely using straps around the upper body to minimize trunk movements (see Figure 1). The seat height and position were adjusted so that the abduction-adduction axis of the participant’s shoulder was aligned with the rotational axis of the dynamometer. The arm being tested was attached by a strap just above the elbow to an armature extending from the dynamometer. The armature was fixed at 30 degrees from vertical, thereby holding the participant’s arm at 30 degrees of shoulder abduction and neutral rotation. All positioning parameters were recorded so that the exact position could be replicated at subsequent assessments. Subjects were informed that this was a test of strength and reaction time, and that they were to abduct their arm as quickly and strongly as they could in response to an audible tone. The duration of the audible tone was between 3 and 5 seconds, and was deliberately different from trial to trial to prevent the participant from anticipating the start or end of the tone. Subjects were instructed to wait for the tone before starting and wait for cessation of the tone before relaxing. After a practice trial, three trials were run, resting 1 minute between trials. Any trials in which the participant was perceived to not have given their best effort, or were not prepared, were repeated. The average abduction torque during the last second of the audible tone was calculated for each trial and those values were averaged over the three trials. Subjects underwent testing of both shoulders, non-paretic side first, and the results are presented as the ratio of the paretic shoulder to the non-paretic shoulder to decrease the influence of intra-participant variability between measurements.
Figure 1.
Illustration of participant positioning for measurement of Isometric shoulder abduction strength and delay of initiation and termination of electromyographic activity of the deltoid.
Delay in initiation and termination of shoulder abduction EMG activity
Electromyographic activity from the deltoid was also measured during the isometric abduction strength trials. Surface EMG recording electrodes (2 cm x 2 cm, Kendall Soft-E H69P) were placed over the deltoid muscle and spaced 4 cm apart. The EMG amplifier gain was adjusted to record as high-fidelity an EMG signal as possible during shoulder abduction. Delay of initiation (DOI) was defined as the duration between onset of the audible tone and the onset of EMG signal while delay of termination (DOT) was defined as the duration between cessation of the audible tone and return of the EMG signal to baseline. Raw EMG signals were analyzed visually to determine the earliest rise in EMG activity relative to steady state for delay of initiation, and return to steady for delay of termination.13, 14 This method has been shown to correlate very highly with various computer-based onset determination techniques, but with greater reliability.15 The mean DOI and DOT of the three trials were calculated, and the ratio of the paretic to non-paretic shoulder DOI and DOT were used as summary metrics.
Pain-free shoulder external rotation range of motion
The participant was supine with the shoulder adducted with hand resting on the abdomen, elbow flexed, and with the humerus supported by the mat. The axis of a universal goniometer was centered on the olecranon process of the ulna projecting through the humeral shaft toward the humeral head. The participant’s shoulder was externally rotated passively to the pain threshold, defined as the start of any pain. Pain at rest was recorded as 0 degrees. Reliability of goniometric measurements has been demonstrated.16
Fugl-Meyer Motor Assessment (Upper Extremity)
The Fugl-Meyer Motor Assessment (FMA) is an impairment measure designed to assess recovery after stroke17 and has been recommended as a clinical and research tool for evaluating changes in motor impairment following stroke.18 Measurement was limited to the upper limb motor impairment component of the FMA. Volitional movement of the upper limb (shoulder, elbow, forearm, wrist, and hand) is examined in and out of synergies. Each item was graded on a 3-point ordinal scale and summed to provide a maximum score of 66, with higher scores indicating lower impairment. The FMA was administered while the participant was seated.
Statistical Analysis
The effect of treatment group over time was analyzed using a linear mixed model for repeated measures for each outcome measure. A random intercept for each participant with a first-order antedependent covariance structure was included since it is reasonable to assume that for each individual there is a greater correlation between assessments that are closer together in time and that variance might be different at different assessments. The analyses were also completed with five discrete time points (baseline, start of treatment, end of treatment, and 6 weeks and 12 weeks post-treatment) so that differences between groups at different assessments could be estimated. This discrete time model failed to converge with the covariance structure for the pain-free shoulder abduction range of motion analysis. Therefore, no assumptions about the variances and covariances were made in this model, and an unstructured covariance structure was utilized. Missing data were handled by a maximum likelihood algorithm under the assumption that the missingness was random. The analyses were conducted by the available-case, intention-to-treat method. When a significant treatment group by time interaction effect was found, pairwise comparisons between groups at the discrete time points of end of treatment (week 4) and follow-up time points (weeks 10 and 16) of the least squares means were computed with Bonferroni correction (alpha=0.017) to control the familywise error rate. We defined α = 0.05 for our level of significance in all statistical tests (before performing any Bonferroni correction, where appropriate). All statistical tests were two-tailed. Sample size was based on the primary outcome for the study, as described previously.6
RESULTS
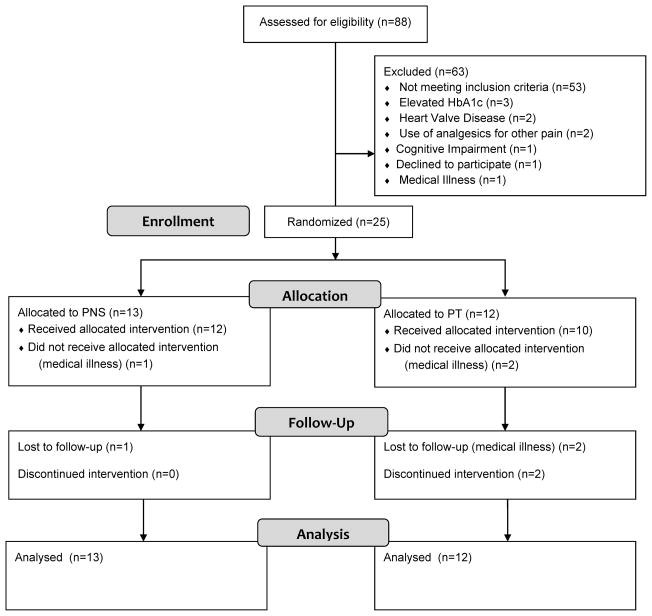
Twenty-five participants were enrolled in the study with 13 randomized to PNS and 12 to PT (see Figure 2). One participant in each group withdrew from the study due to medical illness. Only baseline outcome assessments recorded for these participants. Two participants in the PT group were lost to follow-up after the second outcome assessment. The baseline demographic data are presented in Table 1. The outcomes are presented in Tables 2 and 3.
Figure 2.
Participant flow diagram.
Table 1.
Demographic Information
| PNS | Physical Therapy | |
|---|---|---|
| n=13 | n=12 | |
| Female (%) | 46.2 | 58.3 |
| Age (years, median +/− IQ range) | 54.0 (50.0 – 68.0) | 55.5 (50.0 – 62.5) |
| Subluxation (%) | 61.5 | 66.7 |
| Race/Ethnicity (%) | ||
| White | 46.1 | 41.7 |
| African American | 53.9 | 50.0 |
| Hispanic/Latino | 0 | 8.3 |
| Shoulder Pain > 18 mo. (%) | 61.5 | 58.3 |
| Time Since Stroke (years, median +/− IQ range) | 2.6 (0.9 – 4.0) | 2.3 (0.8 – 4.8) |
| Cortical Stroke (%) | 66.7 | 54.5 |
| Right Hemiplegia (%) | 38.5 | 33.3 |
| Hemorrhagic Stroke (%) | 38.5 | 8.3 |
| Comorbidities at Baseline (%) | ||
| Coronary Artery Disease | 30.8 | 0 |
| Congestive Heart Failure | 0 | 0 |
| Cardiac Arrhythmia | 7.7 | 0 |
| Diabetes Mellitus | 38.5 | 41.7 |
| Hypertension | 76.9 | 100 |
| Renal Dialysis | 0 | 0 |
| Pulmonary Disease | 7.7 | 16.7 |
| Peripheral Vascular Disease | 0 | 8.3 |
| Seizure Disorder | 7.7 | 0 |
| Osteoarthritis | 23.1 | 0 |
| Cancer | 0 | 8.3 |
Demographics for PNS and Physical Therapy groups with chronic hemiplegic shoulder pain. Abbreviations: PNS- peripheral nerve stimulation; IQ- interquartile
Table 2.
Outcomes Assessments of PNS vs. Usual Care for Hemiplegic Shoulder Pain
| Week 0 | Week 1 | Week 4 | Week 10 | Week 16 | |
|---|---|---|---|---|---|
| Shoulder Abductor Moment (+/−SE)* | |||||
| PNS (n=13) | 0.3 (+/− 0.1) | 0.4(+/− 0.1) | 0.4(+/− 0.1) | 0.5(+/− 0.1) | 0.5(+/− 0.1) |
| Usual Care (=12) | 0.3 (+/− 0.1) | 0.3(+/− 0.1) | 0.3(+/− 0.1) | 0.3(+/− 0.1) | 0.4(+/− 0.1) |
| Pain Free Abduction, degrees (+/−SE) | |||||
| PNS (n=13) | 50.5 (+/− 14.4) | 56.2 (+/− 14.6) | 80.8 (+/− 14.6) | 77.0 (+/− 14.6) | 76.9 (+/− 14.6) |
| Usual Care (=12) | 26.7 (+/− 15.0) | 34.0 (+/− 15.2) | 43.7 (+/− 15.9) | 37.4 (+/− 15.6) | 41.5 (+/− 15.9) |
| Delay in EMG Initiation (+/−SE)* | |||||
| PNS (n=13) | 1.4 (+/− 0.2) | 1.3 (+/− 0.2) | 1.2 (+/− 0.2) | 1.2 (+/− 0.2) | 1.4 (+/− 0.2) |
| Usual Care (=12) | 1.6 (+/− 0.2) | 1.3 (+/− 0.3) | 1.4 (+/− 0.3) | 1.2 (+/− 03.2) | 1.2 (+/− 0.3) |
| Delay in EMG Termination (+/−SE)* | |||||
| PNS (n=13) | 2.3 (+/− 0.5) | 2.6 (+/− 0.6) | 1.7 (+/− 0.6) | 1.9 (+/− 0.7) | 1.7 (+/− 0.5) |
| Usual Care (=12) | 1.3 (+/− 0.6) | 3.7 (+/− 0.6) | 2.0 (+/− 0.7) | 1.5 (+/− 0.5) | 2.6 (+/− 0.7) |
| Fugl-Meyer, upper extremity (+/−SE) | |||||
| PNS (n=13) | 50.5 (+/− 14.4) | 56.2 (+/− 14.6) | 80.8 (+/− 14.6) | 77.0 (+/− 14.6) | 76.9 (+/− 14.6) |
| Usual Care (=12) | 26.7 (+/− 15.0) | 34.0 (+/− 15.2) | 43.7 (+/− 15.9) | 37.4 (+/− 15.6) | 41.5 (+/− 15.9) |
The effect of treatment group over time was analyzed using a linear mixed model for repeated measures for each outcome measure with five discrete time points (baseline, start of treatment, end of treatment, and 6 weeks and 12 weeks post-treatment). Least-squares means are presented. Abbreviations: SE- Standard Error; PNS- Peripheral Nerve Stimulation; EMG – Electromyographic
Ratio paretic to non-paretic shoulders
Table 3.
Hypothesis Tests of Model Fixed Effects
| Outcome | Effect | F value (df) | p-value |
|---|---|---|---|
| Shoulder Abductor Moment | group x time | 0.6 (1,66) | 0.46 |
| time | 6.3 (1,21) | 0.02 | |
| group | 0.5 (1,60) | 0.46 | |
| Pain Free Abduction | group x time | 1.0 (1,61) | 0.33 |
| time | 6.5 (1,21) | 0.02 | |
| group | 2.5 (1,61) | 0.12 | |
| Delay in EMG Initiation | group x time | 0.3 (1,32) | 0.59 |
| time | 1.2 (1,13) | 0.30 | |
| group | 0.1 (1,32) | 0.71 | |
| Delay in EMG Termination | group x time | 0.2 (1,32) | 0.69 |
| time | 0.5 (1,13) | 0.50 | |
| group | 0.0 (1,32) | 0.90 | |
| Fugl-Meyer, upper extremity | group x time | 0.3 (1,61) | 0.61 |
| time | 4.9 (1,21) | 0.04 | |
| group | 0.2 (1,61) | 0.69 |
The effect of treatment group over time was analyzed using a linear mixed model for repeated measures for each outcome measure. The model assessed whether the outcomes: 1) change in different ways over time between groups (group by time interaction term); 2) change over time (continuous time effect); or, 3) are different between groups under the assumption that the mean response profiles are parallel (group main effect). Abbreviation: df- degrees of freedom; EMG – Electromyographic
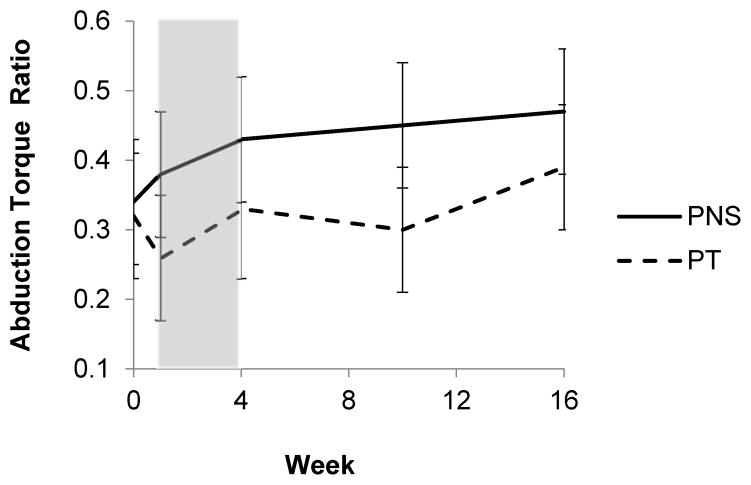
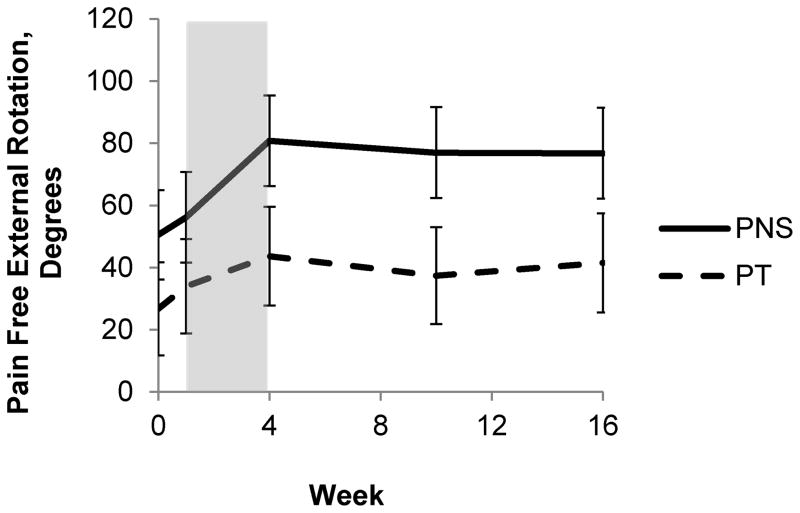
When examining the biomechanical effects of the interventions, participants in both groups experienced significant improvements in some of the outcome measures, but none of the outcomes were significantly different between groups. Across all participants in both the PNS and PT groups there was a significant increase in the maximum abduction strength ratio of paretic:non-paretic shoulders during the study (time effect), though the improvement was not significantly different between groups (time by group interaction effect). Figure 3 illustrates the group changes in the maximum abduction strength ratio. There was no significant main group effect for maximum isometric abduction strength ratio. There was not a significant change in delay of shoulder abduction EMG initiation paretic:non-paretic ratio (time effect), nor was there a significant difference between groups (time by group interaction effect.) There was no significant main group effect for delay of shoulder abduction EMG initiation ratio. There was not a significant change in delay of shoulder abduction EMG termination paretic:non-paretic ratio (time effect), nor was there a significant difference between groups (time by group interaction effect.) There was no significant main group effect for delay of shoulder abduction EMG termination ratio. Across all participants in both the PNS and PT groups there was a significant increase in the pain-free shoulder external rotation ROM of the affected arm during the study (time effect), though the improvement was not significantly different between groups (time by group interaction effect). The group changes in ROM are illustrated in Figure 4. There was no significant main group effect for pain-free external-rotation ROM. Both the PNS and PT group experienced a significant increase in the FMA of the affected arm during the study (time effect), though the improvement was not significantly different between groups (time by group interaction effect). There was no significant main group effect for FMA.
Figure 3.
Graphical comparison of the paretic:non-paretic ratio of maximum isometric shoulder abduction torque. PNS is represented by the solid line and PT the dashed line. The period of treatment is represented by the shaded area. There were significant time effects (F (1,21) =6.5, p=0.02) but not time x group effects (F(1,61)= 0.6, p=0.46.)
Figure 4.
Graphical comparison of the pain-free shoulder external rotation ROM. PNS is represented by the solid line and PT the dashed line. The period of treatment is represented by the shaded area. There were significant time effects (F (1,21) =6.3, p=0.02) but not time x group effects (F(1,66)= 1.0, p=0.33.)
DISCUSSION
This study provides insight into the changes in biomechanics associated with percutaneous PNS and PT treatment for chronic HSP. There were measureable improvements in isometric shoulder abduction strength, pain-free shoulder external rotation range of motion, and global upper limb impairment (FMA) in the affected shoulder after both PNS and PT; however, there was no statistically significant between-group difference in shoulder biomechanics even though the effect of the interventions on 0–10 numeric rating scale of worst pain in the last week, reported in a prior manuscript, showed a difference in pain relief. There was a reduction in worst pain at the end of treatment of 65.3% in the PNS group and 34.3% for the PT, and at 12 weeks after the end of treatment a 60.0% reduction in worst pain for the PNS group compared to 19.7% in the PT group. The differences were clinically and statistically significant. 6 Therefore, pain relief may be caused by a mechanism other than altering the biomechanics of the shoulder.
In this trial, both groups experienced a statistically significant improvement in the strength of their shoulder abductors as measured by the ratio of the maximum isometric shoulder abduction torque of their paretic to non-paretic side. The paretic shoulder abduction strength of both the PNS and PT group was approximately 30% of the non-paretic shoulder abduction strength at baseline and had improved to 50% for the PNS group and 40% for the PT group by 12 weeks after the treatment ended. Whether the greater improvement in the PNS group as compared to the PT group is meaningful is not known since the minimum clinically important difference (MCID) for this outcome has yet to be established. This was the first trial of PNS to include isometric shoulder abductor torque in an analysis, and prior studies only included strength as part of the Fugl-Meyer assessment.
This study was the first trial of PNS for HSP to assess central motor processing. There were no significant changes in the ratio of paretic to non-paretic delay of initiation or termination of shoulder abduction EMG activity with PNS or PT treatment. It is common after stroke for electromyographic (EMG) signals from paretic muscles to have delays in initiation and termination.19 Such delays are correlated with motor impairment and disability14, and might be reflective of impaired central nervous system processes such as slowed sensory processing, disturbed intercortical inhibition, or spastic firing in motoneurons.20 It would be expected for both groups to experience a reduction of EMG delays, or approaching the EMG delay of the unaffected limb, given the improvements in the other outcomes, though variability in the data may have reduced statistical power to detect changes.
There was a statistically significant improvement in pain-free shoulder external rotation ROM for both groups in this study. Both groups experienced approximately 60% improvement in pain-free ROM by the end of the treatment that remained at greater than 50% improvement by 12 weeks after treatment ended. In a prior case series of PNS a significant improvement in external rotation ROM was found21, though a RCT comparing PNS to a shoulder sling did not find a difference in external rotation ROM between groups.22 A change of 25% in ROM has been considered the MCID in at least one prior trial23, indicating that both groups experienced a clinically relevant change in external rotation ROM.
Both groups experienced a statistically significant improvement in the upper extremity portion of the FMA. Improvements in the upper extremity portion of the FMA have been shown in a prior case series of PNS for HSP21, though no differences were found between groups in a RCT comparing PNS to a shoulder sling.22 The changes that occurred within the study are of smaller magnitude than both the conservative estimate for the MCID of 10 points,24 and the less stringent estimate of 4.25 points.25
This study shows that there were statistically and clinically relevant changes as a result of PNS and PT treatment for HSP, though the differences were not as hypothesized. This suggests that both PNS and PT are capable of improving shoulder biomechanics in those with HSP, though changes in biomechanics alone do not account for the greater pain relief associated with PNS than PT. There are a number of reasons why this might be the case. First, shoulder biomechanics and motor function may not be as important in the maintenance of chronic HSP as it is in the development of acute HSP. A study by Roosink, et al. showed a reduction in common initiating factors associated with HSP during the development of chronic HSP.26 Second, it is possible that the outcome measures in this study were inadequate proxy measures for shoulder biomechanics as they relate to HSP. Third, the sample size may have been too small to detect differences in shoulder biomechanics. The trial was powered to detect clinically important differences in pain between the PNS and PT groups6 rather than differences in the secondary measures presented in this article. Fourth, it is possible that the treatment duration was not adequate to achieve lasting results from PT, though the optimal duration is not known. Finally, it is possible that the outcome measures utilized in this study were not of sufficient reliability to allow detection of small differences between groups. For example, the minimum detectable change for the upper extremity portion of the FMA has been found to be 5.2 points27, a greater magnitude the most liberal estimate for minimum clinically important difference of 4.25 points.25 While this trial did not show clinically relevant differences between groups for the FMA, it is possible that the smaller change seen in this study is relevant to reduction in pain with respect to causation.
Additional limitations to those already mentioned deserve consideration. The reliability and responsiveness to change for the measurements of the isometric shoulder abduction strength and the electromyographic recordings have not been established. This makes interpretability of small magnitude change difficult; however, it is apparent that there were no large differences between the PNS and PT groups in the outcomes tested in this study. Additionally, subluxation was not included as an outcome measure in this study. The use of electrical stimulation has not been found to reduce subluxation in those past 6 months from stroke28, nor has PT been shown to reduce subluxation. Finally, the impairment measures in this study are indirect indicators of shoulder biomechanics and the measures chosen may not be important for the development and maintenance of HSP. Such measures are not yet established.
An alternative explanation for the mechanism of PNS in the improvement of HSP is modulation of maladaptive changes in the somatosensory system that are associated with chronic pain. There is evidence that the somatosensory system functions differently in those with HSP9, and in similar ways to the dysfunction of the somatosensory system found in chronic unilateral shoulder pain in an able-bodied population.29 This alteration of function, termed central sensitization, is an enhancement of the processing of sensory signals within the central nervous system that augments the perception of pain that can cause persistent pain in the absence of tissue injury.30 In a study of PNS for chronic pain in those with subacromial impingement syndrome there was indication of a reduction in sensitivity to pain for those who responded to PNS treatment, though additional studies are necessary to determine the mechanism of PNS.31 Future studies of the mechanism of pain relief in PNS should include measures of somatosensory function and central nervous system function, in addition to more refined measures of shoulder impairment and biomechanics, to better address this gap in knowledge.
CONCLUSION
This study suggests that both PNS and PT are capable of improving shoulder biomechanics in those with HSP, though changes in biomechanics alone do not account for the greater pain relief associated with PNS than PT. Both PNS and PT are associated with improvements in shoulder abduction strength, pain-free external ROM, and upper-extremity Fugl-Meyer Motor Assessment. There were no significant changes in delay of initiation or termination of deltoid EMG with either treatment.
Supplementary Material
Acknowledgments
We thank Henry Wu, biomedical engineering student at Case Western Reserve University, for his assistance in data analysis and manuscript preparation.
Footnotes
Disclosures:
This work was sponsored by grant R01HD059777 and K24HD054600 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research.
The author and co-authors have the following disclosures: Richard D. Wilson is a consultant to SPR Therapeutics, LLC. Maria Bennett is an employee of SPR Therapeutics, LLC. John Chae is a consultant and Chief Medical Advisor to SPR Therapeutics, LLC. Dr. Chae also owns equity in SPR Therapeutics, LLC.
Part of this research has presented at the 12th Annual Meeting of the International Neuromodulation Society, Montreal, Quebec, Canada in June, 2015. This article has never been published.
Reprints will not be available from the authors.
IRB approval: IRB09-00502, Clinical Trials Registration: NCT0112338
Contributor Information
Richard D. Wilson, MetroHealth Rehabilitation Institute, MetroHealth Medical Center, Cleveland, OH. Department of Physical Medicine and Rehabilitation, Case Western Reserve University, Cleveland, OH. Cleveland Functional Electrical Stimulation Center, Cleveland, OH.
Jayme S. Knutson, MetroHealth Rehabilitation Institute, MetroHealth Medical Center, Cleveland, OH. Department of Physical Medicine and Rehabilitation, Case Western Reserve University, Cleveland, OH. Cleveland Functional Electrical Stimulation Center, Cleveland, OH.
Maria E. Bennett, SPR Therapeutics, LLC., Cleveland, OH.
John Chae, MetroHealth Rehabilitation Institute, MetroHealth Medical Center, Cleveland, OH. Department of Physical Medicine and Rehabilitation, Case Western Reserve University, Cleveland, OH. Cleveland Functional Electrical Stimulation Center, Cleveland, OH. Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH.
References
- 1.Snels IA, Beckerman H, Lankhorst GJ, Bouter LM. Treatment of hemiplegic shoulder pain in the Netherlands: results of a national survey. Clin Rehabil. 2000;14(1):20–7. doi: 10.1191/026921500668239146. [DOI] [PubMed] [Google Scholar]
- 2.Faghri PD, Rodgers MM, Glaser RM, Bors JG, Ho C, Akuthota P. The effects of functional electrical stimulation on shoulder subluxation, arm function recovery, and shoulder pain in hemiplegic stroke patients. Arch Phys Med Rehabil. 1994;75:73–9. [PubMed] [Google Scholar]
- 3.Chantraine A, Baribeault A, Uebelhart D, Gremion G. Shoulder pain and dysfunction in hemiplegia: effects of functional electrical stimulation. Arch Phys Med Rehabil. 1999;80:328–31. doi: 10.1016/s0003-9993(99)90146-6. [DOI] [PubMed] [Google Scholar]
- 4.Wilson RD, Bennett ME, Lechman TE, Stager KW, Chae J. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case report. Arch Phys Med Rehabil. 2011;92(5):837–40. doi: 10.1016/j.apmr.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chae J, Yu DT, Walker ME, Kirsteins A, Elovic EP, Flanagan SR, et al. Intramuscular electrical stimulation for hemiplegic shoulder pain: a 12-month follow-up of a multiple-center, randomized clinical trial. Am J Phys Med Rehabil. 2005;84(11):832–42. doi: 10.1097/01.phm.0000184154.01880.72. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RD, Gunzler DD, Bennett ME, Chae J. Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93(1):17–28. doi: 10.1097/PHM.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chae J, Ng A, Yu DT, Kirsteins A, Elovic EP, Flanagan SR, et al. Intramuscular electrical stimulation for shoulder pain in hemiplegia: does time from stroke onset predict treatment success? Neurorehabil Neural Repair. 2007;21(6):561–7. doi: 10.1177/1545968306298412. [DOI] [PubMed] [Google Scholar]
- 8.Roy CW, Sands MR, Hill LD. Shoulder pain in acutely admitted hemiplegics. Clin Rehabil. 1994;8:334–40. [Google Scholar]
- 9.Roosink M, Renzenbrink GJ, Buitenweg JR, van Dongen RT, Geurts AC, Ijzerman MJ. Somatosensory symptoms and signs and conditioned pain modulation in chronic post-stroke shoulder pain. J Pain. 2011;12(4):476–85. doi: 10.1016/j.jpain.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Teasell RW, Foley NC, Bhogal SK, Speechley MR. An evidence-based review of stroke rehabilitation. Top Stroke Rehabil. 2003;10(1):29–58. doi: 10.1310/8YNA-1YHK-YMHB-XTE1. [DOI] [PubMed] [Google Scholar]
- 11.Bates B, Choi JY, Duncan PW, Glasberg JJ, Graham GD, Katz RC, et al. Veterans Affairs/Department of Defense Clinical Practice Guideline for the Management of Adult Stroke Rehabilitation Care: executive summary. Stroke. 2005;36(9):2049–56. doi: 10.1161/01.STR.0000180432.73724.AD. [DOI] [PubMed] [Google Scholar]
- 12.Khadilkar A, Phillips K, Jean N, Lamothe C, Milne S, Sarnecka J. Ottawa panel evidence-based clinical practice guidelines for post-stroke rehabilitation. Top Stroke Rehabil. 2006;13(2):1–269. doi: 10.1310/3TKX-7XEC-2DTG-XQKH. [DOI] [PubMed] [Google Scholar]
- 13.Chae J, Quinn A, El-Hayek K, Santing J, Berezovski R, Harley M. Delay in initiation and termination of tibialis anterior contraction in lower-limb hemiparesis: relationship to lower-limb motor impairment and mobility. Arch Phys Med Rehabil. 2006;87(9):1230–4. doi: 10.1016/j.apmr.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Chae J, Yang G, Park BK, Labatia I. Delay in initiation and termination of muscle contraction, motor impairment, and physical disability in upper limb hemiparesis. Muscle & nerve. 2002;25(4):568–75. doi: 10.1002/mus.10061. [DOI] [PubMed] [Google Scholar]
- 15.Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol. 1996;101(6):511–9. doi: 10.1016/s0013-4694(96)95190-5. [DOI] [PubMed] [Google Scholar]
- 16.Hellebrandt FA, Duvall EN, Moore ML. The measurement of joint motion: III the Reliability of Goniometry. Phys Ther Rev. 1949;29(6):302–7. [PubMed] [Google Scholar]
- 17.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1 a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 18.Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther. 1993;73(7):447–54. doi: 10.1093/ptj/73.7.447. [DOI] [PubMed] [Google Scholar]
- 19.Hammond M, Kraft G, Fitts S. Recruitment and termination of EMG activity in the hemiparetic forearm. Arch Phys Med Rehabil. 1998;(69):106–10. [PubMed] [Google Scholar]
- 20.Seo NJ, Rymer WZ, Kamper DG. Delays in grip initiation and termination in persons with stroke: effects of arm support and active muscle stretch exercise. J Neurophysiol. 2009;101(6):3108–15. doi: 10.1152/jn.91108.2008. [DOI] [PubMed] [Google Scholar]
- 21.Renzenbrink GJ, MJIJ Percutaneous neuromuscular electrical stimulation (P-NMES) for treating shoulder pain in chronic hemiplegia. Effects on shoulder pain and quality of life. Clin Rehabil. 2004;18(4):359–65. doi: 10.1191/0269215504cr759oa. [DOI] [PubMed] [Google Scholar]
- 22.Yu DT, Chae J, Walker ME, Kirsteins A, Elovic EP, Flanagan SR, et al. Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: a multicenter randomized clinical trial. Arch Phys Med Rehabil. 2004;85(5):695–704. doi: 10.1016/j.apmr.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 23.van der Windt DA, Koes BW, Deville W, Boeke AJ, de Jong BA, Bouter LM. Effectiveness of corticosteroid injections versus physiotherapy for treatment of painful stiff shoulder in primary care: randomised trial. Bmj. 1998;317(7168):1292–6. doi: 10.1136/bmj.317.7168.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arya KN, Verma R, Garg RK. Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Top Stroke Rehabil. 2011;18(Suppl 1):599–610. doi: 10.1310/tsr18s01-599. [DOI] [PubMed] [Google Scholar]
- 25.Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92(6):791–8. doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- 26.Roosink M, Renzenbrink GJ, Buitenweg JR, Van Dongen RT, Geurts AC, MJIJ Persistent shoulder pain in the first 6 months after stroke: results of a prospective cohort study. Arch Phys Med Rehabil. 2011;92(7):1139–45. doi: 10.1016/j.apmr.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Wagner JM, Rhodes JA, Patten C. Reproducibility and minimal detectable change of three-dimensional kinematic analysis of reaching tasks in people with hemiparesis after stroke. Phys Ther. 2008;88(5):652–63. doi: 10.2522/ptj.20070255. [DOI] [PubMed] [Google Scholar]
- 28.Vafadar AK, Cote JN, Archambault PS. Effectiveness of functional electrical stimulation in improving clinical outcomes in the upper arm following stroke: a systematic review and meta-analysis. Biomed Res Int. 2015;2015:729768. doi: 10.1155/2015/729768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul TM, Soo Hoo J, Chae J, Wilson RD. Central hypersensitivity in patients with subacromial impingement syndrome. Arch Phys Med Rehabil. 2012;93(12):2206–9. doi: 10.1016/j.apmr.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson RD, Harris MA, Gunzler DD, Bennett ME, Chae J. Peripheral Nerve Stimulation for the Treatment of Chronic Subacromial Impingement Syndrome: A Case Series. Neuromodulation. 2014;17(8):771–6. doi: 10.1111/ner.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.