Abstract
Clinical research in the pregnant population allows for delivery of quality, evidence-based care in obstetrics. However, in recent years, the field of obstetrics has faced severe challenges in the recruitment of the pregnant population into clinical trials; a struggle also shared by several other medical disciplines. Herein, we candidly describe our failure to recruit a healthy population of overweight and obese pregnant women in their first trimester. We were then able to glean unsuccessful and successful recruitment approaches and improve our recruitment effort by autopsy of failed strategies and with guidance from a survey disseminated to improve our understanding of community feelings about participating in research while pregnant. These “lessons learned” taught us that active recruitment within this population is a necessity, i.e. direct (face-to-face discussions at obstetric appointments) versus indirect (flyers and general emails) modalities and that prenatal care provider support of the proposed research study is vital to a patient’s willingness to participate. By implementation of “lessons learned”, we describe how we successfully recruited a similar pregnant population one year later. The Clinical Trials related to our article are as follows: 1) Expecting Success: NCT01610752, https://clinicaltrials.gov/ct2/show/NCT01610752 ; 2) MomEE: NCT01954342, https://clinicaltrials.gov/ct2/show/NCT01954342; and 3) Participate While Pregnant Survey: NCT02699632, https://clinicaltrials.gov/ct2/show/NCT02699632.
Undertaking clinical research in the pregnant population is critical for maintaining quality, evidence-based practices in obstetrics and gynecology. Regardless of whether the research is appropriately powered, a well-designed randomized controlled trial, or supported by adequate funding, research questions cannot be answered without access to and willingness of patients to be studied. Embarking on clinical trials to better understand gestational weight gain in an overweight and obese population, within a consecutive four year period (December 2012 – October 2016), our research team unsuccessfully and then successfully recruited a cohort of pregnant women from the same geographical area and with similar eligibility criteria. Herein, we describe recruitment strategies deployed in the pregnant population and present 3 key “lessons learned” which were derived from our experience as well as findings from an online survey completed by over 350 women previously, currently or planning to become pregnant (Box 1).
Box 1. Lesson’s learned for successful recruitment of pregnant women into clinical trials.
Lesson #1: Pregnant women are interested in participating in clinical research trials.
- Lesson #2: Modality of recruitment, “active” vs. “passive”, is critical for success.
- In-person recruitment of specified population, i.e. women pregnant in the first trimester
- Health care provider approval
- Mode of contact, ie, staff-to-patient vs. patient-to-staff
Lesson #3: Obstetrician and midwife support is essential for introducing patients to research studies and for patients to feel comfortable participating in clinical research while pregnant.
The negative impact of maternal obesity and excess gestational weight gain on maternal and infant outcomes is well recognized1–3, yet both conditions are highly prevalent in clinical practice. The latest population level estimates suggest 37% of reproductive-aged women in the United States are obese4, and more than half of these women exceed national recommendations for weight gain during pregnancy5,6. There is a clear need to advance obstetrical practice with evidence-based recommendations to reduce the prevalence of overweight and obesity in women considering pregnancy and, at the same time, to disseminate evidence-based programs during pregnancy to achieve more healthful weight outcomes. To address this need, we launched the Expecting Success Study: Personalized Management of Body Weight during Pregnancy (NCT01610752, a member of the Lifestyle Interventions for Expectant Moms (LIFE-Moms) Consortium, (https://portal.bsc.gwu.edu/web/lifemoms))7, and aimed to enroll 306 healthy, overweight and obese pregnant women to test a personalized gestational weight management intervention delivered in-person or via Smartphone.
In preparation for study launch, investigators considered the available sample population and conservatively estimated 4,000 women out of 8,000 deliveries per year from our collaborating local hospital would qualify for study participation based on the eligibility criteria. Our planned recruitment strategy included distribution of email and social media advertisements to the community, placement of brochures within the hospital’s prenatal packets, and posters in the physician offices, laboratory and ultrasound departments. Study recruitment began in December 2012 and was terminated in April 2014 by the study sponsor for inability to meet enrollment goals (goal of 306 women over two and a half years). Fifty-four participants were enrolled over the 16-month period, only 18% of the planned enrollment target. Approximately eight months following the recruitment failure for Expecting Success, our team launched a second study which aimed to recruit an obese population with identical eligibility criteria (MomEE Study; NCT01954342), and conversely, the enrollment target has been met (goal of 60 women over one year). To evaluate how recruitment strategies targeting a population of pregnant women with overweight and obesity influenced study enrollment, we analyzed the recruitment strategies across these two studies and evaluated their success by examining the number of potential participants who expressed interest in study participation, and then were screened and enrolled, as well as reported reasons for refusal or ineligibility.
Simultaneously, we conducted an open, voluntary survey designed to target a convenience sample of women currently, previously, or planning to become pregnant (NCT02699632, acknowledged and monitored by PBRC IRB #FWA00006218). Our goal was to better understand the willingness and concerns of women for participating in research studies while pregnant. Data utilized herein is from completed questionnaires only, is uncorrected, and was collected from January through June 2016. The survey and data were collected and managed using REDCap electronic data capture tools hosted at Pennington Biomedical Research Center8. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources. The survey was tested for functionality and usability prior to posting on standalone website. Respondents agreed to participate on the first page of the survey, that then led to a second page which listed all questionnaire items (19 total) on a single page and did not allow for reviewing or changing of responses after submission. Occasionally, adaptive questioning was used to reduce the number and complexity of questions. Completeness checks were not employed. Mode of initial contact with potential participants was made on the Internet (email and Facebook posts). Participants were instructed of survey length in these Internet advertisements and study purpose and data storage information on the first page of our electronic survey. No incentives were offered to participants, no protected health information was collected, and no measures were employed for prevention of multiple entries from the same individual. Although number of unique site visitors between January and June 2016 is indeterminable, we had a completion rate of 69% (the percentage of surveys completed out of the total number initiated, i.e. number of page 2 completions/number of page 1 completions).
Lesson #1: Pregnant women are interested in participating in clinical research trials
Pregnancy is recognized as a vulnerable state particularly in the conduct of clinical research. To investigate whether pregnancy may discourage research participation, we asked survey participants about their general willingness to participate in clinical research during pregnancy, including types of studies and selection of reasons for willingness or unwillingness for participation. Importantly, to ensure our respondents did not report biased feelings for participating in research due to previous experience, respondents were surveyed to determine previous research participation. Only 6% of survey respondents reported having participated in a research study during pregnancy in the past.
When asked what types of research respondents were willing to participate in during pregnancy, 89% and 80% reported willingness to participate in observational studies (e.g. collection of observational data without intervention, such as anthropometrics) and retrospective studies (e.g. permission to use access medical records for data collection) respectively. Further supporting these data, 86% of respondents reported being interested in participating in a lifestyle intervention that would involve healthy eating and physical activity in pregnancy. Of the ten options provided, the most common reasons cited for willingness to participate in a lifestyle intervention during pregnancy were “I want my baby to be healthy” (76%), “I want to be healthier for my baby” (71%), and “I enjoy learning about my health” (62%). In response to ten options provided to describe reasons for unwillingness to participate in a lifestyle intervention during pregnancy, the most frequently reported reason was, “I am happy with my exercise habits already” at only 5%. Each of the other 9 reasons were selected by ≤4% of our respondents. Although many studies across the U.S. have recently faced challenges recruiting the pregnant population, these data demonstrate this challenge is not likely a result of a lack of interest or unwillingness from the patient.
Lesson #2: Modality of recruitment is critical
A key difference between the recruitment strategies implemented in our two studies was ‘active’ versus ‘passive’ methods of recruitment. Initially, in the Expecting Success study, we relied on strategies most familiar with our research institution which included poster, banner, and brochure placement in physician offices and hospital departments (laboratory, ultrasound etc.) and advertisements circulated by emails to listserv accounts and social media. Although these passive methods were directed to our target population (e.g., brochures were placed in prenatal packets distributed to all newly pregnant patients, fliers and banners were posted in obstetric waiting rooms and bathrooms), we only met 15% of our monthly recruitment goal using this strategy alone.
After four months of inadequate recruitment in Expecting Success, we established partnerships with local obstetricians’ offices to interact with newly pregnant women at obstetrical appointments face-to-face in an effort to replace these unsuccessful ‘passive’ methods with ‘active’ recruitment strategies. Study staff were provided daily schedules of initial obstetric appointments occurring in the first trimester at participating clinics. Study staff would meet the newly pregnant patients and discuss the study and their participation. Staffing these initial obstetric appointments became our primary method for recruitment in Expecting Success and was later deployed as our primary method for recruitment in the MomEE Study. These meetings were the most fruitful source of study recruitment because they allowed both studies to reach high volumes of potentially eligible patients. However, it was only in the MomEE study that the majority of all study participants enrolled were identified by these in-person interactions.
To better understand the conflicting success for active recruitment at initial obstetric appointments across our two studies, we analyzed and compared the recruitment results over 12 months of active study recruitment in each study. For the first study (Expecting Success), study staff had access to an average of 157 appointments each month, of which 48 (31%) initially met study requirements. Of those, 83% claimed to be interested in study participation after discussing the study in person with study staff. For the second study (MomEE), study staff had access to an average of 99 initial obstetric appointments each month of which 27 (27%) were initially eligible. Of those 27, an average of 88% claimed to be interested in participating after discussing the study in person. In total, 39% of enrolled participants of our first, unsuccessful study resulted from initial obstetric visit recruitment compared to 67% for our second, successful study (Figure 1).
Figure 1.
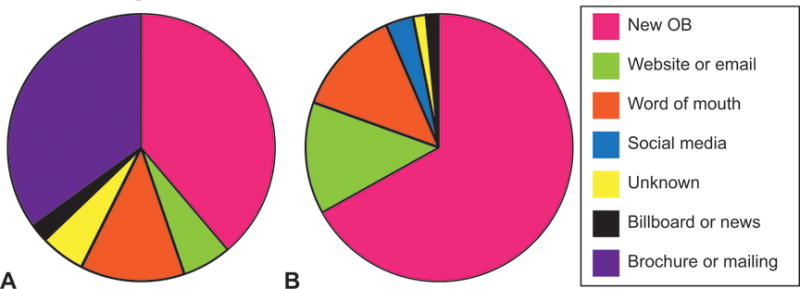
Proportion of recruitment methods which identified the actual enrolled participants in the Expecting Success and MomEE studies; “New OB” = these enrolled participants learned about the study through face-to-face discussions with study staff and prenatal care providers at early obstetric appointments (i.e. “New OB”).
These ‘active’ recruitment strategies of meeting directly with patients and discussing the study in person, were vital to our success; however, it is apparent from the abovementioned data that initial obstetric visit recruitment success was not due solely to access to patients. In our first, unsuccessful study, study staff discussed the study with more patients overall, and more patients seen were initially eligible and claimed to be interested. A key factor we changed between studies was the manner in which study staff and potential participants engaged in follow-up discussions. In the first study (Expecting Success), patient referral to the study was passive. After discussion of the study at the appointment, the patient was provided with contact information for the study team and asked to contact to schedule the first study visit. In the second study (MomEE), we deployed an active referral strategy. At the time of the initial obstetric appointment, patients provided consent to be contacted and clinical or study staff completed a patient contact form including contact information, preferred method of contact (telpephonic, text message, or email) and preferred time of day. This data was accessible electronically via REDCap™. Once this contact information was available, responsibility for follow-up rested on study staff and not on the patient. Study staff were expected to contact eligible patients within 48 hours of receipt of their contact information. This method of active patient referral produced a higher proportion of potentially eligible patients who converted to enrolled study participants.
In support of this observation, respondents to our survey were asked how one would ideally prefer to learn about a research study. Significantly more respondents reported a preference for active forms of recruitment (“at my doctor’s appointment by my health care provider”) over passive forms of recruitment (“I would rather reach out on my own instead of being approached”) (62% vs. 7% respectively, p<0.0001, McNemar’s test).
Lesson #3: Obstetrician and midwife support is essential for introducing patients to research studies and for pregnant patients to feel comfortable with participating in clinical research
Although participating physicians generously provided access to their schedules and patients in the first study (Expecting Success), few discussed or endorsed the study with patients as study staff provided the only avenue to the study. Conversely in our second study (MomEE), obstetricians and midwives frequently discussed the study with patients both before or after the face-to-face conversation with study staff (optimized by real-time reminders from the study staff to health care providers). We credit the success of this endorsement to the following reasons: 1) The prenatal care provider is a trusted source whom the patient knows personally. When survey respondents were asked how they would prefer to learn about a research study, the most cited method was “in person at my doctor’s appointment by my health care provider” (62%), while “in person at my doctor’s appointment by someone I didn’t know (e.g. research staff)” was selected by 36% of respondents. 2) The prenatal care provider is able to answer patient questions, discuss patient concerns, and importantly discuss potential risks of study participation in real time. This observation was supported by the survey. Sixty two percent of survey respondents stated they would feel comfortable participating in a research study while pregnant “only if my physician approved”, and 65% of respondents reported “having my physician or midwife’s approval” would ease their concerns for participating in a research study while pregnant. 3) Recruitment in collaboration with prenatal care providers allows for constant and efficient communication between study and obstetrical staff. Study staff recruiting in-office served as a reminder to obstetrical staff to discuss the study with participants while, at the same time, removed the sole burden of recruitment from obstetrical staff and helped maintain healthy relationships between the research team and obstetrical office. Furthermore, we found pairing the study’s introduction with the primary care provider and study staff allowed an avenue for patients to learn about the study and address risks with their provider, while simultaneously making an introduction between patient and study staff. This introduction to study staff provided an avenue for additional, specific study-related questions and associated “a face with a name” when the staff contacted the patient following her doctor’s appointment to schedule a screening visit. Moreover, a continual presence of study staff in clinics streamlined transmission of medical clearance forms and patient records necessary for determining potential participant eligibility and ultimately enrollment of the patient in the study in a timely manner.
Establishing a new obstetrician-study partnership followed this general procedure: 1) the study principal investigator (PI) made initial contact with the chief obstetrician and requested a meeting along with office/practice manager; 2) with interest established from the clinic, several meetings then occurred with the office manager to determine the best procedure for streamlining the recruitment agenda into the clinic with the least disruption to clinic flow; 3) with the established recruitment plan, a “Lunch and Learn” was scheduled in the office for fellow obstetricians and office staff with the study recruitment team present; and finally 4) with the endorsement of the group’s chief OB, recruitment was launched. For the first few months, the PI remained in contact with the Chief OB to provide recruitment reports and solicit feedback regarding the process which allowed for adjustments to be made if necessary. As, one cannot reimburse clinicians for study recruitment, obstetricians were not compensated or incentivized for participating in the recruitment of our studies. However, a hospital or practice can be compensated for time spent by office staff in helping with recruitment activities, such as locating information in the patient charts, routing forms for physician clearance, etc.. This compensation can reduce burden and make it more appealing to clinicians to agree to participate in the recruitment of research studies.
In summary, an overwhelming majority of women are interested in participating in clinical research while pregnant. Our recent experience shows that the nature of the recruitment strategy (active vs. passive and prenatal care provider involvement), rather than the lack of eligible and willing participants, is the major challenge for enrolling pregnant patients into clinical research studies. Pregnancy itself indeed presents untraditional challenges for recruitment because it is a temporary and time sensitive state with increased patient vulnerability. However, more active recruitment strategies are possible because patients attend specialized appointments with a health care provider and at regular intervals. Yet, while these initial obstetric appointments proved a fruitful medium to access patients, our success in the MomEE study only and not the Expecting Success Study (which both recruited patients at these appointments) demonstrates that simply obtaining access to a provider’s patient pool is not enough. Recruitment success through initial obstetric appointments requires implementation of our additional “lessons learned”: study endorsement by the primary care provider and active referral follow up. This message should be of particular significance to prenatal care providers because both our experience and survey results show in-clinic recruitment by prenatal care providers answering questions, discussing risk, and to not only supporting but encouraging participation when safe and appropriate is absolutely vital to success. We acknowledge that active recruitment strategies are more time consuming, and therefore, more costly; however our team was able to facilitate a process with a pool of well-trained, dependable undergraduate students (pre-medical majors) which allowed for cost-effective implementation. In sum, the optimal strategy for recruitment of the pregnant population is at the prenatal appointment with direct, in-person conversations with a study team member, endorsement by the health care provider, and timely and “active” communication follow-up. This approach permits continual identification of new patients, discussion of patient concerns and health care endorsement in real-time, maintenance of healthy partnerships between research and obstetrical staff, and attainment of recruitment goals.
Acknowledgments
The MomEE trial is supported by the NIDDK (R01DK099175). LIFE-Moms is supported by the NIH through the NIDDK (U01 DK094418 (Redman- Expecting Success), U01 DK094463, U01 DK094416, 5U01 DK094466 (RCU)), NHLBI (U01 HL114344, U01 HL114377), NICHD (U01 HD072834), NCCIH, the NIH Office of Research in Women’s Health (ORWH), the Office of Behavioral and Social Science Research (OBSSR), the Indian Health Service, and the Intramural Research Program of the NIDDK. Also supported in part by 1 U54 GM104940 from NIGMS, which funds the Louisiana Clinical and Translational Science Center. Elizabeth F. Sutton is supported by F31HD084199.
The authors thank the LIFE-Moms consortium members for their contributions to the development and oversight of the common measures and procedures shared across the trials.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has indicated that he/she has met the journal’s requirements for authorship.
References
- 1.Viswanathan M, Siega-Riz AM, Moos MK, et al. Outcomes of maternal weight gain. Evid Rep Technol Assess (Full Rep) 2008 May;(168):1–223. [PMC free article] [PubMed] [Google Scholar]
- 2.Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med. 2003 Oct;26(2):149–159. doi: 10.1207/S15324796ABM2602_07. [DOI] [PubMed] [Google Scholar]
- 3.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol. 2002 Aug;100(2):245–252. doi: 10.1016/s0029-7844(02)02125-7. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama. 2016 Jun 7;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IOM. Weight Gain During Pregnancy: Reexamining the Guidelines. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines; 2009. [PubMed] [Google Scholar]
- 6.DHHS. Pregnancy nutrition survellience 2011 report. 2012 [Google Scholar]
- 7.Clifton GR, Evans M, Cahill AG, et al. Design of lifestyle intervention trials to prevent excessive gestational weight gain in women with overweight or obesity. Obesity (Silver Spring) 2016 Feb;24(2):305–313. doi: 10.1002/oby.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


