Abstract
Background
The Mangled Extremity Severity Score (MESS) was developed 25 years ago in an attempt to utilize the extent of skeletal and soft tissue injury, limb ischemia, shock, and age to predict the need for amputation after extremity injury. Subsequently, there have been mixed reviews as to the utility of this score. We hypothesized that the MESS, when applied to a data set collected prospectively in modern times, would not correlate with the need for amputation.
Methods
We applied the MESS to patient data collected in the American Association for the Surgery of Trauma PROspective Vascular Injury Treatment (PROOVIT) registry. This registry contains prospectively collected demographic, diagnostic, treatment, and outcome data.
Results
Between 2013 and 2015, 230 patients with lower extremity arterial injuries were entered into the PROOVIT registry. The majority were male with a mean age of 34 years (range 4–92) and a blunt mechanism of injury at a rate of 47.4%. A MESS of 8 or greater was associated with a longer stay in the hospital (median 22.5 (15, 29) vs 12 (6, 21), p=0.006) and ICU (median 6 (2, 13) vs 3 (1, 6), p=0.03). 81.3% of limbs were ultimately salvaged (median MESS 4 (3, 5)) and 18.7% required primary or secondary amputation (median MESS 6 (4, 8), p < 0.001). However, after controlling for confounding variables including mechanism of injury, degree of arterial injury, injury severity score, arterial location, and concomitant injuries, the MESS between salvaged and amputated limbs was no longer significantly different. Importantly, a MESS of 8 predicted in-hospital amputation in only 43.2% of patients.
Conclusion
Therapeutic advances in the treatment of vascular, orthopedic, neurologic and soft tissue injuries have reduced the diagnostic accuracy of the MESS in predicting the need for amputation. There remains a significant need to examine additional predictors of amputation following severe extremity injury.
Level of Evidence
Level III evidence, prospective study, prognostic.
Keywords: mangled, trauma, vascular, extremity, amputation
BACKGROUND
The decision on whether to proceed with amputation or reconstruction of a mangled extremity is perhaps one of the most difficult for civilian trauma surgeons, as these types of injuries are seen relatively infrequently. Factors considered in the decision making process include patient age, physiologic condition at presentation, associated injuries, soft tissue factors, and the potential for salvaging a useful limb (1). The Mangled Extremity Severity Score (MESS) was developed 25 years ago at Harborview Medical Center in Seattle by Johansen and colleagues in an attempt to create a tool that accurately predicted the need for amputation (2). The MESS takes into consideration the degree of skeletal and soft tissue injury, limb ischemia, the presence of shock, patient age, and ischemia time. It has been widely utilized since its inception despite continued questions over its prognostic accuracy. The utility of this scoring system, or any other such scoring system, is further questioned given the major advances that have been made in the management of severely mangled extremities, including increased use of tourniquets in both civilian and military settings, numerous new hemostatic agents, advanced tissue transfer techniques, and novel vascular interventions.
In 2013, the AAST Multicenter Trials Committee initiated a prospective registry designed to collect data specific to vascular injuries. The PROspective Observational Vascular Injury Treatment (PROOVIT) registry includes extensive treatment and outcome data from multiple major trauma centers with the aim of informing practice and protocols to improve outcomes (3). The purpose of our study was to utilize the PROOVIT data base to re-evaluate the MESS on data collected prospectively in modern times. The hypothesis was that MESS would be predictive of the need for amputation.
METHODS
Patient data was collected from the AAST Multicenter PROspective Observational Vascular Injury Treatment (PROOVIT) registry. The details describing this large database have been previously described. In brief, it is a prospectively collected database of injuries to named arterial and venous structures from fourteen Level I trauma centers across the country (3). The database includes patient demographics, mechanism of injury, concomitant injuries, intraoperative and postoperative variables for patients entered during the index hospital stay only. The database is actively accruing data from follow up clinic visits and readmissions, and this data was not included in this study.
Lower extremity named arterial injuries were identified between February 2013 and August 2015. Each component of the MESS was obtained prospectively during data collection using the scoring system shown in Table 1. The MESS was calculated for each patient by adding the numerical scores of the skeletal / soft tissue injury, limb ischemia, shock and age scores. If there were greater than 6 hours of ischemia time, the ischemia score was doubled. There were 57 patients in which one component of the MESS (skeletal / soft tissue injury, shock, or ischemia) was missing. The missing data was found to be missing at random with p-value = 0.59 compared to the non-missing variable of age. The missing data was then treated using multiple imputation with 20 imputations. There was no difference in the correlation of MESS or its components before or after use of multiple imputation, suggesting that the bias imposed by the missing data is minimal. The percentage increase in standard error due to the missing values was 6.9% for MESS, 0.03% for shock, 0.02% for skeletal score, and 0.6% for ischemia score.
Table 1.
Mangled Extremity Severity Score (MESS) components prospectively collected in the PROspective Observational Vascular Injury Treatment (PROOVIT) registry
|
Score doubled for ischemia time > 6 hours
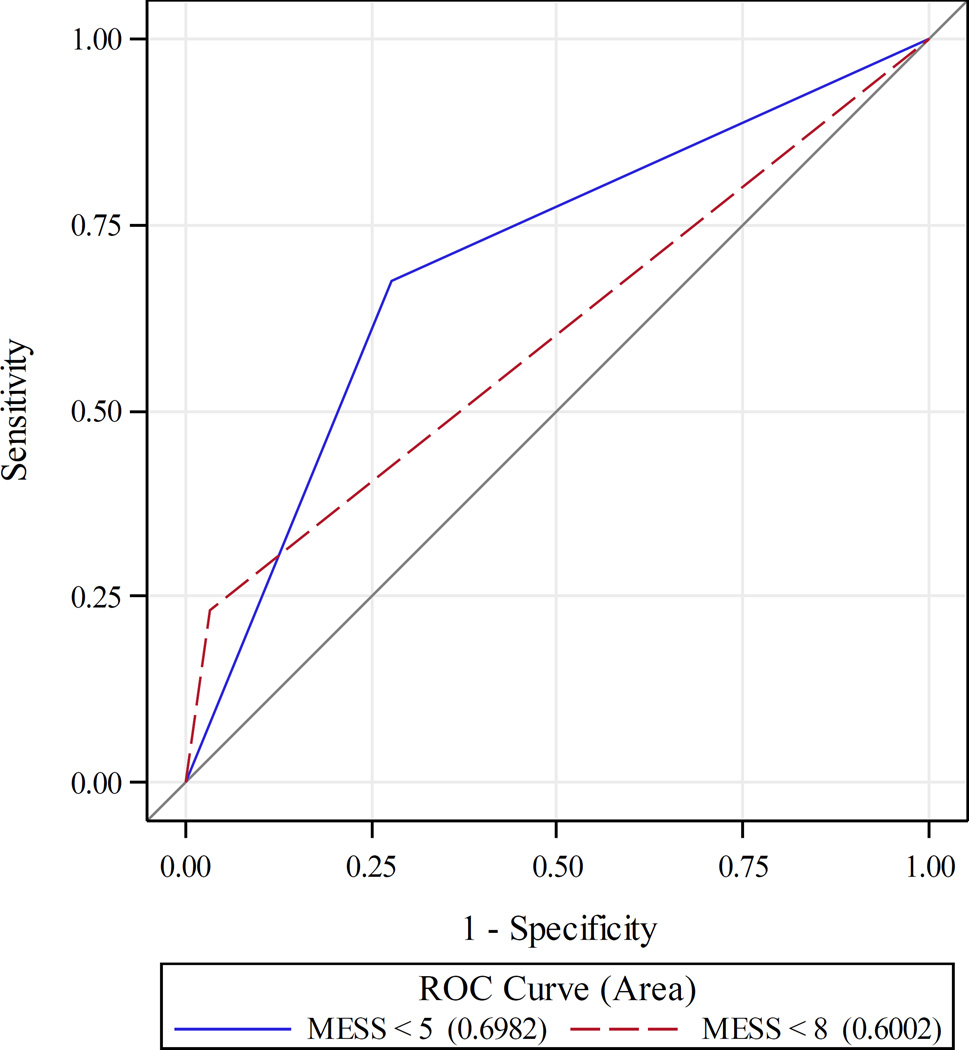
A MESS of 8 was chosen based on a prior study from the original creators of the scoring system, who suggested in their 2016 publication that a threshold of 8 was more appropriate in a modern setting (4). A receiver operating characteristic (ROC) analysis was performed which demonstrated that a MESS of 5 was a better balance of sensitivity and specificity than a MESS of 8. The ROC curves can be found in Figure 1.
Figure 1. Receiver Operator Characteristic (ROC) curves for Mangled Extremity Severity Score cut points.
Receiver operator characteristic (ROC) curve for a Mangled Extremity Severity Score (MESS) cutoff of 5 versus 8. A MESS cutoff of 5 was found to have the best balance of sensitivity and specificity, however, only was predictive of MESS in 20.2% of patients. A MESS of 8, as is used in prior literature, was predictive of amputation in 43.2% of patients.
Statistical analysis was performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA). Univariable logistic regression was used to look at the correlation of the MESS, as well as each MESS component, with the risk of amputation. Odds ratios comparing amputation versus limb salvage were generated. Age, gender, injury mechanism (blunt, penetrating or mixed blunt and penetrating), injury type (transection, flow-limiting lesion, occlusion, pseudoaneurysm or other), arterial injury location (femoral, popliteal, below-popliteal arteries or multi-level injury), use of shunting, pre-hospital tourniquet use, fasciotomy performed at any time during the admission, injury severity score (ISS), and concomitant vein, nerve or orthopedic injury were assessed for confounding. Of note, the database did not distinguish the severity of vein, nerve or orthopedic injury – it reports a binary value of injured or not injured. Independent predictors of amputation were identified by univariable logistic regression. Significant variables (p-value ≤ 0.1) were injury mechanism, the presence of a transection, arterial injury location, ISS, concomitant nerve or orthopedic injury. A multivariable logistic regression with these confounders was performed of the MESS, and separately of the MESS components, with the binary outcome of amputation compared to limb salvage. These were performed separately due to the confounding nature of including both MESS and its components in the same model. The area under the receiver operating characteristic (AUROC) curve for the logistic regression model including MESS was 0.86 [95% CI 0.79 – 0.93]. The Hosmer-Lemeshow (H-L) goodness of fit test had a p = 0.93. The AUROC for the model which included the components age score, skeletal score, ischemia score, and shock score was 0.88 [95% CI 0.82 – 0.94], and the H-L was nonsignificant with p = 0.29. The probability of amputation was modeled using univariable logistic regression to predict amputations with a MESS cutoff of 5 and 8. Finally, demographics of patients with the MESS cutoff of 8 were compared using Wilcoxon rank-sum test and Fisher’s exact test. A p-value < 0.05 was considered statistically significant.
RESULTS
Between February 2013 and August 2015, 230 patients with lower extremity arterial injuries were entered into the PROOVIT registry. The cohort consisted predominantly of men (87.8%) with an average age of 34 years ± 15.3 (range 4–92). The mechanism of injury was reported as blunt in 109 patients (47.4%), penetrating in 114 patients (49.6%), and mixed blunt and penetrating in the remainder (Table 2). Isolated femoral injuries were found in 102 (44.3%) patients, and isolated popliteal injuries in 60 patients (26.1%). Sixty-three injuries to arteries distal to the popliteal artery were identified (27.4%), and 5 injuries were to both the above and below-knee arterial beds. The injury to the artery was most often a transection, present in 45.7% of patients. There were 50 concomitant venous injuries (21.7%). 94% of these venous injuries were repaired at time of initial operation, and the remainder ligated. There were 94 concomitant orthopedic injuries (40.9%) and 33 nerve injuries (14.4%).
Table 2.
Comparison of demographics between patients with Mangled Extremity Severity Score (MESS) < 8 and MESS of 8 or greater.
| MESS Score |
||||
|---|---|---|---|---|
| All | MESS < 8 | MESS ≥ 8 | ||
| Variable | (n = 230) | (n = 214) | (n = 16) | p-value |
| Age (Mean ± SD) | 34 ± 15.3 | 32.8 ± 14.7 | 48.3 ± 15.6 | 0.0003 |
| Male, n (%) | 202 (87.8) | 187 (87.4) | 15 (93.8) | 0.4 |
| Injury Mechanism | 0.004 | |||
| Blunt, n (%) | 109 (47.4) | 96 (44.9) | 13 (81.3) | |
| Penetrating, n (%) | 114 (49.6) | 112 (52.3) | 2 (12.5) | |
| Mixed blunt and penetrating, n (%) | 7 (3.0) | 6 (2.8) | 1 (6.3) | |
| Injured artery: | 0.7 | |||
| Femoral, n (%) | 102 (44.3) | 97 (45.3) | 5 (31.3) | |
| Popliteal, n (%) | 60 (26.1) | 55 (25.7) | 5 (31.3) | |
| Distal to popliteal artery, n (%) | 63 (27.4) | 57 (26.6) | 6 (37.5) | |
| Multiple levels, n (%) | 5 (2.2) | 5 (2.3) | 0 (0) | |
| Transection, n (%) | 105 (45.7) | 93 (43.5) | 12 (75) | 0.01 |
| Flow limiting defect, n (%) | 44 (19.1) | 42 (19.6) | 2 (12.5) | 0.4 |
| Occlusion, n (%) | 38 (16.5) | 36 (16.8) | 2 (12.5) | 0.5 |
| Pseudoaneurysm, n (%) | 9 (3.9) | 9 (4.2) | 0 (0) | 0.5 |
| Other injury type, n (%) | 41 (17.8) | 40 (18.7) | 1 (6.3) | 0.2 |
| Median ISS (Q1, Q3) | 11 (9, 19) | 10.5 (9, 18) | 21 (17, 26) | 0.0003 |
| Median AIS-extremity (Q1, Q3) | 3 (3, 4) | 3 (3, 4) | 3 (3, 4) | 0.1 |
| Mean Admission SBP ± SD | 120.9 ± 30.0 | 121.4 ± 29.9 | 115.9 ± 31.7 | 0.5 |
| Median GCS (Q1, Q3) | 15 (14, 15) | 15 (15, 15) | 15 (14, 15) | 0.7 |
| Concomitant venous injury, n (%) | 50 (21.7) | 46 (21.5) | 4 (25) | 0.5 |
| Vein repaired, n (%) | 47/50 (94.0) | 43/46 (93.4) | 4/4 (100%) | 0.4 |
| Concomitant nerve injury, n (%) | 33 (14.4) | 22 (10.3) | 11 (68.8) | < 0.001 |
| Concomitant orthopedic injury, n (%) | 94 (40.9) | 83 (38.8) | 11 (68.8) | 0.02 |
| Pre-hospital Tourniquet, n (%) | 22 (9.6) | 20 (9.4) | 2 (12.5) | 0.5 |
| Temporary shunt utilized, n (%) | 17 (7.4) | 17 (7.9) | 0 (0) | 0.3 |
| Fasciotomy, n (%) | 94 (40.9) | 89 (41.6) | 5 (31.3) | 0.3 |
ISS = Injury severity score
AIS = Abbreviated injury score
SBP = Systolic blood pressure
GCS = Glasgow coma score
MESS = Mangled extremity severity score
Q1 = Lower quantile (25th percentile)
Q3 = Upper quantile (75th percentile)
Twenty-two patients had a pre-hospital tourniquet applied (9.6%). Ninety-four (40.9%) fasciotomies were performed during the index hospitalization, including 40 prophylactic fasciotomies at the initial procedure, 48 therapeutic fasciotomies at the initial procedure, and 5 delayed fasciotomies (one was not categorized). A temporary shunt was used for damage-control in 17 (7.4%) patients.
We modeled the probability of amputations based on MESS, and determined that MESS greater than or equal to 8 was predictive of in-hospital amputation in only 43.2% of patients. ROC analysis (Figure 1) showed the best balance of sensitivity and specificity was a MESS of 5 (AUROC 0.70 [95% CI 0.62–0.77]), compared to a MESS of 8 (AUROC 0.60 [95% CI 0.54 – 0.67], p = 0.02). However, a MESS of 5 was only predictive of amputation in 20.2% of cases. Based on prior studies and this increase in ability to predict amputation, a MESS of 8 was chosen for further analysis. Sixteen patients had a MESS of greater than or equal to 8 (7.0%). The median MESS was 4 (25th percentile (Q1) 3, 75th percentile (Q3) 6). The median skeletal injury component score was 2 (1, 3), the median ischemia score was 2 (1, 2), the median shock score was 0 (0, 1) and the median age score was 1 (0, 1). Patients with a MESS ≥ 8 were on average older (48.3 years old vs. 32.8, p < 0.0003), and were more likely to have sustained a blunt injury (81.3% vs. 44.9%, p = 0.004). Patients with a MESS of 8 or greater had a higher median ISS (21 vs 10.5, p = 0.0003), though they had no difference in mean abbreviated injury score (AIS) of the extremity, admission systolic blood pressure or GCS (Table 2). There were more concomitant nerve (68.8% vs. 10.3%, p < 0.001) and orthopedic injuries (68.8% vs. 38.8%, p = 0.02) when MESS was greater than or equal to 8. There was no difference in concomitant venous injuries between groups (Table 2).
Primary or secondary amputations were performed in 43 patients (18.7%, median MESS 6 (4, 8)), including 21 primary amputations performed for damage control (9.1%). Limbs were ultimately salvaged in 187 patients (81.3%, median MESS 4 (3, 5), p < 0.001, Table 3). There were 12 deaths (5.2%) in the total cohort.
Table 3.
Mangled Extremity Severity Score (MESS) elements compared between patients who underwent amputations and those who did not; before and after adjustment for significant confounders of injury mechanism, arterial transection, arterial injury location, injury severity score (ISS), and concomitant nerve and orthopedic injuries.
| MESS Elements | Amputations median (Q1, Q3) (n = 43) |
Limb Salvage median (Q1, Q3) (n = 187) |
p-value unadjusted |
p-value adjusted |
|---|---|---|---|---|
| Skeletal / soft tissue score | 3 (2, 3) | 1 (1, 3) | < 0.001 | 0.50 |
| Limb ischemia | 2 (1, 3) | 1 (1, 2) | < 0.001 | 0.79 |
| Shock | 0 (0, 1) | 0 (0, 1) | 0.21 | 0.20 |
| Age score | 1 (0, 1) | 1 (0, 1) | 0.22 | 0.22 |
| Total MESS | 6 (4, 8) | 4 (3, 5) | < 0.001 | 0.18 |
MESS = mangled extremity severity score
Q1 = Lower quantile (25th percentile)
Q3 = Upper quantile (75th percentile)
Univariable logistic regression was performed, looking at age, gender, injury mechanism, injury type, arterial injury location, use of shunting, pre-hospital tourniquet use, fasciotomy performed at any time during the admission, ISS, and concomitant vein, nerve or orthopedic injury for confounding. Blunt injuries were associated with amputation with an odds ratio of 6.4 [95% CI 2.7 – 15.1] compared to penetrating injuries (p < 0.0001). Transection was associated with amputation with an odds ratio of 2.4 [95% CI 1.2 – 4.7] (p = 0.014). Popliteal arterial injuries were associated with a 6.8-fold higher risk of amputation than femoral arterial injuries [95% CI 2.7 – 17.3] (p < 0.001). ISS was only weakly associated with amputation with an odds ratio of 1.02 [95% CI 1.00 – 1.05] (p = 0.08). Concomitant nerve and orthopedic injuries were associated with amputation with an odds ratio of 11.6 [95% CI 5.1 – 26.5] and 6.8 [95% CI 3.2 – 14.7], respectively (p < 0.0001 for each). Age, gender, use of shunting, pre-hospital tourniquet use, fasciotomy performed at any time during the admission, and concomitant vein injury were not significantly associated with amputation and were not included in the final model. After controlling for confounding factors, the overall MESS and its components were no longer different between salvaged and amputated limbs (Table 3). After adjustment, concomitant nerve injury was the only factor that remained an independent predictor of amputation (odds ratio 6.9 [95% CI 2.3 – 21.2], p = 0.001).
A MESS of 8 or greater was associated with a longer stay in the hospital (median 22.5 (15, 29) vs 12 (6, 21), p = 0.006) and intensive care unit (6 (2, 13) vs 3 (1, 6), p = 0.03). There was a higher percentage of both primary traumatic amputations performed for damage control (50.0% vs. 6.1%, p < 0.001) and overall amputations (62.5% vs. 15.4%, p < 0.001) in the group of patients with a MESS of 8 or greater. There was no statistically significant difference in the number of re-interventions or in death between groups (Table 4).
Table 4.
Comparison of outcomes between patients with MESS < 8 and MESS of 8 or greater.
| All (n = 230) |
MESS < 8 (n = 214) |
MESS ≥ 8 (n = 16) |
p-value | |
|---|---|---|---|---|
| Total units packed red blood cells (median (Q1, Q3)) | 3 (0, 8) | 3 (0, 8) | 8 (2.5, 10) | 0.07 |
| Hospital length of stay (median (Q1, Q3)) | 12 (6, 22) | 12 (6, 21) | 22.5 (15, 29) | 0.006 |
| Days in Intensive Care Unit (median (Q1, Q3)) | 3 (1, 6) | 3 (1, 6) | 6 (2, 13) | 0.03 |
| Reintervention required, n (%) | 35 (15.2) | 32 (15) | 3 (18.8) | 0.5 |
| Damage control primary traumatic amputation, n (%) | 21 (9.1) | 13 (6.1) | 8 (50) | < 0.001 |
| All amputations, n (%) | 43 (18.7) | 33 (15.4) | 10 (62.5) | < 0.001 |
| Death, n (%) | 12 (5.2) | 10 (4.7) | 2 (12.5) | 0.2 |
MESS = mangled extremity severity score
Q1 = Lower quantile (25th percentile)
Q3 = Upper quantile (75th percentile)
DISCUSSION
The original MESS was developed in 1990 by a retrospective review of 25 consecutive patients with lower extremity injuries (2). The same authors subsequently applied the scoring system to a group of 26 comparable patients studied prospectively. In the original study, the MESS for salvaged limbs ranged from 3 to 6, whereas the amputated limbs ranged from 7 to 12. These authors concluded that in their hands, a MESS of 7 or greater predicted amputation with 100% accuracy. Subsequent authors were unable to obtain this degree of accuracy, and developed alternative scoring systems. These systems include the Limb Salvage Index (LSI), the Predictive Salvage Index (PSI) the Nerve Injury, Ischemia, Soft-tissue Injury, Skeletal Injury, Shock and Age of Patient Score (NISSA) and the Hannover Fracture Scale (HFS) (1). Each contain various elements of patient characteristics at presentation (e.g. age, presence of shock), structural injury (e.g. concomitant bone, muscle, skin, nerve, vascular, injury, degree of contamination) and treatment factors (e.g. warm ischemia time, time to treatment) (5–8). These five scoring systems were prospectively evaluated in 2001 by Bosse et al. as part of the Lower Extremity Assessment Project (LEAP) study group (9). A total of 556 high-energy injuries were evaluated including ischemic limbs, type III-A, III-B, and III-C tibial fractures, severe distal tibial fractures (open pilon fractures or type III-B ankle fractures), hindfoot fractures and isolated soft-tissue injuries of the lower extremities. This extensive analysis could not validate the clinical utility of any of these scoring systems. The scores did have high specificity in predicting limb-salvage potential, but had a low sensitivity in predicting the need for amputation. A subsequent study by the LEAP group showed that none of these scoring systems were predictive of functional recovery in patients who underwent successful limb reconstruction (10).
Recent re-evaluations of the MESS have continued to question its validity. Menakuru et al. found that of 148 patients, a MESS of > 7 had a sensitivity of only 44% and a specificity of 70% in predicting amputation (11). Recent systematic reviews further confirm the unreliability of the MESS. Fodor et al. concluded that MESS correctly identified the need for amputation in only 25% of cases (12) whereas Schiro et al. found the range of reported accuracy of a MESS > 7 to be anywhere between 0 – 93.4% in the literature (13). The MESS has also been evaluated in combat-related injuries. Sheenan et al. reported on 155 patients treated for type III open tibia fractures in US military service personnel, involving primarily blast injuries (14). 110 had successful limb salvage, and 45 underwent primary amputation. The mean MESS values for amputees was 5.8 and for those that were salvaged was 5.3 (p = 0.057). The sensitivity and specificity of a MESS ≥ 7 in predicting the need for amputation in the combat setting was 35% and 87.8% respectively (positive predictive value of 50%). These military surgeons concluded that the MESS was not useful in battle-field related injuries. Additional studies on battlefield-related extremity vascular injuries did find that those with preserved limbs but high MESS scores (≥ 7) had higher levels of dysfunction as rated with the Short Musculoskeletal Function Assessment tool (15).
In another contemporary analysis of the mangled lower extremity, de Mestral et al. retrospectively examined a cohort of patients entered into the National Trauma Databank (NTDB) between 2007–2009. A total of 1354 patients were identified, with a 21% amputation rate (16). These authors found that the presence of a severe head injury, shock in the emergency room, and a high-energy mechanism of injury were associated with early amputation. Unfortunately, the NTDB does not contain sufficient data to accurately calculate the MESS score, which is why the PROOVIT database project is so important. A recent study from Austria looked at early failed attempts at salvage in open lower limb fractures demonstrating that in addition to MESS, other important predictors of secondary amputations included complex fractures, severe soft tissue damage, and the need for fasciotomy (17). In 60% of these patients, failed limb salvage resulted from infectious complications, and 40% from a failed vascular reconstruction.
In 2015, Aarabi et al. from Seattle presented their data on the utility of MESS 25 years after its creation. In their series of 48 patients with mangled extremities complicated by acute arterial insufficiency, 81% were salvaged (MESS mean of 4.8) and 19% required amputation (MESS mean of 9.1) (4). In their series, those 77% of those that went on to secondary amputation had a popliteal artery injury. These authors also reported that MESS independently predicted the cost and length of hospitalization; on average for every 1-point increase in MESS, the hospital cost increased by almost $6000.
Our study found blunt injuries, vessel transection, popliteal injuries, and concomitant nerve and orthopedic injuries were associated with the need for amputation, and were more predictive than an isolated MESS score. Though patients who underwent limb salvage had a lower MESS score on average, this was not significant after adjustment for confounders. MESS was a very poor predictor of amputation in this cohort, predicting only 43.2% of amputations.
This analysis includes ten patients who died without receiving an amputation. The PROOVIT database does not distinguish if the limb was viable when the patient died, but these are included in the limb salvage category, representing a potential confounding variable. Mangled limbs without arterial injuries are not included in the PROOVIT database. In addition, though this data was prospectively obtained, incomplete or inaccurate data entry is an inherent flaw across all database studies. In this study, patients with missing MESS components were included as missing, meaning that some patients could have a falsely-low total MESS. This was evaluated by correcting the missing values using multiple imputation, and no difference was found in the analysis. The increase in standard error was minimal for the missing component analysis and 6.9% for overall MESS. The missing data was also found to be missing at random compared to non-missing variables, and thus, we conclude that though bias may be present, it is minimal for this study. Furthermore, this study reflects modern practice only among major Level I academic institutions across the country. Practice patterns of the larger enrolling centers may have dictated some of the trends observed.
While our data is robust, prospectively collected and this series is relatively large, we do acknowledge that future investigations will need to examine the long-term outcomes of the patients with salvaged limbs. Late amputations (performed after the first hospitalization) may be required for limb dysfunction, persistent infections/open wounds or in patients with chronic pain as these problems can contribute to significant physical, psychological, financial and social distress for these patients (18). As the LEAP study group has demonstrated, in selected patients, the long term quality of life may be the same in those with amputations and successful prosthetics as it is in patients with limb salvage (19).
Prehospital use of a tourniquet, damage control, balanced resuscitation, the employment of vascular shunts to reduce ischemia time, early fasciotomy, aggressive wound care, microsurgical abilities and advanced tissue coverage techniques have all contributed to our increased ability to care for patients with mangled extremities. At this juncture, we advocate for the use of a team approach to decision making regarding limb salvage rather than the use of a score. Experienced surgeons from vascular, trauma, orthopedic and plastic surgical disciplines evaluating the patient at the bedside and the patient’s limb collaboratively ultimately contributes to the best outcome for the patient and for the extremity. Additionally, continued re-evaluation in the hospital and after discharge with long-term functional outcome data is needed to inform practice decisions and to assure the best quality of life for individual patients with limb-threatening, mangled extremities.
Acknowledgments
Funding: The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through grant #UL1 TR001860.
This work was funded by the National Trauma Institute, Award # NTI-NTRR15-05, and supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Defense Medical Research and Development Program under the prime award #W81XWH-15-2-0089. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense or the National Trauma Institute.
and the AAST PROOVIT Study Group
Tiffany Bee MD; Timothy Fabian, MD
University of Tennessee Health Sciences Center – Memphis, TN, USA
Jay Menaker, MD; Megan Brenner, MD; Thomas M. Scalea, MD, Todd E. Rasmussen, MD
University of Maryland, R Adams Cowley Shock Trauma Center - Baltimore, MD, USA
Jeanette M. Podbielski, RN, CCRP; John B. Holcomb, MD; Garrett Jost
University of Texas Health Sciences Center, Houston - Houston, TX, USA
David Skarupa, MD; Jennifer A. Mull, RN, CCRC; Joannis Baez Gonzalez
University of Florida, Jacksonville – Jacksonville, FL, USA
Jayun Cho, MD; Kenji Inaba, MD
Los Angeles County + University of Southern California Hospital - Los Angeles, CA, USA
Richard D. Catalano, MD; Ahmed M. Abou-Zamzam Jr, MD; Xian Luo-Owen, PhD; Ian S.H. Vannix, BS
Loma Linda University Medical Center - Loma Linda, CA, USA
Nathaniel Poulin, MD
East Carolina Medical Center – Greenville, NC, USA
John K. Bini, MD; Karen Herzig, BSN, RN
Wright State Research Institute - Miami Valley Hospital - Dayton, OH, USA
Scott T. Trexler, MD; Sonya Charo-Griego, RN; Douglas Johnson, LVN
San Antonio Military Medical Center / US Army Institute of Surgical Research - JBSA Fort Sam Houston, TX, USA
John Myers, MD; Michael Johnson, MD; Kristin Rocchi, RN
The University of Texas Health Sciences Center at San Antonio - San Antonio, TX, USA
Stephanie A. Savage, MD, MS
Indiana University School of Medicine - Indianapolis, IN, USA
Ramyar Gilani, MD; Tikesha Smith
Ben Taub General Hospital / Baylor College of Medicine - Houston, TX, USA
George Dulabon, MD; Riyad Karmy-Jones
Peace Health Southwest Washington Medical Center - Vancouver, Washington, USA
Andreas Larentzakis MD; George Velmahos, MD
Massachusetts General Hospital - Boston, Massachusetts, USA
Melissa N. Loja, MD, MAS; Joseph Galante, MD; Misty Humphries, MD; Joseph DuBose, MD
University of California, Davis - Sacramento, CA, USA
Steven R. Shackford, MD; Michael Sise, MD
Scripps Mercy Hospital - San Diego, CA, USA
Forrest O. Moore, MD; Annette Taylor, RN, BSN, CCRC; John Michael Sherman; Jeannette G. Ward, MS-CR
University of Arizona – Chandler Regional Medical Center - Phoenix, AZ, USA
Fausto Y. Vinces, DO; Salvatore Docimo, DO
Lutheran Medical Center - Brooklyn, New York, USA
Ramyar Gilani, MD; Reginva Knight
Ben Taub Hospital / Baylor College of Medicine - Houston, TX, USA
Matthew M. Carrick, MD; Barbara Shaffer, RN, BSN
The Medical Center of Plano - Plano, TX, USA
Jonathan P. Meizoso, MD
Ryder Trauma Center, University of Miami - Miami, FL, USA
Ravi R. Rajani, MD
Emory University School of Medicine, Grady Memorial Hospital - Atlanta, GA, USA
Sameer Hirji, MD; Jonathan D. Gates, MD, MBA
Brigham and Women’s Hospital - Boston, MA, USA
Footnotes
This study was presented at the 75th annual meeting of the American Association for the Surgery of Trauma, September 14–17, 2016, in Waikoloa, Hawaii.
Authorship: This work represents the original efforts of the investigators. All listed authors contributed to study design, data collection, data interpretation, and manuscript development.
Disclosure: The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Melissa N Loja, M.D., M.A.S.; Amanda Sammann, M.D., M.P.H.; Joseph DuBose, M.D.; M. Margaret Knudson, M.D. – Study design, literature search, data analysis, data interpretation, manuscript writing and critical revision.
Chin-Shang Li, Ph.D., Yu Liu, M.S. – Study design, data analysis, critical revision.
Stephanie Savage, M.D., M.S.; Thomas Scalea, M.D.; John B. Holcomb, M.D.; Todd E. Rasmussen, M.D. – Study design, data collection, data interpretation, critical revision.
AAST PROOVIT Study Group – data collection and critical revision.
Contributor Information
Melissa N. Loja, University of California, Davis, Department of Surgery, Divisions of Vascular and Trauma Surgery, 4860 Y Street, Suite 3400, Sacramento, CA 95817, Phone (916) 734-2024, Fax (916) 734-2026.
Amanda Sammann, Department of Surgery, University of California – San Francisco, San Francisco, CA, USA, asammann@yahoo.com.
Joseph DuBose, Department of Surgery, Divisions of Vascular and Trauma Surgery, University of California – Davis, Sacramento, CA, USA, jjd3c@yahoo.com.
Chin-Shang Li, Department of Public Health Sciences, Division of Biostatistics, University of California – Davis, Sacramento, CA, USA, cssli@ucdavis.edu.
Yu Liu, Department of Statistics, University of California – Davis, Sacramento, CA, USA, yzyliu@ucdavis.edu.
Stephanie Savage, Department of Surgery, Indiana University School of Medicine, Indianapolis, IN, USA, saasavage@hotmail.com.
Thomas Scalea, Department of Surgery, R Adams Cowley Shock Trauma Center, University of Maryland, Baltimore, MD, USA, tscalea@umm.edu.
John B. Holcomb, Department of Surgery, Center for Translational Injury Research, University of Texas Health Sciences Center Houston, Houston, TX, USA, John.Holcomb@uth.tmc.edu.
Todd E. Rasmussen, Department of Surgery, R Adams Cowley Shock Trauma Center, University of Maryland, Baltimore, MD, USA, todd.e.rasmussen.mil@mail.mil.
M. Margaret Knudson, Department of Surgery, University of California – San Francisco, San Francisco, CA, USA, Peggy.Knudson@ucsf.edu.
REFERENCES
- 1.Scalea TM, DuBose J, Moore EE, West M, Moore FA, McIntyre R, Cocanour C, Davis J, Ochsner MG, Feliciano D. Western Trauma Association critical decisions in trauma: management of the mangled extremity. J Trauma Acute Care Surg. 2012;72(1):86–93. doi: 10.1097/TA.0b013e318241ed70. [DOI] [PubMed] [Google Scholar]
- 2.Johansen K, Daines M, Howey T, Helfet D, Hansen ST., Jr. Objective criteria accurately predict amputation following lower extremity trauma. J Trauma. 1990;30(5):568–672. doi: 10.1097/00005373-199005000-00007. discussion 72-3. [DOI] [PubMed] [Google Scholar]
- 3.DuBose JJ, Savage SA, Fabian TC, Menaker J, Scalea T, Holcomb JB, Skarupa D, Poulin N, Chourliaras K, Inaba K, et al. The American Association for the Surgery of Trauma PROspective Observational Vascular Injury Treatment (PROOVIT) registry: multicenter data on modern vascular injury diagnosis, management, and outcomes. J Trauma Acute Care Surg. 2015;78(2):215–222. doi: 10.1097/TA.0000000000000520. discussion 22-3. [DOI] [PubMed] [Google Scholar]
- 4.Aarabi S, Kavousi Y, Friedrich J, Singh N, Bulger E. Severe Lower Extremity Injury: MESS (Mangled Extremity Severity Score) Twenty-Five Years Later. J Trauma Acute Care Surg. 2016 in press. [Google Scholar]
- 5.Howe HR, Jr, Poole GV, Jr, Hansen KJ, Clark T, Plonk GW, Koman LA, Pennell TC. Salvage of lower extremities following combined orthopedic and vascular trauma. A predictive salvage index. Am Surg. 1987;53(4):205–208. [PubMed] [Google Scholar]
- 6.McNamara MG, Heckman JD, Corley FG. Severe open fractures of the lower extremity: a retrospective evaluation of the Mangled Extremity Severity Score (MESS) J Orthop Trauma. 1994;8(2):81–87. doi: 10.1097/00005131-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Russell WL, Sailors DM, Whittle TB, Fisher DF, Jr, Burns RP. Limb salvage versus traumatic amputation. A decision based on a seven-part predictive index. Ann Surg. 1991;213(5):473–480. doi: 10.1097/00000658-199105000-00013. discussion 80-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tscherne H, Oestern HJ. [A new classification of soft-tissue damage in open and closed fractures (author's transl)] Unfallheilkunde. 1982;85(3):111–115. [PubMed] [Google Scholar]
- 9.Bosse MJ, MacKenzie EJ, Kellam JF, Burgess AR, Webb LX, Swiontkowski MF, Sanders RW, Jones AL, McAndrew MP, Patterson BM, et al. A prospective evaluation of the clinical utility of the lower-extremity injury-severity scores. J Bone Joint Surg Am. 2001;83-A(1):3–14. doi: 10.2106/00004623-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Ly TV, Travison TG, Castillo RC, Bosse MJ, MacKenzie EJ, Group LS. Ability of lower-extremity injury severity scores to predict functional outcome after limb salvage. J Bone Joint Surg Am. 2008;90(8):1738–1743. doi: 10.2106/JBJS.G.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menakuru SR, Behera A, Jindal R, Kaman L, Doley R, Venkatesan R. Extremity vascular trauma in civilian population: a seven-year review from North India. Injury. 2005;36(3):400–406. doi: 10.1016/j.injury.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Fodor L, Sobec R, Sita-Alb L, Fodor M, Ciuce C. Mangled lower extremity: can we trust the amputation scores? Int J Burns Trauma. 2012;2(1):51–58. [PMC free article] [PubMed] [Google Scholar]
- 13.Schiro GR, Sessa S, Piccioli A, Maccauro G. Primary amputation vs limb salvage in mangled extremity: a systematic review of the current scoring system. BMC Musculoskelet Disord. 2015;16:372. doi: 10.1186/s12891-015-0832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheean AJ, Krueger CA, Napierala MA, Stinner DJ, Hsu JR, Skeletal T, Research C. Evaluation of the mangled extremity severity score in combat-related type III open tibia fracture. J Orthop Trauma. 2014;28(9):523–526. doi: 10.1097/BOT.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 15.Scott DJ, Watson JD, Heafner TA, Clemens MS, Propper BW, Arthurs ZM. Validation of the Short Musculoskeletal Function Assessment in patients with battlefield-related extremity vascular injuries. J Vasc Surg. 2014;60(6):1620–1626. doi: 10.1016/j.jvs.2014.08.060. [DOI] [PubMed] [Google Scholar]
- 16.de Mestral C, Sharma S, Haas B, Gomez D, Nathens AB. A contemporary analysis of the management of the mangled lower extremity. J Trauma Acute Care Surg. 2013;74(2):597–603. doi: 10.1097/TA.0b013e31827a05e3. [DOI] [PubMed] [Google Scholar]
- 17.Fochtmann A, Mittlbock M, Binder H, Kottstorfer J, Hajdu S. Potential prognostic factors predicting secondary amputation in third-degree open lower limb fractures. J Trauma Acute Care Surg. 2014;76(4):1076–1081. doi: 10.1097/TA.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 18.Prasarn ML, Helfet DL, Kloen P. Management of the mangled extremity. Strategies Trauma Limb Reconstr. 2012;7(2):57–66. doi: 10.1007/s11751-012-0137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacKenzie EJ, Bosse MJ, Pollak AN, Webb LX, Swiontkowski MF, Kellam JF, Smith DG, Sanders RW, Jones AL, Starr AJ, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801–1809. doi: 10.2106/JBJS.E.00032. [DOI] [PubMed] [Google Scholar]