Abstract
Anemia is a frequent complication of many inflammatory disorders, including inflammatory bowel disease. Although the pathogenesis of this problem is multifactorial, a key component is the abnormal elevation of the hormone hepcidin, the central regulator of systemic iron homeostasis. Investigations over the last decade have resulted in important insights into the role of hepcidin in iron metabolism and the mechanisms that lead to hepcidin dysregulation in the context of inflammation. These insights provide the foundation for novel strategies to prevent and treat the anemia associated with inflammatory diseases.
I. Introduction
Iron is critical for normal functioning of various biological processes in all kingdoms of life. It is an essential component of several biochemical reactions, including oxygen transport, enzyme catalysis and photosynthesis.1,2 The role of iron in these processes is linked to its ability to transition between different oxidation states (−2 to +6), although within the cell it is commonly found in the ferrous (Fe2+) and ferric forms (Fe3+). The ready movement of iron between oxidation states also renders it extremely toxic due to production of damaging reactive oxygen species. This potentially toxic oxido-reduction is remarkably managed in living systems. Not only are the physiological levels of iron tightly controlled but iron is also usually found complexed to proteins that take part in its absorption, transport, storage and utilization and that help to reduce its toxic effects. About 10–15% of iron in the cell is in the labile form (free, chelatable, metabolically active iron pool), which is loosely associated with water, small organic molecules and proteins. Iron can be stored in the cell in a redox-inactive state in the form of ferritin, which is a multimeric complex consisting of up to 4500 atoms of crystalline ferric iron caged within 24 ferritin heavy and light chain polypeptides. In circulation, ferric iron is bound to a protein called transferrin (forming holo-transferrin) and enters target cells through the type 1 transferrin receptor (TfR1) present on the plasma membrane.
Perturbations of iron homeostasis can lead to deficiency or overload of the metal. The most common abnormality is iron deficiency, which usually results from insufficient dietary intake and manifests as anemia, i.e., a decrease in erythrocyte (red blood cell, RBC) production and hemoglobin content.3 Iron overload can be caused by inherited disorders affecting iron metabolism or RBC turnover, as well as by a number of non-hereditary problems such as viral hepatitis and alchohol-related liver disease.4,5 In these conditions, uncontrolled and excessive entry of iron into the plasma leads to levels higher than those required to sustain iron-dependent processes. As a result, the metal becomes deposited in the liver, heart, pancreas and other organs, ultimately resulting in pathological consequences such as cirrhosis, cardiomyopathy, arthropathy, diabetes and even cancer. A third type of dysregulated iron metabolism occurs secondary to chronic inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease (IBD), and leads to abnormal intracellular sequestration of iron and a decrease in circulating iron concentrations. This ultimately compromises erythropoiesis, producing the disorder known as the anemia of inflammation (AI, also referred to as the anemia of chronic disease). The focus of this article will be on the dysregulation of iron homeostasis that occurs in the context of inflammation, particularly IBD, and how it leads to AI. In order to appreciate the pathogenesis of this problem, it is necessary to understand how iron metabolism is normally regulated.
II. Normal iron metabolism
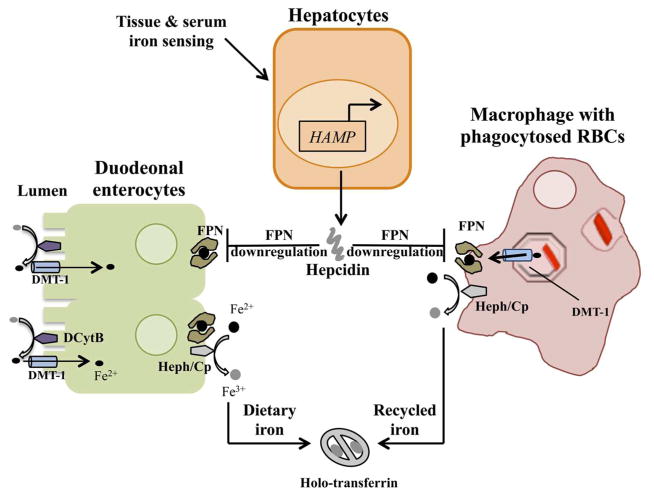
Iron metabolism involves precisely regulated processes of absorption, recycling, transport and cellular utilization (Figure 1).1,6 In adults, a limited portion of the daily iron requirement is obtained via dietary absorption, while most of it is met by recycling of iron from RBCs. During iron recycling, aged erythrocytes are phagocytosed by macrophages in the spleen, bone marrow and liver and degraded to release the heme prosthetic group from hemoglobin. The enzyme heme oxygenase-1 acts on heme to liberate iron (Fe2+), which is then transported into the cytosol via divalent metal transporter-1 (DMT-1, also known as Nramp2) present in the phagolysosomal membrane. The iron released into the cytosol is delivered to various cellular destinations, in part through the action of chaperones belonging to the poly-(rC)-binding protein (PCBP) family.7,8 A significant fraction of cytosolic iron is taken to the mitochondria, the site of synthesis of iron-sulfur clusters and heme. Transport into the mitochondrial inner compartment is mediated by mitoferrin-1, which is found abundantly in erythroid cells, and mitoferrin-2, which is expressed ubiquitously.9 Several lines of evidence suggest that there is a tight link between mitochondrial and cytosolic iron levels and that mitochondrial demand may regulate cytosolic iron metabolism, at least in part.10 Excess iron in the cytosol is diverted towards the cytosolic iron storage protein ferritin.11 Ferritin-associated iron can be released under different circumstances. Autophagic targeting of ferritin to the lysosome appears to be an important pathway for recovering stored iron, particularly when cytosolic iron concentrations fall, but proteasomal degradation of ferritin and direct exit of iron through pores in the ferritin nanocage may also occur.12–14 Iron for systemic distribution is exported out of macrophages via the plasma membrane transporter ferroportin (FPN, encoded by the solute carrier family 40 member 1/SLC40A1 gene), the only known iron export protein.15 Fe2+ iron exported via FPN is oxidised to Fe3+ by the ferroxidases hephaestin and ceruloplasmin and becomes associated with the plasma protein transferrin, which delivers iron to peripheral tissues.6 Cells take up transferrin-bound iron by receptor mediated endocytosis following binding of transferrin to the type 1 transferrin receptor (TfR1). Within endosomes, acidification and the action of membrane bound reductases that convert Fe3+ to Fe2+ result in the disassociation of iron from transferrin and transport via endosomal DMT-1 into the cytosol. Dietary iron is absorbed by enterocytes in the duodenum. Non-heme iron present in the Fe3+ form is converted to Fe2+ by the enzyme duodenal cytochrome B and is taken up by DMT-1 present on the apical plasma membrane. Depending on tissue iron status, the apically absorbed iron can be stored in the cytosol as ferritin or exported via FPN on the basolateral plasma membrane and transported in the plasma bound to transferrin.
Figure 1.
Regulation of systemic iron homeostasis. Hepcidin, which is encoded by the hepcidin anti-microbial peptide (HAMP) gene, is produced by the liver in response to iron status and requirements. It down-regulates ferroportin (FPN) on macrophages and duodenal enterocytes to control the amount of iron entering the circulation from recycled red blood cells (RBCs) and the diet, respectively. Dietary ferric iron is reduced by the action of duodenal cytochrome B (DCytB), imported by divalent metal transporter-1 (DMT-1) expressed on the apical enterocyte membrane, and then exported at the basolateral membrane by FPN. Ferrous iron is converted into the ferric form by hephaestin (Heph) or ceruloplasmin (Cp) and transported in the circulation bound to transferrin (holo-transferrin). Similar transport mechanisms operate in the macrophage to recycle iron from RBCs.
Systemic iron homeostasis is achieved by modulating the expression of FPN and thereby controlling the amount of iron entering the circulation from both the enterocytes that absorb dietary iron and the macrophages that recycle iron from RBCs.6 The key molecule involved in regulating FPN expression is hepcidin, a 25-amino acid hormone that is encoded by the hepcidin anti-microbial peptide (HAMP) gene and secreted by hepatocytes. Hepcidin binds to FPN and induces its internalization and lysosomal degradation, thus decreasing iron export.16 The expression of hepcidin itself is controlled by iron status: elevated tissue and plasma iron concentrations up-regulate the hormone while a decrease in these concentrations or an increase in iron demand inhibit hepcidin expression (see following section for further details). Thus, iron-dependent alterations in circulating hepcidin levels, together with hepcidin-dependent control of FPN-mediated iron efflux, constitute a negative feedback loop that acts as the main regulator of systemic iron homeostasis. This mechanism ensures that the amount of iron entering the circulation is modulated in accordance with iron status and requirements.
Cellular iron homeostasis is maintained by altering the expression of proteins involved in iron uptake, storage and export in response to cytosolic free iron concentrations.1,17 The mRNAs of these proteins contain a conserved 25–30 nucleotide hairpin structure known as the iron response element (IRE) in their 5’ or 3’ untranslated region. The IREs are bound by the iron regulatory proteins (IRPs) IRP1 and IRP2 when cytosolic iron concentration is low, leading to increased stability of the TfR1 and DMT-1 mRNAs (with a consequent increase in iron import) and decreased translation of the FPN and ferritin mRNAs (with a consequent decrease in iron export and storage).17
III. Iron-dependent regulation of hepcidin expression
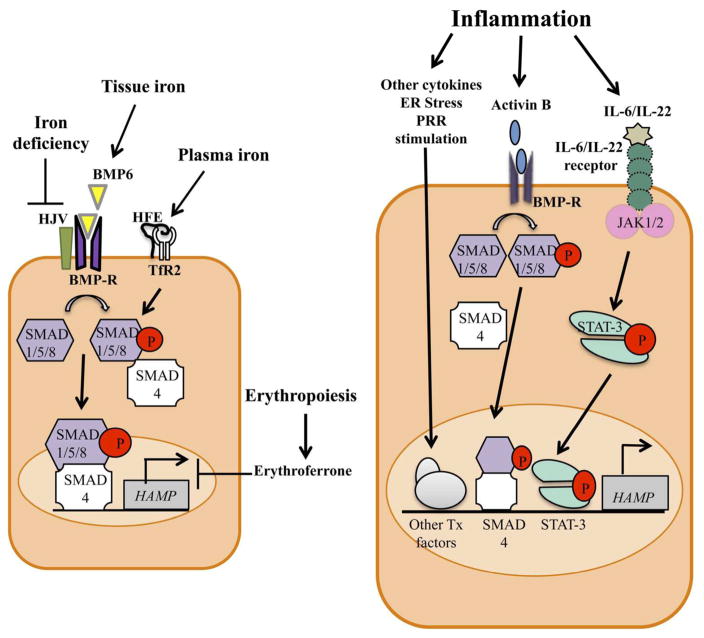
Hepcidin expression is regulated exclusively by transcriptional mechanisms (Figure 2). Bone morphogenetic protein 6 (BMP6) is the major mediator of hepcidin up-regulation in response to increased tissue iron concentrations. BMP6 expression in the liver increases when tissue iron concentrations rise and in turn induces hepcidin expression. Although it is not yet clear how tissue iron influences BMP6 levels, the homeostatic importance of this regulatory mechanism is highlighted by the observation that deficiency of BMP6 leads to progressive iron overload in mice.18,19 Recent studies indicate that conditional deletion of the BMP6 gene in liver sinusoidal endothelial cells mimics the iron overload phenotype of the global knockout, indicating that these cells are the major, physiologically relevant source of BMP6.20 BMP6 produced by the endothelial cells acts in a paracrine manner by binding to the BMP receptor and the co-receptor hemojuvelin (HJV) on adjacent hepatocytes. This interaction leads to the phosphorylation of the receptor-associated small-mothers against decapentaplegic (SMAD) 1/5/8 proteins, which then form a complex with SMAD4. This complex enters the nucleus and activates transcription of the hepcidin gene by binding to BMP-responsive elements in the promoter.21,22 Circulating iron (in the form of holo-transferrin) also influences hepcidin expression. The hemochromatosis protein HFE and the type 2 transferrin receptor (TfR2) expressed on the surface of hepatocytes are required for sensing iron-transferrin but the mechanistic details of this process are not clear. It may involve the formation of an HFE-TfR2 complex, although there is some evidence that the two proteins can act independently.23–27 The mechanisms that act downstream of HFE and TfR2 to up-regulate hepcidin transcription are also not well understood. Several studies indicate that HFE and TfR2 are required for the normal activation of BMP6-induced signals, specifically the phosphorylation of SMAD 1/5/8, that are involved in increasing hepcidin expression.26–29 Recent experiments suggest that TfR2, but not HFE, may have some role in the up-regulation of BMP6 by hepatocyte iron, and that TfR2 and HFE may interact with HJV to facilitate hepcidin induction.27,30 The importance of HFE, TfR2, HJV, as well as hepcidin and FPN, in iron homeostasis is well illustrated by the fact that mutations in genes encoding these proteins are associated with the development of hemochromatosis, a clinically significant iron overload syndrome in humans.4
Figure 2.
Regulation of hepcidin expression in hepatocytes in response to iron status and requirements (left) or inflammation (right). Tissue iron increases the expression of bone morphogenetic protein 6 (BMP6), which acts on the BMP receptor (BMP-R) and co-receptor hemojuvelin (HJV). This interaction leads to the phosphorylation of the receptor-associated small-mothers against decapentaplegic (SMAD) 1/5/8 proteins, which then bind to SMAD4, translocate to the nucleus and up-regulate transcription of the HAMP gene to produce hepcidin. Plasma iron is sensed by the hemochromatosis protein HFE and the type 2 transferrin receptor (TfR2), leading to modulation of BMP/SMAD signals. Iron deficiency inhibits BMP/SMAD signals by inducing degradation of HJV, while erythropoiesis inhibits hepcidin expression through the action of erythroferrone. In inflammatory states, cytokines such as IL-6 and IL-22 act on their respective receptors to activate the Janus kinases (JAK)1/2, leading to phosphorylation and dimerization of signal transducer and activator of transcription 3 (STAT3). Dimeric STAT3 translocates to the nucleus to up-regulate HAMP transcription and increase hepcidin production. Activin B produced during inflammation increases HAMP transcription by activating the BMP/SMAD pathway. Additional inflammatory mediators, including other pro-inflammatory cytokines such as IL-1β, endoplasmic reticulum (ER) stress and agonists of innate pattern recognition receptors (PRRs), induce signals that activate other transcription (Tx) factors and up-regulate HAMP transcription.
In addition to the up-regulation of hepcidin that occurs when tissue and plasma iron levels rise, mechanisms to inhibit hepcidin expression go into effect in states of iron deficiency or increased iron demand. Suppression of hepcidin by low iron levels involves a transmembrane serine protease known as matriptase-2 (encoded by the TMPRSS6 gene) that cleaves HJV and thus attenuates signaling throught the BMP/SMAD pathway.31–33 This effect appears to be mediated by post-translational stabilization of matriptase-2 by low intracellular iron.34 Mice and humans with inactivating mutations in matriptase-2 suffer from a microcytic anemia that is refractory to oral iron therapy and that is associated with abnormally elevated hepcidin levels.31,32,35 Hepcidin expression has to be suppressed in order to meet the increased demand for iron during the state of augmented erythropoiesis that occurs in conditions such as hypoxia, hemolytic anemia and recovery from iron deficiency.36 Hepcidin inhibition under these circumstances involves the hormone erythroferrone, which is produced by RBC precursors in response to erythropoietin.37 The effect of erythroferrone on hepcidin expression has been shown recently to require matriptase-2, presumably in order to suppress BMP/SMAD signaling.38
IV. Iron metabolism and anemia in IBD
IBD is a chronic, relapsing and remitting inflammatory disorder of the gastrointestinal tract. Its exact pathogenesis is not clear but it is believed to be the result of abnormal, microbiota-driven immune responses that develop in genetically predisposed individuals.39 The clinical manifestations range from bloody diarrhea and weight loss to perforation and obstruction of the gastrointestinal tract. IBD affects millions of people worldwide, with rising incidences, especially in urban areas.40 Two major forms of IBD – ulcerative colitis (UC) and Crohn’s disease (CD) – exhibit overlapping symptoms and pathologies. UC involves inflammation that is limited to the colon while CD affects the entire gastrointestinal tract. Many chronic inflammatory diseases, including IBD, are associated with disturbances in iron homeostasis that lead to a significant decrease in circulating iron concentrations.41 The abnormality can be explained to a great extent by the fact that hepcidin expression, in addition to being influenced by iron status and demands, can also be markedly up-regulated by inflammatory signals (Figure 2). The increase in hepcidin levels leads to FPN degradation, with a consequent decrease in iron efflux and a fall in plasma iron concentrations. This response may have evolved as a mechanism to protect against infection since the hypoferremia deprives extracellular pathogens of an essential nutrient.42 Indeed, hepcidin expression is elevated in a number of human infectious diseases,43–45 and hepcidin-induced hypoferremia has been shown to have clear anti-microbial effects in a mouse model of Vibrio vulnificus infection.46 Decreased circulating iron may also have some benefits in terms of reducing the production of tissue-damaging reactive oxygen species. However, persistent hypoferremia will inevitably impair RBC production and lead to anemia. The anemia that develops under these circumstances (AI) is generally mild to moderate, normochromic and normocytic in nature and characterized by normal or elevated serum ferritin concentrations, features that help to distinguish it from the anemia of iron deficiency. AI usually resolves following successful treatment of the underlying inflammation but sometimes it can be significant enough to impair quality of life and may require specific intervention beyond anti-inflammatory therapy.
Anemia is seen in 25–60% of patients with IBD depending on the clinical setting. It is multifactorial in origin, with chronic intestinal bleeding, dietary deficiency of iron and vitamins, inflammation-induced disturbances of iron homeostasis, and medication-associated side effects potentially contributing to its pathogenesis. The most common type of anemia in IBD is a true iron deficiency anemia resulting from intestinal blood loss and inadequate intake of iron. A recent review of European studies of adults with CD or UC reported that 57% of the patients with anemia were iron deficient, while an earlier analysis found that iron deficiency was observed in 36–90% of all patients with CD.47,48 AI is also seen fairly frequently in IBD. It occurs as the sole form of anemia in about 15% of anemic IBD patients, and in combination with iron deficiency anemia in 35–40% of such individuals.49,50 Several studies have examined serum or urine hepcidin concentrations in patients with IBD in order to shed light on the pathogenesis of the associated anemia.51–56 The results generally demonstrate that hepcidin levels are elevated in the patients relative to healthy controls and that they correlate with disease activity and serum ferritin concentrations. The findings are consistent with the idea that increased hepcidin expression is an important factor contributing to iron dysregulation in IBD and to the pathogenesis of AI in this condition. Not surprisingly, there has been a great deal of interest in trying to elucidate the mechanisms that lead to increased hepcidin expression in IBD so that rational interventions to prevent or treat AI can be developed.
V. Inflammation-associated dysregulation of hepcidin expression
An important factor in inflammation-induced hepcidin up-regulation is the cytokine interleukin-6 (IL-6).57–59 IL-6 acts on its receptor expressed on hepatocytes, leading to activation of Janus kinase (JAK) 1/2 and consequent phosphorylation of signal transducer and activator of transcription 3 (STAT3). Phosphorylated STAT3 dimerizes and translocates into the nucleus to transcriptionally activate the hepcidin gene by binding to a specific site in its promoter. Mice deficient in either IL-6 or hepatocyte STAT3 fail to up-regulate hepcidin in response to the injection of stimuli such as lipopolysaccharide (LPS) or turpentine, pointing to the importance of this pathway in inflammation-induced expression of hepcidin.60–62 There is also some evidence to suggest that this pathway is involved in hepcidin up-regulation in human IBD based on correlations between serum hepcidin and IL-6 levels.51,53
Other cytokines that have been implicated in the increased expression of hepcidin associated with inflammation include IL-1β and IL-22. IL-1β produced by macrophages plays an important role in the pathogenesis of intestinal inflammation in at least some forms of human IBD, as well as in mouse models of the disease, and has been shown to up-regulate hepcidin in vitro and in vivo.63–68 Some of this effect could be mediated by IL-1β-induced IL-6 production but IL-6-independent mechanisms are also probably involved. Recent work from our laboratory suggests that IL-1β up-regulates hepcidin by inducing the secretion of members of the transforming growth factor β (TGFβ)/BMP family, BMP2 or activin B, which then act in an autocrine or paracrine fashion to activate the SMAD signaling pathway.68 Activin B is up-regulated in the liver in response to a number of inflammatory stimuli and can increase hepatocyte hepcidin expression in tissue culture and following injection into mice, but recent results indicate that it is not required in vivo for inflammation-induced hepcidin up-regulation.69–72 The exact contribution of IL-1β to increased expression of hepcidin in IBD remains to be determined. The use of antagonists of this cytokine in the clinical treatment of IBD should now make this issue amenable to investigation.64 The role of IL-22 in IBD-associated hepcidin up-regulation is similarly awaiting clarification. IL-22, which activates STAT3, is able to up-regulate hepcidin expression in hepatocytes in vitro and in vivo, but disruption of the IL-22 gene has only a minor effect on the murine hepcidin response to LPS.73–75
Not all inflammatory cytokines induce the increased expression of hepcidin. TNFα, a key mediator of pathology in IBD, has been shown to inhibit hepcidin expression when added to a hepatocyte cell line.61 Moreover, experiments conducted by our group have shown that hepcidin expression is down-regulated in two different mouse models of IBD, and that TNFα is involved in this inhibition.76 Interestingly, treatment of rheumatoid arthritis patients with TNFα inhibitors resulted in a decrease in serum hepcidin levels, possibly via modulation of IL-6.77 It is not clear why TNFα appears to have different effects on hepcidin expression in human rheumatoid arthritis and mouse colitis. The relevance of these findings to human IBD also needs to be clarified, although it is worth noting that anti-TNFα therapy has been shown to improve anemia and increase circulating erythropoietin concentrations in a small number of IBD patients.49
Endoplasmic reticulum (ER) stress is another factor that could influence hepcidin expression in the context of intestinal inflammation. ER stress occurs in intestinal epithelial cells in the inflamed gut and may also affect hepatocytes since liver injury can occur from a variety of causes related to IBD.78,79 The induction of ER stress, either in cultured hepatocyte cell lines or in vivo in mice, leads to increased expression of hepcidin.80–82 This effect involves activation of transcription factors such as C/EBPα and members of the cyclic AMP response element-binding (CREB) family. Interestingly, in vivo experiments have shown that the SMAD signaling pathway is required for ER stress-induced hepcidin up-regulation.82 It is not yet known whether ER stress contributes to increased hepcidin expression in human IBD.
The composition of the gastrointestinal microbiota can also influence hepcidin expression during colitis. Using the dextran sulfate sodium (DSS) induced-colitis mouse model of IBD, we showed recently that wild-type C57BL/6 mice exhibited a significant down-regulation of hepcidin expression following the induction of colitis, whereas IL-10-deficient mice on the same genetic background demonstrated significant up-regulation of hepcidin following colitis induction.83 Interestingly, if the two types of mice were co-housed prior to the induction of colitis, hepcidin expression in the IL-10 knockout mice was suppressed. A similar suppression was observed if the IL-10-deficient animals were transplanted with fecal material from the wild-type mice. These results indicate that IL-10 itself does not directly influence hepcidin expression in the context of colitis. Rather, they suggest that differences in gut microbiota composition between the wild-type and IL-10-deficient mice have a strong effect on inflammation-induced hepcidin expression. The mechanism by which microbiota composition influences hepcidin expression is not yet clear. It could involve changes in the inflammatory cytokine milieu (although we did not observe obvious differences in colonic cytokine levels between the wild-type and IL-10 knockouts in our experiments), or more direct effects of commensal microbes or their products on host pattern recognition receptors (PRRs) and other sensors.73 Clarifying this issue could provide the foundation for microbiota-based strategies to inhibit inflammation-induced hepcidin up-regulation.
It should be remembered that the signals that regulate hepcidin expression in response to iron status and requirements continue to operate in inflammatory states. Accordingly, the net level of expression will reflect the integration of multiple, sometimes opposing, inputs, with the relative strength of the inputs determining how much hepcidin is produced.84 Thus, inflammation occurring in the background of iron deficiency, for instance, will result in lower hepcidin expression than if it were to occur in an iron replete condition. Erythropoietic drive can also attenuate inflammation-induced hepcidin up-regulation as a result of increased production of erythroferrone.37,83
VI. Other factors contributing to abnormal iron metabolism during inflammation
Although it is generally agreed that increased expression of hepcidin is the major factor in the abnormal iron metabolism associated with inflammation, inflammatory mediators can also affect iron homeostasis by mechanisms that do not involve hepcidin. TNFα has been shown in tissue culture experiments to act directly on intestinal epithelial cells to inhibit iron transport by decreasing DMT-1 expression or membrane localization of FPN.85,86 Consistent with these observations, injection of TNFα into mice leads to decreased duodenal uptake of iron and reduced serum iron concentrations without significant changes in liver hepcidin expression.87 Interestingly, recent experiments have demonstrated decreased levels of DMT-1 mRNA and protein in the inflamed ileum and colon of IBD patients, possibly related to a direct effect of TNFα on the intestinal epithelium.88 Whether this abnormality extends to the duodenum and affects iron absorption is not clear. It has also been shown that treatment of human monocyte cell lines with a combination of LPS and the pro-inflammatory cytokine interferon γ results in intracellular sequestration of iron in association with decreases in the levels of FPN mRNA and protein.89 The contribution of this mechanism to inflammation-associated hypoferremia in vivo is not clear. However, it may be relevant that additional studies have shown that injection of wild-type mice with ligands for the PRRs Toll-like receptor (TLR) 2 and TLR6 leads to marked decreases in macrophage FPN mRNA and protein, as well as a reduction in serum iron concentrations, without changes in hepcidin expression.90 Hepcidin-independent mechanisms involved in inflammation-induced hypoferremia have been demonstrated recently using hepcidin knock-out and knock-in mice. Administration of LPS to hepcidin-deficient animals produced a rapid and dramatic reduction of FPN mRNA and protein levels in the duodenum, along with decreases in DMT-1 and duodenal cytochrome B mRNAs in this tissue.91 The associated hypoferremia in the hepcidin-deficient mice was significantly attenuated relative to the wild-type animals but still appreciable. In keeping with these observations, injection of TLR2/TLR6 ligands into knock-in mice expressing a hepcidin-insensitive mutant of FPN resulted in reduced FPN mRNA and protein levels in the liver and spleen and hypoferremia.90 Taken together, these results indicate that there are multiple hepcidin-independent mechanisms that can contribute to the altered iron homeostasis associated with inflammation.
Inflammation-associated hypoferremia compromises erythropoiesis by restricting the amount of iron available for hemoglobin synthesis. In addition, inflammation can directly inhibit RBC generation. In a mouse model of turpentine-induced chronic inflammation, one of the mechanisms contributing to the development of anemia is a hepcidin-independent suppression of intramedullary erythropoiesis.92 Consistent with this observation, a number of inflammatory cytokines, including TNFα and IL-1γ, have been shown to inhibit the proliferation and differentiation of erythroid precursors by reducing production of erythropoietin and/or decreasing sensitivity to this hormone.93 There are very few studies of erythropoiesis in human IBD, although one investigation indicated that erythropoietin levels are inappropriately elevated in children with the disease, possibly because of a failure of the normal bone marrow erythropoietic response.94 Increased RBC destruction may also be involved in the anemia associated with some inflammatory conditions. Injection of heat-killed Brucella abortus into mice produces a marked anemia in conjunction with hypoferremia, reduced erythropoiesis and shortened RBC lifespan.95 These observations may be more relevant to the anemia of critical illness, a poorly understood, moderately severe anemia seen in patients admitted to intensive care units,96 rather than to AI since the latter is milder and not typically associated with hemolysis.
VII. Management of anemia in IBD
Treatment of the underlying inflammatory disorder will usually lead to the resolution of the associated anemia. However, in some cases, the anemia can be significant enough to require specific intervention. The majority of patients with IBD-associated anemia have true iron deficiency and will benefit from iron supplementation.97 Oral iron therapy is successful in replenishing iron stores and increasing hemoglobin in most of these individuals. Oral iron may have unpleasant gastrointestinal side effects, including the potential for exacerbating the intestinal inflammation, and it may be poorly absorbed during acute flares of IBD. It may also cause significant alterations in the gut microbiota, which could have adverse consequences.98 Intravenous iron, available in different formulations that vary in cost, stability, ease of administration and side effects, can be used in individuals who do not respond to or cannot tolerate oral supplementation and is generally safe and effective.97 Iron deficiency in IBD may recur following initially successful oral or intravenous supplementation therapy, so it is important to monitor the patient to determine if additional treatment is required.
Determining the contribution of iron deficiency to the anemia of IBD can be difficult in some circumstances. Typically, iron deficiency anemia is hypochromic and microcytic and is associated with low serum ferritin concentrations. However, ferritin levels may be increased by inflammation, making interpretation of this parameter complicated in the context of an inflammatory state. More recent measurements such as the soluble transferrin receptor to log ferritin ratio or plasma hepcidin concentration may be helpful in distinguishing iron deficiency anemia from AI but may not be definitive.99,100
In the minority of individuals with IBD-associated anemia who have AI, the rational approach to treatment is to inhibit the expression or function of hepcidin. Several types of reagents have been developed for this purpose and are in various stages of pre-clinical or clinical evaluation.101,102 One approach to inhibiting hepcidin expression is to block activation of the BMP signaling pathway. Soluble forms of the BMP co-receptor HJV have been shown to correct hypoferremia and ameliorate anemia in rodent models of AI, including in a mouse model of IBD.103,104 Another molecule that has been shown to reduce hepcidin expression is heparin, a glycosaminoglycan that is able to bind and sequester BMP6.105 The use of heparin is complicated, of course, by its anticoagulant properties. Therefore, modified forms of heparin have been developed that show reduced anticoagulant activity (glycol-split heparin) and have been successful in alleviating AI in a mouse model.106 Inhibition of the BMP co-receptor HJV may be another promising approach to blocking BMP signals leading to hepcidin up-regulation. Monoclonal antibodies targeting HJV reduce hepcidin levels and increase serum iron concentrations when administered to rats or monkeys.107 Pharmacological strategies for inhibiting BMP signaling have also been developed. LDN-193189 is a derivative of dorsomorphin, a small molecule inhibitor of SMAD activation by BMP receptors. It has been demonstrated to inhibit hepcidin expression and reduce hypoferremia in a rat model of AI and in a mouse IBD model.103,104 In addition to inhibiting the expression of hepcidin, blocking its ability to bind to FPN is another strategy for dealing with AI. Reagents that are being developed and evaluated for this purpose include antibodies against hepcidin or FPN, engineered proteins (anticalins) designed to bind hepcidin, and hepcidin-binding structured oligoribonucleotides (spiegelmers).102,103 A spiegelmer with high-affinity binding to hepcidin has been shown to prevent FPN degradation and hypoferremia in a non-human primate model of inflammation.108
Stimulating erythropoiesis with recombinant human erythropoietin has been used in IBD patients whose anemia fails to respond to intravenous iron, although the number of individuals requiring such therapy is small.109,110 When indicated, erythropoietin is administered in combination with intravenous iron and has resulted in significant improvement in RBC parameters.110 Erythropoietin may also be beneficial in AI since it has the ability to lower hepcidin expression secondary to the effects of erythroferrone produced by RBC precursors.37 The value of erythropoietin as treatment for AI in the absence of co-existing iron deficiency has not been specifically evaluated in IBD.
VIII. Conclusion
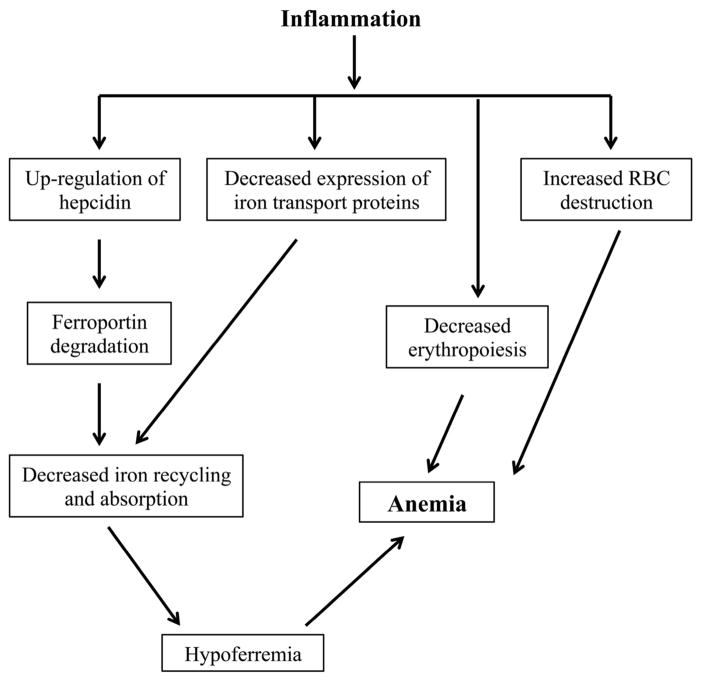
The anemia associated with inflammation is a complex and multifactorial clinical abnormality (Figure 3). Although we now have a better understanding of its general pathogenesis, the mechanisms that contribute to its development in specific inflammatory states such as IBD require further clarification. In this regard, some of the questions that await answers include the following.
Figure 3.
Mechanisms involved in the pathogenesis of the anemia associated with inflammation. The figure shows the various factors that contribute to the development of anemia in the context of inflammation.
Which biomarkers or laboratory parameters are most useful in distinguishing AI from iron deficiency anemia in IBD?
Which of the many inflammatory cytokines produced in IBD are most critical for the up-regulation of hepcidin? Do they act cooperatively or redundantly?
What role do hepcidin-independent mechanisms play in the pathogenesis of the anemia associated with IBD?
How does gut microbiota composition affect hepcidin expression and iron homeostasis in IBD?
What is the optimal treatment for AI in IBD, particularly in regard to the emerging classes of hepcidin inhibiting and blocking reagents? Can manipulation of the microbiota with prebiotics or probiotics be used to prevent or treat AI in IBD?
These issues represent important areas of future research that could yield information relevant to both basic biology and the care of patients with IBD.
Acknowledgments
Work in the authors’ laboratory is supported by United States National Institutes of Health grant R01AI089700.
References
- 1.Silva B, Faustino P. An overview of molecular basis of iron metabolism regulation and associated pathologies. Biochim Biophys Acta - Mol Basis Dis. 2015;1852:1347–1359. doi: 10.1016/j.bbadis.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Soares MP, Hamza I. Macrophages and iron metabolism. Immunity. 2016;44:492–504. doi: 10.1016/j.immuni.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 4.Powell LW, Seckington RC, Deugnier Y. Haemochromatosis. Lancet. 2016;388:706–716. doi: 10.1016/S0140-6736(15)01315-X. [DOI] [PubMed] [Google Scholar]
- 5.Makker J, Hanif A, Bajantri B, Chilimuri S. Dysmetabolic hyperferritinemia: all iron overload is not hemochromatosis. Case Rep Gastroenterol. 2015;9:7–14. doi: 10.1159/000373883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 7.Philpott CC. Coming into view: eukaryotic iron chaperones and intracellular iron delivery. J Biol Chem. 2012;287:13518–13523. doi: 10.1074/jbc.R111.326876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philpott CC, Ryu MS. Special delivery: distributing iron in the cytosol of mammalian cells. Front Pharmacol. 2014;5:173. doi: 10.3389/fphar.2014.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhlenhoff U, Hoffmann B, Richter N, Rietzschel N, Spantgar F, Stehling O, Uzarska MA, Lill R. Compartmentalization of iron between mitochondria and the cytosol and its regulation. Eur J Cell Biol. 2015;94:292–308. doi: 10.1016/j.ejcb.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Richardson DR, Lane DJR, Becker EM, Huang ML-H, Whitnall M, Suryo Rahmanto Y, Sheftel AD, Ponka P. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci U S A. 2010;107:10775–10782. doi: 10.1073/pnas.0912925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theil EC. Ferritin: the protein nanocage and iron biomineral in health and disease. Inorg Chem. 2013;52:12223–12233. doi: 10.1021/ic400484n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linder MC. Mobilization of stored iron in mammals: a review. Nutrients. 2013;5:4022–4050. doi: 10.3390/nu5104022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, Menon S, Wang Z, Honda A, Pardee G, Cantwell J, Luu C, Cornella-Taracido I, Harrington E, Fekkes P, Lei H, Fang Q, Digan ME, Burdick D, Powers AF, Helliwell SB, D'Aquin S, Bastien J, Wang H, Wiederschain D, Kuerth J, Bergman P, Schwalb D, Thomas J, Ugwonali S, Harbinski F, Tallarico J, Wilson CJ, Myver VE, Porter JA, Bussiere DE, Finan PM, Labow MA, Mao X, Hamann LG, Manning BD, Valdez RA, Nicolson T, Schirle M, Knapp MS, Keaney EP, Murphy LO. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16:1069–1079. doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- 15.Drakesmith H, Nemeth E, Ganz T. Ironing out ferroportin. Cell Metab. 2015;22:777–787. doi: 10.1016/j.cmet.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 17.Zhang DL, Ghosh MC, Rouault TA. The physiological functions of iron regulatory proteins in iron homeostasis - an update. Front Pharmacol. 2014;5:124. doi: 10.3389/fphar.2014.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andriopoulos B, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. Nat Genet. 2009;41:482–7. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth M-P. Nat Genet. 2009;41:478–81. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 20.Canali S, Zumbrennen-Bullough KB, Core AB, Wang CY, Nairz M, Bouley R, Swirski FK, Babitt JL. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood. 2016 Nov 18; doi: 10.1182/blood-2016-06-721571. pii: blood-2016-06-721571, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parrow NL, Fleming RE. Bone morphogenetic proteins as regulators of iron metabolism. Annu Rev Nutr. 2014;34:77–94. doi: 10.1146/annurev-nutr-071813-105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Core AB, Canali S, Babitt JL. Hemojuvelin and bone morphogenetic protein signaling in iron homeostasis. Front Pharmacol. 2014;5:104. doi: 10.3389/fphar.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goswami T, Andrews NC. Hereditary hemochromatosis protein HFE interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem. 2006;281:28494–28498. doi: 10.1074/jbc.C600197200. [DOI] [PubMed] [Google Scholar]
- 24.Gao JW, Chen JX, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9:217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos E, Kautz L, Rodriguez R, Hansen M, Gabayan V, Ginzburg Y, Roth MP, Nemeth E, Ganz T. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. 2011;53:1333–1341. doi: 10.1002/hep.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corradini E, Rozier M, Meynard D, Odhiambo A, Lin HY, Feng Q, Migas MC, Britton RS, Babitt JL, Fleming RE. Iron regulation of hepcidin despite attenuated Smad 1,5,8 signaling in mice without transferrin receptor 2 or Hfe. Gastroenterology. 2011;141:1907–1914. doi: 10.1053/j.gastro.2011.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald CJ, Wallace DF, Ostini L, Subramaniam VN. Parenteral vs oral iron: influence on hepcidin signaling pathways through analysis of Hfe/Tfr2-null mice. Am J Physiol Gastrointest Liver Physiol. 2014;306:G132–G139. doi: 10.1152/ajpgi.00256.2013. [DOI] [PubMed] [Google Scholar]
- 28.Corradini E, Garuti C, Montosi G, Ventura P, Andriopoulos B, Jr, Lin HY, Pietrangelo A, Babitt JL. Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis. Gastroenterology. 2009;137:1489–1497. doi: 10.1053/j.gastro.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolondi G, Garuti C, Corradini E, Zoller H, Vogel W, Finkenstedt A, Babitt JL, Lin HY, Pietrangelo A. Altered hepatic BMP signaling pathway in human HFE hemochromatosis. Blood Cells Mol Dis. 2010;45:308–312. doi: 10.1016/j.bcmd.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Alessio F, Hentze MW, Muckenthaler MU. The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. J Hepatol. 2012;57:1052–60. doi: 10.1016/j.jhep.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, Khovananth K, Mudd S, Mann N, Moresco EM, Beutler E, Beutler B. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folgueras AR, de Lara FM, Pendas AM, Garabaya C, Rodriguez F, Astudillo A, Bernal T, Cabanillas R, Lopez-Otin C, Velasco G. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112:2539–2545. doi: 10.1182/blood-2008-04-149773. [DOI] [PubMed] [Google Scholar]
- 33.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao N, Nizzi CP, Anderson SA, Wang J, Ueno A, Tsukamoto H, Eisenstein RS, Enns CA, Zhang AS. Low intracellular iron increases the stability of matriptase-2. J Biol Chem. 2015;290:4432–4446. doi: 10.1074/jbc.M114.611913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heeney MM, Finberg KE. Iron-refractory iron deficiency anemia (IRIDA) Hematol Oncol Clin North Am. 2014;28:637–652. doi: 10.1016/j.hoc.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Parischa SR, McHugh K, Drakesmith H. Regulation of hepcidin by erythropoiesis: the story so far. Annu Rev Nutr. 2016;36:417–434. doi: 10.1146/annurev-nutr-071715-050731. [DOI] [PubMed] [Google Scholar]
- 37.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nai A, Rubio A, Campanella A, Gourbeyre O, Artuso I, Bordini J, Gineste A, Latour C, Besson-Fournier C, Lin HY, Coppin H, Roth MP, Camaschella C, Silvestri L, Meynard D. Limiting BMP-SMAD signaling by matriptase-2 is required for erythropoietin-mediated hepcidin suppression in mice. Blood. 2016;127:2327–2336. doi: 10.1182/blood-2015-11-681494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wlodarska M, Kostic AD, Xavier RJ. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host Microbe. 2015;17:577–591. doi: 10.1016/j.chom.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 41.Cherayil BJ. Pathophysiology of iron homeostasis during inflammatory states. J Pediatr. 2015;167:S15–S19. doi: 10.1016/j.jpeds.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michels K, Nemeth E, Ganz T, Mehrad B. Hepcidin and host defense against infectious diseases. PLoS Pathog. 2015;11:e1004998. doi: 10.1371/journal.ppat.1004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armitage AE, Stacey AR, Giannoulatou E, Marshall E, Sturges P, Chatha K, Smith NM, Huang X, Xu X, Parischa SR, Li N, Wu H, Webster C, Prentice AM, Pellegrino P, Williams I, Norris PJ, Drakesmith H, Borrow P. Distinct patterns of hepcidin and iron regulation during HIV-1, HBV and HCV infections. Proc Natl Acad Sci USA. 2014;111:12187–12192. doi: 10.1073/pnas.1402351111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atkinson SH, Uyoga SM, Armitage AE, Khandwala S, Mugyenyi CK, Bejon P, Marsh K, Beeson JG, Prentice AM, Drakesmith H, Williams TN. Malaria and age variably but critically control hepcidin throughout childhood in Kenya. EBioMedicine. 2015;2:1478–1486. doi: 10.1016/j.ebiom.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darton TC, Blohmke CJ, Giannoulatou E, Waddington CS, Jones C, Sturges P, Webster C, Drakesmith H, Pollard AJ, Armitage AE. Rapidly escalating hepcidin and associated serum iron starvation are features of the acute response to typhoid infection in humans. PLoS Negl Trop Dis. 2015;9:e0004029. doi: 10.1371/journal.pntd.0004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arezes J, Jung G, Gabayan V, Valore E, Ruchala P, Gulig PA, Ganz T, Nemeth E, Bulut Y. Hepcidin-induced hypoferremia is a critical host defense mechanism against siderophilic bacterium Vibrio vulnificus. Cell Host Microbe. 2015;17:47–57. doi: 10.1016/j.chom.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filmann N, Rey J, Schneeweiss S, Ardizzone S, Bager P, Bergamaschi G, Koutroubakis I, Lindgren S, de Morena FL, Moum B, Vavricka SR, Schroder O, Herrmann E, Blumenstein I. Prevalence of anemia in inflammatory bowel diseases in European countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20:936–945. doi: 10.1097/01.MIB.0000442728.74340.fd. [DOI] [PubMed] [Google Scholar]
- 48.Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn's disease. Aliment Pharmacol Ther. 2006;24:1507–1523. doi: 10.1111/j.1365-2036.2006.03146.x. [DOI] [PubMed] [Google Scholar]
- 49.Bergamaschi G, Di Sabatino A, Albertini R, Ardizzone S, Biancheri P, Bonetti E, Cassinotti A, Cazzola P, Markopoulos K, Massari A, Rosti V, Porro GB, Corazza GR. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment. Haematologica. 2010;95:199–205. doi: 10.3324/haematol.2009.009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koutroubakis IE, Ramos-Rivers C, Regueiro M, Koutroumpakis E, Click B, Schwartz M, Swoger J, Baidoo L, Hashash JG, Barrie A, Dunn MA, Binion DG. Five-year period prevalence and characteristics of anemia in a large US inflammatory bowel disease cohort. J Clin Gastroenterol. 2016;50:638–643. doi: 10.1097/MCG.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semrin G, Fishman DS, Bousvaros A, Zholudev A, Saunders AC, Correia CE, Nemeth E, Grand RJ, Weinstein DA. Impaired intestinal absorption in Crohn's disease correlates with disease activity and markers of inflammation. Inflamm Bowel Dis. 2006;12:1101–1106. doi: 10.1097/01.mib.0000235097.86360.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oustamanolakis P, Koutroubakis IE, Messaritakis I, Malliaraki N, Sfiridaki A, Kouroumalis EA. Serum hepcidin and prohepcidin concentrations in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:262–268. doi: 10.1097/MEG.0b013e328343b885. [DOI] [PubMed] [Google Scholar]
- 53.Basseri RJ, Nemeth E, Vassilaki ME, Basseri B, Enayati P, Shaye O, Bourikas LA, Ganz T, Papadakis KA. Hepcidin is a key mediator of anemia of inflammation in Crohn's disease. J Crohns Colitis. 2013;7:286–291. doi: 10.1016/j.crohns.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Bergamaschi G, Di Sabatino A, Albertini R, Costanzo F, Guerci M, Masotti M, Pasini A, Massari A, Campostrini N, Corbella M, Girelli D, Corazza GR. Serum hepcidin in inflammatory bowel diseases: biological and clinical significance. Inflamm Bowel Dis. 2013;19:2166–2172. doi: 10.1097/MIB.0b013e31829a6e43. [DOI] [PubMed] [Google Scholar]
- 55.Mecklenburg I, Reznik D, Fasler-Kan E, Drewe J, Beglinger C, Hruz P Swiss IBD Cohort Study Group. Serum hepcidin concentrations correlate with ferritin in patients with inflammatory bowel disease. J Crohns Colitis. 2014;8:1392–1397. doi: 10.1016/j.crohns.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Martinelli M, Strisciuglio C, Alessandrella A, Rossi F, Auricchio R, Campostrini N, Girelli D, Nobili B, Staiano A, Perrotta S, Miele E. Serum hepcidin and iron absorption in pediatric inflammatory bowel disease. J Crohns Colitis. 2016;10:566–574. doi: 10.1093/ecco-jcc/jjv242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 59.Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C, Trautwein C. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 60.Lee P, Peng H, Gelbart T, Beutler E. The IL-6- and lipopolysaccharide-induced transcription of hepcidin in HFE-, transferrin receptor 2- and beta 2-microglobulin-deficient hepatocytes. Proc Natl Acad Sci USA. 2004;101:9263–9265. doi: 10.1073/pnas.0403108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakamori R, Takehara T, Tatsumi T, Shigekawa M, Hikita H, Hiramatsu N, Kanto T, Hayashi N. STAT3 signaling within hepatocytes is required for anemia of inflammation in vivo. J Gastroenterol. 2010;45:244–248. doi: 10.1007/s00535-009-0159-y. [DOI] [PubMed] [Google Scholar]
- 63.Opipari A, Franchi L. Role of inflammasomes in intestinal inflammation and Crohn's disease. Inflamm Bowel Dis. 2015;21:173–181. doi: 10.1097/MIB.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 64.Shouval DS, Biswas A, Kang YH, Griffith AE, Konnikova L, Mascanfroni ID, Redhu NS, Frei SM, Field M, Doty AL, Goldsmith JD, Bhan AK, Loizides A, Weiss B, Yerushalmi B, Yanagi T, Lui X, Quintana FJ, Muise AM, Klein C, Horwitz BH, Glover SC, Bousvaros A, Snapper SB. Interleukin-1β mediates intestinal inflammation in mice and patients with interleukin-10 receptor deficiency. Gastroenterology. 2016;151:1100–1104. doi: 10.1053/j.gastro.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci USA. 2005;102:1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kramer F, Torzewski J, Kamenz J, Veit K, Hornbach V, Dedio J, Ivashchenko Y. Interleukin-1β stimulates acute phase response and C-reactive protein synthesis by inducing an NF-κB and C/EBPβ-dependent autocrine interleukin-6 loop. Mol Immunol. 2008;45:2678–2689. doi: 10.1016/j.molimm.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 67.Matak P, Chaston TB, Chung B, Srai SK, McKie AT, Sharp PA. Activated macrophages induce hepcidin expression in HuH7 hepatoma cells. Haematologica. 2009;94:773–780. doi: 10.3324/haematol.2008.003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shanmugam NKN, Chen K, Cherayil BJ. Commensal bacteria-induced IL-1β secreted by macrophages up-regulates hepcidin expression in hepatocytes by activating the bone morphogenetic protein signaling pathway. J Biol Chem. 2015;290:30637–30647. doi: 10.1074/jbc.M115.689190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Besson-Fournier C, Latour C, Kautz L, Bertrand J, Ganz T, Roth MP, Coppin H. Induction of activin B by inflammatory stimuli up-regulates expression of the iron-regulatory petptide hepcidin through SMAD 1/5/8 signaling. Blood. 2012;120:431–439. doi: 10.1182/blood-2012-02-411470. [DOI] [PubMed] [Google Scholar]
- 70.Canali S, Core AB, Zumbrennen-Bullough KB, Merkulova M, Wang CY, Schneyer AL, Pietrangelo A, Babitt JL. Activin B induces noncanonical SMAD 1/5/8 signaling via BMP type 1 receptors in hepatocytes: evidence for a role in hepcidin induction by inflammation in male mice. Endocrinology. 2016;157:1146–1162. doi: 10.1210/en.2015-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanamori Y, Sugiyama M, Hashimoto O, Murakami M, Matsui T, Funaba M. Regulation of hepcidin expression by inflammation-induced activin B. Sci Rep. 2016 Dec 6;6:38702. doi: 10.1038/srep38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Besson-Fournier C, Gineste A, Latour C, Gourbeyre O, Meynard D, Martin P, Oswald E, Coppin H, Roth MP. Hepcidin upregulation by inflammation is independent of SMAD 1/5/8 signaling by activin B. Blood. 2016 Nov 30; doi: 10.1182/blood-2016-10-748541. pii: blood-2016-10-748541. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 73.Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend AR, Drakesmith H. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118:4129–4139. doi: 10.1182/blood-2011-04-351957. [DOI] [PubMed] [Google Scholar]
- 74.Smith CL, Arvedson TL, Cooke KS, Dickmann LJ, Forte C, Li H, Merriam KL, Perry VK, Tran L, Rottman JB, Maxwell JR. IL-22 regulates iron availability in vivo through the induction of hepcidin. J Immunol. 2013;191:1845–1855. doi: 10.4049/jimmunol.1202716. [DOI] [PubMed] [Google Scholar]
- 75.Wallace DF, Subramaniam VN. Analysis of IL-22 contribution to hepcidin induction and hypoferremia during the response to LPS in vivo. Int Immunol. 2015;27:281–287. doi: 10.1093/intimm/dxu144. [DOI] [PubMed] [Google Scholar]
- 76.Shanmugam NK, Ellenbogen S, Trebicka E, Wang L, Mukhopadhyay S, Lacy-Hulbert A, Gallini CA, Garrett WS, Cherayil BJ. Tumor necrosis factor α inhibits expression of the iron regulating hormone hepcidin in murine models of innate colitis. PLoS One. 2012;7:e38136. doi: 10.1371/journal.pone.0038136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song SN, Iwahashi M, Tomosugi N, Uno K, Yamana J, Yamana S, Isobe T, Kawabata H, Yoshizaki K. Comparative evaluation of the effects of treatment with tocilizumab and TNFα inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res Ther. 2013;15:R141. doi: 10.1186/ar4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGuckin MA, Eri RD, Das I, Lourie R, Florin TH. ER stress and the unfolded protein response in intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;298:G820–G832. doi: 10.1152/ajpgi.00063.2010. [DOI] [PubMed] [Google Scholar]
- 79.Gisbert JP, Luna M, Gonzalez-Lama Y, Pousa ID, Velasco M, Moreno-Otero R, Mate' J. Liver injury in inflammatory bowel disease: long-term follow-up study of 786 patients. Inflamm Bowel Dis. 2007;13:1106–1114. doi: 10.1002/ibd.20160. [DOI] [PubMed] [Google Scholar]
- 80.Oliveira SJ, Pinto JP, Picarote G, Costa VM, Carvalho F, Rangel M, de Sousa M, de Almeida SF. ER stress-inducible factor CHOP affects the expression of hepcidin by modulating C/EBPα activity. PLoS One. 2009;4:e6618. doi: 10.1371/journal.pone.0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, Pietrangelo A. ER stress controls iron metabolism through the induction of hepcidin. Science. 2009;325:877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Canali S, Vecchi C, Garuti C, Montosi G, Babitt JL, Pietrangelo A. The SMAD pathway is required for hepcidin response during endoplasmic reticulum stress. Endocrinology. 2016;157:3935–3945. doi: 10.1210/en.2016-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shanmugam NK, Trebicka E, Fu LL, Shi HN, Cherayil BJ. Intestinal inflammation modulates expression of the iron-regulating hormone hepcidin depending on erythropoietic activity and the commensal microbiota. J Immunol. 2014;193:1398–1407. doi: 10.4049/jimmunol.1400278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang H, Constante M, Layoun A, Santos MM. Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli. Blood. 2009;113:3593–3599. doi: 10.1182/blood-2008-08-173641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnson D, Bayele H, Johnston K, Tennant J, Srai SK, Sharp P. Tumor necrosis factor alpha regulates iron transport and transporter expression in human intestinal epithelial cells. FEBS Lett. 2004;573:195–201. doi: 10.1016/j.febslet.2004.07.081. [DOI] [PubMed] [Google Scholar]
- 86.Sharma N, Laftah AH, Brookes MJ, Cooper B, Iqbal T, Tselepis C. A role for tumor necrosis factor α in human small bowel iron transport. Biochem J. 2005;390:437–446. doi: 10.1042/BJ20050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laftah AH, Sharma N, Brookes MJ, McKie AT, Simpson RJ, Iqbal TH, Tselepis C. Tumor necrosis factor α causes hypoferraemia and reduced intestinal iron absoprtion in mice. Biochem J. 2006;397:61–67. doi: 10.1042/BJ20060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu W, Song Y, He C, Liu C, Wu R, Fang L, Cong Y, Miao Y, Liu Z. Divalent metal-ion transporter 1 is decreased in intestinal epithelial cells and contributes to the anemia in inflammatory bowel disease. Sci Rep. 2015;5:16344. doi: 10.1038/srep16344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ludwiczek S, Aigner E, Theurl I, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101:4148–4154. doi: 10.1182/blood-2002-08-2459. [DOI] [PubMed] [Google Scholar]
- 90.Guida C, Altamura S, Klein FA, Galy B, Boutros M, Ulmer AJ, Hentze MW, Muckenthaler MU. A novel inflammatory pathway mediating rapid hepcidin-independent hypoferremia. Blood. 2015;125:2265–2275. doi: 10.1182/blood-2014-08-595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deschemin JC, Vaulont S. Role of hepcidin in the setting of hypoferremia during acute inflammation. PLoS One. 2013;8:e61050. doi: 10.1371/journal.pone.0061050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Langdon JM, Yates SC, Femnou LK, McCranor BJ, Cheadle C, Xue QL, Vaulont S, Civin CI, Walston JD, Roy CN. Hepcidin-dependent and hepcidin-independent regulation of erythropoiesis in a mouse model of chronic inflammation. Am J Hematol. 2014;89:470–479. doi: 10.1002/ajh.23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hom J, Dulmovits BM, Mohandas N, Blanc L. The erythroblastic island as an emerging paradigm in the anemia of inflammation. Immunol Res. 2015;63:75–89. doi: 10.1007/s12026-015-8697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsitsika A, Stamoulakatou A, Kafritsa Y, Paleologos G, Panayotou I, Premetis E, Roma E, Papassotiriou I. Erythropoietin levels in children and adolescents with inflammatory bowel disease. J Pediatr Hematol Oncol. 2005;27:93–96. doi: 10.1097/01.mph.0000153441.34407.d9. [DOI] [PubMed] [Google Scholar]
- 95.Kim A, Fung E, Parikh SG, Valore EV, Gabayan V, Nemeth E, Ganz T. A mouse model of anemia of inflammation: complex pathogenesis with partial dependence on hepcidin. Blood. 2014;123:1129–1136. doi: 10.1182/blood-2013-08-521419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Astin R, Puthucheary Z. Anemia secondary to critical illness: an unexplained phenomenon. Extrem Physiol Med. 2014;3:4. doi: 10.1186/2046-7648-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nielsen OH, Ainsworth M, Coskun M, Weiss G. Management of iron-deficiency anemia in inflammatory bowel disease: a systematic review. Medicine (Baltimore) 2015;94:e963. doi: 10.1097/MD.0000000000000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee T, Clavel T, Smirnov K, Schmidt A, Lagkouvardos I, Walker A, Lucio M, Michalke B, Schmitt-Kopplin P, Fedorak R, Haller D. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2016 Feb 4; doi: 10.1136/gutjnl-2015-309940. pii: gutjnl-2015–309940. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weiss G. Anemia of chronic disorders: new diagnostic tools and new treatment strategies. Semin Hematol. 2015;52:313–320. doi: 10.1053/j.seminhematol.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 100.Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood. 2016;127:2809–2813. doi: 10.1182/blood-2015-12-639112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sebastiani G, Wilkinson N, Pantopoulos K. Pharmacological targeting of the hepcidin/ferroportin axis. Front Pharmacol. 2016;7:160. doi: 10.3389/fphar.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blanchette NL, Manz DH, Torti FM, Torti SV. Modulation of hepcidin to treat iron deregulation: potential clinical applications. Expert Rev Hematol. 2016;9:169–186. doi: 10.1586/17474086.2016.1124757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Theurl I, Schroll A, Sonnweber T, Nairz M, Theurl M, Willenbacher W, Eller K, Wolf D, Seifert M, Sun CC, Babitt JL, Hong CC, Menhall T, Gearing P, Lin HY, Weiss G. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. 2011;118:4977–4984. doi: 10.1182/blood-2011-03-345066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang L, Trebicka E, Fu Y, Ellenbogen S, Hong CC, Babitt JL, Lin HY, Cherayil BJ. The bone morphogenetic protein-hepcidin axis as a therapeutic target in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:112–119. doi: 10.1002/ibd.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poli M, Girelli D, Campostrini N, Maccarinelli F, Finazzi D, Luscieti S, Nai A, Arosio P. Heparin: a potent inhibitor of hepcidin expression in vitro and in vivo. Blood. 2011;117:997–1004. doi: 10.1182/blood-2010-06-289082. [DOI] [PubMed] [Google Scholar]
- 106.Poli M, Asperti M, Naggi A, Campostrini N, Girelli D, Corbella M, Benzi M, Besson-Fournier C, Coppin H, Maccarinelli F, Finazzi D, Arosio P. Glycol-split nonanticoagulant heparins are inhibitors of hepcidin expression in vitro and in vivo. Blood. 2014;123:1564–1573. doi: 10.1182/blood-2013-07-515221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Böser P, Seemann D, Liguori MJ, Fan L, Huang L, Hafner M, Popp A, Mueller BK. Anti-repulsive guidance molecule c (RGMc) antibodies increase serum iron in rats and cynomolgus monkeys by hepcidin down-regulation. AAPS J. 2015;17:930–938. doi: 10.1208/s12248-015-9770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schwoebel F, van Eijk LT, Zboralski D, Sell S, Buchner K, Maasch C, Purschke WG, Humphrey M, Zollner S, Eulberg D, Morich F, Pickkers P, Klussmann S. The effects of the anti-hepcidin spiegelmer NOX-H94 on inflammation-induced anemia in cynomolgus monkeys. Blood. 2013;121:2311–2315. doi: 10.1182/blood-2012-09-456756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blumenstein I, Dignass A, Vollmer S, Klemm W, Weber-Mangal S, Stein J. Current practice in the diagnosis and management of IBD-associated anaemia and iron deficiency in Germany: the German AnaemIBD Study. J Crohns Colitis. 2014;8:1308–1314. doi: 10.1016/j.crohns.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 110.Katsanos KH, Tatsioni A, Natsi D, Sigounas D, Christodoulou DK, Tsianos EV. Recombinant human erythropoietin in patients with inflammatory bowel disease and refractory anemia: a 15-year single center experience. J Crohns Colitis. 2012:56–61. doi: 10.1016/j.crohns.2011.07.004. [DOI] [PubMed] [Google Scholar]