Abstract
The key processes of lung development have been elucidated in the past several decades, helping to identify and characterize the resident progenitor cells that ultimately generate the mature organ. The adult lung is a complex organ consisting of scores of different cell lineages that are remarkably quiescent in the absence of injury. Despite low cellular turnover, the lung can respond quickly and dramatically to acute damage when spatially restricted stem and progenitor cells re-enter the cell cycle and differentiate to promote repair. The findings from lung developmental biology are now being used to examine the mechanisms that underlie lung regeneration. The use of in vitro models such as pluripotent stem cells and new methods of gene editing have provided models for understanding lung disease, exploring the mechanisms of lung regeneration, and have raised the prospect of correcting lung dysfunction. We will outline how basic studies into lung developmental biology are now being applied to lung regeneration, opening up new avenues of research that may ultimately be harnessed for treatments of lung disease.
INTRODUCTION
The key processes of lung development have been elucidated in the past several decades, helping to identify and characterize the resident progenitor cells that ultimately generate the mature organ. The adult lung is a complex organ consisting of scores of different cell lineages that are remarkably quiescent in the absence of injury. Despite low cellular turnover, the lung can respond quickly and dramatically to acute damage when spatially restricted stem and progenitor cells re-enter the cell cycle and differentiate to promote repair. The findings from lung developmental biology are now being used to examine the mechanisms that underlie lung regeneration. The use of in vitro models such as pluripotent stem cells and new methods of gene editing have provided models for understanding lung disease, exploring the mechanisms of lung regeneration, and have raised the prospect of correcting lung dysfunction. We will outline how basic studies into lung developmental biology are now being applied to lung regeneration, opening up new avenues of research that may ultimately be harnessed for treatments of lung disease.
DEVELOPMENT OF THE LUNG
Discussion of lung regeneration or disease modeling, requires a thorough understanding of how the lung is generated during normal development. Much of what is known about lung development comes from the extensive studies performed during the last two decades of research on mouse lung development. While differences exist between the mouse and human lung, the power of the mouse genetic model remains preeminent when examining the basics of lung development. The development of the mammalian lung can be broken into three phases: 1) early specification of lung endoderm and mesoderm progenitors within the anterior foregut including initial outpouching of the endoderm tube that generates the lungs, 2) branching morphogenesis which arborizes this primitive lung tube in a highly stereotypical manner that generates hundreds to thousands of branch tips, 3) culminating in sacculation and alveolarization in the last phase of lung development, which generates the functional gas exchange units required for postnatal respiration (Morrisey and Hogan 2010). (Figure 1)
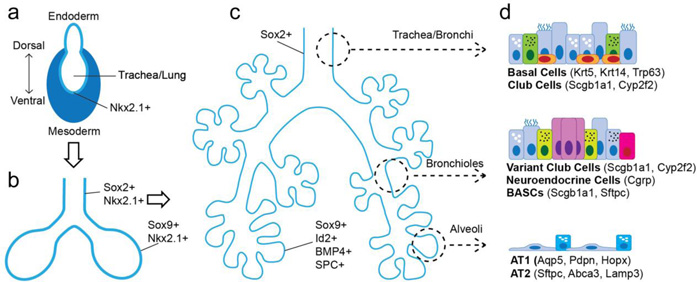
Figure 1. Progenitors of the lung throughout development.
Throughout development, pluripotent cells remain present though to a decreasing capacity for differentiation. The earliest, lung specified progenitors, are the Nkx2.1+ endoderm cells found on the ventral side of the anterior foregut endoderm (A). These endodermal progenitors (Nkx2.1+) then quickly undergo specification for either the proximal airway epithelium (Sox2+) or distal airway epithelium (Sox9+) (B). Following branching morphogenesis, namely after E13.5 in the mouse, the distal progenitors (Sox9+, Id2+, BMP4+, SPC+) are restricted and generate either AT1 and AT2 cells (C). In the fully developed adult lung, multipotent progenitors exist in specific niches across the pulmonary epithelial tree including basal cells, variant Club or secretory cells, BASCs, and AT2 cells (D).
Lung development initiates when the ventral side of the anterior foregut endoderm is specified for lung endoderm fate, which is noted by expression of the transcription factor Nkx2.1 (Cardoso and Lu 2006). In mice this specification event happens at approximately embryonic day 9 (E9.0). The specified lung endoderm (Nkx2.1+) evaginates from the ventral side of the anterior foregut endoderm at approximately E9.5 in the mouse and forms two primitive lung buds. These buds are progressively surrounded by mesenchyme and begin to branch. At the leading tips of the primary lung buds, a process of proximal-distal patterning of the endoderm begins and is noted by differential expression of the transcription factor Sox2, which demarcates the proximal portion of the branching lung epithelium, and Sox9 and Id2, which demarcate the most distal tips of the branching epithelium. The Sox2+ progenitors will generate all of the epithelial lineages within the conducting airways including secretory cells, multi-ciliated cells, and basal cells. The Sox9+/Id2+ cells are initially multipotent, capable of generating both airway as well as alveolar epithelium (Rawlins, Clark et al. 2009). However, after E13.5, Sox9+ progenitors are restricted in their fate and only generate alveolar type 1 (AT1) and type 2 (AT2) cells. These studies have elucidated a model of progressive loss of fate potential in lung endoderm progenitors based on the functional regions of this large and complex organ. This point will become important later when the various modes of lung regeneration are discussed.
Towards late gestation, the differentiation of distal Sox9+/Id2+ progenitors into AT1 and AT2 cells is an important step in the process of generating functional alveolar gas exchange units. Recent evidence suggests the presence of a bipotent progenitor capable of equal differentiation into either AT1 or AT2 cells (Desai, Brownfield et al. 2014). This bipotent progenitor is thought to exist in late gestation and early alveologenesis in the mouse (E18-P0) and has been proposed to be responsible for generation of the lung alveolus. Specific markers for this bipotent progenitor remain unknown, but further characterization of its potential and what pathways regulate its behavior will be important for understanding its full contribution towards generating a functional lung alveolus.
The Sox2+ progenitors give rise to the full complement of airway epithelial lineages. While many of these lineages are fully differentiated functional cells such as multi-ciliated and most secretory cells, several progenitor lineages are also produced during lung development from the Sox2+ early endoderm progenitors. These include basal cells, which express Trp63, Krt5, and Pdpn, and generate multi-ciliated cells and secretory cells after airway injury in the adult trachea and main stem bronchi (Rock, Onaitis et al. 2009). Development of the airway epithelium is regulated by Notch signaling, which plays a key role in promoting secretory epithelial differentiation while inhibiting differentiation of the multi-ciliated epithelium (Tsao, Vasconcelos et al. 2009). There is also a subset of secretory cells that self-renew and differentiate into multi-ciliated after injury (Hong, Reynolds et al. 2001, Giangreco, Reynolds et al. 2002). In addition to these lineages, Sox2+ cells also generate pulmonary neuroendocrine cells, which play an important role in regulating the postnatal immune response in the lung (Branchfield, Nantie et al. 2016).
DEVELOPMENTAL PATHWAYS IN LUNG REGENERATION
Regeneration of the lung after acute injury involves multiple stem/progenitor lineages, thus this process can be thought of as following a “democratic principle” depending on the spatial niches involved (Kotton and Morrisey 2014). The signaling pathways used by these progenitors are often the same used for development of the lung (Shi, Xu et al. 2009, Whitsett, Haitchi et al. 2011, Herriges and Morrisey 2014, Akram, Patel et al. 2016, Bertoncello 2016). In the large airways and trachea, activation of Trp63+ basal cells and their subsequent differentiation into secretory and multi-ciliated epithelial cells is regulated by a complex interplay of Notch signaling, mirroring the role this pathway regulated in airway epithelial development (Guseh, Bores et al. 2009, Rock, Gao et al. 2011, Tsao, Wei et al. 2011, Mori, Mahoney et al. 2015). Variations of Notch have effects from conducting airways, where Notch-3 suppresses basal cell over-expansion (Pardo-Saganta, Law et al. 2015), down to the alveoli where Notch-2 activation is necessary for alveologenesis (Tsao, Matsuoka et al. 2016). Recent work has also demonstrated unique plasticity in certain lung airway epithelial cells, with secretory cells exhibiting dedifferentiation backwards into basal cells to replenish the basal cell population after extensive injury (Tata, Mou et al. 2013). How extensive a role this plasticity plays in physiological regeneration and what molecular pathways regulate it, are important topics that warrant further investigation.
In the more distal lung airways, Wnt signaling has been shown to be important for expansion of a bronchioalveolar stem cell (BASC) lineage and this process is regulated by Gata6, a key transcription factor in lung development (Zhang, Goss et al. 2008). BASC differentiation is also regulated by BMP4 expression, which promotes TSP-1 expression in the lung endothelium, which in turn regulates differentiation of BASCs (Lee, Bhang et al. 2014). These developmentally conserved signaling pathways during regeneration are not limited to the epithelium, but are also seen in the supporting mesenchymal lineages. Hedgehog signaling in the adult lung maintains mesenchymal quiescent, and inhibition of hedgehog can lead to increased epithelial proliferation at homeostasis and after injury (Peng, Frank et al. 2015).
MODELING LUNG DISEASE AND REGENERATION
There are multiple models in which to study lung disease processes and regeneration including both in vivo and in vitro models. The primary in vivo model for the study of lung regeneration is the mouse. While important cellular and physiological differences exist between the mouse and human lung, most if not all of the major cell lineages are shared and, from emerging human lung studies, the expression and function of many of the major transcriptional and signaling pathways are conserved. Importantly, the identification of these major regulators of lung development and regeneration in the mouse system has allowed for exciting new investigations in the human system through derivation of human lung epithelial cells including AT2 cells from pluripotent stem cells and the use of primary human lung cells in lung organoid culturing systems (Rock, Onaitis et al. 2009, Rock, Gao et al. 2011, Longmire, Ikonomou et al. 2012, Mou, Zhao et al. 2012, Barkauskas, Cronce et al. 2013). In addition to these assay systems, emerging techniques in bioengineered systems such as lung-on-a-chip allow for assessment of cell-cell and cell-matrix interactions in a higher throughput manner. (Figure 2)
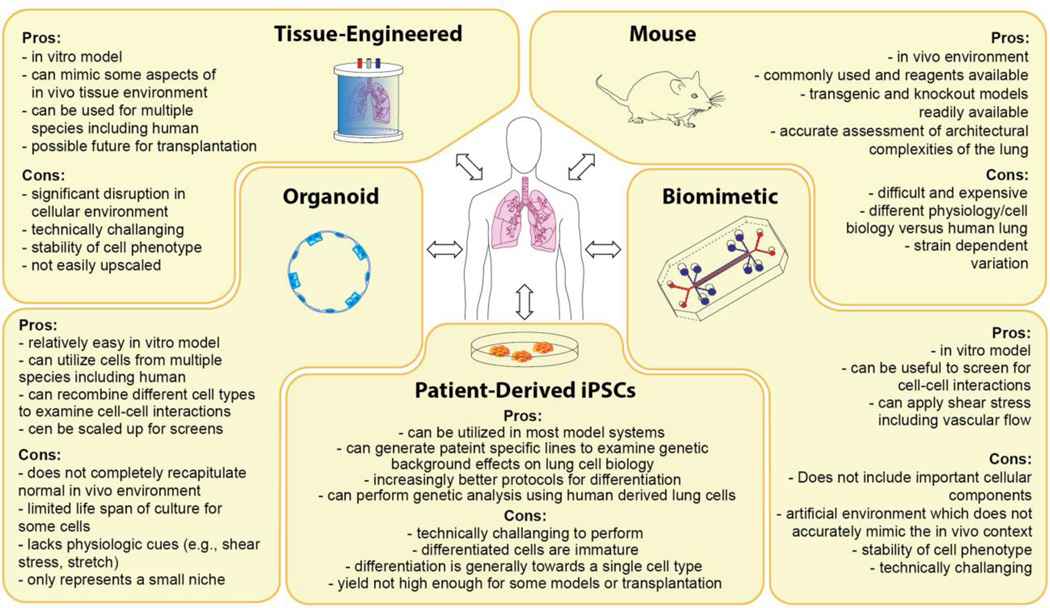
Figure 2. Options for cell behavior and disease modeling in the lung.
Since there are no perfect assays to model all aspects of cell behavior or disease states in the lung, a combination of approaches will be the most efficacious. The in vivo mouse model remains the gold-standard for nearly all aspects of cell and developmental biology as well as disease modeling due to its wide accessibility and the power of mouse genetics. Organoids derived from region-specific cells (e.g., basal, AT2s etc.) allow for assays on specific progenitors of the lung. Patient-derived iPSCs have shown promise in their ability to be used in various models described here. Recent biomimetic devices such as the lung-on-a-chip allow for an analysis of cell-cell interactions that can be perturbed physiologic cues (i.e., shear stress and stretch). Tissue engineering is being applied to create tissue orthologues that could be tested in vitro, to better understand cell-matrix interactions.
IN VIVO ANIMAL MODELS
Given the complexity of the lung and highly heterogeneous cell lineage composition, use of an in vivo system to study lung disease modeling and tissue regeneration remains the gold standard. The genetic power of the mouse makes it the preeminent in vivo model system to date for lung development and regenerative studies. The mouse has also proven to be an excellent model for assessment of drug efficacy, pharmacodynamics, pharmacokinetic and toxicological profiles of novel interventions in the context of the entire body that serve as essential precursors to clinical trials for lung disease treatments (Mercer, Abbott-Banner et al. 2015). Despite its usefulness, there are significant limitations to the modeling of human lung disease and lung regeneration in the mouse, which are outlined below. The various mouse models of lung disease and regeneration do a good job of elucidating general cellular behaviors in disease and states of injury, but are incapable of reflecting all aspects of the human condition.
A model of allergic asthma, which is induced by intertracheal exposure to ovalbumin, can reproduce some features of human asthma such as increased lung eosinophils, but does not give robust lung function responses (Kumar and Foster 2012). The Aspergillus fumigatus model however, induces significant amounts of serum IgE, airway hypereactivity, lung inflammation and a cytokine response (i.e., IL-4&5) which mimics the human response to this allergic fungi (Haczku, Atochina et al. 2001, Kurup and Grunig 2002). Most current models of COPD are designed to induce emphysema, and rarely mimic the disease of the small airways, nor bronchitis or exacerbations (Mercer, Abbott-Banner et al. 2015). Fibrosis is most commonly modeled through administration of the drug bleomycin. While a single intertracheal dose in the mouse reproduces some aspects of bleomycin toxicity in humans, it lacks the persistent fibrotic expansion observed in people (Degryse and Lawson 2011). Other delivery modes for bleomycin including repetitive interperitoneal injections over the course of several weeks may prove to mimic the human response to this drug better than the single intertracheal route (Peng, Frank et al. 2015). In contrast, a mouse model of influenza instigated acute lung injury does mimic many aspects of human influenza infection including the robust immune response and development of keratin 5 expressing epithelium in the alveolar airspaces (Kumar, Hu et al. 2011, Vaughan, Brumwell et al. 2015, Zuo, Zhang et al. 2015). These models can provide critical information regarding the response of the lung to acute injury by delineating the regenerative cell lineages including different progenitor lineages. The knowledge gained from these in vivo models can then be translated to biomimetic in vitro models of repair and regeneration.
ORGANOID MODEL SYSTEMS
In vitro models such as organoids have been employed to help provide alternatives to this research, and have the beneficial utility of being applicable to both development of chronic disease modeling of the lung. Organoids can be generated from multiple cell types and generally self-aggregate into three dimensional structures that can mimic the organization of the native organ (Rock, Onaitis et al. 2009, Sato, Vries et al. 2009, Barker, Huch et al. 2010, Barkauskas, Cronce et al. 2013, Nadkarni, Abed et al. 2016). In the lung, the source of cells used in organoids can include primary cells from human or mouse lungs or cells derived from differentiated pluripotent stem cells. To date, organoids have been generated from at least four distinct regions of the lung: trachea/bronchus (Rock, Onaitis et al. 2009), bronchioles (Vaughan, Ramirez et al. 2006, Peng, Frank et al. 2015), bronchioalveolar duct junction (Kim, Jackson et al. 2005) and the alveolus (Barkauskas, Cronce et al. 2013). Organoid formation from various lung progenitor lineages allows the examination of factors required for both self-renewal and differentiation. For example, Barkauskas et al. showed that primary AT2s, when cultured as organoids called alveolospheres, generate both AT2 and AT1 cells (Barkauskas, Cronce et al. 2013). Similarly, Lee et al. showed that BASCs could generate AT1s and AT2s and in the presence of pulmonary endothelial cells, could form organoids representative of the bronchiolar, bronchioalveolar and alveolar regions in vitro (Lee, Bhang et al. 2014).
Organoid assay systems can also be used to model human diseases, including those that affect the lung such as cystic fibrosis. Forskolin induced swelling of organoids caused by defects in the Cftr gene can be modeled in human intestinal derived organoid cultures (Dekkers, Wiegerinck et al. 2013). Importantly, this defect can be corrected using CRISPR/Cas9 mediated gene editing (Dekkers, Wiegerinck et al. 2013). Similarly, the use of human bronchospheres to study goblet cell metaplasia has resulted in the discovery of the Notch pathway being a potential therapeutic target for the disease (Danahay, Pessotti et al. 2015). In the future, organoid culture assays should be able to identify proliferative cues for human adult lung progenitors as well as assess their ability to differentiate into various daughter cell lineages. Variables including the inclusion of distinct mesenchymal lineages, vascular endothelial lineages, and other cell types will help reveal the cell-cell and cell-matrix interactions required for self-renewal and differentiation of these progenitors.
BIOENGINEERED MODELS
The lung-on-a-chip, a subset of tissue-on-a-chip microfluidic devices, is a bioengineered device that is based on modeling cell-cell interactions that can be explored pharmacologically. The lung-on-a-chip devices are meant to supplement more traditional cell culture approaches, such as the air-liquid-interface (Bals, Beisswenger et al. 2004), that lack aspects of the in vivo scenario (e.g., shear stress, stretch, etc.), while also keeping the cells in a three-dimensional environment that can be monitored in real-time and networked with other organ chips (Esch, King et al. 2011). Through the use of computational fluid dynamics the chips mimic pulmonary capillary gas exchange by controlling partial pressures of oxygen on the arterial and venous ends of the system (Long, Finch et al. 2012). The two major compartments of the lung, the conducting airways and the alveoli, have both been modeled thus far and challenged with certain physiological stimuli such as plug rupture and inflammatory insult (Huh, Fujioka et al. 2007, Huh, Matthews et al. 2010). More importantly, certain physiologic responses have been correlated to in vivo rodent models, such as IL-2 induced pulmonary edema with rescue by angiopoietin-1 administration (Huh, Leslie et al. 2012). To date, there have been a dearth of reports using primary human cells in these devices, whether directly from harvested organs or derived from pluripotent stem cells. These cell types as well as the inclusion of other lineages such as the diverse array of mesenchymal lineages, will need to be included in future studies to validate the importance of this model.
An additional in vitro bioengineered lung model is the decellularized tissue system, where cells are removed using a combination of detergents leaving behind a rigid extracellular matrix that can be subsequently used for reseeding with pulmonary derived cells (Balestrini, Gard et al. 2016). Using this technique, the unique architecture of the lungs, with bifurcating networks of airways and blood vessels, can be maintained following the removal of the living cellular component (Gilpin and Ott 2015). While several studies have used this model to reseed lung cell lineages including endothelial cells and some epithelial lineages (Ren, Moser et al. 2015, Stabler, Caires et al. 2016), most cells do not survive in culture long term (i.e., >2 weeks) and in the case of epithelial cells, many de-differentiate likely due to the absence of a complete repertoire of niche signals (Calle, Mendez et al. 2015).
PLURIPOTENT STEM CELLS
The advent of induced pluripotent stem cells (iPSC) has provided exciting opportunities to assess human lung progenitor cell capacity for self-renewal and differentiation (Takahashi and Yamanaka 2006). In general, generating endodermal derivatives, such as lung epithelium, from ESCs and iPSCs has been more difficult than mesodermal and neural cell lineages. Recent studies have now shown that ESCs and iPSCs can be differentiated into lung epithelial cell lineages including both Sftpc+ AT2s and Scgb1a1+ secretory cell lineages (Longmire, Ikonomou et al. 2012, Mou, Zhao et al. 2012, Huang, Islam et al. 2013). The protocols used for differentiating lung epithelial lineages utilized multiple signaling pathways critical for lung development including Wnt, Bmp, and Fgf. As with many iPSC induced differentiation protocols, precise temporal application of signaling activators and inhibitors is necessary to promote lung epithelial cell differentiation (Green, Chen et al. 2011). The biggest hurdle in the study of ESC or iPSC derived lung epithelium is that the resulting cells are immature and do not represent a fully functional differentiated cell. Despite this and other limitations, such differentiation protocols have been used in various models to explore the differentiation potential of human and mouse ESCs and iPSCs including the kidney capsule formation assay (Mou, Zhao et al. 2012, Liu, Li et al. 2014), lung-on-a-chip type models such as air liquid interface (ALI) culture (Wong, Bear et al. 2012, Firth, Dargitz et al. 2014), and in bioengineered models such as culture within the decellularized lung (Longmire, Ikonomou et al. 2012). The major benefit of patient derived iPSCs is their capacity to harbor genetic mutations that affect respiratory function such as cystic fibrosis or alpha-1 anti-trypsin deficiency. Wong et al. showed that differentiated airway epithelium from iPSCs derived from a human patient with cystic fibrosis exhibited aspects of the CF phenotype which could be rescued following treatment with the small molecule C18 in an ALI culture (Wong, Bear et al. 2012). With the establishment of human biobanks containing hundreds of patient derived iPSCs from lung diseases (Somers, Jean et al. 2010), this technology will only become more useful in the future for screening therapies for lung disease treatments.
PROMOTING ADULT LUNG REGENEATION
DEVELOPMENTAL PATHWAYS AND ADULT LUNG REGENERATION
Multiple developmental pathways have been demonstrated to have important regulatory function in lung regeneration assays. Wnt signaling is a critical pathway in lung endoderm specification and absence of Wnt signaling leads to complete lung agenesis (Goss, Tian et al. 2009, Harris-Johnson, Domyan et al. 2009). Wnt signaling is re-activated in the adult lung after injury and during repair and regeneration (Zhang, Goss et al. 2008, Flozak, Lam et al. 2010, Al Alam, Green et al. 2011, Hashimoto, Chen et al. 2012, Aumiller, Balsara et al. 2013). Wnt signaling is activated upon naphthalene induced airway injury and ectopic activation of Wnt signaling caused by deletion of the transcription factor Gata6, leads to expansion of BASCs (Zhang, Goss et al. 2008). Interestingly, pharmacological inhibition of Wnt signaling reduces bleomycin induced fibrosis in mice suggesting that Wnt signaling could also be used to block this deleterious response to injury (Henderson, Chi et al. 2010). Thus, the ability of Wnt signaling to promote proper repair and regeneration after injury is context dependent and chronic activation could lead to increased fibrosis.
The Notch signaling pathway plays a critical role in promoting secretory cell differentiation during lung endoderm development (Guseh, Bores et al. 2009, Tsao, Vasconcelos et al. 2009). Interestingly, this function of Notch is recapitulated after airway injury and controls basal cell differentiation. Thus, activation of Notch could be used to expand the secretory lineage after airway injury (Rock, Barkauskas et al. 2011, Xing, Li et al. 2012). However, this would come at the expense of decreased multi-ciliated epithelial differentiation, which would result in an imbalance of differentiated cell types in the repaired airway.
Epigenetic regulation by histone acetylation and histone deacetylation has been associated with adult lung diseases including asthma and chronic obstructive pulmonary disease (COPD). Histone acetylation is regulated by multiple enzymes within the histone acetyltransferase (HAT) and histone deacetylase (HDAC) protein families. Asthma patients exhibit increased HAT activity and decreased HDAC activity in their airway epithelium (Gunawardhana, Gibson et al. 2014). In COPD patients, a correlation has been demonstrated between disease severity and progression and decreased HDAC2 expression (Ito, Ito et al. 2005). Genetic knockout models of histone deacetylase (HDAC) deficiency have begun to address the effects of HDACs in lung regeneration. Loss of HDAC1/2 in the postnatal lung secretory epithelium resulted in increased expression of tumor suppressors including Rb1, p21/Cdkn1a, and p16/Ink4a after injury (Wang, Tian et al. 2013). This resulted in a loss of cell proliferation, which is required for secretory cell regeneration. HDAC2 also plays an important role in regulating the expression of the cytokine IL-6, which is important for inhibiting the alveolar response to hyperoxic lung injury (Chokas, Trivedi et al. 2010).
CELL AND GENE THERAPIES FOR LUNG DISEASE AND REGENERATION
The application of adult stem/progenitor cells or patient derived iPSCs for treatments of pulmonary disease is an attractive extension of the basics of developmental biology towards new therapeutic approaches to lung disease. Other cellular options such as mesenchymal stromal/stem cells, have not demonstrated efficacy beyond a small anti-inflammatory paracrine affect (Stabler, Lecht et al. 2015, Ikonomou, Freishtat et al. 2016, McIntyre, Moher et al. 2016). In contrast, the use of iPSCs to generate lung cell lineages for replacement of cells due to acute injury or disease related loss, could eventually be used for regenerative purposes. However, at the current time, there are no acceptable methods for exogenous replacement of any lung cell type. Further research is needed on this topic, including the development of a robust engraftment assay that can be used to assess the feasibility of cell therapy in the lung. The architectural complexity of the respiratory system will likely continue to be a barrier to the development of such an assay.
Recent developments in gene editing could one day be used to correct monogenic mutations that cause lung disease. Advances in the use of CRISPR/Cas9 gene editing techniques could lead to correction of lung diseases such as cystic fibrosis and α1-antitrypsin disorders. Of note, a team of Chinese scientists has been approved for the first use of CRISPR-Cas9 to modify T-cells in order to treat metastatic non-small cell lung cancer (Cyranoski 2016). This application modifies the patient’s T cells ex vivo, and then re-transplants them, a route of treatment that is unlikely to be useful in the lung in the near future. Despite the promise of CRISPR/Cas9 in correction of monogenic diseases, many adult lung diseases are likely polygenic and may require a better understanding of the genetic underpinnings before gene editing can be applied towards their treatment.
SUMMARY AND CONCLUSIONS
The study of basic lung development has been crucial for understanding and manipulating the remodeling and regeneration of the adult organ. While the mouse remains a workhorse for further understanding lung development and regeneration, the emergence of new model systems including lung organoids and patient specific iPSCs provides new models in which to study human lung biology. Future development of gene editing techniques as well as robust engraftment assays should further accelerate research into novel therapeutic approaches for lung disease and injury.
REFERENCES
- Akram KM, Patel N, Spiteri MA, Forsyth NR. Lung Regeneration: Endogenous and Exogenous Stem Cell Mediated Therapeutic Approaches. Int J Mol Sci. 2016;17(1) doi: 10.3390/ijms17010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Alam D, Green M, Tabatabai Irani R, Parsa S, Danopoulos S, Sala FG, Branch J, El Agha E, Tiozzo C, Voswinckel R, Jesudason EC, Warburton D, Bellusci S. Contrasting expression of canonical Wnt signaling reporters TOPGAL, BATGAL and Axin2(LacZ) during murine lung development and repair. PLoS One. 2011;6(8):e23139. doi: 10.1371/journal.pone.0023139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumiller V, Balsara N, Wilhelm J, Gunther A, Konigshoff M. WNT/beta-catenin signaling induces IL-1beta expression by alveolar epithelial cells in pulmonary fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2013;49(1):96–104. doi: 10.1165/rcmb.2012-0524OC. [DOI] [PubMed] [Google Scholar]
- Balestrini JL, Gard AL, Gerhold KA, Wilcox EC, Liu A, Schwan J, Le AV, Baevova P, Dimitrievska S, Zhao L, Sundaram S, Sun H, Rittie L, Dyal R, Broekelmann TJ, Mecham RP, Schwartz MA, Niklason LE, White ES. Comparative biology of decellularized lung matrix: Implications of species mismatch in regenerative medicine. Biomaterials. 2016;102:220–230. doi: 10.1016/j.biomaterials.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R, Beisswenger C, Blouquit S, Chinet T. Isolation and air-liquid interface culture of human large airway and bronchiolar epithelial cells. J Cyst Fibros. 2004;3(Suppl 2):49–51. doi: 10.1016/j.jcf.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123(7):3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6(1):25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Bertoncello I. Properties of Adult Lung Stem and Progenitor Cells. J Cell Physiol. 2016 doi: 10.1002/jcp.25404. [DOI] [PubMed] [Google Scholar]
- Branchfield K, Nantie L, Verheyden JM, Sui P, Wienhold MD, Sun X. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science. 2016;351(6274):707–710. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EA, Mendez JJ, Ghaedi M, Leiby KL, Bove PF, Herzog EL, Sundaram S, Niklason LE. Fate of distal lung epithelium cultured in a decellularized lung extracellular matrix. Tissue Eng Part A. 2015;21(11–12):1916–1928. doi: 10.1089/ten.tea.2014.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133(9):1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Chokas AL, Trivedi CM, Lu MM, Tucker PW, Li S, Epstein JA, Morrisey EE. Foxp1/2/4-NuRD interactions regulate gene expression and epithelial injury response in the lung via regulation of interleukin-6. J Biol Chem. 2010;285(17):13304–13313. doi: 10.1074/jbc.M109.088468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranoski D. Chinese scientists to pioneer first human CRISPR trial. Nature. 2016;535(7613):476–477. doi: 10.1038/nature.2016.20302. [DOI] [PubMed] [Google Scholar]
- Danahay H, Pessotti AD, Coote J, Montgomery BE, Xia D, Wilson A, Yang H, Wang Z, Bevan L, Thomas C, Petit S, London A, LeMotte P, Doelemeyer A, Velez-Reyes GL, Bernasconi P, Fryer CJ, Edwards M, Capodieci P, Chen A, Hild M, Jaffe AB. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. 2015;10(2):239–252. doi: 10.1016/j.celrep.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Degryse AL, Lawson WE. Progress toward improving animal models for idiopathic pulmonary fibrosis. Am J Med Sci. 2011;341(6):444–449. doi: 10.1097/MAJ.0b013e31821aa000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19(7):939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507(7491):190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng. 2011;13:55–72. doi: 10.1146/annurev-bioeng-071910-124629. [DOI] [PubMed] [Google Scholar]
- Firth AL, Dargitz CT, Qualls SJ, Menon T, Wright R, Singer O, Gage FH, Khanna A, Verma IM. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2014;111(17):E1723–E1730. doi: 10.1073/pnas.1403470111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flozak AS, Lam AP, Russell S, Jain M, Peled ON, Sheppard KA, Beri R, Mutlu GM, Budinger GR, Gottardi CJ. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. Journal of Biological Chemistry. 2010;285(5):3157–3167. doi: 10.1074/jbc.M109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161(1):173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin SE, Ott HC. Using nature's platform to engineer bio-artificial lungs. Ann Am Thorac Soc. 2015;12(Suppl 1):S45–S49. doi: 10.1513/AnnalsATS.201408-366MG. [DOI] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17(2):290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MD, Chen A, Nostro MC, d'Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck HW. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nature biotechnology. 2011;29(3):267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardhana LP, Gibson PG, Simpson JL, Powell H, Baines KJ. Activity and expression of histone acetylases and deacetylases in inflammatory phenotypes of asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2014;44(1):47–57. doi: 10.1111/cea.12168. [DOI] [PubMed] [Google Scholar]
- Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136(10):1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haczku A, Atochina EN, Tomer Y, Chen H, Scanlon ST, Russo S, Xu J, Panettieri RA, Jr, Beers MF. Aspergillus fumigatus-induced allergic airway inflammation alters surfactant homeostasis and lung function in BALB/c mice. Am J Respir Cell Mol Biol. 2001;25(1):45–50. doi: 10.1165/ajrcmb.25.1.4391. [DOI] [PubMed] [Google Scholar]
- Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A. 2009;106(38):16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Chen H, Que J, Brockway BL, Drake JA, Snyder JC, Randell SH, Stripp BR. beta-Catenin-SOX2 signaling regulates the fate of developing airway epithelium. Journal of Cell Science. 2012;125(Pt 4):932–942. doi: 10.1242/jcs.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A. 2010;107(32):14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141(3):502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24(6):671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Huang SX, Islam MN, O'Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nature biotechnology. 2013 doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Fujioka H, Tung YC, Futai N, Paine R, 3rd, Grotberg JB, Takayama S. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc Natl Acad Sci U S A. 2007;104(48):18886–18891. doi: 10.1073/pnas.0610868104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4(159):159ra147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328(5986):1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomou L, Freishtat RJ, Wagner DE, Panoskaltsis-Mortari A, Weiss DJ. The Global Emergence of Unregulated Stem Cell Treatments for Respiratory Diseases. Professional Societies Need to Act. Ann Am Thorac Soc. 2016;13(8):1205–1207. doi: 10.1513/AnnalsATS.201604-277ED. [DOI] [PubMed] [Google Scholar]
- Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, Barnes PJ. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352(19):1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med. 2014;20(8):822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang de Y, Lim B, Chow VT, Crum CP, Xian W, McKeon F. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147(3):525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RK, Foster PS. Are mouse models of asthma appropriate for investigating the pathogenesis of airway hyper-responsiveness? Front Physiol. 2012;3:312. doi: 10.3389/fphys.2012.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup VP, Grunig G. Animal models of allergic bronchopulmonary aspergillosis. Mycopathologia. 2002;153(4):165–177. doi: 10.1023/a:1014963600314. [DOI] [PubMed] [Google Scholar]
- Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156(3):440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Li LF, Fu JY, Kao KC, Huang CC, Chien Y, Liao YW, Chiou SH, Chang YL. Induced Pluripotent Stem Cell Therapy Ameliorates Hyperoxia-Augmented Ventilator-Induced Lung Injury through Suppressing the Src Pathway. PLoS One. 2014;9(10):e109953. doi: 10.1371/journal.pone.0109953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C, Finch C, Esch M, Anderson W, Shuler M, Hickman J. Design optimization of liquid-phase flow patterns for microfabricated lung on a chip. Ann Biomed Eng. 2012;40(6):1255–1267. doi: 10.1007/s10439-012-0513-8. [DOI] [PubMed] [Google Scholar]
- Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS, Dowton AA, Serra M, Weiss DJ, Green MD, Snoeck HW, Ramirez MI, Kotton DN. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10(4):398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre LA, Moher D, Fergusson DA, Sullivan KJ, Mei SH, Lalu M, Marshall J, McLeod M, Griffin G, Grimshaw J, Turgeon A, Avey MT, Rudnicki MA, Jazi M, Fishman J, Stewart DJ G. Canadian Critical Care Translational Biology. Efficacy of Mesenchymal Stromal Cell Therapy for Acute Lung Injury in Preclinical Animal Models: A Systematic Review. PLoS One. 2016;11(1):e0147170. doi: 10.1371/journal.pone.0147170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer PF, Abbott-Banner K, Adcock IM, Knowles RG. Translational models of lung disease. Clin Sci (Lond) 2015;128(4):235–256. doi: 10.1042/CS20140373. [DOI] [PubMed] [Google Scholar]
- Mori M, Mahoney JE, Stupnikov MR, Paez-Cortez JR, Szymaniak AD, Varelas X, Herrick DB, Schwob J, Zhang H, Cardoso WV. Notch3-Jagged signaling controls the pool of undifferentiated airway progenitors. Development. 2015;142(2):258–267. doi: 10.1242/dev.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18(1):8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Musunuru K, Cowan C, Rajagopal J. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10(4):385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni RR, Abed S, Draper JS. Organoids as a model system for studying human lung development and disease. Biochem Biophys Res Commun. 2016;473(3):675–682. doi: 10.1016/j.bbrc.2015.12.091. [DOI] [PubMed] [Google Scholar]
- Pardo-Saganta A, Law BM, Tata PR, Villoria J, Saez B, Mou H, Zhao R, Rajagopal J. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell. 2015;16(2):184–197. doi: 10.1016/j.stem.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Frank DB, Kadzik RS, Morley MP, Rathi KS, Wang T, Zhou S, Cheng L, Lu MM, Morrisey EE. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature. 2015;526(7574):578–582. doi: 10.1038/nature14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136(22):3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Moser PT, Gilpin SE, Okamoto T, Wu T, Tapias LF, Mercier FE, Xiong L, Ghawi R, Scadden DT, Mathisen DJ, Ott HC. Engineering pulmonary vasculature in decellularized rat and human lungs. Nat Biotechnol. 2015;33(10):1097–1102. doi: 10.1038/nbt.3354. [DOI] [PubMed] [Google Scholar]
- Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BLM. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial-to-mesenchymal transition. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8(6):639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106(31):12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Shi W, Xu J, Warburton D. Development, repair and fibrosis: what is common and why it matters. Respirology. 2009;14(5):656–665. doi: 10.1111/j.1440-1843.2009.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA, Ying L, Sommer AG, Jean JM, Smith BW, Lafyatis R, Demierre MF, Weiss DJ, French DL, Gadue P, Murphy GJ, Mostoslavsky G, Kotton DN. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells. 2010;28(10):1728–1740. doi: 10.1002/stem.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler CT, Caires LC, Jr, Mondrinos MJ, Marcinkiewicz C, Lazarovici P, Wolfson MR, Lelkes PI. Enhanced Re-Endothelialization of Decellularized Rat Lungs. Tissue Eng Part C Methods. 2016;22(5):439–450. doi: 10.1089/ten.tec.2016.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler CT, Lecht S, Lazarovici P, Lelkes PI. Mesenchymal stem cells for therapeutic applications in pulmonary medicine. Br Med Bull. 2015;115(1):45–56. doi: 10.1093/bmb/ldv026. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tanjore H, Degryse AL, Crossno PF, Xu XC, McConaha ME, Jones BR, Polosukhin VV, Bryant AJ, Cheng DS, Newcomb DC, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. beta-catenin in the alveolar epithelium protects from lung fibrosis after intratracheal bleomycin. Am J Respir Crit Care Med. 2013;187(6):630–639. doi: 10.1164/rccm.201205-0972OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, Medoff BD, Rajagopal J. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503(7475):218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Matsuoka C, Wei SC, Sato A, Sato S, Hasegawa K, Chen HK, Ling TY, Mori M, Cardoso WV, Morimoto M. Epithelial Notch signaling regulates lung alveolar morphogenesis and airway epithelial integrity. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1511236113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136(13):2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Wei SC, Wu MF, Huang MT, Lin HY, Lee MC, Lin KM, Wang IJ, Kaartinen V, Yang LT, Cardoso WV. Notch signaling prevents mucous metaplasia in mouse conducting airways during postnatal development. Development. 2011;138(16):3533–3543. doi: 10.1242/dev.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, Matthay MA, Rock JR, Chapman HA. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517(7536):621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan MB, Ramirez RD, Wright WE, Minna JD, Shay JW. A three-dimensional model of differentiation of immortalized human bronchial epithelial cells. Differentiation. 2006;74(4):141–148. doi: 10.1111/j.1432-0436.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tian Y, Morley MP, Lu MM, Demayo FJ, Olson EN, Morrisey EE. Development and regeneration of Sox2+ endoderm progenitors are regulated by a Hdac1/2-Bmp4/Rb1 regulatory pathway. Dev Cell. 2013;24(4):345–358. doi: 10.1016/j.devcel.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett JA, Haitchi HM, Maeda Y. Intersections between pulmonary development and disease. Am J Respir Crit Care Med. 2011;184(4):401–406. doi: 10.1164/rccm.201103-0495PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol. 2012;30(9):876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Li A, Borok Z, Li C, Minoo P. NOTCH1 is required for regeneration of Clara cells during repair of airway injury. Stem Cells. 2012;30(5):946–955. doi: 10.1002/stem.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, Yang J, DeMayo FJ, Whitsett JA, Parmacek MS, Morrisey EE. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nature genetics. 2008;40(7):862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, Crum CP, Xian W, McKeon F. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517(7536):616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]