Abstract
The lipid transporter, ATP binding cassette, class A3 (ABCA3) is a highly conserved multi-membrane spanning protein that plays a critical role in the regulation of pulmonary surfactant homeostasis. Mutations in ABCA3 have been increasingly recognized as one of the causes of inherited pulmonary diseases. These monogenic disorders produce familial lung abnormalities with pathological presentations ranging from neonatal surfactant deficiency-induced respiratory failure to childhood or adult diffuse parenchymal lung diseases for which specific treatment modalities remain quite limited. More than 200 ABCA3 mutations have been reported to date with approximately three quarters of patients presenting as compound heterozygotes. Recent advances in our understanding of the molecular basis underlying normal ABCA3 biosynthesis and processing, as well as the mechanisms of alveolar epithelial cell dysregulation caused by the expression of its mutant forms, are beginning to emerge. These insights, as well as the role of environmental factors and modifier genes, are discussed in the context of the considerable variability in disease presentation observed in patients with identical ABCA3 gene mutations. Moreover, the opportunities afforded by an enhanced understanding of ABCA3 biology for targeted therapeutic strategies are addressed.
Keywords: ABCA3, Pulmonary surfactant, Alveolar type 2 cells, Lamellar bodies, ABCA3 mutations, Compound heterozygous mutations, Surfactant deficiency, Respiratory distress, Diffuse parenchymal lung disease, Idiopathic pulmonary fibrosis
Introduction
The epithelial monolayer of the distal lung, shown to be critically important for alveolar gas exchange, is coated with a thin film of a surface active agent (=“surfactant”) composed of a complex mixture of lipid and protein that functions to prevent atelectasis by reducing the mechanical forces (i.e. surface tension) that tend to promote the collapse of alveolar units, particularly at end expiration (Schurch and Roach, 1976; Wright and Dobbs, 1991; Batenburg, 1992). Pulmonary surfactant (isolated from various - mammalian species by differential and gradient centrifugation) is composed predominantly of phospholipids, neutral lipids and cholesterol (totaling 90% by weight). In addition, while disproportionately low, the protein constituents of surfactant (10% by weight) have been shown to include several unique proteins that either contribute to the biophysical activity of surfactant lipids or play a key role in lung mucosal host defense (Batenburg, 1992; Wright and Dobbs, 1991). Named in order of their discovery, surfactant proteins (SP)-A, SP-B, SP-C, and SP-D are each synthesized primarily by AT2 cells; however, with the exception of SP-C, they have also been shown to be produced in lesser amounts by other cells types of the distal lung, namely Clara (Club) cells (Rooney et al., 1994; Batenburg and Haagsman, 1998). In addition, SP-A and SP-D have also been shown to be expressed at barrier surfaces of other organs (e.g. stomach, small intestines, colon, kidney, inner ear) (Motwani et al., 1995; Fisher and Mason, 1995; Paananen et al., 2001).
The alveolar surfactant pool size is controlled primarily by AT2 cells that regulate both its biosynthesis and secretion as well as play a contributory role in its re-uptake from the alveolar space. Prior to secretion, synthesized surfactant lipids along with the four surfactant proteins are packaged and stored in multilamellated, lysosome like organelles named lamellar bodies (LBs) which can be readily identified within AT2 cells using transmission electron microscopy. LBs fuse with the AT2 plasma membrane and secrete their contents into the alveolar lumen by classic regulated exocytosis (Ryan et al., 1975). AT2 cells also endocytose surfactant from the alveolar space, some of which is recycled to LBs (Hallman et al., 1981; Fisher and Chander, 1985; Chander et al., 1987; Young et al., 1993) with the remainder subjected to lysosomal degradation (Chander et al., 1987). Alveolar macrophages have also been shown to participate in uptake and degradation of surfactant phospholipid, SP-A, SP-B, and SP-C and thus represent a second cellular component involved in surfactant homeostasis (Wright, 1990; Wright and Youmans, 1995; Bates and Fisher, 1996; Dong and Wright, 1998).
The adenosine triphosphate (ATP) binding cassette subfamily A, member 3 (ABCA3) glycoprotein is a member of the evolutionarily highly conserved multispan transmembrane ABC superfamily of transporters that uses the energy of ATP hydrolysis to translocate a variety of substrates across cell membranes, ranging from small ions (e.g. Cl− by ABCC7 (cystic fibrosis transmembrane conductance regulator (CFTR)) to large molecules (e.g. cholesterol by ABCA1). Twelve human ABC transporters belonging to the A subfamily (ABCA1–ABCA12) and two pseudogenes (ABCA11P and ABCA17P) have been identified to date (Bullard et al., 2005; Piehler et al., 2006; Tusnady et al., 2006). The expression patterns and functional data for ABCA transporters other than ABCA3, such as ABCA1, ABCA2, ABCA4, ABCA6, ABCA7, ABCA9, ABCA10, and ABCA12, suggest a distinct role for each of these transporters in lipid homeostasis functioning in various subcellular compartments (Kelsell et al., 2005; Albrecht and Viturro, 2007; Kaminski et al., 2006). The ABCA3 gene has been mapped to chromosome 16p13.3 and contains 30 exons encoding a 1,704-amino acid (180 kDa) protein (Connors et al., 1997; Klugbauer and Hofmann, 1996). Structure prediction algorithms suggest that ABCA3 is typical of most ABC transporters, consisting of four core domains forming a minimal functional unit having two similarly structured halves (Higgins et al., 1986) (Fig. 1, left and Fig. 3). Each half contains a set of six transmembrane domains and a cytosolic ATP binding cassette (ABC) (nucleotide-binding domain (NBD)) which includes two conserved peptide motifs known as Walker A and Walker B (present in many other proteins that utilize ATP for catalytic energy) and a Walker C signature motif unique to ABCA transporters (Dean et al., 2001). The homology of ABCA3 with other better-characterized ABC transporters suggests that the two sets of transmembrane domains create a conduit within the membrane phospholipid bilayer and that the two NBDs (NBD1 and NBD2) couple the energy of ATP hydrolysis for lipid translocation into the lumen of intracellular compartments (Tusnady et al., 2006; Ban et al., 2007; Kaminski et al., 2006; Kos and Ford, 2009). Although the ABCA3 transporter is found in many tissues including stomach, intestine, liver, kidney, and brain, it is highly expressed in the lung in an AT2-cell specific manner (Mulugeta et al., 2002; Yamano et al., 2001). In AT2 cells, ABCA3 is preferentially trafficked to the limiting membrane of the LBs (Mulugeta et al., 2002; Zen et al., 1998), and thus is optimally positioned to promote lipid transport across this membrane barrier. While the complete substrate repertoire and specificity has eluded characterization, ABCA3 has been shown to inwardly transport a variety of phospholipid species into LBs of AT2 cells and is also potentially implicated in the uptake of cholesterol into other compartments (Cheong et al., 2006; Ban et al., 2007; Fitzgerald et al., 2007; Hammel et al., 2007; Cheong et al., 2007; Zarbock et al., 2015; Matsumura et al., 2007).
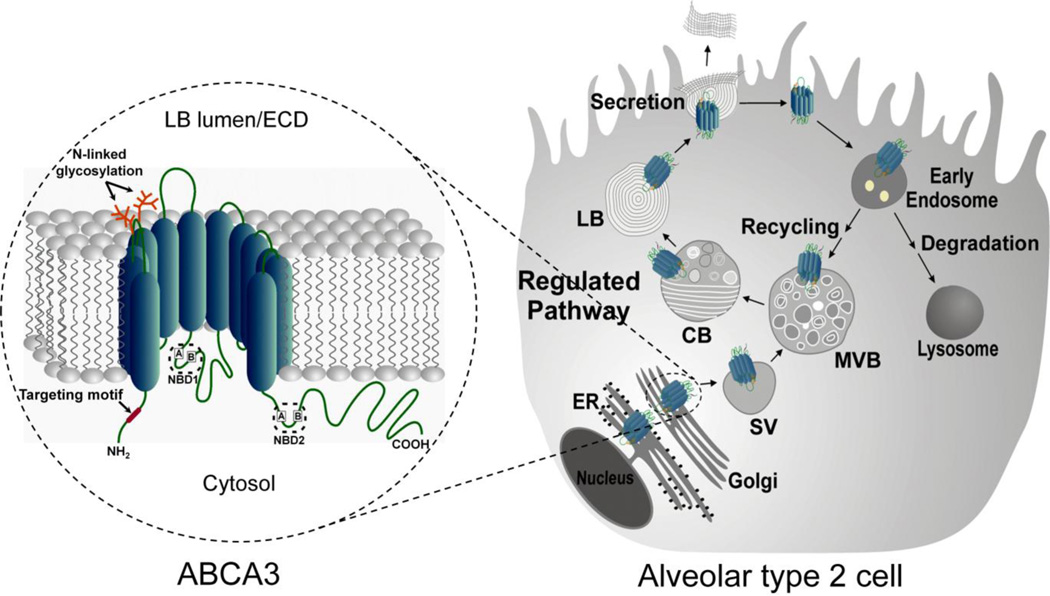
Fig. 1. Schematic representation of structure, biosynthetic processing and life-cycle of ABCA3 by the alveolar type 2 cell.
(Left) Predicted topological structure of the ABCA3 transporter. ABCA3 contains 12 putative membrane-spanning helices where the NH2- and COOH-terminal domains and the two nucleotide binding cassettes (NBD1 and NBD2) as well as the ABC signature motifs, Walker A and Walker B located within each NBD, face the cytosol. The NH2 targeting signal for Golgi exit (red) and the two glycosylation sites within the first luminal loop (orange) are shown. ECD, extracellular domain.
(Right) ABCA3 is synthesized and trafficked along with phospholipids and surfactant proteins (SP-A, SP-B, SP-C, SP-D). ABCA3 is initially routed to the post Golgi sorting vesicle (SV) and subsequently trafficked via the multivesicular body (MVB), composite body (CB) network to LB and plasma membrane. ABCA3 undergoes a posttranslational proteolytic cleavage within the proximal NH2-terminal region at distal post-Golgi compartments. While surfactant phospholipids and surfactant proteins contained in the LB are released by regulated exocytosis, ABCA3 remains in the LB membrane and is recycled or degraded in the lysosomes.
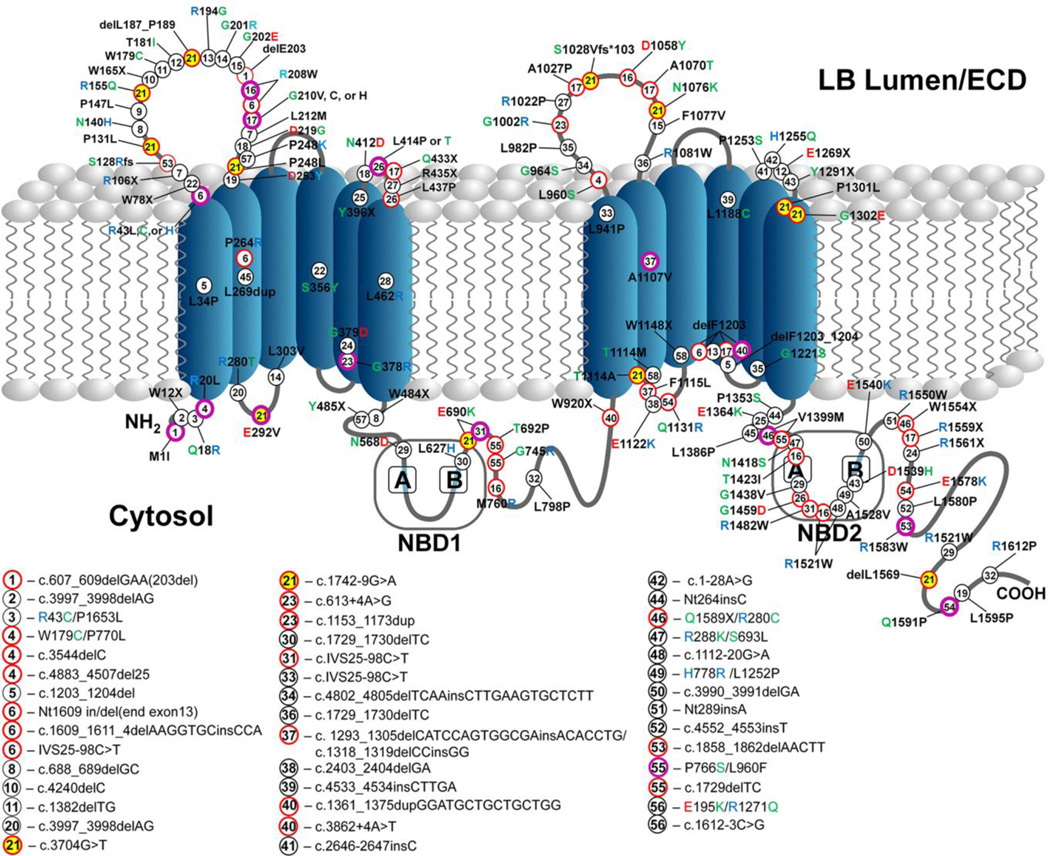
Fig. 3. Lung disease-associated compound heterozygous mutations of ABCA3.
Schematic illustration of published lung disease-associated ABCA3 compound heterozygous mutations with at least one of the mutations in the coding region.
Key:
Number in circle – mutations represented by matching numbers have been reported in a compound heterozygous state
Black circle – mutation reported with only one specific mutation in the second allele
Purple circle – mutation reported with multiple different mutations in the second allele
Red circle – trans allelic mutations corresponding to purple-circled mutations
Yellow highlights – Key example: A common variant, E292V (#21, purple–circled) and the 13 other red-circled mutations (#21) that have been identified in trans
Table beneath figure – Non-coding mutations, mutations that have been reported only by their nucleotide coding location, and mutations reported in cis
Amino acid letter color codes – Black = non polar (or X=stop), Green = polar, Red = negatively charge, Blue = positively charged
ECD, extracellular domain; NBD, nucleotide-binding domain
We have tried to cover as many of the published compound heterozygous mutations in the ABCA3 gene as possible and we apologize for any additional reported mutations we may have missed.
A growing body of evidence indicates that mutations in the ABCA3 gene are associated with familial lung diseases ranging from respiratory failure in term neonates to childhood interstitial lung disease (chILD) to idiopathic pulmonary fibrosis (IPF) and diffuse parenchymal lung disease (DPLD) in adults. In this review, we examine recent developments in understanding the normal biosynthesis of ABCA3 in AT2 cells and the mechanisms underlying lung disorders associated with ABCA3 mutation-induced AT2 cell dysregulation. We also address other contributing factors that are believed to increase lung disease severity in patients with ABCA3 mutations including environmental factors and genetic modifiers. Moreover, potential treatment approaches for patients with ABCA3 mutations (using therapeutic models that show promising outcomes in other disease-causing proteins that have structural similarities to ABCA3 and/or that provoke comparable cellular responses similar to those shown in the presence of ABCA3 mutations) are discussed.
Biosynthesis of ABCA3
As illustrated in Figure 1, studies of ABCA3 biosynthesis using both primary AT2 cells and other cell lines have identified several key domains involved in its intracellular trafficking to LBs that parallel, but remain distinct from, those found in surfactant proteins (Beers, 1998; Beers and Mulugeta, 2005; Mulugeta et al., 2015; Osanai et al., 1998; Guttentag, 2008). Following synthesis of the primary translation product and translocation to the ER, ABCA3 is routed via the Golgi, sorting vesicles (SVs), and multivesicular bodies (MVBs) directly to the outer membrane of LBs in AT2 cells or to lysosomes and lysosomal-related organelles in A549 and HEK293 cell lines (Cheong et al., 2006; Mulugeta et al., 2002; Nagata et al., 2004; Beers et al., 2013). Functionally essential posttranslational modifications including glycosylation and proteolytic cleavage also occur during this trafficking (discussed below). In AT2 cells, ABCA3 is trafficked further from LBs to the plasma membrane and, as for all surfactant-related protein and lipid components, it can be subsequently internalized and either recycled back to LBs and the plasma membrane or shunted to the lysosome for degradation (Schaller-Bals et al., 2000; Schaller-Bals et al., 2000).
Although the exact location remains to be specified, within post-Golgi compartments ABCA3 also undergoes posttranslational proteolysis at the proximal NH2-terminal region generating an ABCA3 product foreshortened by 30–40 kDa (Engelbrecht S et al., 2010). The underlying purpose behind this cleavage is yet to be elucidated since removal of such a large segment (~20% of the primary translation product and including up to three transmembrane domains) is likely to yield a profoundly altered structure of ABCA3 and to disrupt its function. Similar to CFTR, the emergence of a post-translationally modified isoform (in this case a cleaved ABCA3), could eventually serve as a surrogate biochemical marker for anterograde post-Golgi ABCA3 trafficking or its internalization to degradative compartments (Fig. 2, bottom panel).
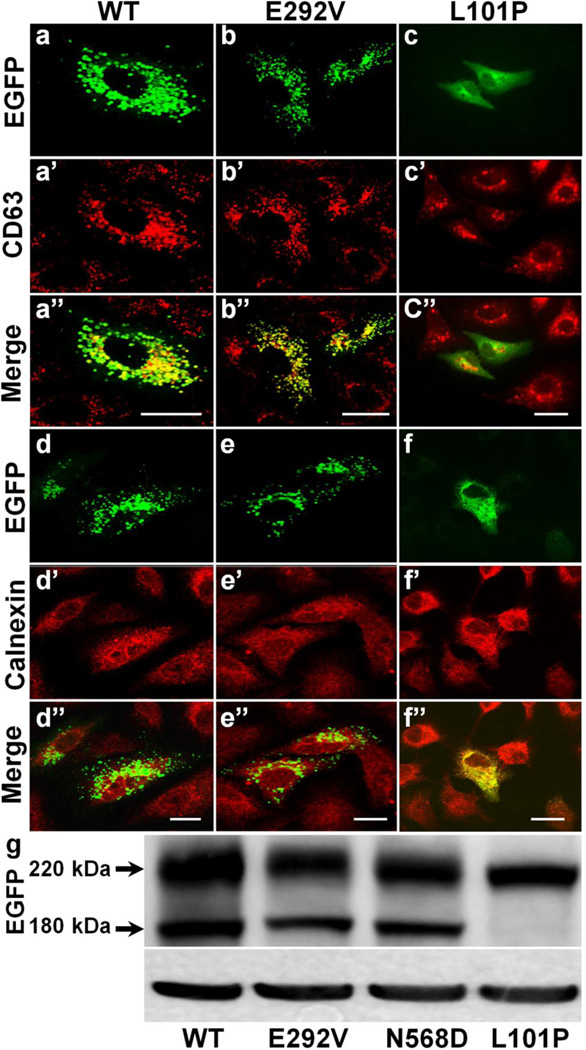
Fig. 2. Type I and Type II ABCA3 mutations.
(a–f) Representative immunostaining images of A549 cells expressing EGFP-tagged ABCA3 isoforms showing typical trafficking patterns of Type I and Type II mutations. Wild type (a, a’, a”, d, d’, d”) and Type II (E292V) functional deficient mutant (b, b’, b”, e, e’, e”) are normally trafficked to distal post-Golgi, CD63 positive, and calnexin negative lysosomal compartments, whereas Type I (L101P) trafficking defective mutant is retained within calnexin-positive and CD63-negative ER compartment (f, f’, f’,’ c, c’, c”). Bar, 5 µm.
(g) Representative anti-EGFP immunoblot of typical ABCA3 protein expression patterns from whole cell lysates of HEK293 cells expressing EGFP-tagged wild type (left lane), Type II mutants (E292V and N568D; middle two lanes), and a Type I mutant (L101P mutant; right lane). The primary translation products of the EGFP-tagged ABCA3 isoforms migrate at ~220 kDa. The presence of processed products (at 180 kDa) in wild type and Type II ABCA3 mutants indicates that similar to the wild type isoform, the Type II mutants are properly targeted to the lysosomes, but not the Type I mutant that is not processed supporting its ER localization shown in the above panels.
Functional domains of ABCA3
The structure of ABCA3 is schematically illustrated in Figure 1, left. Aside from the two nucleotide-binding domains (NBD1 and NBD2) found in every ABC transporter that serve as the functional unit for active substrate transport across membranes by utilizing the chemical energy of ATP hydrolysis (see (Linton, 2007) for review), two additional functional domains have been identified in the ABCA3 protein. First, the NH2 terminal domain of all ABCA transporters, with the exception of one (ABCA10), harbor a signature targeting motif comprising five or six amino acids (xLxxKN or xLxKN) (Mack et al., 2006; Kaminski et al., 2006). This motif was found not to be a targeting determinant for the final deposition of ABCA transporters, but rather it functions to direct proteins to proximal post Golgi sorting vesicles (SVs) (Beers et al., 2011). Because each ABCA transporter has a distinct subcellular destination and function, subsequent targeting of ABCA3 to LBs requires additional unidentified signal(s) (Kaminski et al., 2006; Beers et al., 2011).
Second, two distinct N-linked glycosylation sites at asparagine (N) residues 124 and 140 located within the first NH2-terminal luminal loop of the transporter are essential for ABCA3 stability (Fig. 1, left). N-linked glycosylation is one of the most common co-/posttranslational modifications that occurs during protein synthesis in the ER/Golgi and has been shown to have a pivotal role in the folding, stability, and cellular localization of proteins (Helenius and Aebi, 2001; Beers et al., 2011; Molinari, 2007). Cleavage profiles, generated using endoglycosidase H (Endo H) and N-gylycosidase F (PNGase F) that cleave N-linked glycans including complex carbohydrate chains, have confirmed the structural predictions that ABCA3 undergoes N-linked glycosylation (Cheong et al., 2006; Matsumura et al., 2006; Beers et al., 2013). Subsequent site directed mutagenesis studies have demonstrated that substitution mutations of asparagine to alanine at either or both of the 124 and 140 residues resulted in ER retention of glycan deficient ABCA3 isoforms and showed increased electrophoretic mobility of the expressed products by immunoblot (Beers et al., 2013). Moreover, while the levels of glycan deficient ABCA3 expression were markedly reduced, their levels can be rescued using proteasome inhibitors (Beers et al., 2013). Together, these studies suggest that glycosylation at two specific asparagine sites is critical for proper ABCA3 biosynthesis and that their disruption leads to protein destabilization and proteasomal degradation.
ABCA3: A key component in the formation of lamellar bodies and regulation of surfactant homeostasis
Localized at the outer membrane of LBs, ABCA3 functions as a transporter of lipids into the LBs of AT2 cells and it is now recognized as one of the critical regulators of LB biogenesis and lung surfactant metabolism (Bullard and Nogee, 2007; Bullard et al., 2005; Ban et al., 2007; Cheong et al., 2006). In both in vitro cell line models and in vivo mouse lung models, various studies have demonstrated that ABCA3 is involved in the transport regulation of phosphatidylcholine (PC), phosphatidylglycerol (PG), sphingomyelin, cholesterol, phosphatidylethanolamine, and phosphatidylserine (Cheong et al., 2006; Hammel et al., 2007; Matsumura et al., 2007; Cheong et al., 2007; Ban et al., 2007; Fitzgerald et al., 2007). Among these lipids, PC and PG represent the two most abundant phospholipids in surfactant accounting for 60–70% and ~9% by mass of the total mass of surfactant, respectively. Moreover, the disaturated dipalmytoyl PC (DPPC) constituting 41% of total PC plays a critical role in surface tension lowering properties of surfactant due to its unique properties. The saturated chains of DPPC can be packed at the air-liquid interface to reduce the surface tension required for stabilizing the lung at low lung volume (Hawco et al., 1981; Wustneck et al., 2005; Parra and Perez-Gil, 2015). The importance of ABCA3 in the regulation surfactant homeostasis is underscored by compelling evidence that term neonates with biallelic mutations in the ABCA3 gene suffer from surfactant deficiency and fatal respiratory distress (Shulenin et al., 2004; Garmany et al., 2006; Brasch et al., 2006; Kunig et al., 2007; Bruder et al., 2007; Saugstad et al., 2007; Anandarajan et al., 2009; Wambach et al., 2014). Bronchoalveolar lavage from these infants showed deficiency in both PC and PG with significantly reduced surface activity (Garmany et al., 2006). Ultrastructure examination of lung tissues from these infants also showed a complete lack of mature LBs that were replaced by numerous smaller and denser inclusion bodies lacking well developed lamellae (Shulenin et al., 2004).
Mouse models of ABCA3 deficient mice appear to mirror these findings. Homozygous Abca3 null mice die within a few hours following birth, primarily due to respiratory distress, while heterozygous mice have decreased levels of both PC and PG and fewer LBs (Cheong et al., 2007; Ban et al., 2007; Fitzgerald et al., 2007; Hammel et al., 2007). Other phospholipid species that have been reported to be dramatically reduced in these Abca3 null mice include phosphatidylethanolamine and phosphatidylserine (Cheong et al., 2007; Ban et al., 2007; Fitzgerald et al., 2007). At the ultrastructure level, AT2 cells in Abca3 knockout mice also showed an absence of normal LBs with morphological features similar to those observed in ABCA3 null infants (Cheong et al., 2007; Ban et al., 2007; Hammel et al., 2007; Fitzgerald et al., 2007; Shulenin et al., 2004).
ABCA3 mutations are associated with pulmonary disorders
In addition to their critical function in maintaining surfactant homeostasis, AT2 cells are also recognized as being crucial to the general health of the distal lung, participating in lung repair by serving as a progenitor cell population for replacement of long-lived AT2 and type 1 epithelial (AT1) cells following lung injury (Adamson and Bowden, 1975; Barkauskas et al., 2013; Spencer and Shorter, 1962). This has been supported by the observations from many groups that mutations in genes with predominantly AT2 cell-restricted expression, (e.g. SP-A (SFTPA), SP-C (SFTPC)) or which disrupt AT2 biology, (e.g. Hermansky-Pudlak syndrome, HPS genes), can induce AT2 cell dysfunction and parenchymal lung disease in children and adults (Wert et al., 2009; Stevens et al., 2005; Brasch et al., 2004; Guttentag et al., 2005; Willander et al., 2012).
Similarly, as Figure 3 schematically illustrates, over 200 distinct ABCA3 mutations have been identified and are recognized as the most prevalent group of mutations among genes associated with surfactant related lung disorders (Shulenin et al., 2004; Ota et al., 2016; Peca et al., 2015; Bullard et al., 2005; Garmany et al., 2006; Doan et al., 2008; Flamein et al., 2012; Wambach et al., 2014; Goncalves et al., 2014). Various types of coding and non-coding variants in the ABCA3 gene have been described including missense, nonsense, frameshift, insertion, deletion, and splice site mutations. The vast majority of ABCA3 mutations result in an ABCA3 null phenotype with the aforementioned surfactant deficient neonatal lung disease and death within the first months following birth. The primary mechanism for such consistently poor outcomes appears to be the presence of two null alleles, typically caused by the presence of nonsense or frameshift mutations (Wambach et al., 2014). Histopathological features of these disorders include variable degrees of pulmonary fibrosis with patterns of AT2 cell hyperplasia, interstitial thickening, and airspace infiltration of foamy macrophages that are frequently present with proteinaceous materials (Wert et al., 2009). These features are consistent with disorders that are commonly characterized by pathologists as chronic pneumonitis of infancy (CPI), nonspecific interstitial pneumonia (NSIP), neonatal or congenital pulmonary alveolar proteinosis (PAP), and infantile desquamative interstitial pneumonia (DIP) (Wert et al., 2009; Bullard et al., 2006; Dishop, 2011; Whitsett et al., 2015).
Less frequent are ABCA3 mutations that are associated with a more chronic phenotype that affect older children and adults often resulting in chILD, IPF, or DPLD (Bullard et al., 2005; Bullard and Nogee, 2007; Doan et al., 2008; Wert et al., 2009; Crossno et al., 2010; Epaud et al., 2014; Crossno et al., 2010; Ota et al., 2016). These disease causing ABCA3 mutations have been shown to result in either partial loss-of-function caused by aberrant ABCA3 protein trafficking or impaired ABCA3 lipid-pump function, or alternatively promote a toxic gain-of-function phenotype through induction of cell stress pathways (Matsumura et al., 2006; Cheong et al., 2006; Matsumura et al., 2008). The variable age of onset and overlapping phenotypic features among individuals with these mutations are considered to be due to confounding factors including the nature of the mutations, environmental factors, modifier genes, and clinical interventions (Wert et al., 2009; Young et al., 2008).
Functional classification of disease related ABCA3 mutations
In vitro mechanistic studies have identified three classes of ABCA3 mutations. Type I mutations, such as L101P (Fig. 2), L982P, Q1591P, and L1553P, result in protein misfolding and abnormal intracellular trafficking of the transporter. They are typically initially retained within the ER which results in their impaired processing, generation of an ER stress response, and induction of apoptotic cell death (Matsumura et al., 2006; Young et al., 2008; Weichert et al., 2011; Matsumura et al., 2008). Type II mutations are positioned within or adjacent to the catalytic domains (NBD1 and NBD2) of the transporter and include E292V, N568D, E690K, and T1114M (Fig. 3). While these are trafficked normally to LBs or lysosomal like organelles (LROs) (Fig. 2), these mutations result in a functional deficit in ATP hydrolysis and consequently impaired lipid transfer (Matsumura et al., 2006; Matsumura et al., 2008). Studies have shown that ATP hydrolysis by E292V mutant protein is moderately preserved, whereas the ATP hydrolysis activity in N568D, E690K, and T1114M mutant transporters is dramatically decreased (Matsumura et al., 2006). Finally, Type III mutations are compound heterozygotes (having both Type I and Type II mutations) and frequently exhibit a more severe phenotype. The most prevalent ABCA3 mutation in humans, E292V, is a Type II missense substitution producing an IPF/DPLD phenotype in children and adult patients that is often found in a compound heterozygous state with other Type I or II mutations (Garmany et al., 2008; Matsumura et al., 2008; Wambach et al., 2014; Kitazawa and Kure, 2015) (Fig. 3).
Although these in vitro studies have been instrumental in defining and categorizing broad classes of phenotypes resulting from alterations in the coding region of ABCA3, it has been challenging to model and predict the specific effect of individual mutations based solely on their location within the ABCA3 primary sequence. While some success in genotype-phenotype correlation has been possible with alterations located in the catalytic nucleotide-binding domains (i.e. mutations such as N568D and E690K are likely to disrupt lipid pump function (Matsumura et al., 2006; Matsumura et al., 2008), various studies have revealed limitations in our exact understanding of ABCA3 structure-function where other regions of ABCA3 are concerned. For example, it is unclear how certain functionally deficient mutations such as E292V or T1114M, both located distal to the catalytic domains, disrupt lipid transport. Thus the traditional paradigm for approaching structure-function analysis utilizing in vitro expression to assess trafficking, ATP hydrolysis, and/or lipid transport efficiency remains a reliable but low-throughput approach to understanding the mechanisms by which mutations alter ABCA3 expression and function. Future approaches would benefit from the successful generation of a 3D crystal structure (a highly challenging feat for multi-membrane spanning peptide evaluation), as well as from the development and optimization of high-throughput expression and functional screening assays.
The role of compound heterozygous mutations in ABCA3 mediated lung disease
As illustrated in Figure 3, compound heterozygous variants account for approximately three quarters of all report lung disease-associated ABCA3 mutations to date (Shulenin et al., 2004; Matsumura et al., 2008; Ota et al., 2016; Peca et al., 2015; Bullard et al., 2005; Garmany et al., 2006; Doan et al., 2008; Flamein et al., 2012; Wambach et al., 2014; Goncalves et al., 2014). Many of these mutations are clustered within the first and forth luminal loops as well as in the cytosolic domains, particularly within or adjacent to the second nucleotide-binding domain (NBD2). As previously stated, the presence of a combination of Type I (trafficking) and Type II (lipid pump) variants as compound heterozygous mutations appears to increase the disease severity in children as evidenced by the parents of these children having only one of the mutations and being asymptomatic without apparent respiratory disease (Kitazawa et al., 2013; Wambach et al., 2014; Flamein et al., 2012; Goncalves et al., 2014; Kitazawa and Kure, 2015). While ABCA3-associated lung disease is believed to be inherited in an autosomal recessive manner requiring mutations on both alleles, monoallelic ABCA3 mutations in infants with surfactant deficiency and in children and adults with IPF/DPLD are also common (Shulenin et al., 2004; Agrawal et al., 2012; Peca et al., 2015; Wambach et al., 2012; Naderi et al., 2014). Although the genetic mechanisms underlying the disease in these cases are unclear, it has been proposed that the genetic background of each individual (including potential genetic modifiers) as well as environmental factors may play a role in exacerbating disease severity in the presence of a vulnerable AT2 cell/lung expressing harmful ABCA3 mutant proteins (discussed below).
Moreover, a recent report by Wambach et al. (Wambach et al., 2014) clearly indicates that a number of these compound heterozygotes have two mutations within the same allele, where the contribution of each variant to disease etiology is unclear. However, given that there are some mutations in ABCA3 that lead to partial lipid pump function (Matsumura et al., 2006; Matsumura et al., 2008) or cause inefficient protein trafficking (Beers et al., 2011), the combination of such mutations in cis may have an additive or synergistic effect, with both mutations contributing to the overall dysfunction of the protein.
Impact of environmental factors and modifier genes on disease course
Substantial clinical variability in the age of onset or the severity of lung disease has been observed in children and adults carrying many of the ABCA3 mutations. While some of this may be attributed to variations in the ability of individual ABCA3 mutations to alter cellular homeostasis or trigger specific aberrant signaling pathways, it is also recognized that patients with the identical ABCA3 mutation may develop a very severe form of lung disease, manifest a mild form of disease, or show no symptoms at all suggesting a possible role for modifier genes and/or environmental factors. Since the lung is directly exposed to environmental insults, it has developed a natural ability to protect itself by both immunological and non-immunological mechanisms. While a healthy lung has a highly efficient lung injury/repair system, the risk of lung injury and disease incidence increases with increasing exposure to certain factors such as tobacco smoke, air pollutants, and pathogens. For example, IPF is found more frequently in patients with a history of cigarette smoke exposure (Baumgartner et al., 1997; Schwartz et al., 1994) and is considered one of the strongest associated risk factors for the development of IPF/DPLD (Steele et al., 2005). The most consistent evidence for environmental factors has been from epidemiologic and molecular surveys for exposure to Epstein-Barr virus, cytomegalovirus, hepatitis C, and human herpes virus-8 (Yonemaru et al., 1997; Tang et al., 2003; Lawson et al., 2008). One or more of these viruses have been detected in the lungs of up to 97% of tested patients with IPF (Egan et al., 1995; Tang et al., 2003). An in vitro study using A549 cells, an alveolar epithelial cell line, has shown a potentiating effect by respiratory syncytial virus (RSV) toward a phenotypic shift from epithelial to mesenchymal features (Kaltenborn et al., 2012). Whether the underlying mechanism of this effect is due to a direct interaction between ABCA3 protein and RSV, or is simply a function of global cellular damage by a pathogen, has yet to be elucidated. To date, there are no reports addressing the role viral infection plays in the cell biology of ABCA3 or describing the common mechanisms of disease progression as a result of direct interference of viruses (as well as other pathogens) with ABCA3 trafficking/function, suggesting that further studies in these areas are warranted. Nevertheless, the absence of lung disease in some individuals and the variable disease phenotype in others carrying ABCA3 mutations suggests a contribution by environmental insults, especially in the form of multiple hits (“a two hit model”) such as smoking and viral infection, among others. Thus, depending on the type of mutation, individuals harboring ABCA3 mutation-induced dysfunctional AT2 cells are likely to be more vulnerable to multiple hits that then drive a chronic abnormal lung injury/remodeling process to initiate lung disease and/or accelerate lung disease progression.
The role of modifier genes in exacerbating disease severity has been supported by various studies. In mice, multiple intestinal neoplasia is caused by dominant mutation of the Apc gene where the number of intestinal tumors depends on the Mom-1 (Modifier of Min-1) gene (Dietrich et al., 1993). In humans, a familiar example is sickle cell disease where patients who are homozygous for the sickle hemoglobin mutation can present with remarkably different clinical courses, varying from death in childhood, to multiple organ damage in adults, to being relatively well even until old age (Bunn, 1997; Steinberg, 1999). Various genetic loci have been identified that can modulate the sickle cell disease phenotype, including nucleotide motifs within the beta-globin gene cluster and genes located on different chromosomes (Chui and Dover, 2001). Modifier genes are often suggested to explain clinical variability in other monogenic disorders (Nadeau, 2001; Wolf, 1997). Surfactant protein gene variants, for example, may also act as modifiers. In a Danish cohort, carriers of the 121ins2 SFTPB variant who were also smokers have shown a twofold increased risk for developing COPD (Baekvad-Hansen et al., 2010a). In a separate study, an SFTPC mutation, SP-C A53T, was associated with a twofold increased risk for asthma (Baekvad-Hansen et al., 2010b). Perhaps the most relevant and compelling finding relative to this review is a study reporting that mutations in the ABCA3 gene can modify the severity of lung diseases associated with SFTPC mutation (Bullard and Nogee, 2007). This study examined the families of children carrying the SFTPC I73T mutation suffering from severe pulmonary phenotypes, with asymptomatic parents. They found that three of the four infants were also heterozygous for mutations in the ABCA3 gene, supporting the premise that ABCA3 mutations may act as a genetic modifier in SFTPC mutation-associated lung disorders. The role of specific modifier genes in altering the course of ABCA3 related lung disease is currently unknown but it appears logical that next generation/whole genome sequencing of ABCA3 cohorts approaches could provide additional information.
Strategies for ABCA3 therapeutics
Currently, no specific treatment exists for disorders caused by ABCA3 mutations. Establishing a detailed understanding of the molecular mechanisms by which aberrant expression of these genes induce cellular dysfunction and organ failure is paramount to providing insights for development of targeted therapies. Similar to the approaches to drug development for cystic fibrosis, which has been heavily invested in the correction of specific defects in mutant CFTR protein that emerged from a detailed mechanistic understanding of CFTR biology, ABCA3 mutant isoforms (whether functionally defective or mistargeted) could be subjected to the same therapeutic development strategy. Moreover, in directed cases, approaches aimed at reducing aberrant cellular responses to mutant protein expression including ER stress, inflammatory signaling/cytokine elaboration, and/or cell death could prove beneficial in the search for designing novel therapies for ABCA3-associated pulmonary disorders.
Modulation of protein trafficking by exogenous small molecules (chemical chaperones) is a viable therapeutic approach for disorders caused by mistrafficked and/or dysfunctional mutant proteins, but the feasibility in clinical application is challenging. In vitro, in vivo, and clinical studies have shown that the small molecule, 4-phenylbutyric acid (4-PBA) (an FDA approved drug for urea cycle disorders) enhances proper trafficking of the cystic fibrosis transmembrane conductance regulator variant (CFTRΔF508), as well as mutants of α-1 antitrypsin (α-1AT) and Alzheimer disease amyloid precursor protein (APP) through effects on heat shock protein expression (Wiley et al., 2010; Burrows et al., 2000; Rubenstein and Zeitlin, 2000). Addition of 4-PBA has also been demonstrated to prevent aggregate formation of hSPCΔExon4 mutant protein in vitro (Wang et al., 2003). Nevertheless, although 4-PBA has been recognized as a potential therapeutic drug for disorders including cystic fibrosis and α-1 anti-trypsin deficiency for more than a decade, reports on the development of advanced clinical trials for 4-PBA have been lacking, probably due to the paucity of positive data on improving clinical outcomes. Other small molecules including glycerol and trimethylamine oxide (TMAO) promote protein folding/trafficking and restore function in vitro (Brown et al., 1996; Sato et al., 1996). However, the therapeutic indices for both glycerol and TMAO were shown to be too narrow (toxicity limiting) to attain effective serum levels in preclinical mouse models expressing the CFTRΔF508 variant (Bai et al., 1998).
One promising advance has been in the technology of high-throughput screening that has allowed up to 100,000 small molecules to be tested simultaneously to identify those with the potential to improve the function of defective CFTR protein (Carlile et al., 2007; Galietta et al., 2001). Two main groups of drugs have been identified: “correctors” that correct trafficking of misfolded CFTR protein to the cell membrane and “potentiators” that improve chloride transport of dysfunctional CFTR mutants. Such model systems may have a potential application in analyzing the mistrafficked and dysfunctional mutant ABCA3 isoforms. Furthermore, the structural similarities shared between these two ABC transporters (ABCA3 and CFTR [ABCC7]) could have direct applicability in using and repurposing these existing CFTR compound libraries for directed therapy for ABCA3 mutations.
Concluding remarks
Rare monogenic disorders, such as those caused by ABCA3 mutations, provide unique opportunities to investigate the molecular pathways of cell/lung injury and remodeling. In the case of ABCA3 mutations (as well as other surfactant component mutations), the spatial restriction of their expression to AT2 cells in the lung supports the hypothesis that intrinsic epithelial cell injury and abnormal wound repair initiate and propagate the disruption of normal epithelial homeostasis and epithelial-fibroblast interaction to promote DPLD and fibrosis (see (Uhal and Nguyen, 2013; Mulugeta et al., 2015) for review). Genetic and environmental factors have now been generally implicated as contributors to phenotypic variation in many diseases including ABCA3 related disorders. However, the relative effect of each component is difficult to assess due to the myriad environmental factors and genetic modifiers that are involved. Despite these challenges, progress has been made in deciphering genetic and non-genetic factors underlying disease variability for several of the more common Mendelian disorders which has promoted the development of targeted therapeutic strategies. The challenge remains, especially for rare diseases where blinded, controlled evaluation of therapies has yet to be feasible. Molecular signatures for certain types of mutations that cause cell/lung injury in a mutation-specific fashion and the quality control systems employed by these cells in an attempt to sustain cellular homeostasis are some of the mechanisms that need to be dissected to both enhance our understanding of disease etiology and develop treatments strategies. Moreover, elucidating the contribution of environmental factors and genetic modifiers will further support this effort. In vitro strategies and animal models mimicking both the genotype and phenotype of ABCA3 mediated lung disease may provide the means to overcome some of these challenges.
Acknowledgments
Grants:
This work is supported by the National Institutes of Health (HL129150 to S. Mulugeta; HL119436 to M.F. Beers) and The Department of Veterans Affairs (VA Merit Award BX001176-05A1 to M.F. Beers). M.F. Beers is an Albert M. Rose Established Investigator of the Pulmonary Fibrosis Foundation.
Abbreviations
- AT2
Alveolar type 2
- LB
Lamellar body
- ABCA3
ATP binding cassette class A3
- SP-C
Surfactant Protein C
- SFTP(A, B, C, or D)
Gene encoding surfactant proteins A, B, C, or D
- DPLD
Diffuse parenchymal lung disease
- IPF
Idiopathic pulmonary fibrosis
- NBD
Nucleotide binding domain
Reference List
- Adamson IY, Bowden DH. Derivation of type 1 epithelium from type 2 cells in the developing rat lung. Lab Invest. 1975;32(6):736–745. [PubMed] [Google Scholar]
- Agrawal A, Hamvas A, Cole FS, Wambach JA, Wegner D, Coghill C, Harrison K, Nogee LM. An intronic ABCA3 mutation that is responsible for respiratory disease. Pediatric Research. 2012;71(6):633–637. doi: 10.1038/pr.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht C, Viturro E. The ABCA subfamily--gene and protein structures, functions and associated hereditary diseases. Pflugers Arch. 2007;453(5):581–589. doi: 10.1007/s00424-006-0047-8. [DOI] [PubMed] [Google Scholar]
- Anandarajan M, Paulraj S, Tubman R. ABCA3 Deficiency: an unusual cause of respiratory distress in the newborn. Ulster Med J. 2009;78(1):51–52. [PMC free article] [PubMed] [Google Scholar]
- Baekvad-Hansen M, Dahl M, Tybjaerg-Hansen A, Nordestgaard BG. Surfactant protein-B 121ins2 heterozygosity, reduced pulmonary function, and chronic obstructive pulmonary disease in smokers. Am J Respir Crit Care Med. 2010a;181(1):17–20. doi: 10.1164/rccm.200906-0963OC. [DOI] [PubMed] [Google Scholar]
- Baekvad-Hansen M, Nordestgaard BG, Tybjaerg-Hansen A, Dahl M. Two novel mutations in surfactant protein-C, lung function and obstructive lung disease. Respir Med. 2010b;104(3):418–425. doi: 10.1016/j.rmed.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Bai C, Biwersi J, Verkman AS, Matthay MA. A mouse model to test the in vivo efficacy of chemical chaperones. J Pharmacol. Toxicol. Methods. 1998;40(1):39–45. doi: 10.1016/s1056-8719(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Ban N, Matsumura Y, Sakai H, Takanezawa Y, Sasaki M, Arai H, Inagaki N. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J Biol Chem. 2007;282(13):9628–9634. doi: 10.1074/jbc.M611767200. [DOI] [PubMed] [Google Scholar]
- Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin. Invest. 2013;123(7):3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batenburg JJ. Surfactant phospholipids: synthesis and storage. Am J Physiol. 1992;262(4 Pt 1):L367–L385. doi: 10.1152/ajplung.1992.262.4.L367. [DOI] [PubMed] [Google Scholar]
- Batenburg JJ, Haagsman HP. The lipids of pulmonary surfactant: dynamics and interactions with proteins. Prog. Lipid Res. 1998;37(4):235–276. doi: 10.1016/s0163-7827(98)00011-3. [DOI] [PubMed] [Google Scholar]
- Bates SR, Fisher AB. Surfactant protein A is degraded by alveolar macrophages. Am J Physiol. 1996;271(2 Pt 1):L258–L266. doi: 10.1152/ajplung.1996.271.2.L258. [DOI] [PubMed] [Google Scholar]
- Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155(1):242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- Beers MF. Molecular Processing and Cellular Metabolism of Surfactant Protein C. In: Rooney SA, editor. In Lung Surfactant: Cellular and Molecular Processing. R.G. Landes: Austin, TX; 1998. pp. 93–124. [Google Scholar]
- Beers MF, Hawkins A, Shuman H, Zhao M, Newitt JL, Maguire JA, Ding W, Mulugeta S. A novel conserved targeting motif found in ABCA transporters mediates trafficking to early post-Golgi compartments. Journal of Lipid Research. 2011;52(8):1471–1482. doi: 10.1194/jlr.M013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers MF, Mulugeta S. Surfactant protein C biosynthesis and its emerging role in conformational lung disease. Annual Review of Physiology. 2005;67:663–696. doi: 10.1146/annurev.physiol.67.040403.101937. [DOI] [PubMed] [Google Scholar]
- Beers MF, Zhao M, Tomer Y, Russo SJ, Zhang P, Gonzales LW, Guttentag SH, Mulugeta S. Disruption of N-linked glycosylation promotes proteasomal degradation of the human ATP-binding cassette transporter ABCA3. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2013;305(12):L970–L980. doi: 10.1152/ajplung.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasch F, Griese M, Tredano M, Johnen G, Ochs M, Rieger C, Mulugeta S, Muller KM, Bahuau M, Beers MF. Interstitial lung disease in a baby with a de novo mutation in the SFTPC gene. European Respiratory Journal. 2004;24(1):30–39. doi: 10.1183/09031936.04.00000104. [DOI] [PubMed] [Google Scholar]
- Brasch F, Schimanski S, Muhlfeld C, Barlage S, Langmann T, Aslanidis C, Boettcher A, Dada A, Schroten H, Mildenberger E, Prueter E, Ballmann M, Ochs M, Johnen G, Griese M, Schmitz G. Alteration of the pulmonary surfactant system in full-term infants with hereditary ABCA3 deficiency. Am J Respir Crit Care Med. 2006;174(5):571–580. doi: 10.1164/rccm.200509-1535OC. [DOI] [PubMed] [Google Scholar]
- Brown CR, Hong-Brown LQ, Biwersi J, Verkman AS, Welch WJ. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress & Chaperones. 1996;1(2):117–125. doi: 10.1379/1466-1268(1996)001<0117:ccctmp>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder E, Hofmeister J, Aslanidis C, Hammer J, Bubendorf L, Schmitz G, Rufle A, Buhrer C. Ultrastructural and molecular analysis in fatal neonatal interstitial pneumonia caused by a novel ABCA3 mutation. Mod. Pathol. 2007;20(10):1009–1018. doi: 10.1038/modpathol.3800928. [DOI] [PubMed] [Google Scholar]
- Bullard JE, Nogee LM. Heterozygosity for ABCA3 mutations modifies the severity of lung disease associated with a surfactant protein C gene (SFTPC) mutation. Pediatric Research. 2007;62(2):176–179. doi: 10.1203/PDR.0b013e3180a72588. [DOI] [PubMed] [Google Scholar]
- Bullard JE, Wert SE, Nogee LM. ABCA3 deficiency: neonatal respiratory failure and interstitial lung disease. Semin. Perinatol. 2006;30(6):327–334. doi: 10.1053/j.semperi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Bullard JE, Wert SE, Whitsett JA, Dean M, Nogee LM. ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respir Crit Care Med. 2005;172(8):1026–1031. doi: 10.1164/rccm.200503-504OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N. Engl. J Med. 1997;337(11):762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(4):1796–1801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlile GW, Robert R, Zhang D, Teske KA, Luo Y, Hanrahan JW, Thomas DY. Correctors of protein trafficking defects identified by a novel high-throughput screening assay. Chembiochem. 2007;8(9):1012–1020. doi: 10.1002/cbic.200700027. [DOI] [PubMed] [Google Scholar]
- Chander A, Reicherter J, Fisher AB. Degradation of dipalmitoyl phosphatidylcholine by isolated rat granular pneumocytes and reutilization for surfactant synthesis. J Clin. Invest. 1987;79(4):1133–1138. doi: 10.1172/JCI112929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong N, Madesh M, Gonzales LW, Zhao M, Yu K, Ballard PL, Shuman H. Functional and Trafficking Defects in ATP Binding Cassette A3 Mutants Associated with Respiratory Distress Syndrome. Journal of Biological Chemistry. 2006;281(14):9791–9800. doi: 10.1074/jbc.M507515200. [DOI] [PubMed] [Google Scholar]
- Cheong N, Zhang H, Madesh M, Zhao M, Yu K, Dodia C, Fisher AB, Savani RC, Shuman H. ABCA3 Is Critical for Lamellar Body Biogenesis in Vivo. Journal of Biological Chemistry. 2007;282(33):23811–23817. doi: 10.1074/jbc.M703927200. [DOI] [PubMed] [Google Scholar]
- Chui DH, Dover GJ. Sickle cell disease: no longer a single gene disorder. Curr. Opin. Pediatr. 2001;13(1):22–27. doi: 10.1097/00008480-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Connors TD, Van Raay TJ, Petry LR, Klinger KW, Landes GM, Burn TC. The cloning of a human ABC gene (ABC3) mapping to chromosome 16p13.3. Genomics. 1997;39(2):231–234. doi: 10.1006/geno.1996.4500. [DOI] [PubMed] [Google Scholar]
- Crossno PF, Polosukhin VV, Blackwell TS, Johnson JE, Markin C, Moore PE, Worrell JA, Stahlman MT, Phillips JA, Loyd JE, Cogan JD, Lawson WE. Identification of Early Interstitial Lung Disease in an Individual With Genetic Variations in ABCA3 and SFTPC. Chest. 2010;137(4):969–973. doi: 10.1378/chest.09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. Journal of Lipid Research. 2001;42(7):1007–1017. [PubMed] [Google Scholar]
- Dietrich WF, Lander ES, Smith JS, Moser AR, Gould KA, Luongo C, Borenstein N, Dove W. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell. 1993;75(4):631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- Dishop MK. Paediatric interstitial lung disease: classification and definitions. Paediatr. Respir Rev. 2011;12(4):230–237. doi: 10.1016/j.prrv.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Doan ML, Guillerman RP, Dishop MK, Nogee LM, Langston C, Mallory GB, Sockrider MM, Fan LL. Clinical, radiological and pathological features of ABCA3 mutations in children. Thorax. 2008;63(4):366–373. doi: 10.1136/thx.2007.083766. [DOI] [PubMed] [Google Scholar]
- Dong Q, Wright JR. Degradation of surfactant protein D by alveolar macrophages. Am J Physiol. 1998;274(1 Pt 1):L97–L105. doi: 10.1152/ajplung.1998.274.1.L97. [DOI] [PubMed] [Google Scholar]
- Egan JJ, Stewart JP, Hasleton PS, Arrand JR, Carroll KB, Woodcock AA. Epstein-Barr virus replication within pulmonary epithelial cells in cryptogenic fibrosing alveolitis. Thorax. 1995;50(12):1234–1239. doi: 10.1136/thx.50.12.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht S, Kaltenborn E, Griese M, Kern S. The surfactant lipid transporter ABCA3 is N-terminally cleaved inside LAMP3-positive vesicles. FEBS Letters. 2010 doi: 10.1016/j.febslet.2010.09.026. (1873–3468 (Electronic)) [DOI] [PubMed] [Google Scholar]
- Epaud R, Delestrain C, Louha M, Simon S, Fanen P, Tazi A. Combined pulmonary fibrosis and emphysema syndrome associated with ABCA3 mutations Combined pulmonary fibrosis and emphysema syndrome associated with ABCA3 mutations. Eur. Respir J. 2014;43(2):638–641. doi: 10.1183/09031936.00145213. [DOI] [PubMed] [Google Scholar]
- Fisher AB, Chander A. Intracellular processing of surfactant lipids in the lung. Annu. Rev. Physiol. 1985;47:789–802. doi: 10.1146/annurev.ph.47.030185.004041. [DOI] [PubMed] [Google Scholar]
- Fisher JH, Mason R. Expression of pulmonary surfactant protein D in rat gastric mucosa. Am J Respir Cell Mol Biol. 1995;12(1):13–18. doi: 10.1165/ajrcmb.12.1.7811466. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, Xavier R, Haley KJ, Welti R, Goss JL, Brown CE, Zhuang DZ, Bell SA, Lu N, McKee M, Seed B, Freeman MW. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res. 2007;48(3):621–632. doi: 10.1194/jlr.M600449-JLR200. [DOI] [PubMed] [Google Scholar]
- Flamein F, Riffault L, Muselet-Charlier C, Pernelle J, Feldmann D, Jonard L, Durand-Schneider AM, Coulomb A, Maurice M, Nogee LM, Inagaki N, Amselem S, Dubus JC, Rigourd V, Bremont F, Marguet C, Brouard J, de BJ, Clement A, Epaud R, Guillot L. Molecular and cellular characteristics of ABCA3 mutations associated with diffuse parenchymal lung diseases in children. Hum. Mol Genet. 2012;21(4):765–775. doi: 10.1093/hmg/ddr508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galietta LV, Jayaraman S, Verkman AS. Cell-based assay for high-throughput quantitative screening of CFTR chloride transport agonists. Am J Physiol Cell Physiol. 2001;281(5):C1734–C1742. doi: 10.1152/ajpcell.2001.281.5.C1734. [DOI] [PubMed] [Google Scholar]
- Garmany TH, Moxley MA, White FV, Dean M, Hull WM, Whitsett JA, Nogee LM, Hamvas A. Surfactant composition and function in patients with ABCA3 mutations. Pediatric Research. 2006;59(6):801–805. doi: 10.1203/01.pdr.0000219311.14291.df. [DOI] [PubMed] [Google Scholar]
- Garmany TH, Wambach JA, Heins HB, Watkins-Torry JM, Wegner DJ, Bennet K, An P, Land G, Saugstad OD, Henderson H, Nogee LM, Cole FS, Hamvas A. Population and disease-based prevalence of the common mutations associated with surfactant deficiency. Pediatric Research. 2008;63(6):645–649. doi: 10.1203/PDR.0b013e31816fdbeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves JP, Pinheiro L, Costa M, Silva A, Goncalves A, Pereira A. Novel ABCA3 mutations as a cause of respiratory distress in a term newborn. Gene. 2014;534(2):417–420. doi: 10.1016/j.gene.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Guttentag S. Posttranslational regulation of surfactant protein B expression. Semin. Perinatol. 2008;32(5):367–370. doi: 10.1053/j.semperi.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttentag SH, Akhtar A, Tao JQ, Atochina E, Rusiniak ME, Swank RT, Bates SR. Defective Surfactant Secretion in a Mouse Model of Hermansky-Pudlak Syndrome. American Journal of Respiratory Cell and Molecular Biology. 2005;33(1):14–21. doi: 10.1165/rcmb.2004-0293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallman M, Epstein BL, Gluck L. Analysis of labeling and clearance of lung surfactant phospholipids in rabbit. Evidence of bidirectional surfactant flux between lamellar bodies and alveolar lavage. J Clin. Invest. 1981;68(3):742–751. doi: 10.1172/JCI110310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel M, Michel G, Hoefer C, Klaften M, Muller-Hocker J, de Angelis MH, Holzinger A. Targeted inactivation of the murine Abca3 gene leads to respiratory failure in newborns with defective lamellar bodies. Biochemical and Biophysical Research Communications. 2007;359(4):947–951. doi: 10.1016/j.bbrc.2007.05.219. [DOI] [PubMed] [Google Scholar]
- Hawco MW, Coolbear KP, Davis PJ, Keough KM. Exclusion of fluid lipid during compression of monolayers of mixtures of dipalmitoylphosphatidylcholine with some other phosphatidylcholines. Biochim. Biophys. Acta. 1981;646(1):185–187. doi: 10.1016/0005-2736(81)90286-8. [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291(5512):2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- Higgins CF, Hiles ID, Salmond GP, Gill DR, Downie JA, Evans IJ, Holland IB, Gray L, Buckel SD, Bell AW. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Kaltenborn E, Kern S, Frixel S, Fragnet L, Conzelmann KK, Zarbock R, Griese M. Respiratory syncytial virus potentiates ABCA3 mutation-induced loss of lung epithelial cell differentiation. Hum. Mol Genet. 2012;21(12):2793–2806. doi: 10.1093/hmg/dds107. [DOI] [PubMed] [Google Scholar]
- Kaminski WE, Piehler A, Wenzel JJ. ABC A-subfamily transporters: structure, function and disease. Biochim. Biophys. Acta. 2006;1762(5):510–524. doi: 10.1016/j.bbadis.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Kelsell DP, Norgett EE, Unsworth H, Teh MT, Cullup T, Mein CA, Dopping-Hepenstal PJ, Dale BA, Tadini G, Fleckman P, Stephens KG, Sybert VP, Mallory SB, North BV, Witt DR, Sprecher E, Taylor AE, Ilchyshyn A, Kennedy CT, Goodyear H, Moss C, Paige D, Harper JI, Young BD, Leigh IM, Eady RA, O'Toole EA. Mutations in ABCA12 underlie the severe congenital skin disease harlequin ichthyosis. Am J Hum. Genet. 2005;76(5):794–803. doi: 10.1086/429844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa H, Kure S. Interstitial Lung Disease in Childhood: Clinical and Genetic Aspects. Clin. Med Insights. Circ. Respir Pulm. Med. 2015;9(Suppl 1):57–68. doi: 10.4137/CCRPM.S23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa H, Moriya K, Niizuma H, Kawano K, Saito-Nanjo Y, Uchiyama T, Rikiishi T, Sasahara Y, Sakamoto O, Setoguchi Y, Kure S. Interstitial lung disease in two brothers with novel compound heterozygous ABCA3 mutations. Eur. J Pediatr. 2013;172(7):953–957. doi: 10.1007/s00431-013-1977-8. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Hofmann F. Primary structure of a novel ABC transporter with a chromosomal localization on the band encoding the multidrug resistance-associated protein. FEBS Letters. 1996;391(1–2):61–65. doi: 10.1016/0014-5793(96)00700-4. [DOI] [PubMed] [Google Scholar]
- Kos V, Ford RC. The ATP-binding cassette family: a structural perspective. Cell Mol Life Sci. 2009;66(19):3111–3126. doi: 10.1007/s00018-009-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunig AM, Parker TA, Nogee LM, Abman SH, Kinsella JP. ABCA3 deficiency presenting as persistent pulmonary hypertension of the newborn. J Pediatr. 2007;151(3):322–324. doi: 10.1016/j.jpeds.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, Miller GG, Loyd JE, Blackwell TS. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am. J. Physiol Lung Cell Mol. Physiol. 2008;294(6):L1119–L1126. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- Linton KJ. Structure and function of ABC transporters. Physiology (Bethesda.) 2007;22:122–130. doi: 10.1152/physiol.00046.2006. [DOI] [PubMed] [Google Scholar]
- Mack JT, Beljanski V, Tew KD, Townsend DM. The ATP-binding cassette transporter ABCA2 as a mediator of intracellular trafficking. Biomed. Pharmacother. 2006;60(9):587–592. doi: 10.1016/j.biopha.2006.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Ban N, Ueda K, Inagaki N. Characterization and classification of ATP-binding cassette transporter ABCA3 mutants in fatal surfactant deficiency. J Biol Chem. 2006 doi: 10.1074/jbc.M600071200. (0021–9258 (Print)) [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Ban N, Inagaki N. Aberrant catalytic cycle and impaired lipid transport into intracellular vesicles in ABCA3 mutants associated with nonfatal pediatric interstitial lung disease. AJP - Lung Cellular and Molecular Physiology. 2008;295(4):L698–L707. doi: 10.1152/ajplung.90352.2008. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Sakai H, Sasaki M, Ban N, Inagaki N. ABCA3-mediated choline-phospholipids uptake into intracellular vesicles in A549 cells. FEBS Letters. 2007;581(17):3139–3144. doi: 10.1016/j.febslet.2007.05.078. [DOI] [PubMed] [Google Scholar]
- Molinari M. N-glycan structure dictates extension of protein folding or onset of disposal. Nat. Chem. Biol. 2007;3(6):313–320. doi: 10.1038/nchembio880. [DOI] [PubMed] [Google Scholar]
- Motwani M, White RA, Guo N, Dowler LL, Tauber AI, Sastry KN. Mouse surfactant protein-D. cDNA cloning, characterization, and gene localization to chromosome 14. J Immunol. 1995;155(12):5671–5677. [PubMed] [Google Scholar]
- Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. Journal of Biological Chemistry. 2002;277(25):22147–22155. doi: 10.1074/jbc.M201812200. [DOI] [PubMed] [Google Scholar]
- Mulugeta S, Nureki SI, Beers MF. Lost after translation: Insights From Surfactant For Understanding The Role Of Alveolar Epithelial Dysfunction And Cell Quality Control In Fibrotic Lung Disease. AJP - Lung Cellular and Molecular Physiology. 2015 doi: 10.1152/ajplung.00139.2015. ajplung. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau JH. Modifier genes in mice and humans. Nat. Rev. Genet. 2001;2(3):165–174. doi: 10.1038/35056009. [DOI] [PubMed] [Google Scholar]
- Naderi HM, Murray JC, Dagle JM. Single mutations in ABCA3 increase the risk for neonatal respiratory distress syndrome in late preterm infants (gestational age 34–36 weeks) Am J Med Genet. A. 2014;164A(10):2676–2678. doi: 10.1002/ajmg.a.36660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Yamamoto A, Ban N, Tanaka AR, Matsuo M, Kioka N, Inagaki N, Ueda K. Human ABCA3, a product of a responsible gene for abca3 for fatal surfactant deficiency in newborns, exhibits unique ATP hydrolysis activity and generates intracellular multilamellar vesicles. Biochemical and Biophysical Research Communications. 2004;324(1):262–268. doi: 10.1016/j.bbrc.2004.09.043. [DOI] [PubMed] [Google Scholar]
- Osanai K, Mason RJ, Voelker DR. Trafficking of newly synthesized surfactant protein A in isolated rat alveolar type II cells. American Journal of Respiratory Cell and Molecular Biology. 1998;19(6):929–935. doi: 10.1165/ajrcmb.19.6.3292. [DOI] [PubMed] [Google Scholar]
- Ota C, Kimura M, Kure S. ABCA3 mutations led to pulmonary fibrosis and emphysema with pulmonary hypertension in an 8-year-old girl. Pediatric Pulmonology. 2016;51(6):E21–E23. doi: 10.1002/ppul.23379. [DOI] [PubMed] [Google Scholar]
- Paananen R, Sormunen R, Glumoff V, van Eijk M, Hallman M. Surfactant proteins A and D in Eustachian tube epithelium. American Journal Physiology (Lung Cellular Molecular Physiology) 2001;281(3):L660–L667. doi: 10.1152/ajplung.2001.281.3.L660. [DOI] [PubMed] [Google Scholar]
- Parra E, Perez-Gil J. Composition, structure and mechanical properties define performance of pulmonary surfactant membranes and films. Chem Phys. Lipids. 2015;185:153–175. doi: 10.1016/j.chemphyslip.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Peca D, Cutrera R, Masotti A, Boldrini R, Danhaive O. ABCA3, a key player in neonatal respiratory transition and genetic disorders of the surfactant system. Biochemical Society Transactions. 2015;43(5):913–919. doi: 10.1042/BST20150100. [DOI] [PubMed] [Google Scholar]
- Piehler AP, Wenzel JJ, Olstad OK, Haug KB, Kierulf P, Kaminski WE. The human ortholog of the rodent testis-specific ABC transporter Abca17 is a ubiquitously expressed pseudogene (ABCA17P) and shares a common 5' end with ABCA3. BMC. Mol Biol. 2006;7:28. doi: 10.1186/1471-2199-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney SA, Young SL, Mendelson CR. Molecular and cellular processing of lung surfactant. FASEB Journal. 1994;8(12):957–967. doi: 10.1096/fasebj.8.12.8088461. [DOI] [PubMed] [Google Scholar]
- Rubenstein RC, Zeitlin PL. Sodium 4-phenylbutyrate downregulates Hsc70: implications for intracellular trafficking of DeltaF508-CFTR. Am J Physiol Cell Physiol. 2000;278(2):C259–C267. doi: 10.1152/ajpcell.2000.278.2.C259. [DOI] [PubMed] [Google Scholar]
- Ryan US, Ryan JW, Smith DS. Alveolar type II cells: studies on the mode of release of lamellar bodies. Tissue Cell. 1975;7(3):587–599. doi: 10.1016/0040-8166(75)90028-2. [DOI] [PubMed] [Google Scholar]
- Sato S, Ward CL, Krouse ME, Wine JJ, Kopito RR. Glycerol Reverses the Misfolding Phenotype of the Most Common Cystic Fibrosis Mutation. Journal of Biological Chemistry. 1996;271(2):635–638. doi: 10.1074/jbc.271.2.635. [DOI] [PubMed] [Google Scholar]
- Saugstad OD, Hansen TW, Ronnestad A, Nakstad B, Tollofsrud PA, Reinholt F, Hamvas A, Coles FS, Dean M, Wert SE, Whitsett JA, Nogee LM. Novel mutations in the gene encoding ATP binding cassette protein member A3 (ABCA3) resulting in fatal neonatal lung disease. Acta Paediatr. 2007;96(2):185–190. doi: 10.1111/j.1651-2227.2007.00016.x. [DOI] [PubMed] [Google Scholar]
- Schaller-Bals S, Bates SR, Notarfrancesco K, Tao JQ, Fisher AB, Shuman H. Surface-expressed lamellar body membrane is recycled to lamellar bodies. AJP - Lung Cellular and Molecular Physiology. 2000;279(4):L631–L640. doi: 10.1152/ajplung.2000.279.4.L631. [DOI] [PubMed] [Google Scholar]
- Schurch SF, Roach MR. Interference of bronchographic agents with lung surfactant. Respir Physiol. 1976;28(1):99–117. doi: 10.1016/0034-5687(76)90088-8. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Van Fossen DS, Davis CS, Helmers RA, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149(2 Pt 1):444–449. doi: 10.1164/ajrccm.149.2.8306043. [DOI] [PubMed] [Google Scholar]
- Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M. ABCA3 Gene Mutations in Newborns with Fatal Surfactant Deficiency. The New England Journal of Medicine. 2004;350(13):1296–1303. doi: 10.1056/NEJMoa032178. [DOI] [PubMed] [Google Scholar]
- Spencer H, Shorter RG. Cell turnover in pulmonary tissues. Nature. 1962;194:880. doi: 10.1038/194880a0. [DOI] [PubMed] [Google Scholar]
- Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, Burch LH, Wahidi MM, Phillips JA, III, Sporn TA, McAdams HP, Schwarz MI, Schwartz DA. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172(9):1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MH. Management of sickle cell disease. N. Engl. J Med. 1999;340(13):1021–1030. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- Stevens PA, Pettenazzo A, Brasch F, Mulugeta S, Baritussio A, Ochs M, Morrison L, Russo SJ, Beers MF. Nonspecific interstitial pneumonia, alveolar proteinosis, and abnormal proprotein trafficking resulting from a spontaneous mutation in the surfactant protein C gene. Pediatric Research. 2005;57(1):89–98. doi: 10.1203/01.PDR.0000147567.02473.5A. [DOI] [PubMed] [Google Scholar]
- Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, Brigham KL, Oates JA, Jr, Loyd JE, Stecenko AA. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin. Microbiol. 2003;41(6):2633–2640. doi: 10.1128/JCM.41.6.2633-2640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusnady GE, Sarkadi B, Simon I, Varadi A. Membrane topology of human ABC proteins. FEBS Letters. 2006;580(4):1017–1022. doi: 10.1016/j.febslet.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Uhal BD, Nguyen H. The Witschi Hypothesis revisited after 35 years: genetic proof from SP-C BRICHOS domain mutations. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2013;305(12):L906–L911. doi: 10.1152/ajplung.00246.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambach JA, Casey AM, Fishman MP, Wegner DJ, Wert SE, Cole FS, Hamvas A, Nogee LM. Genotype-phenotype correlations for infants and children with ABCA3 deficiency. Am J Respir Crit Care Med. 2014;189(12):1538–1543. doi: 10.1164/rccm.201402-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambach JA, Wegner DJ, Depass K, Heins H, Druley TE, Mitra RD, An P, Zhang Q, Nogee LM, Cole FS, Hamvas A. Single ABCA3 mutations increase risk for neonatal respiratory distress syndrome. Pediatrics. 2012;130(6):e1575–e1582. doi: 10.1542/peds.2012-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WJ, Mulugeta S, Russo SJ, Beers MF. Deletion of exon 4 from human surfactant protein C results in aggresome formation and generation of a dominant negative. Journal of Cell Science. 2003;116(4):683–692. doi: 10.1242/jcs.00267. [DOI] [PubMed] [Google Scholar]
- Weichert N, Kaltenborn E, Hector A, Woischnik M, Schams A, Holzinger A, Kern S, Griese M. Some ABCA3 mutations elevate ER stress and initiate apoptosis of lung epithelial cells. Respir Res. 2011;12:4. doi: 10.1186/1465-9921-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wert SE, Whitsett JA, Nogee LM. Genetic disorders of surfactant dysfunction. Pediatric and Developmental Pathology. 2009;12(4):253–274. doi: 10.2350/09-01-0586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett JA, Wert SE, Weaver TE. Diseases of pulmonary surfactant homeostasis Diseases of pulmonary surfactant homeostasis. Annu. Rev. Pathol. 2015;10:371–393. doi: 10.1146/annurev-pathol-012513-104644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JC, Meabon JS, Frankowski H, Smith EA, Schecterson LC, Bothwell M, Ladiges WC. Phenylbutyric acid rescues endoplasmic reticulum stress-induced suppression of APP proteolysis and prevents apoptosis in neuronal cells. PLoS. One. 2010;5(2):e9135. doi: 10.1371/journal.pone.0009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willander H, Askarieh G, Landreh M, Westermark P, Nordling K, Keranen H, Hermansson E, Hamvas A, Nogee LM, Bergman T, Saenz A, Casals C, Aqvistg J, Jornvall H, Berglund H, Presto J, Knight SD, Johansson J. High-resolution structure of a BRICHOS domain and its implications for anti-amyloid chaperone activity on lung surfactant protein C. Proc. Natl. Acad. Sci. U. S. A. 2012;109(7):2325–2329. doi: 10.1073/pnas.1114740109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf U. Identical mutations and phenotypic variation. Human Genetics. 1997;100(3–4):305–321. doi: 10.1007/s004390050509. [DOI] [PubMed] [Google Scholar]
- Wright JR. Clearance and recycling of pulmonary surfactant 4. Am J Physiol. 1990;259(2 Pt 1):L1–L12. doi: 10.1152/ajplung.1990.259.2.L1. [DOI] [PubMed] [Google Scholar]
- Wright JR, Dobbs LG. Regulation of pulmonary surfactant secretion and clearance. Annu. Rev. Physiol. 1991;53:395–414. doi: 10.1146/annurev.ph.53.030191.002143. [DOI] [PubMed] [Google Scholar]
- Wright JR, Youmans DC. Degradation of surfactant lipids and surfactant protein A by alveolar macrophages in vitro. Am J Physiol. 1995;268(5 Pt 1):L772–L780. doi: 10.1152/ajplung.1995.268.5.L772. [DOI] [PubMed] [Google Scholar]
- Wustneck R, Perez-Gil J, Wustneck N, Cruz A, Fainerman VB, Pison U. Interfacial properties of pulmonary surfactant layers. Adv. Colloid Interface Sci. 2005;117(1–3):33–58. doi: 10.1016/j.cis.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Yamano G, Funahashi H, Kawanami O, Zhao LX, Ban N, Uchida Y, Morohoshi T, Ogawa J, Shioda S, Inagaki N. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Letters. 2001;508(2):221–225. doi: 10.1016/s0014-5793(01)03056-3. [DOI] [PubMed] [Google Scholar]
- Yonemaru M, Kasuga I, Kusumoto H, Kunisawa A, Kiyokawa H, Kuwabara S, Ichinose Y, Toyama K. Elevation of antibodies to cytomegalovirus and other herpes viruses in pulmonary fibrosis. Eur. Respir J. 1997;10(9):2040–2045. doi: 10.1183/09031936.97.10092040. [DOI] [PubMed] [Google Scholar]
- Young LR, Nogee LM, Barnett B, Panos RJ, Colby TV, Deutsch GH. Usual interstitial pneumonia in an adolescent with ABCA3 mutations. Chest. 2008;134(1):192–195. doi: 10.1378/chest.07-2652. [DOI] [PubMed] [Google Scholar]
- Young SL, Fram EK, Larson E, Wright JR. Recycling of surfactant lipid and apoprotein-A studied by electron microscopic autoradiography. Am J Physiol. 1993;265(1 Pt 1):L19–L26. doi: 10.1152/ajplung.1993.265.1.L19. [DOI] [PubMed] [Google Scholar]
- Zarbock R, Kaltenborn E, Frixel S, Wittmann T, Liebisch G, Schmitz G, Griese M. ABCA3 protects alveolar epithelial cells against free cholesterol induced cell death. Biochim. Biophys. Acta. 2015;1851(7):987–995. doi: 10.1016/j.bbalip.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Zen K, Notarfrancesco K, Oorschot V, Slot JW, Fisher AB, Shuman H. Generation and characterization of monoclonal antibodies to alveolar type II cell lamellar body membrane. Am. J. Physiol. (Lung Cell Mol. Physiol.) 1998;272:L172–L183. doi: 10.1152/ajplung.1998.275.1.L172. [DOI] [PubMed] [Google Scholar]