Abstract
Mechanisms underlying lymphocyte lineage stability and plasticity remain elusive. Recent work indicates that innate lymphoid cells (ILC) possess substantial plasticity. Whereas natural ILC2 (nILC2) produce type-2 cytokines, “plastic” inflammatory ILC2 (iILC2) can co-produce both type-2 cytokines and also the ILC3-characteristic cytokine IL-17. Mechanisms that elicit this lineage plasticity, and the importance in health and disease, remain unclear. Here we show that iILC2 are potent inducers of airway inflammation in response to acute house dust mite challenge. We find that Notch signaling induces lineage plasticity of mature ILC2 and drives the conversion of nILC2 into iILC2. Acute blockade of Notch signaling abolished functional iILC2, but not nILC2, in vivo. Exposure of isolated nILC2 to Notch ligands induced Rorc expression and elicited dual IL-13/IL-17 production, converting nILC2 into iILC2. Together these results reveal a novel role for Notch signaling in eliciting ILC2 plasticity and driving the emergence of highly pro-inflammatory innate lymphocytes.
Introduction
Effector lymphocyte subsets are usually viewed as lineages with unique master transcriptional regulators and distinct phenotype and function profiles. Nevertheless, “hybrid” lymphocytes exerting the functions of multiple effector lineages have been frequently observed, indicating the flexibility of lymphocyte responses. Lymphocyte plasticity underlies the heterogeneity and complexity of human autoimmune and inflammatory disorders, and it may also be harnessed to treat immune-mediated diseases (1). The cellular and molecular mechanisms that control lymphocyte lineage stability and plasticity, however, remain poorly understood.
Recent work indicates that innate lymphoid cells (ILC) possess substantial plasticity. ILC are classified into four lineages -- cytotoxic NK cells and three groups of cytokine-producing helper ILC. Nevertheless, trans-differentiation between group-1 innate lymphoid cells (ILC1) and ILC3 has been frequently observed (2–5). ILC2 can upregulate T-bet expression and acquire ILC1 phenotype and function in the inflamed lungs of COPD patients and in the intestinal samples of Crohn’s disease patients (6–8). A subset of ILC2 from healthy human donors also possesses the capability to co-produce IL-13 and IFN-γ as well as IL-22 (9, 10). The mechanisms that control the lineage stability and flexibility of ILC remain largely unclear.
The relationship between ILC2 and ILC3 is particularly intriguing. In mature ILC2, the transcription factors Bcl11b and Gfi-1 maintain the expression of ILC2 identity genes, and repress the expression of ILC3 signature genes (11, 12). In Bcl11b or Gfi-1 deficient mice, ILC2 gained the ability to produce IL-17, but lost Gata3 expression and the capability to produce IL-5 and IL-13 (11, 12). The lysine methyltransferase G9a plays a similar role in suppressing ILC3-characteristic genes and promoting ILC2 development (13). These studies collectively indicate that ILC2 and ILC3 are closely related subsets, and that the establishment of one lineage fate might require the repression of the other. Nevertheless, recent work also reports the existence of inflammatory type-2 innate lymphoid cells (iILC2) that possess robust functional capabilities of both ILC2 and ILC3 lineages (14). iILC2 emerge in mice after IL-25 treatment or during nematodes infection. iILC2 are phenotypically and developmentally similar to natural ILC2 (nILC2), but they differ from nILC2 in cytokine responsiveness and cytokine production capability. Whereas nILC2 respond to IL-33 and produce type-2 cytokines such as IL-13; iILC2 respond to IL-25 and can co-produce both IL-13 and IL-17. Consistent with their dual ILC2/ILC3 function, iILC2 express high amounts of GATA-3, and also a low level of Rorγt. The presence of iILC2 suggests that the key functions of ILC2 and ILC3 subsets can co-exist in one cell, and that the expression of the signature genes of these two lineages is not always exclusive (14). Indeed, human adult ILC2 from even healthy donors also express low amounts of Rorγt, and can co-produce IL13 and IL-22, indicating that dual ILC2/ILC3 capability might be common in human adult ILC (9, 10). Nevertheless, molecular mechanisms that drive the emergence of iILC2, and their importance in health and disease, remain unknown.
Here we report a new role for Notch signaling, a context-dependent transcriptional regulatory pathway, in eliciting mature ILC2 plasticity and converting nILC2 into iILC2. Acute blockade of Notch signaling abolished iILC2, but not nILC2, in vivo. Exposure of isolated nILC2 to Notch ligands in vitro altered their cytokine responsiveness and elicited co-production of IL-13 and IL-17. In mature ILC2, Notch transcriptional complex directly bound to the Rorc gene locus and promoted its expression, but did not affect the expression of Gata3, thus conferring ILC3-like capability without compromising primary ILC2 function. Together, our data uncover a novel role for Notch signaling in controlling the lineage plasticity of innate lymphocytes.
Materials and Methods
Mice
C57BL/6 (B6, CD45.2), B6-Ly5.2 (CD45.1), and Rag2−/−Il2rg−/− mice were purchased from the Jackson Laboratory or Taconic. To induce the emergence of iILC2, mice were intraperitoneally treated with 400ng recombinant IL-25 (R&D) daily for 4 days. For GSI treatment, 0.2 mg GSI (compound E, Calbiochem) in 200 ul DMSO were intraperitoneally injected at around 4 hours before each IL-25 treatment, daily for 4 days.
Anti-gD (human IgG1 isotype control) or neutralizing monoclonal antibodies specific for Notch1 or Notch2 (15) were injected intraperitoneally (IP) at 5 mg/kg on days 0 and 3. 400ng IL-25 was administrated IP daily for 4 days from days 1 to 4. 25 μg house-dust mite (HDM) extracts (Greer) were administrated intranasally daily for 3 days from days 5 to 8.
For adoptive transfer experiments, iILC2 or control nILC2 were isolated from the lungs of IL-25-treated C57BL/6 mice by flow cytometry cell sorting. 2 × 105 cells were transferred intravenously into Rag2−/−Il2rg−/− mice. Mice were intranasally challenged with 25 ug HDM extracts daily for 3 days. All animal experiments were conducted according to protocols approved by the Institutional Animal Care and Use Committee policies at Albany Medical College.
Flow cytometry, Cell sorting and ELISA
Antibodies used in this study were purchased from eBioscience, Biolegend, or MD Bioproducts. Flow cytometric analysis was performed on a Canto or LSR II Flow Cytometer (BD Biosciences), and cell sorting was performed on a FACSAria II (BD Biosciences). Supernatant of ILC2 culture was taken before PMA/ionomycin re-stimulation, and cytokine release was measured by a standard sandwich ELISA (ebioscience).
Chromatin Immunoprecipitation (CHIP)
To obtain sufficient number of ILC2 for CHIP, an immortalized ILC2 line was established with small intestinal laminal propria (SiLP) ILC2 from C57BL/6 mice by spontaneous mutation and selection (Supplemental Fig. 1). We named this cell line ILC2/b6 line. ILC2/b6 cells were maintained with 10ng/ml of IL-2, IL-7 and IL-33. For CHIP analysis, we grew ILC2/b6 cells on OP9-Dll1 stroma. Around 107 ILC2/b6 cells were re-purified by Magnet-activated cell sorting with CD45 beads (Miltenyi Biotech). CHIP was performed as we described (16). Primers to detect the CSL binding site at the Rorc locus were previously described (17).
Statistics
Comparison between two groups was done via two-sided Student’s t-test. Differences with a P-value less than 0.05 were considered significant.
Results and Discussion
Inflammatory ILC2 are potent inducers of airway inflammation in response to acute House-Dust Mite (HDM) challenge
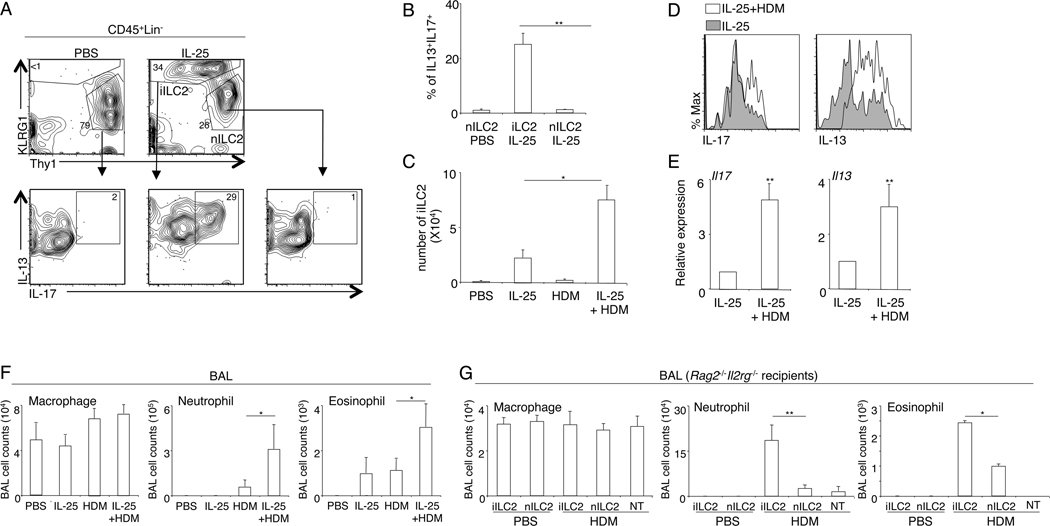
Previous work indicated that IL-25-responsive KLRG1hi iILC2 possess both ILC2 and ILC3-like capabilities (14). We induced the emergence of iILC2 by IL-25 treatment as previously described (14). We confirmed that mouse KLRG1hi iILC2, but not KLRG1int/lownILC2, were capable of co-producing IL-13 and IL-17 in vivo (Fig. 1A, 1B). Because increased airway IL-17 levels have been associated with severe asthma, we examined the capability of iILC2 to induce airway inflammation in response to common allergens. We first treated wild-type C57BL/6 mice with IL-25 to induce the emergence of iILC2 and then challenged the mice with HDM extracts daily for 3 days. Although exposure to HDM extracts alone was not sufficient to induce the development of iILC2, inhalation of HDM extracts increased the number of iILC2 and their expression of both IL-13 and IL-17 (Fig. 1C, D and E). Induction of iILC2 by IL-25 treatment greatly enhanced airway neutrophil infiltration in response to acute HDM challenge, and also increased eosinophil infiltration (Fig. 1F). To determine the role of iILC2 in promoting HDM-induced airway inflammation, we adoptively transferred iILC2 or control nILC2 into Rag2−/−Il2rg−/− mice, and examined their responses to acute HDM challenge. As compared with nILC2, iILC2 elicited much stronger airway inflammation in HDM-challenged mice (Fig. 1G). Much greater neutrophilic inflammation was observed in the BAL fluid of mice transferred with iILC2, and eosinophil infiltration was also slightly elevated (Fig. 1G). Neither iILC2 nor nILC2 induced spontaneous airway inflammation in PBS-treated mice (Fig. 1G). Thus, iILC2 can co-produce both the ILC2 signature cytokine IL-13 and also the ILC3-characteristic cytokine IL-17 in vivo, and they are potent inducers of airway inflammation in response to acute HDM challenge.
Figure 1. iILC2 are potent inducers of airway inflammation in response to acute HDM challenge.
A, C57B/6 mice were treated with PBS or IL-25 daily for 4 days. At 24 hours after the last treatment, lung hematopoietic cells were isolated and re-stimulated with PMA and ionomycin for 3 hours in the presence of monensin. Expression of IL-13 and IL-17 was examined by intracellular staining. Plots were pre-gated on CD45+Lin− cells. nILC2 were identified as CD45+Lin−KLRG1Int/loThy1hi cells. iILC2 were identified as CD45+Lin−KLRG1hiThy1lo/− cells. B, The percentage of cells that can co-produce IL-13 and IL-17 was examined. C, C57BL/6 mice were treated with IL-25 daily for 4 days, and then intranasally challenged with HDM extracts daily for 3 days. Lung iILC2 were examined by flow cytometry analysis at 24 hours after the last HDM challenge (or 96 hours after IL-25 treatment). D. Expression of IL-13 and IL-17 by iILC2 was examined by intracellular staining following PMA and ionomycin re-stimulation. Plots were pre-gated on iILC2 (CD45+Lin−KLRG1hiThy1lo/−). E. mRNA levels were examined by QPCR analysis with freshly isolated lung iILC2 without re-stimulation. Data were normalized to gapdh. F. BAL cell infiltration was examined at 24 hours after the last HDM challenge (or 96 hours after IL-25 treatment). G, 2X105 iILC2 or control nILC2 were were intravenously transferred into Rag2−/− Il2rg−/− mice. Mice were intranasally challenged with PBS or HDM extracts daily for 3 days. BAL immune cell infiltration was examined at 24 hours after the last challenge. NT, no-transfer control. Data are from three independent experiments. Error bars are mean ± SEM. * P<0.05; ** P<0.01.
Notch signaling drives the emergence of iILC2 in vivo
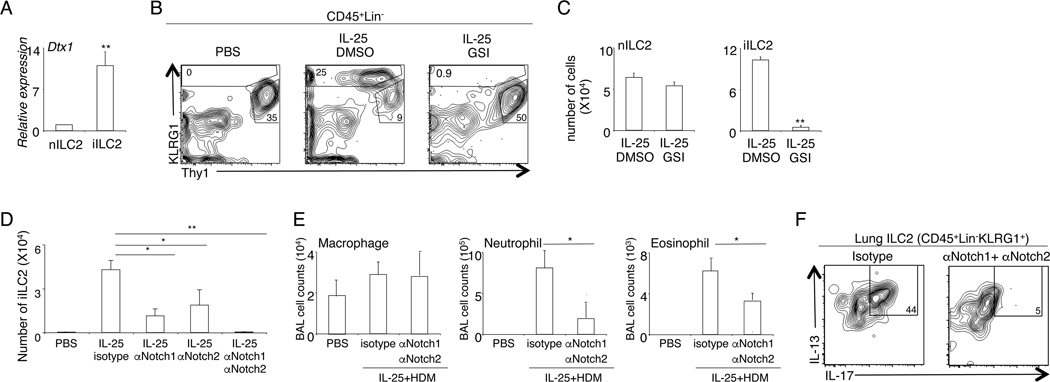
We next sought to understand the transcriptional regulatory mechanisms that drive the emergence of iILC2. Gene expression analysis revealed that, compared with nILC2, iILC2 expressed a much higher amount of Dtx1, a canonical Notch target gene (Fig. 2A). To investigate the role of Notch signaling in regulating the biology of iILC2, we treated the mice with GSI, a pan-Notch inhibitor. Acute GSI treatment did not affect the number of nILC2 in vivo, indicating that Notch signaling is dispensable for the short-time maintenance of nILC2 (Fig. 2B, 2C). Nevertheless, the appearance of KLRG1hi iILC2 was abolished in GSI-treated mice (Fig. 2B, 2C). Thus, Notch signaling is required for the emergence of iILC2 in vivo.
Figure 2. Notch signaling drives the emergence of iILC2.
A, nILC2 and iILC2 were sorted from IL-25-treated mice. The level of Dtx1 mRNA was examined by QPCR analysis. B, Mice were treated with control PBS, or IL-25, or IL-25 with GSI, daily for 4 days. Lung cells were examined by flow cytometry analysis. Plots were pre-gated on CD45+Lin−KLRG1+ cells. C, The number of nILC and iILC2 in mice treated with IL-25, or together with GSI, was quantified. D, C57BL/6 mice were treated with αNotch1 and/or αNotch2 or isotype control on day 0 and day 3. IL-25 was administrated daily for 4 days from day 1 to day 4. The numbers of lung iILC2 were examined by flow cytometry analysis at day 5. E, Mice were treated with αNotch1 and/or αNotch2 or isotype control together with IL-25 as described above. Mice were then challenged with HDM extracts daily for 3 days from day 5 to day 7. BAL cell infiltration was examined at 24 hours after the last HDM challenge. F, Expression of IL-13 and IL-17 by total ILC2 (CD45+Lin−KLRG1+) was examined by intracellular staining following PMA/ionomycin re-stimulation. Data are from three independent experiments. Error bars are mean ± SEM. * P<0.05; ** P<0.01.
To determine the specific Notch receptors implicated in the emergence of iILC2, we treated mice with selective therapeutic antibody antagonists of NOTCH1 (anti-NRR1) or NOTCH2 (anti-NRR2). Anti-NRR1 or anti-NRR2 alone reduced iILC2 numbers whereas administration of both antibodies together nearly completely abolished the emergence of iILC2 cells (Fig. 2D). Co-administration of anti-NRR1 and anti-NRR2 ameliorated both neutrophilic and eosinophilic inflammation in mice pre-treated with IL-25 and challenged with HDM (Fig. 2E). Intracellular cytokine staining confirmed that functional iILC2 capable of co-producing IL-13 and IL-17 were ablated in mice treated with the blocking antibodies (Fig. 2F). Together, these data indicate that both Notch1 and Notch2 receptors are required for the development of iILC2.
Notch signaling alters the cytokine responsiveness of ILC2
We attempted to identify the cells that receive Notch signaling and give rise to iILC2. Gene expression analysis suggested that mature nILC2 expressed both Notch1 and Notch2 (Supplemental Fig. 2A). We thus asked whether Notch signaling can convert mature nILC2 into iILC2. To test this hypothesis, we isolated nILC2 from naïve mice and co-cultured them with OP9-Dll1 or control OP9 stromal cells. The OP9-Dll1 stromal cells have been engineered to constitutively express the Notch ligand Dll1, thus allowing examination of the direct effects of Notch signaling on mature ILC2.
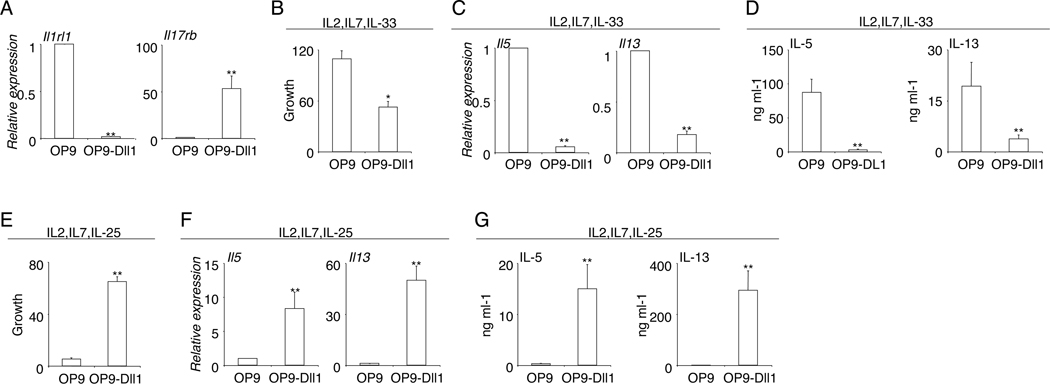
Murine iILC2 and nILC2 differ in cytokine responsiveness (14). Whereas nILC2 express high amounts of IL-33R and respond to IL-33; iILC2 express high levels of IL-25R and respond to IL-25 (14). We thus examined whether Notch signaling can alter the cytokine responsiveness of mature ILC2. Interestingly, exposure to the Notch ligand Dll1 resulted in a drastic reduction in the mRNA expression of Il1rl1, the gene encoding IL-33R (Fig. 3A). In contrast, the expression of Il17rb, the gene encoding IL-25R, was markedly increased in ILC2 co-cultured with OP9-Dll1 (Fig. 3A). Hence, the availability of Notch ligands controls the expression of the two major cytokine receptors, IL-33R and IL-25R, by ILC2, indicating that Notch signaling might alter the cytokine responsiveness of ILC2. We next directly assessed the role of Notch signaling in regulating the cytokine responsiveness of ILC2, by culturing mature nILC2 in the presence of either IL-33 or IL-25. In the presence of IL-33, the Notch ligand Dll1 repressed ILC2 expansion and severely reduced their capability to produce Il5 and Il13, indicating that Notch signaling inhibits ILC2 activation in response to IL-33 (Fig. 3B, 3C, 3D). In contrast, in the presence of IL-25, ILC2 proliferation was greatly enhanced in co-culture with OP9-Dll1 (Fig. 3E). The expression of cytokines Il5 and Il13 per cell was also greatly elevated with the availability of the Notch ligand Dll1, resulting in a considerable increase in cytokine production and release in response to IL-25 (Fig. 3F, 3G). Thus, Notch signaling controls the cytokine responsiveness of ILC2, converting IL-33-responsive nILC2 into IL-25-responsive iILC2.
Figure 3. Notch signaling alters the cytokine responsiveness of ILC2.
A, Sorted nILC2 from the lungs of naïve mice were co-cultured with OP9 or OP9-Dll1 stroma in the presence of 10ng/ml IL-2, IL-7 and Il-33 for 7 days. The expression of mRNA was examined by QPCR analysis. B, Sorted nILC2 from the lungs of naïve mice were co-cultured with OP9 or OP9-Dll1 stroma in the presence of 10ng/ml IL-2, IL-7 and IL-33 for 7 days. The growth of ILC2 was quantified. C, mRNA expression of cytokines was examined by QPCR analysis. D, The release of cytokine in the supernatant was examined by ELISA. F, Sorted nILC2 from the lungs of naïve mice were co-cultured with OP9 or OP9-Dll1 stroma in the presence of 10ng/ml IL-2, IL-7 and IL-25 for 7 days. The growth of ILC2 was quantified. G, mRNA was extracted and examined by QPCR analysis. H, The release of cytokine in the supernatant was examined by ELISA. Data are from three independent experiments. Error bars are mean ± SEM. ** P<0.01.
Notch signaling promotes co-production of IL-13 and IL-17 in ILC2
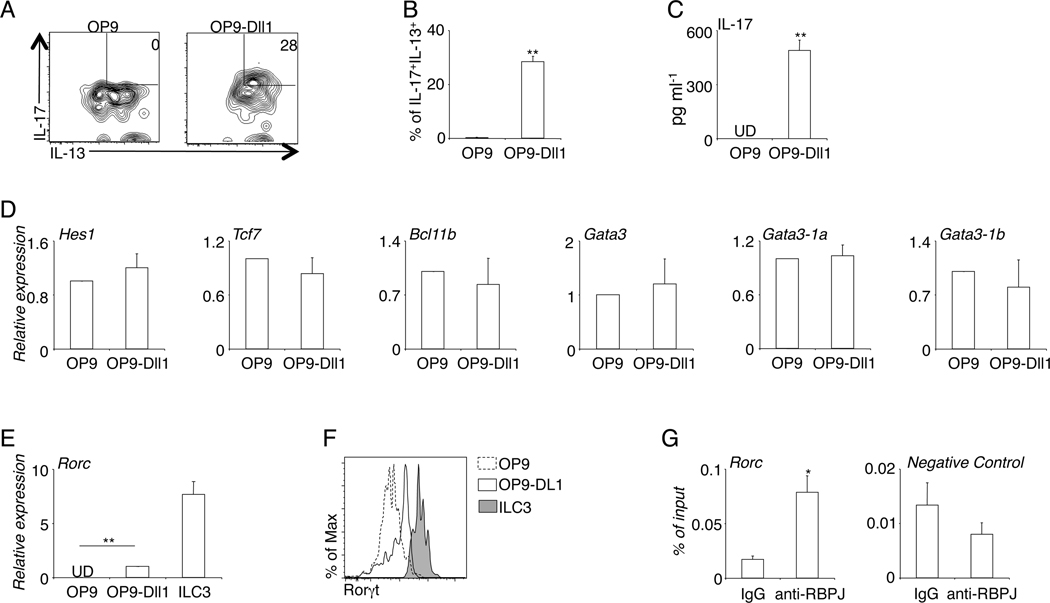
iILC2 differ from nILC2 by their capability to co-produce both type-2 cytokines and also the ILC3-characteristic cytokine IL-17. We investigated whether Notch signaling could promote this dual ILC2/ILC3 capability. We isolated nILC2 from naïve mice and cultured them on OP9-Dll1 or control OP9 stroma, in the presence of a combination of cytokines promoting ILC2 proliferation and Th17 cytokine production. As expected (14), nILC2 produced high amounts of IL-13, but not IL-17, on OP9 stroma (Fig. 4A, 4B). Exposure to the Notch ligand Dll1, however, elicited co-production of IL-13 and IL-17 from ILC2 (Fig. 4A, 4B, 4C). Thus, Notch signaling is sufficient to elicit IL-17 production from ILC2, without compromising their primary function to produce type-2 cytokines.
Figure 4. Notch signaling promotes dual IL-13/IL-17 production by ILC2.
A, Sorted nILC2 from the lungs of naïve mice were co-cultured with OP9 or OP9-Dll1 stroma for 7 days, in the presence of 10ng/ml IL-2, IL-7, IL-25, Il-33, IL-6, IL-1β, IL-23 and 5ng/ml of TGFβ. Cells were re-stimulated with PMA and ionomycin before intracellular staining for IL-13 and IL-17. B, The percentage of cultured ILC2 that co-produce IL-13 and IL-17 was quantified. C, The amount of IL-17 in the culture supernatant was examined by ELISA. D and E, mRNA of cultured ILC2 was extracted and examined by QPCR analysis. F, Expression of Rorγt was examined by intracellular staining. G. CHIP was performed with 107 ILC2/b6 cells. DNA region lacking CSL binding site was used as a negative control. Data are from three independent experiments. Error bars are mean ± SEM. * P<0.05; ** P<0.01.
To investigate the underlying molecular mechanisms by which Notch signaling elicited ILC2 plasticity, we examined and compared the gene expression patterns of ILC2 cultured on OP9-Dlll1 and those cultured on control OP9 stroma. Several transcription factors, including Hes-1, Tcf7 (encoding TCF-1), Bcl11b, and Gata3, are Notch target genes in developing or mature T cells. However, Notch signaling did not affect the expression of any of these transcription factors in mature ILC2 (Fig. 4D). We observed that, in mature ILC2, Notch signaling promoted the expression of Rorc, the ILC3 master transcription factor (Fig. 4E, 4F). ILC2 co-cultured with OP9-Dll1 stroma clearly upregulated Rorc expression as compared with those co-cultured with control OP9 stroma, although the level was lower than the expression by bona fida ILC3 (Fig. 4E, 4F). The intact Gata3 expression but increased Rorc expression might underlie the capability of Notch-elicited iILC2 to co-produce IL-13 and IL-17.
We next performed CHIP to examine whether Notch transcriptional complex might directly bind to the gene locus of Rorc. Because of the rarity of primary ILC2, we established an immortalized ILC2 cell line, named ILC2/b6 line, to obtain sufficient number of ILC2 for CHIP (Supplemental Fig. 1). CHIP with anti-RBPJ polyclonal antibody (Santa Cruz) revealed that Notch transcriptional complex directly bound to the Rorc promoter (Fig. 4G). This region contained a previous described consensus CSL binding site in Th17 cells (17). Together, these results indicate that Notch transcriptional complex directly binds to the Rorc gene locus and promotes its expression, conferring ILC3-like capability without compromising primary ILC2 function.
Our study identifies Notch signaling as an important transcriptional driver in eliciting the functional plasticity of innate lymphoid cells. The flexibility of innate lymphoid cell responses appears to be common in human homeostasis and disease (9, 10). A subset of primary human ILC2 from even healthy donor possess the capability to produce both ILC2- and ILC3- characteristic cytokines (9, 10). In mice, such dual ILC2/ILC3 capability was displayed by IL-25-responsive inflammatory ILC2. We predict that cells with dual ILC2/ILC3 function are important drivers in the pathology of autoimmune and inflammatory diseases such as asthma. Our results have revealed that Notch signaling can elicit ILC plasticity by converting nILC2 into iILC. We show that both Notch1 and Notch2 receptors are required for the development of iILC2, reminiscent of what we previously observed in peripheral mature T cell differentiation (18). We speculate that such transcriptional regulatory pathways that induce lymphocyte lineage flexibility underwrite the heterogeneity and diversity of immune responses in human diseases.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants K22-AI116728 (Q.Y.), RO1-AI091627 (I.M.), and R01-GM105949 (to K.C.M.), Leukemia and Lymphoma Society Grants CDP 1227-14 and TRP 6462-15 (I.M.), the NIH Intramural Research Programs of the National Institute of Allergy and Infectious Diseases (J.Z.), and of the National Cancer Institute, and the Center for Cancer Research (A.B.), and Natural Science Foundation of China (T.L.).
Abbreviations used in this article
- ILC
innate lymphoid cells
- GSI
γ-secretase inhibitor
- CHIP
Chromatin Immunoprecipitation
- BAL
bronchoalveolar lavage
- HDM
house dust mite
References
- 1.DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol. 2016;16:149–163. doi: 10.1038/nri.2015.18. [DOI] [PubMed] [Google Scholar]
- 2.Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed-Nielsen M, Munneke JM, Hazenberg MD, Villaudy J, Buskens CJ, Bemelman WA, Diefenbach A, Blom B, Spits H. Interleukin-12 and -23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity. 2015;43:146–160. doi: 10.1016/j.immuni.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Holscher C, Honig M, Pannicke U, Schwarz K, Ware CF, Finke D, Diefenbach A. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, Mao C, Giovenzana C, Nuovo G, Wei L, Zhang X, Gavrilin MA, Wewers MD, Caligiuri MA. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silver JS, Kearley J, Copenhaver AM, Sanden C, Mori M, Yu L, Pritchard GH, Berlin AA, Hunter CA, Bowler R, Erjefalt JS, Kolbeck R, Humbles AA. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. 2016;17:626–635. doi: 10.1038/ni.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohne Y, Silver JS, Thompson-Snipes L, Collet MA, Blanck JP, Cantarel BL, Copenhaver AM, Humbles AA, Liu YJ. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol. 2016;17:646–655. doi: 10.1038/ni.3447. [DOI] [PubMed] [Google Scholar]
- 8.Lim AI, Menegatti S, Bustamante J, Le Bourhis L, Allez M, Rogge L, Casanova JL, Yssel H, Di Santo JP. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J Exp Med. 2016;213:569–583. doi: 10.1084/jem.20151750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teunissen MB, Munneke JM, Bernink JH, Spuls PI, Res PC, Te Velde A, Cheuk S, Brouwer MW, Menting SP, Eidsmo L, Spits H, Hazenberg MD, Mjosberg J. Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR(+) ILC3 in lesional skin and blood of psoriasis patients. J Invest Dermatol. 2014;134:2351–2360. doi: 10.1038/jid.2014.146. [DOI] [PubMed] [Google Scholar]
- 10.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 11.Califano D, Cho JJ, Uddin MN, Lorentsen KJ, Yang Q, Bhandoola A, Li H, Avram D. Transcription Factor Bcl11b Controls Identity and Function of Mature Type 2 Innate Lymphoid Cells. Immunity. 2015;43:354–368. doi: 10.1016/j.immuni.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spooner CJ, Lesch J, Yan D, Khan AA, Abbas A, Ramirez-Carrozzi V, Zhou M, Soriano R, Eastham-Anderson J, Diehl L, Lee WP, Modrusan Z, Pappu R, Xu M, DeVoss J, Singh H. Specification of type 2 innate lymphocytes by the transcriptional determinant Gfi1. Nat Immunol. 2013;14:1229–1236. doi: 10.1038/ni.2743. [DOI] [PubMed] [Google Scholar]
- 13.Antignano F, Braam M, Hughes MR, Chenery AL, Burrows K, Gold MJ, Oudhoff MJ, Rattray D, Halim TY, Cait A, Takei F, Rossi FM, McNagny KM, Zaph C. G9a regulates group 2 innate lymphoid cell development by repressing the group 3 innate lymphoid cell program. J Exp Med. 2016;213:1153–1162. doi: 10.1084/jem.20151646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, Williamson PR, Urban JF, Jr, Paul WE. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol. 2015;16:161–169. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ, Shelton A, Stawicki S, Watts RJ, Zhang J, Choy R, Howard P, Kadyk L, Yan M, Zha J, Callahan CA, Hymowitz SG, Siebel CW. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, Monticelli LA, Saenz SA, Chi AW, Sonnenberg GF, Tang J, Obaldia ME, Bailis W, Bryson J, Toscano K, Huang J, Haczku A, Pear WS, Artis D, Bhandoola A. T Cell Factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38:694–704. doi: 10.1016/j.immuni.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee S, Schaller MA, Neupane R, Kunkel SL, Lukacs NW. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. J Immunol. 2009;182:7381–7388. doi: 10.4049/jimmunol.0804322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran IT, Sandy AR, Carulli AJ, Ebens C, Chung J, Shan GT, Radojcic V, Friedman A, Gridley T, Shelton A, Reddy P, Samuelson LC, Yan M, Siebel CW, Maillard I. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest. 2013;123:1590–1604. doi: 10.1172/JCI65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.