Dear Editors
Refractory obsessive-compulsive disorder (OCD) is a common and vexing clinical problem. Agents that modulate glutamate, including the NMDA antagonist ketamine, have been the focus of recent interest for the treatment of this population (1), but experience to date has been mixed (2, 3). Ketamine is a rapid-acting antidepressant that enhances cellular mechanisms associated with neural plasticity in prefrontal circuitry associated with extinction learning (4). This raises the intriguing possibility that ketamine may potentiate extinction-based psychotherapy for OCD (5).
CASE
AL1 is a Caucasian male in his late 20s with a principal diagnosis of OCD, comorbid major-depressive disorder (MDD) with chronic suicidal ideation, social anxiety disorder, and a history of bulimia nervosa. AL has been refractory to pharmacological treatments [clomipramine, venlafaxine, fluvoxamine, sertraline, citalopram, fluoxetine (+/− aripiprazole), risperidone, olanzapine, alprazolam, clonazepam, diazepam, bupropion, riluzole, and n-acetylcysteine]. Detailed dosage history was not available for all agents, but fluoxetine (60 mg) and citalopram (60 mg) were given at a therapeutic dose for more than two months with minimal benefit and significant side effects; similarly, 200 mg clomipramine was prescribed, but was discontinued due to serious side effects. AL was also refractory to both residential and outpatient cognitive-behavioral therapy (CBT) with expert providers. Because of the failure of standard treatments, we initiated concurrent ketamine and CBT treatment, seeking a synergistic benefit. AL provided informed consent prior to beginning treatment.
AL was treated for 8 weeks on an inpatient psychiatric unit and for 8 weeks as an outpatient. He received intensive CBT throughout his inpatient stay; he met with his CBT provider every weekday and completed ≈1–2 hrs. of CBT homework every day. CBT focused on exposure with response prevention (ERP). CBT was not manualized. Sessions typically began with homework report and hierarchy updates and ended with therapist assisted exposures. The patient also completed some mindfulness exercises and activity scheduling (i.e. behavioral activation), but the amount of time devoted to these activities was small relative to time spent on ERP. For weeks 3–6 (see Figure 1), therapy was accompanied by twice-weekly administration of racemic ketamine hydrochloride (50mg) delivered in five 10mg doses (5mg/nostril) over a 20-minute period using an LMA intranasal atomizer (6). Racemic ketamine hydrocholoride was used off-label; it is not approved by the FDA for the treatment of OCD. AL returned home 2 weeks after the final ketamine treatment and continued twice-weekly CBT for one month and once-weekly CBT for another month.
Figure 1.
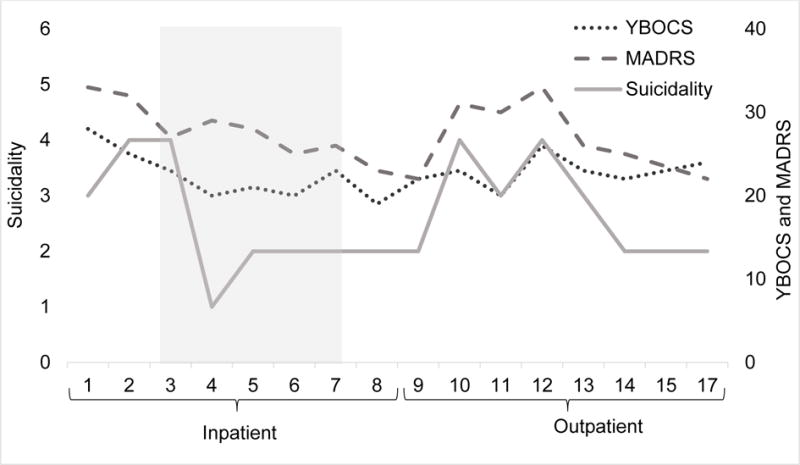
Change in OCD [Yale-Brown Obsessive-Compulsive Scale (YBOCS)] and MDD [Montgomery-Asberg Depression Rating Scale (MADRS)] severity (right axis) were modest following ketamine and CBT (shaded area) but suicidality (MADRS item 10, left axis) drastically reduced following the first week of ketamine treatment.
Pre-treatment OCD and MDD symptoms were severe [Yale-Brown Obsessive-Compulsive Scale (YBOCS) = 28; and Montgomery-Asberg Depression Rating Scale (MADRS) = 33]. OCD symptoms were modestly reduced after 2 weeks of CBT without ketamine (YBOCS = 23); AL attributed these reductions to an absence of many of his symptom triggers on the treatment unit. Intranasal ketamine was well tolerated. Psychotomimetic effects were mild and passed within one hour of drug administration; baseline and 60-min. post ketamine Clinician Administered Dissociative State Scale scores were comparable [(M = 0, SD = 0) and (M = 2.25, SD = 3.19), respectively], t (7) = 1.87, p = .10. Vital signs were monitored during and after ketamine administration and remained stable. Suicidality was rapidly reduced after the first dose of ketamine and was nearly absent following the 3rd administration. OCD symptoms were further reduced following the first week of ketamine (YBOCS = 20) and remained relatively stable until the end of inpatient treatment (see Figure 1). There was a notable improvement in response prevention following the first week of ketamine; while this observation is anecdotal, it corresponded with marked improvements in exposure engagement and homework compliance.
OCD and MDD symptoms, including suicidal ideation, worsened shortly after AL returned home and transitioned to outpatient CBT (see Figure 1), but then improved with ongoing CBT. Despite frequent obsessions and compulsive urges, he reported substantially less distress associated with his OCD symptoms, an improved capacity to dismiss or accept obsessions, better compulsive restraint (particularly delaying) when attempted, and reduced OCD-related functional impairment. Depressive symptoms, while improved, were still severe and often interfered with ERP.
To our knowledge, this is the first report of an OCD patient being treated with intranasal ketamine, the first treated with multiple ketamine administrations, and the first treated with intensive ERP and repeated ketamine administrations. It is of course impossible, in examination of this single case, to determine whether ketamine and intensive CBT synergized to produce clinical benefit. It is possible that the patient benefitted solely from inpatient CBT. Indeed, improvements in OCD symptoms were seen after two weeks of CBT and before ketamine was administered. These improvements may have also resulted from the lack of pertinent obsessive-compulsive triggers on the inpatient unit. Nonetheless, there are several reasons to believe that the patient benefitted from ketamine: He reported additional symptom reductions following the initiation of ketamine treatments (this may have also been due to continued CBT); Compliance with response prevention drastically improved shortly after beginning ketamine, suggesting that ketamine may augment the process of ERP for OCD, and; The patient reported rapid and drastic reductions in suicidal ideation following the first week of ketamine treatments. Further examination of the concurrent use of ketamine and extinction-based ERP for OCD is warranted. Component-controlled trials will be necessary to establish if ketamine and ERP have synergistic effects on OCD symptoms.
Acknowledgments
Disclosures: This work was supported by the State of Connecticut through its support of the Ribicoff Research Facilities and the Clinical Neuroscience Research Unit at the Connecticut Mental Health Center. T. Adams was supported by a National Institute of Mental Health award (5 T32 MH062994 13).
Footnotes
The patient’s real initials were not used in this case report. AL provided verbal consent to publish his treatment details.
References
- 1.Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol Ther. 2011;132:314–332. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez CI, Kegeles LS, Levinson A, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38:2475–2483. doi: 10.1038/npp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloch MH, Wasylink S, Landeros-Weisenberger A, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012;72:964–970. doi: 10.1016/j.biopsych.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez Cl, Wheaton M, Zwerling J, et al. Can exposure-based CBT extend the effects of intravenous ketamine in obsessive-compulsive disorder? an open-label trial. J Clin Psychiatry. 2016;77:408–409. doi: 10.4088/JCP.15l10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapidus KA, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014;76:970–976. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]


