Abstract
Personality traits such as conscientiousness as self-reported by individuals can help predict a range of outcomes, from job performance to longevity. Asking others to rate the personality of their acquaintances often provides even better predictive power than using self-report. Here, we examine whether peer-reported personality can provide a better link between brain function, namely threat-related amygdala activity, and future health-related behavior, namely problem drinking, than self-reported personality. Using data from a sample of 377 young adult university students who were rated on five personality traits by peers, we find that higher threat-related amygdala activity to fearful facial expressions is associated with higher peer-reported, but not self-reported, conscientiousness. Moreover, higher peer-reported, but not self-reported, conscientiousness predicts lower future problem drinking more than one year later, an effect specific to men. Remarkably, relatively higher amygdala activity has an indirect effect on future drinking behavior in men, linked by peer-reported conscientiousness to lower future problem drinking. Our results provide initial evidence that the perceived conscientiousness of an individual by their peers uniquely reflects variability in a core neural mechanism supporting threat responsiveness. These novel patterns further suggest that incorporating peer-reported measures of personality into individual differences research can reveal novel predictive pathways of risk and protection for problem behaviors.
Keywords: Amygdala, Personality, Peer Reports, Conscientiousness, Problem Drinking
1. Introduction
Our peers’ perceptions of us may provide unique insights into our mental health, personality, and even mortality. For example, Jackson et al. (2015) found that friend-reported personality traits were generally a better predictor of mortality than self-reported personality traits. Specifically, when controlling for self-reported personality traits, lower friend-reported conscientiousness and openness predicted higher mortality risk in men while lower friend-reported agreeableness and emotional stability predicted higher mortality in women. Informant reports more broadly have been shown to be useful predictors across a range of other outcomes, including academic achievement (Connelly & Ones, 2010; Kurtz, Puher, & Cross, 2012), physical health in adulthood (Israel, Moffitt, et al., 2014), job performance (Oh, Wang, & Mount, 2011), clinical severity of psychopathology (Verhulst & van der Ende, 1991), and the future emergence of depressive symptoms (Ronning et al., 2011), often predicting additional variance in these outcomes above and beyond self-report. While the nature of such predictive associations is unclear, it has been hypothesized that informant reports may overcome certain biases present in self-report data, and that when multiple informant reports are obtained, averaging across informants increases the reliability of measurements (Jackson et al., 2015; Oh, Wang, & Mount, 2011; Vazire & Mehl, 2008). In this way, it is possible that peer-reports of trait-like personality may also better reflect biological features of an individual than self-report.
In the context of behavioral neuroscience, we often consider associations between individual measures of brain function and self-reported personality or behavior (Hariri, 2009). Peer reports may offer a unique window onto the behavioral correlates of brain function from observers who are familiar with an individual across different contexts and can make judgments based on observations of behavior aggregated over time. Thus, peer reports may map onto inter-individual variability in behaviorally- and clinically-relevant brain function not captured through self-report. As such, peer reports may help us identify previously undetected links between brain function and personality that can subsequently inform relative risk for psychopathology or even optimize treatment strategies (Flory, Lynam, Milich, Leukefeld, & Clayton, 2002; Kotov, Gamez, Schmidt, & Watson, 2010; Naragon-Gainey & Watson, 2014; Ronning et al., 2011; Verhulst & van der Ende, 1991; Wardenaar, Conradi, Bos, & De Jonge, 2014).
The goal of the present work was to examine whether variability in behaviorally- and clinically-relevant brain function, namely threat-related amygdala activity assessed using BOLD fMRI, is associated with peer-reported personality traits including extraversion, conscientiousness, neuroticism, openness, and agreeableness, above and beyond self-reported personality. We further planned to test whether these associations were useful for predicting clinically-relevant behavioral outcomes by testing whether peer-reported personality traits predicted future problem drinking above and beyond self-reported personality traits. We focused our analyses on threat-related amygdala activity because this neural phenotype has been extensively examined in relation to self-reported behaviorally- and clinically-relevant outcomes in this (Nikolova, Knodt, Radtke, & Hariri, 2016; Swartz, Knodt, Radtke, & Hariri, 2015; Swartz et al., 2016) and other samples (Glahn, Lovallo, & Fox, 2007; Marinkovic et al., 2009). In particular, in the present sample, we have found that higher threat-related amygdala activity moderates the experience of stress-related problem drinking associated with reward-related brain function (Nikolova et al., 2016). Furthermore, peer reports of personality specifically were chosen for our analyses because our sample of young adult full-time university students spends a relatively large proportion of time socializing with friends, and thus peers may have more opportunities to observe their behaviors across multiple contexts than other potential informants such as instructors or parents (Eagan et al., 2014). For each participant, we used personality ratings from one to two peers as well as their own self-reported personality.
Our prior work in this sample has demonstrated that individuals with higher threat-related amygdala activity have lower levels of self-reported extraversion (Swartz et al., 2016), as well as higher levels of mood and anxiety symptoms under stress (Swartz et al., 2015). Prior research in other samples has also indicated a positive association between amygdala activity and self-reported neuroticism (Chan, Norbury, Goodwin, & Harmer, 2009; Everaerd, Klumpers, van Wingen, Tendolkar, & Fernandez, 2015; Haas, Omura, Constable, & Canli, 2007). We therefore hypothesized that higher threat-related amygdala activity would be associated with lower peer-reported extraversion but higher peer-reported neuroticism. We did not form directional hypotheses regarding the other personality traits examined given the lack of research in this area to date. Prior research on the personality correlates of problem drinking behavior with both self- and informant-rated personality has indicated that higher extraversion and neuroticism and lower conscientiousness and agreeableness are associated with problem drinking or alcohol use disorders (Flory et al., 2002; Hampson, Goldberg, Vogt, & Dubanoski, 2006; Malouff, Thorsteinsson, Rooke, & Schutte, 2007). Thus, we further hypothesized that peer-reported personality would predict future problem drinking in a similar direction, and that it would explain additional variance above and beyond self-reported personality (Connelly & Ones, 2010; Jackson et al., 2015).
2. Materials and Methods
2.1 Participants
Of the 1,202 participants recruited as part of the ongoing Duke Neurogenetics Study (DNS) as of June, 2015, participants for the present study included 418 young adult university students who had informant data from at least one peer. All procedures were approved by the Duke University Medical Center and participants provided informed consent before study initiation. Participants were college-aged (M=19.8 years, SD=1.3, range: 18 to 22) and 61% were female. Within the current sample, 48% were Caucasian, 32% were Asian, 9% were African American, 7% were bi- or multi-racial, .5% were Native American, and 3.5% self-reported as other. Diagnosis of any past or current DSM-IV Axis I disorder or select Axis II disorders (antisocial personality disorder and borderline personality disorder), assessed with the electronic Mini International Neuropsychiatric Interview (Sheehan et al., 1998) and Structured Clinical Interview for the DSM-IV subtests (First, Spitzer, Gibbon, & Williams, 1996) was not an exclusion. Within the current sample, 67 participants (16%) had at least one past or current psychiatric diagnosis. The most frequent past and current diagnoses were as follows: 35 with alcohol use disorder, 11 with a non-alcoholic substance use disorder, and 18 with major depressive disorder (these include comorbid diagnoses).
As part of the DNS protocol, participants were asked to nominate 3 individuals that knew them well to complete informant reports. Nominated individuals were contacted by e-mail and sent a link to complete the informant questionnaire online. As part of the questionnaire, informants self-selected belonging to one of the following categories: parent, sibling, other relative, close friend, spouse/partner, employer, or other. For the purposes of the present study, we defined a peer as any informant who self-identified as a close friend and who was within 4 years of age to the participant. Peer informants were generally college-aged (M=19.9, SD=1.4, range: 16 to 25 years), 66% were female, and, on a scale from 1 (Not very well) to 3 (Very well), reported knowing the participants very well (M=2.82, SD=0.4). Analyses leveraged personality reports from 1–2 peers for each participant (M=1.30) as relatively few participants had all 3 reports completed by friends (5%). Of the 418 participants with peer-report data, 377 had imaging data meeting quality control criteria (see fMRI methods below). Participant characteristics for this final sample are reported in Table 1.
Table 1.
Participant characteristics.
| Mean (SD) | Min | Max | |
|---|---|---|---|
| Participant Age | 19.8 (1.3) | 18 | 22 |
| Informant familiarity | 2.8 (0.4) | 1.5 | 3.0 |
| Number of peer informants | 1.3 (0.5) | 1 | 2 |
| Self: Extraversion | 119.5 (19.8) | 65 | 173 |
| Self: Conscientiousness | 118.3 (22.1) | 22 | 168 |
| Self: Neuroticism | 86.1 (23.7) | 34 | 169 |
| Self: Agreeableness | 118.0 (18.5) | 61 | 163 |
| Self: Openness | 125.3 (17.8) | 67 | 174 |
| Peer: Extraversion | 11.9 (2.2) | 5 | 15 |
| Peer: Conscientiousness | 12.0 (2.1) | 6 | 15 |
| Peer: Neuroticism | 8.3 (2.3) | 5 | 15 |
| Peer: Agreeableness | 12.8 (1.8) | 7 | 15 |
| Peer: Openness | 12.0 (1.9) | 6 | 15 |
| Problem Drinking (Baseline) | 5.3 (4.2) | 0 | 21 |
| Problem Drinking (Follow-up) | 4.5 (3.8) | 0 | 22 |
| Participant sex (% female) | 59% | ||
| Past or present psychiatric diagnosis | 17% | ||
| Caucasian | 47% | ||
| Asian | 33% | ||
| African American | 8% | ||
| Bi-racial or multi-racial | 7.7% | ||
| Native American | .3% | ||
| Other race/ethnicity | 4% | ||
Note: Informant familiarity was assessed by asking how well peer informants knew the participant on a 3-point scale; self-reported personality was measured with the NEO Personality Inventory Revised; peer-reported personality was measured with an informant-report measure adapted from the Dunedin Multidisciplinary Health and Development Study (Israel et al., 2014); problem drinking was measured with the Alcohol Use Disorders Identification Test; past or present psychiatric diagnosis indicates the percentage of participants with at least one past or present DSM-IV diagnosis as assessed by the electronic Mini International Neuropsychiatric Interview. Participant characteristics are presented for the 377 participants with both peer-report and fMRI data.
2.2 Functional Neuroimaging
Participants were scanned using a research-dedicated GE MR750 3T scanner at the Duke-UNC Brain Imaging and Analysis Center. Amygdala activity to threat was assessed using an emotional face matching paradigm described in detail in previous research (Nikolova et al., 2016; Swartz et al., 2015; Swartz et al., 2016). The paradigm version used in the DNS consisted of four blocks of a face-processing task (one block each for fearful, angry, surprised, and neutral faces; order counter-balanced across participants) interleaved with five blocks of a sensorimotor control task. Each trial in the face matching blocks lasted for 4 seconds with a variable interstimulus interval (ISI) of 2 to 6 seconds (M=4 seconds), for a total block length of 48 seconds. In the shape matching control blocks, each of the six shape trios was presented for 4 seconds with a fixed ISI of 2 seconds for a total block length of 36 seconds.
Imaging data were processed with the standard DNS pre-processing stream. These procedures, as well as quality assurance procedures, have been described in detail in our prior published work (Swartz, Knodt, Radtke, & Hariri, 2015; Swartz et al., 2016) and are available online (https://www.haririlab.com/methods/amygdala.html). Quality control criteria for inclusion of a participant’s imaging data were: <5% volumes exceed quality control criteria for motion or signal intensity outliers, ≥ 90% coverage of signal within the anatomically-defined bilateral amygdala region of interest, and accuracy ≥75% on the matching task performed during scanning.
Following pre-processing and the creation of contrast maps for the main effect of condition at the individual level, individual contrast images were then entered in second-level random effects models to determine mean condition-specific regional responses using one-sample t-tests. We extracted parameter estimates from functional clusters activated within the centromedial and basolateral amygdala at p<.05 family-wise error (FWE) corrected across the search volumes, for the contrasts of the Angry block > Shape blocks and Fearful block > Shape blocks. Amygdala regions were defined from probabilistic cytoarchitectonic mapping of the basolateral and centromedial amygdala sub-regions (Amunts et al., 2005). Because there were 4 amygdala sub-regions for each contrast (left and right basolateral and centromedial amygdala), we averaged these to obtain a mean measure of amygdala activity for each contrast of interest (Angry > Shapes and Fear > Shapes), similar to analytical approaches used in our prior research (Swartz et al., 2015). To control for Type I error, we conducted all analyses first with the mean measures of amygdala activity and only examined effects for sub-regions when effects for the mean measures were significant.
2.3 Self- and peer-reported personality
As part of the DNS protocol, all participants completed the NEO Personality Inventory Revised (NEO-PI-R; Costa & MacCrae, 1992). Total scores on the scales of extraversion, conscientiousness, neuroticism, agreeableness, and openness were used as measures of self-reported personality. Skewness and kurtosis for all self-reported personality measures were <1, with the exception of conscientiousness, which had kurtosis=1.3. Informant reports were adapted from those used in the Dunedin Multidisciplinary Health and Development Study (Israel et al., 2014). In the informant report of personality, there were five items for each of the five traits. Informants were asked to rate the participant on each item (e.g., “talks a lot”) on a scale from 1 (“No, doesn’t apply”) to 3 (“Yes, certainly applies”). Items for each trait were summed to obtain peer measures of extraversion, conscientiousness, neuroticism, agreeableness, and openness that could range from 5 to 15. When there were two peer informants, total scores on each personality trait were averaged across the two informants. Skewness and kurtosis for all peer-reported personality measures were <1. We performed supplementary analyses to examine whether results differed for participants who were rated by one or two peer informants (see Supplement). Reliability, inter-rater agreement (for individuals who had two peer informants), and correlations between self- and peer-report are provided in the results. Intraclass correlation coefficients (ICCs) were estimated with a one-way random effects model; single measures and average measures ICCs are reported. We also report correlations between personality measures and anxiety, as measured by the trait version of the State-Trait Anxiety Inventory (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983).
2.4 Problem drinking at baseline and follow-up
Problem drinking was assessed at baseline and follow-up with the Alcohol Use Disorders Identification Test (AUDIT; Babor, Higgins-Biddle, Saunders, & Monteiro, 2001). The AUDIT consists of items assessing alcohol use that may be considered risky or hazardous. It contains three subscales: hazardous alcohol use, dependence symptoms, and harmful alcohol use. We first ran all analyses using total scores across the three subscales and performed post hoc analyses to examine specific subscales when results with the total score were significant. In addition to completing the AUDIT at baseline, participants were contacted by e-mail every 3 months after having completed their fMRI scan to fill out a follow-up questionnaire online (for further details see Swartz et al., 2015 and Nikolova et al., 2016). Participants completed the AUDIT as part of this follow-up questionnaire; here they indicated how often they had exhibited problem drinking behavior since their last follow-up questionnaire. Similar to our prior research (Swartz et al., 2015), we used data from the most recent follow-up available for each participant. Of the current sample, 235 (56%) completed at least one follow-up and the most recent follow-up was completed on average over a year after the baseline assessment (M=530 days, SD=384, range=90 to 1706 days). Participants who completed the follow-up were more likely to be younger at baseline, t(416)=2.74, p=.01, female, χ2(1)=7.00, p=.01, higher in peer-reported conscientiousness, t(416)=−2.05, p=.04, lower in self-reported extraversion, t(416)=2.36, p=.02, higher in self-reported conscientiousness, t(416)= −2.38, p=.02, and have lower problem drinking behavior at baseline, t(416)=2.71, p=.01.
2.5 Statistical analysis
All analyses were conducted with Mplus v7 software using full information maximum likelihood estimation with 1,000 bootstrapped samples to account for non-normality in the data. Analyses proceeded in the following order: 1) testing the association between threat-related amygdala activity and self-reported personality; 2) testing the association between threat-related amygdala activity and peer-reported personality; 3) testing the association between threat-related amygdala activity and peer-reported personality, controlling for self-reported personality; 4) testing the association between peer-reported personality and future problem drinking behavior, controlling for self-reported personality and baseline problem drinking behavior; and 5) constructing an indirect effects model to assess the indirect effect of threat-related amygdala activity on future problem drinking via peer-reported personality. In addition, for all significant effects, we re-ran analyses winsorizing outliers for all continuous variables to +/− 3 SD from the mean.
In the first set of analyses, associations were examined between threat-related amygdala activity and self-reported personality. For these analyses, mean amygdala activity to fearful facial expressions and mean amygdala activity to angry facial expressions were mean-centered and entered as predictors. Sex (0=male 1=female) was entered as a covariate, as well as presence of a psychiatric diagnosis (0=no psychiatric diagnosis 1=past or present psychiatric diagnosis), and baseline AUDIT total scores (mean centered). The five self-reported personality traits were modeled as the outcome variables in five separate regressions. We tested whether the association between amygdala activity and each personality trait differed for amygdala activity to fearful faces compared to angry faces. To do so, we used a nested model comparison approach in Mplus. Specifically, we first constructed a model in which the associations between amygdala activity and the personality trait were freed to vary for fearful and angry faces; we next constructed a nested model in which the association between amygdala activity to angry faces and a personality trait was constrained to be equal to the association between amygdala activity to fearful faces and a personality trait. When constraining effects to be equal results in a significant reduction in model fit, this indicates the effects significantly differ by emotional stimulus type. Because we performed five of these comparisons (for each of the 5 personality traits), we performed a false-discovery rate (FDR) correction to control for multiple comparisons. Moderation by sex was tested through a similar approach using multi-group modeling to test whether parameter estimates significantly differed by sex.
A similar approach was used to model effects of amygdala activity on peer-reported personality traits. In addition, when significant associations between mean amygdala activity and peer-reported personality were detected, follow-up analyses were conducted to determine whether they were specific to one hemisphere or amygdala sub-region through a similar parameter constraint approach. Next, to test whether associations with personality were unique to peer-report, we tested whether effects held while controlling for self-reported personality traits, as measured by the NEO-PI-R. Exploratory analyses were also conducted to examine whole-brain associations between peer-reported personality traits controlling for self-reported personality (see Supplement for whole-brain methods and results).
After having established that amygdala activity was associated with peer-reported conscientiousness and extraversion, we entered these traits as predictors of future problem drinking behavior controlling for baseline problem-drinking behavior, as well as self-reported conscientiousness and extraversion. The following were also entered as covariates in these analyses: psychiatric diagnosis, age at baseline, amygdala activity, time since scanning, and time since the last follow-up assessment. Similar to the procedures described above, we used multi-group models to examine moderation of these effects by sex. Finally, we constructed an indirect effects model to test the indirect effect of threat-related amygdala activity on future problem drinking via peer-reported personality. The indirect effect was assessed by estimating bias-corrected 95% confidence intervals using 1,000 bootstrapped samples; confidence intervals that do not include 0 are considered to be significant at p<.05. We report results for the indirect effect using self-reported personality for comparison.
3. Results
3.1 Characteristics of peer reports
The reliability of the peer-reported personality measures, assessed using Cronbach’s alpha, were acceptable as follows: extraversion, α = .77, agreeableness, α = .70, conscientiousness, α = .76, neuroticism, α =.78, and openness, α = .74. Based on commonly used cutoffs (Hallgren, 2012), inter-rater agreement (for cases that had two peer informants) was fair for extraversion, average measure ICC=.70, single measure ICC=.54; agreeableness, average measure ICC=.65, single measure ICC=.48; and conscientiousness, average measure ICC=.65, single measure ICC=.48; but poor for neuroticism, average measure ICC=.48, single measure ICC=.32; and openness, average measure ICC=.53, single measure ICC=.36. We continued to test the association between amygdala activity and all 5 traits, noting the caveat that agreement was poor for neuroticism and openness. Correlations between self- and peer-reported personality were in the moderate to large range for all subscales: extraversion, r=.55, p<.001, conscientiousness, r=.45, p<.001, agreeableness, r=.43, p<.001, neuroticism, r=.35, p<.001 and openness, r=.33, p<.001 (Table 2).
Table 2.
Correlations between self- and peer-reported personality traits
| Self: Extraversion |
Self: Conscientiousness |
Self: Neuroticism |
Self: Agreeableness |
Self: Openness |
Peer: Extraversion |
Peer: Conscientiousness |
Peer: Neuroticism |
Peer: Agreeableness |
Peer: Openness |
Self: Anxiety |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Self: Extraversion | 1 | r=.03, p=.49 | r=−.15, p=.002 | r=.19, p<.001 | r=.27, p<.001 | r=.55, p<.001 | r=−.12, p=.02 | r=−.01, p=.83 | r=.13, p=.01 | r=.04, p=.44 | r=−.22, p<.001 |
| Self: Conscientiousness | 1 | r=−.45, p<.001 | r=.06, p=.26 | r=−.08, p=.10 | r=−.15, p=.003 | r=.45, p<.001 | r=.07, p=.18 | r=−.03, p=.56 | r=−.06, p=.27 | r=−.43, p<.001 | |
| Self: Neuroticism | 1 | r=−.05, p=.36 | r=.12, p=.01 | r=−.02, p=.74 | r=−.03, p=.56 | r=.35, p<.001 | r=−.01, p=.81 | r=.004, p=.93 | r=.81, p<.001 | ||
| Self: Agreeableness | 1 | r=.19, p<.001 | r=−.05, p=.31 | r=.09, p=.08 | r=−.01, p=.80 | r=.43, p<.001 | r=.02, p=.74 | r=−.01, p=.78 | |||
| Self: Openness | 1 | r=.21, p<.001 | r=−.09, p=.07 | r=.05, p=.28 | r=.08, p=.09 | r=.33, p<.001 | r=.13, p=.01 | ||||
| Peer: Extraversion | 1 | r=−.15, p=.002 | r=−.09, p=.10 | r=.17, p<.001 | r=.24, p<.001 | r=−.05, p=.34 | |||||
| Peer: Conscientiousness | 1 | r=.08, p=.09 | r=.18, p<.001 | r=.18, p<.001 | r=−.05, p=.34 | ||||||
| Peer: Neuroticism | 1 | r=−.29, p<.001 | r=−.08, p=.12 | r=.27, p<.001 | |||||||
| Peer: Agreeableness | 1 | r=.22, p<.001 | r=.04, p=.41 | ||||||||
| Peer: Openness | 1 | r=.07, p=.20 |
Note: Self-reported personality was measured with the NEO Personality Inventory Revised and peer-reported personality was measured with an informant-report measure adapted from the Dunedin Multidisciplinary Health and Development Study (Israel et al., 2014). Self-reported anxiety was measured with the trait version of the State-Trait Anxiety Inventory (Spielberger et al., 1983).
3.2 Amygdala activation: Main effects of task
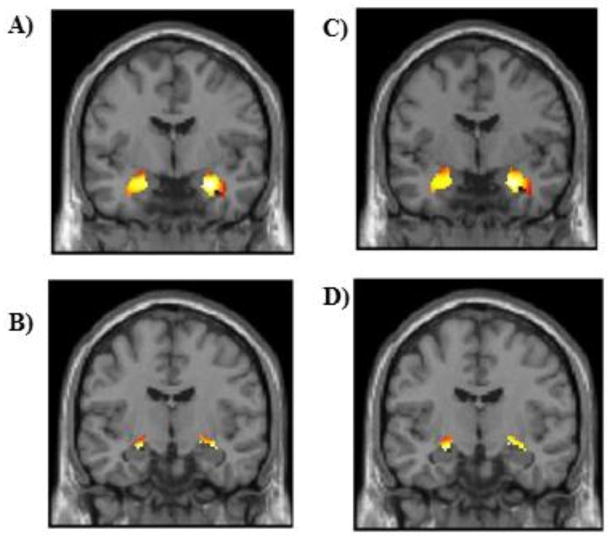
As expected the contrasts of the Fearful block > Shapes blocks and Angry block > Shapes blocks elicited significant amygdala activation within the left and right basolateral and centromedial regions (Table 3 and Figure 1). Parameter estimates were extracted from these functional clusters, averaged across sub-regions, and submitted to statistical analyses in Mplus.
Table 3.
Main effects of task within the basolateral and centromedial amygdala
| t(376)= | p-corrected= | Cluster size | Peak coordinates | |
|---|---|---|---|---|
|
Fearful Faces > Shapes
| ||||
| Left centromedial | 10.02 | <.001 | 28 | −24, −10, −14 |
| Right centromedial | 8.25 | <.001 | 62 | 32, −8, −12 |
| Left basolateral | 14.30 | <.001 | 200 | −20, −8, −20 |
| Right basolateral | 15.78 | <.001 | 224 | 22, −6, −20 |
|
| ||||
| Angry Faces > Shapes | ||||
|
| ||||
| Left centromedial | 12.62 | <.001 | 58 | −24, −10, −14 |
| Right centromedial | 11.79 | <.001 | 67 | 22, −10, −10 |
| Left basolateral | 15.67 | <.001 | 201 | −22, −8, −20 |
| Right basolateral | 19.50 | <.001 | 233 | 22, −6, −20 |
Note: Corrected p-values are small-volume family-wise error (FWE) corrected across the volume of the region of interest. Coordinates reported in MNI space.
Figure 1. Main effects of task within the basolateral and centromedial amygdala regions of interest for the contrasts of Fearful Faces > Shapes and Angry Faces > Shapes.
The contrast of Fearful Faces > Shapes resulted in significant activation within the left and right basolateral (A) and centromedial amygdala (B). The contrast of Angry Faces > Shapes resulted in significant activation within the left and right basolateral (C) and centromedial amygdala (D). All figures are thresholded at p<.05 family-wise error corrected across the volume of the region of interest.
3.3 Association between amygdala activity and self-reported personality traits
We first examined associations between amygdala activity and self-reported personality, and tested whether effects differed for amygdala activity to angry and fearful faces. There was a significant difference in the association between extraversion and amygdala activity to angry and fearful facial expressions, Δχ2(1)=8.66, p=.003, FDR-corrected p=.015. This difference was driven by a negative association between amygdala activity to fearful facial expressions and self-reported extraversion, B=−11.43, SE=3.96, Beta=−.14, p=.004 ΔR2=.02, total R2 for model=.13. This effect remained significant when winsorizing continuous variables to mitigate the influence of outliers, B=−11.84, SE=4.13, Beta=−.14, p=.004, ΔR2=.02. The effect for angry facial expressions was not significant, B=4.28, SE=3.36, Beta=.06, p=.202, ΔR2=.003. No interactions or main effects survived correction for multiple comparisons for the other self-reported personality traits. Likewise, there were no significant moderating effects of sex.
3.4 Association between amygdala activity and peer-reported personality traits
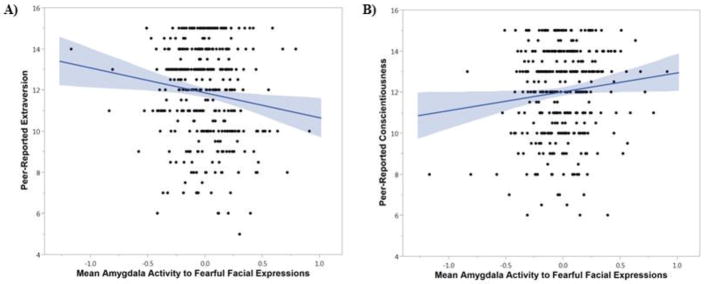
There were significant differences by expression type for peer-reported extraversion, Δχ2(1)=9.18, p=.003, FDR-corrected p=.015, and peer-reported conscientiousness, Δχ2(1)=6.18, p=.013, FDR-corrected p=.033. Similar to what was observed with self-report, amygdala activity to fearful facial expressions was negatively associated with peer report of extraversion, B=−1.10, SE=.47, Beta=−.12, p=.020, ΔR2=.01, total R2 for model=.09, with higher amygdala activity to fearful facial expressions predicting lower peer-reported extraversion (Figure 2A). This effect remained significant when winsorizing continuous variables, B=−1.20, SE=.47, Beta=−.13, p=.011, ΔR2=.02. The association went in the opposite direction for amygdala activity to angry facial expressions and peer-reported extraversion, B=.79, SE=.41, Beta=.09, p=.057, ΔR2=.01. For peer-reported conscientiousness, the significant difference by expression type was due to a positive association between amygdala activity to fearful facial expressions and peer-reported conscientiousness, B=.91, SE=.43, Beta=.10, p=.035, ΔR2=.01, total R2 for model=.08 (Figure 2B). Here, higher amygdala activity to fearful facial expressions was associated with higher peer reports of conscientiousness. This effect remained significant when winsorizing continuous variables, B=.93, SE=.44, Beta=.10, p=.035, ΔR2=.01. The effect was not significant for amygdala activity to angry faces, B=−.57, SE=.39, Beta=−.07, p=.147, ΔR2=.005. Interactions and main effects for other personality traits were not significant. Effects were also not moderated by sex. Constraining effects to be equal across sub-regions and hemispheres did not result in reductions in model fit for either extraversion or conscientiousness, indicating that effects were generally of similar magnitude for all sub-regions and hemispheres.
Figure 2. Associations between amygdala activity to fearful facial expressions and peer-reported extraversion and conscientiousness.
Scatterplots demonstrate associations between amygdala activity to fearful facial expressions and (A) peer-reported extraversion and (B) peer-reported conscientiousness (n=377). Mean amygdala activity represents the mean of the four sub-regions of amygdala activity examined (left and right centromedial and basolateral amygdala). Amygdala activity is mean-centered. Shaded region represents 95% confidence intervals.
3.5 Association between amygdala activity and peer-reported personality traits controlling for self-reported personality traits
Next, to determine whether peer-reported personality was uniquely associated with amygdala activity above and beyond self-report, we examined associations between amygdala activity and peer-reported personality controlling for self-reported personality. Critically, when controlling for self-reported extraversion, the association between amygdala activity to fearful facial expressions and peer-reported extraversion dropped to non-significance, B=−.43, SE=.38, Beta=−.05, p=.258, indicating that amygdala activity was associated with overlapping variance in self- and peer-reported extraversion. In contrast, when controlling for self-reported conscientiousness, amygdala activity to fearful facial expressions remained significantly associated with peer-reported conscientiousness, B=.97, SE=.40, Beta=.11, p=.014, suggesting that the peer reports were uniquely associated with amygdala activity taking into account self-report data. This association also remained significant when winsorizing continuous variables, B=.97, SE=.41, Beta=.11, p=.018. Whole-brain results for associations with peer-reported personality controlling for self-reported personality are reported in the Supplement.
3.6 Peer-reported personality traits predicting future problem drinking controlling for self-reported personality traits
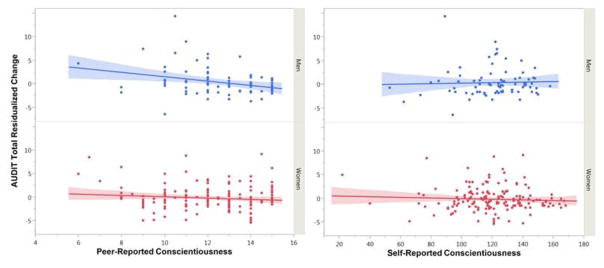
Next, we tested whether associations between threat-related amygdala activity and peer-reported personality were relevant for predicting future health-related behavior by examining whether peer-reported personality predicted future problem drinking, as measured by the AUDIT. Multi-group analyses indicated the effect of peer-reported conscientiousness was moderated by sex, Δχ2(1)=5.37, p=.021, FDR-corrected p=.042. Specifically, in men only, lower peer-reported conscientiousness predicted higher future problem drinking, B=−.61, SE=.20, Beta=−.31, p=.002, ΔR2=.08. total R2 for model=.62 (Figure 3). Indeed, this was the only significant predictor of future problem drinking in the model other than baseline problem drinking (Table 4). Moreover, peer-reported conscientiousness remained a significant predictor of problem drinking in men when winsorizing continuous variables, B=−.59, SE=.19, Beta=−.32, p=.002, ΔR2=.09. When examining sub-scales as outcomes, peer-reported conscientiousness was a significant predictor of hazardous alcohol use, B=−.25, SE=.10, Beta=−.22, p=.010, harmful alcohol use, B=−.30, SE=.13, Beta=−.35, p=.020, and marginally, dependence symptoms, B=−.10, SE=.05, Beta=−.26, p=.066, in men. The association between peer-reported extraversion and problem drinking was not significantly moderated by sex, Δχ2(1)=.05, p=.816, and was not a significant predictor of future problem drinking. Additionally, all significant effects reported above remained significant when controlling for self-reported trait anxiety.
Figure 3. Peer-reported, but not self-reported conscientiousness, predicts future problem drinking in men but not women.
Scatterplots demonstrate associations between conscientiousness and future problem drinking behavior in men (n=78) and women (n=157). Residualized change was calculated by obtaining the residuals from a regression with AUDIT baseline scores predicting AUDIT follow-up scores. Thus, residualized change scores represent the variance in follow-up AUDIT scores not predicted by baseline AUDIT. Peer-reported conscientiousness was a significant predictor of future AUDIT scores in men, whereas self-reported conscientiousness was not.
Table 4.
Results of regression predicting future problem drinking as measured by AUDIT total scores at follow-up
| Predictor | B | S.E. | Beta | p |
|---|---|---|---|---|
|
Men
| ||||
| Baseline AUDIT | .71 | .10 | .70 | <.001 |
| Age | −.02 | .40 | −.01 | .96 |
| Psychiatric diagnosis | .37 | .85 | .03 | .67 |
| Time since scanning | −.01 | .04 | −.03 | .76 |
| Time since last follow-up | .03 | .08 | .04 | .74 |
| NEO-PI-R Conscientiousness | .04 | .02 | .19 | .08 |
| NEO-PI-R Extraversion | −.01 | .02 | −.04 | .70 |
| Amygdala activity to angry faces | 1.07 | 1.57 | .07 | .50 |
| Amygdala activity to fearful faces | 1.80 | 1.96 | .11 | .36 |
| Peer-reported conscientiousness | −.61 | .20 | −.31 | .002 |
| Peer-reported extraversion | .12 | .18 | .06 | .52 |
|
| ||||
|
Women
| ||||
| Baseline AUDIT | .54 | .07 | .64 | <.001 |
| Age | −.08 | .15 | −.03 | .60 |
| Psychiatric diagnosis | −.65 | .71 | −.07 | .36 |
| Time since scanning | .01 | .02 | .04 | .59 |
| Time since last follow-up | .02 | .04 | .03 | .72 |
| NEO-PI-R Conscientiousness | −.002 | .01 | −.01 | .86 |
| NEO-PI-R Extraversion | −.01 | .01 | −.03 | .71 |
| Amygdala activity to angry faces | .18 | .94 | .01 | .85 |
| Amygdala activity to fearful faces | −.16 | .88 | −.01 | .86 |
| Peer-reported conscientiousness | −.11 | .13 | −.07 | .37 |
| Peer-reported extraversion | .17 | .11 | .11 | .12 |
Note: AUDIT=Alcohol Use Disorders Identification Test; NEO-PI-R=NEO Personality Inventory Revised.
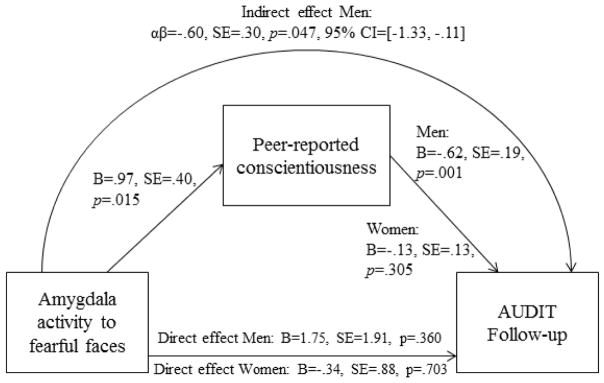
3.7 Indirect effect of threat-related amygdala activity on future problem drinking via peer-reported conscientiousness
Based on the results of the analyses reported above, we modeled an indirect effect of threat-related amygdala activity on future problem drinking via peer-reported conscientiousness. The indirect effect using peer-reported conscientiousness was significant in men, αβ= −.60, SE=.30, p=.047, 95% bias corrected confidence intervals = [−1.33, −.11] (Figure 4). Thus, higher amygdala activity to fearful facial expressions predicted higher peer-reported conscientiousness, which predicted lower future problem drinking. In contrast, the indirect effect was not significant in men when using self-reported conscientiousness, αβ= −.20, SE=.20, p=.32, [−.73, .08]. The indirect effect was not significant in women using either peer-reported or self-reported conscientiousness.
Figure 4. Indirect effect of amygdala activity to fearful facial expressions on future problem drinking in men via peer-reported conscientiousness.
The indirect effect was modeled using a multi-group model with participant sex as the grouping factor. The association between amygdala activity and peer-reported conscientiousness was constrained to be equal between men and women, given no significant moderation. The effect of peer-reported conscientiousness on AUDIT follow-up scores was freed to vary between men and women, given evidence for significant moderation. Accordingly, the indirect effect was estimated separately for men and women. 95% CI=95% bias-corrected bootstrapped confidence intervals.
4. Discussion
Young adults, especially university students, spend a relatively large amount of time interacting with their peers, and as such, peers can provide unique information about a given individual’s personality based on repeated interactions across multiple contexts. In the present study, we found that university students exhibiting relatively higher amygdala activity to fearful facial expressions were rated by their peers as being relatively lower in extraversion but higher in conscientiousness. Critically, the unique association between peer-reported conscientiousness and amygdala function was independent of self-reported conscientiousness. The association between amygdala activity and peer-reported conscientiousness is further noteworthy because peer-reported but not self-reported conscientiousness was a significant predictor of future problem drinking in men. This unique mapping of peer- but not self-reported conscientiousness to behaviorally- and clinically-relevant brain function and, in men, to future health-related outcomes could reflect that, in comparison with self-report, peer-report of conscientiousness may be less subject to social desirability bias (John & Robins, 1993).
Notably, we observed different associations between peer-reported personality and amygdala activity to fearful facial expressions compared to amygdala activity to angry facial expressions. Specifically, higher amygdala activity to fearful facial expressions, but not angry facial expressions, predicted lower extraversion and higher conscientiousness. It has been suggested that fearful facial expressions with directed eye gaze, as used in our work, represent a more ambiguous threat, as the source of the threat is not evident from the expression (Adams Jr., Gordon, Baird, Ambady, & Kleck, 2003). Therefore, examining amygdala activity separately for ambiguous (i.e., fearful facial expression with directed eye gaze) relative to overt (i.e., angry facial expression with directed eye gaze) threat may result in different associations with personality assessed by both self- and peer-report. Moreover, compared to angry facial expressions, fearful facial expressions have different effects on skin conductance response (Soussignan et al., 2013), the startle reflex (Springer, Rosas, McGetrick, & Bowers, 2007), attention (Taylor & Whalen, 2014), and memory (Davis et al., 2011). For example, fearful faces increase detection of changes in peripheral stimuli (Taylor & Whalen, 2014), and memory is enhanced for neutral words presented after fearful, but not angry, faces (Davis et al., 2011), potentially because the source of threat is ambiguous when viewing fearful faces, necessitating greater attention to the surrounding context to determine the source of threat. These differences in the physiological and cognitive consequences of processing fearful compared to angry facial expressions could all contribute to the pattern of results observed here. In addition to replicating the emotion-specific behavioral association observed in the present study, determining the cognitive mechanisms underlying this association is an important direction for future research.
Although higher risk for mood and anxiety disorders associated with heightened threat-related amygdala activity is well-documented in prior research, the present results highlight a potential positive outcome associated with heightened amygdala activity. Namely, relatively higher fear-related amygdala activity may confer an advantage in certain contexts by making individuals more careful or cautious of potential danger in their environment. The observed association between relatively higher amygdala activity to fearful facial expressions and higher peer-reported conscientiousness is novel and requires replication in independent samples before firm conclusions may be drawn. One potential explanation for this association is that enhanced processing of potential threat in the environment, as indexed by fearful facial expressions, may increase sensitivity to the negative consequences of risky actions, in which case individuals with higher amygdala activity to fearful facial expressions may be more careful and responsible in order to avoid negative consequences or disappointing others.
Moreover, these results align with the finding that individuals in the DNS with higher threat-related amygdala activity were relatively protected from developing problem drinking behavior associated with relatively high reward-related activity of the ventral striatum (Nikolova et al., 2016). In prior research with participants in the DNS, individuals with higher reward-related activity of the ventral striatum, which is associated with higher impulsivity, were found to exhibit higher stress-related problem drinking behavior. Thus, one route to problem drinking appears to involve higher impulsivity and sensation-seeking behavior. However, this effect was not observed in individuals with higher threat-related amygdala activity, suggesting that enhanced processing of threat-related stimuli, including fearful facial expressions, may buffer risk associated with greater reward-related processing (Nikolova et al., 2016). The present results may help explain why this buffering effect is observed; namely, higher amygdala activity to fearful facial expressions is associated with higher conscientiousness, which may facilitate resistance to impulsive risky decision-making related to drinking behavior. In sum, greater sensitivity to potential threat in the shared environment, as indexed by higher amygdala activity specifically to fearful facial expressions, may be associated with higher conscientiousness in order to avoid the perceived negative consequences associated with risky or careless behavior. This, in turn, may predict lower likelihood of developing problematic drinking behavior.
It is interesting to note the areas of convergence and divergence in which peer raters agreed or disagreed with each other, with self-report, and correlations with amygdala activity. Agreement between different peer raters, between peer-report and self-report, and the overlap in the association between peer-report, self-report, and brain function, was highest for the measure of extraversion. Because items assessing extraversion generally rely on readily observable behaviors (e.g., “talks a lot”), this may explain why we see the most convergence between different raters and brain function for this aspect of personality. Counter to expectations, we did not observe an association between amygdala function and peer-reported neuroticism. This may reflect the fact that many items assessing neuroticism require an assessment of mental state (e.g., “gets nervous easily”) that may be more difficult for peers to observe. This may be even more difficult in our relatively high-functioning participants, who likely possess effective behavioral strategies for regulating negative mood particularly in the presence of peers. Our results also conform with those of prior research, which finds higher levels of informant agreement on personality traits that are easier to observe, such as extraversion, and lower levels of agreement on personality traits that require insight into mental states, such as neuroticism and openness (Connelly & Ones, 2010; John & Robins, 1993; Ready, Clark, Watson, & Westerhouse, 2000). Overall, this suggests that peer-reports may be particularly useful when examining the neural correlates of aspects of personality that are observable and that may be subject to social desirability bias when assessed through self-report.
Discrepancies in brain and behavior associations of the peer- and self-report measures of personality can also be compared to the discrepancies in predictive utility noted when comparing self- and clinician-rated symptoms in individuals with psychiatric disorders. For instance, prior studies have noted that agreement between self- and clinician-rated depression severity is modest at the baseline of clinical trials (Dunlop et al., 2010) and that lower agreement predicts decreased likelihood of remission after undergoing treatment (Dunlop et al., 2010; Dunlop et al., 2011). Indeed, it is interesting to speculate that in the present study, individuals with the lowest levels of conscientiousness may be the least likely to report (or be aware of) their carelessness, which could have contributed to the finding that peer-reported personality was a better predictor of future changes in problem drinking relative to self-reported personality. Overall, then, in studies of the neural correlates of personality or psychopathology, a combination of self-, peer-, and clinician-rated measures could each provide complementary yet unique insights into behavior and future clinical outcomes.
These results should be interpreted with respect to several limitations. First, we did not collect additional data on the informants themselves, and so we were unable to examine potential moderating factors such as whether nominated peers were attending the same university as the participants, or the personality or problem drinking behavior of the informants (Ready et al., 2000). Second, inter-rater agreement was poor for neuroticism and openness, which also exhibited the weakest correlations between peer- and self-report. As discussed above, this may be related to the difficulty in assessing mental states from observation. Third, we used two different instruments with different psychometric properties, including the wording and number of items, to assess self- and peer-reported personality. This may have contributed to lower correlations between peer- and self-report for some personality traits and differences in the predictive associations between peer- and self-report. Future research using identical measures for peer- and self-report will be necessary to address these possibilities. Fourth, because these analyses required that a participant nominate a peer informant who actually completed the online questionnaire, the present sample likely excludes DNS participants who may be socially isolated individuals lacking close friends to either nominate or who are willing to complete the questionnaire. Likewise, participants who completed the follow-up questionnaire to assess future problem drinking were generally more conscientious and had lower problem-drinking behavior at baseline. Given this pattern of attrition and the overall high-functioning nature of our university student sample, it is likely that the effects reported here are an underestimate of what would be observed in an at-risk sample with less attrition. Fifth, our analyses are limited to associations at one developmental period with a relatively brief informant assessment of personality. It remains to be determined whether the associations observed here exist at other stages of the life span or would be observed with different measures of personality. Sixth, the effect sizes observed were small, with amygdala activity generally explaining an additional 1% of variance in peer-reported personality. These effect sizes are consistent with prior brain-behavior associations observed in this sample using similar analytic methods (Swartz et al., 2015; Swartz et al., 2016; Nikolova & Hariri, 2012; Nikolova, Singhi, Drabant, & Hariri, 2013). These small effect sizes for a single neuroimaging measure point to the difficulty in explaining variation in a complex human behavior such as conscientiousness, and suggest that identifying a broader network with which the amygdala interacts to process threat, as well as networks associated with other processes such as self-control, will likely be necessary to explain clinically-relevant levels of variance. Finally, although amygdala activity to fearful facial expressions was associated with peer-reported conscientiousness in both men and women, peer-reported conscientiousness predicted future problem drinking in men only. While the nature of this sex difference remains to be determined, we speculate that it may reflect differences in drinking motives between male and female university students. For example, low conscientiousness is associated with drinking to enhance positive emotions (Stewart, Loughlin, & Rhyno, 2001), and men tend to exhibit higher enhancement motives than women in university samples (Kuntsche, Knibbe, Gmel, & Engels, 2006).
These limitations notwithstanding, our results provide initial evidence that peer reports can reveal unique associations between brain function and personality left undetected using self-report measures. Specifically, we observed a unique association between amygdala activity to ambiguous environmental threat as represented by fearful facial expressions and peer-reported conscientiousness, which in turn predicted problem drinking over one year later in men. If confirmed in future research, these findings may have implications for predicting a range of outcomes including physical health and longevity. More broadly these results suggest there may be much to be gained by incorporating informant reports of personality and behavior into future research on individual differences in the neural basis of behavior.
Supplementary Material
Highlights.
Neural correlates of self- and peer-reported personality are examined.
Higher amygdala activity is associated with peer-reported conscientiousness.
Peer-reported conscientiousness predicts lower future problem drinking in men.
Peer report may reveal novel associations between brain, personality, and behavior.
Acknowledgments
The Duke Neurogenetics Study is supported by Duke University and NIH grant DA033369. JRS received support through the Center for the Study of Adolescent Risk and Resilience (P30DA023026) and through NIH grant R01AG049789. ARH is supported by NIH grants R01DA033369 and R01AG049789.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RB, Jr, Gordon HL, Baird AA, Ambady N, Kleck RE. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300:1536. doi: 10.1126/science.1082244. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N, … Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology. 2005;210(5):343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identificaton Test: Guidlines for Use in Primary Care. 2. Switzerland: World Health Organization; 2001. [Google Scholar]
- Chan SWY, Norbury R, Goodwin GM, Harmer CJ. Risk for depression and neural responses to fearful facial expressions of emotion. The British Journal of Psychiatry. 2009;194:139–145. doi: 10.1192/bjp.bp.107.047993. [DOI] [PubMed] [Google Scholar]
- Connelly BS, Ones DS. An other perspective on personality: Meta-analytic integration of observers’ accuracy and predictive validity. Psychol Bull. 2010;136(6):1092–1122. doi: 10.1037/a0021212. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, MacCrae RR. Manual for the Revised NEO Personality Inventory (NEO-PIR) and the NEO Five-Factor Inventory (NEO-FFI) Odessa, FL: Psychological Assessment Resources, Inc; 1992. [Google Scholar]
- Davis FC, Somerville LH, Ruberry EJ, Berry AB, Shin LM, Whalen PJ. A tale of two negatives: Differential memory modulation by threat-related facial expressions. Emotion. 2011;11(3):647–655. doi: 10.1037/a0021625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Li T, Kornstein SG, Friedman ES, Rothschild AJ, Pedersen R, … Keller M. Correlation between patient and clinician assessments of depression severity in the PREVENT study. Psychiatry Res. 2010;177:177–183. doi: 10.1016/j.psychres.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Li T, Kornstein SG, Friedman ES, Rothschild AJ, Pedersen R, … Trivedi MH. Concordance between clinician and patient ratings as predictors of response, remission, and recurrence in major depressive disorder. J Psychiatr Res. 2011;45:96–103. doi: 10.1016/j.jpsychires.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagan K, Bara Stolzenberg E, Ramirez JJ, Aragaon MC, Ramirez Suchard M, Hurtado S. The American freshman: National norms fall 2014. Los Angeles: Higher Education Research Institute, UCLA; 2014. [Google Scholar]
- Everaerd D, Klumpers F, van Wingen G, Tendolkar I, Fernandez G. Association between neuroticism and amygdala responsivity emerges under stressful conditions. NeuroImage. 2015;112:218–224. doi: 10.1016/j.neuroimage.2015.03.014. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBM. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-patient Edition. New York: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- Flory K, Lynam D, Milich R, Leukefeld C, Clayton R. The relations among personality, symptoms of alcohol and marijuana abuse, and symptoms of comorbid psychopathology: Results from a community sample. Experimental and Clinical Psychopharmacology. 2002;10(4):425–434. doi: 10.1037//1064-1297.10.4.425. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: Studies from the Oklahoma Family Health Pattern Project. Biological Psychiatry. 2007;61(11):1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: Personality-dependent activation in the amygdala and subgenual anterior cingulate. Behavioral Neuroscience. 2007;121(2):249–256. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Hallgren KA. Computing inter-rater reliability for observational data: An overview and tutorial. Tutor Quant Methods Psychol. 2012;8(1):23–34. doi: 10.20982/tqmp.08.1.p023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson SE, Goldberg LR, Vogt TM, Dubanoski JP. Forty years on: Teachers’ assessments of children’s personality traits predict self-reported health behaviors and outcomes at midlife. Health Psychology. 2006;25(1):57–64. doi: 10.1037/0278-6133.25.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annual Review of Neuroscience. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel S, Moffit TE, Belsky DW, Hancox RJ, Poulton R, Roberts B, Thomson WM. Translating personality psychology to help personalize preventive medicine for young adult patients. Journal of Personality and Social Psychology. 2014;106(3):484–498. doi: 10.1037/a0035687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JJ, Connolly JJ, Garrison SM, Leveille MM, Connolly SL. Your friends know how long you will live: A 75-year study of peer-rated personality traits. Psychological Science. 2015;26(3):335–340. doi: 10.1177/0956797614561800. [DOI] [PubMed] [Google Scholar]
- John OP, Robins RW. Determinants of interjudge agreement on personality traits: The Big Five domains, observability, evaluativeness, and the unique perspective of the self. Journal of Personality. 1993;61(4):521–551. doi: 10.1111/j.1467-6494.1993.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorder: A meta-analysis. Psychological Bulletin. 2010;136(5):768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, Engels R. Who drinks and why? A review of socio-demographic, personality, and contextual issues behind the drinking motives in young people. Addictive Behaviors. 2006;31(10):1844–1857. doi: 10.1016/j.addbeh.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Kurtz JE, Puher MA, Cross NA. Prospective prediction of college adjustment using self- and informant-rated personality traits. Journal of Personality Assessment. 2012;94:630–637. doi: 10.1080/00223891.2012.672506. [DOI] [PubMed] [Google Scholar]
- Malouff JM, Thorsteinsson EB, Rooke SE, Schutte NS. Alcohol involvement and the five-factor model of personality: A meta-analysis. J Drug Education. 2007;37(3):277–294. doi: 10.2190/DE.37.3.d. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O’Reilly CE, Howard JA, Sawyer K, Harris GJ. Alcoholism and dampened temporal limbic activation to emotional faces. Alcoholism: Clinical and Experimental Research. 2009;33(11):1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naragon-Gainey K, Watson D. Consensually defined facets of personality as prospective predictors of change in depression symptoms. Assessment. 2014;21(4):387–403. doi: 10.1177/1073191114528030. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Hariri AR. Neural responses to threat and reward interact to predict stress-related problem drinking: A novel protective role of the amygdala. Biology of Mood & Anxiety Disorders. 2012;2:19. doi: 10.1186/2045-5380-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Knodt AR, Radtke SR, Hariri AR. Divergent responses of the amygdala and ventral striatum predict stress-related problem drinking in young adults: Possible differential markers of affective and impulsive pathways of risk for alcohol use disorder. Molecular Psychiatry. 2016;21(3):348–356. doi: 10.1038/mp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Singhi EK, Drabant EM, Hariri AR. Reward-related ventral striatum reactivity mediates gender-specific effects of a galanin remote enhancer haplotype on problem drinking. Genes, Brain and Behavior. 2013;12:516–524. doi: 10.1111/gbb.12035. [DOI] [PubMed] [Google Scholar]
- Oh I, Wang G, Mount MK. Validity of observer ratings of the five-factor model of personality traits: A meta-analysis. Journal of Applied Psychology. 2011;96:762–773. doi: 10.1037/a0021832. [DOI] [PubMed] [Google Scholar]
- Ready RE, Clark LA, Watson D, Westerhouse K. Self- and peer-reported personality: Agreement, trait ratability, and the “self-based heuristic”. Journal of Research in Personality. 2000;34:208–224. [Google Scholar]
- Ronning JA, Haavisto A, Nikolakaros G, Helenius H, Tamminen T, Moilanen I, … Sourander A. Factors associated with reported childhood depressive symptoms at age 8 and later self-reported depressive symptoms among boys at age 18. Social Psychiatry and Psychiatric Epidemiology. 2011;46(3):207–218. doi: 10.1007/s00127-010-0182-6. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Soussignan R, Chadwick M, Philip L, Conty L, Dezecache G, Grezes J. Self-relevance appraisal of gaze direction and dynamic facial expressions: Effects on facial electromyographic and autonomic reactions. Emotion. 2013;13(2):330–337. doi: 10.1037/a0029892. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Springer US, Rosas A, McGetrick J, Bowers D. Differences in startle reactivity during the perception of angry and fearful faces. Emotion. 2007;7(3):516–525. doi: 10.1037/1528-3542.7.3.516. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Loughlin HL, Rhyno E. Internal drinking motives mediate personality domain -- drinking relations in young adults. Personality and individual differences. 2001;30:271–286. [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85(3):505–511. doi: 10.1016/j.neuron.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Waller R, Bogdan R, Knodt AR, Sabhlok A, Hyde LW, Hariri AR. A common polymorphism in a Williams syndrome gene predicts amygdala reactivity and extraversion in healthy adults. Biological Psychiatry. 2016 doi: 10.1016/j.biopsych.2015.12.007. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Whalen PJ. Fearful, but not angry, expressions diffuse attention to peripheral targets in an attentional blink paradigm. Emotion. 2014;14(3):462–468. doi: 10.1037/a0036034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazire S, Mehl MR. Knowing me, knowing you: The accuracy and unique predictive validity of self-ratings and other-ratings of daily behavior. Journal of Personality and Social Psychology. 2008;95(5):1202–1216. doi: 10.1037/a0013314. [DOI] [PubMed] [Google Scholar]
- Verhulst FC, van der Ende J. Assessment of child psychopathology: Relationships between different methods, different informants and clinical judgment of severity. Acta Psychiatr Scand. 1991;84:155–159. doi: 10.1111/j.1600-0447.1991.tb03120.x. [DOI] [PubMed] [Google Scholar]
- Wardenaar KJ, Conradi HJ, Bos EH, De Jonge P. Personality modulates the efficacy of treatment in patients with major depressive disorder. Journal of Clinical Psychiatry. 2014;75(9):e916–e923. doi: 10.4088/JCP.13m08855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.