Abstract
Intestinal fibrosis is an intractable complication of Crohn's disease (CD), and, when occurring excessively, causes severe intestinal obstruction that often necessitates surgical resection. The fibrosis is characterized by an imbalance in the turnover of extracellular matrix (ECM) components, where intestinal fibroblasts/myofibroblasts play active roles in ECM production, fibrogenesis and tissue remodeling, which eventually leads to the formation of stenotic lesions. There is however a great paucity of knowledge about how intestinal fibrosis initiates and progresses, which hampers the development of effective pharmacotherapies against CD. Recently, we explored the potential implications of transient receptor potential (TRP) channels in the pathogenesis of intestinal fibrosis, since they are known to act as cellular stress sensors/transducers affecting intracellular Ca2+ homeostasis/dynamics, and are involved in a broad spectrum of cell pathophysiology including inflammation and tissue remodeling. In this review, we will place a particular emphasis on the intestinal fibroblast/myofibroblast TRPC6 channel to discuss its modulatory effects on fibrotic responses and therapeutic potential for anti-fibrotic treatment against CD-related stenosis.
Keywords: Inflammatory Bowel disease, myofibroblast, TRP channel, fibrotic stenosis, Ca2+
Methods
The details of the procedures used for cell culture, [Ca2+]i measurement, immunostaining, and real-time RT-PCR are described elsewhere (1). Biopsy samples were obtained from CD patients according to their informed consents. Statistical analysis was performed as described previously (1). The Fukuoka University Hospital Ethics Committee approved the protocol, and written informed consent was obtained from all patients.
Expression array analysis
Total RNA isolation for array: The total RNA was isolated from the cerebellum of each individual animal using TRIzol Reagent (nitrogen) and purified using the SV Total RNA Isolation System (Promega) according to the manufacturer's instructions. RNA samples were quantified by an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) and the quality was confirmed with an Experion System (Bio-Rad Laboratories, Hercules, CA).
Gene expression microarrays: The cRNA was amplified, labeled using GeneChip® WT Terminal Labeling and Control Kit, and hybridized to an Affymetrix Human Genome U133 Plus 2.0 array according to the manufacturer's instructions. All hybridized microarrays were scanned by an Affymetrix scanner. Relative hybridization intensities and background hybridization values were calculated using the Affymetrix Expression ConsoleTM.
Data analysis and filter criteria: Raw signal intensities for respective probes were calculated from hybridization intensities. Then the raw signal intensities of two samples were log2-transformed and normalized by RMA (Robust Multi-array Average) and quantile algorithm [P] with Affymetrix® Expression ConsoleTM 1.1 software. To identify up- or down-regulated genes, we calculated Z-scores [Z] and ratios (non-log scaled fold-change) from the normalized signal intensities of respective probes for comparison between control and experiment sample. Finally, we established the criteria for regulated genes: (up-regulated genes) Z-score ≥ 2.0 and ratio ≥ 1.5-fold, (down-regulated genes) Z-score ≤ –2.0 and ratio ≤ 0.66.
Intestinal Fibroblast/Myofibroblast and fibrosis
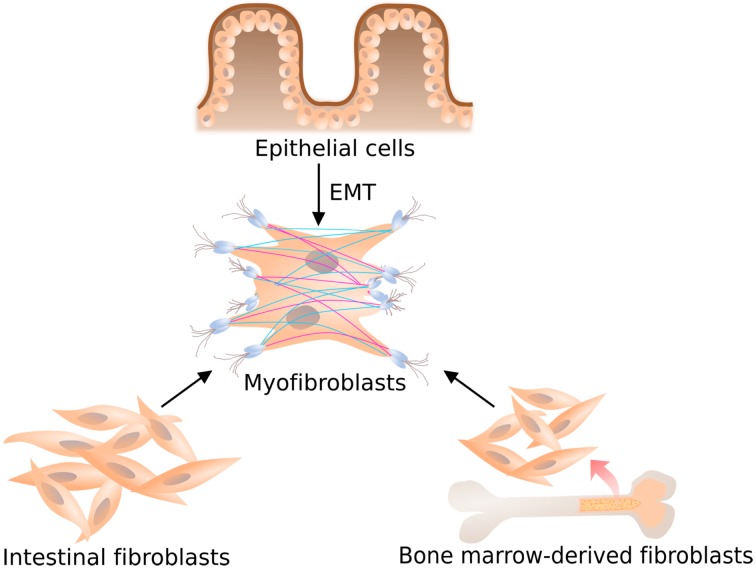
Fibrosis is the common final pathway to organ failure in diseases of the heart, kidney, liver, lung, and intestine. It has been estimated that about 45% of human deaths are associated with fibroproliferative disorders including fibrosis (2). Intestinal fibrosis is a major complication of inflammatory bowel disease (IBD) and can occur in both ulcerative colitis (UC) and CD, but is much more prevalent in CD (3). Approximately 40% of CD patients with ileal disease develop clinically apparent strictures throughout their lifetime, which significantly influences the quality of life (4, 5). The excessive presence of fibrous tissue increases the thickness of bowel wall, reducing the elasticity and the function over the affected area. Even surgical removal performed to eliminate the fibrotic stenosis and obstructing strictures often fails to prevent a recurrence in the same patient (6). Anti-inflammatory therapies are not efficient in resolving the fibrosis, CD patients treated with biologics still develop strictures and associated complications (7). Currently, there is not much information known about pathogenic mechanisms associated with detrimental fibrosis, but a few clues have been hinted by experimental models (8,9,10). The critical role of intestinal fibroblasts/myofibroblasts in wound healing and development of fibrosis is well recognized (11,12,13). Persistent myofibroblastic activity can underlie hypertrophic scarring, loss of tissue compliance, and even rampant fibrosis that is the basis for fibrotic disorders of the heart, skin, lung, kidney, skeletal muscle, and liver (8, 14, 15). As shown in Fig. 1, the origin of fibroblasts and myofibroblasts is very controversial and potentially includes resident fibroblasts, bone marrow derived mesenchymal precursors (fibrocytes), and epithelial cells undergoing the epithelial-to-mesenchymal transition (EMT). Fibroblasts isolated from IBD mucosa proliferate faster than normal, and this increase occurs after exposure to growth factors and proinflammatory cytokines, and after direct cell-to-cell contact with inflammatory cells (10, 16). During the inflammatory process, to repair and regenerate homeostasis, these tissue-resident fibroblasts are activated and transformed into myofibroblasts, contractile cells expressing α-SMA and myosin bundles. Myofibroblasts secrete ECM and collagens, and are vital players in the fibrotic stenotic tissue, by aiding tissue contracture and healing. In the wound-healing program, a substantial portion of myofibroblasts could also arise from regenerating epithelial or endothelial cells or from epithelial stem cell progenitors via EMT. Circulating fibrocytes appear universally involved in organ fibrosis. A complex array of cytokines, chemokines and growth factors regulate fibrocyte biology, and these are associated with fibrogenesis in CD. The cytokines transforming growth factor β1 (TGF-β1), connective tissue growth factor and interleukin 13 (IL-13), overexpressed in the strictured Crohn's intestine, promote fibrocyte generation and/or differentiation (17, 18). Increased resident fibroblast/myofibroblast populations are pivotal to fibrosis development. During inflammatory process, profibrotic cytokines and chemokines (TGF-β, IL-13, IL-17), the peptide hormone Angiotensin II, growth factors (CTGF and PDGF) and matrix factors (hyaluronan fragments, mechanical stress and/or stiffness) are secreted from mesenchymal and inflammatory cells to induce or augment myofibroblast transformation (8, 19). These changes subsequently induce extracellular matrix deposition, metalloproteinase inhibition, and fibroblast activation.
Fig. 1.
Schematic illustration showing the evolution of the myofibroblast phenotype. Distinct cell types are involved in intestinal fibrosis, such as ECM-producing cells derived from epithelial, local or bone marrow-derived fibroblasts. Following an increase in mechanical tension, and fibrotic cytokine such as TGF-β1 further differentiation occurs to a contractile phenotype, termed a differentiated myofibroblast, characterized by the expression of alpha smooth muscle actin.
TGF-β is central to the development of fibrotic stenosis in CD. In numerous cell types, TGF-β secretion augments myofibroblast transformation. There are three TGFβ isoforms in mammals, namely TGFβ1, TGFβ2 and TGFβ3 which are expressed in myofibroblasts, vascular smooth muscle cells, endothelial cells, and macrophages (20). The human TGFβ1 gene produces a 390 amino acid propeptide which is cleaved intracellularly into two identical 112 amino acid peptide subunits joined together by a disulphide bond (21). The canonical TGF-β signaling pathway commences with binding of TGF-β to a TGF-β type 2 receptor, which subsequently heterodimerizes with a TGF-β type l receptor to form an active TGFβR1 complex. TGF-β and its receptors are up-regulated in CD strictures, and abnormal TGF-β signaling impairs the intestinal immune tolerance and tissue repair (22). Blockade of TGFβR1 signaling by an injectable inhibitor (SD-208) was evaluated in two experimental animal models of intestinal fibrosis: anaerobic bacteria- and trinitrobenzensulphonic acid-induced colitis (TNBS). SD-208 reduced fibroblast activation, phosphorylation of Smad2 and Smad3 proteins, and intestinal wall collagen deposition in both models (23). Although TGFβ1 and mechanical stress (generated by ECM stiffness) are recognized as major mediators of myofibroblast differentiation, the molecular signals in these soluble and mechanical signals are still elusive. Furthermore, intestinal stricture formation in CD is driven by local excessive production of TGF-β (13, 24). In addition to TGF-β1, emerging evidence has shown that IL-13 and IL-17 are involved in intestinal fibrosis. In TNBS-induced colitis, inhibition of IL-13 signaling by administration of small interfering RNA targeting the IL-13Rα2, reduces fibrosis and expression of TGFβ (25). IL-17A expression was found to be increased in the inflamed areas of patients with inflammatory bowel disease (26). But, blockade of IL-17A by administration of the anti-IL-17A antibody, secukinumab, failed to meet its primary endpoint, in a clinical trial of CD patients (27).
Myofibroblasts synthesize ECM components and generate high contractile forces for wound retraction or tissue remodeling in developmental processes. It is well known that fibrosis is associated with excessive accumulation of ECM components, such as collagens, matrix metalloproteinases (MMPs), and tissue inhibitors of metalloproteinases (TIMPs) (2, 28, 29). This is mainly owing to increased synthesis and decreased degradation of ECM components. Notably, MMPs that degrade the ECM are upregulated, whereas TIMPs are down-regulated (30). In addition, other ECM proteins, such as fibronectins, elastin, and fibrillins, are upregulated during the development of fibrosis. In response to tissue injury and profibrotic mediators, including TGF-β, IL-13 and IL-17, fibroblasts differentiate into myofibroblasts, and the activation and/or recruitment of fibroblasts resistant to apoptosis result in fibrogenesis and subsequent fibrosis (31, 32). A defining feature of fibroblast to myofibroblast differentiation is the formation of αSMA stress fibers that provide a structural network for generating contractile forces (10, 16, 33). The α-SMA expression is suppressed by extracellular fibrotic collagen and by anti-fibrotic cytokines, such as IL-10 and IL-11 (34, 35, 36). Increased constitutive N-cadherin expression in fibroblasts has been shown to potentiate stricture formation in CD patients (37).
Fibroblast/Myofibroblast TRP channels and tissue remodeling
Interestingly, calcium signaling has recently gained much attention as a regulator of myofibroblast contractile activity but it is not known whether calcium signaling is required for the differentiation of fibroblasts to myofibroblasts (32, 38). Ca2+ is an essential signaling molecule implicated in various long-term cellular consequences, such as differentiation, gene expression, and cell proliferation, growth and death, and it plays a significant role in regulating fibroblast functions (39,40,41). In general, there are two distinct sources of Ca2+ for elevating intracellular Ca2+ levels: Ca2+ influx across the plasma membrane and Ca2+ release from the endoplasmic reticulum. Ca2+ influx can occur through three functionally distinct classes of Ca2+-permeable channels, i.e. voltage-gated Ca2+ channels, receptor-operated Ca2+–permeable channels (ROC) and store-operated Ca2+ channels (SOC). Through extensive survey of their molecular identification, the majority of ROCs and some of SOCs have been closely linked to the transient receptor potential (TRP) superfamily (42, 43). Several lines of evidence suggest that fibrosis-associated events in myofibroblasts are controlled by the cytosolic Ca2+ concentration ([Ca2+]i), which is mediated by some members of TRP superfamily (44,45,46,47). TRP channels are cellular sensors for a variety of physical and chemical stimuli (48,49,50). Gastrointestinal TRP channels are involved in the sensation of smell, taste, touch, temperature, and pain (48, 51,52,53). TRP channels also play essential roles in cell signaling and responses to benign or harmful environmental changes (54,55,56,57). In addition to Ca2+, TRP channels change the membrane potential, translocate important signaling ions across the cell membrane, change enzymatic activities, and initiate endocytosis or exocytosis (48, 58, 59). The TRPC family consists of seven distinct isoforms designated as TRPC1–TRPC7 (44, 58, 60, 61). TRPC family members can be transcriptionally induced and/or are directly activated by G-protein coupled-receptor (GPCR) signaling through diacylgylcerol, and are susceptible to the depletion of intracellular Ca2+ stores or to the stretch of the plasma membrane (55). TRPC channel-mediated Ca2+ influx can directly activate the Ca2+-sensitive protein phosphatase calcineurin to induce diverse intracellular responses through its downstream transcriptional effector nuclear factor of activated T-cells (NFAT) (46). TRPC1-mediated Ca2+ influx is essential for intestinal homeostasis/inflammation and progesterone-induced endometrial decidualization (57, 62, 63). Low intensity irradiation with 635 ± 5 nm diode laser inhibited TGF-β1/Smad3-mediated fibroblast-myofibroblast transition and this effect involved the modulation of TRPC1 ion channels (64). Ca2+ signaling via the TRPM7 channel likely plays a key role in TGF-β1-elicited fibrogenesis in human atrial fibroblasts (47). Cell-cell contact formation is governed by Ca2+ signaling via TRPC4, which co-immunoprecipitates with the junction proteins β-catenin and cadherin in vascular endothelial cells (65). However, whether TRP channels play roles in intestinal fibrosis remains to be investigated.
Intestinal Fibrotic stenosis and Myofibroblast TRPC6 channels
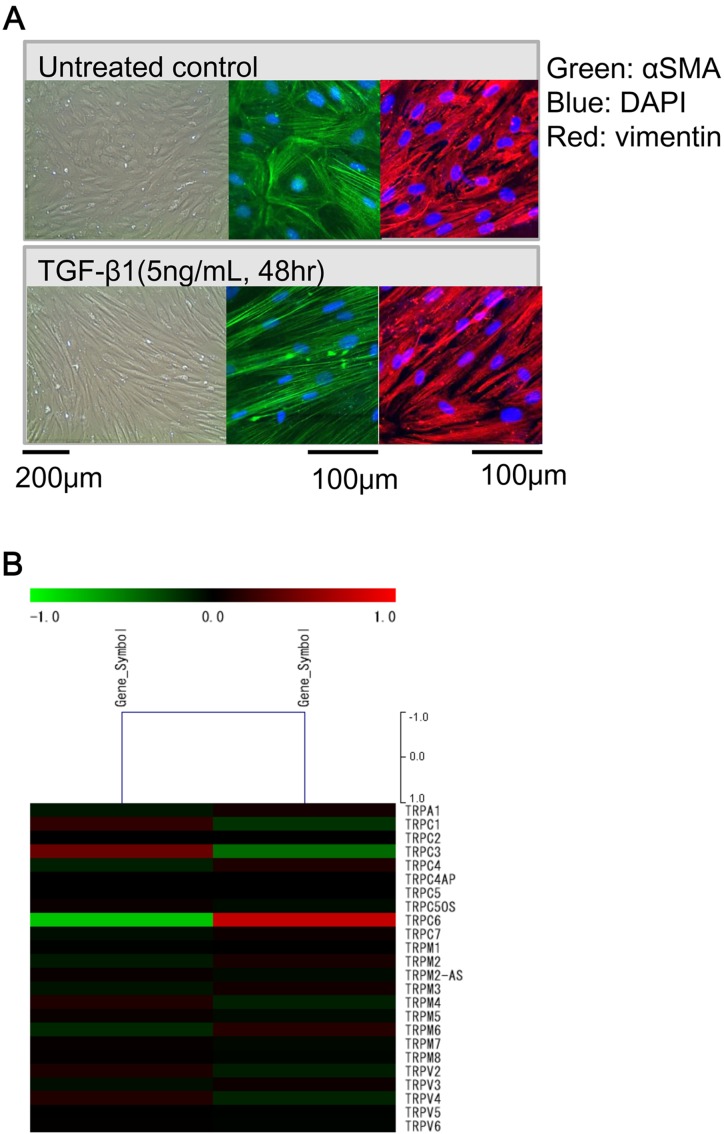
We investigated whether TRP channels are involved in the expression of fibrosis-associated molecules and TGF signaling in InMyoFib cells. At first, we examined TGF-β1-induced morphological changes and TRP channel expression in InMyoFib (intestinal myofibroblast cell line: Fig. 2A). We found that TGF-β1 significantly upregulates TRPC6 mRNA (Fig. 2B) and protein expression. Upregulated TRPC6 expression is essential for the formation of α-SMA stress fibers and N-cadherin-mediated adherens junctions, which respectively enable myofibroblasts to gain contractility and reinforce mutual intercellular connections (6, 8, 66). The hallmarks of myofibroblast differentiation are stress fiber development and de novo α-SMA expression. Incorporation of α-SMA into stress fibers confers high contractility to myofibroblasts, promoting the formation of specialized contacts within extracellular matrix regions termed "supermature focal adhesions" in vitro and "fibronexus" in vivo (67). Fibroblasts from the strictured regions of CD patients show increased constitutive expression of N-cadherin and exhibit enhanced basal cell migration (37). TGF-β1 potently induces N-cadherin expression in intestinal fibroblasts and cell's migration ability (37, 68). Interestingly, adherens junctions appear in fibrotic tissue, but are absent in normal tissue in which fibroblasts do not develop stress fibers (67). Thus, direct links between subcellular stress fibers and cell surface cadherins may serve to maintain the tension created between adjacent cells. In the current review, it is well accepted that cellular adhesion molecules, integrins and cadherins, may contribute to the development of tissue fibrosis (69, 70).
Fig. 2.
TGF-β1-induced morphological changes and TRPC6 expression in InMyoFib cells. A: Phase-contrast (left) or immunostaining images of InMyoFib cells stained with anti-α-SMA (green) and anti-vimentin (red) antibodies on the day of plating (untreated control), or 48-h post-treatment with TGF-β1 (5 ng/ml). Modified from (1) B: Fold change of TRP isoform mRNAs in InMyoFibs by expression array analysis. Control vs TGF-β1 (5 ng/ml, 24 h) treated InMyoFib.
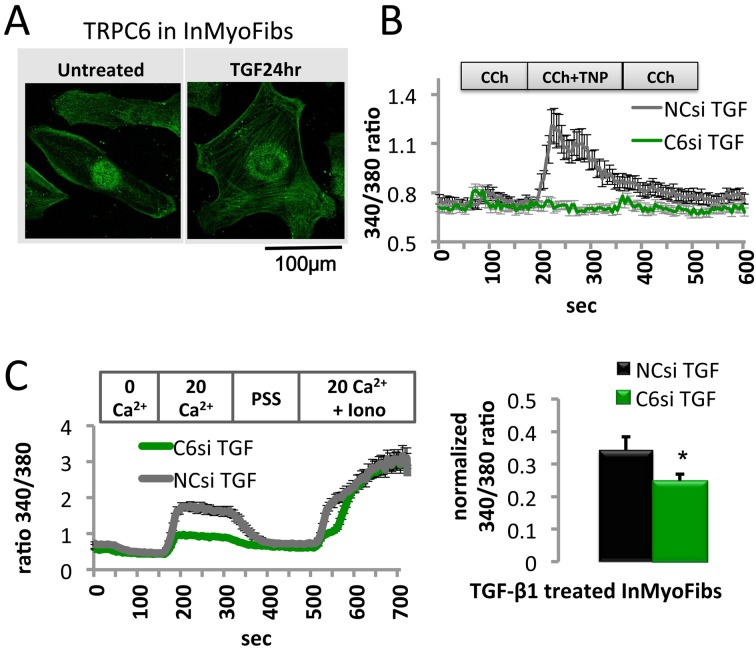
Among the members of the TRP channel family, TRPC6 is a receptor-operated cation channel that can be activated by angiotensin II or endothelin I through stimulation of their corresponding receptors and secondary generation of diacylglycerol. Additionally, TRPC6 participates in the development and pathogenesis of fibrotic diseases, such as hepatic, renal, pulmonary, and cardiac fibrosis (45, 71, 72). TRPC6 and calcineurin are required to promote myofibroblast differentiation, suggesting the presence of a comprehensive pathway for the differentiation associated with TGFβ, p38 MAPK and serum response factor (33). We also tested the abilities of the muscarinic agonist carbachol (CCh) and a membrane-bulging agent TNP to induce Ca2+ influx and potentiation (73). The magnitude of the CCh-induced Ca2+ influx and its enhancement by TNP were nearly abolished by TRPC6-si treatment (Fig. 3). These results strongly suggested that TRPC6 makes a critical contribution to TGF-β1-mediated enhancement of both basal and biochemically/mechanically-induced Ca2+ influxes in InMyoFibs. Overexpression of TRPC6 siRMA, dominant-negative TRPC6 mutants (Δ130-TRPC6 and 3A-TRPC6) (46) or administration of SKF resulted in the enhanced phosphorylation of SMAD-2, ERK1/2, and p38-MAPK (1). Moreover, treatment with cyclosporin A or FK506 significantly enhanced TGF-β1-induced phosphorylation of SMAD-2, ERK1/2, and p38-MAPK (1). These lines of evidence suggest that Ca2+ influx through this channel negatively regulates TGF-β1-SMAD/p38-MAPK/ERK1/2 signaling via calcineurin activation.
Fig. 3.
TRPC6-associated Ca2+ influx by TGF-β1 treatment. A: InMyoFib plasma membrane expression of TRPC6. Immunofluorescence double staining of untreated or TGF-β1-treated (5 ng/ml) InMyoFib cells with TRPC6-Alexa488 (green) primary antibodies. Scale bar = 100 µm. B: TRPC6si pre-treatment diminished shear stress-induced Ca2+ entry. Representative [Ca2+]i responses of TGF-β1-treated (5 ng/ml) cells. Cells were exposed to CCh (10 μM), CCh + TNP (500 μM), or CCh (10 μM). C: Representative [Ca2+]i responses of TGF-β1 (5 ng/ml)-treated cells that were transfected with negative control siRNA (NCsi) or TRPC6 siRNA (C6si) during a pre-treatment step. Data points represent mean ± S.E.M. from > 30 cells. Modified from (1).
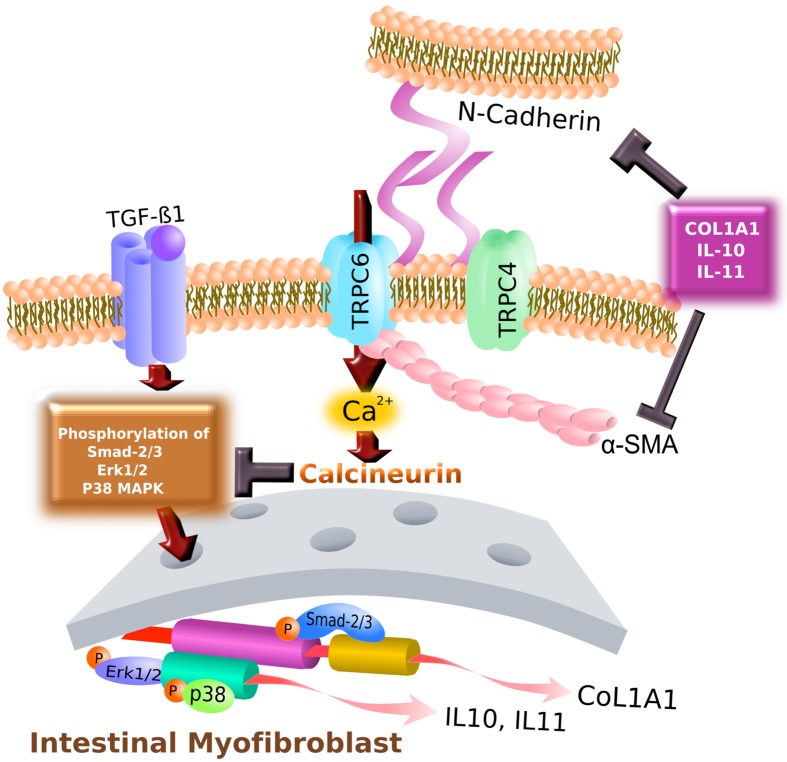
As summarized in Fig. 4, while TGF-β1-mediated increase in TRPC6 activity promotes the expression of α-SMA and N-cadherin and strengthened their interactions with TRPC6 protein, it also negatively regulates collagen synthesis and the secretion of anti-inflammatory/anti-fibrotic factors. These pleiotropic effects appear to be mediated by distinct downstream pathways of TGF-β1 signaling, suggesting that TRPC6 may be involved in fibrosis in a very intricate way. Obviously, more in-depth investigation is necessary to decipher how stimulation of TGF receptor(s) leads to the activation of distinct TRPC6-mediated signaling cascades linked to both anti- and pro-fibrotic consequences during the transition from wound healing to fibrosis. This may open an avenue of discovering a new TRPC6-targeting therapy which would be more appropriate for CD patients with intestinal fibrosis (1).
Fig. 4.
Hypothetical TRPC4- / TRPC6-mediated signaling pathway downstream of TGF-β1 in InMyoFib cells. TRPC6 interacted directly with α-SMA, N-cadherin, activating its expression, and indirectly supported α-SMA, N-cadherin expression by downregulating the negative regulators COL1A1, IL-10, and IL-11. The TRPC6 channel negatively regulates COL1A1, IL-10, and IL-11 expression, as well as Smad-2, ERK, and p38-MAPK phosphorylation in intestinal myofibroblasts. AKT signal transduction may involve TRPC6 upregulation downstream of the TGF-β1 receptors.
TGF-β1-induced secretion of collagen, IL-10, and IL-11 appears to negatively regulate α-SMA and N-cadherin expression. This mechanism may serve as negative feedback regulation by anti-fibrotic factors. The observed negative regulation of TGF-β1-SMAD signaling via calcineurin, which is activated through increased TRPC activity, may be an important anti-fibrotic mechanism. Indeed, in cultured mesangial cells, the calcineurin inhibitors, cyclosporin A and FK506, were found to activate this signaling pathway, thereby initiating fibrogenic gene expression [25]. Calcineurin dephosphorylates a variety of kinase substrates (74). In fact, calcineurin dephosphorylates several phosphorylated proteins involved in TGF-β1 signaling (e.g., SMAD, ERK1/2, or p38-MAPK) independently of NFAT activation (75). This raises an intriguing possibility that targeting the TRPC6-calcineurin signaling axis may be a useful therapeutic strategy for reinforcing the anti-fibrotic potential in fibrotic diseases, such as CD. In fact, it is known that inhibition of calcineurin has healing effects on erosions/ulcers in ulcerative colitis (UC), and a calcineruin inhibitor FK506 (tacrolimus) has been used in clinical practice for treating the patients with UC (76). Moreover, clinical trials of tacrolimus treatment for fistulas in CD are already underway (77, 78). However, our results have indicated that this compound may also facilitate fibrogenic processes in the gut. These "double-edged sword" effects of tacrolimus tell us that, besides its primary therapeutic goal of wound healing, a simple strategy to inhibit calcineurin would also bring about the undesired adverse effect, fibrosis.
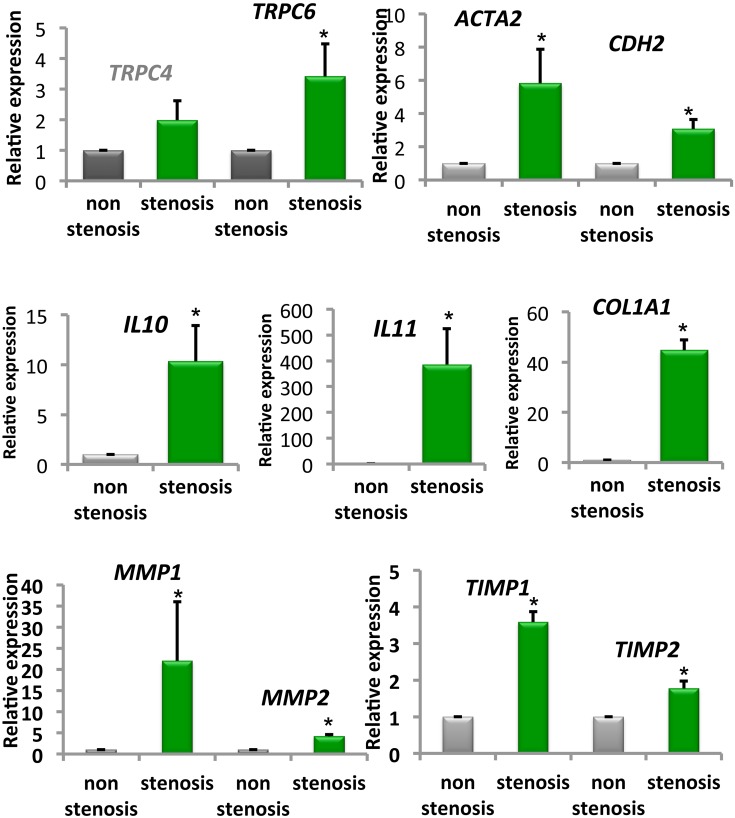
Additionally, we obtained 12 paired biopsy samples from stenotic and non-stenotic ileal regions of six CD patients, five of which received anti-TNF agents. We examined the expression levels of TRP channels and fibrosis-associated factors. Stenotic lesions can be inflammatory, fibrogenic, or neoplastic, or can possess all of these characteristics (1, 7). The mRNA levels of TRPC6, ACTA2, CDH2, IL-10, IL-11, and COL1A1 were significantly higher in stenotic areas than in non-stenotic mucosal areas in CD patients (Fig. 5). This new finding indicates that TRPC6 vitally contributes to the progression of excessive fibrosis in both an experimental model and human tissues, which should help elucidate the mechanism underlying the fibrotic process. These mechanisms may be relevant not only to intestinal fibrosis, but also to other fibrotic lesions of the skin, lung, and liver, where these channels are expressed at significant levels.
Fig. 5.
Crohn's disease (CD) patient biopsies from non-stenotic or stenotic intestinal areas. mRNA levels of TRPC4, TRPC6, ACTA2 (α-SMA), CDH2 (N-cadherin), IL10, IL11, COL1A1, MMP1, MMP2, TIMP1, and TIMP2 in biopsies were examined by real-time RT-PCR in non-stenotic or stenotic inflamed mucosal tissues of CD patients. *P < 0.05 vs. non-stenotic sample (12 paired biopsy samples obtained from 6 patients). Modified from (1).
Summary
We focused on TGF-β1-mediated signaling and regulation by TRPC6 channels, which modulate myofibroblast functions associated with wound repair, such as stress fiber formation, cell-cell adhesion, ECM synthesis, and cytokine secretion. Our results showed that TGF-β1-mediated signaling in intestinal myofibroblasts comprises several phosphorylation events, and forms an intricate network involving TRPC6-mediated signaling pathways, the result suggesting a new anti-fibrotic strategy for treating chronic intestinal inflammatory diseases. The final consequence of the activation of this network appears to be a complex balance between pro-fibrotic and anti-fibrotic activities. Further studies investigating the spatiotemporal heterogeneity of TGF-β1-mediated signaling may help to elucidate the pathways underlying progression of CD-associated fibrosis.
The above findings are consistent in part with a previous study that TRPC6-mediated Ca2+ influx was obligatory for myofibroblast differentiation in dermal and cardiac wound healing, but simultaneously suggest a greater complexity of TRPC6-mediated signaling in the intestinal fibrotic processes. Furthermore, the expression profile in CD patient samples indicated similarities between the strictured regions in CD patients and InMyoFibs in terms of pro- and anti-fibrogenic factors. Collectively, the present results suggest that actual signaling pathways activated during TGF-β1-induced fibrosis include many factors that interact via an interconnected network, as recapitulated in the scheme shown. Although some improvement has been made in elucidating the patho-mechanism for fibrosis, the knowledge is still devoid of effective anti-fibrotic agents (79, 80). In this regard, it is highly probable that TRP channels are attractive therapeutic candidates involved in a large spectrum of human intestinal health and diseases, including infectious, indefinite complaint, and inflammatory diseases, which will deserve continuous investigation in future.
Conflict of interest
The authors have no conflict of interest directly relevant to the content of this article.
Acknowledgments
This study was supported by MEXT/JSPS KAKENHI Grant Number (B) 22790677 and (B) 25860571; a MEXT-supported program for research activities of female researchers; the Kaibara Morikazu Medical Science Promotion Foundation; the Clinical Research Foundation; and the Central Research Institute of Fukuoka University.
References
- 1.Kurahara LH, Sumiyoshi M, Aoyagi K, Hiraishi K, Nakajima K, Nakagawa M, Hu Y, Inoue R. Intestinal myofibroblast TRPC6 channel may contribute to stenotic fibrosis in Crohn’s disease. Inflamm Bowel Dis. 2015; 21(3): 496–506 doi: 10.1097/MIB.0000000000000295 [DOI] [PubMed] [Google Scholar]
- 2.Ghosh AK, Vaughan DE. Fibrosis: is it a coactivator disease? Front Biosci (Elite Ed). 2012; 4: 1556–70. doi: 10.2741/e480 [DOI] [PubMed] [Google Scholar]
- 3.Bettenworth D, Rieder F. Medical therapy of stricturing Crohn’s disease: what the gut can learn from other organs - a systematic review. Fibrogenesis Tissue Repair. 2014; 7 (1): 5 doi: 10.1186/1755-1536-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spinelli A, Correale C, Szabo H, Montorsi M. Intestinal fibrosis in Crohn’s disease: medical treatment or surgery? Curr Drug Targets. 2010; 11(2): 242–8. doi: 10.2174/138945010790309984 [DOI] [PubMed] [Google Scholar]
- 5.Jacob N, Targan SR, Shih DQ. Cytokine and anti-cytokine therapies in prevention or treatment of fibrosis in IBD. United European Gastroenterol J. 2016; 4(4): 531–40 doi: 10.1177/2050640616649356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speca S, Giusti I, Rieder F, Latella G. Cellular and molecular mechanisms of intestinal fibrosis. World J Gastroenterol. 2012; 18(28): 3635–61. doi: 10.3748/wjg.v18.i28.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Kuemmerle JF. Mechanisms that mediate the development of fibrosis in patients with Crohn’s disease. Inflamm Bowel Dis. 2014; 20(7): 1250–8. doi: 10.1097/MIB.0000000000000043; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010; 2: 78. doi: 10.3410/B2-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latella G, Sferra R, Speca S, Vetuschi A, Gaudio E. Can we prevent, reduce or reverse intestinal fibrosis in IBD? Eur Rev Med Pharmacol Sci. 2013; 17(10): 1283–304. [PubMed] [Google Scholar]
- 10.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007; 127(3): 526–37. doi: 10.1038/sj.jid.5700613 [DOI] [PubMed] [Google Scholar]
- 11.Meier JK, Scharl M, Miller SN, Brenmoehl J, Hausmann M, Kellermeier S, Schölmerich J, Rogler G. Specific differences in migratory function of myofibroblasts isolated from Crohn’s disease fistulae and strictures. Inflamm Bowel Dis. 2011; 17(1): 202–12 doi: 10.1002/ibd.21344 [DOI] [PubMed] [Google Scholar]
- 12.Valentich JD, Popov V, Saada JI, Powell DW. Phenotypic characterization of an intestinal subepithelial myofibroblast cell line. Am J Physiol. 1997; 272(5 Pt 1): C1513–24. [DOI] [PubMed] [Google Scholar]
- 13.Biancheri P, Giuffrida P, Docena GH, Macdonald TT, Corazza GR, Di Sabatino A. The role of transforming growth factor (TGF)-beta in modulating the immune response and fibrogenesis in the gut. Cytokine Growth Factor Rev. 2014; 25(1): 45–55. doi: 10.1016/j.cytogfr.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 14.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008; 214(2): 199–210. doi: 10.1002/path.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YS, Li SH, Guo J, Mihic A, Wu J, Sun L, Davis K, Weisel RD, Li RK. Role of miR-145 in cardiac myofibroblast differentiation. J Mol Cell Cardiol. 2014; 66: 94–105. doi: 10.1016/j.yjmcc.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 16.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002; 3(5): 349–63. doi: 10.1038/nrm809 [DOI] [PubMed] [Google Scholar]
- 17.Sahebally SM, Burke JP, Chang KH, Kiernan MG, O’Connell PR, Coffey JC. Circulating fibrocytes and Crohn’s disease. Br J Surg. 2013; 100(12): 1549–56. doi: 10.1002/bjs.9302 [DOI] [PubMed] [Google Scholar]
- 18.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012; 18(7): 1028–40. doi: 10.1038/nm.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010; 106(11): 1675–80. doi: 10.1161/CIRCRESAHA.110.217737 [DOI] [PubMed] [Google Scholar]
- 20.Agrotis A, Kalinina N, Bobik A. Transforming growth factor-beta, cell signaling and cardiovascular disorders. Curr Vasc Pharmacol. 2005; 3(1): 55–61. [DOI] [PubMed] [Google Scholar]
- 21.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol Genet Metab. 2000; 71(1-2): 418–35. doi: 10.1006/mgme.2000.3032 [DOI] [PubMed] [Google Scholar]
- 22.Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996; 110(4): 975–84. doi: 10.1053/gast.1996.v110.pm8613031 [DOI] [PubMed] [Google Scholar]
- 23.Medina C, Santos-Martinez MJ, Santana A, Paz-Cabrera MC, Johnston MJ, Mourelle M, Salas A, Guarner F. Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J Pathol. 2011; 224(4): 461–72. doi: 10.1002/path.2870 [DOI] [PubMed] [Google Scholar]
- 24.Di Sabatino A, Jackson CL, Pickard KM, Buckley M, Rovedatti L, Leakey NA, Picariello L, Cazzola P, Monteleone G, Tonelli F, Corazza GR, MacDonald TT, Pender SL. Transforming growth factor beta signalling and matrix metalloproteinases in the mucosa overlying Crohn's disease strictures. Gut. 2009; 58(6): 777–89. doi: 10.1136/gut.2008.149096 [DOI] [PubMed] [Google Scholar]
- 25.Fichtner-Feigl S, Young CA, Kitani A, Geissler EK, Schlitt HJ, Strober W. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology. 2008; 135(6): 2003–13, 13 e1-7. doi: 10.1053/j.gastro.2008.08.055 [DOI] [PubMed] [Google Scholar]
- 26.Biancheri P, Pender SL, Ammoscato F, Giuffrida P, Sampietro G, Ardizzone S, Ghanbari A, Curciarello R, Pasini A, Monteleone G, Corazza GR, Macdonald TT, Di Sabatino A. The role of interleukin 17 in Crohn's disease-associated intestinal fibrosis. Fibrogenesis Tissue Repair. 2013; 6(1): 13. doi: 10.1186/1755-1536-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, Karczewski J, Pezous N, Bek S, Bruin G, Mellgard B, Berger C, Londei M, Bertolino AP, Tougas G, Travis SP, Secukinumab in Crohn’s Disease Study Group Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012; 61(12): 1693–700. doi: 10.1136/gutjnl-2011-301668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, Baralle FE, Toews GB, White ES. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008; 177(6): 638–45. doi: 10.1164/rccm.200708-1291OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol. 2013; 304(3): C216–25. doi: 10.1152/ajpcell.00328.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol. 2007; 46(5): 955–75. doi: 10.1016/j.jhep.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 31.Arribillaga L, Dotor J, Basagoiti M, Riezu-Boj JI, Borrás-Cuesta F, Lasarte JJ, Sarobe P, Cornet ME, Feijoó E. Therapeutic effect of a peptide inhibitor of TGF-β on pulmonary fibrosis. Cytokine. 2011; 53(3): 327–33. doi: 10.1016/j.cyto.2010.11.019 [DOI] [PubMed] [Google Scholar]
- 32.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011; 29(5): 196–202. doi: 10.3109/08977194.2011.595714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012; 23(4): 705–15. doi: 10.1016/j.devcel.2012.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrlich HP, Allison GM, Leggett M. The myofibroblast, cadherin, alpha smooth muscle actin and the collagen effect. Cell Biochem Funct. 2006; 24(1): 63–70. doi: 10.1002/cbf.1188 [DOI] [PubMed] [Google Scholar]
- 35.Stangou M, Bhangal G, Lai PC, Smith J, Keith JC, Jr, Boyle JJ, Pusey CD, Cook T, Tam FW. Effect of IL-11 on glomerular expression of TGF-beta and extracellular matrix in nephrotoxic nephritis in Wistar Kyoto rats. J Nephrol. 2011; 24(1): 106–11. doi: 10.5301/JN.2010.5094 [DOI] [PubMed] [Google Scholar]
- 36.Shi JH, Guan H, Shi S, Cai WX, Bai XZ, Hu XL, Fang XB, Liu JQ, Tao K, Zhu XX, Tang CW, Hu DH. Protection against TGF-β1-induced fibrosis effects of IL-10 on dermal fibroblasts and its potential therapeutics for the reduction of skin scarring. Arch Dermatol Res. 2013; 305(4): 341–52. doi: 10.1007/s00403-013-1314-0 [DOI] [PubMed] [Google Scholar]
- 37.Burke JP, Cunningham MF, Sweeney C, Docherty NG, O’Connell PR. N-cadherin is overexpressed in Crohn’s stricture fibroblasts and promotes intestinal fibroblast migration. Inflamm Bowel Dis. 2011; 17(8): 1665–73. doi: 10.1002/ibd.21543 [DOI] [PubMed] [Google Scholar]
- 38.Follonier Castella L, Gabbiani G, McCulloch CA, Hinz B. Regulation of myofibroblast activities: calcium pulls some strings behind the scene. Exp Cell Res. 2010; 316(15): 2390–401. doi: 10.1016/j.yexcr.2010.04.033 [DOI] [PubMed] [Google Scholar]
- 39.Berridge MJ. Calcium signalling remodelling and disease. Biochem Soc T. 2012; 40: 297–309. doi: 10.1042/BST20110766 [DOI] [PubMed] [Google Scholar]
- 40.Yue LX, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011; 89(4):7 44–53. doi: 10.1093/cvr/cvq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saliba Y, Karam R, Smayra V, Aftimos G, Abramowitz J, Birnbaumer L, Fares N. Evidence of a Role for Fibroblast Transient Receptor Potential Canonical 3 Ca2+ Channel in Renal Fibrosis. J Am Soc Nephrol. 2015; 26(8): 1855–76. doi: 10.1681/ASN.2014010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nilius B, Flockerzi V. Mammalian transient receptor potential (TRP) cation channels. Preface. Handbook Exp Pharmacol. 2014; 223: v–vi. [PubMed] [Google Scholar]
- 43.Salido GM, Jardín I, Rosado JA. The TRPC ion channels: association with Orai1 and STIM1 proteins and participation in capacitative and non-capacitative calcium entry. Adv Exp Med Biol. 2011; 704: 413–33 doi: 10.1007/978-94-007-0265-3_23 [DOI] [PubMed] [Google Scholar]
- 44.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006; 99(2): 119–31. doi: 10.1161/01.RES.0000233356.10630.8a [DOI] [PubMed] [Google Scholar]
- 45.Nishida M, Onohara N, Sato Y, Suda R, Ogushi M, Tanabe S, Inoue R, Mori Y, Kurose H. Galpha12/13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J Biol Chem. 2007; 282(32): 23117–28. doi: 10.1074/jbc.M611780200 [DOI] [PubMed] [Google Scholar]
- 46.Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, Mori Y, Nagao T, Kurose H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006; 25(22): 5305–16. doi: 10.1038/sj.emboj.7601417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, Liang B, Yue L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res. 2010; 106(5): 992–1003. doi: 10.1161/CIRCRESAHA.109.206771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng J. Molecular mechanism of TRP channels. Compr Physiol. 2013; 3(1): 221–42. doi: 10.1002/cphy.c120001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoue R, Ito Y, Mori Y. [TRP-related proteins as new target molecules: their correspondence to native receptor-operated cation channels]. Tanpakushitsu Kakusan Koso. 2000; 45(6 Suppl): 1038–46. [PubMed] [Google Scholar]
- 50.Inoue R, Ito Y, Mori Y. [The TRP proteins, a rapidly expanding Ca2+ entry channel family and a new molecular target for drug development]. Nihon Rinsho. 2002; 60(1): 18–24. [PubMed] [Google Scholar]
- 51.Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat Rev Neurosci. 2001; 2(6): 387–96. doi: 10.1038/35077544 [DOI] [PubMed] [Google Scholar]
- 52.Clapham DE. TRP channels as cellular sensors. Nature. 2003; 426(6966): 517–24. doi: 10.1038/nature02196 [DOI] [PubMed] [Google Scholar]
- 53.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006; 68: 619–47. doi: 10.1146/annurev.physiol.68.040204.100431 [DOI] [PubMed] [Google Scholar]
- 54.Cheng Y, Nash HA. Drosophila TRP channels require a protein with a distinctive motif encoded by the inaF locus. Proc Natl Acad Sci USA. 2007; 104(45): 17730–4. doi: 10.1073/pnas.0708368104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inoue R, Jian Z, Kawarabayashi Y. Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Ther. 2009; 123(3): 371–85. doi: 10.1016/j.pharmthera.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 56.Inoue R, Shi J, Jian Z, Imai Y. Regulation of cardiovascular TRP channel functions along the NO-cGMP-PKG axis. Expert Rev Clin Pharmacol. 2010; 3(3): 347–60. doi: 10.1586/ecp.10.15 [DOI] [PubMed] [Google Scholar]
- 57.Numata T, Takahashi K, Inoue R. “TRP inflammation” relationship in cardiovascular system. Semin Immunopathol. 2016; 38(3): 339–56. doi: 10.1007/s00281-015-0536-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flockerzi V. An introduction on TRP channels. Handb Exp Pharmacol. 2007(179): 1–19. doi: 10.1007/978-3-540-34891-7_1 [DOI] [PubMed] [Google Scholar]
- 59.Xu T, Wu BM, Yao HW, Meng XM, Huang C, Ni MM, Li J. Novel insights into TRPM7 function in fibrotic diseases: a potential therapeutic target. J Cell Physiol. 2015; 230(6): 1163–9. doi: 10.1002/jcp.24801 [DOI] [PubMed] [Google Scholar]
- 60.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay BC, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium. 2007; 42(2): 213–23. doi: 10.1016/j.ceca.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 61.Putney JW. Physiological mechanisms of TRPC activation. Pflugers Arch. 2005; 451(1): 29–34. doi: 10.1007/s00424-005-1416-4 [DOI] [PubMed] [Google Scholar]
- 62.Hai L, Kawarabayashi Y, Imai Y, Honda A, Inoue R. Counteracting effect of TRPC1-associated Ca2+ influx on TNF-alpha-induced COX-2-dependent prostaglandin E2 production in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2011; 301(2): G356–67. doi: 10.1152/ajpgi.00354.2010 [DOI] [PubMed] [Google Scholar]
- 63.Kawarabayashi Y, Hai L, Honda A, Horiuchi S, Tsujioka H, Ichikawa J, Inoue R. Critical role of TRPC1-mediated Ca2+ entry in decidualization of human endometrial stromal cells. Mol Endocrinol. 2012; 26(5): 846–58. doi: 10.1210/me.2011-1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sassoli C, Chellini F, Squecco R, Tani A, Idrizaj E, Nosi D, Giannelli M, Zecchi-Orlandini S. Low intensity 635 nm diode laser irradiation inhibits fibroblast-myofibroblast transition reducing TRPC1 channel expression/activity: New perspectives for tissue fibrosis treatment. Lasers Surg Med. 2016; 48(3): 318–32. doi: 10.1002/lsm.22441 [DOI] [PubMed] [Google Scholar]
- 65.Graziani A, Poteser M, Heupel WM, Schleifer H, Krenn M, Drenckhahn D, Romanin C, Baumgartner W, Groschner K. Cell-cell contact formation governs Ca2+ signaling by TRPC4 in the vascular endothelium: evidence for a regulatory TRPC4-beta-catenin interaction. J Biol Chem. 2010; 285(6): 4213–23. doi: 10.1074/jbc.M109.060301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ina K, Kitamura H, Tatsukawa S, Fujikura Y. Significance of α-SMA in myofibroblasts emerging in renal tubulointerstitial fibrosis. Histol Histopathol. 2011; 26(7): 855–66. [DOI] [PubMed] [Google Scholar]
- 67.Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Biol Cell. 2004; 15(9): 4310–20. doi: 10.1091/mbc.E04-05-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Welch MP, Odland GF, Clark RA. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990; 110(1): 133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agarwal SK. Integrins and cadherins as therapeutic targets in fibrosis. Front Pharmacol. 2014; 5: 131. doi: 10.3389/fphar.2014.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Conroy KP, Kitto LJ, Henderson NC. αv integrins: key regulators of tissue fibrosis. Cell Tissue Res. 2016; 365(3): 511–9. doi: 10.1007/s00441-016-2407-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ilatovskaya D, Palygin O, Lowing A, Levchenko V, Staruschenko A. Angiotensin II Dependent Regulation of TRPC6 Calcium Channels in the Podocytes of the STZ-induced Type 1 Diabetic Dahl SS Rats Daria. Faseb J. 2015; 29. WOS:000361722706427. [Google Scholar]
- 72.Ilatovskaya DV, Staruschenko A. TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases. Am J Physiol-Renal. 2015; 309(5): F393–7. doi: 10.1152/ajprenal.00186.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, Henriksen FH, Salomonsson M, Morita H, Kawarabayashi Y, Mori M, Mori Y, Ito Y. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res. 2009; 104(12): 1399–409. doi: 10.1161/CIRCRESAHA.108.193227 [DOI] [PubMed] [Google Scholar]
- 74.Genazzani AA, Carafoli E, Guerini D. Calcineurin controls inositol 1,4,5-trisphosphate type 1 receptor expression in neurons. Proc Natl Acad Sci U S A. 1999; 96(10): 5797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lim HW, New L, Han J, Molkentin JD. Calcineurin enhances MAPK phosphatase-1 expression and p38 MAPK inactivation in cardiac myocytes. J Biol Chem. 2001; 276(19): 15913–9. doi: 10.1074/jbc.M100452200 [DOI] [PubMed] [Google Scholar]
- 76.Matsuoka K, Saito E, Fujii T, Takenaka K, Kimura M, Nagahori M, Ohtsuka K, Watanabe M. Tacrolimus for the Treatment of Ulcerative Colitis. Intest Res. 2015; 13(3): 219–26. doi: 10.5217/ir.2015.13.3.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sandborn WJ, Present DH, Isaacs KL, Wolf DC, Greenberg E, Hanauer SB, Feagan BG, Mayer L, Johnson T, Galanko J, Martin C, Sandler RS. Tacrolimus for the treatment of fistulas in patients with Crohn’s disease: a randomized, placebo-controlled trial. Gastroenterology. 2003; 125(2): 380–8. [DOI] [PubMed] [Google Scholar]
- 78.Marzo M, Felice C, Pugliese D, Andrisani G, Mocci G, Armuzzi A, Guidi L. Management of perianal fistulas in Crohn’s disease: an up-to-date review. World J Gastroenterol. 2015; 21(5): 1394–403. doi: 10.3748/wjg.v21.i5.1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pellino G, Pallante P, Selvaggi F. Novel biomarkers of fibrosis in Crohn’s disease. World J Gastrointest Pathophysiol. 2016; 7(3): 266–75. doi: 10.4291/wjgp.v7.i3.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gasparetto M, Angriman I, Guariso G. The multidisciplinary health care team in the management of stenosis in Crohn’s disease. J Multidiscip Healthc. 2015; 8: 167–79. doi: 10.2147/JMDH.S38729 [DOI] [PMC free article] [PubMed] [Google Scholar]