Abstract
Aging is associated with reductions in gray matter volume and cortical thickness. One factor that may play a role in mitigating age-associated brain decline is cardiorespiratory fitness (CRF). Although previous work has identified a positive association between CRF and gray matter volume, the relationship between CRF and cortical thickness, which serves as a more sensitive indicator of gray matter integrity, has yet to be assessed in healthy young and older adults. To address this gap in the literature, 32 young and 29 older adults completed treadmill-based progressive maximal exercise testing to assess CRF (peak VO2), and structural magnetic resonance imaging (MRI) to determine vertex-wise surface-based cortical thickness metrics. Results indicated a significant CRF by age group interaction such that Peak VO2 was associated with thicker cortex in older adults but with thinner cortex in young adults. Notably, the majority of regions demonstrating a positive association between peak VO2 and cortical thickness in older adults overlapped with brain regions showing significant age-related cortical thinning. Further, when older adults were categorized as high or low fit based on normative data, we observed a stepwise pattern whereby cortex was thickest in young adults, intermediate in high fit older adults and thinnest in low fit older adults. Overall, these results support the notion that CRF-related neuroplasticity may reduce although not eliminate age-related cortical atrophy.
Keywords: aerobic fitness, physical activity, lifespan, aging, brain maintenance, MRI
1. Introduction
Cerebral volume loss is a well-documented correlate of the aging process, accompanied by a reliable pattern of regional cortical thinning with advancing age (Fjell et al., 2009b; Salat et al., 2004; Shaw et al., 2016). Brain regions most susceptible to age-related cortical degeneration largely coincide with heteromodal association areas that support higher-level processing, and include lateral temporal, inferior parietal, and frontal regions, as well as the precuneus, temporoparietal junction, and fusiform gyrus (Fjell et al., 2009a; Fjell et al., 2009b; Shaw et al., 2016). Despite a general trend of cortical atrophy in healthy aging, individual differences have been observed in the rate and extent of degeneration, with some adults maintaining relatively more intact structural integrity throughout late life than same-aged peers (Pfefferbaum and Sullivan, 2015). Thus, in an effort to promote optimal brain health within a society where the population distribution is increasingly shifting towards older ages (Vincent and Velkoff, 2010), identifying relevant lifestyle factors that may protect against age-related neurostructural decline is warranted.
One factor that may play a role in mitigating age-associated brain decline is cardiorespiratory fitness ◆(CRF). CRF reflects the efficiency of the circulatory and respiratory systems to provide oxygenated blood to musculature during sustained aerobic physical activity. As a modifiable health factor, CRF can be optimized through regular engagement in physical activity of moderate to vigorous intensity such as jogging, swimming, or biking. The gold standard of CRF assessment is treadmill-based graded maximal exercise testing, which yields a measure of peak volume of oxygen consumption (peak VO2) at maximum intensity exercise. Compared to self-report or estimated CRF metrics, peak VO2 provides an objective and reliable indicator of CRF. Whereas systemic benefits of enhanced CRF to both emotional and physical health are well documented (DiLorenzo et al., 1999; Myers et al., 2015; Papasavvas et al., 2016; Tozzi et al., 2016; Warburton et al., 2006), only recently have studies begun to explore the relation between CRF and brain structure, particularly in the context of aging.
Cross-sectional studies of older adults have generally reported a positive association of CRF with gray matter volume (Boots et al., 2015; Bugg and Head, 2011; Erickson et al., 2007; Gordon et al., 2008; Weinstein et al., 2012) as well as with cognitive performance in the domains of executive function and memory (Barnes et al., 2003; Hayes et al., 2016). Interestingly, reliable CRF effects have been observed primarily within lateral prefrontal and parietal gray matter regions (Erickson et al., 2014; Hayes et al., 2014), cortical areas most vulnerable to age-related atrophy (Fjell et al., 2009b). Given this apparent regional overlap between CRF and aging effects, it follows that enhanced CRF could lessen the degree of cortical decline in aging. In support of this concept, aerobic exercise intervention studies have provided evidence of CRF-related neuroplasticity, as exercising older adults show greater regional brain volume than age-matched controls (Colcombe et al., 2006; Erickson et al., 2011).
Although positive associations between CRF and gray matter volume have been previously reported, it is less clear how CRF may relate to cortical thickness in older adult populations. Given that the surface area component used to define volume captures additional variance related to head size and the degree of regional cortical folding, volumetric approaches have been shown to yield less sensitive estimates of age-associated cortical atrophy when compared to surface-based cortical thickness techniques (Hutton et al., 2009; Lemaitre et al., 2012; Panizzon et al., 2009). Studies of patient populations (mild cognitive impairment, heart failure, and schizophrenia) have demonstrated positive associations between cortical thickness and CRF (Alosco et al., 2013; Reiter et al., 2015; Scheewe et al., 2013), yet the association between CRF and cortical thickness in the context of healthy aging remains unknown. One recent study (Lee et al., 2016) demonstrated a positive relation between self-reported physical activity and cortical thickness in healthy older adults. However, given that subjective CRF measures do not align well with objectively quantified peak VO2 (Tager et al., 1998), additional study is warranted.
In the current cross-sectional study, we investigated age-dependent associations between objectively quantified CRF (peak VO2) and cortical thickness in healthy older and young adults using a whole-brain cortical surface-based approach. The primary goals of this study were 1) to assess whether CRF is differentially associated with cortical thickness in young and older adults, 2) to determine the spatial overlap between observed CRF and aging effects across the cortex, and 3) to examine whether higher CRF in late life may eliminate age-related cortical thickness decline. Based on the previously reported beneficial effects of CRF on gray matter volume in aging, as well as positive associations between cortical thickness and CRF in patient populations, we expected to observe similar positive associations between CRF and cortical thickness in our older adult cohort. In particular, we anticipated that regions where CRF is positively associated with cortical thickness in older adults would overlap with frontal and temporoparietal regions most susceptible to age-related cortical thinning. Given the dearth of studies in young adults, we had no strong prediction about the association between CRF and cortical thickness for this cohort. A recent study of early adolescents (aged 9–11 years) found that greater CRF was associated with thinner cortex within regions of superior frontal, superior temporal and lateral occipital cortex (Chaddock-Heyman et al., 2015), a finding that likely reflects successful maturational development and neural pruning. The fact that in some brain regions protracted maturational cortical thinning has been observed throughout young adulthood (Tamnes et al., 2013) raises the possibility that young adults may show a negative association between CRF and cortical thickness similar to that observed in adolescents. By examining young and older adults in the same study, we were able to directly assess age-dependent associations between CRF and cortical thickness, and additionally, whether age-related differences in cortical thickness are impacted by CRF.
2. Material and Methods
2.1 Participants
Thirty-four young adults and 35 older adults were enrolled in the current study. Six older adults (four with incidental findings on MRI and two with excessive head motion) and two young adults (one with excessive head motion, the other whose peak VO2 value was a statistical outlier at greater than 3 standard deviations above the mean) were excluded from the current analyses. The final sample consisted of 32 young adults (age = 18–31 years) and 29 older adults (age = 55–82 years; see Table 1 for participant characteristics).
Table 1.
Characteristics (mean and standard deviation) of young and older adults, as well as high fit (ACSM percentile ≥50) and low fit (ACSM percentile <50) older adults.
| YA | OA | LFOA | HFOA | |
|---|---|---|---|---|
| Number of Participants | 32 (17 F) | 29 (15 F) | 14 (6 F) | 15 (9 F) |
| Age (years) | 21.0 (3.1)a | 63.7 (6.5) | 64.8 (7.0)b | 62.7 (6.1)b |
| Race (% Caucasian) | 75 a | 89 | 92 a | 87 a |
| Education (years) | 14.4 (1.8) a | 16.3 (2.6)* | 15.4 (2.7) a | 17.1 (2.4) b |
| WTAR (raw score) | 43.2 (4.0) a | 42.5 (6.2) | 39.6 (6.5) b | 45.2 (4.6) a |
| WTAR (standard score) | 117.5 (6.6) a | 115.0 (9.3) | 110.6 (9.8) b | 119.1 (6.8) a |
| MoCA | 28.5 (1.5) a | 27.8 (1.8) | 26.9 (2.1) b | 28.5 (1.0) a |
| CES-D | 6.2 (4.1) a | 5.2 (4.3) | 5.0 (4.3) a | 5.3 (4.5) a |
| BMI (kg/m2) | 23.0 (2.9) a | 25.9 (4.5)* | 28.4 (5.0) b | 23.5 (2.2) a |
| Peak VO2 (ml/kg/min) | 39.0 (7.1) a | 30.4 (7.4)* | 24.9 (4.7) b | 35.6 (5.4) a |
| Peak VO2 ACSM Percentile | 35.9 (25.8) a | 42.1 (31.7) | 13.6 (16.0) b | 68.7 (14.1) c |
| RER (VCO2/VO2) | 1.26 (0.02) a | 1.16 (0.08)* | 1.15 (0.03) b | 1.16 (0.02) b |
Significant difference between YA and OA (p<0.05).
Identical alphabetic superscripts denote the groups did not differ on this measure. American College of Sports Medicine (ACSM); Center for Epidemiological Studies Depression Scale (CES-D); Female (F); High Fit Older Adults (HFOA); Low Fit Older Adults (LFOA); Montreal Cognitive Assessment (MoCA); Older Adults (OA); Respiratory Exchange Ratio (RER); Wechsler Test of Adult Reading (WTAR); Young Adults (YA).
To ensure our sample represented a wide range of cardiorespiratory fitness levels, participants were recruited from general participant pools (Boston University for young adults, and the Boston University Memory Disorders Research Center at VA Boston, Boston University Alzheimer’s Disease Center, the Massachusetts Alzheimer’s Disease Research Center, and the Alzheimer’s Association TrialMatch for older adults) as well as through local libraries, YMCAs, and track (running) meets. Participants included in the study obtained at least a 12th grade education, were free from contraindications to cardiopulmonary testing or Magnetic Resonance Imaging (MRI), and did not have major medical (e.g., myocardial infarction, vascular disease), neurological (e.g., Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, head trauma), psychiatric (e.g., bipolar disorder, schizophrenia), or substance abuse issues that might affect cognition, as determined by a comprehensive health screen. Participants were screened for depression using a cut-off score of 16 on the Center for Epidemiologic Studies Depression Scale (CES-D) 20-item version. Mental status was assessed using the Montreal Cognitive Assessment (MOCA; http://www.mocatest.org/), where participants were excluded for cognitive impairment based on a cut-off score of 23 (Luis et al., 2009).
2.2 Cardiopulmonary Exercise Testing
An exercise physiologist and a cardiologist of the VA Boston Healthcare System supervised all sessions. Graded maximal exercise testing in association with air-gas-exchange was conducted using a two-minute Bruce protocol (Bruce et al., 1973) on a motor driven Woodway Barimill treadmill. A light-weight disposable pneumotach device was positioned in the participant’s mouth during exercise for gas exchange assessment (MedGraphics Ultima II). Peak volume of oxygen consumption and respiratory exchange ration (RER) were measured, as well as maximum heart rate, blood pressure, and ECG waveforms. Self-reported ratings of perceived exertion were collected at 1-minute intervals using the 20-point Borg Scale. Peak VO2 was considered valid if at least two of the following criteria were met: 1) respiratory exchange ratio greater than or equal to 1.05; 2) maximum heart rate equivalent to 85% of the age-predicted maximum (i.e., 220 – age); and 3) rating of perceived exertion greater or equal to 17, corresponding to an exertion level of “very hard” (a rating of 20 represents “maximal exertion”).
2.3 MRI Data Acquisition and Processing
Structural MRI was collected on a 3 Tesla Siemens Tim Trio scanner with a 12-channel head coil located at the VA Boston Healthcare System, Jamaica Plain Campus. A whole-brain T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence, empirically optimized for a high signal to noise ratio, was acquired in the sagittal plane: TR = 2530 ms, TE = 3.32 ms, TI = 1100 ms, flip angle = 7°, slices = 176, slice thickness = 1 mm, FOV = 256, matrix = 256×256, voxel size = 1 mm isotropic. To aid in tissue classification during semi-automated post-processing procedures, a T2-weighted Fluid-Attenuated Inversion Recovery (FLAIR) image was also acquired: TR = 6000 ms, TE = 475 ms, TI = 2100 ms, flip angle = 120°, slices = 176, slice thickness = 1 mm, FOV = 256, matrix = 256×256, voxel size = 1mm isotropic.
2.4 Surface-Based Cortical Thickness Measurement
A high-resolution T1-weighted volume, in conjunction with a T2-weighted FLAIR volume (to aid in tissue classification), was processed to obtain cortical thickness measures using the FreeSurfer image analysis suite (version 5.3.0; http://surfer.nmr.mgh.harvard.edu). Briefly, this process included motion correction of volumetric T1-weighted images, removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated Tailarach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (Fischl et al., 2002; Fischl et al., 2004; Segonne et al., 2004), intensity normalization (Segonne et al., 2004; Sled et al., 1998), tessellation of the gray matter/white matter boundary, automated topology correction, and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid (CSF) borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale et al., 1999; Fischl and Dale, 2000; Segonne et al., 2004). For each participant, the derived pial and white matter surface boundaries were manually inspected and edited by a trained rater (VJW) to assure proper segmentation of tissue class and to remove erroneously labeled non-gray matter tissue (e.g., dura) from the cortical surface. Surface boundaries were then recomputed following manual intervention as needed.
Once the models outlining cortical boundaries were complete, several deformable procedures were performed for further data processing and analysis including surface inflation (Fischl et al., 1999a), registration to a spherical atlas which optimizes individual cortical folding patterns to match cortical geometry across participants (Fischl et al., 1999b), and the creation of a variety of surface-based data including maps of curvature and sulcal depth. This method used both intensity and continuity information from the entire three-dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface (Fischl and Dale, 2000). The maps were created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. Also, the maps were not restricted to the voxel resolution of the original data and were thus capable of detecting sub-millimeter differences between groups.
Thickness measurements were mapped on the inflated surface of each participant’s reconstructed brain (Dale et al., 1999; Fischl et al., 1999a). This procedure allowed visualization of data across the entire cortical surface (i.e., both the gyri and sulci) without interference from cortical folding. Maps were subsequently smoothed using a circularly symmetric Gaussian kernel across the surface with a full width half max (FWHM) of 20 mm, supported by prior work suggesting that greater smoothing in a sample of healthy elderly adults increases reliability, sensitivity, and statistical power in FreeSurfer surface-based analyses (Liem et al., 2015). Next, cortical maps were averaged across participants using a non-rigid high-dimensional spherical averaging method to align cortical folding patterns (Fischl et al., 1999a). This procedure provides accurate matching of morphologically homologous cortical locations among participants on the basis of each individual’s anatomy while minimizing metric distortion, resulting in a mean measure of cortical thickness at each point on the reconstructed surface.
2.5 Statistical Analyses
Whole-brain surface-based analyses were implemented using the FreeSurfer command line tool “mri_glmfit” to perform regression analyses at each vertex along the cortical mesh. To assess age-dependent differential associations between cortical thickness and peak VO2, the Age Group by Peak VO2 interaction was tested, controlling for sex and education. To determine the extent to which associations between cortical thickness and peak VO2 overlapped with age-related differences in cortical thickness, a second regression analysis examined vertex-wise thickness metrics between the two age groups (older adults < young adults), controlling for sex and education. Resulting z-statistic maps were thresholded using a vertex-wise threshold of p < 0.05. Following whole-brain regression analyses, multiple comparison correction was performed using a clusterwise procedure described previously (Hagler et al., 2006; Segonne et al., 2004) and adapted for cortical surface analysis. This procedure, which is available as part of the FreeSurfer processing stream (mri_glmfit-sim), utilizes a pre-cached data simulation to obtain a distribution of the maximum cluster size under the null hypothesis. Briefly, a normal distribution z-map was synthesized using the matched smoothing (20 FWHM) and thresholding parameters (p < 0.05) as specified in the original analysis. Areas of maximum cluster size were recorded under these specifications, and the procedure was repeated for 10,000 iterations per hemisphere. Only clustered vertices are retained under the assumption that vertices demonstrating false positive effects would not appear next to each other. Once the distributions of the maximum cluster size across simulations were obtained, correction for multiple comparisons was accomplished by identifying clusters in the original statistical maps where the clusterwise p-value is the probability of seeing a maximum cluster of that size or larger during the simulation (p < 0.05).
3. Results
3.1 Participant Characteristics
Demographic characteristics of the young and older adult samples are provided in Table 1. No study participants had hypertension (blood pressure greater than 140/90 mmHg), as assessed by the exercise physiologist and cardiologist performing the cardiorespiratory exercise assessment. Groups were matched in sex, χ2 (1) = 0.01, p = 0.91, and race, χ2(1) = 2.21, p = 0.14, with both age groups consisting mostly of Caucasians. There were no group differences in estimates of pre-morbid intellectual functioning on the Wechsler Test of Adult Reading (WTAR), t (59) = 1.21, p = 0.23, self-reported depression symptoms (CES-D), t (59) < 1, p = 0.33, or performance on a test of mental status (MOCA), t (59) = 1.91, p = 0.06. Older adults completed a greater number of years of formal education, t (59) = 3.31, p <0.01, and had a higher body mass index (BMI), t (59) = −2.96, p <0.01, than young adults. As expected, peak VO2 (ml/kg/min) was significantly lower in older than in young adults, t (59) = 4.62 p <0.01. However, there was no difference in the American College of Sports Medicine (ACSM) mean peak VO2 percentile scores for young and older adults, t (59) < 1, p = 0.41, indicating that both groups were equivalently fit relative to their age and sex-based peers (Pescatello and American College of Sports, 2014). Furthermore, young and older adults demonstrated a similar range of values on both raw peak VO2 scores (young adults: 30.8 ml/kg/min; older adults: 29.3 ml/kg/min) and peak VO2 percentile scores corrected for age and sex using ACSM normative data (young adults: 0–90th percentile; older adults: 0–90th percentile), indicating representative and comparable samples in terms of CRF.
3.2. Differential Effects of Peak VO2 on Cortical Thickness in Older and Young Adults
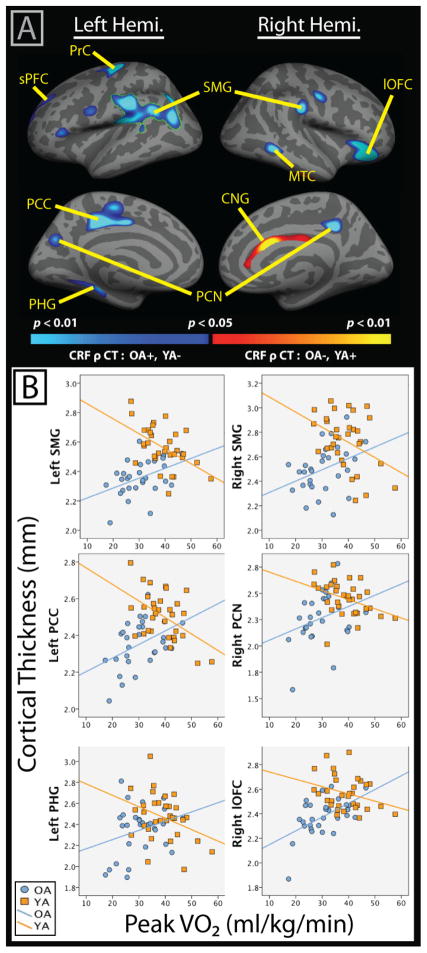
A significant peak VO2 by Age Group interaction was observed in several cortical regions (Figure 1a, Table 2). The strongest effect was observed in left lateral temporal-parietal cortex (outlined in green in Figure 1a), which remained significant following cluster-based multiple comparison correction. Additional regions that did not survive the cluster-based correction but were statically significant at p < 0.05 uncorrected with a minimum cluster extent of 250 mm2 included the left paracentral gyrus extending into the posterior cingulate, left parahippocampal gyrus, left precentral cortex, right lateral orbitofrontal cortex, right middle temporal gyrus, and bilateral precuneus. These areas are also visualized in Figure 1a and reported in Table 2. Scatterplots demonstrating the differential associations between cortical thickness and peak VO2 for young and older adults within select regions of interest are presented in Figure 1b.
Figure 1.
A) Results of whole-brain vertex-wise analysis evaluating the differential association between peak VO2 (CRF) and cortical thickness (CT) in older (OA) versus young adults (YA), controlling for sex and education. Results that survived a cluster-based correction for multiple comparisons (clusterwise threshold of p<0.05) are outlined in green and include the left SMG. Blue color scale represents interaction effects where peak VO2 and cortical thickness were positively correlated in OA and negatively associated in YA. The opposite pattern was observed in the right CNG, although within-group correlations between CRF and thickness were not significant in follow-up partial correlations. B) Scatterplots visualizing association of cortical thickness and peak VO2 by Age Group. Note: CNG= cingulate; lOFC=lateral orbitofrontal cortex; MTC=middle temporal cortex; PCC= Paracentral/Posterior Cingulate Cortex; PCN=Precuneus; PHG= parahippocampal gyrus; PrC= Precentral Cortex; SMG= supramarginal gyrus; sPFC= Superior Prefrontal Cortex;
Table 2.
Size of significant clusters (p<0.05, uncorrected) from whole-brain analysis evaluating the interaction between Age Group and peak VO2 on cortical thickness controlling for sex and education, as well as partial correlations illustrating the strength of the association between peak VO2 and cortical thickness (controlling for age, in addition to sex, and education) within each age group.
| Hemi. | Cluster Size (mm2) | Peak VO2 Partial Correlation (pr) | ||
|---|---|---|---|---|
| YA | OA | |||
| Supramarginal/Inf. Parietal | LH | 4215 | −0.49** | 0.45* |
| Lateral Orbitofrontal | RH | 1256 | −0.44* | 0.54** |
| Paracentral/Post. Cingulate | LH | 1235 | −0.51** | 0.42* |
| Precentral | LH | 794 | −0.45* | 0.55** |
| Superior Frontal | LH | 687 | −0.38* | 0.35 |
| Cingulate | RH | 505 | 0.25 | −0.36 |
| Parahippocampal | LH | 481 | −0.41* | 0.43* |
| Supramarginal | RH | 387 | −0.51** | 0.37 |
| Precuneus | RH | 361 | −0.40* | 0.38 |
| Middle Temporal | RH | 356 | −0.11 | 0.51** |
| Parstriangularis | LH | 286 | −0.28 | 0.41* |
| Precuneus | LH | 277 | −0.26 | 0.46* |
p < 0.05,
p < 0.01
To illustrate the strength of significant map-wise interaction effects, mean cortical thickness within these regions was extracted for each participant, and follow-up partial correlations were performed to evaluate the association between CRF and cortical thickness [controlling for age (years), sex, and education (years)] within young and older cohorts separately (Table 2). This follow-up analysis was not a replication of the previous vertex-wise analysis, as here we account for age within each group as a continuous variable. Nevertheless, it should be noted that these values may represent an over-estimation because the vertices were selected based on the results of a related surface-based analysis. One advantage of this approach is that it allows for an estimate of the strength of the relationship between CRF and cortical thickness in older and young adults in familiar statistical terms, while controlling for age, sex, and education. Results revealed significant positive associations between peak VO2 and cortical thickness in older adults, with the strongest effects observed in regions of the left precentral, right lateral orbitofrontal, and right middle temporal cortices. In contrast, young adults showed negative associations between CRF and cortical thickness, with the most prominent effects observed in left paracentral/posterior cingulate and bilateral supramarginal cortices. An inverse interaction was observed in the right anterior cingulate such that higher peak VO2 was associated with thinner cortex in older adults and thicker cortex in young adults, but follow-up partial correlations with peak VO2 within this region were not significant for either age group and thus, this region was not included in follow-up analyses in section 3.4 below.
3.3 Whole-brain Conjunction Analysis of Aging and CRF Effects
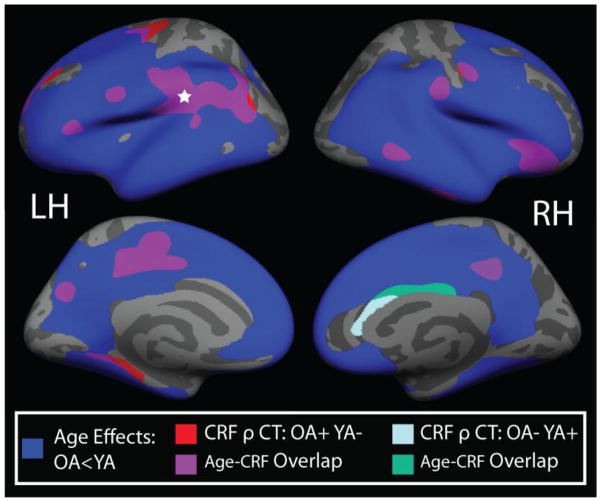
In order to compare the regional overlap of CRF and age effects, a second whole-brain regression analysis was conducted to determine age-related differences in cortical thickness between young and older adults (see Supplementary Figure 1). After controlling for sex and years of education, widespread reductions in cortical thickness were evident with aging in regions of medial and lateral prefrontal and parietal cortex, as well as inferior and lateral temporal cortex. Conversely, occipital, primary motor, and somatosensory cortices were relatively spared. No regions showed thicker cortex in older adults relative to young adults. To illustrate the spatial overlap between regions showing significant age group differences and regions showing a significant Peak VO2 by Age Group interaction effect (reported in section 3.2), a whole-brain conjunction map is presented in Figure 2. Of the 12 clusters showing a significant Peak VO2 by Age Group interaction, only the left parahippocampal cortex and left precentral clusters did not overlap with areas exhibiting significant age-related cortical thinning.
Figure 2.
Conjunction map demonstrating the spatial overlap between 1) age-group by peak VO2 interaction AND 2) age differences in cortical thickness (OA < YA). The blue label represents areas where cortical thickness was significantly lower in OA compared to YA. Red labels indicate regions where a significant interaction effect was noted such that CRF was positively associated with cortical thickness in OA, but negatively associated with thickness in YA. The light blue label represents regions where CRF was negatively associated with cortical thickness in OA, but positively associated with thickness in YA. Purple and green labels indicate regions of spatial overlap between the interaction effect and the age effects. All analyses controlled for sex and years of education, with a vertex-wise threshold of p<0.05. All age effects (blue labels) remained significant following cluster-based multiple comparison correction. For the CRF by age-group interaction analysis, the left supramarginal region (indicated by white star) remained significant following multiple comparison correction. LH=left hemisphere; RH=right hemisphere.
3.4. Comparison of Cortical Thickness in Young Adults and Older Adults Stratified by CRF Level
To determine whether age-related changes in cortical thickness were driven by CRF level in older adults, the older adult cohort was divided into two groups based on their American College of Sports Medicine (ACSM) normative peak VO2 scores adjusted for age and sex (Pescatello and American College of Sports, 2014). We defined as low fit older adults (LFOA; n=14) individuals scoring below the 50th percentile for normative peak VO2, and as high fit older adults (HFOA; n=15) those scoring above the 50th percentile. High and low fit older adults did not significantly differ in age, t(27) = 0.85, p = 0.41, education, t(27) = 1.82, p = 0.08, depression scores on the CES-D, t(27) = 0.21, p = 0.84, sex, χ2 (1) = 0.85, p = 0.36, race (p = 1.00, Fisher’s Exact Test 2-sided), or RER, t(27) = −0.32, p = 0.75. However, high fit older adults scored higher on a measure of estimated pre-morbid functioning (WTAR), t(27) = 2.70, p < 0.05, and had higher performance on a cognitive screening measure (MOCA), t(27) = 2.52, p < 0.05.
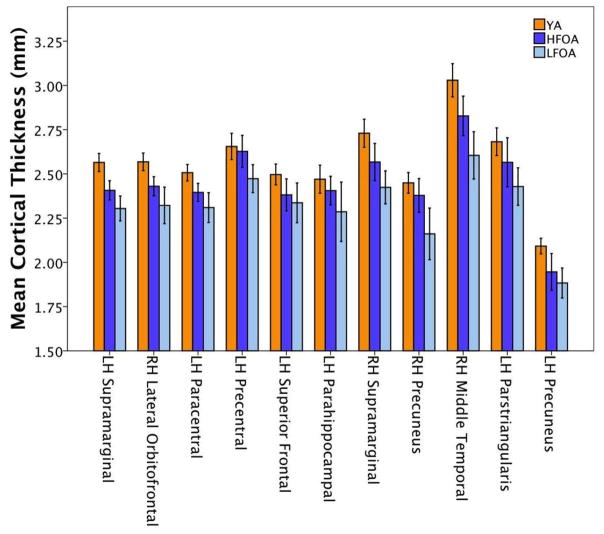
Mean cortical thickness was extracted for young adults, high fit older adults, and low fit older adults from the regions in which a significant Age Group by Peak VO2 interaction was observed (see section 3.2). These values are reported in Table 3 and visualized as a bar graph in Figure 3. The aim of this analysis was to evaluate whether CRF reduced age-related cortical decline among older adults. Cortical thickness in the young adults is thought to reflect optimal values, prior to the onset of age-related cortical atrophy; therefore, the young adults served as control group, regardless of CRF status. To compare the overall pattern of cortical thickness by group (young adults, low or high fit older adults) across these regions of interest, we conducted a follow-up repeated measures ANOVA with a Greenhouse-Geisser correction, with sex and education included as covariates of no interest. A significant main effect of region was observed, indicating that mean cortical thickness differed significantly between groups, F(6.49, 363.39) = 3.63, p = 0.001. Post hoc tests using the Bonferroni correction revealed a stepwise pattern such that young adults (mean = 2.56 ±0.02 mm) demonstrated thicker cortex than high fit older adults (mean = 2.46 ±0.03 mm; p = 0.027), and high fit older adults demonstrated thicker cortex than low fit older adults (mean = 2.33 ±0.03 mm; p = 0.006).
Table 3.
Mean cortical thickness for young adults (YA), high fit older adults (HFOA), and low fit older adults (LFOA) within significant regions of interest. Effect sizes evaluating the magnitude of cortical thickness differences between groups are additionally reported as Hedge’s g, with bold text indicating a large effect size. Regions are listed in order of largest to smallest.
| Hemi. | Mean Cortical Thickness (mm) | Effect Size (Hedge’s g) | |||||
|---|---|---|---|---|---|---|---|
| YA | HFOA | LFOA | YA > LFOA | YA > HFOA | HFOA > LFOA | ||
| Supramarginal/Inf. Parietal | LH | 2.56 (0.14) | 2.41 (0.10) | 2.30 (0.12) | 1.901 | 1.144 | 0.971 |
| Lateral Orbitofrontal | RH | 2.57 (0.14) | 2.43 (0.10) | 2.32 (0.18) | 1.607 | 1.068 | 0.742 |
| Paracentral/Post. Cingulate | LH | 2.51 (0.13) | 2.39 (0.09) | 2.31 (0.15) | 1.443 | 0.991 | 0.634 |
| Precentral | LH | 2.66 (0.21) | 2.63 (0.16) | 2.47 (0.14) | 0.973 | 0.151 | 1.032 |
| Superior Frontal | LH | 2.50 (0.16) | 2.38 (0.16) | 2.33 (0.19) | 0.986 | 0.737 | 0.278 |
| Parahippocampal | LH | 2.47 (0.22) | 2.41 (0.15) | 2.29 (0.29) | 0.729 | 0.294 | 0.511 |
| Supramarginal | RH | 2.73 (0.22) | 2.57 (0.19) | 2.42 (0.16) | 1.493 | 0.745 | 0.827 |
| Precuneus | RH | 2.45 (0.16) | 2.38 (0.17) | 2.16 (0.25) | 1.492 | 0.422 | 1.007 |
| Middle Temporal | RH | 3.03 (0.26) | 2.83 (0.20) | 2.60 (0.23) | 1.660 | 0.809 | 1.040 |
| Parstriangularis | LH | 2.68 (0.22) | 2.57 (0.25) | 2.43 (0.18) | 1.175 | 0.471 | 0.621 |
| Precuneus | LH | 2.09 (0.12) | 1.95 (0.19) | 1.89 (0.15) | 1.517 | 0.947 | 0.339 |
Figure 3.
Mean cortical thickness within regions of interest for young adults (YA), high fit older adults (HFOA) and low fit older adults (LFOA). Error bars represent a 95% confidence interval.
Finally, to evaluate the regional variability in the magnitude of absolute cortical thickness differences between groups, standardized effect sizes were calculated using Hedge’s g equation and reported in Table 3. Hedge’s g is considered a robust estimate for unequal sample sizes given that it weights each group’s standard deviation by its sample size (Hedges, 1981). Similar to Cohen’s d, effect sizes greater than 0.5 are considered moderate, whereas values above 0.8 are considered large (Cohen, 1988). Large effects sizes were observed in ten of the eleven brain regions when comparing cortical thickness between young and low fit older adults, whereas only six of eleven regions showed large effect sizes when comparing thickness between young and high fit older adults, suggesting that CRF may attenuate age-related differences in cortical thickness in some brain areas. In particular, a subset of brain regions (left precentral, left parstriangularis, and right precuneus) showed small effect sizes when comparing cortical thickness between young and high fit older adults, but large effects sizes when comparing thickness between high and low fit older adults. Thus, in these regions, cortical thickness in the high fit older adults appeared more similar to young adults than their low fit peers.
4. Discussion
In the current study, we evaluated age-dependent associations between an objective indicator of CRF (peak VO2) and vertex-wise cortical thickness in a sample of healthy young and older adults. We observed a significant age group by CRF interaction in several cortical areas, with the strongest effect noted in the left supramarginal cortex that remained significant following multiple comparison correction. Follow-up analyses indicated that higher CRF was related to thicker cortex in older adults, but thinner cortex in young adults, even though young adults had higher cortical thickness overall. Among older adults, higher peak VO2 was positively associated with cortical thickness primarily within the same brain regions that showed significant age-associated cortical decline. When comparing cortical thickness between young adults and older adults stratified by CRF level, a stepwise pattern emerged such that the cortex was thickest in young adults, intermediate in high fit older adults, and thinnest in low fit older adults. Thus, higher CRF was associated with thicker cortex in older adults, but not to the extent to eliminate age-related differences in cortical thickness.
4.1. Greater CRF is Associated with Thicker Cortex in Healthy Aging
Within brain regions demonstrating a significant age group by CRF interaction, CRF was positively associated with cortical thickness among older adults in left parahippocampal, paracentral, precuneus, and supramarginal cortices, as well as right middle temporal and lateral orbitofrontal areas, after additionally correcting for the continuous effects of age. These regional findings align with results of previous studies evaluating CRF and cortical thickness in patient populations (Alosco et al., 2013; Scheewe et al., 2013) and mild cognitive impairment (Reiter et al., 2015), as well as with a study relating cortical thickness and subjectively reported exercise duration in normal aging (Lee et al., 2016). Notably, associations between CRF and cortical thickness reliably appear within multimodal association regions across both healthy and abnormal aging studies, suggesting that higher order cortices may be most sensitive to variation in CRF.
Furthermore, as the present study included both young and older adults, we were able to directly demonstrate that regional associations between CRF and cortical thickness share considerable overlap with cortical areas vulnerable to age-related decline. Following a direct between-group age comparison, older adults showed widespread cortical thinning compared to young adults in ventrolateral, dorsolateral and dorsomedial prefrontal cortex, lateral and inferior temporal cortex, and inferior parietal regions. This pattern of cortical thinning in older adults is consistent with a prior meta-analysis of cortical thickness studies in aging (Fjell et al., 2009b), reflecting a particular vulnerability of higher order association areas to age-related atrophy.
Considering the spatial overlap of regions that showed reduced cortical thickness in older adults relative to young adults, coupled with positive associations between cortical thickness and CRF in older adults, the question arises to what extent CRF may attenuate age-related cortical thinning. When we classified older adults as high or low fit and directly compared cortical thickness to young adults (an age range in which the cortex is approaching optimal thickness, prior to the onset of age-related atrophy), our results revealed a stepwise progression across regions, such that the cortex was thickest in young adults, intermediate in high fit older adults, and thinnest in low fit older adults. Interestingly, we noted regional variability in the magnitude of cortical thickness differences between groups, such that in some brain regions (left precentral, right precuneus, and right parstriangularis) small effect sizes were noted when comparing cortical thickness between high fit older adults and young adults, whereas large effect sizes were noted when comparing high fit older adults to low fit older adults. In other words, the cortex of high fit older adults appeared more similar to young adults than to their low fit peers matched on age, gender, and education, supporting the notion that higher CRF may support brain maintenance by attenuating age-related cortical decline in some areas. Nonetheless, some degree of age-related cortical thinning was apparent across most cortical regions regardless of CRF status, indicating that although CRF may contribute to successful brain aging, CRF alone is not enough to completely eliminate age-related cortical decline in older adults.
Notably, the spatially overlapping yet directionally opposing effects of aging and CRF on cortical thickness were observed primarily in multimodal association cortices. One reason why multimodal cortices are considered to be highly predisposed to age-related degeneration and disease pathology in late life is the high degree of life-long plasticity that is required within these areas to meet complex integrative processing demands (Fjell et al., 2014). On a cellular level, processes leading to age-related gray matter atrophy are likely multifactorial and include a combination of vascular changes leading to subclinical ischemia and reduced perfusion, cerebral inflammation that may preferentially impact association and limbic cortices, increased oxidative stress and corticosteroids, a loss of synaptic spines and synapses, as well as shrinkage of cell bodies (Raz and Rodrigue, 2006). Conversely, prior work in humans has demonstrated a cascade of beneficial effects of enhanced CRF on brain structure as evidenced by increased cerebral profusion (Chapman et al., 2013), hippocampal neurogenesis (Pereira et al., 2007), increased markers of neuronal viability such as N-acetyl-aspartate (Gonzales et al., 2013), and greater white matter structural integrity (Johnson et al., 2012; Voss et al., 2013; Hayes et al., 2015). Likewise, aerobic exercise in rodents has been linked to increased neuronal growth, synaptic density, and neurovascularization (van Praag, 2008, 2009). Thus, neurostructural changes associated with CRF may directly oppose mechanisms of cortical degeneration observed in aging. As age-related cortical atrophy frequently precipitates cognitive decline in the elderly (Kaup et al., 2011), interventions aimed at improving CRF may help to preserve neuronal integrity, benefit cognition, and ultimately prolong functional independence in late life.
4.2. Greater CRF is Associated with Thinner Cortex Among Young Adults
In contrast to the pattern observed in older adults, higher peak VO2 was associated with thinner cortex in young adults. The differential association between CRF and cortical thickness in young and older adults can be understood in the context of dynamic changes in cortical thickness across the lifespan. Work by Tamnes and colleagues (Tamnes et al., 2010; Tamnes et al., 2013) has shown that cortical thickness peaks during early childhood, and subsequently undergoes a period of accelerated maturational cortical thinning throughout adolescence that slows and stabilizes upon entering young adulthood. Cortical thickness then remains relatively stable until mid- to late-life when regional cortical atrophy is commonly observed. Moreover, these fluctuations in cortical thickness across the lifespan have been further linked to cognitive ability. A recent longitudinal study showed that whereas in early adulthood, increased cortical thinning is associated with higher IQ, this relationship reverses around age 42, such that attenuated cortical thinning (and in some cases even thickening) is associated with higher IQ (Schnack et al., 2015). Intriguingly, in the current study, the strongest negative associations between CRF and cortical thickness in young adults included higher order association areas (particularly within orbitofrontal cortex and temporal-parietal regions), which are among those that tend to be the last to undergo maturational cortical thinning (Sowell et al., 2003). Thus, whereas a positive association of CRF and thickness in older adults is generally interpreted as reflecting preservation of gray matter in the context of aging, a negative association between peak VO2 and cortical thickness in young adulthood may reflect a beneficial effect of CRF on cortical development.
In line with our finding in young adults, a recent study of preadolescent children (aged 9–10 years) similarly demonstrated that higher peak VO2 was associated with thinner cortex within regions of superior frontal, superior temporal and lateral occipital cortex (Chaddock-Heyman et al., 2015). Results of the current study extend findings of a negative association between CRF and cortical thickness to young adults and demonstrate that young adulthood is also a period in which enhanced CRF may benefit brain structure. Nevertheless, such findings may be region-specific, as a recent voxel-based morphometry study focusing on the medial temporal lobes observed a positive association between peak VO2 and right entorhinal volume in young adults (Whiteman et al., 2016). Additional studies are needed to further delineate the relationship between CRF and brain structure in young adults.
4.3. Limitations of the Current Study
The current study was cross-sectional and does not allow inferences about a causal relationship between CRF and cortical thickness. Other cohort factors such as genetics, cardiovascular health, or nutrition could have influenced the results. This study employed a modest sample size, which may limit statistical power. Replication of the observed effects in larger samples is needed. Given this caveat, we report both corrected and uncorrected results for comprehensive data presentation. We additionally provide the corresponding effect sizes for uncorrected clusters to provide insight into the magnitude of the observed effects. Although all regions did not survive a cluster-based multiple comparison procedure, we were encouraged by the regional similarity of observed effects in our study and previous studies in age restricted samples (i.e., older adults only). Furthermore, the bilateral distribution of several of our regional findings (i.e., precuneus, supramarginal gyrus) additionally suggests that these regions do not reflect type I error. Finally, the sample was relatively homogeneous in regards to racial composition, and participants were healthier and more educated than the general population, which may limit generalization of the findings to other samples. Future longitudinal studies assessing alterations in cortical thickness throughout development as a function of CRF would provide direct insight into the age-dependent associations observed in the current study.
4.4. Conclusions
We observed differential associations between CRF and cortical thickness in young and older adults. After additionally controlling for age as a continuous variable within each group, CRF was positively associated with cortical thickness in older adults largely within multimodal association areas. These regional findings largely overlapped with cortical areas also vulnerable to age-related cortical decline. Higher fit older adults had greater cortical thickness than their lower fit peers, but CRF alone was not enough to eliminate age differences in cortical thickness, as high fit older adults had decreased cortical thickness relative to young adults. In young adults, CRF was associated with thinner cortex, possibly reflecting accelerated neural pruning that may benefit the developing young adult brain. Overall, these data support the notion that CRF is associated with neurostructural integrity in older, as well as young adults.
Supplementary Material
Results of whole-brain vertex-wise analysis evaluating the main effect of age group on cortical thickness after controlling for sex and education. Blue colored regions indicate where older adults (OA) show significantly thinner cortex than young adults (YA). Vertex-wise significance maps are thresholded at p<0.05, and all regions survived a cluster-based multiple comparison correction using a clusteriwise p-value of 0.05. Colored outlines depicted as an overlay on top of the significance map reflect automatic anatomical parcellations based on the destrieux atlas available within the FreeSurfer processing suite. LH=left hemisphere; RH=right hemisphere.
Acknowledgments
This work was supported by the Department of Veterans Affairs, Rehabilitation, Research & Development Service (Career Development Award e7822w awarded to S.M.H.), the Department of Veterans Affairs, Clinical Science Research & Development Services (M.V.), and the National Institute of Aging (K24AG035007 awarded to R.A.S.). Assistance with participant recruitment was provided by the Massachusetts Alzheimer’s Disease Research Center (P50-AG005134) and the Boston University Alzheimer’s Disease Center (P30-AG13846). This work was further supported with resources and use of facilities at the Neuroimaging Research for Veterans Center, VA Boston Healthcare System. The authors thank Kelly Allsup, M.A., Pantel Vokonas, M.D., Huiting Liu, M.A., Margaret Cadden, and Rebecca Lysiak for assistance with data collection. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs, the National Institutes of Health, or the United States Government. The authors have no conflicts of interest to report.
In Text Abbreviations
- CRF
Cardiorespiratory fitness
- LFOA
Low fit older adult
- HFOA
High fit older adult
- YA
Young adult
- OA
Older adult
- ACSM
American College of Sports Medicine
- WTAR
Wechsler Test of Adult Reading
- MoCA
Montreal Cognitive Assessment
- CES-D
Center for Epidemiologic Studies Depression Scale
- BMI
Body Mass Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alosco ML, Brickman AM, Spitznagel MB, Griffith EY, Narkhede A, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Hughes J, Rosneck J, Gunstad J. Poorer physical fitness is associated with reduced structural brain integrity in heart failure. J Neurol Sci. 2013;328:51–57. doi: 10.1016/j.jns.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- Boots EA, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, Koscik RL, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, LaRue A, Asthana S, Hermann BP, Sager MA, Johnson SC, Okonkwo OC. Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease. Brain Imaging Behav. 2015;9:639–649. doi: 10.1007/s11682-014-9325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging. 2011;32:506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock-Heyman L, Erickson KI, Kienzler C, King M, Pontifex MB, Raine LB, Hillman CH, Kramer AF. The role of aerobic fitness in cortical thickness and mathematics achievement in preadolescent children. PLoS One. 2015;10:e0134115. doi: 10.1371/journal.pone.0134115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, Defina LF, Keebler MW, Didehbani N, Lu H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci. 2013;5:75. doi: 10.3389/fnagi.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- DiLorenzo TM, Bargman EP, Stucky-Ropp R, Brassington GS, Frensch PA, LaFontaine T. Long-term effects of aerobic exercise on psychological outcomes. Prev Med. 1999;28:75–85. doi: 10.1006/pmed.1998.0385. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Elavsky S, McAuley E, Korol DL, Scalf PE, Kramer AF. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging. 2007;28:179–185. doi: 10.1016/j.neurobiolaging.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging. 2014;35(Suppl 2):S20–28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB. What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog Neurobiol. 2014;117:20–40. doi: 10.1016/j.pneurobio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci. 2009a;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009b;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales MM, Tarumi T, Kaur S, Nualnim N, Fallow BA, Pyron M, Tanaka H, Haley AP. Aerobic fitness and the brain: increased N-acetyl-aspartate and choline concentrations in endurance-trained middle-aged adults. Brain Topogr. 2013;26:126–134. doi: 10.1007/s10548-012-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, McAuley E, Kramer AF, Colcombe S, Gratton G, Fabiani M. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45:825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Alosco ML, Forman DE. The Effects of Aerobic Exercise on Cognitive and Neural Decline in Aging and Cardiovascular Disease. Curr Geriatr Rep. 2014;3:282–290. doi: 10.1007/s13670-014-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Forman DE, Verfaellie M. Cardiorespiratory Fitness Is Associated With Cognitive Performance in Older But Not Younger Adults. J Gerontol B Psychol Sci Soc Sci. 2016;71:474–482. doi: 10.1093/geronb/gbu167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Salat DH, Forman DE, Sperling RA, Verfaellie M. Cardiorespiratory fitness is associated with white matter integrity in aging. Ann Clin Transl Neurol. 2015;2:688–698. doi: 10.1002/acn3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV. Distribution theory for Glass’ estimator of effect size and related estimators. Journal of Educational Statistics. 1981;6:106–128. [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59:1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup AR, Mirzakhanian H, Jeste DV, Eyler LT. A review of the brain structure correlates of successful cognitive aging. J Neuropsychiatry Clin Neurosci. 2011;23:6–15. doi: 10.1176/appi.neuropsych.23.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Shin HY, Kim HJ, Jang YK, Jung NY, Lee J, Kim YJ, Chun P, Yang JJ, Lee JM, Kang M, Park KC, Na DL, Seo SW. Combined effects of physical exercise and education on age-related cortical thinning in cognitively normal individuals. Sci Rep. 2016;6:24284. doi: 10.1038/srep24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 2012;33:617, e611–619. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem F, Merillat S, Bezzola L, Hirsiger S, Philipp M, Madhyastha T, Jancke L. Reliability and statistical power analysis of cortical and subcortical FreeSurfer metrics in a large sample of healthy elderly. Neuroimage. 2015;108:95–109. doi: 10.1016/j.neuroimage.2014.12.035. [DOI] [PubMed] [Google Scholar]
- Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry. 2009;24:197–201. doi: 10.1002/gps.2101. [DOI] [PubMed] [Google Scholar]
- Myers J, McAuley P, Lavie CJ, Despres JP, Arena R, Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis. 2015;57:306–314. doi: 10.1016/j.pcad.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasavvas T, Bonow RO, Alhashemi M, Micklewright D. Depression Symptom Severity and Cardiorespiratory Fitness in Healthy and Depressed Adults: A Systematic Review and Meta-Analysis. Sports Med. 2016;46:219–230. doi: 10.1007/s40279-015-0409-5. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescatello LS American College of Sports M. ACSM’s guidelines for exercise testing and prescription. 9. Wolters Kluwer/Lippincott Williams & Wilkins Health; Philadelphia: 2014. [Google Scholar]
- Pfefferbaum A, Sullivan EV. Cross-sectional versus longitudinal estimates of age-related changes in the adult brain: overlaps and discrepancies. Neurobiol Aging. 2015;36:2563–2567. doi: 10.1016/j.neurobiolaging.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter K, Nielson KA, Smith TJ, Weiss LR, Alfini AJ, Smith JC. Improved Cardiorespiratory Fitness Is Associated with Increased Cortical Thickness in Mild Cognitive Impairment. J Int Neuropsychol Soc. 2015;21:757–767. doi: 10.1017/S135561771500079X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Scheewe TW, van Haren NE, Sarkisyan G, Schnack HG, Brouwer RM, de Glint M, Hulshoff Pol HE, Backx FJ, Kahn RS, Cahn W. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomised controlled trial in patients with schizophrenia and healthy controls. Eur Neuropsychopharmacol. 2013;23:675–685. doi: 10.1016/j.euroneuro.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Schnack HG, van Haren NE, Brouwer RM, Evans A, Durston S, Boomsma DI, Kahn RS, Hulshoff Pol HE. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb Cortex. 2015;25:1608–1617. doi: 10.1093/cercor/bht357. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shaw ME, Sachdev PS, Anstey KJ, Cherbuin N. Age-related cortical thinning in cognitively healthy individuals in their 60s: the PATH Through Life study. Neurobiol Aging. 2016;39:202–209. doi: 10.1016/j.neurobiolaging.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Tager IB, Hollenberg M, Satariano WA. Association between self-reported leisure-time physical activity and measures of cardiorespiratory fitness in an elderly population. Am J Epidemiol. 1998;147:921–931. doi: 10.1093/oxfordjournals.aje.a009382. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Dale AM, Ostby Y, Grydeland H, Richardson G, Westlye LT, Roddey JC, Hagler DJ, Jr, Due-Tonnessen P, Holland D, Fjell AM. Brain development and aging: overlapping and unique patterns of change. Neuroimage. 2013;68:63–74. doi: 10.1016/j.neuroimage.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi L, Carballedo A, Lavelle G, Doolin K, Doyle M, Amico F, McCarthy H, Gormley J, Lord A, O’Keane V, Frodl T. Longitudinal functional connectivity changes correlate with mood improvement after regular exercise in a dose-dependent fashion. Eur J Neurosci. 2016 doi: 10.1111/ejn.13222. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent GK, Velkoff VA. The next four decades: The older population in the United States: 2010 to 2050. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010. [Google Scholar]
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL, Wojcicki TR, White SM, Gothe N, McAuley E, Sutton BP, Kramer AF. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp. 2013;34:2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein AM, Voss MW, Prakash RS, Chaddock L, Szabo A, White SM, Wojcicki TR, Mailey E, McAuley E, Kramer AF, Erickson KI. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun. 2012;26:811–819. doi: 10.1016/j.bbi.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman AS, Young DE, Budson AE, Stern CE, Schon K. Entorhinal volume, aerobic fitness, and recognition memory in healthy young adults: A voxel-based morphometry study. Neuroimage. 2016;126:229–238. doi: 10.1016/j.neuroimage.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of whole-brain vertex-wise analysis evaluating the main effect of age group on cortical thickness after controlling for sex and education. Blue colored regions indicate where older adults (OA) show significantly thinner cortex than young adults (YA). Vertex-wise significance maps are thresholded at p<0.05, and all regions survived a cluster-based multiple comparison correction using a clusteriwise p-value of 0.05. Colored outlines depicted as an overlay on top of the significance map reflect automatic anatomical parcellations based on the destrieux atlas available within the FreeSurfer processing suite. LH=left hemisphere; RH=right hemisphere.