Abstract
Aside from cortical damage associated with age, cerebrovascular and neurodegenerative diseases, it's an outstanding question if factors of global health, including normal variation in blood markers of metabolic and systemic function, may also be associated with individual variation in brain structure. This cross-sectional study included 138 individuals between 40 to 86 years old who were physically healthy and cognitively intact. Eleven markers (total cholesterol, HDL, LDL, triglycerides, insulin, fasting glucose, glycated hemoglobin, creatinine, blood urea nitrogen, albumin, total protein) and five derived indicators (estimated glomerular filtration rate, creatinine clearance rate, insulin-resistance, average glucose, and cholesterol/HDL ratio) were obtained from blood sampling of all participants. T1-weighted 3T MRI scans were used to evaluate gray matter cortical thickness. The markers were clustered into five factors, and factor scores were related to cortical thickness by general linear model. Two factors, one linked to insulin/metabolic health and the other to kidney function (KFF) showed regionally selective associations with cortical thickness including lateral and medial temporal, temporoparietal, and superior parietal regions for both factors and frontoparietal regions for KFF. An association between the increasing cholesterol and greater thickness in frontoparietal and occipital areas was also noted. Associations persisted independently of age, presence of cardiovascular risk factors and ApoE gene status. These findings may provide information on distinct mechanisms of inter-individual cortical variation as well as factors contributing to trajectories of cortical thinning with advancing age.
Keywords: Magnetic Resonance Imaging, gray matter, aging, insulin, kidney, cholesterol
1 Introduction
Progressive age-related changes in gray matter volume and cortical thickness in healthy individuals follow a complex pattern and include noticeable changes in the prefrontal cortex, as well as lateral temporal lobes, medial parietal cortex, primary sensorimotor and visual cortices (Carmichael et al., 2013; Fjell et al., 2014; Salat et al., 2004). However, little is known on which factors may contribute to individual differences in brain structure prior to changes associated with senescence as well as what influence such factors might play in the trajectory of typical brain aging.
Outstanding attention has been drawn to identify structural brain changes that differentiate healthy aging from disease-related processes such as lesions of vascular origin and Alzheimer's disease (AD). Additionally, cardiovascular risk and disease (CVD) comprise a highly prevalent set of conditions in older adults that contribute to accelerated and altered trajectories of structural brain changes that are above and beyond those of typical aging and dementia and have been studied in detail (Gearing et al., 1995; Petrovitch et al., 2005; Sachdev et al., 2014). CVD can eventually modify brain structure through variable mechanisms, ranging from microvascular insults that present as white matter lesions to large infarcts (Gearing et al., 1995; Pantoni and Garcia, 1995; Sachdev et al., 2014).
It is now clear that health ‘risk’ states (states predictive of future disease) additionally influence brain structure even prior to a significant cardiovascular event. For example, reduction in gray matter volume is linked to hypertension, high cholesterol levels, poor peripheral glucose regulation and diabetes mellitus (Akintola et al., 2015; Meyer et al., 2000; Chen et al., 2006; Enzinger et al., 2005; Heijer et al., 2003; Jongen et al., 2007; Raz and Rodrigue, 2006; Schmidt et al., 2004; Tiehuis et al., 2008). Hippocampal and medial temporal atrophy has been shown in patients with diabetes and hypertension (Korf et al., 2007, 2005; Musen et al., 2006), and frontal and prefrontal cortices also seem to be particularly vulnerable to various CVD risk factors (Heijer et al., 2003; Raz et al., 2007, 2003; Reed et al., 2004). Taken together, these studies suggest that an overall higher risk of CVD is related to structural brain changes even without well-defined vascular lesions (Enzinger et al., 2005; Seshadri et al., 2004; Skoog et al., 1996). In older cognitively intact or near-normal participants, poorer kidney function was related to brain imaging measures of small vessel disease (Ikram et al., 2007), and lower brain volume has been related to insulin resistance and higher levels of visceral fat (Debette et al., 2010; Tan et al., 2011).
Aside from CVD, the study of the influence of other systemic diseases on individual variation and brain aging has been minimal, especially within normal limits of cognition. Particular regional relationships between higher blood pressure within or near “normal range” and reduced cortical thickness in prefrontal and temporal-parietal have been described (Leritz et al., 2011). It is noteworthy that such deleterious effects of systemic health on the brain do not seem to be restricted to disease or to individuals who fall into pre-clinical “abnormal ranges”.
Nevertheless, investigations linking variation in a diverse range of systemic health markers and variation in brain structure are lacking, particularly in the “normal range” spectrum of cognition and general health. We hypothesized that variation in systemic health would be associated with brain morphometry in an age-independent manner in individuals considered to be of typical health and aging. In this sense, “disease-free” or “disease-controlled” young and older brains could have some regional cortical differences according to each one's systemic health. Since healthier behavior and lower risks of CVD may be related to a lower development of cognitive impairment in older adults (Langa, 2015), this could contribute to our understanding of individual differences in brain aging within the wide range of “normality” prior and during typical senescence. Also, some of these various metabolic functions and organ systems may decline normally with aging and could contribute to alterations in brain structure.
The present work builds on one of our recent studies investigating associations between white matter integrity and a large variety of clinical laboratory parameters obtained from a fasting venous blood sample in a cohort of generally healthy and cognitively intact individuals in different stages of life, ranging from middle-aged to older individuals (Ryu et al., 2016; Ryu et al., 2014). These clinical blood markers were grouped into five factors using factor analysis, which were related to insulin and metabolic regulation, peripheral glucose levels, kidney function, lipid regulation and blood protein levels. Given the differential physiological relevance of these markers, we hypothesized that we would see differential associations between blood factors and cortical thickness depending on the system. In the present cross-sectional study, we investigated the relationship between these five factors indicative of systemic health and cortical thickness in healthy young and older adults. Our main goal was to examine if and how common blood markers of general health relate to gray matter structural measures. Secondarily, we further defined the relationship between those factors and age and investigated possible impacts of cardiovascular risk factors (hypertension, type 2 diabetes and dyslipidemia) and Apolipoprotein E (ApoE) gene status on this relationship. Our findings suggest associations between cortical thickness and measures of lipid metabolic health and kidney function in the range of typical variation. These relationships tend to be present also prior to later aging. Our results provide novel insights into potential mechanisms influencing differential trajectories of the brain structure through the aging process.
2 Materials and Methods
2.1 Study design and participants
A sample of 250 cognitively healthy middle-aged and older adults were recruited through the Massachusetts General Hospital, the local community, and local senior centers; these individuals form part of a longitudinal cohort to evaluate vascular contributions to brain aging. A total of 138 middle-aged and older adults (55 men/83 women) aged 40-86 years was selected for this cross-sectional study based on the availability of fasting venous blood sample and T1-weighted MRI data. All participants were physically healthy, cognitively intact, and literate with at least a high school education. Participants were excluded if they had major neurologic or psychiatric illnesses, history of stroke, significant head trauma, brain surgery or substance abuse, unstable medical illness, cancer within the nervous system or contraindications for an MRI scan. One hundred twenty-five participants were Caucasian (90.57%), 11 were African American (7.97%), and two were Asian.
Participants with controlled hypertension (HT), dyslipidemia (DLP), or type 2 diabetes (DM) were not excluded. Individuals with these characteristics represented 55.8% of the sample (total N = 77; HT alone = 18, DLP alone = 23, DM = 2; HT + DM = 6, HT + DLP= 14, DM + DLP= 1, HT + DM + DLP= 13).
The present work shares most of the participants with a prior investigating the effects of systemic health factors on white matter integrity with diffusion tensor imaging, with the exception of one subject lacking cortical thickness data (Ryu et al., 2016). Part of the sample (127 out of 138 individuals) also overlaps with a prior study investigating the impact of insulin resistance in white matter integrity (Ryu et al., 2014). There was no overlap in imaging methods analyses with any of our previous papers. The study was approved by the Partners Healthcare Internal Review Board (#2008P001486/MGH) and followed the Ethical Principles and Guidelines for the Protection of Human Subjects of Research, generally known as the Belmont Report. All participants provided written informed consent to participate in this research.
2.2 Clinical procedures
Assessments included ascertainment of medical history as well as general medical, physical, and neurologic examinations. Overnight fasting venous blood samples were collected on the day of the MRI session for estimation of 11 markers: total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride, fasting serum insulin, fasting glucose, glycosylated hemoglobin A1C (HbA1C), creatinine, blood urea nitrate (BUN), albumin, and total protein. Serum insulin was measured using electrochemiluminescence immunoassay (Mayo Medical Laboratories, Andover, MA). Five indicators of systemic health – homeostasis model assessment of insulin resistance (HOMA-IR), average glucose level, estimated glomerular filtration rate (eGFR), creatinine clearance (CCL) and cholesterol to HDL ratio (Chol/HDL ratio) – were calculated as per our previous study and will be further referenced in the text as blood markers (Ryu et al., 2016).
We also assessed the presence of the epsilon 4 allele of the Apolipoprotein E (ApoE E4), in a subset of the individuals. ApoE E4 is a well-know risk factor for the development of AD and an additional analysis of the relationship between cortical thickness and factors regarding the presence of this gene was performed. DNA for this analysis was extracted from frozen blood samples on the Autogen FlexStar instrument using Qiagen's FlexiGene chemistry. The DNA was quantified by Quant-iT PicoGreen assay (Invitrogen) and normalized to 10 ng/ul. ApoE genotyping was performed via Applied Biosystems Taqman SNP Genotyping assays rs7412 and rs329458.
2.3 Factor analysis of blood markers
Factor analysis was performed to identify the latent structure of the blood markers. The resulting factors were deemed significant based on eigenvalue criteria (> 1.0). Varimax orthogonal rotation was used and variables with absolute factor loadings at 0.40 or above were considered major contributors to a factor, as described previously (Ryu et al., 2016). Secondary confirmatory analyses including factor analyses both without component rotation and indicators including age in their calculation were also performed. Structure of the factors is presented in Table 2.
Table 2.
Components of the five Factors used in the general linear model regressions versus cortical thickness in the study.
| Factor | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Interpretation | Insulin/Metabolic | Glucose | Kidney function | Cholesterol | Protein |
| % variance explained | 20.78 | 18.42 | 18.03 | 13.78 | 8.41 |
| Insulin | 0.82 | 0.19 | −0.01 | −0.08 | 0.06 |
| HDL | −0.81 | 0.01 | 0.00 | 0.19 | 0.02 |
| Chol/HDL ratio | 0.81 | −0.04 | 0.02 | 0.45 | 0.00 |
| HOMA-IR | 0.75 | 0.47 | 0.03 | −0.10 | 0.03 |
| Triglycerides | 0.69 | 0.04 | 0.16 | 0.28 | −0.01 |
| Average Glucose | 0.07 | 0.93 | 0.17 | −0.13 | −0.07 |
| HbA1C | 0.07 | 0.93 | 0.17 | −0.13 | −0.07 |
| Fasting Glucose | 0.17 | 0.86 | 0.10 | −0.07 | −0.10 |
| eGFR | −0.03 | −0.14 | −0.92 | 0.02 | −0.02 |
| Creatinine | 0.23 | 0.12 | 0.86 | −0.11 | 0.11 |
| BUN | 0.15 | 0.27 | 0.77 | −0.03 | 0.01 |
| CCL | 0.40 | 0.06 | −0.76 | −0.12 | 0.13 |
| Cholesterol | −0.08 | −0.13 | −0.01 | 0.97 | 0.08 |
| LDL | 0.13 | −0.17 | −0.06 | 0.92 | 0.09 |
| Total Protein | 0.10 | 0.01 | 0.11 | 0.02 | 0.84 |
| Albumin | −0.07 | −0.20 | −0.08 | 0.12 | 0.76 |
Bolded values indicate an absolute loading value of 0.40 or above. HDL = high density lipoprotein; LDL = low density lipoprotein; Chol =cholesterol; HbA1C = glycated hemoglobin; HOMA-IR = homeostatic model assessment of insulin resistance; BUN = blood urea nitrogen; eGFR = estimated glomerulus filtration rate; CCL = creatinine clearance
2.4 MRI acquisition
All MRI studies were performed on the same 3 Tesla Siemens Trio scanner (Siemens Medical Solutions, Erlangen, Germany) with a 12-channel phased-array head coil reception and body coil transmission. T1-weighted whole-brain high-resolution (1×1×1 mm) multi-echo MPRAGE were obtained using the following parameters: TI =1000 ms, TR = 2530 ms, TE = 1.64, 3.50; 5.36 and 7.22 ms, field of view = 256×256 mm (sagittal), matrix size = 256×256×176, bandwidth = 651 Hz/pixel, and GRAPPA factor = 2, empirically optimized for high contrast between gray and white matter, as well as gray matter and cerebrospinal fluid (CSF) for optimal structural and surface segmentation.
2.5 Cortical thickness and hippocampal volume processing
Automated subcortical and WM segmentation as well as cortical surface reconstruction were obtained from the T1-weighted MRI images using the FreeSurfer image suite version 5.3.0 (https://surfer.nmr.mgh.harvard.edu). The same program was further used to obtain cortical thickness measurements. The technical details of these procedures were described in prior publications (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2004a, 2004b; et al., 2002; Fischl et al., 2001, 1999).
Thickness measures were mapped on the ‘inflated’ surface of each participant's reconstructed brain (Fischl et al., 1999). This procedure allows visualization of data across the entire cortical surface without interference from cortical folding. As opposed to volumetric spatial filtering prone to partial-volume bias in the estimation of gray matter volumes, thickness values at each surface vertex were smoothed here across the cortical mantle using a circularly symmetric Gaussian kernel with a standard deviation of 30 mm and averaged across participants using a non-rigid high-dimensional spherical averaging method to align cortical folding patterns (Fischl et al., 1999).
The FreeSurfer processing stream also provides automatically individual volumetric values for cortical and subcortical structures (anatomic segmentation) (Fischl et al., 2002). Since the hippocampus is not on the cortical surface, and due to its significance in Alzheimer's disease and other brain disorders, we included an additional volumetric directed analysis of this structure. The volumes of the left and right hippocampi of each subject were averaged and represented as a percentage of intracranial volume.
2.5 Statistical analyses and results display
We initially explored the relationship between each of the factors and age, and also of hippocampal volumes and the factors while controlling for the effects of age. These analyses were performed using linear and rank correlation methods with Matlab R2014b (The Mathworks Inc., U.S.A.). We tested variables for normal distribution through a simulation method (Jarque-Berra test), and significance of the associations found was tested with Pearson's (for data with normal distribution) and Spearman's (for data without normal distribution) correlation tests.
A general linear model (GLM) analysis was performed to examine regional associations between the five health factors and cortical thickness at each vertex. These analyses were then repeated regressing out the following potential confounders: age, sex, the other factors and motion parameters (translation and rotation) obtained from diffusion-weighted volumes in our previous study (Ryu et al., 2016). Secondary analyses with the intention to explore specific effects of sex and age were also performed.
For illustrative purposes, results were presented with and without correction for multiple comparisons at p< 0.05 on a semi-inflated surface provided with FreeSurfer. Correction for multiple comparisons was performed using a clusterwise approach procedure described previously (Hagler et al., 2006), adapted for cortical surface analysis and part of the FreeSurfer processing stream. Surface data were initially corrected for multiple comparisons with a threshold of p<0.05 for the clusterwise p value, for both hemispheres and in both directions (taking into account positive and negative effects), using a Monte Carlo simulation approach. When values of p <0.05 were found, then another analysis was performed with a threshold of p<0.01.
After the clusterwise correction for multiple comparisons, specific regions of interest (ROI) were also drawn in the areas of significant correlation between cortical thickness and each factor, to build dispersion plot graphics that provides information on the individual behavior of individuals in each analysis. These values were used also for two additional exploratory analyses to clarify the impact of individuals with cardiovascular risk factors (hypertension, diabetes mellitus and dyslipidemia) and the influence of ApoE gene status in the associations.
3 Results
3.1 Participants
Table 1 presents demographics and average blood markers for all participants. The group average for each blood marker was within the normative values, except for CCL, which was slightly lower than normal. However, CCL is known to decrease with age and our sample included both middle-aged and older adults. Although a small portion of the participants was outside of the normative range for each marker, results were examined with these individuals as well as with exclusion of outliers to confirm that the associations were not primarily due to individuals with extreme values.
Table 1.
Demographics and sample averages of blood markers, blood pressure and indicators of all individuals (N = 138).
| Variables | Reference valuesa | Mean (standard deviation) | Range |
|---|---|---|---|
| Age, y | 62.08 (11.20) | 40-86 | |
| Female, % (n) | 59.42 (82) | ||
| Cholesterol, mg/dL | < 200 | 186.71 (36.28) | 106-313 |
| HDL, mg/dL | 40 to 60 | 59.60 (17.82) | 27-116 |
| LDL, mg/dL | < 130 | 106.76 (30.67) | 42-204 |
| Triglycerides, mg/dL | < 150 | 101.75 (54.87) | 34-313 |
| Chol/HDL Ratio | ≤ 5 | 3.35 (1.02) | 1.7-6.6 |
| Average Glucose, mg/dL | 77 to 137 | 115.51 (19.37) | 91-217 |
| HbA1C, % | 4.3 to 6.4 | 5.65 (0.67) | 4.8-9.2 |
| Insulin, uIU/mL | 2.6 to 25.0 | 8.75 (5.74) | 1.8-33 |
| Fasting Glucose, mg/dL | 70 to 110 | 91.13 (20.11) | 52-200 |
| HOMA-IR | < 2.5 | 2.04 (1.55) | 0.33-8.39 |
| Albumin, g/dL | 3.3 to 5.0 | 4.40 (0.22) | 3.8-4.9 |
| BUN, mg/dL | 8 to 25 | 16.58 (6.66) | 7 - 64 |
| Creatinine, mg/dL | 0.6 to 1.5 | 0.94 (0.26) | 0.58-2.14 |
| eGFR, mL/min/1.73 m2 | ≥ 60 | 77.76 (17.32) | 26.2-107.2 |
| CCL, mL/min | 97 to 137 for men, 88 to 128 for women | 86.10 (31.13) | 29.5-109.1 |
| Total Protein, g/dL | 6.0 to 8.3 | 7.23 (0.39) | 6.2-8.3 |
| Blood pressure | |||
| Systolic | - | 122.40 (14.43) | 96-172 |
| Diastolic | - | 77.43 (9.97) | 50-112 |
from the Massachusetts General Hospital core laboratory reference ranges and the National Cholesterol Education Program; these values are provided for informational purpose only and may vary based on clinical practice, ethnicity, age, sex, and other factors.
3.2 Factor analysis of blood markers
As described in Ryu et al. (2016), five significant primary factors were extracted from the 16 blood markers (Table 2). Briefly, Factor 1 was weighted by several of the variables with the strongest weighting for insulin and HDL, therefore, defined as the ‘insulin/metabolic factor’, and increased with greater fasting insulin, total cholesterol/HDL ratio, HOMA-IR, triglyceride, CCL and lower HDL. Factor 2, defined as a ‘glucose factor’, increased with greater HOMA-IR, average and fasting blood glucose and HbA1C. Factor 3, defined as a ‘kidney function factor’, increased with greater creatinine and BUN, and lower eGFR and CCL. Factor 4, defined as a ‘cholesterol factor’, increased with higher Chol/HDL ratio, total cholesterol, and LDL. Factor 5, defined as a ‘protein factor’, increased with total protein and albumin. Except for Factor 5, which is related to nutrition and liver function (responsible for albumin synthesis), greater factor scores were related to marker levels usually linked to poorer health or increased risk of disease overall. These five factors cumulatively accounted for 79.42% of the variance all blood markers and indicators and were tested in the surface-based general linear models with cortical thickness.
A more conservative set of factors that did not include variables with an age component (i.e. eGFR and CCL) was also created. This analysis resulted in factors with a similar structure to the original ones and similar associations with cortical thickness. This was particularly important for the kidney function, which included only BUN and creatinine as major components, from now on called unadjusted kidney function factor (Ryu et al., 2016).
3.2.1 Analyses of possible correlations between Factors and age
Except for factor 4 (cholesterol), all factors were correlated with age, with different degrees of significance (insulin/metabolic: Rho= 0.200, p= 0.018; glucose: Rho= 0.256, p= 0.0024; kidney function: Rho= 0.504, p= 2.7 × 10−10; protein: R= 0.265, p= 0.002). Even when using the factor analysis excluding age-adjusted indicators (eGFR and CCL), the unadjusted kidney function factor remained correlated with age (R = 0.263, p = 0.0018). These associations demonstrate the need to carefully examine correlations between health factors and cortical measures while accounting for age.
We also performed a secondary analysis after splitting the cohort into two different age groups (younger and equal to or older than 60 years old) in order to further explore this relationship. After this split, only Factor 3 (kidney function) remained correlated to age in individuals older than 60 (Rho= 0.328; p= 0.004).
3.3 Surface-based associations between factors and cortical thickness
Maps of the associations between each factor and cortical thickness are shown in Figure 1 with and without controlling for the effects of age. We also performed analyses controlling for sex, the other blood factors and estimated motion in the scanner. These variables had little impact in the results and therefore are not shown. Plots of the average cortical thickness and the residuals for the areas that remained statistically significant after correcting for multiple comparisons and controlling for the effects of age (seen in the insulin/metabolic, kidney function and cholesterol factors) are presented in Figure 2, as well as the areas used for extraction of the cortical thickness data. Specific GLM analyses of the associations between cortical thickness and the factors splitting the cohort into two groups (younger and older than 60 years old) and sex were also performed. Results for Factor 3 were the most informative and are disclosed as supplement material (supplementary figure 1). We found no significant statistic differences in the correlations between cortical thickness and factors between men and women in a posteriori analyses. Due to the period of time of imaging acquisition, we examined any associations between scan date and primary variables. Although there was an association between scan date and Factor 3, it was not related to any of the imaging markers and use of scan date as a covariate did not alter the associations reported.
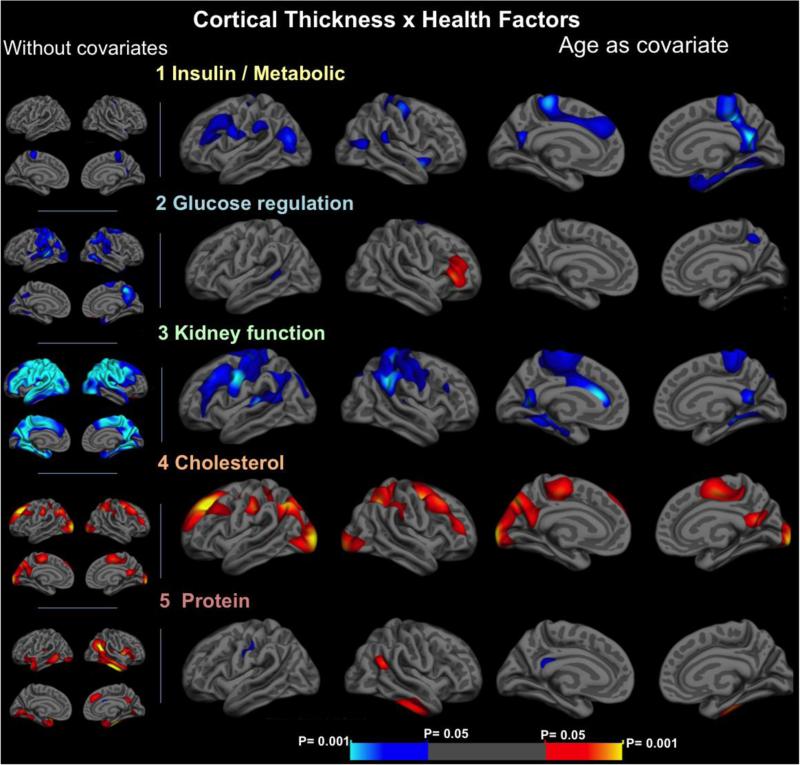
Figure 1.
statistical maps of the correlations between cortical thickness and each of the health factors, without covariates (left column, smaller images) and accounting for the effects of age (right – larger images). Maps are presented on the semi-inflated cortical surfaces of an average brain. The color scale at the bottom represents the statistical significance of the correlations with yellow and light blue indicating the most significant regions; blue indicates a negative association between the factor score and thickness, red/yellow indicates positive relationships.
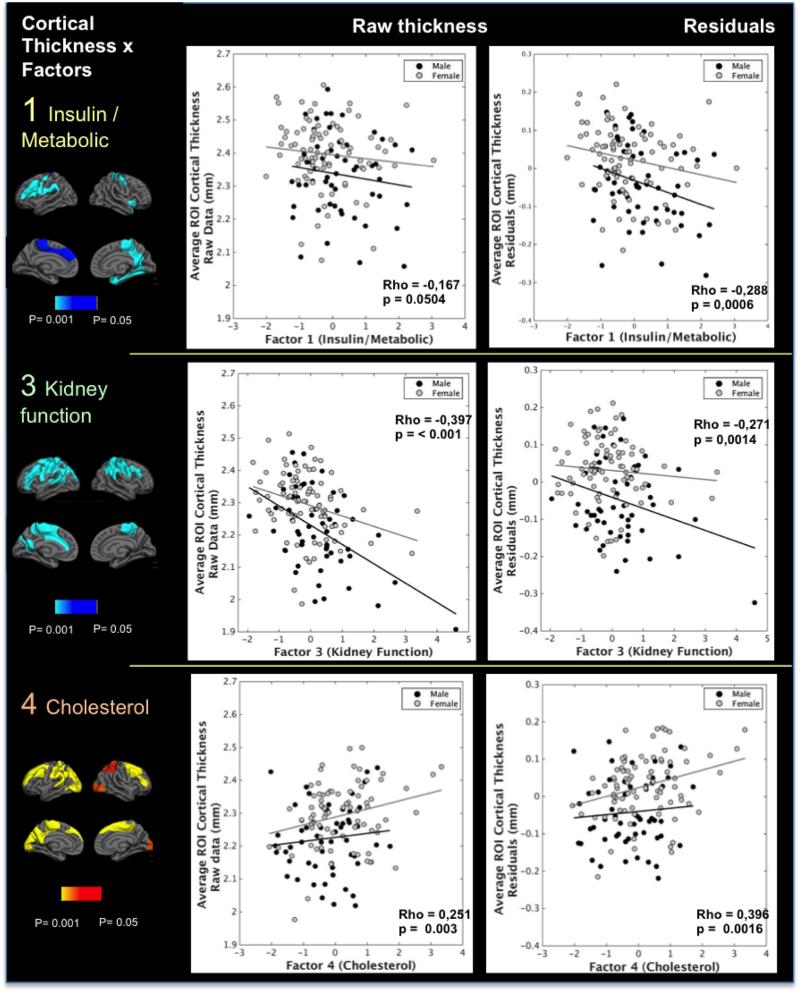
Figure 2.
scatterplots of the associations between factors 1, 3 and 4 and cortical thickness. Raw cortical thickness values (left column) and residuals (right column) of the regressions between thickness and age were extracted from ROIs defined by the significant voxel-wise associations with each of the factors after multiple comparisons. These values were used for examination of the distribution of the effects. Male: black spheres and lines; Female: gray spheres and lines. Associations for factor 1 were significant only after accounting for age as a covariate; other factors were significant in all sub analyses. The differences noted in the effects related to sex are not statistically significant. These effects, however, are always in the same direction in both genders.
A brief description of the main findings for each factor is given below.
Factor 1 – insulin/metabolic
In Factor 1 an increase in insulin, insulin resistance (HOMA/IR), cholesterol and triglycerides, as well as a decrease in HDL and creatinine clearance were associated with decreased cortical thickness in regions including the right posterior cingulate, precuneus, insula, medial temporal lobe and left sensorimotor and prefrontal cortices. Effects on the the right posterior cingulate, precuneus, insula and medial temporal lobe (including enthorhinal cortex) and left sensorimotor and prefrontal cortex persisted after correction for multiple comparisons with p< 0.05 and were enhanced after accounting for the effects of age, which acted as a confounder (Figures 1 and 2). An area of association in the right precuneus also survived multiple comparisons at the 0.01 threshold level (p = 0.0004).
Factor 2 – glucose
An increase in this factor was associated with decreased cortical thickness in several areas in the temporoparietal transition bilaterally in the analyses without covariates. However, when controlling for the effects of age and other covarates, these areas were dramatically reduced and did not survive multiple comparisons.
Factor 3 – kidney function factor
Kidney function factor was the most strikingly related to cortical thickness in widespread association and sensory-motor areas. An almost whole-brain negative association between kidney function factor and cortical thickness was found before accounting for covariates. A significant reduction of the aforementioned effects, particularly in the lateral and medial temporal lobes was seen when controlling for the effects of age. However, some widespread clusters of negative association of the factor with cortical thickness persisted in parietal areas, temporoparietal transitions and medial temporal areas. The areas in the parietal, frontoparietal, and in left prefrontal and medial frontal regions persisted after correction for multiple comparisons and after controlling for the other factors and sex, with a p threshold of < 0.05. An additional analysis with a derived factor of kidney function without age-adjusted variables showed similar areas of negative association with cortical thickness, even when accounting for the effects of age. Negative associations also persisted when splitting the participants into two groups: younger and older than 60 years old, even though the effects were more pronounced in older subjects, and also when investigating an unadjusted kidney function factor without age-derived variables (supplementary figure 1).
Factor 4 – cholesterol
An increase in total cholesterol and LDL values was associated with increased cortical thickness bilaterally in the prefrontal cortex, superior parietal (including right precuneus) and frontoparietal areas, but also in some bilateral parietooccipital and lateraloccipital areas. The areas with a higher consistency of this association were in the prefrontal cortices. These effects persisted after correction for multiple comparisons (p<0.05) and were not influenced by age, sex and other covariates. Two areas of association in the left superiorfrontal (p = 0.0004) and lateral occipital giri (p = 0.0002) also survived multiple comparisons at the 0.01 threshold level.
Factor 5 – Protein
Increases in total protein and albumin values were associated with higher cortical thickness in specific temporal and temporoparietal areas. However, most of these effects did not persist after considering the effects of age in the analysis (Figure 1). No major effects of sex and other covariates were seen.
3.4 Associations between factors and hippocampal volume
Overall there was no major statistically significant association between corrected hippocampal volume and any of the factors while considering age as a covariate. Factor 3, however, presented some outliers (values above 3 standard deviations of the Factor) and after excluding these individuals, the kidney function factor showed a negative correlation with corrected hippocampal volume (r= −0.168, p= 0.049, Pearson's correlation, corrected for age), meaning that worse kidney function may be linked to smaller hippocampi.
3.5 Surface-based associations between factors and cortical thickness after excluding individuals with cardiovascular risk factors
Considering that the presence of cardiovascular risk factors among some individuals could influence the relationship between cortical thickness and the factors (even when under specific medication), an additional analysis was performed after excluding those with hypertension, dyslipidemia and type 2 diabetes using the cortical thickness data extracted from the ROIs seen in figure 2 for Factors 3 (kidney function), 4 (cholesterol), and the residuals of the relations between age and thickness for Factor 1, taking age into account as a covariate. The correlation between thickness and all the three factors remained significant in this analysis and are shown in the Supplementary figure 3(Factor 1– R= −0,2687 / p = 0,0379; Factor 3 – R = −0.3665 / 0.004; Factor 4 – R = 0,2592 / p = 0,0455).
3.6 Surface-based associations between factors and cortical thickness considering ApoE e4 status
Additional plots and analyses were generated to investigate the potential influence of APOE genotype on factor by thickness associations using thickness measures from the ROIs shown in figure 2 for factors 1, 3, and 4. For Factor 1 we used the residuals of the regression between cortical thickness and age, since age acted a confounder in this case. In total, 109 individuals had information on ApoE e4 status. Individuals in the two subgroups (ApoE e4 positive, n = 26/ApoE e4 negative, n = 83) did not differ in age and sex.
Analyses demonstrated that ApoE e4 status did not seem to influence the associations between blood factors and thickness (supplementary figure 3). This was confirmed by comparing the correlation coefficient obtained for both subgroups using a Fisher's r-to-z transformation, which showed no differences between the slopes of the subgroups for the three factors. However, we emphasize that the subanalyses performed has reduced statistical power relative to the primary analyses of this work.
4 Discussion
In this cross-sectional study, we assessed the relationship between brain cortical thickness and inter-individual variation across a diverse collection of standard physiological blood markers commonly used to monitor systemic health in clinical medicine. Some of these associations are described here for the first time, especially for participants in the middle-age range. All participants were cognitively healthy and represented a sample of what is expected to be within ‘typically’ or even ‘optimally’ healthy adulthood for the given age. By exploring this, we address an underexplored issue of physiological processes that may contribute to normative cortical variation as well as conditions that may contribute to variation in trajectories of brain aging.
Among our principal findings were the consistent associations of lower cortical thickness with increasing levels of insulin and lipid metabolism markers (factor 1), and also with decreasing kidney function (factor 3). These survived multiple comparisons corrections, controlling for age and other covariates, and were very consistent in all of our sub-analyses, i.e. after excluding individuals with cardiovascular risk factors and when considering ApoE status. Other results such as the increase in cortical thickness with increasing cholesterol levels replicate prior work by Leritz and colleagues in a different set of older adults (Leritz et al., 2011). These findings provide insight into inter-individual cortical variations that may have implications for understanding disease processes.
The most statistically robust effect in this work is the finding of an inverse correlation between kidney function and cortical thickness. Associations between markers of kidney function with cerebral blood flow and markers of white matter integrity in the range of healthy variation or slightly abnormal levels have already been demonstrated (Ryu et al., 2016; Sedaghat et al., 2015). In end-stage renal disease, central nervous system impairment is common and could be due to retention of toxic solutes such as urea, creatinine, parathyroid hormone, and myoinositol (Fraser and Arieff, 1988). Also, due to anatomical and physiological similarities, the kidneys are sensitive to microvascular and ischemic damage in a similar way as brain tissue (Ikram et al., 2007; O'Rourke and Safar, 2005; S Sedaghat et al., 2015). Small vessel disease is also correlated to mediators such as homocystein, endothelin-1 (Love and Miners, 2015), which could have some influence on kidney function. The current findings in a cohort comprising kidney function in the normal range of individual variation suggest that these relations detected in disease states may extend to variation in function of healthier individuals.
The renal system is responsible for several critical functions including blood filtration, ion homeostasis and blood pressure regulation, and hydration levels, all of which may contribute to the results reported here. Indeed, simple hydration status is linked to acute changes in cortical thickness and other structural indices of the brain from MRI (Biller et al., 2015; Streitbürger et al., 2012). The kidney function factor was partly defined by GFR, which is known to decrease with age (Glassock and Winearls, 2009). Furthermore, we showed a strong correlation between this and age, which could mean that the effects seen could be due to age and not the factor itself. However, even though less significant and less widespread, effects of kidney function persisted when splitting participants in two age groups, especially in those younger than 60, where the factor no longer correlates with age, also persisted after excluding outliers, subjects with dyslipidemia, diabetes and hypertension, and while investigating a derivative factor without age-adjusted variables and correcting for age. Therefore, after these analyses we consider that the kidney function factor was still related to inter-individual regional variation in cortical thickness, independently of age.
Increases in measures related to insulin and lipid metabolism (factor 1) were also inversely correlated with cortical thickness in temporal and parietal areas and some areas of the prefrontal cortex, but also in mesial temporal lobes. These effects were independent of age and other covariates. They are consistent with previous studies showing changes in gray and white matter particularly in temporal and parietal areas in individuals with diabetes mellitus, insulin resistance, and central obesity (Akintola et al., 2015; Debette et al., 2010; Ryu et al., 2014; Tan et al., 2011). Incidence of type 2 diabetes mellitus (DM), insulin resistance (IR), CVD and neurodegenerative diseases are frequently concomitant and increase with age (Vagelatos and Eslick, 2013). Interestingly, insulin resistance and diabetes are also a well-known risk factor for AD (Schilling, 2016). None of the previous studies focused on disease-free middle-aged and older adults with ‘normal’ or slightly abnormal systemic markers in their cohort as we did.
The results of Factors 1 and 3 overall suggest that some degree of brain tissue variations may be linked to systemic/organ health even in younger people without established kidney disease and diabetes mellitus. These results add information to the findings already published by our group, in which the same factors had qualitative differential impacts on the integrity of white-matter fibers (Ryu et al., 2016). In that work, the insulin factor was more qualitatively associated with long projection fibers, whereas kidney function had a more widespread association with periventricular white matter classically associated with aging and CVD (Pantoni and Garcia, 1995). Such findings, especially for factor 3, are in agreement with the areas of reduced cortical thickness seen in the present study, which presented an overlap of regions prone to reduce its thickness and have reduced blood flow and metabolism in normal aging but also vulnerable to CVD and AD, such as prefrontal, temporal parietal and medial temporal cortices (Korf et al., 2005; Carmichael et al., 2013; Dickerson et al., 2009; Dukart et al., 2011; Fjell et al., 2014; Love and Miners, 2015; McEvoy et al., 2009; Reed et al., 2004; Salat et al., 2004).
We note that the associations between health factors and morphometric measurements reported here may have technical implications for our prior work examining health factor associations with white matter microstructural properties using diffusion imaging (Ryu et al., 2016). Namely, prior work has demonstrated the potential for partial volume contamination of diffusion measures (Jbabdi et al., 2010; Bach et al., 2014) and it is possible that individuals with reduced brain tissue volumes may be more susceptible to such effects. In light of the information provided in the present work, our previous results should, therefore, be considered with caution. Nevertheless, given the microstructural and anatomical specificity of the health effects reported in that work, we believe that the results reported also occurred due to true associations and not only by technical limitations. It will be important to examine this possibility more closely in future work. Additionally, it is possible that the effects are linked in a biological sense- e.g. the cortical and white matter systems showing associations with a particular health factor are also structurally connected. We hope to examine such questions in the future.
An association of higher values of the cholesterol factor and higher cortical thickness in frontal and parietal areas was noted. Similar results have been previously observed in older adults (Leritz et al., 2011). In Leritz et al. (2011), the authors noted this was an unforeseen finding since high cholesterol is related to atherosclerosis and could be a risk factor for AD and other neurodegenerative diseases (Ricciarelli et al., 2012). However, it is clear that cholesterol ranges considered risk factors for cardiovascular disease are very different than having values in normal range. It was recently reported that higher cholesterol levels are associated with better performance on memory tests in cognitively healthy middle-older adults (Leritz et al., 2016). Such higher values of blood cholesterol could even be related to a release of excessive quantities of these molecules from the central nervous system. Alternatively, the inability of the brain to process cholesterol in excess can be prejudicial and may be related to pathologic states such as AD. Therefore, it appears plausible in light of these results that greater cholesterol may be related to a thicker cortex in the disease-free reference range. At this time, mechanisms of such associations are highly speculative given the distinction between serum and brain cholesterol dynamics (Björkhem and Meaney, 2004).
Finally, it should be highlighted that even though Factor 4 (cholesterol) is related to lipid metabolism, it is fundamentally different from factor 1 (insulin/metabolic). Indeed, Factor 1 included HDL, triglycerides and other markers such as insulin and creatinine clearance, which are related to lipid, glucose and protein metabolism, while Factor 4 was uniquely related to LDL and total cholesterol. Furthermore, while the total cholesterol to HDL ratio was related to both factors, the associations between these factors and cortical thickness were in opposite directions (an increasing in Factor 1 was linked to lower cortical thickness while an increasing in Factor 4 was related to higher cortical thickness) and were associated with different cortical regions. This suggests that this ratio is related to two competing underlying physiological processes affecting different regions of the brain in opposite ways. It is possible that Factor 4 is linked to the positive effects of cholesterol on brain function while Factor 1 may be more strongly related to the potentially early negative effects of excess insulin resistance and dyslipidemia, even though our sample was generally in the normal reference range.
We shall address several limitations to the present work. First, the results presented here are cross-sectional, and therefore, no direct causal inferences can be drawn. The findings provide suggestive links and still need follow-up work to clarify the role of each of the presented health factors on gray matter structure over time. Another limitation is that the sample used in this study consists predominantly of white Caucasian middle-aged and older adults from the Boston metropolitan area, which may not be generalized to other populations. The blood markers available for this work may also not reflect the ideal set, and future work will require larger marker sets. Finally, given the complexity of the current project, we did not examine associations between cortical thickness and cognitive function, which would require careful consideration, yet limits the functional interpretation of the current findings. The authors plan follow-up work and further investigations to clarify the relationship between the present findings and cognition.
Lastly, although we consider the control of subject motion in the MRI analysis one strength of our analysis, it can only correct for patient specific motion during the entire imaging acquisition. This correction does not take into account correction for motion during specific sequences, i.e. the T1 sequence used for cortical thickness measurements in the present work.
Despite these limitations, our results present novel insights and suggest that gray matter structure may be influenced by systemic health even in the absence of disease, and as early as middle age. From a theoretical perspective, these findings could give some hints on how and why people age differently and why different patterns of degeneration are seen in the spectrum of a particular disease. More practically, since there is evidence that the incidence of dementia is lowering in some high-income countries that intensified the modification of specific CVD risk factors (Langa, 2015), it is possible that the relations found in the present work could have a public-health impact and may help encourage proper prevention and care for all organ systems. Future interventional and longitudinal studies should focus on whether optimizing these systemic markers can delay or prevent cognitive decline.
Supplementary Material
Highlights.
Cortical thickness was associated with blood markers in younger and older adults.
Associations were present even within the “normal range” of blood markers.
Lower cortical thickness was associated with higher levels of insulin and lipids.
Lower regional cortical thickness was associated with decreasing kidney function.
Higher levels of cholesterol were associated with higher cortical thickness.
Acknowledgments
The authors would like to thank Chang-woo Ryu and Seon-Young Ryu for their previous work on the factorial analysis of the blood markers, and also Paul Wilkens and Kimberly Stephens for their substantial help with Unix and FreeSurfer scripting.
Funding:
This work was supported by the National Institutes of Health – NIH (grant number R01NR010827, using resources provided by NIH grants NS042861, NS058793); by the Center for Functional Neuroimaging Technologies (P41RR14075), a P41 Regional Resource supported by the Biomedical Technology Program of the National Center for Research Resources (NCRR), NIH; and by the NCRR Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program (grant numbers S10RR021110, S10RR023401, S10RR019307, S10RR019254 and S10RR023043).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akintola A, Berg A. van den, Altmann-Schneider I, Jansen S, Buchem M. van, Slagboom E, Westendorp R, Heemst D. van, Grond J. van der. Parameters of glucose metabolism and the aging brain: a magnetization transfer imaging study of brain macro- and micro-structure in older adults without diabetes. Age. 2015;37:9802. doi: 10.1007/s11357-015-9802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller A, Reuter M, Patenaude B, Homola G, Breuer F, Bendszus M, Bartsch A. Responses of the Human Brain to Mild Dehydration and Rehydration Explored In Vivo by 1H-MR Imaging and Spectroscopy. Am J Neuroradiol. 2015;36:2277–2284. doi: 10.3174/ajnr.A4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M, Laun FB, Leemans A, Tax CM, Biessels GJ, Stieltjes B, Maier-Hein KH. Methodological considerations on tract-based spatial statistics (TBSS). Neuroimage. 2014;15:358–369. doi: 10.1016/j.neuroimage.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Björkhem I, Meaney S. Brain Cholesterol: Long Secret Life Behind a Barrier. Arteriosclerosis Thrombosis Vasc Biology. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- Carmichael O, McLaren D, Tommet D, Mungas D, Jones R, Initiative A. Coevolution of brain structures in amnestic mild cognitive impairment. NeuroImage. 2013;66:449456. doi: 10.1016/j.neuroimage.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wen W, Anstey K, Sachdev P. Effects of cerebrovascular risk factors on gray matter volume in adults aged 60–64 years: A voxel-based morphometric study. Psychiatry Res Neuroimaging. 2006;147:105–114. doi: 10.1016/j.pscychresns.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Dale A, Fischl B, Sereno M. Cortical Surface-Based Analysis. I Segmentation and Surface Reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Debette S, Beiser A, Hoffmann U, DeCarli C, O'Donnell C, Massaro JM, Au R, Himali JJ, Wolf PA, Fox CS, Seshadri S. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Annals of Neurology. 2010;68:136–144. doi: 10.1002/ana.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B, Bakkour A, Salat D, Feczko E, Pacheco J, Greve D, Grodstein F, Wright C, Blacker D, Rosas H, Sperling R, Atri A, Growdon J, Hyman B, Morris J, Fischl B, Buckner R. The Cortical Signature of Alzheimer's Disease: Regionally Specific Cortical Thinning Relates to Symptom Severity in Very Mild to Mild AD Dementia and is Detectable in Asymptomatic Amyloid-Positive Individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart J, Schroeter ML, Mueller K. Age correction in dementia--matching to a healthy brain. PLoS ONE. 2011;6:e22193. doi: 10.1371/journal.pone.0022193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzinger Fazekas, Matthews Ropele, Schmidt Smith, Schmidt Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64:1704–11. doi: 10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale A. Measuring the thickness of the human cerebral cortex from magnetic resonance images. P Natl Acad Sci Usa. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat D, Busa E, Seidman L, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale A. Automatically Parcellating the Human Cerebral Cortex. Cereb Cortex. 2004a;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale A. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. Ieee T Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat D, Busa E, Albert M, Dieterich M, Haselgrove C, Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale A. Whole Brain Segmentation Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat D, Kouwe A, Makris N, Ségonne F, Quinn B, Dale A. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004b;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno M, Dale A. Cortical Surface-Based Analysis. II Infation, Flattening, and a Surface-Based Coordinate System. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fjell A, McEvoy L, Holland D, Dale A, Walhovd K, Initiative A. What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Progress in neurobiology. 2014;117:20–40. doi: 10.1016/j.pneurobio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C, Arieff A. Nervous system complications in uremia. Annals of internal medicine. 1988:143–153. doi: 10.7326/0003-4819-109-2-143. [DOI] [PubMed] [Google Scholar]
- Gearing M, Mirra S, Hedreen J, Sumi S, Hansen L, Heyman A. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part X. Neuropathology Confirmation of the Clinical Diagnosis of Alzheimer's Disease. Neurology. 1995;45:461–466. doi: 10.1212/wnl.45.3.461. [DOI] [PubMed] [Google Scholar]
- Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truthsand consequences. TRANSACTIONS OF THE AMERICAN CLINICAL AND CLIMATOLOGICAL ASSOCIATION. 2009;120:419–428. [PMC free article] [PubMed] [Google Scholar]
- Hagler D, Saygin A, Sereno M. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijer T, Skoog I, Oudkerk M, Leeuw F-E, Groot J, Hofman A, Breteler M. Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiol Aging. 2003;24:307–313. doi: 10.1016/s0197-4580(02)00088-x. [DOI] [PubMed] [Google Scholar]
- Ikram Vernooij, Hofman Niessen, van der Lugt Breteler, M.B. Kidney Function Is Related to Cerebral Small Vessel Disease. Stroke. 2007;39:55–61. doi: 10.1161/STROKEAHA.107.493494. [DOI] [PubMed] [Google Scholar]
- Jbabdi S, Behrens TE, Smith SM. Crossing fibres in tract-based spatial statistics. Neuroimage. 2010;49:249–256. doi: 10.1016/j.neuroimage.2009.08.039. [DOI] [PubMed] [Google Scholar]
- Jongen C, Grond J, Kappelle L, Biessels G, Viergever M, Pluim J, Group U. Automated measurement of brain and white matter lesion volume in type 2 diabetes mellitus. Diabetologia. 2007;50:1509–16. doi: 10.1007/s00125-007-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf ES, Straaten E.C. van, Leeuw F.-E.E. de, Flier W.M. van der, Barkhof F, Pantoni L, Basile AM, Inzitari D, Erkinjuntti T, Wahlund L-OO, Rostrup E, Schmidt R, Fazekas F, Scheltens P. Diabetes mellitus, hypertension and medial temporal lobe atrophy: the LADIS study. Diabet. Med. 2007;24:166–71. doi: 10.1111/j.1464-5491.2007.02049.x. [DOI] [PubMed] [Google Scholar]
- Korf ESC, Scheltens P, Barkhof F, Leeuw F-E. Blood Pressure, White Matter Lesions and Medial Temporal Lobe Atrophy: Closing the Gap between Vascular Pathology and Alzheimer's Disease? Dement Geriatr Cogn. 2005;20:331–337. doi: 10.1159/000088464. [DOI] [PubMed] [Google Scholar]
- Langa K. Is the risk of Alzheimer's disease and dementia declining? Alzheimer's Research & Therapy. 2015;7:34. doi: 10.1186/s13195-015-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz E, McGlinchey R, Salat D, Milberg W. Elevated levels of serum cholesterol are associated with better performance on tasks of episodic memory. Metab Brain Dis. 2016;31:465–73. doi: 10.1007/s11011-016-9797-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, Fischl B, McGlinchey RE, Milberg WP. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. NeuroImage. 2011;54:2659–2671. doi: 10.1016/j.neuroimage.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S, Miners J. Cerebrovascular disease in ageing and Alzheimer's disease. Acta Neuropathol. 2015;131:645–658. doi: 10.1007/s00401-015-1522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Roddey JC. Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment 1. Radiology. 2009 doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Rauch G, Rauch R, Haque A. Risk factors for cerebral hypoperfusion, mild cognitive impairment, and dementia. Neurobiol Aging. 2000;21:161–9. doi: 10.1016/s0197-4580(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Musen G, Lyoo I, Sparks C, Weinger K, Hwang J, Ryan C, Jimerson D, Hennen J, Renshaw P, Jacobson A. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Nestle Nutr Works Se. 2006;55:326–33. doi: 10.2337/diabetes.55.02.06.db05-0520. [DOI] [PubMed] [Google Scholar]
- O'Rourke M, Safar M. Relationship Between Aortic Stiffening and Microvascular Disease in Brain and Kidney Cause and Logic of Therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia J. The Significance of Cerebral White Matter Abnormalities 100 Years After Binswanger's Report: A Review. Upd Int Car. 1995;26:1293–1301. doi: 10.1161/01.str.26.7.1293. [DOI] [PubMed] [Google Scholar]
- Petrovitch H, Ross W, Steinhorn S, Abbott R, Markesbery W, Davis D, Nelson J, Hardman J, Masaki K, Vogt M, Launer L, White L. AD lesions and infarcts in demented and non- demented Japanese- American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue K. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue K, Acker J. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117:1169–80. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue K, Kennedy K, Acker J. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–57. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Reed B, Eberling J, Mungas D, Weiner M, Kramer J, Jagust W. Effects of White Matter Lesions and Lacunes on Cortical Function. Arch Neurol-chicago. 2004;61:1545–1550. doi: 10.1001/archneur.61.10.1545. [DOI] [PubMed] [Google Scholar]
- Ricciarelli R, Canepa E, Marengo B, Marinari U, Poli G, Pronzato M, Domenicotti C. Cholesterol and Alzheimer's disease: A still poorly understood correlation. IUBMB Life. 2012;64:931–935. doi: 10.1002/iub.1091. [DOI] [PubMed] [Google Scholar]
- Ryu C, Coutu J, Greka A, Rosas H, Jahng G, Rosen B, Salat D. Differential associations between systemic markers of disease and white matter tissue health in middle-aged and older adults. J Cereb Blood Flow Metabolism. 2016 doi: 10.1177/0271678X16653613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SY, Coutu J-P, Rosas HD, Salat DH. Effects of insulin resistance on white matter microstructure in middle-aged and older adults. Neurology. 2014;82:1862–70. doi: 10.1212/WNL.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P, Kalaria R, O'Brien J, Skoog I, Alladi S, Black S, Blacker D, Blazer D, Chen C, Chui H, Ganguli M, Jellinger K, Jeste D, Pasquier F, Paulsen J, Prins N, Rockwood K, Roman G, Scheltens P, Disorders I. Diagnostic Criteria for Vascular Cognitive Disorders: A VASCOG Statement. Alz Dis Assoc Dis. 2014;28:206. doi: 10.1097/WAD.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the Cerebral Cortex in Aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Schilling M. Unraveling Alzheimer's: Making Sense of the Relationship between Diabetes and Alzheimer's Disease1. J Alzheimer's Dis. 2016;51:961–977. doi: 10.3233/JAD-150980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Launer L, Nilsson L-G, Pajak A, Sans S, Berger K, Breteler M, Ridder M, Dufouil C, Fuhrer R, Giampaoli S, Hofman A, Consortium C. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Nestle Nutr Works Se. 2004;53:687–92. doi: 10.2337/diabetes.53.3.687. [DOI] [PubMed] [Google Scholar]
- Sedaghat S, Cremers L, Groot M, Hoorn E, Hofman A, Lugt A, Franco O, Vernooij M, Dehghan A, Ikram M. Kidney function and microstructural integrity of brain white matter. Neurology. 2015;85:154–61. doi: 10.1212/WNL.0000000000001741. [DOI] [PubMed] [Google Scholar]
- Sedaghat S, Vernooij M, Loehrer E, Mattace-Raso F, Hofman A, Lugt A, Franco O, Dehghan A, Ikram M. Kidney Function and Cerebral Blood Flow: The Rotterdam Study. J Am Soc Nephrol. 2015;27:715–721. doi: 10.1681/ASN.2014111118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Wolf P, Beiser A, Elias M, Au R, Kase C, D'Agostino R, DeCarli C. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63:1591–9. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- Skoog I, L, B., G, S., B, B., S, L.-A., B, L., Göran Anders, A, A. 15-year longitudinal study of blood pressure and dementia. The Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- Streitbürger D-P, Möller H, Tittgemeyer M, Hund-Georgiadis M, Schroeter M, Mueller K. Investigating structural brain changes of dehydration using voxel-based morphometry. Plos One. 2012;7:e44195. doi: 10.1371/journal.pone.0044195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZS, Beiser AS, Fox CS, Au R, Himali JJ, Debette S, DeCarli C, Vasan RS, Wolf PA, Seshadri S. Association of Metabolic Dysregulation With Volumetric Brain Magnetic Resonance Imaging and Cognitive Markers of Subclinical Brain Aging in Middle-Aged Adults. Diabetes Care. 2011;34:1766–1770. doi: 10.2337/dc11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiehuis A, Graaf Y, Visseren F, Vincken K, Biessels G, Appelman A, Kappelle L, Mali W, Group S. Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke J Cereb Circulation. 2008;39:1600–3. doi: 10.1161/STROKEAHA.107.506089. [DOI] [PubMed] [Google Scholar]
- Vagelatos N, Eslick G. Type 2 Diabetes as a Risk Factor for Alzheimer's Disease: The Confounders, Interactions, and Neuropathology Associated With This Relationship. Epidemiol Rev. 2013;35:152–160. doi: 10.1093/epirev/mxs012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.