Abstract
Objectives
Spectral resolution is a correlate of open-set speech understanding in post-lingually deaf adults as well as pre-lingually deaf children who use cochlear implants (CIs). In order to apply measures of spectral resolution to assess device efficacy in younger CI users, it is necessary to understand how spectral resolution develops in NH children. In this study, spectral ripple discrimination (SRD) was used to measure listeners’ sensitivity to a shift in phase of the spectral envelope of a broadband noise. Both resolution of peak to peak location (frequency resolution) and peak to trough intensity (across-channel intensity resolution) are required for SRD.
Design
SRD was measured as the highest ripple density (in ripples per octave) for which a listener could discriminate a 90 degree shift in phase of the sinusoidally-modulated amplitude spectrum. A 2X3 between subjects design was used to assess the effects of age (7-month-old infants versus adults) and ripple peak/trough “depth” (10, 13, and 20 dB) on SRD in normal hearing listeners (Experiment 1). In Experiment 2, SRD thresholds in the same age groups were compared using a task in which ripple starting phases were randomized across trials to obscure within-channel intensity cues. In Experiment 3, the randomized starting phase method was used to measure SRD as a function of age (3-month-old infants, 7-month-old infants, and young adults) and ripple depth (10 and 20 dB in repeated measures design).
Results
In Experiment 1, there was a significant interaction between age and ripple depth. The Infant SRDs were significantly poorer than the adult SRDs at 10 and 13 dB ripple depths but adult-like at 20 dB depth. This result is consistent with immature across-channel intensity resolution. In contrast, the trajectory of SRD as a function of depth was steeper for infants than adults suggesting that frequency resolution was better in infants than adults. However, in Experiment 2 infant performance was significantly poorer than adults at 20 dB depth suggesting that variability of infants’ use of within-channel intensity cues, rather than better frequency resolution, explained the results of Experiment 1. In Experiment 3, age effects were seen with both groups of infants showing poorer SRD than adults but, unlike Experiment 1, no significant interaction between age and depth was seen.
Conclusions
Measurement of SRD thresholds in individual 3 to 7-month-old infants is feasible. Performance of NH infants on SRD may be limited by across-channel intensity resolution despite mature frequency resolution. These findings have significant implications for design and stimulus choice for applying SRD for testing infants with CIs. The high degree of variability in infant SRD can be somewhat reduced by obscuring within-channel cues.
Introduction
Spectral resolution can be defined as the sensitivity of a listener to a change in the number, location, or height of peaks in the amplitude spectrum of a complex acoustic sound. Spectral resolution is important for discrimination of speech sounds, including vowels as well as consonants differing in place-of-articulation. It is also important for understanding speech in noisy listening environments. Various psychophysical measures of spectral resolution have been shown to be correlated with speech perception performance in adults who use cochlear implants (CIs) or hearing aids (Henry and Turner 2003, Henry et al. 2005, Litvak et al. 2007, Won et al. 2007, Saoji et al. 2009, Anderson et al. 2011, Won et al. 2011, Won et al. 2011). Moreover, spectral resolution is related to non-linguistic abilities such as pitch discrimination, melody, and timbre identification in CI users (Jung et al. 2012, Drennan et al. 2015), and the degree of spread of excitation due to channel interactions (Won et al. 2014).
Spectral resolution has shown promise as a proxy measure of speech perception to assess device candidacy and measure effectiveness of signal processing strategies (Drennan et al. 2014, Shim et al. 2014, Drennan et al. 2015). Given that spectral resolution is robustly related to speech perception for adult CI listeners, some investigators have suggested that it could be useful for testing device efficacy in infants with CIs or HAs who are too young for clinical measures of speech perception (Won et al. 2011, Drennan et al. 2014). One implicit assumption is that non-linguistic measures of spectral resolution are minimally affected by experience and learning (Drennan et al. 2015).
Spectral resolution depends on at least two sensory factors. First, spectral resolution requires resolution of location of peaks in the amplitude spectrum (which we will refer to as frequency resolution). Second, spectral resolution requires resolution of the relative intensity of peaks and troughs in the amplitude spectrum (which we will refer to as across-channel intensity resolution). The central aim of the present study is to test whether these two factors have different developmental trajectories. Whether or not such differences exist would have potential implications for the use of spectral resolution as a measure of auditory acuity in very young listeners. Although normal hearing (NH) human infants are born with immature sensitivity to acoustic variation (Weir 1979, Bull et al. 1984), they are able to perceive changes in spectral patterns and shape (Trehub 1973, Tsang and Trainor 2002). Measures of frequency resolution, including critical bandwidth and psychophysical tuning curve width, mature by 6 months of age (Olsho et al. 1987, Schneider et al. 1990, Spetner and Olsho 1990). Despite the fact that infants and young children are particularly reliant on spectral detail for speech discrimination in studies using CI simulations (Warner-Czyz et al. 2014, Nittrouer et al. 2015), spectral resolution remains immature in NH listeners through 7–10 years of age (Blagosklonova et al. 1989, Allen and Wightman 1994, Peter et al. 2014). One possible explanation for this is that development of spectral resolution in NH listeners is mainly due to maturation of across-channel intensity resolution.
Previous research of maturation of intensity resolution in NH children has mainly focused on listeners’ sensitivity to intensity changes across time and have employed stimuli in which changes are constant across the spectrum or are within a frequency channel (Olsho et al. 1987, Trehub 1988, Werner and Gillenwater 1990, Schneider et al. 1991, Tharpe and Ashmead 2001). In these studies, adult-like resolution has generally been shown to occur as late as school-age. To contrast with the relative intensity resolution across the spectrum, we will refer to this type of intensity resolution as “within-channel intensity resolution.” Development of across-channel intensity resolution, such as in spectral profile analysis (Green and Mason 1985) has not been well characterized. If across-channel and within-channel intensity resolution develop over similar timeframes, spectral resolution might develop much more slowly in infants than maturation of frequency resolution would suggest. For instance, unlike frequency resolution, within-channel intensity resolution matures in NH children through 5–8 years of age (Maxon and Hochberg 1982). This may limit the utility of spectral resolution as a meaningful measure of CI efficacy in infants with CIs or HAs.
One measure of spectral resolution that has received considerable attention in the adult CI literature is spectral ripple discrimination (SRD). In SRD, noises with modulated, or “rippled,” power spectra are presented and listeners are asked to respond when the ripple is shifted in phase so that the peaks and troughs in the power spectrum of a target stimulus are inverted relative to a reference stimulus (Lopez Valdes et al. 2014, Jeon et al. 2015, Won et al. 2015). The task can be made progressively more difficult either by increasing the ripple density (density approach) or by reducing the peak to trough depth intensity (depth approach) until the listener is unable to discriminate the target and reference stimuli. For the density approach, listener performance is typically reported as the highest ripple density they could discriminate. In contrast, the depth approach measures the smallest peak to trough depth they could discriminate. While the depth approach has been used in studies of NH listeners (Supin et al. 1994, Supin et al. 1994, Supin et al. 1999), the density approach has primarily been used in studies of CI listeners (Henry and Turner 2003, Henry et al. 2005, Won et al. 2007).
Studies of NH children suggest that SRD develops gradually through 7–10 years of age (Blagosklonova et al. 1989, Allen and Wightman 1994, Peter et al. 2014). In an early brief report, Blagosklonova et al. (1989) found adult-like SRD thresholds in children aged 7–15 years. Allen and Wightman (1994) tested school-age children and adults on several measures of spectral resolution including SRD. Children demonstrated poorer, and more variable, performance than adults at 4–5 years, but adult-like performance was seen at by 9 years old. More recently, Peter et al. (2014) found that SRD was adult-like in 12–18 year old NH children, but not in 8–11 year old children. Given early maturation of frequency resolution in NH listeners (Olsho et al. 1987, Schneider et al. 1990, Spetner and Olsho 1990), it is possible that maturation of across channel intensity resolution is the source of prolonged development of SRD in NH children.
One study of pre-lingually deaf children with CIs suggests that SRD is developed at least by 8–16 years old. Jung et al. (2012) measured SRD using the density approach in 11 pre-lingually deaf children, aged 8–16 years old, who were implanted prior to 5 years of age. Mean SRD of the children was not significantly different from post-lingually deaf adults with similar device and signal processing strategy constraints. Thus, by 8–16 years old, pre-lingually deaf children showed mature spectral resolution relative to adults with the same peripheral limitations of the CI. As in adult CI users, children’s SRD was significantly correlated with spondee identification in steady state noise.
In addition to frequency resolution and across-channel intensity resolution, maturation of listening strategy or perceptual weighting could affect SRD performance in young children. For instance, young listeners have been shown to rely more than adults on spectral speech discrimination cues (Nittrouer et al. 2015). Furthermore, based on their susceptibility to informational masking, (Allen and Wightman 1995, Oh et al. 2001, Leibold and Neff 2011), young listeners might be less likely to focus on available within-channel temporal or intensity cues than adults. Listeners with impoverished spectral resolution, such as CI users, may learn to focus on non-spectral cues (Moberly et al. 2014, Moberly et al. 2016). Thus, age effects could arise from young listeners adopting different strategies, or being more variable in the strategies they adapt, than adults. In other words, differences in SRD between young listeners and adults might not have anything to do with differences in spectral resolution per se.
The spectral modulation transfer function (SMTF) defines the relationship between spectral ripple density and depth (Supin et al. 1999, Saoji et al. 2009, Anderson et al. 2012). When the dependent variable is spectral ripple depth, the SMTF is exponential (Supin et al. 1999). At low modulation densities, listeners are able to discriminate SRD stimuli at very small modulation depths. Modulation depth thresholds begin to increase at spectral ripple densities above 6 ripples per octave in NH listeners (Supin et al. 1999) as opposed to 1 ripple per octave in post-lingually deaf adult CI listeners (Saoji et al. 2009, Anderson et al. 2012). The spread of the function across the frequency axis reflects frequency resolution whereas the position along the depth axis reflects across-channel peak/trough intensity resolution (Saoji et al. 2009). Both Saoji et al. (2009) and Anderson et al. (2012) found significant individual variability across adult CI listeners in both components of the SMTF and concluded that frequency resolution and across-channel intensity resolution were unique factors underlying spectral ripple perception.
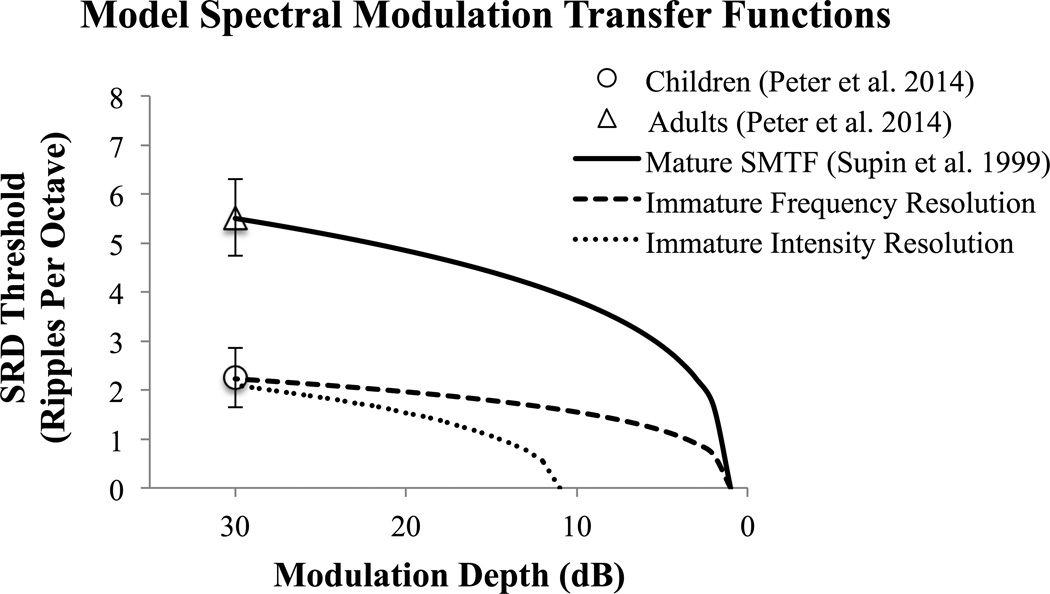
Whether development of SRD is due to maturation of frequency resolution, intensity resolution or both could be determined by comparing SMTF shapes across development. In Figure 1, three hypothetical SMTFs are shown based on the adult and 8–11 year old SRD thresholds from Peter et al. (2014) and the previously described SMTF function (Supin et al. 1999). Compared to the “mature” SMTF, the SMTF for listeners with immature frequency resolution should be flatter along the frequency axis. In contrast, the SMTF for listeners with immature across-channel intensity resolution should be parallel, but with a shifted x-intercept, compared to the mature SMTF.
Figure 1.
Hypothetical spectral modulation transfer functions (SMTFs) for spectral ripple discrimination density threshold as a function of modulation depth. Symbols represent mean and +/− 1 standard error SRD thresholds for 8–11 year old normal hearing children and adults (from (Peter et al. 2014). SMTFs were derived using the function: f(x) = B*√((√(X/A))-1), where f(x) = the SRD ripple density threshold at ripple depth X, B is related to the inverse of the spectral filter width and A represents the modulation depth at f(x) = 0 (inverse of Eq 9 from Supin et al. 1999). The “mature” SMTF was fit using A = 1dB (from Supin et al. 1999) and the adult mean SRD threshold at 30dB depth (Peter et al. 2014). The “immature frequency resolution” SMTF was then fit using A = 1dB and the 8–11 year old mean SRD threshold at 30dB depth. The “immature intensity resolution” SMTF was fit by using the derived value of B for the “mature” SMTF and mean SRD threshold at 30dB. Relative to mature listeners, listeners with immature across-channel intensity resolution should have parallel SMTFs with a greater x-intercept whereas listeners with immature frequency resolution would have a flatter SMTF along the frequency axis.
The main goal of the present study was to compare the SMTF from NH infants to young adults. In Experiment 1, we tested 7-month-old infants who would be expected to have mature frequency resolution. It was hypothesized that differences in performance between infants and adults would be attributable mainly to infants’ poor sensitivity to across-channel peak/trough intensity differences. Hence, we expected the infant SMTF to resemble the “immature intensity resolution” SMTF from Figure 1. To test this hypothesis, we examined SRD thresholds as a function of age group and modulation depth. A main effect of age but no significant interaction would be consistent with an infant SMTF that was parallel to the adult SMTF but x-axis shifted (resembling the “immature intensity resolution” SMTF from Figure 1). In contrast, a significant interaction between age and depth would suggest differences in frequency resolution between infants and adults.
In Experiments 2 and 3, we investigated whether infants or adults were relying on within-channel intensity cues to discriminate spectral ripple stimuli. In contrast to Experiment 1, in which the ripple phase (i.e. spectral modulation starting phase) was constant, listeners in the latter experiments were tested with randomized ripple phase. This condition was meant to eliminate within-channel intensity cues for SRD (Won et al. 2011). It was hypothesized that both infant and adult SRD threshold would be poorer when within-channel cues were removed. In Experiment 2, mean SRD was examined as a function of age and starting phase condition (constant versus random) at a single ripple depth (20 dB) using a between subjects design. In Experiment 3, a repeated measures design was used to measure SRD at 10 dB and 20 dB in individual listeners from one of 3 age groups: 3-month-olds, 7-month-olds, and young adults.
EXPERIMENT 1 – Effect of Ripple Depth (Between-Subjects) and Age on SRD
The study was conducted in the Department of Speech and Hearing Sciences at the University of Washington. All experimental procedures followed National Institutes of Health regulations and were approved by the Human Subject Institutional Review Board of the University of Washington.
Materials and Methods
Participants
Fifty-eight 7-month-old infants and 36 young adults were initially recruited. For infants, inclusion criteria were full term birth (38 weeks gestation), no medical or developmental concerns per parental report, no history of otitis media within 3 weeks of the test date, no more than 2 previous episodes of acute otitis media, no risk factors for hearing loss (Joint Commission on Infant Hearing, 2007), and newborn hearing screening passed bilaterally. Infants were screened on each test day using tympanometry with a 226Hz probe and had peak admittance of ≥ 0.2 mmhos and peak pressure between −200 and +50 daPa. Infants were tested in 3 visits over 7–14 days. Adults ranged from 18–30 years old, reported no hearing loss, had no history of noise exposure, and had no prior psychoacoustic experience. Adults passed the tympanometric screen with a peak admittance of ≥ 0.9 mmhos. Data from 23 infants were excluded due to failed tympanometry (n=9), failure to complete scheduled visits (n=10), or experimenter error (n=4) leaving 35 infants who completed testing. Data from 3 adults were excluded due to experimenter error (n=2) and failure to complete scheduled visit (n=1) leaving 33 adults.
Stimuli
Stimuli were constructed from 1s noise carriers consisting of 2555 random phase pure tone frequency components between 100 and 5000 Hz. Spectral envelope was determined by a full-wave rectified sinusoid with peaks and troughs uniformly spaced logarithmically in the frequency domain. Both “standard” and “inverted” ripples were created using spectral envelope phases of zero and π/2 radians respectively. Two types of experimental stimuli were created, standard-standard “no-change” stimuli and standard-inverted “change” stimuli. Thus, each stimulus was 2-seconds in duration, consisting of two concatenated 1-s noises.
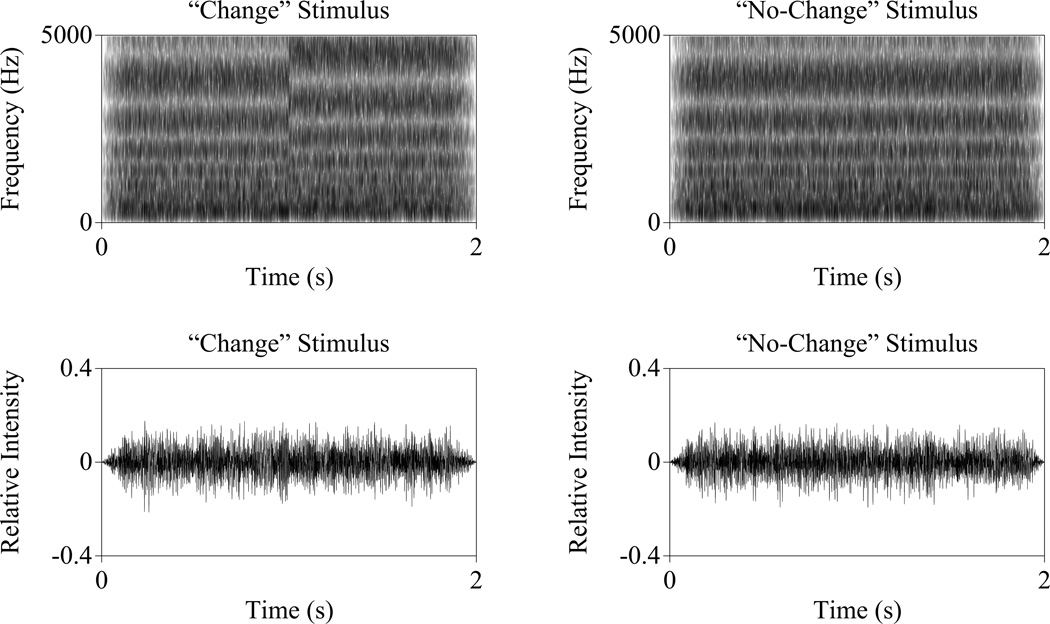
For change stimuli, the root-mean-square amplitude of the first and last noise segments were matched, 150ms rise/fall ramps were added, and a long-term speech-spectrum-shaped filter applied (Byrne et al. 1994). To eliminate high frequency artifact at the point of concatenation, a 5kHz low-pass filter was applied to each stimulus. Sets of 10 change and 10 no-change stimuli, each with randomly generated noise carriers, were created with ripple densities ranging from 0.5 ripples per octave to 45 ripples per octave in steps of 1.414 ripples per octave. Each ripple density stimulus was created with peak-trough depths of 10, 13, and 20 dB. Figure 2 shows the time-waveforms (1a, b) and the spectrograms (1c, d) for the 20 dB depth, 1-ripple-per-octave change and no-change stimuli. Stimuli were similar to those used to elicit cortical evoked acoustic change responses (Won et al. 2011, Lopez Valdes et al. 2014) except they were shortened from 4 to 2 seconds to increase the efficiency of infant testing. Pilot testing of NH adults indicated that shorter trials did not affect thresholds and that these stimuli were sufficient to elicit electrophysiological responses in a NH adult.
Figure 2.
Example acoustic spectrograms (top) and time waveforms (bottom) for “change” (left) and “no change” (right) stimuli. Both stimuli have identical ripple densities (2 ripples per octave) and depths (20 dB peak/trough depth). The spectral envelope change occurred at 1s in the change stimulus.
Procedure
A single-interval forced choice paradigm for SRD was adopted based on the behavioral task employed by Won et al. (2011). This task is easier and less memory intensive than the multiple-alternative forced-choice procedure typically used with adults and is therefore ideal for comparing auditory acuity of infants and adults. Moreover, SRD thresholds with the two procedures are strongly correlated in adults with CIs (Won et al. 2011).
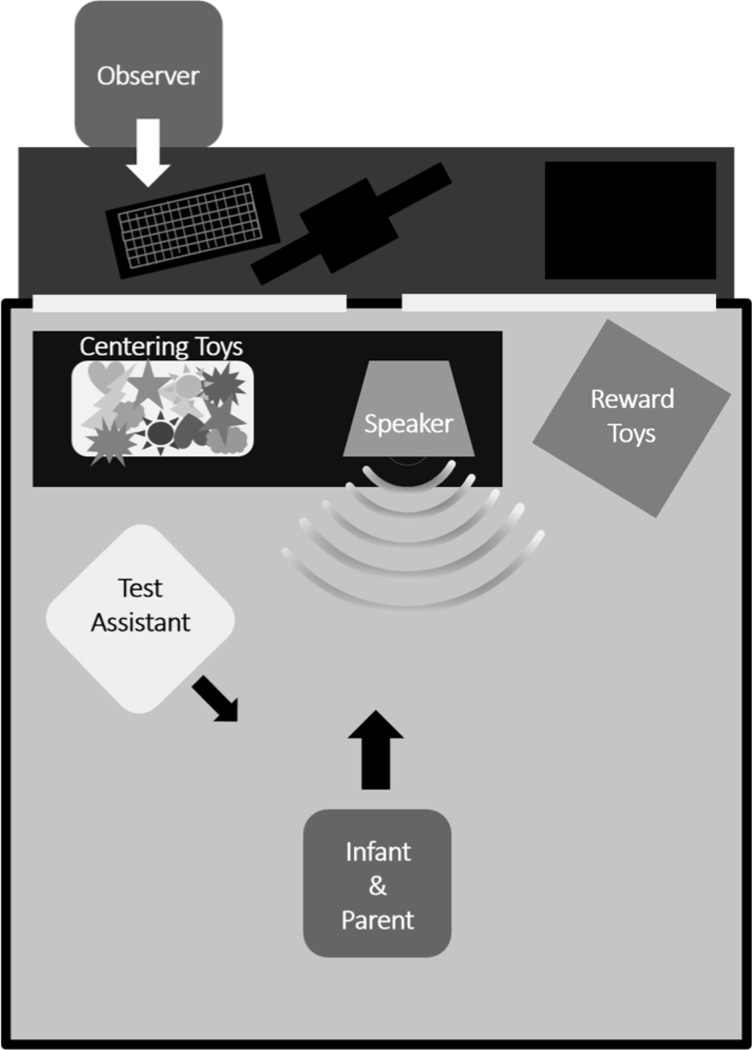
Subjects were tested using an observer based psychoacoustic procedure (OPP) as described by Werner (Olsho et al. 1987, Werner 1995). The booth setup is schematized in Figure 3. Testing was carried out in a single-walled sound booth while sitting on a caregiver’s lap facing a loudspeaker 1.6m away at zero azimuth. An assistant sat toward the infant’s left side manipulating toys to direct the infant’s attention forward midline. Two mechanical animal toys with lights sat in a dark plexiglass box to the participant’s right. On top of the plexiglass box was a 15in monitor connected to a DVD player. Stimuli were presented via the loudspeaker at 65dBA at an ISI of 1s. The observer sat outside the test booth and observed the participant’s behavior through a glass window. The caregiver, assistant, and observer all wore circumaural headphones that played masking stimuli, repeating trains of no-change stimuli. Maskers and stimuli were calibrated prior to each test visit. Participants were randomized within blocks to ripple depths of 10, 13 or 20 dB to obtain an equal number of participants of each age in each ripple depth group.
Figure 3.
Test-booth setup for observer-based psychoacoustic procedure.
Participants were presented with a repeating stream of background no-change stimuli and the observer initiated a trial whenever the participant was quiet and facing midline. To the listener, no-change trials were indistinguishable from the background stimuli. The observer initiated a trial by pressing a button on a computer keyboard when the infant was in a calm state. The type of trial, “change” or “no-change”, was determined randomly. The trial began 1s after the most recent background stimulus with a 4s response window. During this window, the observer decided whether a change trial had occurred and indicated this decision by pressing a button on a computer keyboard. Behavioral cues for individual infants varied between participants and across test sessions. While most infants made a head turn toward the reinforcer for part of the procedure, interest in the reinforcer often waned over time. The observer was also able to use a number of other cues including eye movements, facial expressions, head turns or sudden changes in overall motor activity. Often, the response behavior noted by the observer changed over time for a given infant. Regardless of what the observer used, the observer was blind to trial type and, therefore, the decision was based only on the listener’s behavior. Feedback was provided to the observer at the end of each trial. Participants’ responses were reinforced for each correct identification of a change trial by a 4s activation of the mechanical toy or DVD clip.
The study began with an initial training phase followed by a variable number of test phases. During training, the ripple density was fixed at 1 RPO and 75% of trials were change trials. Trial type varied pseudorandomly with 6 change and 2 no-change trials in each block of 8 trials. The initial training phase was meant to teach an association between the change stimulus and the reinforcer. Thus, listeners were reinforced after each change trial regardless of the observer’s response. Training ended when the observer reached a criterion of 80% hit rate and 20% correct rejection rate over 5 consecutive change and no-change trials, respectively. If criterion was not reached after 20 trials, the session was ended.
Testing differed from training in several respects. First, ripple density increased successively in phases. Second, change and no-change trials were presented pseudorandomly with equal frequency in blocks of 20 trials. Third, listeners were reinforced only on change trials on which the observer scored a hit. Each phase ended after the observer reached a criterion of 80% hit rate and 80% correct rejection rate over 5 consecutive change and 5 consecutive no-change trials or after 40 trials at a fixed ripple density. If the criterion was not reached after 40 trials the session was ended. In each subsequent session, testing began at the highest ripple density at which the infant had previously been tested. The SRD threshold was defined as the highest ripple density at which the 80%/80% criterion was reached.
To ensure that non-sensory factors related to attention and arousal were not responsible for a failure to reach criterion, a “reminder” procedure was used (Clarkson and Clifton 1995, Lau and Werner 2012). If the observer was incorrect on 3 consecutive trials, the infant was tested with up to 20 reminder trials at a previously tested ripple density until they achieved a criterion of 5 correct responses out of 6 consecutive trials. If this criterion was met, the test resumed at the current ripple density. If not, the session was discontinued. Up to 2 reminder procedures were allowed at each ripple density
On each visit, infants were tested on 1–3 sessions depending on time and infant disposition. Between sessions, infants were given a break to feed, be changed, or interact with the caregiver. Subsequent sessions began with several trials at the lowest ripple density to ensure that infants were able to reach the 5/6 criteria. If this criterion was not met, the session was ended. If this criterion was met, testing resumed at the last incomplete ripple density. If infants were not able to reach criterion at that ripple density after being tested on at least 12 trials, it was assumed that the infant was not able to resolve that ripple density. If, however, the infant did not complete at least 12 trials, then the participant’s data were excluded, because the upper limit of SRD discrimination could not be estimated. Of infants who completed testing, the average number of test trials per session was 40 trials (SD = 26.3) and the average number of trials per visit was 88.4 (SD = 54.6).
Adults were tested over 1 or 2 sessions completed in a single 60 to 90-minute visit. Adult listeners sat alone in the test booth and were instructed to raise their hand when they “heard the sound that activates the toy.” The observer recorded only the adult hand-raising responses. Due to the fact that false-alarms were extremely unusual for adults, they would rarely trigger “reminder” trials based on the 3-consecutive incorrect rule. Therefore, reminder trials were given to adults after 3-consecutive incorrect signal trials. In all other aspects, the stimuli and procedure were the same for adults and infants.
All statistical analyses were conducted using SPSS Version 23 (Armonk, NY: IBM Corp) with the exception of exact binomial tests which were conducted using an online calculator (Lowry). Sizes of significant effects and/or interactions are reported as ηp2 or cohen’s d for 2-way ANOVAs and independent-samples t tests respectively.
Results
As described above, each participant was tested under one of three ripple depth conditions: 10, 13, or 20 dB depth. Of 35 infants who completed testing, 9 were tested at 10 dB depth, 16 at 13 dB depth and 10 at 20 dB depth. The numbers of adults tested at each ripple depth were 10, 10 and 13 for 10 dB, 13 dB and 20 dB depths, respectively. All adults and most infants (29 of 35) completed training in fewer than 15 trials. The average number of training trials was 10.7 (SD = 6.36). The average number of test phase trials per session was 39.3 trials (SD = 21.5).
A simulation of 1000 test sessions was conducted to determine what percentage of infant-observer pairs could reach criterion based on chance performance alone. The testing software used in the experiment was modified so the response on each test trial was selected pseudorandomly (with the rate of “change” trial selection equal to the observed rate of 54%). The proportion of simulated sessions in which criterion was reached for one ripple density was 12.5%. The proportion of simulated sessions in which criterion was reached for two ripple densities was 2%.
All adults and 33 of 35 infants reached criterion at the lowest ripple density. Both infants who did not reach criterion at any phase were tested at 13 dB depth. An exact binomial test with an assumed probability of .125 was conducted for each age group to test whether the observed probability was greater than the simulated probability. For both infants and adults this test was significant (p < 0.0001) indicating that more listeners passed at least one test phase than would be predicted by responding at random. We then repeated the analyses stratified by age and modulation depth. For both age groups and across all modulation depths, the observed probability that a participant reached criterion at the lowest ripple density was significantly greater than 0.125 (exact binomial p <0.0001). Similarly, when the observed probability of listeners reaching criterion for two or more test phases (26 of 31 infants) was compared to the assumed probability of 0.02, the overall and stratified (by age group and modulation depth) binomial exact tests were all significant (all p < 0.015).
In the second set of analyses, SRD thresholds for individual listeners were examined. For each infant, the highest SRD density at which they met criteria was used as the threshold. SRD thresholds for infants who reached criteria at two or more ripple depths (achieved by only 2% of simulated sessions) were considered above chance performance. SRD thresholds for infants who only reached criteria at one ripple depth could have occurred based on chance for 12.5% of participants. Rather than exclude these infant thresholds, which could bias the average infant SRD to artificially higher levels, we chose to include these data and note chance performance in the figure. Importantly, statistical results were unaffected even if these infant SRD thresholds were excluded from the analyses. SRD thresholds of 4 infants (all at 13 dB depth) could not be determined either because of failure to reach criterion at the easiest ripple density of 1 ripple per octave (n=2) or an insufficient number of trials at the highest ripple density (n=2). The latter two infants reached criterion at 1.4 and 2.8 ripples per octave respectively but did not complete sufficient trials at the next highest density due to fussiness. One way to handle this would be to assign a threshold of 1 ripple per octave (the easiest density) to the infants who did not reach criterion and 1.4 or 2.8 ripples per octave to the infants who reached criterion. Alternatively, and most conservatively, these data could be excluded from the remaining analyses. It was decided to go with the latter approach and exclude data from these 4 infants. One infant reached criterion at the highest ripple depth (45 RPO) and threshold was considered 45 RPO for this participant. Thus, of the 35 infants who completed testing, thresholds were determined for 88.6%. Thresholds were determined for 32 of 33 adults who completed testing. The adult for whom a threshold was not obtained did not complete a sufficient number of trials at the highest ripple depth.
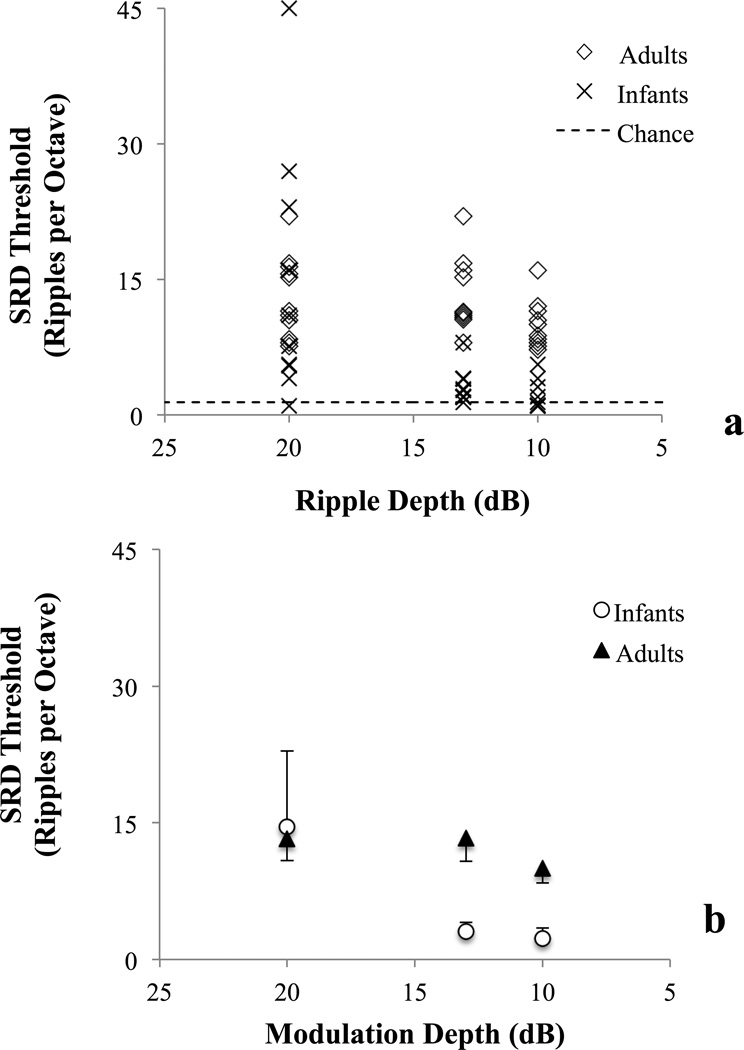
Individual SRD thresholds for infants and adults are shown in Figure 4a as a function of modulation depth. Adults tended to have higher (better) thresholds than infants at lower ripple depths but not at 20 dB depth. SRD thresholds of infants at 20 dB overlapped the adult range with 2 infants performing well above the best adult listener. Mean SRD threshold as a function of ripple depth and age group is shown in Figure 4b. Thresholds were best at 20 dB depth for both age groups; however, the degree to which thresholds worsened with decreasing depth appears greater for infants than for adults. A 2-way (2X3) ANOVA revealed significant main effects of Depth [F(2,57) = 8.686, p = 0.001, ηp2 = 0.237] and Age [F(1,61) = 12.581, p = 0.001, ηp2 = 0.183]. In addition, the interaction between Age and Depth was significant [F(2,61) = 5.083, p = 0.009, ηp2 = 0.154]. Two-tailed independent-samples t-tests were conducted to analyze the effect of Age at each ripple depth. Levene’s test reached significance, revealing unequal variance between age groups, at 20 dB depth [F(21) = 5.999, p = 0.0115] but not at 10 or 13 dB depth [p’s > 0.05]. Thus, equal variance t-tests were conducted at 10 and 13 dB depth whereas an unequal variance t-test was conducted at 20 dB depth. Significant effects of Age were found at 10 dB [t(18) = −7.863, p < 0.0001, cohen’s d = 3.611] and at 13 dB depth [t(21) = −4.194, p < 0.0001, cohen’s d = 1.796], but not at 20 dB depth [t(13.681) = 0.010, p = .496].
Figure 4.
Individual spectral ripple discrimination (SRD) thresholds stratified by age group and ripple depth (4a). Each marker indicates performance of an individual listener with up to 5% jitter to highlight overlapping values. Thresholds above dotted line are above chance performance based on 1000 simulated sessions. Mean spectral ripple discrimination (SRD) threshold as function of ripple depth and age group (4b). Up error bars and down error bars indicate 95% CI for infants and adults respectively.
It is possible that the high variance of SRD threshold in the 20 dB depth condition may have skewed the data resulting in the observed Age X Depth interaction. Therefore, in a post-hoc analysis, we recomputed the Age X Depth ANOVA excluding the 20 dB depth condition. In this analysis there was a main effect of Age [F(1,37) = 110.3, p < 0.0001, ηp2 = 0.749] and depth [F(1,37)=5.66, p = 0.023, ηp2 = 0.133] but the Age X Depth interaction did not reach significance [F(1,37)=2.24, p = 0.143].
Discussion
Like their adult counterparts, nearly all normal-hearing 7-month infants were able to discriminate spectral ripple stimuli at 13 and 20 dB depth. Using OPP, we were able to obtain SRD thresholds from the majority of 7-month infants with a very low attrition rate. Thus, unlike many infant psychoacoustic measures, the current method appears to be efficient and potentially useful for studying spectral resolution of individual infants.
Mean SRD thresholds were significantly poorer for infants than adults at the lower ripple depths. The performance of the majority of infants was not significantly above chance at 10 dB depth. Poorer infant performance at low ripple depths suggests that their SMTF x-intercept is greater than adults. This result is consistent with the first hypothesis that infants’ across-spectrum peak/trough intensity resolution is immature. Contrary to the predictions of the second hypothesis, however, we found a significant interaction between age and modulation depth. This result suggests that SMTFs are not parallel between age groups. Moreover, the steeper SMTF of infants would be consistent with better frequency resolution in NH infants than adults. However, this conclusion would be inconsistent with literature on maturation of adult-like frequency resolution based on measures of cochlear tuning (Olsho et al. 1987, Schneider et al. 1990, Spetner and Olsho 1990).
An alternative explanation of this finding is based on the observation of high variability in infant performance in the 20 dB depth condition. High across-subject variability is a known hallmark of auditory perceptual development often attributed to immature perceptual efficiency rather than sensory acuity (Allen and Wightman 1994, Werner and Boike 2001, Moore et al. 2011). However, immature efficiency alone would be expected to affect infant performance equally across ripple depths. The fact that infant SRD threshold variance was so much greater at 20 dB depth than at 10 or 13 dB depth suggests that the mechanisms underlying individual infant performance may have been different across ripple depths. Furthermore, the 3 highest infant SRD thresholds were well above what would be expected based on spatial resolving capacity alone (Supin et al. 1994, Supin et al. 1994, Supin et al. 1999). At ripple densities above 14 ripples per octave (RPO), discrimination is likely based on within-channel intensity or temporal cues (Anderson et al. 2011, Anderson et al. 2012). It is possible, therefore, that performance of listeners utilizing within-channel cues in the 20 dB depth condition skewed the data resulting in the observed interaction between age and ripple depth and leading to higher average performance. This seems likely given that, when the data were reanalyzed with the 20 dB depth condition removed, the interaction was no longer significant.
The availability of within-channel intensity cues for SRD raises the possibility that listeners could focus on a narrow-band area of the spectrum to complete the task. Thus, the task may not always depend on a listener’s ability to discriminate the spectral envelope shape. One way to reduce this possibility is to randomly vary the phase of the ripple envelope from trial to trial so that any within-channel cues are spread randomly across the spectrum (Anderson et al. 2011, Anderson et al. 2012). Experiment 2 was, therefore, designed to compare infant and adult SRD at 20 dB depth using this procedural modification: unlike Experiment 1 where the starting phase of the spectral envelope was fixed at zero degrees, in Experiment 2 starting phase was randomized across trials. Thus, within-channel cues were inconsistent and unpredictable across the spectrum. Although randomization of spectral envelope phase was not shown to affect mean SRD in adult CI listeners (Won et al. 2011), this has not been investigated in NH adults or in younger listeners. It was hypothesized that, with obscured within-channel cues, mean infant SRD at 20 dB depth would be poorer than mean adult SRD.
EXPERIMENT 2 - Effect of Starting Phase Randomization and Age on SRD
Materials and Methods
Participants
Twenty-two 7-month-old infants and 16 young adults were initially recruited. Inclusion criteria and tympanometric screening were identical to Experiment 1. Infants were tested over 3 visits and 10–14 days and adults tested at one visit. Twelve infants (2 due to failed tympanometry, 3 due to experimenter error, and 7 due to failure to complete scheduled visits) and two adults (both due to experimenter error) were excluded leaving 10 and 14 participants in each age group respectively.
Stimuli
Stimuli were constructed from 1s wide-band noise carriers just as in Experiment 1 with one important difference. Instead of using a constant starting phase of 0 radians, standard ripple starting phases varied from 0 to 2π radians. Inverted ripple starting phases were equal to the corresponding standard starting phase plus π/2 radians. Standard and inverted ripples were concatenated to create change stimuli, and stimuli were filtered and ramped as in Experiment 1. Sets of 10 change and 10 no-change stimuli, each with randomly generated noise carrier and starting phases, were created with ripple densities ranging from 0.5 ripples per octave to 45 ripples per octave in steps of 1.414 ripples per octave. Ripple peak-trough depth was set at 20 dB.
Procedure
Subjects were tested using the same procedure and setup as in Experiment 1. Individual SRD thresholds were determined as described in Experiment 1.
Results
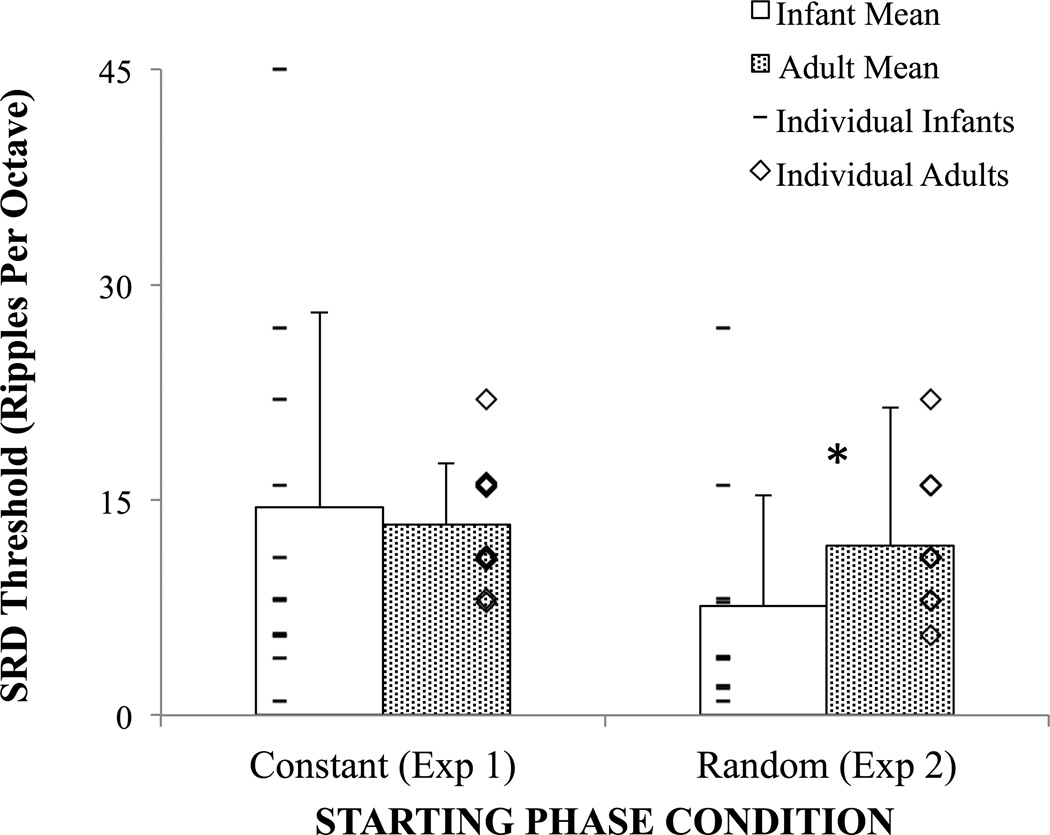
SRD thresholds of individual infants tested with random starting phase are shown in Figure 5. Similar to infants tested with constant starting phase at 20 dB depth in Experiment 1, all but one infant performed above chance as previously defined. However, infant performance tended to be poorer than adults. Mean SRD threshold as a function of age group for both the constant-phase (20 dB depth from Experiment 1) and randomized-phase conditions are shown as data bars in Figure 5. As described previously, mean infant and adult SRD thresholds were not significantly different at 20 dB depth in the constant-phase condition. In contrast, infant mean SRD performance was significantly poorer than adults in the randomized phase condition. Levene’s test did not reach significance [F(23) = 3.278, p > 0.05] and the equal variance independent samples 2-tailed t-test reached significance [t(23) = −2.056, p = 0.049, cohen’s d = 0.772].
Figure 5.
Mean spectral ripple discrimination (SRD) threshold as a function of age group with random versus constant spectral envelope starting phase. Error bars indicate 1 standard deviation from the mean. Individual thresholds are shown with up to 5% jitter to highlight overlapping values. * Indicates significant effect of age, Independent Samples t-test, 2-tailed p = 0.049.
Discussion
Given that infant frequency resolution, and therefore steepness of the SMTF, should be adult-like (Olsho et al. 1987, Schneider et al. 1990, Spetner and Olsho 1990), the finding that infant SMTF appeared steeper than adults in Experiment 1 requires explanation. In Experiment 2, we explored the possibility that variability in infants’ sensitivity or attention to within-channel cues at 20 dB depth led to the observed interaction. Although such cues were equally available at lower ripple depths, it may be that non-spectral cues become more salient (and therefore used by some listeners) for the higher ripple densities perceived at 20 dB depth (Anderson et al. 2011, Anderson et al. 2012).
The results of Experiment 2 support this hypothesis. Average performance of 7-month-old NH infants on SRD is poorer than adults when within-channel intensity cues are reduced. The fact that this age effect emerged when spectral ripple starting phase was randomized supports the hypothesis that some infants in Experiment 1 may have been utilizing within-channel cues on the task, particularly at the high ripple depths. In order to lend further support to this hypothesis, a third Experiment was carried out in which the SMTF was measured in 3 age groups: 3-month-olds, 7-month-olds, and young adults. In Experiment 3, the starting phase of the spectral envelope was randomized across trials for all ripple depths. Furthermore, ripple depth was treated as a within-subjects variable in order to remove between-subjects contributions to the SRD variance across ripple depths.
EXPERIMENT 3 – Effect of Ripple Depth (Within-Subjects) and Age on SRD
Materials and Methods
Participants
Forty-four normal-hearing infants (20 three-month-olds and 24 seven-month-olds) and 24 young adults were initially recruited. Inclusion criteria and tympanometric screening were identical to Experiments 1 and 2. Infants were tested over 3–4 visits and 10–14 days and adults tested at one visit. Twelve infants were excluded due to failed tympanometry (1 three-month-old, 5 seven-month-olds), experimenter error (1 three-month-old, 3 young adults) or failure to complete scheduled visits (4 seven-month-olds), leaving 18, 15, and 21 participants in the 3-month-old, 7-month-old, and young adult groups respectively.
Stimuli
Stimuli were constructed as described in Experiment 2 with two ripple peak-trough depths: 10 dB or 20 dB.
Procedure
Subjects were tested using the same OPP procedure and setup as in Experiments 1 and 2. Individual SRD thresholds were determined as described in Experiment 1. Each listener was tested at both ripple depths. Order of ripple depth was counterbalanced across listeners.
Results
The percentages of participants who reached criterion for at least one and two test phases are shown in Table 1. For all age groups, the number who reached criterion at either ripple depth exceeded chance performance, exact binomial test p’s < 0.001 (based on 0.125 and 0.02 probabilities of reaching criterion for one or two or more test phases respectively). Thresholds were calculated for all but four infants (3 three-month olds, 1 seven-month-old). These infants were excluded because they failed to reach criterion on at least one test phase at both 10 and 20 dB depths. Thus, the remaining participants (15 three-month-olds, and 14 seven-month-olds, 21 adults) each provided two SRD thresholds. A repeated measures ANOVA was conducted to examine the effect of ripple depth (within-subjects, 2 levels), test order (between-subjects, 2-levels), and age group (between subjects, 3-levels) on SRD threshold. Similar to Experiment 1, there were significant main effects of both Depth and Age. Mean SRD was significantly higher in the 20 dB versus the 10 dB condition [F(1,44) = 27.839, p < 0.0001, ηp2 = 0.388] and in older versus younger participants [F(2,44) = 14.608, p < 0.0001, ηp2 = 0.399]. Order was not a significant main effect [F(1,44) = 2.245, p = 0.141]. Unlike Experiment 1, there were no significant interactions between any variables. In particular, the interaction between Age X Depth did not reach significance [F(2,44) = 0.397, p = 0.675].
Table 1.
Frequency of reaching test criterion per age group and ripple depth
| 10 dB | 20 dB | |
|---|---|---|
| 3-month-olds (n=18) | 88.9% (33.3%) | 100% (72.2%) |
| 7-month-olds (n=15) | 93.3% (46.7%) | 100% (86.7%) |
| Young adults (n=14) | 100% (100%) | 100% (100%) |
Note: Percent of participants who reached criterion for at least one test phase shown (percent who reached criterion for at least two test phases shown in parentheses). Based on simulated probabilities of 0.125 for reaching criterion for one test phase, each percentage was greater than chance (binomial exact p’s < 0.0001).
Mean SRD as a function of age group and depth is shown in Figure 6. Unlike Experiment 1, the degree to which SRD changes with ripple depth is visually similar across age groups. However, these mean data may not reflect the degree to which slope of the SMTF varies across individuals and ages. To examine this directly, we computed the slope between the 2 SRD thresholds as a function of ripple depth for each participant. The effect of age group on SMTF slope was then assessed using a 3X2 ANOVA with Age and Order as independent variables. Neither main effect, nor the interaction reached significance [p’s > 0.05] indicating that mean SMTF slopes were similar across age groups.
Figure 6.
Mean spectral ripple discrimination (SRD) threshold as a function of ripple depth and age group. Ripple depth was a within-subjects variable. Up and down error bars indicate 95% CI for adults and infants respectively.
General Discussion
The present study demonstrates that measurement of SRD with the OPP method is feasible in NH infants. This is not surprising given that OPP has been used to measure auditory acuity on a variety of psychoacoustic tasks in listeners ranging in age from 2 weeks old (Werner and Gillenwater 1990) through toddlerhood (Dasika et al. 2009). OPP has already been used to test auditory detection (Dasika et al. 2009) and sound localization (Grieco-Calub et al. 2008) in young CI users making it ideal for application for future studies of SRD in infants with CIs.
Compared to other behavioral methods used to assess infant auditory discrimination, such as visual habituation (Houston et al. 2007, Warner-Czyz et al. 2014) or the head turn preference procedure (Bertoncini et al. 2009, Bertoncini et al. 2011), OPP requires a much longer testing time and number of trials (Olsho et al. 1987, Werner 1995). That being said, the attrition rate in the present study was at least as favorable as that reported for either visual habituation or the head turn preference procedure. Moreover, there are three main advantages to OPP over these other methods. First, OPP can be more easily adapted to measure threshold. Second, OPP can measure discrimination capacity of individual infants. In visual habituation and the head turn preference procedure, the small numbers of test trials make it difficult to extrapolate individual differences in looking times to perceptual capacities, although some attempts have been made (Houston et al. 2007). Thus, OPP might be used in future studies to examine relationships between individual infant SRD and speech perception capacity. Third, OPP does not require a particular stereotyped behavioral response. Therefore, infants can be tested beyond the point at which these responses habituate or at ages when these responses are not robust or consistent.
Spectral ripple discrimination is much poorer in adult post-lingually deaf CI users, as well as in pre-lingually deaf children with CIs, than in their NH age cohorts due to limited spectral resolution provided by the devices and electroneural interface (Henry and Turner 2003, Henry et al. 2005, Won et al. 2007, Jung et al. 2012, Peter et al. 2014). Although spectral ripple discrimination has recently been shown to be a promising clinical marker of auditory acuity in adult, post-lingually deaf, CI users (Drennan et al. 2014, Shim et al. 2014, Drennan et al. 2015), little is known about how performance on this measure changes with development. Although spectral resolution might prove to be a relatively stable and acute measure of device efficacy for adults, it is important to understand how spectral resolution is affected by development before measures of spectral resolution can be applied effectively to young listeners with CIs. The present study demonstrates that NH infants’ SRD thresholds, at least at low ripple depths, are poorer than adults. Furthermore, infant thresholds at the highest ripple depth tested in this study were poorer than adults when within-channel cues were minimized. Taken with the limited literature on SRD in older children, this would place the age of maturation for SRD somewhere between 7-months-old and 8–11 years for NH listeners (Blagosklonova et al. 1989, Allen and Wightman 1994, Jung et al. 2012, Peter et al. 2014). Recently, similar rates of spectral resolution maturation have been reported for tasks involving minimum-phase difference detection (Rayes et al. 2014) as well as dynamic spectro-temporal phase detection (Rayes et al. 2014, Kirby et al. 2015).
The present study sought to examine whether age differences in SRD thresholds were related to differences in frequency resolution or across-channel intensity resolution. Supin and colleagues first demonstrated that SRD thresholds were quantitatively related to equivalent rectangular bandwidths and, hence, to a listener’s frequency resolving capacity (Supin et al. 1994, Supin et al. 1999). Supin and colleagues also recognized that across-channel peak-trough intensity resolution was a separate limiting factor for SRD (Supin et al. 1999). Based on early maturation of cochlear frequency tuning in NH infants (Spetner and Olsho 1990), we had hypothesized that differences in SRD performance between 7-month-old infants and adults would primarily be due to immature across-channel intensity resolution. Thus, it was expected that infant SMTF, relative to adults, would be parallel and shifted on the x-axis as shown for the “immature intensity resolution” curve plotted in Figure 1.
Taken together, the results from the three Experiments are consistent with that hypothesis. In Experiment 1, the significant interaction between ripple depth and age indicated that the SMTF shapes of NH infants and young adults were significantly different. This could arise either from differences in SMTF spread on the frequency axis or on the x-axis. Infant SRD was poorer than adults at low ripple depths but not at higher depths suggesting that infant SMTF x-intercept is greater than adults. Thus, these data are suggestive of an approximately 10-fold difference in across-channel intensity resolution (10 dB versus 1dB in infants and adults respectively). In Experiment 3, when within-channel cues were obscured, infant SMTFs were similar to adults in terms of spread on the frequency axis. Moreover individual listener SMTF slopes did not differ significantly across ages. Therefore, the main effect of Age on SRD in Experiment 3 is best explained by age differences in across-channel intensity resolution.
The present findings have important implications for future studies of SRD development in infants and children. At the very least, SRD thresholds cannot be assumed to reflect the same auditory capacities in immature listeners and adults. While frequency resolution should mature during infancy, across-channel intensity resolution likely matures more gradually. In previous studies of SRD in older children (Jung et al. 2012, Peter et al. 2014), thresholds at lower modulation depths were not determined. As shown in Figure 1, differences in SRD threshold at a single, large ripple depth could be explained by a number of different hypothetical SMTFs. Until the age of maturation of the across-channel intensity resolution is understood, interpretation of SRD thresholds in very young listeners should be made with caution.
One important remaining question is whether frequency resolution or across-channel intensity resolution are both important factors for speech understanding in CI listeners. Currently, there are few clues from the existing literature. For SRD, there are few studies that have examined the SMTF in individual listeners with CIs. SRD thresholds are significantly better at 30dB depth than at 10 dB depth and thresholds at these two depths are strongly correlated in adult CI listeners (Won et al. 2011). Strong correlations between speech perception and SRD thresholds have been found at both large (Won et al. 2007) and small (Drennan et al. 2014) ripple depths. However, to date no studies have carefully examined whether SRD SMTF parameters are correlated with speech perception in CI listeners.
In contrast, at least two studies have investigated the SMTF for spectral ripple detection (Saoji et al. 2009, Anderson et al. 2012). In both studies, the depth approach was used to obtain modulation depth thresholds at fixed ripple densities and exponential SMTFs fit to the threshold data. Saoji and colleagues reported that the spread of the SMTF along the intensity/depth axis, but not along the frequency/density axis, was strongly correlated with phoneme perception in adult CI listeners (Saoji et al. 2009). Similarly, Anderson et al. (2012) found that spectral ripple detection at low ripple density was most robustly related to speech perception in both noise and quiet. Thus, both studies would imply that across-channel intensity resolution, and not spectral frequency resolution per se, is the important factor underlying the relationship between SRD and speech perception.
Anderson et al. (2012) further investigated whether across-channel intensity resolution was related to intensity difference limens with broadband noise. Interestingly, the difference limen did not correlate significantly with either spectral ripple detection or discrimination thresholds. This finding was similar to earlier work by Green and Mason showing no relationship between spectral profile analysis and broadband intensity discrimination (Green and Mason 1985). Anderson et al. concluded that sensitivity to intensity differences across frequency was mediated by different mechanisms than sensitivity to intensity across time. Perhaps more importantly, intensity discrimination did not correlate with any speech perception metrics. This finding is in agreement with research on slow-rate amplitude modulation detection in adult CI listeners (Won et al. 2011).
Mean SRD threshold of infants was adult-like, on average, at 20 dB depth when ripple phase was held constant but not when phase varied across trials. This finding suggests that eliminating within-channel cues affects SRD performance more in infants than adults. It is possible, therefore, that some subset of infant listeners use different cues than the majority of listeners in the constant phase condition. If presence of redundant within-channel cues is beneficial for a subset of infants, this could explain the difference in infant 20 dB depth SRD across Experiments 1–3.
It is known that available within-channel cues may contribute to SRD performance at ripple densities beyond the ripple frequency resolution (Anderson et al. 2011, Anderson et al. 2012). While we attempted to control for high-frequency temporal cues by low pass filtering of the stimuli, we cannot rule out the possibility that some listeners with very high SRD thresholds were responding to within-channel cues. Given that NH listeners behavioral temporal resolution is mature at least by preschool age (Hall and Grose 1994), and temporal encoding at the level of the auditory brainstem is mature by 3 months of age (Werner et al. 2001), we should expect that 7-month-old infants have the ability to encode such within-channel cues. The fact that age differences in SRD emerged when starting phase was randomized in Experiments 2 and 3 suggests that this procedure modification can reduce the effect of within-channel cues on SRD in immature listeners.
In conclusion, spectral resolution as assessed by spectral ripple discrimination is immature in normal hearing 7-month-old infants. The present study supports the hypothesis that infants’ resolution in the frequency domain matures earlier than peak/trough intensity resolution. These basic findings suggest at least 3 important implications for research into spectral resolution of young children who use cochlear implants. First, it should not be assumed that device and electroneural interface factors are the only important limitations to spectral resolution in young children with CIs. The finding that some aspects of spectral resolution develop gradually in NH children would suggest that similarly prolonged maturation would be present in pre-lingually deaf children with CIs. For instance, we might expect early maturation of frequency resolution in these children, and thus for spectral resolution of implanted children to be similar to post-lingually deaf adults during the first year of implant experience. In contrast, the time course for maturation of peak-trough intensity resolution might be prolonged in CI children as it is in NH children.
Second, adapting SRD for measurement of spectral resolution and auditory acuity in very young children with CIs may not be straightforward. If peak-trough intensity resolution at low ripple densities is the primary factor relating SRD thresholds and speech perception, then this may be difficult to ascertain in very young CI patients who have immature across-channel intensity resolution. Further research into how the SMTF changes with development is needed before the appropriate stimulus conditions for assessing device efficacy in these patients can be determined.
Third, measures of SRD in young listeners should carefully control for within-channel cues when possible. Variable use of these cues by immature listeners might be a particular challenge for interpretation of SRD thresholds. Randomization of ripple phase across test trials is one accepted practice as is level roving across trials. Level roving was not employed in this study due to previous pilot testing in the senior authors lab suggesting that young infants have particular difficulty with level-roved signals. However even with these controls, within-channel and temporal cues are not completely eliminated. An alternative approach, such as temporally modulated spectral ripples, might be considered to eliminate these confounding cues (Aronoff and Landsberger 2013).
Acknowledgments
The authors would like to acknowledge the American Otological Society (Clinician-Scientist Award), National Institutes of Health (NIDCD R01DC00396, K23DC013055, and P30DC04661), and private donations to the Virginia Merrill Bloedel Hearing Research Center particularly from Motors Northwest for funding the present studies. We also thank Ward Drennan and Gary Jones for collaboration and assistance with stimulus generation, Mariette Verschaeve and Joanne Huang for assistance with data collection, and Vasant Dasika and Rosemary Lovett for collection of pilot data.
Footnotes
Financial Disclosures/Conflicts of Interest:
The co-authors have no relevant financial disclosures or conflicts of interest.
References
- Allen P, Wightman F. Psychometric functions for children's detection of tones in noise. J Speech Hear Res. 1994;37(1):205–215. doi: 10.1044/jshr.3701.205. [DOI] [PubMed] [Google Scholar]
- Allen P, Wightman F. Effects of signal and masker uncertainty on children's detection. J Speech Hear Res. 1995;38(2):503–511. doi: 10.1044/jshr.3802.503. [DOI] [PubMed] [Google Scholar]
- Anderson ES, et al. Comparing spatial tuning curves, spectral ripple resolution, and speech perception in cochlear implant users. J Acoust Soc Am. 2011;130(1):364–375. doi: 10.1121/1.3589255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ES, et al. Assessing the role of spectral and intensity cues in spectral ripple detection and discrimination in cochlear-implant users. J Acoust Soc Am. 2012;132(6):3925–3934. doi: 10.1121/1.4763999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff JM, Landsberger DM. The development of a modified spectral ripple test. J Acoust Soc Am. 2013;134(2):EL217–EL222. doi: 10.1121/1.4813802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoncini J, et al. Six-month-old infants discriminate voicing on the basis of temporal envelope cues (L) J Acoust Soc Am. 2011;129(5):2761–2764. doi: 10.1121/1.3571424. [DOI] [PubMed] [Google Scholar]
- Bertoncini J, et al. Discrimination of speech sounds based upon temporal envelope versus fine structure cues in 5- to 7-year-old children. J Speech Lang Hear Res. 2009;52(3):682–695. doi: 10.1044/1092-4388(2008/07-0273). [DOI] [PubMed] [Google Scholar]
- Blagosklonova NK, et al. Auditory frequency resolving power in normal hearing children and ones with hearing loss. International Journal of Psychophysiology. 1989;7(2–4):148–149. [Google Scholar]
- Bull D, et al. Infants' discrimination of intensity variation in multisyllabic stimuli. J Acoust Soc Am. 1984;76(1):13–17. doi: 10.1121/1.391110. [DOI] [PubMed] [Google Scholar]
- Byrne D, et al. An International Comparison of Long-Term Average Speech Spectra. Journal of the Acoustical Society of America. 1994;96(4):2108–2120. [Google Scholar]
- Clarkson MG, Clifton RK. Infants' pitch perception: inharmonic tonal complexes. J Acoust Soc Am. 1995;98(3):1372–1379. doi: 10.1121/1.413473. [DOI] [PubMed] [Google Scholar]
- Dasika VK, et al. Measuring sound detection and reaction time in infant and toddler cochlear implant recipients using an observer-based procedure: a first report. Ear Hear. 2009;30(2):250–261. doi: 10.1097/AUD.0b013e3181986dfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan WR, et al. Validation of a clinical assessment of spectral-ripple resolution for cochlear implant users. Ear Hear. 2014;35(3):e92–e98. doi: 10.1097/AUD.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan WR, et al. Clinical evaluation of music perception, appraisal and experience in cochlear implant users. Int J Audiol. 2015;54(2):114–123. doi: 10.3109/14992027.2014.948219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan WR, et al. Nonlinguistic Outcome Measures in Adult Cochlear Implant Users Over the First Year of Implantation. Ear Hear. 2015 doi: 10.1097/AUD.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Mason CR. Auditory profile analysis: frequency, phase, and Weber's law. J Acoust Soc Am. 1985;77(3):1155–1161. doi: 10.1121/1.392179. [DOI] [PubMed] [Google Scholar]
- Grieco-Calub TM, et al. Using the observer-based psychophysical procedure to assess localization acuity in toddlers who use bilateral cochlear implants. Otol Neurotol. 2008;29(2):235–239. doi: 10.1097/mao.0b013e31816250fe. [DOI] [PubMed] [Google Scholar]
- Hall JW, 3rd, Grose JH. Development of temporal resolution in children as measured by the temporal modulation transfer function. J Acoust Soc Am. 1994;96(1):150–154. doi: 10.1121/1.410474. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW. The resolution of complex spectral patterns by cochlear implant and normal-hearing listeners. J Acoust Soc Am. 2003;113(5):2861–2873. doi: 10.1121/1.1561900. [DOI] [PubMed] [Google Scholar]
- Henry BA, et al. Spectral peak resolution and speech recognition in quiet: normal hearing, hearing impaired, and cochlear implant listeners. J Acoust Soc Am. 2005;118(2):1111–1121. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- Houston DM, et al. Assessing speech discrimination in individual infants. Infancy. 2007;12(2):119–145. doi: 10.1111/j.1532-7078.2007.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Jeon EK, et al. Cochlear implant users' spectral ripple resolution. J Acoust Soc Am. 2015;138(4):2350. doi: 10.1121/1.4932020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, et al. Psychoacoustic Performance and Music and Speech Perception in Prelingually Deafened Children with Cochlear Implants. Audiol Neurootol. 2012;17(3):189–197. doi: 10.1159/000336407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BJ, et al. Spectro-temporal modulation detection in children. J Acoust Soc Am. 2015;138(5):EL465–EL468. doi: 10.1121/1.4935081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BK, Werner LA. Perception of missing fundamental pitch by 3- and 4-month-old human infants. J Acoust Soc Am. 2012;132(6):3874–3882. doi: 10.1121/1.4763991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold LJ, Neff DL. Masking by a remote-frequency noise band in children and adults. Ear Hear. 2011;32(5):663–666. doi: 10.1097/AUD.0b013e31820e5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak LM, et al. Relationship between perception of spectral ripple and speech recognition in cochlear implant and vocoder listeners. J Acoust Soc Am. 2007;122(2):982–991. doi: 10.1121/1.2749413. [DOI] [PubMed] [Google Scholar]
- Lopez Valdes A, et al. Objective assessment of spectral ripple discrimination in cochlear implant listeners using cortical evoked responses to an oddball paradigm. PLoS One. 2014;9(3):e90044. doi: 10.1371/journal.pone.0090044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry R. Binomial Probabilities. 2001 Retrieved July 20, 2016, from http://vassarstats.net/binomialX.html.
- Maxon AB, Hochberg I. Development of psychoacoustic behavior: sensitivity and discrimination. Ear Hear. 1982;3(6):301–308. doi: 10.1097/00003446-198211000-00003. [DOI] [PubMed] [Google Scholar]
- Moberly AC, et al. Word Recognition Variability With Cochlear Implants: "Perceptual Attention" Versus "Auditory Sensitivity". Ear Hear. 2016;37(1):14–26. doi: 10.1097/AUD.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly AC, et al. Do adults with cochlear implants rely on different acoustic cues for phoneme perception than adults with normal hearing? J Speech Lang Hear Res. 2014;57(2):566–582. doi: 10.1044/2014_JSLHR-H-12-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, et al. Development of auditory processing in 6- to 11-yr-old children. Ear Hear. 2011;32(3):269–285. doi: 10.1097/AUD.0b013e318201c468. [DOI] [PubMed] [Google Scholar]
- Nittrouer S, et al. Measuring the effects of spectral smearing and enhancement on speech recognition in noise for adults and children. J Acoust Soc Am. 2015;137(4):2004–2014. doi: 10.1121/1.4916203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EL, et al. Children's detection of pure-tone signals with random multitone maskers. J Acoust Soc Am. 2001;109(6):2888–2895. doi: 10.1121/1.1371764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsho LW, et al. Level and age effects in infant frequency discrimination. J Acoust Soc Am. 1987;82(2):454–464. doi: 10.1121/1.395446. [DOI] [PubMed] [Google Scholar]
- Olsho LW, et al. An observer-based psychoacoustic procedure for use with young infants. Developmental Psychology. 1987;23(5):627–640. [Google Scholar]
- Peter V, et al. Assessing spectral and temporal processing in children and adults using temporal modulation transfer function (TMTF), Iterated Ripple Noise (IRN) perception, and spectral ripple discrimination (SRD) J Am Acad Audiol. 2014;25(2):210–218. doi: 10.3766/jaaa.25.2.9. [DOI] [PubMed] [Google Scholar]
- Rayes H, et al. Discrimination of static and dynamic spectral patterns by children and young adults in relationship to speech perception in noise. Audiol Res. 2014;4(1) doi: 10.4081/audiores.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoji AA, et al. Spectral modulation detection and vowel and consonant identifications in cochlear implant listeners. J Acoust Soc Am. 2009;126(3):955–958. doi: 10.1121/1.3179670. [DOI] [PubMed] [Google Scholar]
- Schneider BA, et al. Size of critical band in infants, children, and adults. J Exp Psychol Hum Percept Perform. 1990;16(3):642–652. doi: 10.1037//0096-1523.16.3.642. [DOI] [PubMed] [Google Scholar]
- Schneider BA, et al. Developmental perspectives on the localization and detection of auditory signals. Percept Psychophys. 1991;49(1):10–20. doi: 10.3758/bf03211611. [DOI] [PubMed] [Google Scholar]
- Shim HJ, et al. Can unaided non-linguistic measures predict cochlear implant candidacy? Otol Neurotol. 2014;35(8):1345–1353. doi: 10.1097/MAO.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetner NB, Olsho LW. Auditory frequency resolution in human infancy. Child Dev. 1990;61(3):632–652. [PubMed] [Google Scholar]
- Supin A, et al. Frequency resolving power of the human's hearing. Neurosci Lett. 1994;165(1–2):195–198. doi: 10.1016/0304-3940(94)90743-9. [DOI] [PubMed] [Google Scholar]
- Supin A, et al. Frequency resolving power measured by rippled noise. Hear Res. 1994;78(1):31–40. doi: 10.1016/0378-5955(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Supin A, et al. Ripple depth and density resolution of rippled noise. J Acoust Soc Am. 1999;106(5):2800–2804. doi: 10.1121/1.428105. [DOI] [PubMed] [Google Scholar]
- Tharpe AM, Ashmead DH. A longitudinal investigation of infant auditory sensitivity. Am J Audiol. 2001;10(2):104–112. doi: 10.1044/1059-0889(2001/011). [DOI] [PubMed] [Google Scholar]
- Trehub A. Stimulus intensity and modulation of brain output. Biophys J. 1973;13(7):705–710. doi: 10.1016/S0006-3495(73)86016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehub A. Auditory Sensitivity in school-age children. Journal of Experimental Child Psychology. 1988;46(2):273–285. doi: 10.1016/0022-0965(88)90060-4. [DOI] [PubMed] [Google Scholar]
- Tsang CD, Trainor LJ. Spectral slope discrimination in infancy: sensitivity to socially important timbres. Infant Behavior and Development. 2002;25(2):183–194. [Google Scholar]
- Warner-Czyz AD, et al. Vowel discrimination by hearing infants as a function of number of spectral channels. J Acoust Soc Am. 2014;135(5):3017–3024. doi: 10.1121/1.4870700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir C. Auditory frequency sensitivity of human newborns: some data with improved acoustic and behavioral controls. Percept Psychophys. 1979;26(4):287–294. doi: 10.3758/bf03199882. [DOI] [PubMed] [Google Scholar]
- Werner LA. Observer-based approaches to human infant psychoacoustics. In: Klump GM, Dooling RJ, Fay RR, Stebbins WC, editors. Methods in Comparative Psychoacoustics. Boston: Birkhauser Verlag; 1995. pp. 135–146. [Google Scholar]
- Werner LA, Boike K. Infants' sensitivity to broadband noise. J Acoust Soc Am. 2001;109(5 Pt 1):2103–2111. doi: 10.1121/1.1365112. [DOI] [PubMed] [Google Scholar]
- Werner LA, et al. Human auditory brainstem response to temporal gaps in noise. J Speech Lang Hear Res. 2001;44(4):737–750. doi: 10.1044/1092-4388(2001/058). [DOI] [PubMed] [Google Scholar]
- Werner LA, Gillenwater JM. Pure-tone sensitivity of 2- to 5-week-old infants. Infant Behavior and Development. 1990;13:355–375. [Google Scholar]
- Won JH, et al. Relationship between behavioral and physiological spectral-ripple discrimination. J Assoc Res Otolaryngol. 2011;12(3):375–393. doi: 10.1007/s10162-011-0257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JH, et al. Acoustic temporal modulation detection and speech perception in cochlear implant listeners. J Acoust Soc Am. 2011;130(1):376–388. doi: 10.1121/1.3592521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JH, et al. Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users. J Assoc Res Otolaryngol. 2007;8(3):384–392. doi: 10.1007/s10162-007-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JH, et al. Relationship among the physiologic channel interactions, spectral-ripple discrimination, and vowel identification in cochlear implant users. J Acoust Soc Am. 2014;136(5):2714–2725. doi: 10.1121/1.4895702. [DOI] [PubMed] [Google Scholar]
- Won JH, et al. Evidence of across-channel processing for spectral-ripple discrimination in cochlear implant listeners. J Acoust Soc Am. 2011;130(4):2088–2097. doi: 10.1121/1.3624820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JH, et al. Spectral and temporal analysis of simulated dead regions in cochlear implants. J Assoc Res Otolaryngol. 2015;16(2):285–307. doi: 10.1007/s10162-014-0502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]