Abstract
Background
Observational studies have identified an association between body mass index (BMI) and incident atrial fibrillation (AF). Inferring causality from observational studies, however, is subject to residual confounding, reverse causation, and bias. The primary objective of this study was to evaluate the causal association between BMI and AF using genetic predictors of BMI.
Methods
We identified 51 646 individuals of European ancestry without AF at baseline from seven prospective population-based cohorts initiated between 1987 and 2002 in the United States, Iceland, and the Netherlands with incident AF ascertained between 1987 and 2012. Cohort-specific mean follow-up ranged 7.4 to 19.2 years, over which period there were a total of 4178 cases of incident AF. We performed a Mendelian randomization with instrumental variable analysis to estimate a cohort-specific causal hazard ratio for the association between BMI and AF. Two genetic instruments for BMI were utilized: FTO genotype (rs1558902) and a BMI gene score comprised of 39 single nucleotide polymorphisms identified by genome-wide association studies to be associated with BMI. Cohort-specific estimates were combined by random-effects, inverse variance weighted meta-analysis.
Results
In age- and sex-adjusted meta-analysis, both genetic instruments were significantly associated with BMI (FTO: 0.43 [95% CI: 0.32 – 0.54] kg/m2 per A-allele, p<0.001); BMI gene score: 1.05 [95% CI: 0.90-1.20] kg/m2 per 1 unit increase, p<0.001) and incident AF (FTO – HR: 1.07 [1.02-1.11] per A-allele, p=0.004; BMI gene score – HR: 1.11 [1.05-1.18] per 1-unit increase, p<0.001). Age- and sex-adjusted instrumental variable estimates for the causal association between BMI and incident AF were HR 1.15 [1.04-1.26] per kg/m2, p=0.005 (FTO) and 1.11 [1.05-1.17] per kg/m2, p<0.001 (BMI gene score). Both of these estimates were consistent with the meta-analyzed estimate between observed BMI and AF (age- and sex-adjusted HR 1.05 [1.04-1.06] per kg/m2, p<0.001). Multivariable adjustment did not significantly change findings.
Conclusions
Our data are consistent with a causal relationship between BMI and incident AF. These data support the possibility that public health initiatives targeting primordial prevention of obesity may reduce the incidence of AF.
Keywords: fibrillation, epidemiology, genetics, obesity, prevention
Introduction
Atrial fibrillation (AF) is the most common heart rhythm disturbance in the world with an estimated global prevalence of 34 million.1,2 The sheer size of this population represents a major public health burden underscored by the myriad consequences of AF, which include thromboembolic stroke,3 heart failure (HF),4 cognitive dysfunction,5 and increased mortality.6 The incidence of AF also appears to be increasing,7,8 even after accounting for the ageing population,7,9,10 suggesting that the increasing prevalence of other AF risk factors may be driving at least part of this observed trend.
Epidemic increases in the prevalence of obesity have coincided with the expanding global burden of AF,8,11 and consistent associations between obesity and incident AF have been reported in several prospective cohort studies.12-17 Longitudinal changes in body mass index (BMI) have been associated with concordant change in AF risk,15,16 further suggesting a potential to modify AF risk with weight interventions. Given that approximately one third of the world’s population is estimated to be overweight or obese,11 targeting obesity – through both preventive and weight reduction strategies – has the potential to greatly lower the incidence of AF if the observed associations between BMI and AF are causal.18 However, it is not possible to establish causality solely on the basis of observational analyses, which remain vulnerable to residual confounding, reverse causation, and bias.19 To date, there have been no ‘gold-standard’ randomized controlled trials of obesity interventions for the primary prevention of AF.
In this context, Mendelian randomization analysis – using genetic instruments which are randomly assigned during meiosis and therefore unlikely to be related to potential confounders – is an alternative approach that can be used to infer the extent of causality between a proposed risk factor and outcome (e.g. BMI and incident AF).20 Therefore, in our study, we employ Mendelian randomization techniques to test the hypothesis that the known positive observational relationship between BMI and incident AF is causal.
Methods
Study Population
The study population was drawn from seven prospective cohorts studies conducted in the United States and Europe: the Age, Gene/Environment Susceptibility Reykjavik Study (AGES), the Atherosclerosis Risk in Communities (ARIC) study, the Framingham Heart Study (FHS), the Prevention of Renal and Vascular End-stage Disease (PREVEND) study, the Rotterdam Study (RS-I and RS-II), and the Women’s Genome Healthy Study (WGHS). Detailed information for the participating cohorts is provided in the Supplemental Material. Inclusion criteria for our study required complete genome-wide genotyping data and European ancestry verified by principle components analysis. Exclusion criteria included the presence of AF at enrollment and missing covariates of interest. The Institutional Review Boards at each participating institution approved the individual studies and study participants provided written informed consent.
Assessment of AF
AF was assessed using cohort-specific methodology including physician-adjudication, electrocardiography, and diagnosis codes for AF or flutter (ICD-9-CM 427.3, 427.31 or 427.32; ICD-10 I48) present in hospitalization discharges or death certificates. Further details of AF ascertainment in each cohort are available in the Supplemental Material.
Assessment of BMI and Covariates
BMI was measured at study baseline and calculated as the ratio of weight (kg) and height squared (m2). Anthropomorphic measures were assessed directly in all studies with the exception of WGHS, in which measures were assessed using a questionnaire.21,22 Other covariates relevant to incident AF including self-reported smoking history and alcohol consumption, systolic and diastolic blood pressure, anti-hypertensive medication use, height, and history of medical comorbidities (diabetes, HF, coronary heart disease) were collected according to study-specific protocols (see Supplemental Material). Protocols for covariate ascertainment and definitions were comparable across studies.
Genotyping and Genetic Risk Score (BMI Gene Score)
Genotyping methodology within the AFGen consortium has been described elsewhere, and an overview is provided in the Supplemental Material (Supplemental Table 1).23 We first derived a genetic instrument using the FTO single-nucleotide polymorphism (SNP; rs1558902) given that this locus demonstrated the strongest association with BMI in previous genetic analyses.24 To improve the precision of instrumental variable estimates,25 we then selected 38 additional SNPs previously validated in replication cohorts to be significantly associated with BMI at genome-wide significance (Supplemental Table 2).24,26 A weighted genetic score (BMI gene score) comprised of these 39 SNPs was constructed for each individual by summing the number of inherited BMI-increasing alleles of each SNP weighted by their effect size.27
Statistical Analysis
The FTO SNP (rs1558902) and the BMI gene score were used as genetic instrumental variables for all analyses, and measures of BMI taken at study baseline were used as the observational exposure variable. Follow-up time was defined from study baseline until the first occurrence of AF, death, loss to follow-up, or end of study period. Within each cohort, the association of both instrumental variables (FTO, BMI gene score) with BMI was assessed using linear regression. Cox proportional hazards models were used to evaluate the observational association between BMI at study enrollment and incident AF. Cox models were also used to estimate the relative risk of incident AF associated with both instruments (BMI gene score and FTO). All models were initially adjusted for age and sex. Given that the validated SNPs associated with BMI at genome-wide significance were identified in models adjusted for age and age-squared,24,26 instrument-BMI models were additionally adjusted for age-squared. Subsequently, the following covariates were added to this model in a sequential fashion. Model 2 additionally adjusted for smoking status and alcohol intake. Model 3 additionally adjusted for comorbidities that may either mediate or confound the BMI-AF relationship including hypertension (systolic and diastolic blood pressure, anti-hypertensive medication use), diabetes mellitus, and history of HF or coronary heart disease at study entry. The final model (Model 4) further adjusted for height to account for the known effects of height on AF risk, distinct from its implicit contribution to BMI.28,29 In the FHS cohort, incident analyses were performed using Cox proportional hazards regression with a robust variance estimator clustering by family to adjust for relatedness. In the ARIC cohort, as participants were enrolled at more than one site, all models were additionally adjusted for study site. To assess if the associations between the genetic instruments and AF were mediated by BMI, instrument-AF models were adjusted for BMI measured at study baseline. There were no violations of the proportional hazards assumption in all models.
A causal hazard ratio for the association of BMI with incident AF was derived using the Wald-type estimator with standard errors estimated by the delta method.30,31 Briefly, the Wald estimator is the ratio of the log of the hazard ratio of the genetic instrument-AF association and the linear regression coefficient of the genetic instrument -BMI association. Exponentiation of this ratio yields a hazard ratio estimating the causal relationship between BMI and incident AF. Cohort-specific estimates were pooled using random-effects, inverse variance weighted meta-analysis. The pooled effect estimates for the BMI gene score were derived per 1-unit change and additionally reported per 1-standard deviation change, using a global standard deviation derived as the square root of the sample-size weighted mean of cohort-specific squared standard deviations. Heterogeneity was assessed with the I2 and Cochrane Q statistics (Qp),32 and potential sources of heterogeneity were explored with meta-regression.33,34 A Wald test was used to assess the significance of the difference between the observational and instrumental variable estimates. To assess the stability of estimating the cohort-specific standard errors of the Wald estimator, the overall instrumental estimate was also constructed with an alternative strategy using initial meta-analysis of the instrument:BMI and instrument:AF associations across cohorts followed by derivation of the Wald estimator. As results did not differ significantly between these approaches (Supplemental Table 4), presentation of the meta-analysis of cohort-specific instrumental variable estimates was prioritized. Statistical analysis was performed using R software version 3.2.1 (R Project for Statistical Computing) or SAS version 9.3 (SAS Institute, Cary, NC). A 2-tailed P < 0.05 was considered to indicate statistical significance.
Results
Study Cohorts
Baseline characteristics for the seven contributing populations totaling 51 646 participants free of AF at baseline are shown in Table 1. The mean age at study enrollment ranged from 49 to 76 years whereas mean BMI at study enrollment ranged from 25.9 to 27.2 kg/m2. Overall, more women than men were included (N=37 430; 72% of study cohort). Cohort-specific mean follow-up ranged 7.4 to 19.2 years, over which period there were a total of 4178 cases of incident AF.
Table 1.
Baseline Characteristics Study Cohorts
| AGES (n=2953) |
ARIC (n=9276) |
FHS (n=7509) |
PREVEND (n=3515) |
RS-I (n=5729) |
RS-II (n=2087) |
WGHS (n=20577) |
|
|---|---|---|---|---|---|---|---|
| Age, years | 76 (5) | 54 (6) | 51 (15) | 49 (12) | 69 (9) | 65 (8) | 54 (7) |
| Men | 1341 (42%) | 4356 (47%) | 3459 (46%) | 1809 (51%) | 2302 (40%) | 949 (46%) | - |
| Height, cm | 167 (9) | 169 (9) | 169 (10) | 174 (9) | 167 (9) | 170 (9) | 164 (6) |
| Current cigarette smoking | 378 (13%) | 2293 (25%) | 1183 (16%) | 1235 (35%) | 1324 (23%) | 470 (23%) | 2315 (11%) |
| Body mass index, kg/m2 | 27.1 (4.4) | 27.0 (4.9) | 27.0 (5.1) | 26.1 (4.2) | 26.3 (3.7) | 27.2 (4.0) | 25.9 (4.9) |
| SBP, mmHg | 143 (20) | 118 (17) | 123 (18) | 129 (20) | 139 (22) | 143 (21) | 123 (14) |
| DBP, mmHg | 74 (10) | 72 (10) | 75 (10) | 74 (10) | 74 (11) | 79 (11) | 77 (9) |
| Hypertensive medication | 1834 (62%) | 2344 (25%) | 1578 (21%) | 416 (14%) | 1730 (30%) | 566 (27%) | 2610 (13%) |
| Diabetes mellitus | 326 (11%) | 795 (9%) | 443 (6%) | 133 (4%) | 571 (10%) | 219 (11%) | 497 (2%) |
| Previous heart failure | 49 (2%) | 334 (4%) | 40 (0.5%) | 6 (0.2%) | 139 (2%) | 18 (1%) | - |
| Previous coronary heart disease | 495 (17%) | 454 (5%) | 419 (6%) | 28 (1%) | 431 (8%) | 128 (6%) | - |
| EtOH Consumption ≥ 2 drinks/day | 23 (1%) | 594 (6%) | 7243 (96%)* | 943 (27%)† | 916 (16%) | 390 (19%) | 838 (4%) |
| Body mass index gene score | 4.1 (0.5) | 4.1 (0.5) | 4.1 (0.5) | 4.1 (0.5) | 4.1 (0.5) | 4.1 (0.5) | 4.1 (0.5) |
| FTO variant (rs1558902) CAF, % | 40 | 41 | 42 | 40 | 38 | 40 | 41 |
| # Atrial fibrillation cases | 422 (14%) | 1373 (15%) | 555 (7%) | 113 (3%) | 693 (12%) | 80 (4%) | 942 (5%) |
| Mean follow-up time, years | 7.4 (2.5) | 19.2 (5.5) | 8.4 (3.2) | 9.6 (2.3) | 12.7 (5.7) | 8.4 (1.9) | 18.0 (3.3) |
| Baseline years | 2002-2006 | 1987-1989 | 1987-2007 | 1997-1998 | 1990-93 | 2000-01 | 1992 - 1995 |
| End of follow-up | 2012 | 2010 | 2011 | 2008 | 2010 | 2010 | 2011 |
Values correspond to N (%) or mean (standard deviation). Body mass index (BMI) gene score reflects a weighted sum of BMI increasing alleles of 39 single nucleotide polymorphisms (see Methods).
>0 drinks/day;
≥1 drink/day.
AGES, indicates the Age, Gene/Environment Susceptibility—Reykjavik study; ARIC, Atherosclerosis Risk in Communities; FHS, Framingham Heart Study; PREVEND, Prevention of Renal and Vascular End-Stage Disease; RS, Rotterdam Study; WGHS, Women's Genome Health Study; SBP, systolic blood pressure; DBP, diastolic blood pressure; EtOH, alcohol; CAF, coded allele frequency for FTO SNP (rs1558902) associated with increasing body mass index.
BMI and Incident AF: Observational Risk Estimates
In observational analysis, increasing BMI was associated with uniformly significant increased risk of incident AF. Study-specific increments in risk ranged from 3 to 6% per 1 kg/m2 increase in BMI (Table 2). In age- and sex-adjusted analysis, the pooled hazard ratio for incident AF was 1.05 per kg/m2 [95% CI: 1.04-1.06] (p<0.001) without significant heterogeneity (I2 = 24.1%, Qp = 0.37). Additional adjustment for potential confounders (Model 2: smoking, alcohol use), potential intermediaries in the causal pathway between BMI and AF (Model 3: hypertension, diabetes mellitus, HF, coronary heart disease), and height (Model 4) minimally changed the association between BMI and AF (Model 4: HR 1.04 [95% CI: 1.03-1.05], p<0.001; I2 = 0%, Qp = 0.78) (Table 2).
Table 2.
Association of BMI and Incident AF: Observational Estimates
|
Model 1
(HR, 95% CI) |
Model 2
(HR, 95% CI) |
Model 3
(HR, 95% CI) |
Model 4
(HR, 95% CI) |
|
|---|---|---|---|---|
| Per1 kg/m2 ↑ BMI | ||||
|
| ||||
| AGES | 1.03 (1.01-1.05) | 1.03 (1.01-1.06) | 1.03 (1.01-1.05) | 1.03 (1.01-1.05) |
| ARIC | 1.06 (1.05-1.07) | 1.07 (1.06-1.08) | 1.05 (1.03-1.06) | 1.05 (1.04-1.06) |
| FHS | 1.05 (1.03-1.06) | 1.05 (1.03-1.07) | 1.04 (1.02-1.06) | 1.04 (1.02-1.06) |
| PREVEND | 1.06 (1.01-1.10) | 1.05 (1.01-1.10) | 1.05 (1.00-1.11) | 1.06 (1.00-1.12) |
| RS-I | 1.05 (1.03-1.07) | 1.04 (1.02-1.07) | 1.03 (1.00-1.05) | 1.04 (1.01-1.06) |
| RS-II | 1.05 (1.00-1.11) | 1.05 (1.00-1.11) | 1.03 (0.97-1.11) | 1.03 (0.97-1.10) |
| WGHS | 1.05 (1.04-1.06) | 1.05 (1.04-1.06) | 1.03 (1.02-1.05) | 1.04 (1.03-1.05) |
|
| ||||
| Meta-Analysis | ||||
| Observational estimate | 1.05 (1.04-1.06) | 1.05 (1.04-1.06) | 1.04 (1.03-1.05) | 1.04 (1.03-1.05) |
| Test for overall effect Heterogeneity [I2, Qp] |
p<0.001 [24%, 0.37] |
p<0.001 [47.5%, 0.10] |
p<0.001 [5.1%, 0.68] |
p<0.001 [0%, 0.78] |
I2 reflects heterogeneity between studies with higher values reflecting greater heterogeneity. Qp reflects Cochran’s Q statistic, a test for heterogeneity. AF, atrial fibrillation; BMI, body mass index; HR, hazard ratio; CI, confidence interval; AGES: Age, Gene/Environment Susceptibility—Reykjavik study; ARIC, Atherosclerosis Risk in Communities; FHS, Framingham Heart Study; PREVEND, Prevention of Renal and Vascular End-stage Disease; RS, Rotterdam Study; WGHS, Women's Genome Health Study. Model 1: adjustment for age and sex. Model 2: Model 1 + smoking status, alcohol intake. Model 3: Model 2 + potential mediators of BMI-AF association (systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, diabetes, previous coronary heart disease and previous heart failure). Model 4: Model 3 + height.
Genetic Instruments and BMI
Cohort-specific genetic instruments (FTO, BMI gene score) are summarized in Table 1. The coded allele frequency (CAF) for the FTO SNP (rs1558902) associated with increasing BMI was comparable across studies (range: 38-42%). The BMI gene score had a cohort-specific mean of 4.1±0.5 in all cohorts. In age- and sex-adjusted analysis, each instrument was associated with significant increase in BMI with effect sizes ranging 0.21 to 0.55 kg/m2 per A-allele of the FTO SNP and 0.78 to 1.25 kg/m2 per 1-unit increase in BMI gene score (p < 0.001 for both meta-analyzed instrument-BMI associations; Figure 1). The association between both instruments and BMI demonstrated moderate heterogeneity across studies (FTO: I2 = 67.9%, Qp < 0.01; BMI gene score: I2 = 72.3%, Qp <0.01 in age- and sex-adjusted models) with greater instrument-BMI effect size in cohorts with younger populations (p, for association of mean cohort age and instrument-BMI effect size = 0.01 (FTO) and 0.004 (BMI gene score); Supplemental Figure 1). Heterogeneity was substantially attenuated following meta-regression accounting for differences in mean age between cohorts (residual heterogeneity: FTO: I2 = 37.5%, Qp = 0.17; BMI gene score: I2 = 37.5%, Qp = 0.16 in age- and sex-adjusted models). There was no material change in the instrument-BMI effect size in multivariable-adjusted models (Supplemental Figure 2) and moderate heterogeneity in these models was similarly attenuated in meta-regression accounting for mean cohort age (Supplemental Table 3).
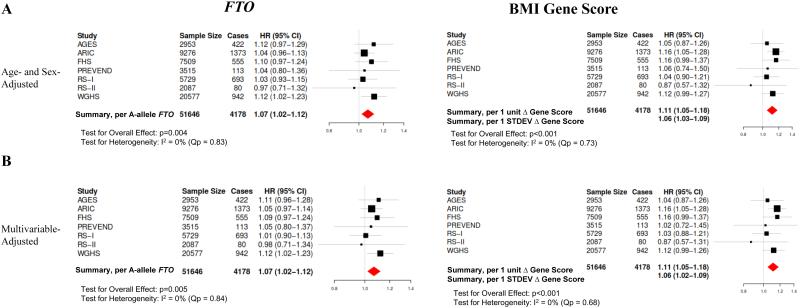
Figure 1. Association between Genetic Instruments and BMI.
Study-specific and meta-analyzed pooled associations between each genetic instrument (FTO, BMI gene score) and BMI (kg/m2) are shown, adjusted for age, age-squared, and sex. Effect estimates are per A-allele of FTO or change in BMI gene score (1-unit increase and 1 STDEV change). I2 reflects heterogeneity across studies with greater values reflecting greater heterogeneity. Qp reflects Cochran’s Q statistic, a test for heterogeneity. BMI, body mass index. STDEV, standard deviation.
Genetic Instruments and Incident AF
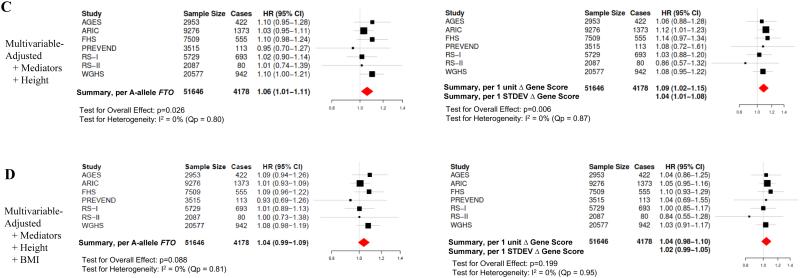
We next examined the relationship between the genetic instruments and incident AF. Study-specific associations and pooled estimates from meta-analysis between both instruments (FTO, BMI gene score) and incident AF are shown in Figure 2. In meta-analysis of age- and sex-adjusted models, increase in both instruments was associated with a significantly increased risk of incident AF (FTO: HR 1.07 [95% CI: 1.02-1.12] per A-allele of the FTO SNP, p=0.004); BMI gene score: HR 1.11 [95% CI: 1.05-1.18] per 1-unit increase in BMI gene score, p<0.001) with minimal heterogeneity across studies (FTO: I2 = 0%, Qp=0.83; BMI gene score: I2 = 0%, Qp=0.73). Adjustment for potential confounders, potential causal intermediaries, and height did not meaningfully change the instrument-AF associations (Figure 2) with minimal heterogeneity across studies in adjusted analyses (Supplemental Table 3). In contrast, adjustment of the instrument-AF associations for BMI measured at study baseline yielded non-significant associations between the genetic instruments and incident AF in all models, consistent with mediation of the instrument-AF association by BMI (Figure 2D; Supplemental Figure 3).
Figure 2. Association between Genetic Instruments and AF.
Shown are study-specific and meta-analyzed pooled estimates for the associations between each genetic instrument (FTO, BMI gene score) and risk of incident AF. Effect estimates are per A-allele of FTO or change in BMI gene score (1-unit increase and 1 STDEV change). (A) Shown are age- and sex-adjusted associations (Model 1) as well as (B) multivariable models which include additional adjustment for smoking status and alcohol intake (Model 2). (C) Multivariable models were subsequently adjusted for possible mediators of the BMI-AF association (systolic and diastolic blood pressure, anti-hypertensive medication use, diabetes mellitus, previous heart failure or coronary heart disease) as well as height (Model 4). (D) Finally, to assess if the genetic instrument-AF association was mediated by BMI, models were adjusted for BMI measured at the time of cohort baseline. I2 reflects heterogeneity across studies with greater values reflecting greater heterogeneity. Qp reflects Cochran’s Q statistic, a test for heterogeneity. BMI; body mass index; STDEV, standard deviation.
BMI and Incident AF: Causal Inference Using Instrumental Variable Analysis
Given the separate and significant associations of the genetic instruments with BMI and incident AF, we combined these effects to derive an instrumental variable estimate of the association between BMI and AF for each cohort (Table 3). In meta-analysis of instrumental variable estimates from age- and sex-adjusted models, each 1 kg/m2 increase in BMI was associated with a significantly increased risk of incident AF for both FTO (HR 1.15 [95% CI: 1.05-1.27], p=0.004) and the BMI gene score (HR 1.11 [95% CI: 1.05-1.17], p<0.001) with minimal heterogeneity across studies (FTO: I2=0%, Qp=0.91; BMI gene score: I2=0%, Qp=0.91). Both of these estimates were numerically larger than the observational estimates from age- and sex-adjusted models (HR=1.05 [95% CI: 1.04-1.06] per 1 kg/m2 increase; Table 2) although not statistically significantly different (p, for comparison of instrumental vs. observational estimates: FTO: 0.063, BMI gene score: 0.061). Adjustment for confounders, potential causal intermediaries, and height did not change the instrumental estimates (Table 3). To assess the robustness of our approach, we derived the instrumental estimates of the BMI-AF association using study-combined estimates of the genetic instrument-BMI and genetic-instrument-AF associations. The estimated causal effects of BMI and incident AF with this alternative approach were similar to meta-analysis of study-specific instrumental variable estimates (Supplemental Table 4).
Table 3.
Association of BMI and Incident AF: Instrumental Variable Estimates
|
Model 1
(HR, 95% CI) |
Model 2
(HR, 95% CI) |
Model 3
(HR, 95% CI) |
Model 4
(HR, 95% CI) |
|
|---|---|---|---|---|
| Instrument = FTO Score Per1 kg/m2 ↑ BMI |
||||
| AGES | 1.37 (0.86-2.17) | 1.30 (0.86-1.96 | 1.33 [0.83-2.13) | 1.36 (0.82-2.24) |
| ARIC | 1.08 (0.93-1.25) | 1.10 (0.95-1.28) | 1.07 (0.90-1.27) | 1.07 (0.90-1.27) |
| FHS | 1.19 (0.95-1.49) | 1.18 (0.94-1.47) | 1.20 (0.94-1.52) | 1.21 (0.94-1.56) |
| PREVEND | 1.12 (0.56-2.24) | 1.13 (0.55-2.33) | 0.80 (0.36-1.80) | 0.86 (0.37-1.96) |
| RS-I | 1.15 (0.68-1.94) | 1.03 (0.64-1.67) | 1.04 (0.58-1.85) | 1.08 (0.59-1.96) |
| RS-II | 0.90 (0.36-2.27) | 0.93 (0.36-2.39) | 1.06 (0.35 -3.21) | 1.05 (0.34-3.23) |
| WGHS | 1.23 (1.03-1.47) | 1.23 (1.03-1.47) | 1.24 (0.99-1.56) | 1.25 (0.99-1.57) |
|
| ||||
| Meta-Analysis | ||||
| IV Estimate | 1.15 (1.05-1.27) | 1.15 (1.05-1.27) | 1.14 (1.02-1.28) | 1.15 (1.03-1.29) |
| Test for overall effect Heterogeneity [I2, Qp] |
p=0.004 [0%, 0.91] |
p=0.003 [0%, 0.95] |
p=0.03 [0%, 0.87] |
p=0.019 [0%, 0.88] |
|
| ||||
| Instrument = BMI Gene Score Per1 kg/m2 ↑ BMI |
||||
|
| ||||
| AGES | 1.05 (0.86-1.29) | 1.05 (0.85-1.30) | 1.06 (0.85-1.32) | 1.06 (0.87-1.30) |
| ARIC | 1.13 (1.04-1.23) | 1.13 (1.04-1.23) | 1.10 (1.01-1.21) | 1.10 (1.01-1.21) |
| FHS | 1.14 (0.99-1.30) | 1.13 (0.99-1.30) | 1.13 (0.98-1.31) | 1.12 (0.97-1.30) |
| PREVEND | 1.05 (0.75-1.47) | 1.02 (0.72-1.44) | 1.01 (0.69-1.50) | 1.08 (0.72-1.62) |
| RS-1 | 1.06 (0.88-1.27) | 1.05 (0.84-1.30) | 1.03 (0.82-1.29) | 1.04 (0.83-1.30) |
| RS-2 | 0.84 (0.50-1.43) | 0.84 (0.50-1.41) | 0.84 (0.47-1.50) | 0.82 (0.46-1.47) |
| WGHS | 1.10 (0.99-1.21) | 1.09 (0.99-1.21) | 1.08 (0.96-1.21) | 1.07 (0.95-1.20) |
|
| ||||
| Meta-Analysis | ||||
| IV Estimate | 1.11 (1.05-1.17) | 1.10 (1.05-1.17) | 1.09 (1.03-1.15) | 1.09 (1.03-1.15) |
| Test for overall effect Heterogeneity [I2, Qp] |
p<0.001 [0%, 0.91] |
p<0.001 [0%, 0.89] |
p=0.004 [0%, 0.96] |
p=0.004 [0%, 0.96] |
I2 reflects heterogeneity between studies with higher values reflecting greater heterogeneity. Qp reflects Cochran’s Q statistic, a test for heterogeneity. AF, atrial fibrillation; BMI, body mass index; HR, hazard ratio; CI, confidence interval; AGES: Age, Gene/Environment Susceptibility—Reykjavik study; ARIC, Atherosclerosis Risk in Communities; FHS, Framingham Heart Study; PREVEND, Prevention of Renal and Vascular End-stage Disease; RS, Rotterdam Study; WGHS, Women's Genome Health Study. IV, instrumental variable. Model 1: adjustment for age and sex. Model 2: Model 1 + smoking status, alcohol intake. Model 3: Model 2 + potential mediators of BMI-AF association (systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, diabetes, previous coronary heart disease and previous heart failure). Model 4: Model 3 + height.
Discussion
In our prospective cohorts of over 50 000 individuals without AF at baseline, genetic variants associated with increasing BMI were significantly associated with incident AF. We show that measured BMI mediates at least a portion of the effect of these genetic variants on incident AF, whereas accounting for other AF risk factors had no impact on the genetic instrument-AF risk estimates. Taken together, these data support a causal relationship between obesity and incident AF and further suggest an important role for obesity-targeted public health interventions to combat the expanding global epidemic of AF.
There are several lines of evidence from observational and small-scale randomized studies in support of a causal relationship between obesity and AF.35 First, strong and consistent dose response relationships between BMI and incident AF have been documented in several large-scale prospective cohort studies (~3-5% increased risk of AF per kg/m2 increase in BMI).12,13,15,17,35,36 A remarkably similar association was identified in meta-analysis of observational estimates in the present study. Second, prospective studies have shown an association between longitudinal reductions in BMI and decreased incident AF.15,16 Third, among patients with established AF, sustained weight loss has been associated with reductions in AF in both observational studies37,38 and randomized trials.18 For example, in the recently reported LEGACY-AF (Long-Term Effect of Goal Directed Weight Management on Atrial Fibrillation Cohort) study of obese patients (BMI ≥27 kg/m2) with AF, weight loss of ≥10% was associated with a 6-fold decrease in AF recurrence at 5 years.38 Extending these observational findings, a recent small, randomized controlled trial in 150 obese patients with AF demonstrated significant reductions in AF burden and symptom severity scores with weight-loss intervention compared to general lifestyle advice.18 In contrast, the efficacy of lifestyle intervention for the primary prevention of AF is less well established. For example, randomization of obese individuals with diabetes mellitus to an intensive lifestyle intervention was not associated with a reduced incidence of AF in the presence of modest weight loss.39
Compelling as these data may be, observational risk estimates of the BMI-AF relationship are intrinsically subject to important limitations in causal inference. First, all observational studies are vulnerable to the risk of residual confounding which may bias estimates. More specifically, participants who are overweight or who do not lose weight likely differ from those who maintain or attain a normal BMI in many other respects that may be related to AF. By comparison, the use of BMI-associated genotypes which are randomly assigned at meiosis significantly attenuates this source of bias. For example, the FTO SNP and the genetic variants utilized in our BMI gene score have been previously shown to be unrelated to known cardiovascular disease risk factors which may confound the relationship between BMI and AF.40 Second, observational risk estimates associated with obesity and weight loss are subject to ‘reverse causation’ bias in which subclinical manifestations of the disease of interest (eg. AF) may lead to illness-induced weight change.41 In our study, use of genetic instruments, which are immutable and randomly allocated at conception, almost entirely eliminates this potential source of bias.
Third, observational studies which account for cumulative BMI exposure have identified greater cardiovascular risk estimates when compared to cross-sectional BMI assessment.42-45 Taken further, BMI-associated genetic variants may better reflect lifetime exposure to obesity (i.e. ‘area under the BMI curve’) when compared to a one-time measure or even repeated measures of BMI recorded during cohort studies.46 This might account for the numerically, albeit non-significantly, greater estimated causal effect of BMI on incident AF we obtained using genetic instruments compared to observational estimates. Similar discordance in instrumental and observational estimates has been reported for the association between BMI-increasing genetic variants and coronary disease risk.40 Alternatively, pleiotropy – in which the selected genetic variants mediate AF risk through a non-BMI pathway – might account for some of the discordance in risk estimates.46 The association between the genetic instruments and AF, however, remained significant after adjustment for several possible pleiotropic mediators including hypertension and coronary heart disease. In addition, our finding that adjustment for observed BMI nullified the statistical association between the genetic instruments and AF supports BMI as the causal mediator of the association between the genetic instruments and incident AF, and argues against a major contribution from pleiotropic effects.
There are several potential mechanisms which may underlie a causal relationship between BMI and incident AF. Obesity has been linked to both cardiac structural pathology and systemic processes associated with the development of AF.35 For example, obesity has been associated with left atrial enlargement and left ventricular diastolic impairment, both of which predispose to AF.13,18 Similarly, obesity has been associated with a systemic inflammatory state47 which, in turn, has been associated with an increased risk of AF.48,49 More proximate to the anatomic substrate of AF, increasing BMI has also been associated with epicardial fat which is a risk factor for incident AF in community cohorts.50,51 Epicardial fat may directly modify the electrical atrial substrate through local perturbation of autonomic balance,52 elaboration of pro-fibrotic adipokines, and induction of oxidative stress pathways implicated in AF pathogenesis.53,54 Weight loss has been associated with improvements in diastolic function,18 decrease in systemic inflammatory markers,38 and a decline in pericardial fat volume,50 each reflecting possible mechanisms of the salutary effects of obesity interventions in AF. Finally, increased BMI is associated with incident cardiovascular disease that may further increase the risk of AF (eg. hypertension, HF, myocardial infarction).8,55
Despite major advances in stroke prophylaxis56 and ablative therapy,57 AF remains a significant cause of morbidity and mortality.58 To the extent that obesity is causally associated with AF as suggested by our study, the impact of obesity-focused public health interventions for the primary prevention of AF could be quite substantial.59 Observational studies have estimated that up to 12-18% of AF may be attributable to obesity, 8,15,35,60,61 and recent data suggest that the attributable risk associated with obesity has increased over the past 50 years in conjunction with the rising prevalence of obesity.8 Our data also support a potential cumulative impact of BMI exposure on AF15,62 which might serve to reinforce the importance of durable weight maintenance after obesity interventions63 and place further emphasis on the primordial prevention of obesity.64-66
Limitations
The results of our study should be interpreted in the context of its study design. Causal inference using Mendelian randomization is predicated on several specific design criteria including consistent genotype-to-exposure effect estimates.67 In our study, the magnitude of the significant associations between genetic instruments and increasing BMI were moderately heterogeneous and stronger in cohorts with a lower mean age. BMI at older ages is more likely to be influenced by the development of comorbid illness, which may confound the BMI-AF association and decrease the specificity of BMI as a pathophysiological surrogate of AF risk (eg. for adiposity, epicardial fat). Second, AF ascertainment was not systematically harmonized across studies, although the large sample size (> 50 000 participants) augmented the precision of effect estimates, and associations between observational and genetic instruments with AF were consistent across cohorts. Third, genetic variants associated with BMI are known to explain only a minority of the variation in BMI,24 thus decreasing the power of these instruments to detect significant exposure-outcome association and potentially inflating bias related to violations of instrumental variable assumptions (e.g. absent pleiotropy).68 The use of a weighted genetic score, as employed in our study, mitigated both of these risks.27 Fourth, additional SNPs not included in our gene score have been recently associated with BMI in GWAS.69 Given the reported per allele effect estimates, we would estimate that these SNPs in toto would account for no more than 0.6% of the variance in BMI (assuming an additive contribution) and therefore inclusion of these identified gene variants would be unlikely to alter the findings of our study. Finally, our study included participants of European ancestry, and therefore our findings may not be generalizable to non-European individuals.
Conclusion
In summary, in this Mendelian randomization study of over 50 000 individuals of European ancestry, genetic variants associated with BMI were significantly associated with an increased risk of incident AF and instrumental variable analysis supported a causal relationship between BMI and AF. These data augment support for the primordial prevention of obesity as a public health target to combat the expanding global burden of AF.
Supplementary Material
Clinical Perspective.
What Is New?
Mendelian randomization utilizes genetic variants associated with a proposed risk factor to infer the causal association between a risk factor and an outcome.
In this study of more than 50,000 European individuals without atrial fibrillation (AF) at baseline, genetic variants associated with increasing body mass index (BMI) were significantly associated with an increased risk of AF.
The association between genetically determined obesity and AF persisted even after adjustment for traditional AF risk factors including hypertension, diabetes mellitus, coronary artery disease, and heart failure.
Taken together, these data are consistent with a causal association between increasing BMI and AF.
What Are the Clinical Implications?
Our findings augment support for the primordial prevention of obesity as a significant public health target to combat the expanding global burden of AF.
Our findings also highlight the potential impact of cumulative exposure to obesity and risk of AF which, in turn, has implications for both the timing and durability of obesity interventions.
Acknowledgments
Funding
Dr. Alonso is supported by American Heart Association grant 16EIA26410001. Dr. Chatterjee is supported by NHLBI T-32 HL-007575 (Bethesda, Maryland). Dr. Ellinor is supported by an Established Investigator Award from the American Heart Association (13EIA14220013) and by the Foundation Leducq (14CVD01). Dr. Lubitz is supported by NIH grant K23HL114724 and a Doris Duke Charitable Foundation Clinical Scientist Development Award 2014105. Dr. Rienstra is supported by a grant from the Netherlands Organization for Scientific Research (Veni grant 016·136·055).
AGES: The Age, Gene/Environment Susceptibility-Reykjavik Study was supported by National Institutes of Health (contracts N01-AG-12100 and HHSN271201200022C), the National Institute on Aging Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). ARIC: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. The authors thank the staff and participants of the ARIC study for their important contributions. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. FHS: The Framingham Heart Study is supported by National Heart, Lung, and Blood Institute contracts HHSN268201500001I; N01-HC 25195; 2R01HL092577; 1R01HL128914; completed 1R01 HL102214; 1RC1HL101056; K24HL105780. PREVEND: The Prevention of Renal and Vascular End-Stage Disease study is supported by the Dutch Kidney Foundation (grant E0.13) and the Netherlands Heart Foundation (NHS2010B280). Rotterdam Study: The Rotterdam Study is supported by the Erasmus MC University Medical Center, the Netherlands Organization for Scientific Research (NWO), the Netherlands Organization for Health Research and Development, the Netherlands Genomics Initiative, and the municipality of Rotterdam. The authors thank the staff and participants from the Ommoord district. WGHS: The Women’s Genome Health Study and AF endpoint confirmation was supported by HL043851, HL080467, HL093613, and HL099355 (completed) from the National Heart, Lung, and Blood Institute, CA047988 (completed) and UM1CA182913 from the National Cancer Institute, and the Watkin’s Foundation, with collaborative scientific support and funding for genotyping provided by Amgen.
Footnotes
Disclosures: Dr. Ellinor is the principle investigator on a grant from Bayer HealthCare to the Broad Institute focused on the genetics and therapeutics of atrial fibrillation.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 5.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke. 1997;28(2):316–321. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 7.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 8.Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsang TS, Petty GW, Barnes ME, et al. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol. 2003;42(1):93–100. doi: 10.1016/s0735-1097(03)00500-x. [DOI] [PubMed] [Google Scholar]
- 10.Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D'Agostino RB. Secular trends in the prevalence of atrial fibrillation: The Framingham Study. Am Heart J. 1996;131(4):790–795. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 11.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32(9):1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 12.Vermond RA, Geelhoed B, Verweij N, et al. Incidence of Atrial Fibrillation and Relationship With Cardiovascular Events, Heart Failure, and Mortality: A Community-Based Study From the Netherlands. J Am Coll Cardiol. 2015;66(9):1000–1007. doi: 10.1016/j.jacc.2015.06.1314. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 14.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: The Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–495. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Tedrow UB, Conen D, Ridker PM, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS. J Am Coll Cardiol. 2010;55(21):2319–2327. doi: 10.1016/j.jacc.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huxley RR, Misialek JR, Agarwal SK, et al. Physical activity, obesity, weight change, and risk of atrial fibrillation: the atherosclerosis risk in communities study. Circulation Arrhythm Electrophysiol. 2014;7(4):620–625. doi: 10.1161/CIRCEP.113.001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azarbal F, Stefanick ML, Salmoirago-Blotcher E, et al. Obesity, physical activity, and their interaction in incident atrial fibrillation in postmenopausal women. J Am Heart Assoc. 2014;3(4):e001127. doi: 10.1161/JAHA.114.001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310(19):2050–2060. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 19.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad T, Lee IM, Pare G, et al. Lifestyle interaction with fat mass and obesity-associated (FTO) genotype and risk of obesity in apparently healthy U.S. women. Diabetes Care. 2011;34(3):675–680. doi: 10.2337/dc10-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi Q, Chu AY, Kang JH, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367(15):1387–1396. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinner MF, Tucker NR, Lunetta KL, et al. Integrating genetic, transcriptional, and functional analyses to identify 5 novel genes for atrial fibrillation. Circulation. 2014;130(15):1225–1235. doi: 10.1161/CIRCULATIONAHA.114.009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer TM, Lawlor DA, Harbord RM, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21(3):223–242. doi: 10.1177/0962280210394459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berndt SI, Gustafsson S, Magi R, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. 2013;45(5):501–512. doi: 10.1038/ng.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies NM, von Hinke Kessler Scholder S, Farbmacher H, Burgess S, Windmeijer F, Smith GD. The many weak instruments problem and Mendelian randomization. Stat Med. 2015;34(3):454–468. doi: 10.1002/sim.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg MA, Kaplan RC, Siscovick DS, et al. Genetic variants related to height and risk of atrial fibrillation: the cardiovascular health study. Am J Epidemiol. 2014;180(2):215–222. doi: 10.1093/aje/kwu126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg MA, Patton KK, Sotoodehnia N, et al. The impact of height on the risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J. 2012;33(21):2709–2717. doi: 10.1093/eurheartj/ehs301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas DC, Conti DV. Commentary: the concept of 'Mendelian Randomization'. Int J Epidemiol. 2004;33(1):21–25. doi: 10.1093/ije/dyh048. [DOI] [PubMed] [Google Scholar]
- 31.Palmer TM, Sterne JA, Harbord RM, et al. Instrumental variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am J Epideiol. 2011;173(12):1392–1403. doi: 10.1093/aje/kwr026. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 33.Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI. Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract. 2009;63(10):1426–1434. doi: 10.1111/j.1742-1241.2009.02168.x. [DOI] [PubMed] [Google Scholar]
- 34.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nalliah CJ, Sanders P, Kottkamp H, Kalman JM. The role of obesity in atrial fibrillation. Eur Heart J. 2015;37(20):1565–1572. doi: 10.1093/eurheartj/ehv486. [DOI] [PubMed] [Google Scholar]
- 36.Dublin S, French B, Glazer NL, et al. Risk of new-onset atrial fibrillation in relation to body mass index. Arch Intern Med. 2006;166(21):2322–2328. doi: 10.1001/archinte.166.21.2322. [DOI] [PubMed] [Google Scholar]
- 37.Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64(21):2222–2231. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 38.Pathak RK, Middeldorp ME, Meredith M, et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY) J Am Coll Cardiol. 2015;65(20):2159–2169. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Alonso A, Bahnson JL, Gaussoin SA, et al. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the Look AHEAD randomized trial. Am Heart J. 2015;170(4):770–777. doi: 10.1016/j.ahj.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordestgaard BG, Palmer TM, Benn M, et al. The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a Mendelian randomisation approach. PLoS Med. 2012;9(5):e1001212. doi: 10.1371/journal.pmed.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flegal KM, Graubard BI, Williamson DF, Cooper RS. Reverse causation and illness-related weight loss in observational studies of body weight and mortality. Am J Epidemiol. 2011;173(1):1–9. doi: 10.1093/aje/kwq341. [DOI] [PubMed] [Google Scholar]
- 42.Adams KF, Leitzmann MF, Ballard-Barbash R, et al. Body mass and weight change in adults in relation to mortality risk. Am J Epidemiol. 2014;179(2):135–144. doi: 10.1093/aje/kwt254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 44.Abdullah A, Amin FA, Stoelwinder J, et al. Estimating the risk of cardiovascular disease using an obese-years metric. BMJ. 2014;4(9):e005629. doi: 10.1136/bmjopen-2014-005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdullah A, Wolfe R, Stoelwinder JU, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40(4):985–996. doi: 10.1093/ije/dyr018. [DOI] [PubMed] [Google Scholar]
- 46.Millard LA, Davies NM, Timpson NJ, Tilling K, Flach PA, Davey Smith G. MR-PheWAS: hypothesis prioritization among potential causal effects of body mass index on many outcomes, using Mendelian randomization. Sci Rep. 2015;5:16645. doi: 10.1038/srep16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahajan R, Lau DH, Sanders P. Impact of obesity on cardiac metabolism, fibrosis, and function. Trends Cardiovasc Med. 2015;25(2):119–126. doi: 10.1016/j.tcm.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Schnabel RB, Larson MG, Yamamoto JF, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121(2):200–207. doi: 10.1161/CIRCULATIONAHA.109.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dewland TA, Vittinghoff E, Harris TB, et al. Inflammation as a Mediator of the Association Between Race and Atrial Fibrillation: Results from the Health, Aging, and Body Composition Study. JACC Clinical Electrophysiol. 2015;1(4):248–255. doi: 10.1016/j.jacep.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abed HS, Nelson AJ, Richardson JD, et al. Impact of weight reduction on pericardial adipose tissue and cardiac structure in patients with atrial fibrillation. Am Heart J. 2015;169(5):655–662. doi: 10.1016/j.ahj.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Wong CX, Abed HS, Molaee P, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011;57(17):1745–1751. doi: 10.1016/j.jacc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 52.Balcioglu AS, Cicek D, Akinci S, et al. Arrhythmogenic evidence for epicardial adipose tissue: heart rate variability and turbulence are influenced by epicardial fat thickness. Pacing Clin Electrophysiol. 2015;38(1):99–106. doi: 10.1111/pace.12512. [DOI] [PubMed] [Google Scholar]
- 53.Salgado-Somoza A, Teijeira-Fernandez E, Fernandez AL, Gonzalez-Juanatey JR, Eiras S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol. 2010;299(1):H202–209. doi: 10.1152/ajpheart.00120.2010. [DOI] [PubMed] [Google Scholar]
- 54.Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw045. doi: 10.1093/eurheartj/ehw045. [DOI] [PubMed] [Google Scholar]
- 55.Movahed MR, Lee JZ, Lim WY, Hashemzadeh M, Hashemzadeh M. Strong independent association between obesity and essential hypertension. Clin Obes. 2016;6(3):189–192. doi: 10.1111/cob.12139. [DOI] [PubMed] [Google Scholar]
- 56.Boriani G, Laroche C, Diemberger I, et al. 'Real-world' management and outcomes of patients with paroxysmal vs. non-paroxysmal atrial fibrillation in Europe: the EURObservational Research Programme-Atrial Fibrillation (EORP-AF) General Pilot Registry. Europace. 2016 doi: 10.1093/europace/euv390. [DOI] [PubMed] [Google Scholar]
- 57.Latchamsetty R, Morady F. Catheter Ablation of Atrial Fibrillation. Heart Fail Clin. 2016;12(2):223–233. doi: 10.1016/j.hfc.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 58.Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013. doi: 10.1136/bmj.h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perez MV, Wang PJ, Larson JC, et al. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the Women's Health Initiative Observational Study. Heart. 2013;99(16):1173–1178. doi: 10.1136/heartjnl-2013-303798. [DOI] [PubMed] [Google Scholar]
- 61.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123(14):1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grundvold I, Bodegard J, Nilsson PM, et al. Body weight and risk of atrial fibrillation in 7,169 patients with newly diagnosed type 2 diabetes; an observational study. Cardiovasc Diabetol. 2015;14:5. doi: 10.1186/s12933-014-0170-3. doi: 10.1186/s12933-014-0170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inge TH, Courcoulas AP, Jenkins TM, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med. 2016;374(2):113–123. doi: 10.1056/NEJMoa1506699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faith MS, Stettler N, Pietrobelli A. Engaging Primary Care Clinicians in Early Obesity Prevention Research. JAMA. 2015;314(8):823–824. doi: 10.1001/jama.2015.6262. [DOI] [PubMed] [Google Scholar]
- 65.Lumeng JC, Taveras EM, Birch L, Yanovski SZ. Prevention of obesity in infancy and early childhood: a National Institutes of Health workshop. JAMA Pediatr. 2015;169(5):484–490. doi: 10.1001/jamapediatrics.2014.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Penalvo JL, Santos-Beneit G, Sotos-Prieto M, et al. The SI! Program for Cardiovascular Health Promotion in Early Childhood: A Cluster-Randomized Trial. J Am Coll Cardiol. 2015;66(14):1525–1534. doi: 10.1016/j.jacc.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 67.Nitsch D, Molokhia M, Smeeth L, DeStavola BL, Whittaker JC, Leon DA. Limits to causal inference based on Mendelian randomization: a comparison with randomized controlled trials. Am J Epidemiol. 2006;163(5):397–403. doi: 10.1093/aje/kwj062. [DOI] [PubMed] [Google Scholar]
- 68.Hernan MA, Robins JM. Instruments for causal inference: an epidemiologist's dream? Epidemiology. 2006;17(4):360–372. doi: 10.1097/01.ede.0000222409.00878.37. [DOI] [PubMed] [Google Scholar]
- 69.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.