Abstract
Host immune response leading to fibrosis of implanted biomaterials and devices can significantly limit implant performance and life span. The surface physicochemical properties of implanted materials play an important role in modulating this fibrotic response. Here, we demonstrate the surface modification of model devices with zwitterionic phosphorylcholine polymer through mussel-mimetic catecholamine polymer thin films. Using this method, we successfully modify the surfaces of alginate hydrogel microspheres to reduce the tissue reaction by reducing the fibrosis in immunocompetent C57BL/6J mice We demonstrate that this facile coating method can be extended to other solid biomaterials, such as polystyrene microspheres, a model material known to produce robust foreign body responses and fibrosis, as a platform approach to improve biocompatibility and reduce fibrosis in vivo.
Keywords: alginate, dopamine, fibrosis, foreign body response, zwitterionic polymers
Graphical Abstract
The surface modification of model biomaterials with zwitterionic phosphorylcholine polymer is demonstrated through mussel-mimetic catecholamine polymer thin films. Using this method, the surfaces of alginate hydrogel microspheres are successfully modified to reduce the tissue reaction by reducing the fibrosis in immunocompetent C57BL/6J mice. It is also demonstrated that this facile coating method can be used as a platform approach to improve biocompatibility and reduce fibrosis in vivo.
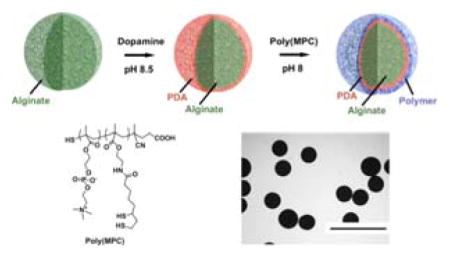
The success of an implanted device is often dependent on a favorable interaction with the host immune system. Implanted materials can elicit a cascade of host immune responses, including acute/chronic inflammation, a foreign body reaction, and formation of a fibrotic capsule around the implant.[1–3] This fibrotic tissue growth can lead to isolation of the implant from the host, compromising device performance and ultimately leading to device failure. Therefore, strategies to mitigate the fibrotic foreign body response are imperative in the design of long-term use of implanted biomaterials and medical devices. Several methods have been reported to modify the implant surface with coatings that have anti-inflammatory properties and/or resist fibrosis.[4–6] Among the many natural and synthetic materials used as coatings for implantable devices, zwitterionic polymers have received much attention as a result of their ultra-low fouling properties.[7–11] Recently, Jiang et al. reported that zwiterionic hydorogels uniquly are able to supress foreign body and fibrotic responses in robust rodent models of fibrosis.[12] However, surface modification of materials can be challenging, requiring surface-specific multi-step protocols. For example, a coating procedure may necessitate a step-wise progression of surface hydrophilization via plasma treatment, silanization to immobilize handles for attachment, followed by surface grafting and/or polymerization to the device surface.[13,14] Therefore, the development of simple and universal coating methods to modify a broad range of surfaces with nonfouling zwitterionic polymers is of great interest.
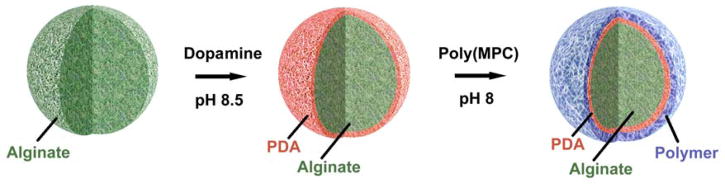
Here we develop a platform approach to coating biomaterial surfaces with anti-biofouling zwitterionic polymers using facile and scalable methodology. We then assess the effect of this coating on in vivo biocompatibility. This approach employs, first, modification of the surface with mussel-inspired polydopamine (PDA) films by oxidative self-polymerization of dopamine, and followed by conjugation of thiol-containing zwitterionic polymers to this PDA layer (Figure 1). [15–17] Although there is a reported one pot approach to immobilize thiol or amine free molecules on PDA layer, we preferred using a thiol containing polymer to be immobilized on PDA layer through micheal addition reaction for stable covalent attachment.[18] Catechol groups have been used as anchoring moiety for various types of natural and synthetic polymers to be coated on surfaces.[19] Among all those polymers used for coating via self-polymerization of dopamine, anti-biofouling zwitterionic polymers constitute a very small percantage. Some of these examples include coating of zwitterrionic polymers onto various substrates such as silica, gold, and iron oxide, and these showed excellent non-fouling properties.[20–22] However, majority of these studies have focused their evaluation of biocompatability in vitro. Despite progress in the development of catechol-mediated zwitterionic surface coatings, evaluation of in vivo biocompatability is important to further assess the potential utility of these coatings.
Figure 1.
Cartoon representation of surface coating of biomaterials (e.g. Alginate microspheres)
Using a dopamine-mediated conjugation method, we attached zwitterionic polymers on to the surface of biomaterials and examined the efficacy of our coating approach to reduce host immune responses and fibrosis to implanted biomaterials in vivo. We conjugated zwitterionic polymers onto the surface of alginate hydrogel microspheres. Alginate, a naturally occurring anionic biopolymer, forms hydrogels in aqueous conditions in the presence of divalent cations such as Ca2+and Ba2+. Commonly prepared as gelled microspheres, alginate has been broadly used as biomaterials for drug delivery, tissue engineering, and cell transplantation.[23] However, following implantation, alginate microspheres can promote the formation of fibrous overgrowth around the microspheres, compromising function of the implant.[24,25] Polycations, such as poly-L-lysine (PLL), are commonly used to coat alginate and other material surfaces, but in general do not block fibrosis.[26,27] A library of cationic poly(β-amino alcohols) was also developed and some members were shown to reduce the immune response to polystyrene microparticles.[5] Using combinatorial methods our group has recently developed a large library of alginate hydrogels and identified chemical modifications that substantially reduce the inflammatory effects of alginate microspheres in non-human primates.[28] However, there continues to be a need to develop covalent surface treatments to reduce the fibrosis of hydrogels.
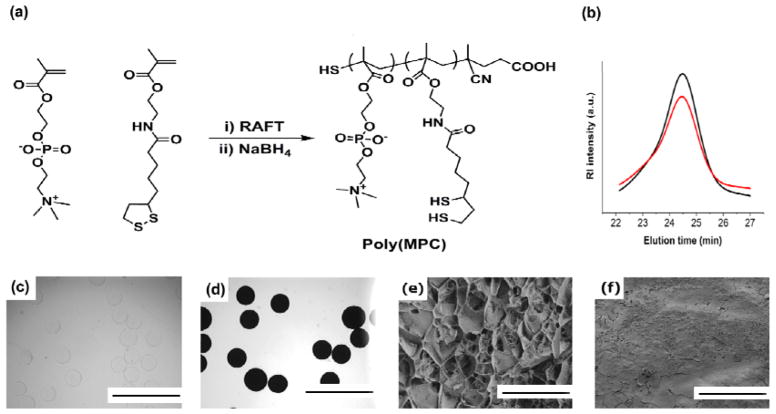
To assess the effect of zwitterionic coatings on the biocompatibility of alginate microspheres, we first synthesized a zwitterionic phosphorylcholine polymer with pendant dithiol-containing comonomers. Phosphorylcholine polymers have several advantages as coating materials, including hydrophilicity, high water solubility and anti-biofouling properties.[29,30] Reversible addition-fragmentation chain transfer (RAFT) polymerization of methacryloyloxyethyl phosphorylcholine (MPC) and lipoic acid methacrylate monomers followed by disulfide reduction yielded poly(MPC) polymer with free pendant thiol groups along the backbone (Figure 2a). GPC analysis showed no change in the polymer molecular weight and/or PDI after reduction (Figure 2b). After successfully synthesizing poly(MPC) copolymers with free thiol groups (Mn: 27 kDa, PDI: 1.3) we next immobilized these polymers onto Ba2+-crosslinked alginate hydrogel microspheres (~0.5 mm diameter, Figure 2c), a size shown to produce higher level of fibrosis in vivo.[31] For our studies, we utilized Barium ions to make alginate beads as it was shown that Barium-alginate beads have higher mechanical stability than Calcium beads.[32] First, alginate microspheres were coated with PDA by immersion for 18–20 hours in a 3 mg/mL dopamine solution prepared in 10 mM Tris buffered saline (pH 8.5), followed by multiple rinses with Tris buffer. PDA coated alginate microspheres were then treated with poly(MPC) polymer in Tris buffer (pH 8.0) at room temperature for 18–24 hours (Figure 2d). Since it was previously reported that polycations such as PLL and dopamine coatings on alginate enhanced the physical stability of alginate micropsheres,[33,34] it is also reasonable to envison that dopamine-zwittterionc coating might exhibit similar mechanical stabilities.
Figure 2.
Surface coating of alginate microspheres with zwitterionic polymers. (a) Synthesis and structure of thiol-containing phosphorylcholine zwitterionic copolymer. (b) Aqueous GPC traces of phosphorylcholine copolymers before (black line) and after (red line) reduction with NaBH4. Bright field microscope images of alginate microspheres (c) before and (d) after surface coating procedure. Scale bars, 2 mm. Representative freeze-fracture cryo-SEM imaging of Alginate (e) and Alginate-MPC (f) microspheres. Scale bars, 10 μm
Cryogenic scanning electron microscopy (cryo-SEM) images of surface coated alginate microspheres (Alg-MPC) revealed non-porous surface topology, whereas the surface of alginate capsules appeared highly porous (Figure 2e,f).
Using X-ray photoelectron spectroscopy (XPS), the characteristic N 1s peak of PDA at 399.5 eV and a distinct P 2p peak of poly(MPC) polymer at 134 eV were observed, confirming successful coating of alginate microspheres (Figure S1).[17]
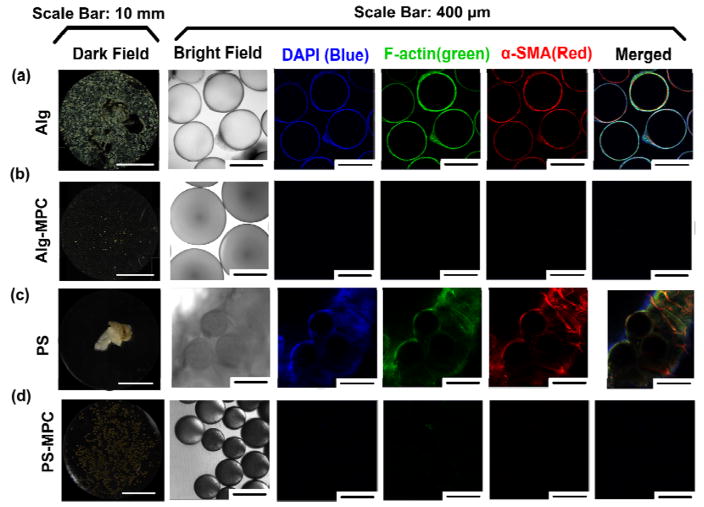
To investigate the effect of poly(MPC) coating on the foreign body reactions, MPC-modified alginate microspheres were implanted into the intraperitoneal space of immunocompetent C57BL/6J mice (n=5). Based on our previous studies where we evaluated the kinetics of fibrosis formation in the intraperitoneal space, we determined that 14 days would be indicative of longer term performance.[28,31,35] Therefore, after 14 days, the capsules were retrieved and examined for cellular overgrowth and fibrosis. Dark field phase contrast microscopy of retrieved unmodified capsules showed extensive cellular deposition on unmodified microspheres, whereas poly(MPC) coated microspheres showed little cellular deposition (Figure 3a,bFigure S2a,b). Immunofluorescence imaging was used to further characterize the cellular deposition on the microsphere surface. Staining of the retrieved microspheres using DAPI (nuclear stain), F-actin (cellular cytoskeleton marker) and α-smooth muscle actin (α-SMA, fibrosis-associated myofibroblast marker) revealed very little cellular staining on zwitterionic-coated microspheres (Figure 3b).[31] In contrast, the unmodified alginate microspheres stained extensively for these markers, indicating significant fibrosis formation on the unmodified spheres (Figure 3a).
Figure 3.
Zwitterionic surface modification of microspheres reduces fibrosis in vivo. The first column (a–d) shows representative dark field microscope images for retrieved alginate and PS microspheres, 14 days after intraperitoneal implantation into C57BL/6J mice. The second column shows bright field images for stained microspheres, with their corresponding immunofluorescence confocal images (columns 3–6).
To ensure that the reduction in cellular deposition on modified alginate microspheres was attributable to coating with poly(MPC), PDA-coated alginate microspheres without subsequent zwitterionic modification were also evaluated. 14 days following implantation, phase contrast microscopy of these PDA microspheres showed clumping and extensive cellular deposition (Figure S2c). This supports the function of the zwitterionic poly(MPC) coating in mitigating the fibrotic tissue response.
We next sought to evaluate the versatility of this anti-fibrotic effect for other biomaterials. Since the PDA coating procedure can be used on effectively nearly all types of materials, the coating strategy should likewise be largely material-independent.
Polystyrene (PS), when used as a biomaterial, is known to induce a fibrotic reaction when implanted.[36,37] Therefore, polystyrene microspheres (~0.5 mm) were coated with poly(MPC) polymer and implanted into mice to explore the versatility of our approach to reducing fibrosis of implanted materials. Phase contrast microscopy of retrieved PS microspheres confirms that unmodified materials become heavily encased in fibrotic tissue. However, poly(MPC) coated PS microspheres remained completely free of fibrosis (Figure 3c, Figure S2d).
Immunofluorescence imaging of retrieved PS microspheres confirmed poly(MPC) coating resulted in a dramatic reduction in cellular depositions on PS microspheres (Figure 3d, Figure S2e). These results support the versatility of this coating methodology to a variety of implantable materials in order to control the fibrotic tissue response.
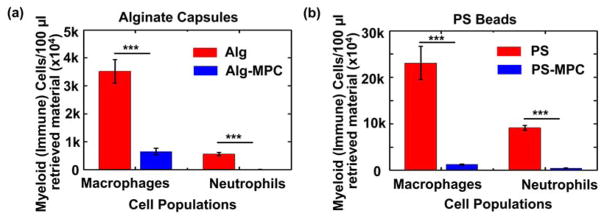
Flow cytometry analysis was performed on the cellular deposition from retrieved microspheres to quantify immune cells (macrophages and neutrophils) present onto the microsphere surface. It was found that poly(MPC) coating reduced the macrophage adhesion by ~4 fold for alginate and ~25 fold for PS microspheres. Interestingly, zwitterionic coating seems to completely abrogate neutrophil attachment to both materials. (Figure 4). The reduction of immune cells on the material surface confirms findings from microscopy analysis for a reduction in cellular deposition attributable to poly(MPC) coating.
Figure 4.
Flow cytometry for cells isolated from the surface layer were analyzed using specific markers for host macrophages and neutrophils present on alginate (a) and PS microparticles (b). ***P < 0.0001
In summary, we have demonstrated a facile and versatile method for surface modification of biomaterials with anti-fouling zwitterionic polymers. This approach improved the biocompatibility of implanted materials by reducing the surface-mediated fibrotic reaction in vivo. This methodology is broadly applicable to endowing a variety of materials with zwitterionic polymer surface coatings, as demonstrated in data collected for both alginate and PS microspheres. We believe that this approach to surface modification with zwitterionic polymers can be applied to virtually any implantable biomaterial, with broad use for cell transplantation, drug delivery, tissue engineering, and biomedical device implantation.
Supplementary Material
Figure S1. Representative XPS charts of original Alginate spheres and Alginate modified with PDA and MPC polymers
Figure S2. Dark field phase contrast images of the retrieved capsules (from Figure 3) at higher magnifications for (a) Alginate, (b) Alginate-MPC, (c) Alginate-PDA, (d) PS, (e) PS-MPC
Acknowledgments
This work was supported by the Juvenile Diabetes Research Foundation (JDRF) (Grant 17-2007-1063), the Leona M. and Harry B. Helmsley Charitable Trust Foundation (Grant 2014PG-T1D002), the Koch Institute Support (core) Grant P30-CA 14051 from the National Cancer Institute, and also by a generous gift from the Tayebati Family Foundation. Additionally, this work made use of the MRSEC shared experimental facilities at MIT, supported by the National Science Foundation under award number DMR-1419807. O. V was supported by JDRF and DOD/CDMRP postdoctoral fellowships (Grants 3-2013-178 and W81XWH-13-1-0215, respectively). J. C. D and S. B were supported by JDRF postdoctoral fellowship (Grants 3-PDF-2015-91-A-N, and 3-PDF-2015-90-A-N, respectively). M. J. W is extremely grateful for the financial support from the National Institutes of Health through Ruth L. Kirschstein National Research Service Award (No. F32DK101335). We also thank the Koch Institute Swanson Biotechnology Center for technical support, specifically Microscopy and Flow Cytometry. The authors would like to acknowledge the use of resources at the W. M. Keck Biological Imaging Facility (Whitehead Institute). The authors would also like to thank W. Salmon for assistance with confocal microscopy and E. L. Shaw for assistance with XPS.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Dr. Volkan Yesilyurt, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Department of Anesthesiology, Boston Children’s Hospital, Boston, MA 02115, USA
Dr. Omid Veiseh, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA, 02139, USA
Dr. Joshua C. Doloff, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Department of Anesthesiology, Boston Children’s Hospital, Boston, MA 02115, USA
Jie Li, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Department of Anesthesiology, Boston Children’s Hospital, Boston, MA 02115, USA.
Dr. Suman Bose, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Department of Anesthesiology, Boston Children’s Hospital, Boston, MA 02115, USA
Dr. Xi Xie, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
Andrew R. Bader, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Department of Anesthesiology, Boston Children’s Hospital, Boston, MA 02115, USA
Michael Chen, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Department of Anesthesiology, Boston Children’s Hospital, Boston, MA 02115, USA.
Dr. Matthew J. Webber, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Department of Anesthesiology, Boston Children’s Hospital, Boston, MA 02115, USA
Dr. Arturo J. Vegas, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Department of Anesthesiology, Boston Children’s Hospital, Boston, MA 02115, USA
Prof. Robert Langer, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Department of Anesthesiology, Boston Children’s Hospital, Boston, MA 02115, USA. Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA, 02139, USA. Harvard–Massachusetts Institute of Technology Division of Health Sciences and Technology, Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
Prof. Daniel G. Anderson, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Department of Anesthesiology, Boston Children’s Hospital, Boston, MA 02115, USA. Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA, 02139, USA. Harvard–Massachusetts Institute of Technology Division of Health Sciences and Technology, Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
References
- 1.Anderson JM, Rodriguez A, Chang DT. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward WK. J Diabetes Sci Technol. 2008;2:768–777. doi: 10.1177/193229680800200504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu K, Mei Y, Hadjesfandiari N, Kizhakkedathu JN. Colloids Surf B. 2014;124:69–79. doi: 10.1016/j.colsurfb.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Yang C, Ding X, Ono RJ, Lee H, Hsu LY, Tong YW, Hedrick J, Yang YY. Adv Mater. 2014;26:7346–7351. doi: 10.1002/adma.201402059. [DOI] [PubMed] [Google Scholar]
- 5.Ma M, Liu WF, Hill PS, Bratlie KM, Siegwart DJ, Chin J, Park M, Guerreiro J, Anderson DG. Adv Mater. 2011;23:H189–H194. doi: 10.1002/adma.201100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma X, Wu T, Robich MP, Wang X, Wu H, Buchholz B, McCarty S. Int J Clin Exp Med. 2010;3(3):192–201. [PMC free article] [PubMed] [Google Scholar]
- 7.Laschewsky A. Polymers. 2014;6:1544–1601. [Google Scholar]
- 8.Lowe AB, McCormick CL. Chem Rev. 2002;102:4177. doi: 10.1021/cr020371t. [DOI] [PubMed] [Google Scholar]
- 9.Smith RS, Zhang Z, Bouchard M, Li J, Lapp HS, Brotske GR, Lucchino DL, Weaver D, Roth LA, Coury A, Biggerstaff J, Sukavaneshvar S, Langer R, Loose C. Sci Transl Med. 2012;4:153ra132. doi: 10.1126/scitranslmed.3004120. [DOI] [PubMed] [Google Scholar]
- 10.Yang W, Bai T, Carr LR, Keefe AJ, Xu J, Xue H, Irvin CA, Chen S, Wang J, Jiang S. Biomaterials. 2012;33:7945–7951. doi: 10.1016/j.biomaterials.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Wei Q, Becherer T, Angioletti-Uberti S, Dzubiella J, Wischke C, Neffe AT, Lendlein A, Ballauff M, Haag R. Angew Chem Int Ed. 2014;53:8004–8032. doi: 10.1002/anie.201400546. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Cao Z, Bai T, Carr L, Ella-Menye JR, Irwin C, Ratner BD, Jiang S. Nat Biotech. 2013;31:553–557. doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- 13.Ikada Y. Biomaterials. 1994;15:725–736. doi: 10.1016/0142-9612(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 14.Chu PK, Chen JY, Wang LP, Huang N. Mat Sci Eng R. 2002;36:143–206. [Google Scholar]
- 15.Lee H, Dellatore SM, Miller WM, Messersmith PB. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Rho J, Messersmith PB. Adv Mater. 2009;21:431–434. doi: 10.1002/adma.200801222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ham HO, Liu Z, Lau KHA, Lee H, Messersmith PB. Angew Chem Int Ed. 2011;50:732–736. doi: 10.1002/anie.201005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang SM, Hwang NS, Yeom J, Park SY, Messersmith PB, Choi IS, Langer R, Anderson DG, Lee H. Adv Funct Mater. 2012;22:2949. doi: 10.1002/adfm.201200177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blaszykowski C, Sheikh S, Thompson M. Biomater Sci. 2015;3:1335. doi: 10.1039/c5bm00085h. [DOI] [PubMed] [Google Scholar]
- 20.Li G, Cheng G, Xue H, Chen S, Zhang F, Jiang S. Biomaterials. 2008;29:4592. doi: 10.1016/j.biomaterials.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Gao C, Li G, Xue H, Yang W, Zhang F, Jiang S. Biomaterials. 2010;31:1486. doi: 10.1016/j.biomaterials.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Sundaram HS, Han X, Nowinski AK, Ella-Menye J-R, Wimbish C, Marek P, Senecal K, Jiang S. ACS Appl Mater Interfaces. 2014;6:6664. doi: 10.1021/am500362k. [DOI] [PubMed] [Google Scholar]
- 23.Lee KY, Mooney DJ. Prog Poly Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King A, Sandler S, Anderson A. J Biomed Mater Res. 2001;57:374–383. doi: 10.1002/1097-4636(20011205)57:3<374::aid-jbm1180>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Scharp DW, Marchetti P. Adv Drug Deliv Rev. 2014;67–68:35–73. doi: 10.1016/j.addr.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Spasojevic M, Paredes-Juarez GA, Vorenkamp J, de Haan BJ, Schouten AJ, de Vos P. PLos One. 2014;9:e109837. doi: 10.1371/journal.pone.0109837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim F, Sun AM. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 28.Vegas AJ, et al. Nat Biotechnol. 2016;34:345–352. doi: 10.1038/nbt.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seetho K, Zhang S, Pollack KA, Zou J, Raymound JE, Martinez E, Wooley KL. ACS Macro Lett. 2015;4:505–510. doi: 10.1021/mz500818c. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Lawrence J, Sangram P, Emrick T. Macromolecules. 2013;46:119–127. [Google Scholar]
- 31.Veiseh O, et al. Nat Mater. 2015;14:643–652. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponce S, Orive G, Hernandez R, Gascon AR, Pedraz JL, de Haan BJ, Faas MM, Mathieu HJ, de Vos P. Biomaterials. 2006;27:4831. doi: 10.1016/j.biomaterials.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Spasojevic M, Bhujpal S, Paredes G, de Haan BJ, Schouten AJ, de Vos P. J Biomed Mater Res Part A. 2014;102A:1887. doi: 10.1002/jbm.a.34863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim BJ, Park T, Moon HC, Park SY, Hong D, Ko EH, Kim JY, Hong JW, Han SW, Kim YG, Choi IS. Angew Chem Int Ed. 2014;53:14443. doi: 10.1002/anie.201408454. [DOI] [PubMed] [Google Scholar]
- 35.Vegas AJ. Nat Med. 2016;22:306. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akilbekova D, Bratlie KM. PLoS ONE. 2015;10(6):e0130386. doi: 10.1371/journal.pone.0130386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bratlie KM, Dang TT, Leyle S, Nahrendorf M, Weissleder R, Langer R, Anderson DG. PLoS ONE. 2010;5(4):e10032. doi: 10.1371/journal.pone.0010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative XPS charts of original Alginate spheres and Alginate modified with PDA and MPC polymers
Figure S2. Dark field phase contrast images of the retrieved capsules (from Figure 3) at higher magnifications for (a) Alginate, (b) Alginate-MPC, (c) Alginate-PDA, (d) PS, (e) PS-MPC