Highlights
-
•
There is big concern about reflux appearance after sleeve gastrectomy.
-
•
Chronic reflux increases risk of esophageal adenocarcinoma.
-
•
We present a case of an esophageal adenocarcinoma after sleeve gastrectomy.
-
•
Relationship between sleeve gastrectomy and reflux needs further study.
Keywords: Esophageal adenocarcinoma, Sleeve gastrectomy, Bariatric surgery, Gastroesophageal reflux disease, Barrett’s esophagus, Case report
Abstract
Introduction
Laparoscopic sleeve gastrectomy has become the most popular bariatric procedure worldwide. However, postoperative gastroesophageal reflux disease appearance is a matter of concern. Only two cases of esophageal adenocarcinoma after gastric sleeve have been described, none of them with preoperative endoscopic evaluation.
Presentation of case
We report a case of a 48-year-old male with morbid obesity and normal preoperative endoscopy and esophagram who underwent a laparoscopic sleeve gastrectomy and developed an esophageal adenocarcinoma five years later.
Discussion
Despite promising results in terms of weight loss and resolution of comorbidities, the onset or worsening of gastroesophageal reflux and its related complications, such as Barrett's esophagus or esophageal adenocarcinoma, is a matter of concern and need further study.
Conclusion
We present a case of an esophageal adenocarcinoma five years after a laparoscopic sleeve gastrectomy for morbid obesity. There is need to better determine the relationship between sleeve gastrectomy and gastroesophageal reflux disease in order to prevent its related complications, such as esophageal adenocarcinoma.
1. Introduction
Obesity is one of the leading health problems worldwide and has already reached epidemic proportions. Obese patients are at increased risk of developing comorbidities such as gastroesophageal reflux disease (GERD), with a potential increase in reflux symptoms, esophagitis and esophageal adenocarcinoma [1].
Bariatric surgery is the most effective therapy available for morbid obesity, and provides long-term weigth loss as well as improvement or complete resolution of comorbidities [2].
Gastric bypass is considered the gold standard surgery for the treatment of refractary morbid obesity, due to its very good long terms results, with mean loses of 50–70% of excess bodyweight and a sound control of obesity related diseases [3].
During the last decade, Laparoscopic Sleeve Gastrectomy (LSG) has become more and more popular among surgeons and patients, and it is nowadays, perhaps, the preferred bariatric technique in many countries. Its results are also excellent in terms of weight loss and improvement of comorbidities, with the advantage of being a simpler operation from the technical standpoint.
However, there is one shortcoming of LSG in the long-term follow-up and this is the onset of de novo GERD. Available data about the relationship between sleeve gastrectomy and gastroesophageal reflux disease is both limited and contradictory. Several series have demonstrated significant increase on GERD and hiatal hernia post sleeve, and there is also an increase of revisional surgeries associated with this refractory reflux [4], [5], [6]. Many surgeons ponder LSG as a good operation even in GERD patients, and the rationale for this is that sleeve improves symptoms and reduces reflux in obese patients with preexisting GERD, that there is low incidence of Barrett’s esophagus reported in obese patients, and that there are only a few reports of esophageal adenocarcinoma after sleeve gastrectomy published in the literature [7], [8].
Now, fanning the flames of this controversy, we report a case of an esophageal adenocarcinoma five years after LSG, in a patient with preoperative normal studies. This work has been reported following the SCARE criteria [9].
2. Presentation of case
48-Year-old man that underwent a laparoscopic sleeve gastrectomy for morbid obesity. Preoperative BMI was 48,5 kg/m2 and he had a history of hypertension, dyslipidemia, insulin resistance and obstructive sleep apnea. He also had a 30 pack-year history of smoking and no previous GERD symptoms.
Our rutine preoperative evaluation included an upper gastrointestinal series and esophagogastroduodenoscopy (EGD), which showed absence of reflux or hiatal hernia, no esophagitis or Barrett́s esophagus. Helicobacter pylori was negative.
The surgery was uneventful and the patient had an excellent recovery. On post-operative day one a gastrograffin swallow study ruled out any leak or stricture.
Subsequent follow-up occurred at one, three, six, nine and twelve months postoperatively. At one year he had achieved an excess weight loss of 70%. On the following visit, 15 months after the operation, he complained of new onset of typical GERD symptoms. He was put on proton pump inhibitors (PPI) and a new EGD and barium swallow were requested, but he did not do the studies and was then lost to follow up.
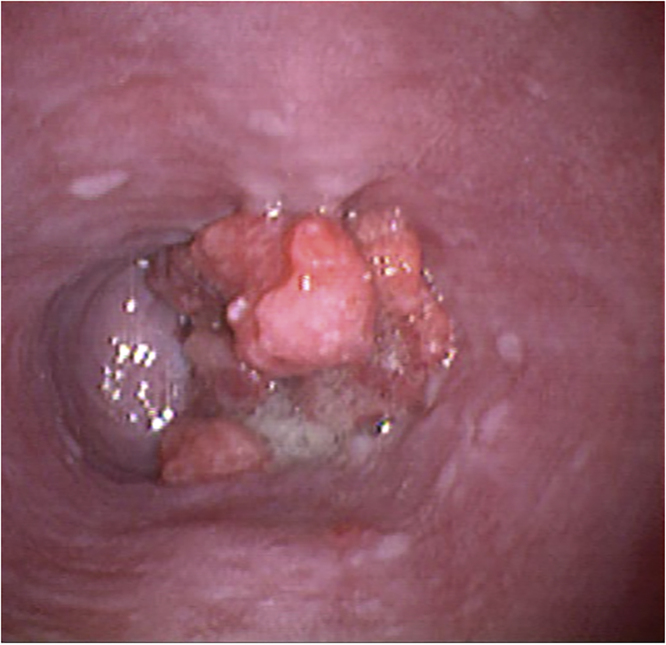
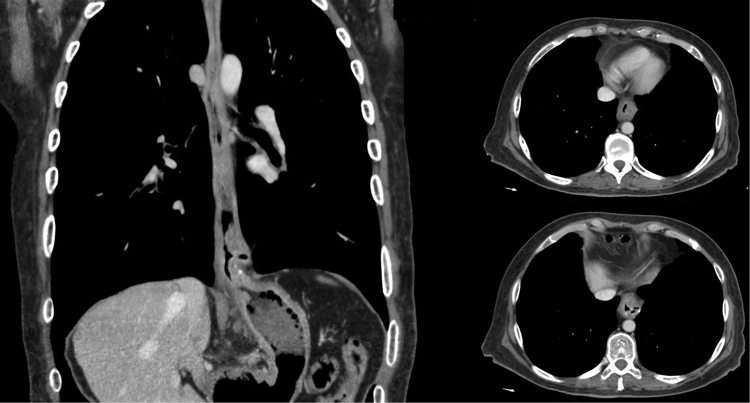
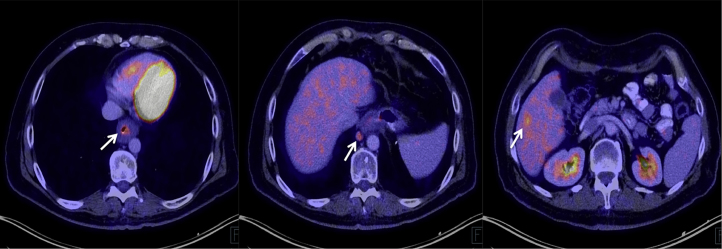
He returned 4 years later (fifth postoperative year) referring dysphagia to solids and a 10 kg weight loss. A new endoscopy revealed a solid mass on the lower third of the esophagus (Fig. 1). Histopathological evaluation demonstrated a moderately differentiated adenocarcinoma of the esophagus. Staging was completed with a multislice computed tomography scan, using a distention technique with carbon dioxide, which confirmed the presence of a large tumor in the lower esophagus with evidence of regional lymph node involvement (Fig. 2). He started neoadjuvant chemoradiotherapy, with five courses of carboplatin and paclitaxel and 41.4 Gy of concomitant radiotherapy. During re-staging prior to surgery, a Positron Emission Tomography/Computed Tomography (PET/CT) showed two metabolically active liver images with Standardized Uptake Values (SUV) of 5.8 and 4.1, compatible with metastatic disease (Fig. 3). After multidisciplinary consultation, further chemotherapy was decided.
Fig. 1.
Endoscopic image of the tumor in the lower esophagus.
Fig. 2.
Pneumo-computed tomography showing thickening of the esophageal wall and regional lymph nodes.
Fig. 3.
Primary tumor, metastatic locoregional lymph node and liver metastasis on PET-CT scan.
3. Discussion
Sleeve gastrectomy has become the most common bariatric procedure in the United States in 2015, accounting for 53.8% of the 196,000 surgeries performed, followed by gastric bypass (23,1%) and gastric banding (5,7%) [10]. The success and popularity of this technique is supported by the excellent short and medium term results in relation to weigth loss and resolution of comorbidities, with a very low rate of complications, without promoting changes in intestinal anatomy and nutrient absortion.
However, there is big concern about the impact of LSG on the physiology of the gastroesophageal junction and the antireflux barrier. Several mechanisms of impairment of cardial continence are attributed to sleeve, like lost of angle of His’ flap valve, reduced lower esophageal sphincter (LES) pressure, cardial dilatation, damage of sling fibers, alteration of esophageal outflow secondary to increased intragastric pressure, etc.
The data published is confounding and inconsistent.
Some studies conclude that LSG improves the antireflux mechanism, and hence consider LSG a good option even in obese patients with GERD.
Rebecchi et al. analized a cohort of 71 morbid obese patients with LSG who were studied with GERD questionnaire, endoscopy, manometry and ph monitoring, before and two years after surgery. In those patients with GERD symptoms and pathologic ph monitoring (n = 28), these authors found improvement in both symptoms and ph monitoring. In the group without preoperative reflux, only 5% developed de novo GERD [7].
Chiu et al. published a systematic review of the effect of LSG on GERD and found that four publicatons showed an increase, while seven showed a decrease in GERD symptoms after LSG. But the quality of these studies did not allow to reach a consensus: no randomized controlled trials analized GERD after sleeve as a primary outcome, and there was lack of standarization in terms of surgical technique, severity of symptoms, and follow-up (follow-up was short in most of the studies) [11].
Likewise, several reports described the development or even worsening of GERD after LSG.
In a prospective randomized trial that compared LSG with gastric banding, Himpens et al. found development of de novo reflux symptoms in 21.5% of the LSG cohort at one-year follow-up [12].
DuPree et al. analyzed 4832 patients submitted for LSG from the Bariatric Outcomes Longitudinal Database (BOLD). They found that 84.1% of the LSG patients with preexisting GERD continued to have GERD symptoms after LSG, while only 15.9% showed GERD resolution; and 8.6% of the LSG patients with no previous GERD developed symptoms postoperatively [13].
Tai et al. found significant increase in GERD symptoms (12.1% vs. 47%), erosive esophagitis (16.7% vs 66.7%) and hiatal hernia (6.1% vs 27.3%) one year after LSG [4].
Braghetto et al. analized 231 patients without reflux symptoms, esophagitis or Barrett, who underwent a LSG. In the follow-up, GERD symptoms were found in 23.2%, erosive esophagitis in 15.5%, and Barrett’s esophagus (intestinal metaplasia) in 1.2% [5].
The available data is not conclusive so as to consider GERD as a contraindication for LSG, but is robust enough to claim that gastric bypass is a better option in obese patients with GERD symptoms or GERD related complications [4], [5], [14].
This concept is also validated by the significant proportion of the LSG patients who are on PPI treatment and by the increasing rate of revisional surgery on sleeve patients who are converted to gastric bypass because of intractable reflux [6].
Gagner emphazised that “the benefits of LSG definitely outweighs its potential and actual risks”, and compared the sleeve with the Duodenal Switch, pointing out that after 25 years there are not reports of esophageal adenocarcinoma after Duodenal Switch [8]. The big difference between these operations is that in the Duodenal Switch a Roux en Y is performed and all the alkaline secretions are diverted distally to the stomach.
The incidence of Barrett’s esophagus in morbid obese patients is about 1.3% [5]. But there is no data to establish the rate of Barrett’s esophagus ten o more years after LSG, and this should be a matter of concern, considering that many of the patients being offered a sleeve gastrectomy are young.
The importance of reflux after LSG is being underestimated, and moreover, bariatric sugeries are being performed in some centers without a preoperative upper endoscopy.
Esophagogastric cancer after bariatric procedures was reported in less than 40 cases, and to our knowledge, only two cases of esophageal adenocarcinoma after LSG have been published. Scheepers et al. described the case of a 57-year-old woman who was submitted for a LSG and was diagnosed with esophageal adenocarcinoma four months after bariatric surgery [15]. In another case reported by Sohn et al., a 44-year-old woman who underwent LSG developed an adenocarcinoma of the gastroesophageal junction 2.5 years after surgery [16]. Surprisingly, in both cases the sleeve gastrectomy was performed without previous endoscopic evaluation, making it impossible to rule out the presence of early or premalignant esophageal lesions. As a matter of fact, in the first case, the esophageal adenocarcinoma diagnosed four months after the sleeve had been probably present at the time of the bariatric surgery.
In our case, the patient did not complain of GERD symptoms and had a completely normal preoperative upper endoscopy and esophagogram, making our case, to our knowledge, the first report of this type in the literature. Unfortunately the patient refused to be studied when GERD symptoms started 15 months after surgery and was lost to follow-up. Although there were other cofactors like smoking and obesity, and we cannot state that GERD directly caused the esophageal cancer, we think it might have played some role in its pathogenesis.
4. Conclusion
We report the occurrence of an esophageal adenocarcinoma five years after LSG in a patient with normal preoperative esophageal evaluation. The relationship between GERD and LSG should be better determined in order to prevent reflux related complications, such as esophageal adenocarcinoma.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
This research did not receive any grant or funding.
Ethical approval
Does not apply.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Fernando Gabriel Wright: data collection and analysis, writing the paper.
Agustin Duro: data collection and analysis, writing the paper.
Juan Rodolfo Medici: data collection and analysis, writing the paper.
Santiago Lenzi: data analysis and interpretation.
Axel Federico Beskow: data collection and analysis, writing the paper.
Demetrio Cavadas: data collection and analysis, writing the paper.
Guarantor
Demetrio Cavadas, M.D., Ph.D.
Contributor Information
Fernando Gabriel Wright, Email: fernando.wright@hospitalitaliano.org.ar.
Agustin Duro, Email: agustin.duro@hospitalitaliano.org.ar, agustinduro@hotmail.com.
Juan Rodolfo Medici, Email: juan.medici@hospitalitaliano.org.ar.
Santiago Lenzi, Email: santiago.lenzi@hospitalitaliano.org.ar.
Axel Federico Beskow, Email: axel.beskow@hospitalitaliano.org.ar.
Demetrio Cavadas, Email: demetrio.cavadas@hospitalitaliano.org.ar.
References
- 1.Hampel H., Abraham N.S., El-Serag H.B. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann. Intern. Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 2.Picot J., Jones J., Colquitt J.L. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol. Assess. 2009;13(1-190):215–357. doi: 10.3310/hta13410. [DOI] [PubMed] [Google Scholar]
- 3.Powell M.S., Fernandez A.Z., Jr. Surgical treatment of morbid obesity: the laparoscopic Roux-en-Y gastric bypass. Surg. Clin. N. Am. 2011;91:1203–1224. doi: 10.1016/j.suc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Tai C.M., Huang C.K., Lee Y.C., Chang C.Y., Lee C.T., Lin J.T. Increase in gastroesophageal reflux disease symptoms and erosive esophagitis 1 year after laparoscopic sleeve gastrectomy among obese adults. Surg. Endosc. 2013;27:1260–1266. doi: 10.1007/s00464-012-2593-9. [DOI] [PubMed] [Google Scholar]
- 5.Braguetto I., Csendes A. Prevalence of Barretts’s esophagus in bariatric patients undergoing sleeve gastrectomy. Obes. Surg. 2016;26:710–714. doi: 10.1007/s11695-015-1574-1. [DOI] [PubMed] [Google Scholar]
- 6.Langer F.B., Bohdjalian A., Shakeri-Leidenmühler S., Schoppmann S.F., Zacherl J., Prager G. Conversion from sleeve gastrectomy to Roux-en-Y gastric bypass: indications and outcome. Obes. Surg. 2010;20:835–840. doi: 10.1007/s11695-010-0125-z. [DOI] [PubMed] [Google Scholar]
- 7.Rebecchi F., Allaix M.E., Giaccone C., Ugliono E., Scozzari G., Morino M. Gastroesophageal reflux disease and laparoscopic sleeve gastrectomy: a physiopathologic evaluation. Ann. Surg. 2014;260:909–914. doi: 10.1097/SLA.0000000000000967. [DOI] [PubMed] [Google Scholar]
- 8.Gagner M. Is sleeve gastrectomy always an absolute contraindication in patients with Barrett́s? Obes. Surg. 2016;26:715–717. doi: 10.1007/s11695-015-1983-1. [DOI] [PubMed] [Google Scholar]
- 9.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 10.American Society for Metabolic and Bariatric Surgery 2016. Estimate of bariatric surgery numbers 2011–2015. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers (Accessed 18 10 2016).
- 11.Chiu S., Birch D.W., Shi X. Effect of sleeve gastrectomy on gastroesophageal reflux disease: a systematic review. Surg. Obes. Relat. Dis. 2011;7:510–515. doi: 10.1016/j.soard.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Himpens J., Dapri G., Cadière G.B. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes. Surg. 2006;16:1450–1456. doi: 10.1381/096089206778869933. [DOI] [PubMed] [Google Scholar]
- 13.DuPree C.E., Blair K., Steele S.R., Martin M.J. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease: a national analysis. JAMA Surg. 2014;149:328–334. doi: 10.1001/jamasurg.2013.4323. [DOI] [PubMed] [Google Scholar]
- 14.Csendes A., Burgos A.M., Smok G., Burdiles P., Henriquez M.T. Effect of gastric bypass on Barrett’s esophagus and intestinal metaplasia of the cardia in patients with morbid obesity. J. Gastrointest. Surg. 2006;10:259–264. doi: 10.1016/j.gassur.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Scheepers A.F., Schoon E.J., Nienhuijs S.W. Esophageal carcinoma after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2011;7:e11–12. doi: 10.1016/j.soard.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Sohn S., Fischer J., Booth M. Adenocarcinoma of the gastro-oesophageal junction after sleeve gastrectomy: a case report. ANZ J. Surg. 2015;(April) doi: 10.1111/ans.13064. [DOI] [PubMed] [Google Scholar]