Abstract
Hypoxia-inducible factor-1α (HIF-1α) in adipose tissue is known to promote obesity. We hypothesized that HIF-1α interferes with brown fat thermogenesis, thus decreasing energy expenditure. To test this hypothesis, we compared transgenic mice constitutively expressing HIF-1α in adipose tissues (HIF-1α++) at usual temperature (22°C) where brown fat is somewhat active, or at thermoneutrality (30°C), where brown fat is minimally active. HIF-1α++ mice or control litter mates were separated into room temperature (22°C) or thermoneutrality (30°C) groups. We assessed weight gain, food intake, calorimetry, activity; and oxygen consumption and transcriptional changes in isolated white and brown adipocytes. At 22°C, HIF-1α++ mice exhibited accelerated weight gain, cold and glucose intolerance, hyperglycemia, and decreased energy expenditure without changes in food intake or activity. These changes were absent or minimal at thermoneutrality. In brown adipocytes of HIF-1α++ mice, oxygen consumption decreased ~50% in association with reduced mitochondrial content, uncoupling protein 2, and peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1α). In conclusion, adipose HIF-1α overexpression inhibits thermogenesis and cellular respiration in brown adipose tissue, promoting obesity in the setting of reduced ambient temperature.
Keywords: brown adipose, thermogenesis, HIF, obesity, hypoxia
INTRODUCTION
Over the last two decades, there has been growing interest in roles of hypoxia inducible factor 1 (HIF-1) in mediating metabolism. HIF-1 is a heterodimer comprised of an oxygen sensitive HIF-1α subunit and a constitutively produced HIF-1β subunit[1]. In the presence of oxygen, HIF-1α undergoes rapid ubiquitination and proteasome degradation. Hypoxia prevents HIF-1α hydroxylation by prolyl hydroxylases, allowing HIF-1α to bind with HIF-1β and activate hypoxia response elements on target genes such as vascular endothelial growth factor (VEGF), glucose transporters, and glycolytic enzymes [2-4].
Although essential to cope with hypoxia, HIF-1α may also aggravate obesity and associated metabolic dysfunction. White adipose tissue (WAT) HIF-1α increases after high fat diet (HFD) presumably due to compromised O2 diffusion into hypertrophic adipocytes outstripping their blood supply [5, 6]. A HIF-1α knockout in adipocytes [7, 8] and HIF-1α antisense oligonucleotides [9] improved insulin sensitivity and reduced adiposity in HFD fed mice. Constitutive expression of HIF-1α in adipose tissue induced insulin resistance, accelerated weight gain, and caused WAT fibrosis and inflammation [10]. HFD acutely increases nitric oxide, inhibiting insulin signaling and promoting chemokines that lead to macrophage infiltration in a HIF-1α dependent manner [8]. HIF-1α also up-regulates angiopoietin-like 4 which in turn inhibits lipoprotein lipase in WAT, which might link hypoxia from sleep apnea to increased triglycerides [11]. Thus HIF-1α is necessary and sufficient to induce WAT hypertrophy, inflammation and insulin resistance. However, less is known regarding systemic mechanisms of adipose HIF-1α induced weight gain.
We wondered whether the impact of adipose HIF-1α on weight gain might be related to effects in BAT, which is much more metabolically active than WAT, particularly at unadjusted laboratory temperatures of ~22°C rather than at thermoneutrality (30°C) [12-15]. Therefore, we undertook this study to determine whether HIF-1α activation in adipose tissues causes weight gain in a temperature dependent manner. Since humans are less sensitive than rodents to hyper-metabolic effects of cold and typically reside at thermoneutrality [12], a temperature dependent effect of adipose HIF-1α could have significant clinical implications.
MATERIALS AND METHODS
Ethical Approval
The study was approved by the Johns Hopkins University Animal Care and Use Committee.
Experimental animals
HIF-1α transgenic mice (HIF-1α++) were generated by sub-cloning the human HIF-1α gene containing a deletion of the oxygen degradation domain (amino acids 403-603) under control of the aP2 promoter into a plasmid, and injecting the construct into FVB-derived blastocysts[10]. This deletion promotes stability of HIF-1α in adipose tissues under normoxic conditions. Mice were genotyped for presence of transgene using primers: 5’CAAGAAGCCCTAACGTGTTAT and 5’GTGATGTAGTAGCTGCATGA. In addition, expression of the transgene was confirmed by real-time reverse transcriptase PCR using these primers and SYBR Green detection method (Life Technologies). Littermates lacking the transgene were used as controls. Transgenic HIF-1α++ mice have HIF-1α transgene in inguinal, mesenteric, epididymal, perirenal, and brown fat depots with minimal expression in other tissues [10]. Mice were maintained on an ad libitum chow diet or on a high fat diet consisting of 20% protein, 36.1% carbohydrate, and 35.2% fat by weight (Harlan Teklad TD.03584). Room lights were programmed to maintain a 12:12-hr. light-dark cycle, with lights on from 09:00 to 21:00. To maintain thermoneutrality, cages were placed in a Draeger IC 8000 incubator set to 30°C. Mice maintained at 22°C were housed in an adjacent space in a thermostat-controlled room. We have previously demonstrated the presence of transgene RNA, protein, and HIF binding activity in adipose tissue of HIF-1α++ mice and lack of transgene expression in non-adipose tissues[10, 11].
Calorimetry
Calorimetry was performed in an open-circuit indirect calorimeter (Oxymax CLAMS unit, Columbus Instruments). Data were collected in a subset of transgenic mice or controls (n = 5/group) at age 12 weeks. The same animals underwent testing at 22°C for 1 week, followed by a second week of testing at 30°C (using a temperature controlled room) and the final 3 days of each condition were used for analysis. Rates of oxygen consumption (VO2, ml/kg/hr.) and carbon dioxide production (VCO2) were measured for each chamber every 16 minutes. Energy expenditure was calculated as EE = O2 × (3.815 + (1.232×RER)).
Telemetry
Mice were anesthetized with isoflurane and implanted with TA-F10 transmitters (Data Sciences International, St. Paul, MN) in the peritoneal cavity. After at least 5 days of recovery, body temperature and activity data were captured using PowerLabs 16/35 interfaced with LabChart Pro software from ADInstruments (Colorado Springs, CO). For cold challenge testing, a subset of telemetry-monitored mice (n=4 for each genotype) was transferred for 6 hours to a 4° C cold room, then transferred back to 30° C for recovery.
Gene expression, Western blotting, and Assays
Total RNA was extracted from tissues using Trizol (Life Technologies, Rockville, MD), and cDNA was synthesized using AdvantageRT for PCR kit from Clontech (Palo Alto, CA). Real-time reverse transcriptase PCR was performed with primers from Invitrogen (Carlsbad, CA) and Taqman probes from Applied Biosystems (Foster City, CA).
Results were quantified according to the 2−ΔΔCt method, using 18s as an internal housekeeping gene. To assess mitochondrial content, we measured ratio of mitochondrial to genomic DNA. This was achieved by PCR using primers for NADH dehydrogenase subunit 2 (Nd2) to amplify mitochondrial DNA, and primers for nucleoside diphosphate kinase 1 (Nme1) to amplify genomic DNA as previously described [16].Adipose tissue pyruvate dehydrogenase phosphorylation was assessed by Western blot, probing for extent of E1 subunit phosphorylation. Primary antibody used was against phosphorylated Pyruvate Dehydrogenase E1-alpha subunit [p Ser293, Novus Biologicals NB110-93479] at a dilution of 1:1000. Secondary antibody was goat anti-rabbit at 1:5000 dilution. The same blots were stripped and re-probed for actin as a normalizing protein using rabbit antibody and goat anti-mouse-HRP. The results were expressed as ratios of optical density of the bands to actin.
Histology
Adipose tissue was fixed in 10% buffered formalin, embedded in paraffin and sectioned into 5 μm slices. Histological assessment of tissue morphology was performed using an Olympus microscope (Olympus, Tokyo, Japan). The diameter of adipocytes was measured using image-J (National Institutes of Health, Bethesda, MD), and a histogram was created from the diameter of 500 cells (10 mice/group).
Intraperitoneal glucose tolerance test (GTT)
Mice were injected with 1g/kg glucose in the peritoneal cavity. Blood was collected from the tail in un-anesthetized mice 0, 10, 20, 30, 60, 90, and 120 min after glucose injection. Blood glucose was tested with Accu-Chek kits from Roche Diagnostics (Indianapolis, IN).
Oxygen consumption in adipocytes
Krebs-Ringer HEPES bicarbonate buffer was prepared containing 120 mM NaCl, 4.8 mM KCl, 25 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 15mM NaHCO3, 30 mM HEPES, and 10 mM glucose. Fatty-acid free bovine serum albumin was added (4%) and titrated to pH 7.4. Adipocyte wash buffer was prepared by adding 500 nM adenosine to this solution. Fat pads were harvested from HIF-1α++ or control mice housed at 22°C, and minced into pieces using scissors, followed by digestion in collagenase, 2mg/mL in BSA-Krebs solution (approx. 3 mL/mg tissue). Fat was transferred to a 50 mL conical tube and incubated at 37°C at 120 RPM 1 hr. The fat slurry was filtered through 250 micron gauze mesh and centrifuged at 500 RPM for 1 minute. The infranatant was removed and adipocytes were washed twice in adipocyte wash buffer. Cells were then re-suspended in wash buffer in 5-8 volumes of the final amount of fat cake. Typical yield of this procedure was about 5000 cells/μL. Adipocyte mixtures were pipetted into micro-respiration chambers with a Clarke electrode (Unisense, Denmark), 10 minutes/chamber. An aliquot of adipocytes was diluted 1:2 in tryptan blue and 8 μL placed on a hemocytometer to determine cell number. Oxygen consumption was normalized to cells per chamber.
Statistical analysis
Two-way ANOVA was performed to examine effects of ambient temperature and genotype, followed by Bonferroni posttests. Statistics were calculated using GraphPad Prism (GraphPad Software, La Jolla, CA). For GTT data, we calculated the area under the curve (AUC) using a trapezoidal rule. Values are reported as mean ± SEM. A p-value of <0.05 was considered significant.
RESULTS
Weight gain, adiposity, and food intake
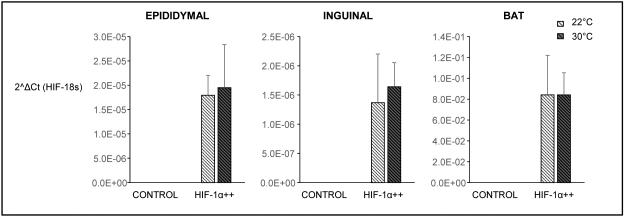
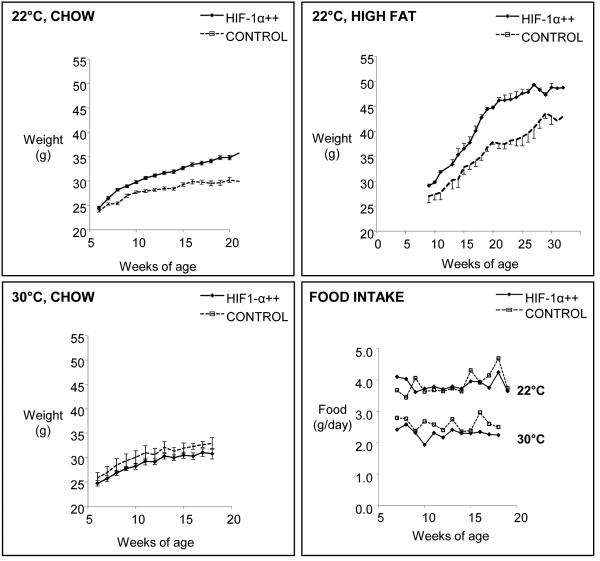
We verified that the human HIF-1α gene lacking the oxygen degradation domain was expressed in adipose depots of HIF-1α++ mice, unaffected by ambient temperature, and absent in control littermates (Fig. 1). At 22°C, when fed either a chow diet or a high fat diet (HFD), HIF-1α++ mice gained more weight than controls (Fig. 2). At 30°C, HIF-1α++ mice did not differ in weight compared to controls. As expected, mice housed at 30°C consumed less food [14], but food intake did not differ between HIF-1α++ and control mice (Fig. 2). In terms of adiposity, mice housed at thermoneutrality had decreased brown, epididymal, and inguinal fat mass (Fig. 3). At the cooler temperature HIFα++ mice had more inguinal fat mass. There was also a trend towards greater epididymal fat weight independent of temperature (p=0.074). Plasma glucose and lipid profiles demonstrated a decrease in cholesterol at thermoneutrality, while triglycerides were modestly increased in HIF-1α++ mice regardless of temperature. Fasting glucose was only increased in HIF-1α++ mice at 22°C.
Fig. 1.
Gene expression in adipose tissues of HIF-1α++ and control littermates housed at 22°C or 30°C. Fold change in RNA expression is graphed as 2^ΔCt value. Transgene was present in all adipose depots, unaffected by ambient temperature, and absent in control mice. (Two-way ANOVA, effect of genotype, p<0.0001, effect of temperature p=0.36)
Fig. 2.
Weight gain in HIF-1α++ (n=15) and control mice (n=15) housed at 22°C fed a chow or high fat diet; and in HIF-1α++ (n=10) and control mice (n=10) housed at 30°C fed a chow diet. HIF-1α++ mice gained more weight (p<0.01) regardless of diet, but only at 22°C. Mice housed at 30°C consumed less food (p<0.001 by two-way repeated measures ANOVA), but there was no effect of genotype on food intake.
Fig. 3.
Tissue weights and plasma metabolic profile of chow-fed HIF-1α++ and control mice housed at 22°C or 30°C, at 20 weeks of age. Cooler ambient temperature independently increased fat mass and cholesterol. HIF-1α++ increased triglycerides regardless of temperature, but increased inguinal fat weight and glucose only at 22°C. Error bars denote standard error. By two-way ANOVA: *p<0.05 for effect of temperature; †p<0.05 for effect of HIF-1α++; #p<0.01 for HIF-1α++ versus control at 22°C.
Consistent changes were evident in adipose tissue histology. As shown in Fig. 4, adipocytes were markedly enlarged in the inguinal fat of HIF-1α++ mice, but only at 22°C. There was a modest enlargement of epididymal adipocytes regardless of ambient temperature. The most pronounced enlargement of inguinal fat was also observed in a previous study of these transgenic mice, who exhibit the highest expression of HIF-1α++ in this fat depot relative to epididymal and brown fat [10]. BAT was infiltrated with white adipocytes at 30°C, but did not differ in adipocyte size between HIF-1α++ and control mice (Fig. 5). In addition, the number and diameter of BAT intracellular lipid droplets (median 4.2 μm in all groups) did not exhibit differences between genotypes. Oral glucose tolerance was impaired at 22°C in HIF-1α++ mice (Supplemental Fig. 1). Hence, adipose HIF-1α over-expression causes weight gain, expansion of primarily subcutaneous fat, hyperglycemia, and glucose intolerance but only below thermoneutrality.
Fig.4.
Representative H&E sections of epididymal and inguinal fat from HIF-1α++ and control mice housed at 22°C or 30°C, and histogram of adipocyte diameters. In epididymal adipocytes, there was a modest increase in cell size regardless of ambient temperature. In inguinal fat, adipocytes of HIF-1α++ mice were markedly enlarged, but only at 22°C.
Fig. 5.
Representative H&E sections of brown fat from HIF-1α++ and control mice housed at 22°C or 30°C. No obvious differences were observed in cell morphology other than an expected infiltration of white adipocytes at thermoneutrality.
Calorimetry, thermoregulation, and glucose tolerance in vivo
Based on these results, we hypothesized that HIF-1α++ mice would be cold sensitive and fail to increase energy expenditure in response to sub-thermoneutral temperature. Indeed, at 22°C energy expenditure (EE) was reduced in HIF-1α++ mice throughout the day, but the defect was abolished at 30°C, where EE decreased to basal levels in both groups (Fig. 6). HIF-1α++ mice also maintained a body temperature of roughly 1°C cooler when housed below thermoneutrality. Supplemental Fig. 2 demonstrates the averaged O2 and CO2 production rates of mice, revealing the temperature-dependent metabolic defect in HIF-1α++ mice. Since the body weights of mice differed at the time of calorimetry (controls: 26.2 ± 0.6 g; HIF-1α++: 29.4 ± 0.5 g, p<0.01) we also analyzed O2 and VO2 without normalizing to body weight. The effects of temperature and HIF-1α++ remained significant without normalization. During calorimetry, food consumption and activity were not statistically different between genotypes (Supplemental Fig. 3). Furthermore, HIF-1α++ mice became more hypothermic when challenged acutely in 4°C (Supplemental Fig. 4).
Fig. 6.
Energy expenditure (EE) and core body temperature of HIF-1α++ or control mice (n=5/group, individually housed) over 48 hours. Gray regions denote circadian dark (wake) phase. At 22°C, HIF-1α++ mice exhibited lower EE and body temperature (p<.001, two-way ANOVA of averaged values in Supplemental Fig. 2). At 30°C, energy expenditure decreased to basal levels in both genotypes, and differences in EE or body temperature were no longer evident. Similar results were obtained when calorimetry results were expressed per mouse, rather than per kg (Supplemental Fig. 2).
Adipocyte respiration in vitro
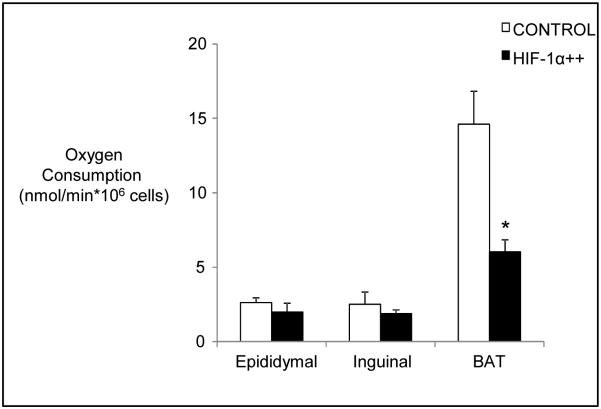
Next, we determined whether the reduced energy expenditure of HIF-1α++ mice could be detected in isolated adipocytes. As shown in Fig. 7, respiration rates of brown adipocytes was reduced by over 50% in HIF-1α++ mice versus control mice. As expected, white adipocytes exhibited markedly less oxygen consumption than brown adipocytes. However, there was no effect of HIF-1α++ on epididymal or inguinal adipocyte respiration.
Fig. 7.
Oxygen consumption in white and brown adipocytes isolated from HIF-1α++ and control mice housed at 22°C. Oxygen consumption was reduced in brown, but not white, adipocytes of HIF-1α++ mice. Oxygen consumption in brown adipocytes was over fourfold higher than in white adipocytes (p<0.001 by one-way ANOVA). *p<0.05 for HIF-1α++ versus control.
Gene expression and metabolic pathways
We assessed genes of respiration under established or potential transcriptional control by HIF-1α, including pyruvate dehydrogenase kinase (PDK), uncoupling proteins UCP1 and UCP2, Carnitine palmitoyltransferase I (CPT1), and peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1α). As seen in Fig. 8, cooler ambient temperature increased expression of BAT UCP1, PDK2, PDK4, CPT1, and PGC-1α. This pattern is consistent with more severe cold exposure or hibernation [17, 18]. PDK1 was not detectable in BAT under any conditions, consistent with previous studies localizing PDK1 chiefly to mammalian cardiac cells [19]. Unexpectedly, VEGF also decreased. Mitochondrial content was reduced by HIF-1α overexpression at 22°C, but was unaffected at 30°C.
Fig. 8.
Transcriptional changes in BAT of HIF-1α++ and control mice. By two-way ANOVA, *p<0.05 for effect of temperature; †p<0.05 for effect of HIF-1α++; #p<0.05 for HIF-1α++ versus control at 22°C.
Decreased BAT PDK4 would be expected to reduce phosphorylation of pyruvate dehydrogenase (PDH), thereby facilitating entry of pyruvate into the TCA cycle. Since this was contrary to our hypothesis, we probed for the extent of PDH phosphorylation of E1 (the primary site of inactivation by PDK’s) by western blot. PDH phosphorylation was not altered by HIF-1α overexpression (Supplemental Fig. 5). These results confirm that respiratory suppression by HIF-1α does not occur via PDH phosphorylation/inactivation.
In epididymal fat, there was no impact of HIF-1α overexpression on PDK1, PDK2, or mitochondrial content, although we observed a fall in UCP2 expression. Cooler temperature also independently decreased PGC-1α and VEGF (Fig. 9). A similar pattern of decreased UCP2 was evident in inguinal fat (Fig. 10) but no other significant changes were observed for HIF-1α overexpression.
Fig. 9.
Transcriptional changes in epididymal fat of HIF-1α++ and control mice. By two-way ANOVA, *p<0.05 for effect of temperature; †p<0.05 for effect of HIF-1α++.
Fig. 10.
Transcriptional changes in inguinal fat of HIF-1α++ and control mice. By two-way ANOVA, *p<0.05 for effect of temperature; †p<0.05 for effect of HIF-1α++.
DISCUSSION
The main finding of this study is that constitutive expression of adipose HIF-1α caused obesity, hypothermia, hyperglycemia, and glucose intolerance at cool ambient temperatures, but minimal metabolic changes at thermoneutrality. In parallel, BAT oxygen consumption and mitochondrial content were reduced, and UCP2, CPT1, and PGC1α were downregulated. In WAT we did not observe HIF-1α mediated changes in oxygen consumption, nor transcriptional changes that would be expected to impede respiration. Therefore, HIF-1α suppresses thermogenesis in BAT, causing obesity in the setting of reduced ambient temperature.
Loss and gain-of-function studies have shown that adipose HIF-1α promotes obesity. In WAT, HIF-1α is detectable under normoxic conditions [6] and increases after HFD [7, 20-22]. Increases in HIF-1α might be caused by poorly vascularized adipocytes [5]. Saturated fatty acids also stabilize WAT HIF-1α by reducing intracellular oxygen content from mitochondrial uncoupling [8]. Hyperinsulinemia, an expected response to insulin resistance from HFD, is also an agonist of HIF-1α in adipocytes [6]. Regardless of the cause of WAT HIF-1α activation, its inhibition diminished weight gain in HFD obese mice [5, 7-9, 23]. There was no effect of HIF-1α inhibition in mice fed chow diets, suggesting basal HIF-1α activity does regulate weight gain. In mice on a HFD, adipose HIF-1α knockout (using a tamoxifen inducible aP2-Cre excision model) increased VO2, lowered the respiratory exchange ratio, raised body temperature, and increased WAT mitochondrial biogenesis. HIF-1α was found to repress transcription of Sirtuin 2, a deactylase that facilitates fatty acid oxidation [5]. In terms of gain-of-function experiments, Halberg et al showed that HIF-1α++ mice were more obese than controls, with markedly hypertrophied inguinal fat pads exhibiting macrophage infiltration, fibrosis, and the upregulation of extracellular matrix proteins and lysyl oxidase[10]. By using thermoneutrality and measuring respiration in isolated white and brown adipocytes, we localized the energy defect to BAT. We confirmed that adipose HIF-1α constitutive expression predominantly enlarged subcutaneous fat, where the HIF-1α transgene is most abundantly expressed [10], but this phenomenon was abrogated by thermoneutrality. Thus, we hypothesize that hypo-metabolic consequences of BAT HIF-1α synergize with local WAT HIF-1α to promote subcutaneous obesity and metabolic dysfunction. To confirm this hypothesis, it will be necessary to specifically target HIF-1α in BAT or WAT.
A more complex picture of HIF-1α’s role in BAT emerges considering findings of two other studies. First, Zhang et al. showed that inhibition of adipose HIF-1α (using transgenic dominant negative expression) impaired BAT thermogenesis and fatty acid oxidation, downregulated oxidative pathways, and caused loss of mitochondria[24]. Authors ascribed these surprising findings to loss of HIF-1α dependent BAT vascularization. Of note, HIF-1α inhibition in their model reduced PDK expression, potentially increasing oxidative phosphorylation, while the buildup of acetyl-coA and NADH might impair fatty acid oxidation. Second, in the aforementioned study showing amelioration of HFD-induced obesity by adipose HIF-1α knockout [5] authors went on to specifically knock out BAT HIF-1α (using UCP1-Cre excision). Interestingly, loss of BAT HIF-1α by this method did not affect weight gain. Apparently, HIF-1α has a modest role in BAT under physiological conditions, while high and low extremes of HIF-1α activity, at least under normoxic conditions, impair thermogenesis.
Guided by prior studies [25], we examined pathways by which HIF-1α might impair BAT respiration. We first examined the impact of HIF-1α on PDK isoforms, since HIF-1α has been shown to suppress oxygen consumption in hypoxic cells via increases in PDK [26]. PDKs phosphorylate and inactivate PDH, inhibiting pyruvate entry into the TCA cycle. In BAT, cooler ambient temperature increased PDK2 and PDK4, and increased PDH phosphorylation reducing its activity, consistent with reduced oxygen consumption observed in hibernating mammals [17, 18]. Interestingly, HIF-1α++ decreased BAT PDK4 transcription and did not alter PDH phosphorylation, exonerating this as the main pathway for the suppression of respiration. This was surprising, given that HIF-1α is necessary for PDK1 upregulation and reduced respiration during hypoxia in renal cell carcinoma cells [25]. Perhaps these divergent results are explained by the method of HIF-1α manipulation (in vivo hypoxia induction, versus transgenic expression), cell type, or the presence of normoxic conditions in this present study. Second, we hypothesized that HIF-1α++ might inhibit the expression of uncoupling proteins. HIF-1α++ suppressed transcription of UCP1 at 30°C, but cold stimulation of UCP1 at 22°C was not affected. This suggests that there is not a direct interaction of HIF-1α with UCP1 and that suppression of respiration in BAT occurs through alternative pathways. Furthermore, since UCP1 and HIF-1α are both upregulated by norepinephrine in BAT [27, 28], these findings suggest opposing roles of these molecules under conditions of sympathetic stimulation. Interestingly, HIF-1α++ lowered UCP2, an uncoupling protein with roles in reactive oxygen species scavenging and an unclear role in thermogenesis [29, 30]. Third, we examined BAT CPT1β, which transports fatty acids from the cytosol to the inner mitochondrial membrane, the rate-limiting step of beta-oxidation [31]. Similar to other reports [32] cooler housing increased BAT CPT1; there was a trend towards inhibition by HIF-1α. Finally, we examined mitochondrial DNA content and expression of the central inducer of mitochondrial biogenesis, PGC-1α. PGC-1α was first discovered in BAT, where it dramatically increased in response to cold leading to increases in PPAR-gamma and mitochondrial DNA [33, 34]. Mitochondrial DNA and PGC-1α were significantly decreased by HIF-1α over-expression. Interestingly, HIF-1α lowered BAT mitochondria DNA to a level similar to that of thermoneutral conditions. HIF-1α has been shown to inhibit mitochondrial biogenesis [35-37] and induce autophagy via induction of BNIP3 [16] and BNIP3L [38]. In addition, HFD-associated HIF-1α activation inhibited WAT fatty acid oxidation, via PGC-1α [5]. Hence, BAT HIF-1α inhibits respiration by several potentially overlapping pathways including reductions in mitochondria, PGC-1α, CPT, and UCP2.
The complex pattern of transcriptional changes induced by HIF-1α in this study point to cross-talk between redundant cellular oxygen sensing mechanisms. The reduced mitochondrial mass in BAT may increase intracellular oxygen levels (as observed in other contexts of mitochondrial inhibition [39]). Increased oxygen availability reduces PGC-1α [40] [41] potentially via 5’-AMP-activated protein kinase (AMPK) [42] which in turn reduces transcription of VEGF [43] and CPT1 [44]. In addition, accumulations of pyruvate and reductions in acetyl coA serve as feedback signals to reduce PDK transcription [45]. Hence, the direct effects of HIF-1α on PDK and VEGF transcription could be overridden by the indirect consequences of increasing intracellular oxygen (schematic proposed in Supplemental Fig. 6).
In mammals, cold upregulates thermogenic capacity of BAT, facilitated by increases in UCP1, sympathetic innervation, and adrenergic receptors. The increase in UCP1 is dependent upon adrenergic-cAMP signaling and enhanced by interactions of PGC-1α with PPAR-gamma [28]. Brown adipocytes may also have evolved mechanisms to reduce oxygen consumption to facilitate hibernation and torpor, in which HIF-1α may play a role. HIF-1α mRNA is upregulated in brown adipocytes by norepinephrine [27] while HIF-1α protein and binding activity greatly increased in BAT of hibernating mammals [46] via a post-translational, hypoxia-independent pathway [47]. Interestingly, early cold adaptation (<7 days) increased BAT HIF-1α while levels of proteins involved in oxidative phosphorylation, glycolysis, and beta oxidation were reduced. Later, as shivering is replaced by non-shivering thermogenesis [48], BAT HIF-1α decreased and oxidative pathways were restored [49]. Since BAT is a major plasma lipid-clearing organ in rodents [50], its inactivation led to several metabolic defects including obesity, increased triglycerides, and impaired glucose homeostasis. It is not clear why the inguinal (subcutaneous) depot in particular was more prone to hypertrophy in this study without an obvious defect in the oxygen consumption of these adipocytes. Subcutaneous fat has a much higher thermogenic potential than visceral fat, so it is possible that HIF-1α inhibited “browning” of this region without altering its basal respiration.
There are several limitations to this study. First, we utilized animals with levels of adipose HIF-1α that might not occur under physiological conditions, particularly in BAT. Constitutive expression of HIF-1α under these non-physiologic conditions may alter the interplay between HIF-1α and other oxygen sensors such as PGC-1α, as described above. Second, we could not clearly distinguish between local and systemic effects of HIF-1α in each adipose depot. However, we have determined that BAT is the relevant site for reduced EE and obesity in the HIF-1α++ model. Third, we did not determine the precise pathway by which HIF-1α reduced BAT respiration. Modifications to the diet of the mice and culture media in which oxygen consumption was measured might have revealed differential effects of BAT HIF-1α on glucose and lipid oxidation. As these assays require relatively large numbers of adipocytes, we plan these experiments in a subsequent analysis.
In summary, adipose HIF-1α upregulation induces obesity in a temperature dependent manner. The obesity results from the suppression of thermogenesis in BAT, in association with reduced BAT mitochondria. Future studies of HIF-1α should consider potential interactions of this molecule with ambient temperature and its physiologic role in mammalian temperature regulation.
Supplementary Material
Key Messages.
Constitutive HIF-1α activation in adipose tissue promotes weight gain in mice.
The weight gain is associated with reduced brown adipose tissue function and oxygen consumption.
Reduced oxygen consumption may be mediated by reductions in mitochondria.
Acknowledgments
Funding Sources:
J. Jun: National Institutes of Health 1K08-HL109475
V. Polotsky: National Institutes of Health R01-HL080105, R01-HL128970, R01-HL133100, P50-ES018176, American Sleep Foundation 133-BS-15
P. Scherer: National Institutes of Health R01-DK55758; P01-DK088761; R01-DK099110
REFERENCES CITED
- 1.Semenza GL, Shimoda LA, Prabhakar NR. Regulation of gene expression by HIF-1. NovartisFoundSymp. 2006;272:2–8. [PubMed] [Google Scholar]
- 2.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza GL. Transcriptional regulation by hypoxia-inducible factor 1 molecular mechanisms of oxygen homeostasis. Trends Cardiovasc Med. 1996;6:151–157. doi: 10.1016/1050-1738(96)00039-4. [DOI] [PubMed] [Google Scholar]
- 4.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan J, Danzer C, Simka T, Ukropec J, Walter KM, Kumpf S, Mirtschink P, Ukropcova B, Gasperikova D, Pedrazzini T, et al. Dietary obesity-associated Hif1alpha activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev. 2012;26:259–270. doi: 10.1101/gad.180406.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1{alpha} activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab. 2011;300:E877–885. doi: 10.1152/ajpendo.00626.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Mol Cell Biol. 2013;33:904–917. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YS, Kim JW, Osborne O, Oh da Y, Sasik R, Schenk S, Chen A, Chung H, Murphy A, Watkins SM, et al. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell. 2014;157:1339–1352. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin MK, Drager LF, Yao Q, Bevans-Fonti S, Yoo DY, Jun JC, Aja S, Bhanot S, Polotsky VY. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1alpha. PLoS One. 2012;7:e46562. doi: 10.1371/journal.pone.0046562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drager LF, Yao Q, Hernandez KL, Shin MK, Bevans-Fonti S, Gay J, Sussan TE, Jun JC, Myers AC, Olivecrona G, et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med. 2013;188:240–248. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maloney SK, Fuller A, Mitchell D, Gordon C, Overton JM. Translating Animal Model Research: Does It Matter That Our Rodents Are Cold? Physiology (Bethesda) 2014;29:413–420. doi: 10.1152/physiol.00029.2014. [DOI] [PubMed] [Google Scholar]
- 13.Uchida K, Shiuchi T, Inada H, Minokoshi Y, Tominaga M. Metabolic adaptation of mice in a cool environment. Pflugers Arch. 2010;459:765–774. doi: 10.1007/s00424-010-0795-3. [DOI] [PubMed] [Google Scholar]
- 14.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. JExpBiol. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 15.Jun JC, Shin MK, Yao Q, Devera R, Fonti-Bevans S, Polotsky VY. Thermoneutrality modifies the impact of hypoxia on lipid metabolism. Am J Physiol Endocrinol Metab. 2013;304:E424–435. doi: 10.1152/ajpendo.00515.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Hao Q, Yadav R, Basse AL, Petersen S, Sonne SB, Rasmussen S, Zhu Q, Lu Z, Wang J, Audouze K, et al. Transcriptome profiling of brown adipose tissue during cold exposure reveals extensive regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2015;308:E380–392. doi: 10.1152/ajpendo.00277.2014. [DOI] [PubMed] [Google Scholar]
- 18.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 19.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329:191–196. doi: 10.1042/bj3290191. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 21.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 22.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 23.Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 2011;60:2484–2495. doi: 10.2337/db11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Lam KS, Ye H, Chung SK, Zhou M, Wang Y, Xu A. Adipose tissue-specific inhibition of hypoxia-inducible factor 1{alpha} induces obesity and glucose intolerance by impeding energy expenditure in mice. J Biol Chem. 2010;285:32869–32877. doi: 10.1074/jbc.M110.135509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813:1263–1268. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Nikami H, Nedergaard J, Fredriksson JM. Norepinephrine but not hypoxia stimulates HIF-1alpha gene expression in brown adipocytes. Biochem Biophys Res Commun. 2005;337:121–126. doi: 10.1016/j.bbrc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Klingenspor M. Cold-induced recruitment of brown adipose tissue thermogenesis. Exp Physiol. 2003;88:141–148. doi: 10.1113/eph8802508. [DOI] [PubMed] [Google Scholar]
- 29.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 30.Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki N, Yamanaka Y, Hashimoto Y, Shinohara Y, Shima A, Terada H. Structural features of the gene encoding human muscle type carnitine palmitoyltransferase I. FEBS Lett. 1997;409:401–406. doi: 10.1016/s0014-5793(97)00561-9. [DOI] [PubMed] [Google Scholar]
- 32.Eddy SF, Morin P, Jr., Storey KB. Differential expression of selected mitochondrial genes in hibernating little brown bats, Myotis lucifugus. J Exp Zool A Comp Exp Biol. 2006;305:620–630. doi: 10.1002/jez.a.294. [DOI] [PubMed] [Google Scholar]
- 33.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 34.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Mason SD, Rundqvist H, Papandreou I, Duh R, McNulty WJ, Howlett RA, Olfert IM, Sundberg CJ, Denko NC, Poellinger L, et al. HIF-1alpha in endurance training: suppression of oxidative metabolism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2059–2069. doi: 10.1152/ajpregu.00335.2007. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagen T. Oxygen versus Reactive Oxygen in the Regulation of HIF-1alpha: The Balance Tips. Biochem Res Int. 2012;2012:436981. doi: 10.1155/2012/436981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Hagan KA, Cocchiglia S, Zhdanov AV, Tambuwala MM, Cummins EP, Monfared M, Agbor TA, Garvey JF, Papkovsky DB, Taylor CT, et al. PGC-1alpha is coupled to HIF-1alpha-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc Natl Acad Sci U S A. 2009;106:2188–2193. doi: 10.1073/pnas.0808801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu L, Wang Q, Zhang L, Fang Z, Zhao F, Lv Z, Gu Z, Zhang J, Wang J, Zen K, et al. Hypoxia induces PGC-1alpha expression and mitochondrial biogenesis in the myocardium of TOF patients. Cell Res. 2010;20:676–687. doi: 10.1038/cr.2010.46. [DOI] [PubMed] [Google Scholar]
- 42.Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5'-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 44.Louet JF, Hayhurst G, Gonzalez FJ, Girard J, Decaux JF. The coactivator PGC-1 is involved in the regulation of the liver carnitine palmitoyltransferase I gene expression by cAMP in combination with HNF4 alpha and cAMP-response element-binding protein (CREB) J Biol Chem. 2002;277:37991–38000. doi: 10.1074/jbc.M205087200. [DOI] [PubMed] [Google Scholar]
- 45.Sugden MC, Holness MJ. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch Physiol Biochem. 2006;112:139–149. doi: 10.1080/13813450600935263. [DOI] [PubMed] [Google Scholar]
- 46.Morin P, Jr., Storey KB. Cloning and expression of hypoxia-inducible factor 1alpha from the hibernating ground squirrel, Spermophilus tridecemlineatus. Biochim Biophys Acta. 2005;1729:32–40. doi: 10.1016/j.bbaexp.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Maistrovski Y, Biggar KK, Storey KB. HIF-1alpha regulation in mammalian hibernators: role of non-coding RNA in HIF-1alpha control during torpor in ground squirrels and bats. J Comp Physiol B. 2012;182:849–859. doi: 10.1007/s00360-012-0662-y. [DOI] [PubMed] [Google Scholar]
- 48.Jansky L, Hart JS. Cardiac output and organ blood flow in warm- and cold-acclimated rats exposed to cold. Can J Physiol Pharmacol. 1968;46:653–659. doi: 10.1139/y68-096. [DOI] [PubMed] [Google Scholar]
- 49.Vucetic M, Otasevic V, Korac A, Stancic A, Jankovic A, Markelic M, Golic I, Velickovic K, Buzadzic B, Korac B. Interscapular brown adipose tissue metabolic reprogramming during cold acclimation: Interplay of HIF-1alpha and AMPKalpha. Biochim Biophys Acta. 2011;1810:1252–1261. doi: 10.1016/j.bbagen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. Brown adipose tissue activity controls triglyceride clearance. NatMed. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.